Abstract
Heterocyclic aromatic amines (HCAA) are listed by the US Food and Drug Administration (FDA) as harmful or potentially harmful constituents of tobacco smoke. However, quantifying HCAA exposure is challenging. In this study, we developed a sensitive, precise and accurate isotope dilution, liquid chromatography-tandem mass spectrometry (LC-MS/MS) method to quantify urinary HCAAs in smokers and nonsmokers. The high throughput robotic sample preparation system could handle a throughput of over 300 samples per day, while maintaining intra-day and inter-day imprecision and bias ≤10%. The limits of detection of carcinogenic HCAAs ranged from 0.31-0.83 pg/mL. The validated method was applied to measure HCAAs in urine collected from smokers and non-smokers. This sensitive and efficient analytical method is ideal to support large-scale biomonitoring studies of HCAA exposure.
Keywords: heterocyclic aromatic amines, urine, automation, high throughput, LC-MS/MS
Introduction
Tobacco use is the single largest preventable cause of illness and death in the United States.[1] Heterocyclic aromatic amines (HCAAs) comprise an important class of carcinogens formed during combustion processes (e.g. smoking tobacco), as well as cooking meats at high temperature (see Electronic Supplementary Material Figure S-1).[2-4] The International Agency for Research on Cancer (IARC) lists a number of HCAAs as possible and probable carcinogens, including 2-amino-9H-pyrido[2,3-b] indole (AαC), 2-amino-3-methyl-9H-pyrido[2,3-b] indole (MeAαC), 3-amino-1, 4-dimethyl-5H-pyrido [4,3-b ]indole (Trp-P-1), 3-amino-1-methyl-5H-pyrido [4,3-b] indole (Trp-P-2), 2-amino-6-methyldipyrido[1,2-a:3′,2′-d]imidazole (Glu-P-1), 2-aminodipyrido[1,2-a:3′,2-d]imidazole (Glu-P-2), 2-amino-3-methyl-3H-imidazo[4,5-f]quinolone (IQ) and 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP).[5] Both toxicological and epidemiological studies link HCAA exposure with elevated cancer risk.[6-10] Two beta-carboline HCAAs, 1-Methyl-9H-pyrido[3,4-b]indole (harman) and 9H-Pyrido[3,4-b]indole (norharman) are the most abundant HCAAs in cigarette smoke condensate.[2, 11] Additionally, harman and norhaman may modulate nicotine action.[12] Because of their associated health risks, it is important to assess exposure to both potentially carcinogenic HCAAs, as well as harman and norharman.
HCAAs are excreted in urine, which, owing to its noninvasive collection, is a convenient biofluid for large-scale epidemiological investigation of HCAA exposure.[13-16] However, measurement of HCAAs in urine is challenging, as their concentrations are often low. Fluorescence and ultraviolet methods were insufficiently sensitive to detect HCAAs at ppt levels.[14, 17] Gas chromatography mass spectrometry (GC/MS) significantly lowers the limit of detection (LOD) for HCAAs, but requires complex derivatization and cleanup, which reduce analytical throughput.[16] Although LC-MS/MS avoids the difficulties of derivatization and achieves a LOD in low pg/mL levels,[15, 18, 19] previously reported methods are insufficiently sensitive to detect MeAαC in smokers, which is roughly tenfold lower than AαC in cigarette smoke condensate.[2, 20, 21] Another challenge for analysis of urinary HCAAs is the labor intensive and time consuming nature of previously reported sample preparation methods, a factor that significantly reduces analytical throughput.[15, 16, 18, 22-24] To satisfy the requirement of large-scale studies, we developed and validated a sensitive, precise and accurate method that uses isotope dilution LC-MS/MS for simultaneous analysis of structurally diverse HCAAs in human urine samples. The use of a robotic automation system substantially speeded sample preparation and improved throughput, while reducing human errors. Overall, this method is robust, efficient and labor-saving for urinary HCAA analysis.
Experimental Procedures
Materials
Harman, Norharman, AαC, MeAαC, Trp-P-1, Trp-P-2, Glu-P-1, Glu-P-2, IQ, PhIP and their corresponding isotope-labeled analogs—Harman-13C2, 15N; Norharman-D7; AαC-15N3; MeAαC-D3; Trp-P-1-13C2,15N; Trp-P-2-13C2,15N; Glu-P-1-13C3; Glu-P-2-13C2,15N; IQ-D3; and PhIP-D3—were purchased from Toronto Research Chemicals Inc. (North York, ON, Canada). LC/MS grade acetonitrile (ACN) from Fisher Scientific (Pittsburgh, PA, USA) and LC/MS grade water from J.T. Baker (Center Valley, PA, USA) were used for mobile phase preparation. LC/MS grade ammonium hydroxide from Sigma-Aldrich (St. Louis, MO, USA) was used for pH adjustment. All other reagents were at least high performance liquid chromatography (HPLC) grade. Ninety-six-well plates of ISOLUTE SLE+ (supported liquid extraction) and EVOLUTE® CX (mix mode of cation exchange resin, CX) for sample preparation were purchased from Biotage Inc (Charlotte, NC, USA).
Instrumentation
LC–MS/MS
Samples were analyzed using Shimadzu LC-30AD HPLC module (Columbia, MD, USA) in tandem with AB Sciex API 5500 QTRAP system (Framingham, MA, USA). Chromatographic separation was conducted on a reversed phase column (Agilent Zorbax Eclipse Plus C18, 2.1×100 mm 3.5 μm; CA, USA). Sample was eluted by a linear gradient of mobile phase A (0.05% ammonium hydroxide in H2O, v/v) and mobile phase B (100% ACN) at a flow rate of 0.4 mL/min. Mobile phase B was increased from 5 to 36% (v/v) from 0-5 min, kept at 36% over 1.5 min, changed from 36 to 98% over 1 min, and kept at 98% for 2.5 min, then changed back to 5% in 0.01 min and kept at equilibration for 2 min. Post-column infusion of ACN at 0.2 mL/min was used to increase the signal response of HCAAs. The mass spectrometric analysis was carried out in the positive ion mode with the following parameters: IonSpray voltage at 2000 V, source heater temperature at 650 °C, curtain gas at 35 psi, ion source gas 1 at 60 psi, ion source gas 2 at 70 psi, and collision gas at high level. The compound dependent parameters are summarized in Table 1. All LC-MS/MS data were recorded at unit mass resolution in multiple reaction monitoring (MRM) mode. Scheduled MRM algorithm acquisition method was also applied in analysis. Analyst® software 1.6 (Applied Biosystems) was used for operation of the LC–MS/MS system.
