Abstract
The Lone Star tick, Amblyomma americanum, is endemic to the southeastern United States and capable of transmitting pathogenic diseases and causing non-pathogenic conditions. To remain firmly attached to the host, the tick secretes a proteinaceous matrix termed the cement cone which hardens around the tick’s mouthparts to assist in the attachment of the tick as well as to protect the mouthparts from the host immune system. Cement cones collected from ticks on a host are commonly contaminated with host skin and hair making analysis of the cone difficult. To reduce the contamination found in the cement cone, we have adapted an artificial membrane feeding system used to feed long mouthpart ticks. Cones collected from in vivo and membrane fed ticks are analyzed to determine changes in the cone morphology. Comparisons of the cement cones using light microscopy shows similar structures and color however using scanning electron microscopy the cones have drastically different structures. The in vivo cones contain fibrils, sheets, and are heavily textured whereas cones from membrane fed ticks are remarkably smooth with no distinct structures. Analysis of the secondary protein structures using FTIR-ATR show both in vivo and membrane fed cement cones contain β sheets but only in vivo cement cones contain helical protein structures. Additionally, proteomic analysis using LC–MS/MS identifies many proteins including glycine rich proteins, metalloproteases, and protease inhibitors. Proteomic analysis of the cones identified both secreted and non-secreted tick proteins. Artificial membrane feeding is a suitable model for increased collection of cement cones for proteomic analysis however, structurally there are significant differences.
Keywords: Amblyomma americanum, Cement proteins, Artificial membrane feeding, Proteome
1. Introduction
In order to reach the nutritious bloodmeal, ticks must penetrate the host’s skin with the hypostome and are assisted by the presence of recurved teeth along the hypostome surface. Adult Ixodid ticks stay attached to the host for 7–21 days dependent on the species, and consequently firm attachment to the host by way of a cement cone is required. Tick feeding is divided into three general stages: attachment, slow feeding, and fast feeding (Anderson and Magnarelli, 2008; Kemp et al., 1982). The attachment phase has multiple steps including identification of a vertebrate host, penetration of the barbed mouthparts deep into the dermis, encasement of the hypostome in a narrow secreted cement cone for a stealthy but secure attachment and to provide a conducive environment for the injection of pathogenic microbes during the bloodmeal (Alekseev et al., 1995; Anderson and Magnarelli, 2008). The cement cone protects the mouthpart from the host immune system (Alekseev et al., 1995) while also anchoring the tick into the host dermis. The proteinaceous matrix of the cement cone is secreted by both longirostra (long mouthparts) and brevirostra (short mouthparts) tick species (Kemp et al., 1982).
Ticks secrete two types of cement, a primary “core” cement and a secondary “cortex” cement. The primary secretion, or the core, has been noted as early as 5–30 min after attachment (Gregson, 1960; Kemp et al., 1982). The core cement hardens almost instantaneously once in place, while the cortex cement secretes from the tick for multiple days and has a graduate hardening process (Kemp et al., 1982). Although both the core and cortex cement are predominantly proteinaceous, the core cement also contains lipids while the cortex cement has more carbohydrates than lipids (Kemp et al., 1982; Moorhouse and Tatchell, 1966; Stone et al., 1977). The relative quantities of amino acids in the cement cone revealed that small amino acids such as glycine, serine, and leucine were the most abundant, followed by tyrosine, an amino acid known for its cross-linking properties (Kemp et al., 1982). Although tyrosine is present, there is currently no evidence regarding its role in the protein structure or the aggregation of proteins for the formation of the cement cone.
The majority of research regarding the cement cone structure comes from the study of tick bite site biopsies. Using common histological stains, hematoxylin and eosin, the cement cone can be identified by its bright pink color (Chinery, 1973). Differences in the core and cortex cement are clearly visible after histology staining and can give some insight into the formation of the cement and how feeding takes place through the cement. The core cement lies close to the hypostome and forms a tapered tube in which the hypostome fits. Transverse cuts of the biopsy indicate that the primary cement is almost perfectly circular indicating that full coverage of the hypostome is important in preventing host detection of the hypostome (Chinery, 1973). The cortex cement is layered on the exterior of the core cement, coming into direct contact with the host skin (Gregson, 1960; Kemp et al., 1982). The strands of the fully cured cortex cement are intertwined with the surrounding skin tissue (Chinery, 1973) allowing for a more secure attachment.
Adhesive protein secretions are produced in many other invertebrates for structures such as egg casing adhesives, spider silk, and barnacle adhesive. Analysis of the protein composition of these other invertebrate adhesive secretions revealed over-representation of small amino acids, glycine and serine being the most prevalent (Li et al., 2008) similar to the amino acid composition found in tick cement proteins. Insect egg casing adhesives can be found as either hydrogel with high elasticity and tack or as a glue which becomes tough and dry through the evaporation of the solvent (Li et al., 2008). Proteomic analysis of the egg casing adhesives revealed mostly large molecular weight proteins (more than 75 kDa) except for shield bugs which contain proteins spanning the entire range of molecular weights according to SDS-PAGE (Li et al., 2008). Various spider silk formulations are used as a cocoon to protect the spider from the environment during development, adhesive to secure the spider webbing and egg casings to the substrate, and webbing to provide safety and assist in capturing food (Winkler and Kaplan, 2000). The most commonly studied spider silk is dragline silk which has high tensile strength and is commonly comprised of only a few proteins. These proteins self-assemble into crystalline repeats which are bound together by disulfide bond formation, glycosylation, or cation interactions (Winkler and Kaplan, 2000). Barnacles also secrete an adhesive cement which is used to firmly adhere to their substrate however, barnacle cement differs from the other adhesives listed here as the protein composition of barnacle cement contains cysteine repeats (He et al., 2013) rather than a large number of glycine repeats. However, the two-cement process of barnacles (Burden et al., 2012) mimics the two-cement composition of tick cement.
The focus of tick cement cone research has shifted in the last 40 years. Original research of the topic focused on identifying structural characteristics of the cone and using histological staining to uncover bits of information regarding its composition (Kemp et al., 1982; Moorhouse and Tatchell, 1966; Stone et al., 1977). As molecular biology based techniques improved, the focus began to shift towards identifying probable cement proteins from sialotranscriptomes and understanding the proteins responsible for the makeup of the cement cone to exploit these proteins for vaccine development (Binnington and Kemp, 1980; Bishop et al., 2002; Havlíková et al., 2009; Karim and Ribeiro, 2015; Kemp et al., 1982; Kim et al., 2014; Maruyama et al., 2010; Zhou et al., 2006). However, structural and proteomic research of the cement cone has been largely lacking in the last 10–15 years due to the difficulty in collecting cement cones which remain embedded in the skin of the host. Another complication in cement cone research is the solubility of the cement cone. The curation process for the cement formation results in an extremely hard cone which is difficult to solubilize. Solubilization has been most successful in hot acids or bases (Kemp et al., 1982), however at these extreme conditions identification of proteins is difficult as such harsh conditions can undergo hydrolysis. The use of molecular techniques and the development of an artificial membrane feeding system allow the tick research community to circumvent this problem. Common artificial feeding methods involve feeding the tick via a capillary tube fitted on its mouthparts; however, this does not simulate a natural feeding environment, and feeding cannot proceed for multiple days as with in vivo feeding. To better replicate natural feeding, membranes are now being used to simulate skin and the ticks feed on a blood pool supplied underneath. This method has been used to feed multiple tick species with varying mouthpart sizes(Andrade et al., 2014; Fourie et al., 2013; Kröber and Guerin, 2007a, 2007b; Oliver et al., 2016). Previous studies using Amblyomma hebraeum have shown cement cones produced on the underside of the membrane to be easily collectable without the inference of the host dermis (Kröber and Guerin, 2007b). Here, we utilized an artificial membrane feeding system to farm cement cones from adult female Amblyomma americanum ticks. We have used multiple techniques to compare the cones collected from in vivo fed and membrane fed ticks to identify the strengths and weaknesses of artificial membrane feeding for cement cone research.
2. Materials and methods
2.1. Materials
All common laboratory supplies and chemicals were purchased from Sigma-Aldrich (St. Louis, Missouri) or Fisher Scientific (Grand Island, New York) unless otherwise specified.
2.2. Tick rearing
Adult A. americanum ticks were purchased from the Tick Rearing Facility at Oklahoma State University and maintained at the University of Southern Mississippi according to established methods (Patrick and Hair, 1975). The adult ticks were maintained at room temperature with 90% relative humidity and a long light cycle of 14 h light 10 h dark. All animal work was conducted according to the approved protocol by the institutional Animal Use and Care Committee (IACUC) of the University of Southern Mississippi (protocol# 15101501).