Table 1. Compound dependent MS parameters and ion transitions for HCAA analysis.a.
| Precursor ions (m/z) | Product ions (m/z) | DPb (V) | EPc (V) | CEd (V) | CXPe (V) | ||
|---|---|---|---|---|---|---|---|
| Harman | Quantitation ion | 183.1 | 115.0 | 260 | 10 | 68 | 14 |
| Confirmation ion | 183.1 | 88.9 | 260 | 10 | 63 | 14 | |
| Internal standard ion | 186.1 | 117.1 | 260 | 10 | 68 | 14 | |
| Norharman | Quantitation ion | 169.1 | 115.1 | 170 | 10 | 82 | 14 |
| Confirmation ion | 169.1 | 88.9 | 170 | 10 | 105 | 10 | |
| Internal standard ion | 176.2 | 120.0 | 170 | 10 | 82 | 14 | |
| AαC | Quantitation ion | 184.1 | 167.1 | 51 | 10 | 31 | 22 |
| Confirmation ion | 184.1 | 140.0 | 51 | 10 | 43 | 20 | |
| Internal standard ion | 187.0 | 169.1 | 51 | 10 | 31 | 22 | |
| MeAαC | Quantitation ion | 198.1 | 181.1 | 36 | 10 | 31 | 16 |
| Confirmation ion | 198.1 | 127.1 | 36 | 10 | 53 | 12 | |
| Internal standard ion | 201.1 | 184.1 | 36 | 10 | 31 | 16 | |
| IQ | Quantitation ion | 199.1 | 184.1 | 56 | 10 | 35 | 18 |
| Confirmation ion | 199.1 | 157.1 | 56 | 10 | 47 | 12 | |
| Internal standard ion | 202.2 | 184.2 | 56 | 10 | 35 | 18 | |
| Trp-P-1 | Quantitation ion | 212.1 | 167.1 | 41 | 10 | 55 | 16 |
| Confirmation ion | 212.1 | 195.0 | 41 | 10 | 32 | 16 | |
| Internal standard ion | 215.1 | 168.0 | 41 | 10 | 55 | 16 | |
| Trp-P-2 | Quantitation ion | 198.1 | 181.1 | 36 | 10 | 31 | 16 |
| Confirmation ion | 198.1 | 127.0 | 36 | 10 | 53 | 12 | |
| Internal standard ion | 201.1 | 183.1 | 36 | 10 | 31 | 16 | |
| Glu-P-1 | Quantitation ion | 199.1 | 92.0 | 121 | 10 | 45 | 12 |
| Confirmation ion | 199.1 | 172.1 | 121 | 10 | 34 | 12 | |
| Internal standard ion | 202.0 | 92.0 | 121 | 10 | 45 | 12 | |
| Glu-P-2 | Quantitation ion | 185.1 | 168.1 | 126 | 10 | 35 | 10 |
| Confirmation ion | 185.1 | 78.0 | 126 | 10 | 49 | 10 | |
| Internal standard ion | 188.1 | 171.1 | 126 | 10 | 35 | 10 | |
| PhIP | Quantitation ion | 225.1 | 210.1 | 111 | 10 | 39 | 28 |
| Confirmation ion | 225.1 | 115.0 | 111 | 10 | 61 | 10 | |
| Internal standard ion | 228.2 | 210.1 | 111 | 10 | 39 | 28 |
Parameters for Harman and Norharman have been detuned to avoid saturating detector.
Declustering potential.
Entrance potential.
Collision energy.
Collision cell exit potential.
High throughput robotic automation system for sample preparation
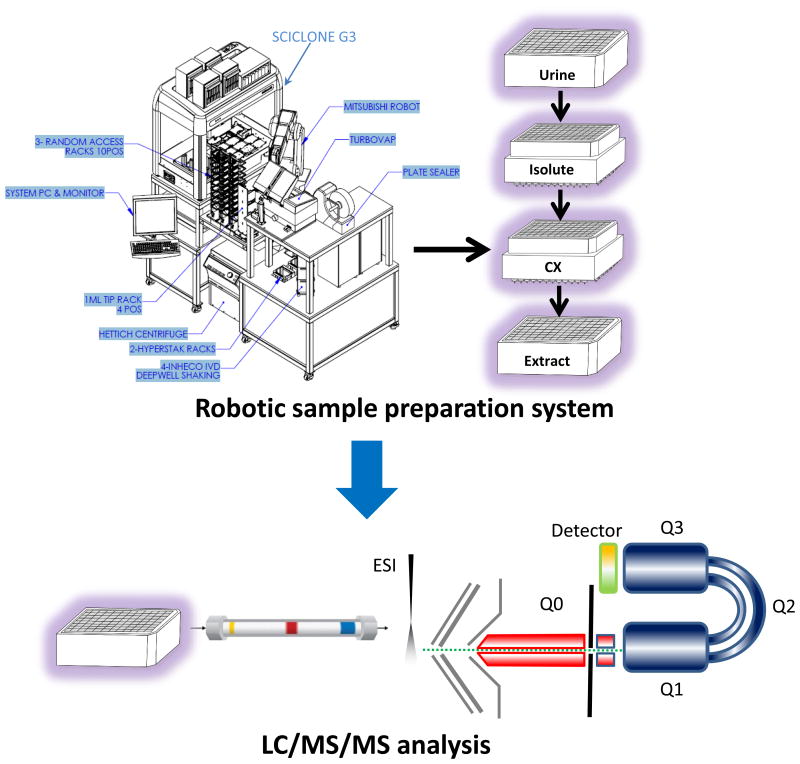
Sample dispensing and tube–to–96-well plate reformatting were conducted on Microlab Star Liquid Handling Workstation from Hamilton Robotics (Reno, NV, USA). The Perkin Elmer Staccato system for sample preparation was purchased from PerkinElmer (Boston, MA, USA). The Staccato automation system consisted of a Caliper SciClone G3 Automated Liquid Handing Workstation, Mitsubishi S Series Melfa RV-6SDL Industrial Robot, BIOTAGE Turbovap 96 Automated Evaporation system, FluidX Impression Enhanced Rack Scanner, Thermo Scientific ALPS 3000 sealer, Hettich Gmbh & Co. KG Rotanta 460 Robotic centrifuge, and FluidX Capping/Decapping System (Figure 1).
Figure 1.
High throughput robotic sample preparation system coupled with LC-MS/MS.
Preparation of standard solutions
Individual stock solutions of native and isotopically-labeled HCAAs were prepared by accurately weighing and dissolving each pure standard in methanol. Calibration curve standards at 11 concentration levels were prepared by serial dilution and mixing of stock solutions in 10% methanol in H2O containing 0.1% formic acid (Table 2). Aliquots of 10 μL of each calibration standard were injected in the LC-MS/MS for analysis. Calibration curves were constructed by plotting the peak area ratios of the standards and IS against the concentrations ratio of standards and IS using weighted linear regression (weight = 1/X). Only values within the linear range of the assay (Table 2) were reported.
Table 2. Minimal matrix effects on calibration curves of HCAA prepared in urine.
| Water calibration curves on-column (pg) | Urine calibration curves (pg/ml) | IS conc. in urine (pg/ml) | Calibration curves | R2 | Difference in slopes % | |
|---|---|---|---|---|---|---|
| AαC | 0.098-19.7 | 0.98-197 | 50 | Water: y=0.984x+0.00167 | 0.999 | 2.6 |
| Urine: y=1.01x+0.00394 | ||||||
| MeAαC | 0.1-20 | 1-200 | 50 | Water: y=0.857x+0.000561 | 0.999 | 1.7 |
| Urine: y=0.872x+0.000126 | ||||||
| IQ | 0.1-20 | 1-200 | 30 | Water: y=1.24x+0.00386 | 0.999 | 0.8 |
| Urine: y=1.25x+0.00787 | ||||||
| Trp-P-1 | 0.078-15.6 | 0.78-156 | 39 | Water: y=0.984x+0.00153 | 0.999 | 0.5 |
| Urine: y=0.989x+0.000628 | ||||||
| Trp-P-2 | 0.067-13.5 | 0.67-135 | 38.4 | Water: y=0.923x+0.00158 | 1.000 | 3.3 |
| Urine: y=0.954x+0.0012 | ||||||
| Glu-P-1 | 0.073-14.7 | 0.73-147 | 78.4 | Water: y=1.22x+0.00152 | 1.000 | 0.81 |
| Urine: y=1.23x+0.00282 | ||||||
| Glu-P-2 | 0.066-13.3 | 0.66-133 | 107.4 | Water: y=1.10x+0.00035 | 1.000 | 0.92 |
| Urine: y=1.09x+0.00428 | ||||||
| PhIP | 0.1-20 | 1-200 | 25 | Water: y=1.04x+0.0216 | 0.998 | 2.8 |
| Urine: y=1.07x+0.00116 | ||||||
| Harman | 1-400 | 10 -4000 | 300 | Water: y=1.14 x+0.00458 | 1.000 | 2.6 |
| Urine: y=1.17x+4.05 | ||||||
| Norharman | 1-400 | 10 -4000 | 300 | Water: y=1.43x+0.00875 | 0.999 | 0.7 |
| Urine: y=1.42x+2.03 | ||||||
Sample preparation
Sample preparation was conducted on a fully automated and integrated robotic system without the need of manual intervention. Aliquots of 50 μL of isotopically-labelled solution containing 150, 150, 25, 25, 19.5, 19.2, 39.2, 53.7, 15, 12.5 pg of harman, norharman, AαC, MeAαC, Trp-P-1, Trp-P-2, Glu-P-1, Glu-P-2, IQ, PhIP, respectively were spiked into 0.5 mL of urine samples in a polypropylene 96-well plate. The urine samples were then basified by 50 μL of 10 N NaOH. To measure total urinary HCAAs—including free and conjugated forms—basified urine samples were hydrolyzed at 70 °C for 5 hours, followed by two-step solid phase extraction.[15] Because the recommended capacity of each well of 96-well Isolute SLE+ plate is 0.4 mL, which is less than our sample volume (0.5 mL specimen plus 50 μL of IS and 50 μL of NaOH), each basified urine sample was split into two equal portions and dispensed into two adjacent wells in the 96-well Isolute SLE+ plate. Each split sample was then recombined by elution into a single well of a 48 deep well plate before proceeding to the EVOLUTE® CX SPE step. After transferring samples into Isolute SLE+ plates, positive pressure of 1 psi was applied on Isolute SLE+ plate for 20 seconds to initiate sample loading. Four minutes were allowed for the urine sample to be absorbed by Isolute SLE+ resin. Then, 2.25 mL of dichloromethane (DCM) was added to each well of the SLE+ plate, and HCAAs were eluted by gravity. The collected DCM eluent was acidified by addition of 40 μL of formic acid. The 96-well EVOLUTE® CX plate was pre-washed with 5% ammonia hydroxide in methanol (1 mL/well) and conditioned sequentially with methanol (1 mL/well) and 2% formic acid in methanol (1 mL/well). Acidified DCM eluent from Isolute SLE+ plate was loaded onto conditioned EVOLUTE® CX plate by gravity. EVOLUTE® CX plate was washed sequentially with 2% formic acid in methanol (1 mL/well), water (1 mL/well) and 30% methanol in water containing 2% ammonia hydroxide (1 mL/well). Positive pressure of 3 psi was applied at each wash for 2 min. EVOLUTE® CX plate was then dried under nitrogen flow of 30 psi for 10 min. Analytes were eluted from EVOLUTE® CX plate by 2 × 0.5 mL of 5% ammonia hydroxide in methanol under gravity for 10 min and then at a positive pressure of 1 psi for 3 min. The eluent was collected into a new polypropylene 96-well plate, followed by complete drying under nitrogen. The residue was reconstituted with 50 μL of 10% methanol in water containing 0.1% formic acid (v/v), followed by thorough vortex mixing. An aliquot of 10 μL was injected into the LC-MS/MS for analysis (Figure 1).
Method validation
Precision and accuracy
Urine samples containing low, medium or high levels of HCAAs were prepared by spiking blank urine with different levels of HCAA standard solutions. The intra-day precision and accuracy were evaluated by analyzing replicates of HCAA-spiked urine samples at three different levels within one day. The inter-day precision and accuracy were evaluated by analyzing the spiked urine samples at three different levels on three to six separate days. The intra-day and inter-day precision were calculated as the relative standard deviation (RSD). Accuracy was calculated using the equation: (determined value - nominal value)/nominal value ×100%.
Sensitivity
The LOD and limit of quantification (LOQ) for all HCAAs were calculated based on the extrapolated standard deviation at zero concentration.[25] Four to five spiked urine pools at low concentrations (see Electronic Supplementary Material Table S-1) were prepared and analyzed repeatedly on different days (N>34). The standard deviation of each pool was plotted against the concentration. S0 is the estimated standard deviation when the urinary concentration is extrapolated to zero concentration. HCAA LODs and LOQs were 3 times and 10 times S0, respectively. Because endogenous urinary harman and norharman in human urine are significantly higher than the LOD and LOQ of the present method, estimation of LOD and LOQ for harman and norharman was conducted by using synthetic urine (Ricca Chemical Company, Arlington, TX).
Matrix effect
Calibration curves of HCAAs in urine matrix were prepared by spiking standards of HCAAs at different levels in blank urine matrix (Table 2). Equivalent on-column amounts of HCAAs in water calibration curves were injected for the LC-MS/MS analysis. The calibration curves built in urine matrix after sample extraction were run in parallel, with calibration curves built in water matrix. The averages of the slopes of the calibration curves for urine matrix and water matrix were compared to assess the influence of matrix effect on the curves. Ion suppression or enhancement was estimated by comparing the peak area of un-extracted isotopically-labeled IS dissolved in extract of blank urine with the peak areas of an equivalent amount of IS dissolved in water matrix.
Extraction recovery
Amounts of 150, 150, 25, 25, 19.5, 19.2, 39.2, 53.7, 15, 12.5 pg of isotopically-labeled harman, norharman, AαC, MeAαC, Trp-P-1, Trp-P-2, Glu-P-1, Glu-P-2, IQ, PhIP, respectively, were spiked into 0.5 mL of urine samples. The urine samples were extracted by following the sample preparation procedure described above. Extraction recovery for each analyte was calculated by comparing the peak areas of isotopically-labeled internal standard in extracted urine samples with that of equivalent amounts of the un-extracted internal standard solutions dissolved in the extract of blank urine.