2.3. Assembly of an artificial membrane feeding apparatus
Acrylic chambers were sculpted to fit precisely into the well of a six-well microplate (Kröber and Guerin, 2007a). Silicone membranes were prepared by mixing 3.3 mL of silicone oil and 10 g of Elasatosil E4 silicone glue (Wacker, Munchen, Germany), with 26 mL toluene (Andrade et al., 2014). This mixture was stirred on a magnetic stir-plate for 20 min at 600 rpm. Lens paper was cut into 3 cm by 3 cm squares. The silicone mixture was poured into a petri dish for a larger surface area and the lens paper squares were carefully swiped over the surface of the silicone in order to coat one side of the lens paper. The one-sided silicone squares were hung to dry for 48 h in a vented chemical hood. After the silicone membranes were dried, fiberglass mesh which had been precisely cut to fit inside the acrylic chamber was carefully glued to the non-coated side of the silicone membrane using Elastosil E4. The reinforced membranes were then glued to the bottom of the acrylic chambers with the same silicone glue. This glue was allowed to dry for at least 24 h before used for feeding. Excess membrane was trimmed from the circumference of each chamber to ensure a proper fit into the six-well microplate. Chambers were also submerged in 70% ethanol to test for membrane integrity before feeding (Andrade et al., 2014).
2.4. Collection of cement cones from in vivo fed ticks
A total of 50 female and 25 male ticks were placed onto the back of a sheep and enclosed in a sock glued to the sheep. Ticks were manually removed at regular intervals as needed for other ongoing experiment using forceps. All non-treatment ticks are inspected for cement cone formation. Pictures of intact cement cones were taken with a Dino Light camera. Cones were carefully removed by pulling the cone from the tick mouthparts using sharp point forceps and stored dry at −80 °C until analyzed. Skin contaminate and hair can often be identified and care is taken to remove what is clearly not cement however, it difficult to remove all layers of skin as the color of the cement and skin are similar.
2.5. Collection of cement cones from membrane fed ticks
A total of 15 females and 5 males were placed into an artificial feeding chamber. Animal hair collected from a local pet grooming facility was then placed on top the silicone membrane to simulate host fur and animal scent to the membrane. The animal hair is washed with a non-medicated pet shampoo prior to being sheared from the animal. Using a cotton stopper, the total volume of the chamber was reduced to force the ticks to interact with the membrane. Blood was collected from a local abattoir who regularly slaughters bovine and porcine animals. Blood is defibrinated by manual agitation using a plastic stirring rod, large clots were removed manually and small clots by straining. Defibrinated blood was stored for up to two weeks at 4 °C as 25 mL aliquots until used and 3–4 mL aliquots were pre-warmed to 37 °C, added to a single well of a 6-well plate and the feeding chamber was placed into the well so that the membrane comes into direct contact with the blood. To maintain the optimal temperature for feeding, the feeding system was placed in a 37 °C incubator. The blood was changed twice daily and the membrane was rinsed with a PBS solution with 2% Penicillin/Streptomycin at each blood change (Andrade et al., 2014). The ticks were monitored daily for attachment and changes in engorgement. Ticks can be fed up to 20 days and cones can be collected at any time. The outside of the silicone membrane was examined daily for the presence of cement cones and the cones were removed with sharp pointed forceps during the course of blood feeding. Cement cones were collected from various time points including 24 h, 3 days, 5 day, and 7 days after tick attachment. All cones collected are from feeding females except for one male cone which was used for FTIR. Cones from membrane fed ticks are rinsed with PBS with penicillin/Streptomycin prior to removal and are placed in 1.5 mL microcentrifuge tubes and placed at room temperature for 24 h to dry the cones and are then stored at −80 °C.
2.6. Scanning electron microscopic analysis
Cement cone samples were oriented and mounted onto standard aluminum scanning electron microscopy (SEM) mounts with carbon conductive adhesive tabs and were coated with a thin deposit of silver using a Quorum Emitech K550X (East Sussex, United Kingdom) sputter coater to remove any charging that may occur on the surface of the sample. Electron micrographs were captured with an FEI Quanta 200 (Hillsboro, Oregon) environmental scanning electron microscope (ESEM) operating in high vacuum mode at an accelerating voltage range of 10 kV to 20 kV.
2.7. Fourier transform infrared spectroscopy – attenuated total reflectance
The Fourier Transform Infrared Spectroscopy-Attenuated Total Reflectance (FTIR-ATR) spectra were obtained for in vivo and membrane fedcones. More than 300 scans were taken to increase the signal to noise ratio. Spectra were deconvoluted using Fityk 0.9.8 software (Wojdyr, 2010). Gaussian equations were applied to the Amide II peak until the deconvoluted spectrum matched the original curve. Secondary peaks were identified and peak wavelengths matched to known secondary structures.
2.8. Proteome
Cement cones collected from in vivo and membrane fed ticks were grouped accordingly and each group placed in a 1.5 mL micro-centrifuge tube with 250 μL of 8 M urea. The tubes were secured to a vortex and allowed to vortex overnight. The samples were centrifuged briefly on a small tabletop centrifuge to sedimentate the remaining insoluble cement fragments and the supernatant was removed. The supernatant was mixed in a 1:1 ratio with reducing Laemmali sample buffer (Bio-rad, Hercules, California) and subjected to SDS-PAGE using AnykD gels (Bio-Rad). After electrophoresis, the gel was stained using GelCode Blue overnight and destained with 3–5 washes of 20 mL water. Gel images were obtained using Bio-Rad Versa Doc white light transillumination.
Bands were manually excised from the gels and washed with 100 mM ammonium bicarbonate buffer (pH 8.5) and 100% acetonitrile mix (v/v, 1:1) until the color disappeared. The gel slices were washed with HPLC grade water followed by 100% acetonitrile, and then dried in a speedvac as described by Chao et al. (2004). The proteins were digested with trypsin (0.5 mg/mL, Promega, Madison, WI, USA) using a 50:1 ratio (protein:trypsin) overnight at 37° C. Hydrophilic peptides were eluted using NANOpure water, followed by the elution of hydrophobic peptides with 50% acetonitrile with 5% trifluoroacetic acid. The eluted peptides were dried in a speedvac and resuspended in water/acetonitrile (50:50) and 0.1% formic acid to a final peptide concentration of 1 mg/mL. The digested samples were analyzed on a LTQ Vello mass spectrometer (Thermo Electron) with in-line HPLC as described by Chao et al. (2004).
2.9. Data analysis
Protein identification was performed using the Sequest algorithm (Eng et al., 1994) in the Protein Discoverer v. 1.4 (Thermo Electron, Woburn, MA, USA) and the tick database containing 3500 tick specific polypeptides (Francischetti et al., 2009; Karim et al., 2011). The identified peptides were further evaluated using the charge state versus cross-correlation number (Xcorr). The criteria for positive identification of peptides were Xcorr >1.5 for singly charged ions, Xcorr >2.0 for doubly charged ions, and Xcorr >2.5 for triply charged ions. Only the best peptides were considered. To positively identify a protein, one tryptic peptides had to detect in all three analysis, or at least two different peptides had to be detected in a single analysis. Nonspecific matches (i.e., false positive matches) were eliminated by searching with publically available rabbit and sheep databases on NCBI (to eliminate any host proteins) and a reversed protein sequence database generated from A. americanum sialome sequence (Karim and Ribeiro, 2015).
3. Results
3.1. Feeding of tick using artificial membrane feeding
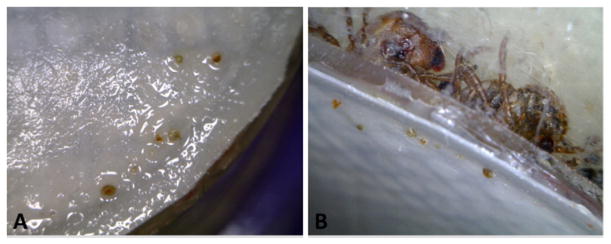
Ticks were placed inside the acrylic chamber and inspected every 24 h to gage attachment success. During the first inspection, it is common to find a majority of the tick have climbed to the cotton stopper and these ticks are placed back to the bottom of the chamber underneath the animal hair to force interaction with the membrane. Ticks which are not attached after 48 h are removed from the chamber as they are unlikely to attach after that point. After 48 h, the attached ticks are monitored daily for changes in female size indicating successful feeding. The underside of the membrane is examined at each cleaning to verify tick attachment and to search for the presence of fungal contamination or cement cones which can be removed as experimental design dictates (Fig. 1).
Fig. 1.
Hypostomes and cement cones on the underside of the membrane feeding apparatus. (A) Six hypostomes are visible piercing through the surface of the membrane. (B) The same region of the membrane from another angle shows the ticks in the chamber attached to the membrane.