Stability of spiked urine samples at low, medium and high levels
Freeze–thaw (-70 °C–room temperature) stability was evaluated by measuring spiked urine samples at high and low concentrations subjected to four freeze–thaw cycles before sample preparation. Stability at room temperature was evaluated by maintaining spiked urine samples at high and low concentrations at room temperature for 24 h before sample preparation. The stability of samples in auto-sampler was evaluated by analyzing extracted samples at high and low concentrations after being placed in the auto-sampler at 15 °C for 24 h.
Method application
This method was applied to the analysis of HCAAs in urine samples from 28 nonsmokers and 48 smokers who had been exposed to mainstream cigarette smoke. Spot urine samples of all anonymous smoker and nonsmoker were collected by Tennessee Blood Services (Memphis, TN, USA) with Institutional Review Board (IRB) approval. No dietary restrictions were applied on participants. See Electronic Supplementary Material Table S-2 for the detail information.
Results and Discussion
Robotic automation system for sample preparation
A number of HCAAs are classified as possible and probable carcinogens by IARC.[5] Large-scale population biomonitoring studies could provide valuable information about human exposure to HCAAs from various sources, such as inhaled tobacco smoke and ingested well-done meats. However, the low sensitivity and throughput of previous analytical methods for measurement of urinary HCAAs have been major obstacles for large-scale epidemiologic studies. Recently, automatic, on-line, in-tube, solid-phase microextraction (SPME) was used to increase the throughput of urinary HCAA analysis.[26] Compared with previously reported manual methods, SPME significantly improved analytical throughput, but at the cost of higher background noise in the chromatogram.[26] In the present study, a two-step SPE with multiple intensive washing procedures was used to minimize chromatographic background noise from matrix. At the same time, we enhanced throughput via use of a robotic automation system (Perkin Elmer Staccato system) for sample preparation and increased the sensitivity by using API 5500 QTRAP LC-MS/MS for sample analysis. The automated sample preparation system could process over 300 urine samples per day unattended, and thus meet the throughput needs of large-scale epidemiology studies.
Chromatography optimization
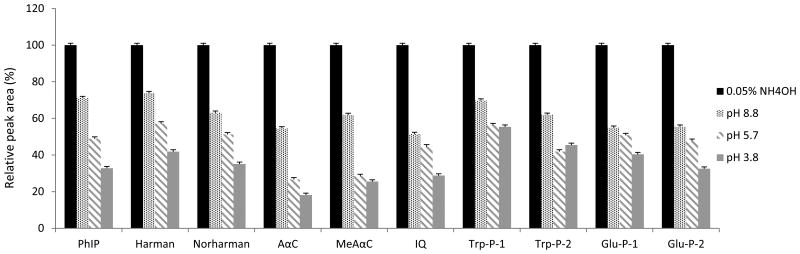
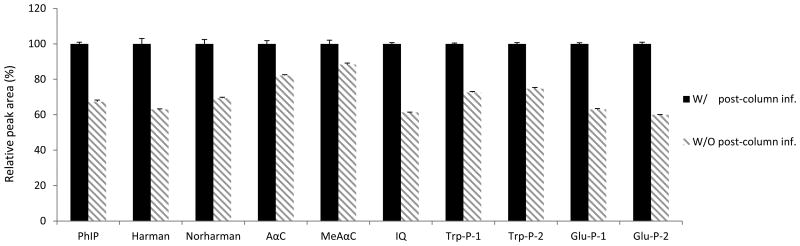
The selection of appropriate HPLC columns and mobile phase are critical to HCAA analysis. Among different type of columns, Agilent Zorbax Eclipse Plus C18 2.1×100 mm 3.5 μm gave the best peak shapes and minimal column adsorption for most HCAAs (see Electronic Supplementary Material Figure S-2). HCAAs are weak bases, and the chromatography could be substantially influenced by the pH of the mobile phase. Optimal chromatography, in terms of separation, retention, and peak shapes, was obtained by using 0.05% NH4OH in water (pH 10.5) as aqueous phase. The signal response of the targeted HCAAs was further enhanced at base mobile phase (Figure 2). Post-column infusion of ACN also improved the signal-to-noise ratio for the targeted HCAAs (Figure 3).
Figure 2.
Effects of pH of mobile phases on the signal responses of HCAAs. HCAA standards (on-column amount of 10 pg) were applied for LC-MS/MS analysis. The gradient program was run as described in the experimental section. Four types of mobile phase A (5 mM ammonium formate at pH 3.8, 5.7, 8.8 and 0.05% NH4OH in H2O at pH 10.5) were tested for best signal responses of HCAAs.
Figure 3.
Effects of post-column infusion of ACN (at 0.2 mL/min) on the signal responses of HCAAs.
Method validation
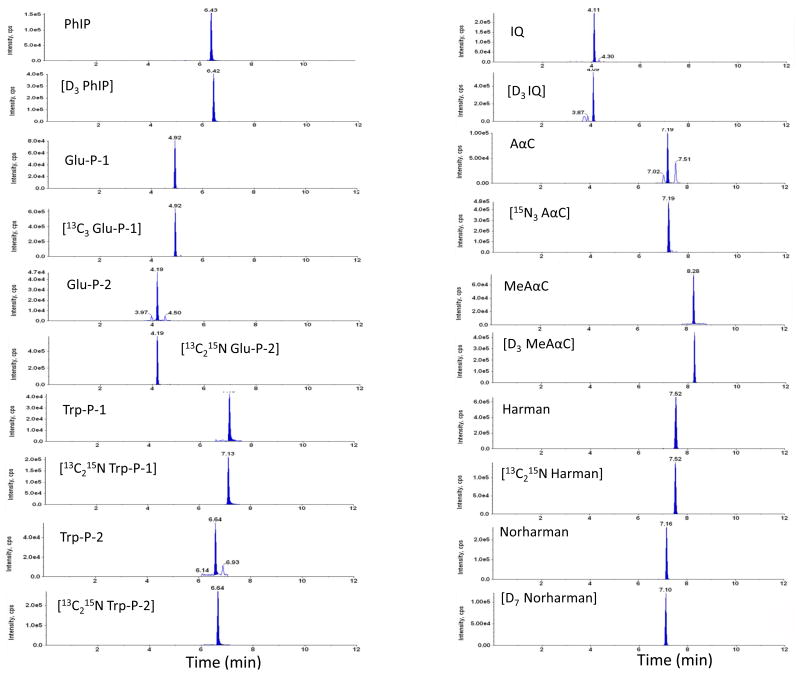
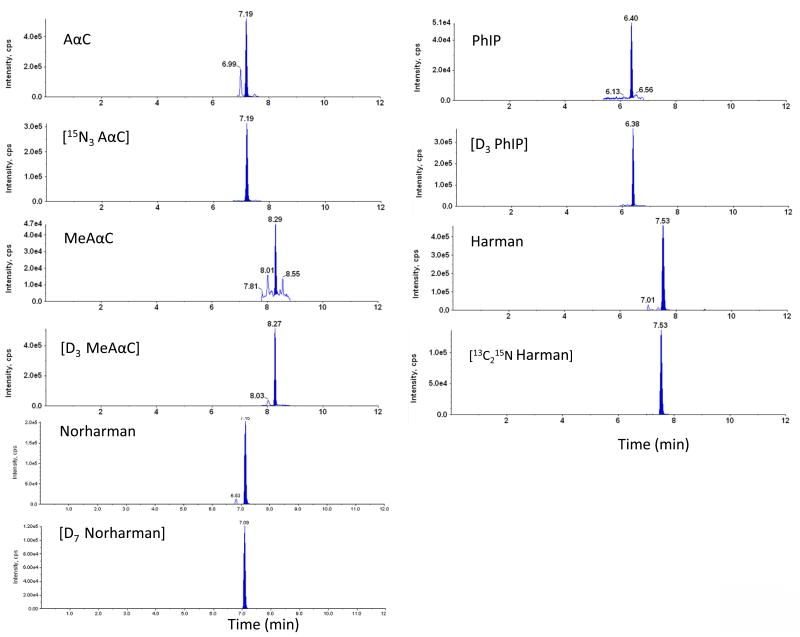
The current method featured an intensive sample cleanup procedure (diatomaceous earth SPE in tandem with mixed mode of cation exchange SPE) using robotic automation system for extraction of HCAAs from urine samples. Urine samples were basified with 10N NaOH for extraction of HCAAs on Isolute SLE+ plates. The sample pH was tested at different volume ratios of sample over 10N NaOH. At range from 1:50 to 1:10 (10N NaOH: urine sample; v/v) the sample pH plateaued at pH 14. And we finally chose ratio at 1:10 as reported previously.[15] The methanol percentage in washing solution for HCAA extraction on EVOLUTE® CX plate was optimized. 30% methanol in washing solution that we used in our method could maximize the retention of polar HCAAs on EVOLUTE® CX plate, while maintain sufficient washing efficiency. (see Electronic Supplementary Material Figure S-3) Representative chromatograms of spiked urine samples and urine samples from smokers are shown in Figure 4 and Figure 5, respectively. Under the described chromatographic conditions, HCAAs were well separated from endogenous interferences in the urine matrix.