By checking the membrane each day, cement cone growth can be monitored and documented. As seen in Fig. 1, the ticks cluster together when feeding. Because of this, tick attachment does not always happen at straight through the skin or membrane, often times happening at an angle where the hypostome takes a longer path to the blood supply. The two ticks visible through the chamber wall are a good example of this. The tick to the right has attached directly through the membrane and the hypostome is protruding through the membrane more than the surrounding hypostomes. The tick to the left has attached at an angle most likely because of crowding. The hypostome of this tick does not protrude through the membrane as extensively as the first tick. It would be interesting to correlate the angle of attachment and consequently the amount of hypostome present on the other side of the membrane with the engorgement size and engorgement time to determine if there is any change in the ability to feed.
The daily observation of the attached ticks revealed some differences between in vivo and membrane fed ticks. Ticks which are fed on live animals attach within the first 24 h; however, tick which are fed using the in vitro feeding system show a delayed attachment with the highest attachment rates after 48 h. Along with the increased attachment period, there is also a decrease in total tick attachment. Feeding of ticks on live animals during the proper seasons often results in attachment rates close to 90%. However, when ticks are fed using the membrane feeding system, attachment rates range between 50 and 75%.
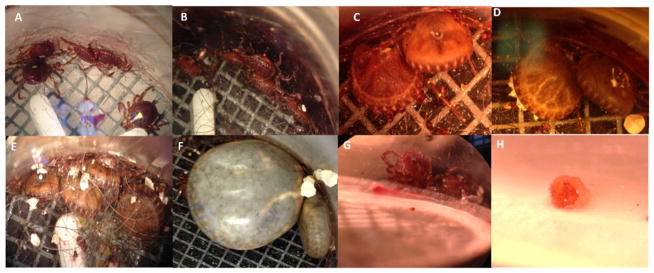
A. americanum fed on live animals reach an engorged state after 12 days of feeding. The size of female ticks changes slowly during the first 5–7 days with drastic changes taking place in the final 3–4 days of feeding. Ticks fed using an in vitro feeding system require a longer feeding period before reaching the fast feeding stage (Fig. 2). The ticks fed using artificial membrane feeding can be examined in lab using a dissecting microscope so changes in tick size can be more clearly distinguished. Ticks that are fed in vivo can only be examined with a microscope after being removed from the host. The progress of feeding from 2 to 10 days (Fig. 2A–E) shows that it takes more than 10 days for the tick to enter the fast feeding stage. Fig. 2F shows a tick that has been attached to the membrane for 24 days. After 15 days of feeding changes in size slowed. Eggs were visible underneath the cuticle however, the tick was unable to detach. The tick was forcibly removed and placed in a humid chamber for ovipositioning however, no eggs were deposited.
Fig. 2.
Feeding progression of ticks using artificial membrane feeding. Pictures were taken to document changes in tick size as feeding continued. Ticks have been feeding for (A) 2 days, (B) 4 days, (C) 6 days, (D) 8 days, (E) 10 days, and (F) 24 days. (G) Attached tick can be seen through the chamber wall and the hypostome is visible through the membrane. (H) Close examination of the hypostome shows a small cement cone that has been secreted from the tick.
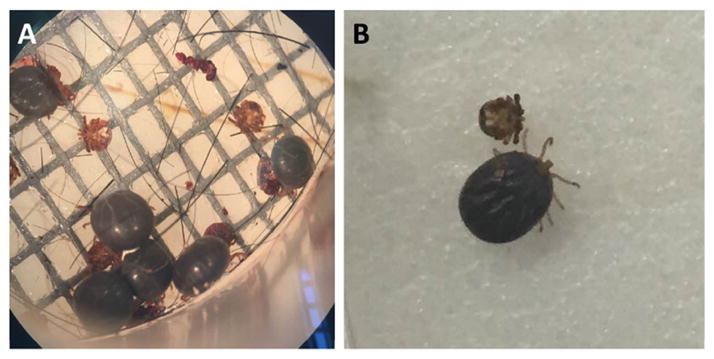
Nymphal ticks were also fed using the artificial membrane feeding system (Fig. 3). In laboratory settings, nymphs are commonly fed on small mammals such as hamsters or mice. Nymphs fed in the artificial membrane feeding system were weighed and stored in a plastic vial inside a high humidity chamber to monitor the molting process. The nymphal ticks that fed to engorgement weights higher than 10.0 mg (one exception of 7.30 mg) molted into female adult ticks (Table 1). Of the 11 nymphs that successfully fed and molted, seven molted into adult females.
Fig. 3.
A. americanum nymphs fed using an artificial membrane feeding apparatus. (A) Engorged nymphal ticks can be seen attached to the membrane ready to drop off. (B) Comparison of unfed and fully engorged nymph ticks.
Table 1.
Sex of molted adult ticks and the weights of nymphal ticks fed using an artificial membrane feeding system.
| Engorged nymphs that molted into adult females (mg) | Engorged nymphs that molted into adult males (mg) |
|---|---|
| 13.7 | 8.9 |
| 12.2 | 7.3 |
| 10.9 | 6.6 |
| 10.5 | 5.5 |
| 10.3 | – |
| 10.0 | – |
| 7.30 | – |
| Mean 10.7 ± 1.8 | Mean 7.1 ± 1.0 |
3.2. Description of cement cone
In Fig. 4 two female ticks are shown which have been removed from a host sheep. The cement cone is visible from the dorsal and ventral views (Fig. 4A and B, respectively) and the hypostome is visible through the cone. The tip of the cone is tinted a slight red color indicating that it may have been in direct contact with blood. The cone appears to be relatively smooth with a pointed tip. It is important to note that it is difficult to remove all layers of skin on the cement cone and that some of what is visible is likely residual skin layers.
Fig. 4.
In vivo fed adult female Amblyomma americanum ticks removed from a sheep during feeding. The cement cone is visibly attached to the hypostome of the right tick from both the dorsal (A) and ventral (B) views. The cone located on the hypostome of the tick on the right covers the hypostome for the purpose of tick attachment and protection of the hypostome.
Ticks fed using an artificial membrane feeding system also form a cement cone that surrounds the mouthparts (Fig. 5). Inspection of these cones can take place much earlier than in vivo fed ticks (Fig. 5A) and the same cement cone can be observed the course of the bloodmeal. The general morphological characteristics of the cone remain regardless of feeding type. The cone is slightly transparent with the mouthparts still visible through the cement (Fig. 5B) as seen in the in vivo fed cones (Fig. 4) and the cement cone base is wide across the surface of the membrane. However, there is a lack of structural definition in the membrane fed cement cones as observed when removing the cones from the tick’s mouthparts. When the cone is grasped with the forceps, there is more give to the cone indicating that the cone has not completed the hardening process. To better preserve the microstructures on the cement cone surface, ticks can also be removed from the membrane by cutting the membrane away and the cones can be visualized on the mouthparts (Fig. 5C) and removed the same as in vivo fed cones. By removing cement cones in this way differences in cement cone size and shape can clearly be seen. Often the cones are flat and widespread on the tick mouthparts (Fig. 5C).
Fig. 5.
Arificial membrane fed ticks with visible hypostomes. The external view of the artificial membrane feeding apparatus membrane (A) allows for the visualization of the cement cone (B) as the cone is forming. Ticks can be removed from the feeding apparatus with cones intact (C) and without contamination for further analysis.
3.3. Comparison of SEM images
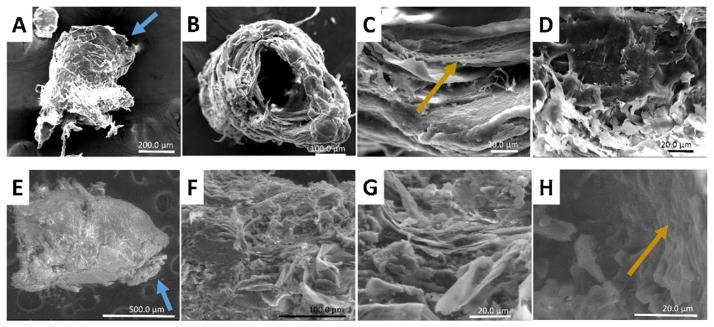
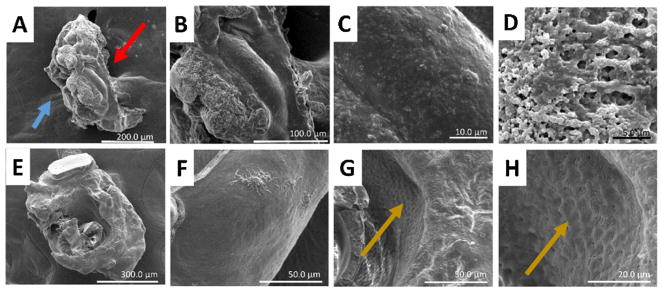
Cement cones collected from both in vivo (Fig. 6) and membrane (Fig. 7) fed ticks were subjected to SEM for further analysis of the cone surface. Fig. 6 examines two in vivo cones (Fig. 6A–D and E–H). Each in vivo cone is approximately 250 μm at the base and extends between 300 and 700 μm. The outer surface has regions which appear relatively smooth near the tip of the cone (blue arrow) as well as highly textured regions near the base. In Fig. 6B the cone is positioned such that the opening in which the mouthparts fit is visible. In this view, the layering of the cone can easily be seen. The layering of the protein secretions causes a basket weaving structure consisting of both sheet like arrangements and fiber arrangements (Fig. 6C). Interestingly, this view also shows the presence of small circular indentations aligned along the inner edge of the cone opening (yellow arrow). Close examination of the outer regions of the cone shows an overall smooth appearance with extensive flaking near the edges. Similar structures can be found in the second cone (Fig. 6E–H). Images 6F and 6 G exhibit the same sheet and fiber basket weaving as seen in Fig. 6B. In addition to these assemblies, Fig. 6G also reveals clusters of clumps similar to cauliflower. The inner edge of the mouthpart opening visualized in Fig. 6H also contains the indentations (yellow arrow) as seen in Fig. 6C, although the definition is decreased.
Fig. 6.
Scanning Electron Microscopy (SEM) images for cones collected from in vivo sheep fed ticks after five or more days of feeding. These cones contain multiple morphological characteristics such as fibers (B), layers (C and G), flaking (D) and indentions (C and H). The blue arrows indicate the tip of the cement cone which comes in contact with the bloodmeal. The yellow arrows identifies the areas of hypostome indentions. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7.
Scanning Electron Microscopy (SEM) of cement cones collected from membrane fed ticks. Comparison of a cone collected from a tick which had only been feeding 24 h (A–D) and shows different morphology than longer fed cones (E–H). In these cones, a distinction can be made between primary (D) and secondary (C) cement. The base of the cement cone is indicated with red arrow. Blue arrow identifies the tip of the cement cone. Also, the layering of the recurved teeth on the hypostome is visible along the inner walls of the cone (G and H) and indicated by yellow arrows. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 7A–D shows the structural morphology of an artificially membrane fed cone collected 24 h after attachment and compared to a membrane fed cone 7 days post-attachment (Fig. 7E–H). The 24 h membrane fed cone is much smaller than the in vivo fed cones, measuring 250 μm across and approximately 100 μm long (Fig. 7A). The overall topography of the cone exhibits large mounds (Fig. 7A), which are not present in any of the cones collected from later time points. Closer examination of the cone reveals two distinct textures (Fig. 7B), a remarkably smooth surface located closest to the base (red arrow) and a more porous region near the tip of the cone (blue arrow). Close examination of the cone base (Fig. 7C) shows areas which were previously heavily textured prior to the full curation of the primary cement.
As the feeding progresses, the cement additional layers of cortex cement adds bulk to the cement cone. In Fig. 7E, the cone is turned upward so that the location of the hypostome can be clearly seen. This cone has a smooth micro surface with a varied topography of the cone as a whole. The outer surface (Fig. 7F) displays a smooth surface similar to that seen in Fig. 7C. However, irregular features are observed on the surface, which maybe the result of contamination from immune cells found in the blood or a fungal contaminant resulting from the constant moist conditions. Inspection of the inner surface of the cone, where the hypostome would have been located (Fig. 7G), reveals a pattern of indentions (yellow arrow). Close examination (Fig. 7H) of this area shows that the indentions (yellow arrow) are formed in rows down the length of the cone opening. Interestingly, the fibrous/basket weaving formations seen in in vivo formed cement cones (Fig. 6) are completely absent from membrane fed cones.
3.4. Comparison of fourier transform infrared spectroscopy attenuated total reflectance (FTIR-ATR) images
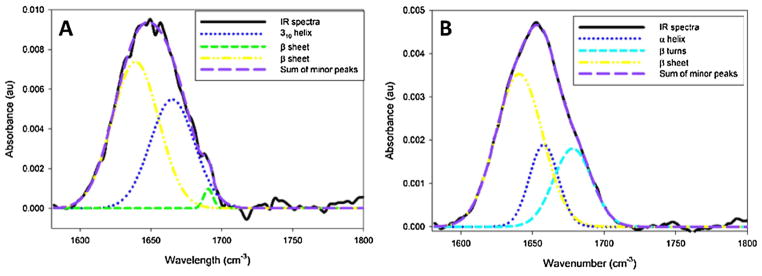
In order to obtain information regarding the protein composition of the cement cones, FTIR-ATR was used to determine the secondary structures of the proteins present on the outer surface of the cone. Using this method, it was determined that all cement cones (in vivo and membrane fed) contain to some extent β-sheet structures and most of the cement cones contain β turns (Figs. 8 and 9, respectively). The cones collected from in vivo fed ticks also possess proteins in a helical confirmation (310 helix in Fig. 8A and α helix in Fig. 8B). In each in vivo cone, β sheet structures represent over half of the total structures found (Fig. 8A–B). The percent of helical structures present varies between the in vivo fed cones, with more helix formation in the lab fed cement cone (Fig. 8A) compared to the field collected cement cones (Fig. 8B). The remaining secondary structure identified in Fig. 8B corresponds to spectra from β turns.
Fig. 8.
FT-IR spectra with Gaussian deconvolution for the identification of protein secondary structure. In vivo cones contain a mixture of β sheet and helical structural components.
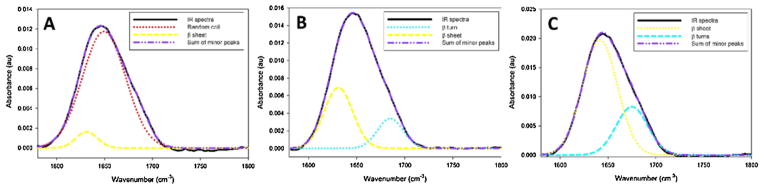
Fig. 9.
FT-IR spectra with Gaussian deconvolution of membrane collected cement cones. Cones collected later in feeding contain primarily β sheet structures while a cone which was collected during the first 3 days of feeding is primairly random coil in structure.
Cones collected from in vitro fed ticks have a markedly different spectrum from in vivo fed cones. Each of the membrane fed cones (Fig. 9A–C) contains only two secondary structures. Analysis of a cement cone collected from a membrane fed female tick after just 72 h of feeding reveals almost exclusively random coil structures with a minor portion of the spectra assigned to β-sheet structures (Fig. 9A). The remaining two cones collected from membrane fed ticks (female after seven days of feeding and male after five days of feeding) show less variation in their structural analysis (Fig. 9B and C, respectively). These cones contain predominantly β sheet structures (Fig. 9B–C). The remaining structures of the cement cones analyzed in Fig. 9B–C consists of β-turns.
3.5. Comparative list of identified proteins
The solubilization of tick cement cones in 8 M urea does not dissolve the cone completely but does allow for the investigation of the soluble fraction of the cement cone. SDS-PAGE analysis of the soluble proteins shows multiple proteins ranging from 10 to 250 kDa (Fig. 10). To identify these proteins, the bands were subjected to trypsin digestion in gel segments as indicated by the boxes in Fig. 7. The digested protein fragments were subjected to LC–MS/MS and mapped using a previously generated transcriptome from the salivary glands of A. americanum (Karim and Ribeiro, 2015). It is important to note that there are many more proteins present in the SDS-PAGE than are identified by LC–MS/MS. The peptides identified by LC–MS/MS are first mapped using host based databases. The remaining unidentified peptides are then mapped using to the tick sialotranscriptome. Any peptide that does not meet the strict criteria is removed from the analysis. This causes a large number of peptides to remain unidentified.
Fig. 10.
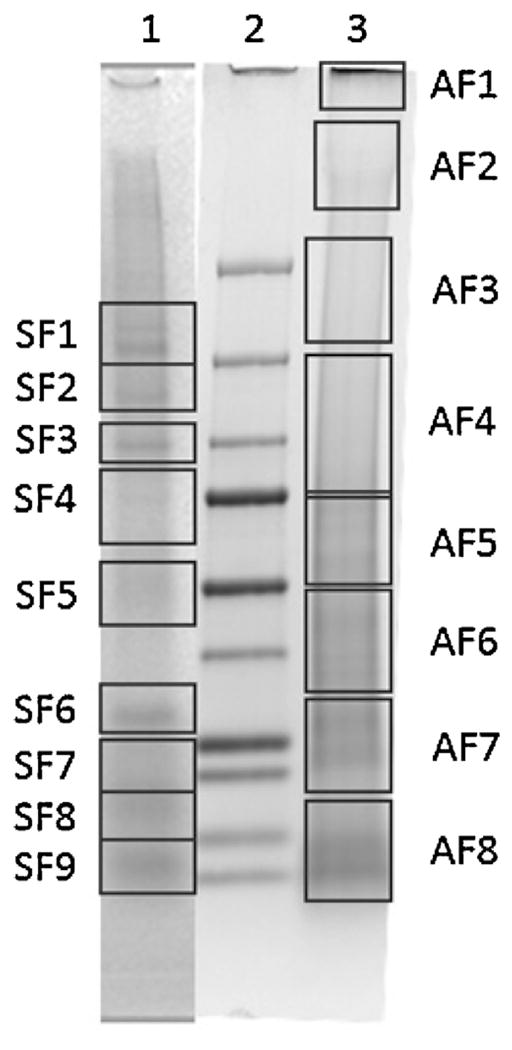
SDS-PAGE of cement soluble fraction. The cones were dissolved in 8 M urea overnight and subsequently run on a SDS-PAGE. The bands were excised as indicated by the boxes and digested using trypsin. Lane 1—in vivo fed cone, Lane 2—molecular weight marker (from bottom: 10 kDa, 15 kDa, 20 kDa, 25 kDa, 37 kDa, 50 kDa, 75 kDa, 100 kDa, 150 kDa, 250 kDa), Lane 3—membrane fed cone.