Figure 4.
MRM chromatograms of blank urine sample spiked with 10 pg/mL of HCAAs. The urine samples were hydrolyzed at 70 °C for 5 hours under base conditions and prepared by robotic sample preparation system prior to LC-MS/MS analysis.
Figure 5.
Representative MRM chromatograms of total urinary HCAAs detected in smokers with AαC at 69.8 pg/mL, MeAαC at 4.58 pg/mL, PhIP at 3.35 pg/mL, Harman at 774 pg/mL, Norharman at 371 pg/mL. The urine samples were hydrolyzed at 70 °C for 5 hours with basic condition and prepared by robotic sample preparation system prior to LC-MS/MS analysis.
Calibration curves of ten HCAAs were linear over the ranges specified in Table 2 (R2>0.998). The influence of urine matrix on the calibration curves was estimated by comparing the slopes of calibration curves built by using urine matrix and water matrix. These slopes differed by <5%, indicating that urine matrix has minimal impact on the quantification of HCAAs based on calibration curves prepared in water (Table 2). Relatively large y-intercept values were found in urine calibration curves of harman and norharman, resulting from high endogenous levels of harman and norharman in the blank urine pool. Since harman and norharman were found in both smoker and nonsmoker urine pools, and there is no analyte-free blank urine, water calibration curves were used to quantify HCAAs in urine samples as described previously.[15, 18]
Intra-day and inter-day RSD at low, medium and high concentrations for all HCAAs were below 10% (Table 3). Intra-day and inter-day accuracy ranged between 90-110% (Table 3). Except for harman and norharman, HCAA LOQ ranged from 1.0-2.8 pg/mL, and LOD ranged from 0.31-0.83 pg/mL. Harman and norharman exhibited superior sensitivity on API 5500 QTRAP at their optimal MS parameters. To allow linear quantification of all analytes following a single sample injection, compound-specific parameters of the MS instrument for harman and norharman were “de-tuned” to avoid saturation of the MS detector. De-tuning compound-specific parameters (mainly through de-tuning collision energy) was performed to reduce the MS signal responses of harman and norharman by about 25-fold. As a result of de-tuning, the LOD of harman and norharman were higher than the LOD of the other HCAAs analyzed. HCAA extraction recoveries varied from 44 to 93% (Table 3). In sample preparation, adsorption of some HCAAs to the container was noticed during the last step of solvent evaporation under N2. Glass containers that are even silanized showed high tendency of HCAA adsorption. Polypropylene containers could reduce the extent, but could not completely prevent the adsorption. Adsorption of HCAAs to the container may in part contribute to the low recoveries of some HCAAs. Ion suppression reduced signal responses by 15 to 49% (Table 3). Because the extent of ion suppression and HCAA recoveries varied among analytes, corresponding isotopically-labeled internal standards were used to assure accurate measurement of each HCAA.
Table 3. Intra-day, inter-day precision and accuracy, LOD, LOQ, recoveries, ion suppression for HCAA analysis.
|
|
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Analyte | Intraday | Interday | ||||||||||||||
| Nominal Concentration (pg/mL) | Determined Concentration (pg/mL) | Accuracy (%) |
RSD (%) |
Nominal Concentration (pg/mL) | Determined Concentration (pg/mL) | Accuracy (%) |
RSD (%) |
LOQ (pg/mL) |
LOD (pg/mL) |
Recovery (%) |
Ion suppression (%) |
|||||
|
|
|
|||||||||||||||
| n=8 | n=6 | |||||||||||||||
| AαC | 150 | 151 | ± | 2.3 | 101 | 1.5 | 150 | 148 | ± | 5.7 | 98 | 3.9 | 2.1 | 0.62 | 64 | 47 |
| 40 | 41 | ± | 1.6 | 103 | 4 | 40 | 40 | ± | 1.8 | 100 | 4.5 | |||||