The proteins identified in the segments SF1-SF9 from in vivo cones show interesting results (Table 2). Only seven A. americanum proteins were identified from only four gel segments (SF2, SF3, SF4, and SF9). Four of these proteins are considered intracellular proteins with functions necessary for cell maintenance (Putative deSUMOylating isopeptidase, putative cytochrome p450, putative ribosomal protein I6, and putative Histone H2B). The remaining proteins identified from the cement cones include a glycine rich protein of nearly 70 kDa, a metalloprotease, and a hypothetical secreted protein with no known function. Also identified from the cement cones were host proteins. This is typical in in vivo cones as there is skin and hair embedded within the cement structure and it is possible for proteomic analysis of in vivo cones to contain more host proteins than tick proteins. It is also important to note that the remaining cement present after solubilization may contain the bulk of cement proteins and only the outer layers and host contaminates were solubilized by the urea. This would account for the low identification of tick proteins. The use of artificial membrane feeding reduces the amount of host contamination present in the cones however, proteins from the blood can also be sources of contamination. Cones that were collected from membrane fed ticks resulted in the identification of many more proteins than those found in in vivo cones. From the eight segments (AF1–AF8) excised from Lane 3 of Fig. 10 from an artificial membrane collected cone, six of these segments (AF2, AF4–AF8) led to the identification of a mixture of 26 secreted and non-secreted proteins (Table 3). Identified secreted proteins include glycine rich proteins, serine protease inhibitors, metalloproteases, and unclassified secreted proteins.
Table 2.
Proteins identified from cement cones collected from in vivo fed ticks.
| Gel Slice | Protein ID | Peptide Sequences | % Coverage | MW (kDa);pI |
|---|---|---|---|---|
| SF2 | Putative deSUMOylating isopeptidase 2, partial GI: 759085692 |
YHLMNK | 3.53 | 19.0; 8.19 |
| Putative cytochrome p450 4w1, partial GI: 759084668 |
GRKLpK | 11.93 | 12.7; 9.99 | |
| SF3 | Putative Glycine-rich secreted cement protein, partial GI: 759090220 |
YPGLSGLYGR | 4.25 | 69.2; 9.41 |
| SF4 | Putative ribosomal protein I6 GI: 759086918 |
TGLLMVTGpYGINGcPLRR | 13.12 | 32.1; 11.05 |
| AamerSigP-2853 | TWWSRWLSRDIFIAVVIASMSATFSWLWR | 27.62 | 12.2; 10.46 | |
| SF9 | Putative secreted metalloprotease, partial GI: 759089918 |
LLGYLCVMVNSANLRYQDTVAPRVK | 10.78 | 26.0; 7.02 |
| Putative Histone h2b, partial GI: 759084736 |
LLLPGELAK | 7.89 | 12.8; 10.54 |
SF—sheep fed sample.
Table 3.
Proteins identified from cement cone collected from membrane fed ticks.
| Gel Slice | Protein ID | Peptide sequences | % Coverage | MW (kDa);pI |
|---|---|---|---|---|
| AF2 | Serine protease inhibitor GI:805449283 |
LIDTPVDLALPK LLSKLIDTpVDLALPK MTILLpR |
15.75 | 15.9; 6.96 |
| Putative RNA recognition motif 1, partial GI:759085422 |
VATSRAIR KPRLIVR |
9.68 | 17.1; 9.99 | |
| Hypothetical protein, partial GI:759085430 |
ELFDEIWTLLR | 7.14 | 18.4; 4.48 | |
| Putative p1 ap, partial GI:759087220 |
VGCPMxxxxLSARIIQCYLATRMHFYAR KSAKMTDCSDCHATLVAASDVPPAAILTELR |
17.56 | 37.5; 8.35 | |
| AF4 | Putative tick metalloprotease, partial GI:759089644 |
MEGLVGpRHRIEPLSVSEK LIVLVVLVTVPTKGLEQpMLVYpRLLEER |
24.37 | 22.5; 6.07 |
| Putative metallopeptidase, partial GI:759086118 |
IDGEKSIIQNPTEAQRK | 8.37 | 22.8; 5.54 | |
| Hypothetical protein, partial GI:759086210 |
LFAKQQGNVGAQALSPALTGKR | 10.38 | 22.9; 9.20 | |
| Serine protease inhibitor, partial GI:805448403 |
RSLAIFVpAPSSNLAALEK VSAAKHLAVFRAGHR |
13.13 | 28.5; 6.38 | |
| Serine protease inhibitor, partial GI:805449067 |
RAQPPppVEFRVEHp TGGKIPK |
5.71 | 42.4; 9.20 | |
| AF5 | Hypothetical protein GI:759090180 |
ENLVANTVAGPALLDTAATTVR | 5.58 | 39.1; 9.19 |
| Putative coiled-coil domain-containing protein, partial GI:759087600 |
TEKLQFTKDEPK | 2.62 | 52.3; 8.91 | |
| Putative tick metalloprotease, partial GI:759089956 |
QLNVSNSTFEEK | 4.98 | 27.0; 5.08 | |
| AF6 | Putative cement protein RIM36, partial GI:196476756 |
VITDPSTGLPIAQAVYIGIVR | 12.65 | 16.8; 9.41 |
| Putative purine nucleoside phosphorylase, partial GI:759086528 |
VFGLSLISNECISNYDTQQVANHEEVLETGQKRK | 14.47 | 26.2; 8.76 | |
| Putative secreted protein precursor, partial GI:759089510 |
NARDYEcNNHHEENYCPGQSpLQCKGGNVCVCDR | 18.78 | 21.0; 8.40 | |
| AF7 | Hypothetical protein GI:759090180 |
ENLVANTVAGPALLDTAATTVR | 5.58 | 39.1; 9.19 |
| AF8 | Putative DNA replication licensing factor mcm4 component GI:759087732 |
KAIACLLFGGSVKR ETVPNVPIKPGLEGYALPR ITAIGVYSIK KGPQEK cNSDRAGQPKCPVDpFFIVpDK HLASSPNIYERIAKSIApSIYGFADVK GEIQHR cGpRLSATAAEK |
15.85 | 82.1; 7.91 |
| Putative transcription factor a mitochondrial, partial GI:759085292 |
KPRSPRSAYAFFCIEAR | 11.64 | 17.1; 8.95 | |
| Hypothetical protein GI:759088860 |
mRARSVAVFSLLLHSTSppSQK | 17.05 | 14.0; 10.29 | |
| Putative methylthioadenosine phosphorylase mtap, partial GI:759086166 |
QLQIpHHEAGTVVTIEGPR | 9.09 | 22.9; 8.15 | |
| Putative secreted protein precursor, partial GI:759089400 |
LLVCISSASNQAKTLLSK QKVSSR |
14.12 | 19.0; 9.04 | |
| Putative n-acetylglucosamine kinase, partial GI:759087196 |
NAGLSEEVR GATLSK LAKLAKEGDELSK |
8.48 | 35.9; 6.61 | |
| Putative myosin class vi heavy chain, partial GI:759085786 |
ELLDTIFSFLAR | 6.78 | 20.3; 5.07 | |
| Putative golgi vesicular membrane trafficking protein p18 GI:759084714 |
MLQDmNTDFDAGEGILKSTmGRLVK | 22.52 | 12.8; 9.23 | |
| Putative tick cystatins 1 GI:759088844 |
MARSVSVVAVLAVcIAAcVASIPGGWSAQEPQSSPKYK | 29.46 | 14.1; 5.48 | |
| Putative secreted protein precursor GI:759088682 |
SLDmLLPVDTDpQVIIRR RGHKPK |
19.01 | 13.5; 9.35 |
AF—artificially fed sample.
4. Discussion
4.1. Cement cones from both feeding systems have similar characteristics
The tick hypostome has recurved teeth which are believed to assist the tick in piercing the host skin and initial attachment to the host. The firm attachment of the tick to the host is necessary for the tick to maintain access to the bloodmeal. Soon after attachment, the tick begins to secrete a highly proteinaceous saliva into the host. Some components of this saliva begin to aggregate and form a hardened cement surrounding the mouthparts, referred to as a cement cone. While fluid, this cement intertwines itself between the layers of the host’s skin and fits firmly within the bite cavity. Cement cones are secreted in the first days of feeding and harden through the following days. This would imply that after a certain point in feeding the cement cone has completed its formation. Although the cones here have been collected at different time points, the cement cone formation is complete after approximately four days (Moorhouse and Tatchell, 1966). The complete formation of the cement cone allows for the comparison of the cement cones collected after any point after four days of feeding. As the tick is forcibly removed, the cement cone is often left buried in the skin of the host as seen with the left tick presented in Fig. 4. The cone extends past the hypostome in both in vivo and membrane fed ticks (Fig. 4A and B, respectively). Upon examination the cement cone completely encompasses the tick mouthparts. This indicates that there must be some opening of the cone to allow for the uptake of the blood-meal. Histological staining of tick bite site biopsies has revealed an opening at the tip of the cone (Chinery, 1973). This opening was present through the entirety of the cone so that the blood was drawn into the cone and then taken into the tick by its hypostome. This way, the hypostome never comes into direct contact with the in vivo blood pool reducing the chances of immune system activation (Binnington and Kemp, 1980; Bishop et al., 2002). Our finding of cone discoloration near the tip of the in vivo cone supports the histology data. Many of the proteins previously identified from solubilized cement cones are host contaminate proteins even when the cone has been rinsed with PBS and ethanol. The presence of these proteins after washing is a direct consequence of the blood present within the center of the cone.
One significant difference observed between the two cones types is the changes in the curing process. Although the exact curing processes is not understood, in vivo fed ticks use air flow as a means of drying the cement (Kemp et al., 1982). This allows for both the primary and secondary cement to harden albeit at different rates. However, due to the lack of a dermis in the artificial membrane system, air flow to the cement is minimal which causes the cones to remain soft. The cone is still intact indicating some level of aggregation or cross-linking involved in the curation process. In order to collect accurate structural data from the membrane fed cement cones, great care is taken to remove the cone from the hypostome and the cone is placed into a 1.5 mL tube and the drying process is allowed to finish at room temperature for at least 24 h before long term storage.
It is important to note that during the cone removal process there is significant pulling of the hypostome. This stress on the hypostome would occur in nature when a tick is attached to an unsuitable location for feeding. It would be advantageous for the tick to quickly detach from the host to prevent possible death by being pressed against a hard surface or by being pulled apart. When the hypostome is pulled during the cone removal, the tick secretes a few microliters of saliva which is able to completely dissolve the cement cone in less than one minute (unpublished data). The components of this secretion have not yet been elucidated due to the small volume present however the use of this secretion is of interest. After the secretion of this compound, the cone does still persist which indicates that the tick uses this secretion only to loosen the hypostome for removal. This quick solubilization is remarkable, as the cones are otherwise insoluble in anything other than hot acids. The identification of the compounds present in the secretion could have many applications in the biomedical field such as dissolution of blood clots and plaques.
4.2. SEM reveals differences at the cone surface
The skin of a host can be millimeters in thickness and the tick hypostome must reach through the full thickness to reach the bloodmeal. In vivo cones are embedded in the host skin and therefore create an interlocking network of host tissue and cement. Impressions of this network are recognizable by SEM of in vivo fed cones (Fig. 6). The layering and texture surrounding these cones could easily allow for a more secure attachment to the host. The membranes of the artificial feeding system are typically less than one millimeter and therefore the cone is present solely on the outer surface of the membrane removing the rigid surrounding matrix of the skin. This could explain the lack of layering and fibrous textures easily visualized in the in vivo cones.
Along the inner surfaces of the cones examined in this work, the impressions left by the hypostome teeth are clearly visible (yellow arrows in Figs. 6 and 7). It is a reasonable conclusion that the indentions found are caused by these structures on the hypostome. When the cement cone is forcibly removed from the mouthparts, the hypostome remains imbedded in the cement and the teeth can be seen extending out of the cement (Data not shown). As the tick is feeding the hypostome is filled with fluid, either saliva or blood. Because of the presence of this extra fluid, the hypostome is slightly swollen making it fit directly into the sides of the cement (Chinery, 1973). As the tick finishes feeding, the hypostome no longer contains the same amount of fluid and therefore is smaller in size. This allows for the easy removal of the hypostome from the bite lesion.
4.3. Identification of two types of cement from membrane fed cones
Artificial membrane feeding of the ticks allows for the examination and collection of the cement cone at a much earlier point in tick feeding when compared to in vivo feeding giving us a unique look at the early stages of cone development. In Fig. 7, a cone collected approximately 24 h after attachment contains cement with two distinct differences in structure which could indicate at two different stages of the curation process. The smooth region of the cone near the base has likely been present longer and represents cement that has cured for a longer time. It has been documented that a primary cement is secreted in the early hours of feeding that cures very quickly followed by a secondary cement secreted after the initial attachment and has a longer curing time (reviewed in (Kemp et al., 1982)).
It is unknown how the cement cone fully hardens. It is known that insect cuticle hardens by a sclerotizing process which is believed to take place by protein cross-linking and dehydration. In insects, cuticle proteins are cross-linked together at tyrosine positions through interactions with quinone compounds such as catechols (Andersen, 2010). Previous proteomic analysis of in vivo fed cement cones from our lab (data not shown) has also found tick tissue transglutaminase which is able to cross-link proteins at the glutamine residues. Although tissue transglutaminase was not found in this study, this could be due to different solublization techniques or changes in the identification of transglutaminase peptides from the in-gel digestion.
4.4. Secondary structures of cement proteins exhibit minor differences
Early research noted that the cone is only soluble in hot acidic or alkali solutions however this makes proteomic analysis difficult. This lack of proteomic data has hindered cement research and many current conclusions about tick cement are purely speculative. The commonalities of tick glycine rich proteins and spider silk GRPs is a prime example of this (Maruyama et al., 2010). Spider GRPs are known to be extremely long in length with 10–20 amino acid long repeats (Tokareva et al., 2013). However, tick GRPs are smaller and repeats are typically 3–7 amino acids long or the repeats are lacking (Maruyama et al., 2010). To gather more information about the proteins present on the surface of the cement cone face, FTIR-ATR is used to determine protein secondary structure. The spectra for two in vivo fed cones (Fig. 8) show similar structural components although the amounts in which these secondary structures are found differ. The lack of helical structures in the membrane collected cones is likely due to the cone not being contaminated with host skin. The interlocking nature of the cement cone with the layers of the host skin would allow for skin cells and extracellular proteins to become imbedded within the cured cone. The fact that these structures are missing from the membrane fed cones goes to show that this method does allow for the study of tick cement cones without interfering host proteins. Comparison of the later fed membrane cones also shows that there is no significant difference between the secondary structures found in cones from male and female ticks. The presence of a full sclerotized scutum covering their backs, males do not expand during feeding as females do, and therefore they feed intermittently on the host taking in multiple small bloodmeals from the same host. The shorter feeding time of males also contributes to the difficult task of collecting cement cones from in vivo sources. Proteins of the male salivary glands have only recently begun to be investigated.
Along with time comparable cones from in vivo and artificial membrane sources, a cone removed from a membrane fed female was removed after just 3 days of feeding. This cone did not show a predominance of β-sheet structures as seen with both in vivo and longer feeding membrane cones but rather contained almost exclusively random coil structures (Kong and Yu, 2007). As seen with early cones in the SEM (Fig. 7A–D), there is a curing process that occurs which hardens the cone. Although it is not currently known how this process occurs, the data shown here indicates that a conformational shift in the proteins may play a role in this hardening process. This needs to be further investigated by measuring the secondary structures of cones collected at multiple time points of the feeding.
4.5. Proteins of in vivo and membrane fed cement cones contain the same protein families
Identification of proteins from the cement cone is a difficult process. To date, the only proteins known to be present in the cement cone include GRPs isolated from two different tick species (Rhipicephalus appendiculatus and Haemaphysalis longicornis) using immunochemistry (Bishop et al., 2002; Havlíková et al., 2009) and chitinase which was experimentally proven using RNA interference (Kim et al., 2014). However, direct solubilization of the cone for proteomic studies typically reveals more host proteins than tick proteins. The interlocking nature of the cement cone with the host dermis makes the collection of a cone free of host proteins impossible. Artificial membrane feeding removes the host dermis and therefore allows for a cement cone completely free of host skin cells.