| 10 | 10 | ± | 0.2 | 101 | 1.7 | 10 | 10 | ± | 0.5 | 100 | 5 | |||||
| MeAαC | 150 | 150 | ± | 1.1 | 100 | 0.8 | 150 | 144 | ± | 6.9 | 96 | 4.8 | 1.1 | 0.33 | 54 | 49 |
| 40 | 40 | ± | 0.8 | 101 | 2 | 40 | 38 | ± | 2.1 | 96 | 5.5 | |||||
| 10 | 10 | ± | 0.1 | 98 | 0.8 | 10 | 10 | ± | 0.4 | 95 | 4.2 | |||||
| IQ | 150 | 152 | ± | 1.8 | 102 | 1.2 | 150 | 152 | ± | 11.2 | 102 | 7.3 | 2 | 0.37 | 75 | 26 |
| 40 | 42 | ± | 1 | 105 | 2.4 | 40 | 41 | ± | 3.2 | 103 | 7.7 | |||||
| 10 | 11 | ± | 0.3 | 106 | 3.2 | 10 | 10 | ± | 0.8 | 102 | 7.8 | |||||
| Trp-P-1 | 117 | 117 | ± | 2.1 | 100 | 1.8 | 117 | 112 | ± | 4.9 | 96 | 4.3 | 2.6 | 0.79 | 93 | 39 |
| 31 | 32 | ± | 1.8 | 101 | 5.7 | 31 | 30 | ± | 1.7 | 96 | 5.7 | |||||
| 7.8 | 7.5 | ± | 0.2 | 97 | 3.2 | 7.8 | 7.2 | ± | 0.7 | 93 | 9.9 | |||||
| Trp-P-2 | 101 | 104 | ± | 2.4 | 103 | 2.3 | 101 | 100 | ± | 4.1 | 99 | 4.1 | 1.2 | 0.63 | 90 | 38 |
| 27 | 28 | ± | 1.2 | 104 | 4.3 | 27 | 26 | ± | 1.5 | 98 | 5.8 | |||||
| 6.7 | 6.6 | ± | 0.2 | 98 | 2.8 | 6.7 | 6.4 | ± | 0.4 | 95 | 6.3 | |||||
| Glu-P-1 | 105 | 104 | ± | 0.8 | 98 | 0.8 | 105 | 104 | ± | 1.4 | 99 | 1.3 | 1 | 0.31 | 44 | 15 |
| 28 | 28 | ± | 0.2 | 100 | 0.8 | 28 | 28 | ± | 0.4 | 98 | 1.4 | |||||
| 7 | 7 | ± | 0.2 | 100 | 2.7 | 7 | 6.8 | ± | 0.2 | 98 | 2.3 | |||||
| Glu-P-2 | 107 | 106 | ± | 0.8 | 99 | 0.7 | 107 | 105 | ± | 2.1 | 98 | 2 | 2.8 | 0.83 | 55 | 24 |
| 29 | 29 | ± | 0.2 | 100 | 0.8 | 29 | 28 | ± | 0.7 | 97 | 2.6 | |||||
| 7.2 | 7.4 | ± | 0.3 | 103 | 4.1 | 7.2 | 7.1 | ± | 0.3 | 99 | 4.2 | |||||
| PhIP | 150 | 149 | ± | 1.8 | 100 | 1.2 | 150 | 147 | ± | 3.9 | 98 | 2.7 | 1.1 | 0.34 | 79 | 21 |
| 40 | 40 | ± | 0.5 | 99 | 1.3 | 40 | 39 | ± | 1 | 97 | 2.6 | |||||
| 10 | 10 | ± | 0.2 | 100 | 2 | 10 | 10 | ± | 0.4 | 96 | 4.2 | |||||
|
|
|
|||||||||||||||
| Intraday | Interday | |||||||||||||||
| n=4 | n=3 | |||||||||||||||
|
|
|
|||||||||||||||
| Harman | 3530 | 3825 | ± | 205.3 | 108 | 5.4 | 3530 | 3583 | ± | 302.8 | 102 | 8.5 | 93 | 28 | ||
| 313 | 335 | ± | 18.1 | 107 | 5.4 | 313 | 341 | ± | 32.6 | 109 | 9.6 | |||||
| NorHarman | 2132 | 2120 | ± | 37.4 | 99 | 1.8 | 2132 | 2171 | ± | 118.9 | 102 | 5.5 | 86 | 24 | ||
| 662 | 690 | ± | 27.3 | 104 | 4 | 662 | 710 | ± | 63 | 107 | 8.9 | |||||
| Harman a | 4000 | 3816 | ± | 205.2 | 95 | 5.4 | 4000 | 3832 | ± | 176.8 | 96 | 4.6 | 15.3 | 4.6 | ||
| 400 | 401 | ± | 6.9 | 100 | 1.7 | 400 | 389 | ± | 17.5 | 97 | 4.5 | |||||
| 100 | 100 | ± | 2 | 100 | 2 | 100 | 97 | ± | 5.5 | 97 | 5.7 | |||||
| NorHarman a | 4000 | 4024 | ± | 96 | 101 | 2.4 | 4000 | 4073 | ± | 137 | 102 | 3.4 | 42 | 12.6 | ||
| 400 | 416 | ± | 3.6 | 104 | 0.9 | 400 | 418 | ± | 16.8 | 104 | 4 | |||||
| 100 | 105 | ± | 1.3 | 105 | 1.2 | 100 | 104 | ± | 5.1 | 104 | 4.9 | |||||
Prepared in synthetic urine.
The stability of ten HCAAs was evaluated under various conditions. As shown in Table 4, all HCAAs were stable in urine after four freeze–thaw cycles at -70 °C and stable at room temperature for at least 24 hours. In addition, all HCAAs were stable in the extracted samples at autosampler for at least 24 hours.
Table 4. The stability of HCAAs at various conditions.
| Nominal concentrations (pg/mL) | Determined concentrations (pg/mL) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Room temperature for 24 hrs | Four freeze–thaw cycles | Auto-sampler for 24 hrs | ||||||||
| AαC | 150 | 149.7 | ± | 3.1 | 142.7 | ± | 2.9 | 152.7 | ± | 1.5 |
| 20 | 21.1 | ± | 0.5 | 18.9 | ± | 0.4 | 21.0 | ± | 0.7 | |
| MeAαC | 150 | 151.3 | ± | 2.1 | 139.0 | ± | 2.0 | 163.7 | ± | 3.2 |
| 20 | 20.5 | ± | 0.5 | 18.8 | ± | 0.1 | 21.2 | ± | 0.2 | |
| IQ | 150 | 155.0 | ± | 2.6 | 152.7 | ± | 1.5 | 156.7 | ± | 2.5 |
| 20 | 21.6 | ± | 0.7 | 21.1 | ± | 0.2 | 20.8 | ± | 0.7 | |
| Trp-P-1 | 150 | 149.3 | ± | 2.5 | 149.3 | ± | 3.8 | 152.3 | ± | 2.1 |
| 20 | 19.6 | ± | 0.9 | 18.8 | ± | 0.6 | 20.5 | ± | 0.5 | |
| Trp-P-2 | 150 | 149.3 | ± | 2.5 | 148.0 | ± | 1.0 | 148.7 | ± | 2.1 |
| 20 | 19.6 | ± | 0.2 | 18.6 | ± | 0.2 | 19.5 | ± | 0.3 | |
| Glu-P-1 | 150 | 151.7 | ± | 6.1 | 148.3 | ± | 4.2 | 153.3 | ± | 2.1 |
| 20 | 21.0 | ± | 0.2 | 19.3 | ± | 0.2 | 21.2 | ± | 0.5 | |
| Glu-P-2 | 150 | 148.7 | ± | 2.3 | 146.0 | ± | 2.6 | 150.0 | ± | 1.7 |
| 20 | 19.9 | ± | 0.6 | 20.1 | ± | 0.5 | 21.1 | ± | 0.5 | |
| PhIP | 150 | 152.3 | ± | 4.9 | 149.7 | ± | 2.1 | 151.3 | ± | 4.7 |
| 20 | 21.2 | ± | 0.5 | 20.7 | ± | 0.5 | 20.0 | ± | 0.2 | |
| Harman | 1487 | 1563 | ± | 21 | 1507 | ± | 23 | 1493 | ± | 15.3 |
| 257 | 282 | ± | 9.5 | 266 | ± | 9.5 | 261 | ± | 8.5 | |
| Norharman | 1493 | 1580 | ± | 10 | 1537 | ± | 5.8 | 1477 | ± | 15.3 |
| 287 | 278 | ± | 2.9 | 297 | ± | 11 | 272 | ± | 5.1 | |
Method application
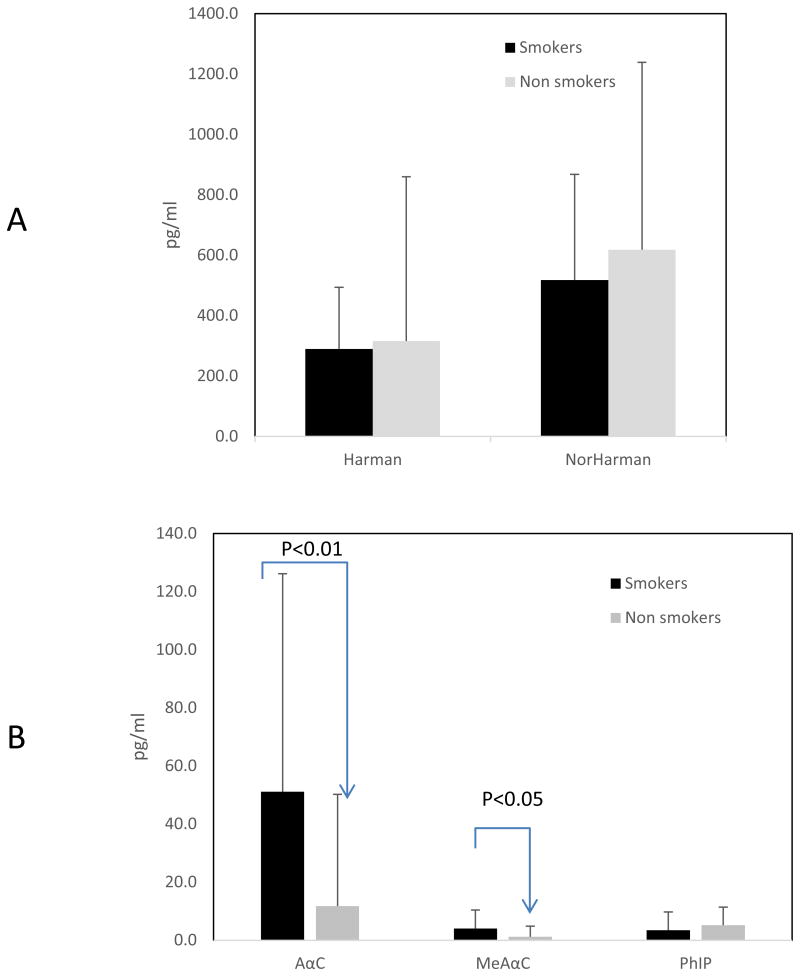
The current method was successfully used to quantify targeted HCAAs in urine collected from smokers and non-smokers. AαC and MeAαC are the predominant carcinogenic HCAAs in cigarette smoke. [2] Urinary concentrations of AαC were significantly higher in smokers (51.2±75 pg/ml) than in non-smokers (11.8±38 pg/ml) (P<0.05) (Figure 6). Similarly, about 4-fold difference in urinary AαC was found between smokers (around 26 pg/ml) and non-smokers (around 6.5 pg/ml) in previous study. [18] Urinary MeAαC in smokers were not characterized before because of the insufficient sensitivity.[18] In the present study, MeAαC could be detected in 46 out of 48 smokers at 4.0±6.4 pg/ml and in 6 out of 28 non-smokers at 2.3±6.4 pg/ml. Urinary concentrations of PhIP and the two beta-carboline HCAAs evaluated, harman and norharman, were similar in smokers and non-smokers. As there are reports that the half-lives of HCAA are relatively short (< 24 hours), the urinary HCAA levels probably reflect the short-term exposure to HCAA [27, 28]. Measurement of HCAA in hair could be an alternative analysis to determine the long-term exposure to HCAA.[29, 30]
Figure 6.
Comparison of total urinary HCAAs in smokers and nonsmokers. (A) Urinary harman and norharman; (B) urinary AαC, MeAαC, PhIP. The urine samples were hydrolyzed at 70 °C for 5 hours with basic condition and prepared by robotic sample preparation system prior to LC-MS/MS analysis.
Conclusion
We have developed a sensitive, high throughput analytical method using isotope dilution tandem mass spectrometry coupled with robotic sample preparation for quantification of urinary heterocyclic aromatic amines in smokers and non-smokers. The sensitivity, precision, accuracy and throughput are adequate for use in large-scale biomonitoring studies. Such studies can provide population-scale data about the magnitude and prevalence of exposure to these compounds, as well as relative source contributions (e.g., cigarette smoke vs. diet) and exposure trends.
Supplementary Material
Acknowledgments
The authors would like to thank the Center for Tobacco Products, Food and Drug Administration for providing funds for this project. We also thank Tonya Guillot, Katie Wozniak, Dana Freeman, Jason Terranova, Binnian Wei, John Lee, Justin Brown, and Christina Brosius for their suggestions and support during this study.
Footnotes
Notes: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention. Use of trade names and commercial sources is for identification only and does not constitute endorsement by the U.S. Department of Health and Human Services or the Centers for Disease Control and Prevention.
Conflict of Interest: The authors declare no competing financial interest.