When investigating the protein composition of cement cones, a large focus is placed on finding the structural components. However, in the comparative proteomes listed here (Tables 2 and 3) there are few proteins which would serve as a scaffolding for the cement. Proteomic analysis of the in vivo cement cones results in only seven A. americanum proteins identified from four gel regions. These proteins include both intracellular proteins such as cytochrome p450, ribosomal proteins, and histone components. There are two explanations for the presence of these proteins in the cement cone. The first is that pieces of the tick hypostome remain embedded in the cement and the cells are lysed during the solublization process releasing intracellular proteins. The second possible explanation involves the unconventional secretion of intracellular proteins using exosomes. It has been hypothesized that the secretion of these intracellular proteins could help identify new functions for these proteins (Díaz-Martín et al., 2013). Proteins identified from the cement cone which are either secreted or have enzymatic function which could be used to the benefit of the tick include deSUMOylating isopeptidase, glycine rich proteins, and a metalloprotease. DeSUMOylating isopeptidases are involved in the removal of small ubiquitin-like modifiers which can affect cellular processes and act as gene expression modifiers (Li et al., 2005). The presence of this protein in the cement cone may be the result of residual tick cells or it may be secreted from the salivary glands into the host to modulate the protein functionality of host immune proteins. A glycine rich protein was also identified in the in vivo cement cone proteome. GRPs are a class of proteins which contain more than 20% glycine in the primary sequence. These proteins are the major component of spider silks (Tokareva et al., 2013; Winkler and Kaplan, 2000)and are also found abundant in plant cell walls(Sachetto-Martins et al., 2000). It is hypothesized that the GRPs present in the cement cone give the cone its strength and insoluble characteristics. The third protein identified from the in vivo cement cone proteome which has probable function is a metalloprotease. Metalloproteases assist in the prevention of blood clot formation throughout the bloodmeal. The presences of this protein in the cement cone could be a remnant of the saliva secreted from the tick during feeding. It is also possible that the metalloprotease is necessary at the location of the cement cone to prevent blood clotting specifically at the cone by the activation of platelets by collagen like proteins in the cement.
The proteome assembled from membrane collected cones revealed many more A. americanum proteins (26 proteins from six gel sections). The membrane fed cones also contained intracellular proteins such as nucleoside phosphorylase, DNA replication factors, transcription factors, and Golgi membrane proteins. As mentioned above, these intracellular proteins are the result of tick hypostome cells embedded into the cement of the cone. Other proteins found in the membrane fed cones include metalloproteases, serine protease inhibitors, multiple hypothetical proteins with unknown functions, and a RIM36 like glycine rich protein. Thrombin is a serine protease found in the blood which is necessary to the formation of blood clots. The inhibition of thrombin by the protease inhibitors likely reduces the amount of clot formation around the cement cone. There is a high incidence of hypothetical proteins in the artificial membrane fed cement cone. These proteins have been identified as RNA transcripts from the sialotranscriptome generated for A. americanum (Karim and Ribeiro, 2015). However, these proteins have not been characterized in any other organism and so it is not possible to predict a function for these proteins.
The presence of functional proteins within the cement cone has been investigated previously. Tick tissues, saliva, and cement cones were collected from R. appendiculatus and the lysozyme activity of the tissues was measured (Alekseev et al., 1995). Although the activity did not differ greatly throughout the samples, there was a distinct difference between the cones of female and male ticks. There was also a significant increase of the lysozyme activity in cones collected from ticks infected with tick-borne encephalitis virus (Alekseev et al., 1995). This added activity found in the cone itself leaves open the possibility for many other functions embedded in the cone. The presence of proteins with known enzymatic functions within the cement cone structure supports the idea that the cone may play more than just a structural role. It should be noted that the cross-linking of the scaffolding proteins would likely make them impenetrable to the solubilization techniques used here. The interaction of 8 M urea with the cement cone is likely to only occur with proteins which are present along the surface or that are not tightly bound to each other. To further investigate these proteins, the cross-linking nature of the scaffolding would need to be disrupted.
5. Conclusions
The feeding biology of adult Ixodid ticks requires their attachment onto the host for more than 10 days. This level of attachment is facilitated by the formation of a proteinaceous cement cone which is secreted from the tick’s salivary glands in the early stages of feeding. New approaches to tick feeding include artificial methods which utilize silicone membranes as host mimics and allow for more controlled experimental designs. The use of these artificial membrane feeding methods allows for a higher success rate of cement cone collection; however, there has been little work thus far comparing the tick biology between in vivo and membrane feeding. Here, we compare the cement cones from in vivo and artificial membrane feeding methods to determine the suitability of membrane feeding methods as a comparative feeding model. Comparison of the cones under magnification shows distinct differences in the appearance of the cones however, the composition of the cone is unlikely to change due to the change in feeding method. The structures of the cones are further investigated using SEM to examine the surface topography. Using SEM, a difference can be distinguished between the two feeding types. The formation of the cone in in vivo feeding takes places within the dermal layers of the host which are likely responsible for many of the morphological changes seen here. Another aspect of cement cone research is to determine the proteins which are responsible for the cone’s composition. To do this, we utilized FTIR-ATR to determine the secondary structures found on the cone’s surface. Each of the cones contained a significant proportion of β-sheet structures. In each of the in vivo cones, a helical structure is also present. It is possible that these helical structures are due to proteins synthesized by the host skin. One final evaluation compared the proteins identified from each cone type. Cones collected from in vivo fed ticks yielded fewer identifiable tick-specific proteins than cones collected from membrane fed ticks. The presence of host skin cells on the in vivo cones are more abundant and the protein sequence database for mammals is more developed making them easier to identify. The study of cement cones collected from membrane fed ticks is an important advancement for the proteomic study of cement cones. Artificial membrane feeding systems also yields an opportunity to collect and study cement cones much earlier than in vivo feeding allows.
Acknowledgments
This work was supported by grants from the National Institute of Allergy and Infectious Diseases award #AI099919, National Science Foundation’s Experimental Program to Stimulate Competitive Research (EPSCoR) under Cooperative Agreement No. IIA1430364, United States Department of State award #PGA-P21049 (Pakistan-United States Science and Technology Cooperation Program) to SK and the National Institutes of General Medical Sciences award # P20RR016476 to the MS-INBRE core facility. The funders had no role in study design, data collection, analysis, decision to publish, or manuscript preparation.