References
- 1.US DHHS USDoHaHS. The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General — Executive Summary. T US Department of Heath and Human Services, Centers for Disease Control and Prevention, Coordinating Center for Health Promotion, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health 2006; [PubMed] [Google Scholar]
- 2.Zhang L, Ashley DL, Watson CH. Quantitative analysis of six heterocyclic aromatic amines in mainstream cigarette smoke condensate using isotope dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Nicotine Tob Res. 2011;13:120–6. doi: 10.1093/ntr/ntq219. [DOI] [PubMed] [Google Scholar]
- 3.Turesky RJ. Heterocyclic aromatic amine metabolism, DNA adduct formation, mutagenesis, and carcinogenesis. Drug Metab Rev. 2002;34:625–50. doi: 10.1081/dmr-120005665. [DOI] [PubMed] [Google Scholar]
- 4.Turesky RJ, Le Marchand L. Metabolism and biomarkers of heterocyclic aromatic amines in molecular epidemiology studies: lessons learned from aromatic amines. Chem Res Toxicol. 2011;24:1169–214. doi: 10.1021/tx200135s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.IARC Monographs on the Evaluation of Carcinogenic Risks to Humans: Some NaturallyOccurring Substances - Food Items and Constituents, Heterocyclic Aromatic Amines and Mycotoxins. IARC Monogr Eval Carcinog Risk Chem Hum. 1993;56:165–242. [Google Scholar]
- 6.Cross AJ, Sinha R. Meat-related mutagens/carcinogens in the etiology of colorectal cancer. Environ Mol Mutagen. 2004;44:44–55. doi: 10.1002/em.20030. [DOI] [PubMed] [Google Scholar]
- 7.Dai Q, Shu XO, Jin F, Gao YT, Ruan ZX, Zheng W. Consumption of animal foods, cooking methods, and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 2002;11:801–8. [PubMed] [Google Scholar]
- 8.Le Marchand L, Hankin JH, Wilkens LR, Pierce LM, Franke A, Kolonel LN, et al. Combined effects of well-done red meat, smoking, and rapid N-acetyltransferase 2 and CYP1A2 phenotypes in increasing colorectal cancer risk. Cancer Epidemiol Biomarkers Prev. 2001;10:1259–66. [PubMed] [Google Scholar]
- 9.Sugimura T. Overview of carcinogenic heterocyclic amines. Mutat Res. 1997;376:211–9. doi: 10.1016/s0027-5107(97)00045-6. [DOI] [PubMed] [Google Scholar]
- 10.Shirai T, Sano M, Tamano S, Takahashi S, Hirose M, Futakuchi M, et al. The prostate: a target for carcinogenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) derived from cooked foods. Cancer Res. 1997;57:195–8. [PubMed] [Google Scholar]
- 11.Totsuka Y, Ushiyama H, Ishihara J, Sinha R, Goto S, Sugimura T, et al. Quantification of the co-mutagenic beta-carbolines, norharman and harman, in cigarette smoke condensates and cooked foods. Cancer Lett. 1999;143:139–43. doi: 10.1016/s0304-3835(99)00143-3. [DOI] [PubMed] [Google Scholar]
- 12.Sofuoglu M, LeSage MG. The reinforcement threshold for nicotine as a target for tobacco control. Drug Alcohol Depend. 2012;125:1–7. doi: 10.1016/j.drugalcdep.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Andres F, Zougagh M, Castaneda G, Rios A. Determination of heterocyclic amines in urine samples by capillary liquid chromatography with evaporated light-scattering detection. Anal Bioanal Chem. 2010;397:223–31. doi: 10.1007/s00216-009-3370-z. [DOI] [PubMed] [Google Scholar]
- 14.Shah FU, Barri T, Jönsson JÅ, Skog K. Determination of heterocyclic aromatic amines in human urine by using hollow-fibre supported liquid membrane extraction and liquid chromatography-ultraviolet detection system. Journal of Chromatography B. 2008;870:203–8. doi: 10.1016/j.jchromb.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Holland RD, Taylor J, Schoenbachler L, Jones RC, Freeman JP, Miller DW, et al. Rapid biomonitoring of heterocyclic aromatic amines in human urine by tandem solvent solid phase extraction liquid chromatography electrospray ionization mass spectrometry. Chem Res Toxicol. 2004;17:1121–36. doi: 10.1021/tx049910a. [DOI] [PubMed] [Google Scholar]
- 16.Reistad R, Rossland OJ, Latva-Kala KJ, Rasmussen T, Vikse R, Becher G, et al. Heterocyclic aromatic amines in human urine following a fried meat meal. Food Chem Toxicol. 1997;35:945–55. doi: 10.1016/s0278-6915(97)00112-9. [DOI] [PubMed] [Google Scholar]
- 17.De Andrés F, Zougagh M, Castañeda G, Sánchez-Rojas JL, Ríos A. Screening of non-polar heterocyclic amines in urine by microextraction in packed sorbent-fluorimetric detection and confirmation by capillary liquid chromatography. Talanta. 2011;83:1562–7. doi: 10.1016/j.talanta.2010.11.060. [DOI] [PubMed] [Google Scholar]
- 18.Fu Y, Zhao G, Wang S, Yu J, Xie F, Wang H, et al. Simultaneous determination of fifteen heterocyclic aromatic amines in the urine of smokers and nonsmokers using ultra-high performance liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2014;1333:45–53. doi: 10.1016/j.chroma.2014.01.057. [DOI] [PubMed] [Google Scholar]
- 19.Turesky RJ, Yuan JM, Wang R, Peterson S, Yu MC. Tobacco smoking and urinary levels of 2-amino-9H-pyrido[2,3-b]indole in men of Shanghai, China. Cancer Epidemiol Biomarkers Prev. 2007;16:1554–60. doi: 10.1158/1055-9965.EPI-07-0132. [DOI] [PubMed] [Google Scholar]
- 20.Manabe S, Tohyama K, Wada O, Aramaki T. Detection of a carcinogen, 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), in cigarette smoke condensate. Carcinogenesis. 1991;12:1945–7. doi: 10.1093/carcin/12.10.1945. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto T, Yoshida D, Tomita H. Determination of mutagens, amino-alpha-carbolines in grilled foods and cigarette smoke condensate. Cancer Lett. 1981;12:105–10. doi: 10.1016/0304-3835(81)90045-8. [DOI] [PubMed] [Google Scholar]
- 22.Frandsen H. Biomonitoring of urinary metabolites of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) following human consumption of cooked chicken. Food Chem Toxicol. 2008;46:3200–5. doi: 10.1016/j.fct.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 23.Stillwell WG, Kidd LC, Wishnok JS, Tannenbaum SR, Sinha R. Urinary excretion of unmetabolized and phase II conjugates of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine and 2-amino-3,8-dimethylimidazo[4,5-f]quinoxaline in humans: relationship to cytochrome P4501A2 and N-acetyltransferase activity. Cancer Res. 1997;57:3457–64. [PubMed] [Google Scholar]
- 24.Ushiyama H, Wakabayashi K, Hirose M, Itoh H, Sugimura T, Nagao M. Presence of carcinogenic heterocyclic amines in urine of healthy volunteers eating normal diet, but not of inpatients receiving parenteral alimentation. Carcinogenesis. 1991;12:1417–22. doi: 10.1093/carcin/12.8.1417. [DOI] [PubMed] [Google Scholar]
- 25.Taylor JK. Quality Assurance of Chemical Measurements. Chapter 9. Lewis Publishers; Boca Raton, FL: 1987. pp. 78–82. [Google Scholar]
- 26.Kataoka H, Inoue T, Ikekita N, Saito K. Development of exposure assessment method based on the analysis of urinary heterocyclic amines as biomarkers by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2014;406:2171–8. doi: 10.1007/s00216-013-7420-1. [DOI] [PubMed] [Google Scholar]
- 27.Turesky RJ, Konorev D, Fan X, Tang Y, Yao L, Ding X, et al. Effect of Cytochrome P450 Reductase Deficiency on 2-Amino-9H-pyrido[2,3-b]indole Metabolism and DNA Adduct Formation in Liver and Extrahepatic Tissues of Mice. Chem Res Toxicol. 2015;28:2400–10. doi: 10.1021/acs.chemrestox.5b00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boobis AR, Lynch AM, Murray S, de la Torre R, Solans A, Farre M, et al. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994;54:89–94. [PubMed] [Google Scholar]
- 29.Kataoka H, Inoue T, Saito K, Kato H, Masuda K. Analysis of heterocyclic amines in hair by on-line in-tube solid-phase microextraction coupled with liquid chromatography-tandem mass spectrometry. Anal Chim Acta. 2013;786:54–60. doi: 10.1016/j.aca.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 30.Bessette EE, Yasa I, Dunbar D, Wilkens LR, Le Marchand L, Turesky RJ. Biomonitoring of carcinogenic heterocyclic aromatic amines in hair: a validation study. Chem Res Toxicol. 2009;22:1454–63. doi: 10.1021/tx900155f. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.