References
- Alekseev AN, Burenkova L, Podboronov aVM, Chunikhin SP. Bacteriocidal qualities of ixodid tick (Acarina: Ixodidae) salivary cement plugs and their changes under the influence of a viral tick-borne pathogen. J Med Entomol. 1995;32:578–582. doi: 10.1093/jmedent/32.5.578. [DOI] [PubMed] [Google Scholar]
- Andersen SO. Insect cuticular sclerotization: a review. Insect Biochem Mol Biol. 2010;40:166–178. doi: 10.1016/j.ibmb.2009.10.007. http://dx.doi.org/10.1016/j.ibmb.2009.10.007. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Magnarelli LA. Biology of ticks. Infect Dis Clin North Am. 2008;22:195–215. doi: 10.1016/j.idc.2007.12.006. http://dx.doi.org/10.1016/j.idc.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Andrade JJ, Xu G, Rich SM. A silicone membrane for in vitro feeding of Ixodes scapularis (Ixodida: Ixodidae) J Med Entomol. 2014;51:878–879. doi: 10.1603/me13180. [DOI] [PubMed] [Google Scholar]
- Binnington KC, Kemp DH. Role of tick salivary glands in feeding and disease transmission. Adv Parasitol. 1980;18:315–339. doi: 10.1016/s0065-308x(08)60403-0. [DOI] [PubMed] [Google Scholar]
- Bishop R, Lambson B, Wells C, Pandit P, Osaso J, Nkonge C, Morzaria S, Musoke A, Nene V. A cement protein of the tick Rhipicephalus appendiculatus, located in the secretory e cell granules of the type III salivary gland acini, induces strong antibody responses in cattle. Int J Parasitol. 2002;32:833–842. doi: 10.1016/s0020-7519(02)00027-9. S0020751902000279[pii] [DOI] [PubMed] [Google Scholar]
- Burden DK, Barlow DE, Spillmann CM, Orihuela B, Rittschof D, Everett RK, Wahl KJ. Barnacle Balanus amphitrite adheres by a stepwise cementing process. Langmuir. 2012;28:13364–13372. doi: 10.1021/la301695m. http://dx.doi.org/10.1021/la301695m. [DOI] [PubMed] [Google Scholar]
- Chao CC, Wu SL, Ching WM. Using LC–MS with de novo software to fully characterize the multiple methylations of lysine residues in a recombinant fragment of an outer membrane protein from a virulent strain of Rickettsia prowazekii. Biochim Biophys Acta. 2004;1702:145–152. doi: 10.1016/j.bbapap.2004.08.013. http://dx.doi.org/10.1016/j.bbapap.2004.08.013, S1570-9639(04)00212-2[pii] [DOI] [PubMed] [Google Scholar]
- Chinery WA. The nature and origin of the cement substance at the site of attachment and feeding of adult Haemaphysalis spinigera (Ixodidae) J Med Entomol. 1973;10:355–362. doi: 10.1093/jmedent/10.4.355. [DOI] [PubMed] [Google Scholar]
- Díaz-Martín V, Manzano-Román R, Valero L, Oleaga A, Encinas-Grandes A, Pérez-Sánchez R. An insight into the proteome of the saliva of the argasid tick Ornithodoros moubata reveals important differences in saliva protein composition between the sexes. J Proteomics. 2013;80C:216–235. doi: 10.1016/j.jprot.2013.01.015. http://dx.doi.org/10.1016/j.jprot.2013.01.015. [DOI] [PubMed] [Google Scholar]
- Eng J, McCormack A, Yates J. An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J Am Soc Mass Spectrom. 1994;5:976–989. doi: 10.1016/1044-0305(94)80016-2. [DOI] [PubMed] [Google Scholar]
- Fourie JJ, Stanneck D, Luus HG, Beugnet F, Wijnveld M, Jongejan F. Transmission of Ehrlichia canis by Rhipicephalus sanguineus ticks feeding on dogs and on artificial membranes. Vet Parasitol. 2013;197:595–603. doi: 10.1016/j.vetpar.2013.07.026. http://dx.doi.org/10.1016/j.vetpar.2013.07.026. [DOI] [PubMed] [Google Scholar]
- Francischetti IMB, Sa-Nunes A, Mans BJ, Santos IM, Ribeiro JMC. The role of saliva in tick feeding. Front Biosci. 2009;14:2051–2088. doi: 10.2741/3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregson J. Morphology and functioning of the mouthparts of Dermacentor andersoni Stiles. Part II. The feeding mechanism in relation to the host. Acta Trop. 1960;17:72–79. [PubMed] [Google Scholar]
- Havlíková S, Roller L, Koci J, Trimnell AR, Kazimírová M, Klempa B, Nuttall PA. Functional role of 64P, the candidate transmission-blocking vaccine antigen from the tick, Rhipicephalus appendiculatus. Int J Parasitol. 2009;39:1485–1494. doi: 10.1016/j.ijpara.2009.05.005. http://dx.doi.org/10.1016/j.ijpara.2009.05.005. [DOI] [PubMed] [Google Scholar]
- He LS, Zhang G, Qian PY. Characterization of two 20 kDa-cement protein (cp20k) homologues in Amphibalanus amphitrite. PLoS One. 2013;8:e64130. doi: 10.1371/journal.pone.0064130. http://dx.doi.org/10.1371/journal.pone.0064130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Ribeiro JM. An insight into the sialome of the Lone Star tick, Amblyomma americanum, with a glimpse on its time dependent gene expression. PLoS One. 2015;10:e0131292. doi: 10.1371/journal.pone.0131292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim S, Singh P, Ribeiro JMC. A deep insight into the sialotranscriptome of the gulf coast tick, Amblyomma maculatum. PLoS One. 2011;6:e28525. doi: 10.1371/journal.pone.0028525. http://dx.doi.org/10.1371/journal.pone.0028525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp DH, Stone BF, Binnington KC. Tick attachment and feeding: role of the mouthparts, feeding apparatus, salivary gland secretions and the host response. In: Obenchain FD, Galun R, editors. Physiology of Ticks. Pergamon Press; Elmsford: 1982. pp. 119–168. [Google Scholar]
- Kim TK, Curran J, Mulenga A. Dual silencing of long and short Amblyomma americanum acidic chitinase forms weakens the tick cement cone stability. J Exp Biol. 2014 doi: 10.1242/jeb.107979. http://dx.doi.org/10.1242/jeb.107979. [DOI] [PMC free article] [PubMed]
- Kong J, Yu S. Fourier Transform Infrared Spectroscopic Analysis of Protein Secondary Structures Protein FTIR Data Analysis and Band Assignment. 2007;39:549–559. doi: 10.1111/j.1745-7270.2007.00320.x. http://dx.doi.org/10.1111/j.1745-7270.2007.00320.x. [DOI] [PubMed] [Google Scholar]
- Kröber T, Guerin P. The tick blood meal: from a living animal or from a silicone membrane? ALTEX. 2007;24(Spec No):39–41. [PubMed] [Google Scholar]
- Kröber T, Guerin PM. In vitro feeding assays for hard ticks. Trends Parasitol. 2007b;23:445–449. doi: 10.1016/j.pt.2007.07.010. http://dx.doi.org/10.1016/j.pt.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Li S-J, Hankey W, Hochstrasser M. Preparation and characterization of yeast and human desumoylating enzymes. Methods Enzymol. 2005;398:457–467. doi: 10.1016/S0076-6879(05)98037-3. http://dx.doi.org/10.1016/S0076-6879(05)98037-3. [DOI] [PubMed] [Google Scholar]
- Li D, Huson MG, Graham LD. Proteinaceous adhesive secretions from insects, and in particular the egg attachment glue of Opodiphthera sp. moths. Arch Insect Biochem Physiol. 2008;69:85–105. doi: 10.1002/arch.20267. http://dx.doi.org/10.1002/arch.20267. [DOI] [PubMed] [Google Scholar]
- Maruyama SR, Anatriello E, Anderson JM, Ribeiro JMJMCJM, Brandão LG, Valenzuela JG, Ferreira BR, Garcia GR, Szabó MP, Patel S, Bishop R, de Miranda-Santos IK, Brandao LG, Valenzuela JG, Ferreira BR, Garcia GR, Szabo MP, Patel S, Bishop R, de Miranda-Santos IK, Brandão LG, Valenzuela JG, Ferreira BR, Garcia GR, Szabó MP, Patel S, Bishop R, de Miranda-Santos IK. The expression of genes coding for distinct types of glycine-rich proteins varies according to the biology of three metastriate ticks, Rhipicephalus (Boophilus) microplus, Rhipicephalus sanguineus and Amblyomma cajennense. BMC Genomics. 2010;11:363. doi: 10.1186/1471-2164-11-363. http://dx.doi.org/10.1186/1471-2164-11-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhouse D, Tatchell R. The feeding processes of the cattle tick Boophilus microplus (Canestrini): a study into the host-parasite relations. Part I. Attachment to the host. Parasitology. 1966;56:623–632. doi: 10.1017/s003118200007164x. [DOI] [PubMed] [Google Scholar]
- Oliver J, Lynn G, Burkhardt N, Price L, Nelson C, Kurtti T, Munderloh U. Infection of immature Ixodes scapularis (Acari: Ixodidae) by membrane feeding. J Med Entomol. 2016;53:409–415. doi: 10.1093/jme/tjv241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick CD, Hair JA. Laboratory rearing procedures and equipment for multi-host ticks (Acarina: Ixodidae) J Med Entomol. 1975;12:389–390. doi: 10.1093/jmedent/12.3.389. [DOI] [PubMed] [Google Scholar]
- Sachetto-Martins G, Franco LO, de Oliveira DE. Plant glycine-rich proteins: a family or just proteins with a common motif? Biochim. Biophys Acta—Gene Struct Expr. 2000;1492:1–14. doi: 10.1016/s0167-4781(00)00064-6. http://dx.doi.org/10.1016/S0167-4781(00)00064-6. [DOI] [PubMed] [Google Scholar]
- Stone B, Binnington KC, Court R. The attachment cement of the cattle tick Boophilus microplus: formation and composition. Abstracts of the Proceedings of 8th Int. Conf; WAAVP Sydney, Australia. 1977. [Google Scholar]
- Tokareva O, Jacobsen M, Buehler M, Wong J, Kaplan DL. Structure-function-property-design interplay in biopolymers: spider silk. Acta Biomater. 2013;10:1612–1626. doi: 10.1016/j.actbio.2013.08.020. http://dx.doi.org/10.1016/j.actbio.2013.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler S, Kaplan DL. Molecular biology of spider silk. J Biotechnol. 2000;74:85–93. doi: 10.1016/s1389-0352(00)00005-2. [DOI] [PubMed] [Google Scholar]
- Wojdyr M. Fityk: a general-purpose peak fitting program. J Appl Cryst. 2010;43:1126–1128. [Google Scholar]
- Zhou J, Gong H, Zhou Y, Xuan X, Fujisaki K. Identification of a glycine-rich protein from the tick Rhipicephalus haemaphysaloides and evaluation of its vaccine potential against tick feeding. Parasitol Res. 2006;100:77–84. doi: 10.1007/s00436-006-0243-7. http://dx.doi.org/10.1007/s00436-006-0243-7. [DOI] [PubMed] [Google Scholar]