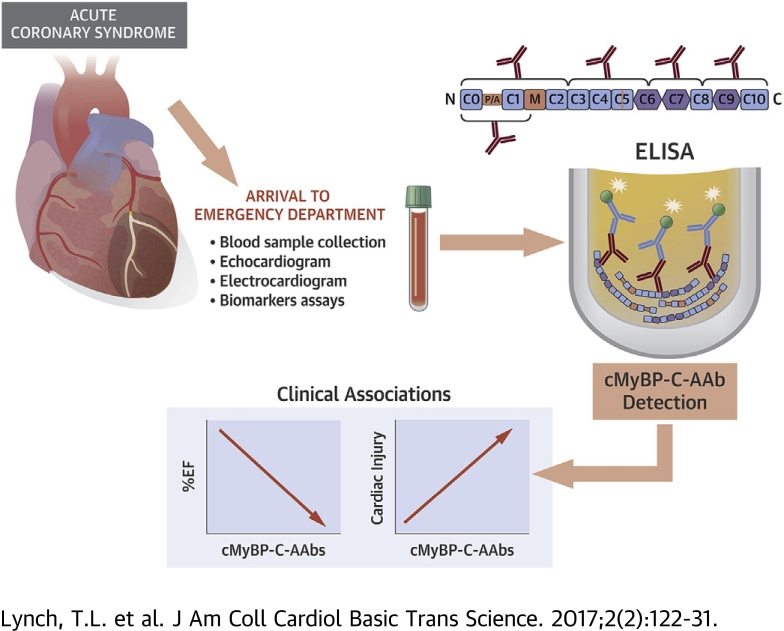
Visual Abstract
Key Words: acute myocardial infarction, autoantibodies, cardiac myosin binding protein-c, cardiomyopathy
Abbreviations and Acronyms: AAb, autoantibody; ACS, acute coronary syndrome; AMI, acute myocardial infarction; cMyBP-C, cardiac myosin binding protein-C; cTnI, cardiac troponin-I; DCM, dilated cardiomyopathy; ELISA, enzyme-linked immunosorbent assay; HCM, hypertrophic cardiomyopathy; LVEF, left ventricular ejection fraction; NSTEMI, non–ST-segment elevation myocardial infarction; NT-proBNP, N-terminal pro-brain natriuretic peptide; OD, optical density; STEMI, ST-segment elevation myocardial infarction; UA, unstable angina
Highlights
-
•
ACS remains a leading cause of death worldwide.
-
•
cMyBP-C-AAbs were detected in ACS patients' sera upon arrival to the emergency department.
-
•
The presence of cMyBP-C-AAbs was negatively associated with cardiovascular function and positively associated with the degree of myocardial injury as determined by circulating levels of serum proteins in ACS patients.
-
•
The development of novel indicators to predict infarction severity and cardiovascular function following ischemic injury may provide early treatment recommendations for ACS patients to limit myocardial damage.
Summary
The degradation and release of cardiac myosin binding protein-C (cMyBP-C) upon cardiac damage may stimulate an inflammatory response and autoantibody (AAb) production. We determined whether the presence of cMyBP-C-AAbs associated with adverse cardiac function in cardiovascular disease patients. Importantly, cMyBP-C-AAbs were significantly detected in acute coronary syndrome patient sera upon arrival to the emergency department, particularly in ST-segment elevation myocardial infarction patients. Patients positive for cMyBP-C-AAbs had reduced left ventricular ejection fraction and elevated levels of clinical biomarkers of myocardial infarction. We conclude that cMyBP-C-AAbs may serve as early predictive indicators of deteriorating cardiac function and patient outcome in acute coronary syndrome patients prior to the infarction.
Acute myocardial infarction (AMI) remains a leading cause of morbidity and mortality worldwide often as a result of prolonged, irreversible myocardial cell damage or death (1). Upon myocardial damage, the release of previously “hidden” cardiac proteins into the circulation may trigger an immune response and the production of autoantibodies (AAbs) that target cardiac antigens (2). Indeed, such AAbs have been critically linked to heart failure 2, 3, 4. The myocardial injury that occurs in patients with AMI may cause leakage of cardiac antigens into the circulation and the production of AAbs that associate with AMI severity (5).
Cardiac myosin binding protein-C (cMyBP-C) is a sarcomeric regulatory protein that controls cardiac contractile function 1, 6, 7. cMyBP-C contains 8 immunoglobulin-like domains, 3 fibronectin type III domains, a proline-alanine-rich region between C0 and C1, and an M domain (1). Recently, it was demonstrated that cMyBP-C is extensively proteolyzed during AMI, and can be released into the circulation potentially serving as a biomarker of AMI 6, 8. Interestingly, previous work has shown a strong reactivity and immunogenicity for AAbs against fragments of murine and human cMyBP-C that could generate experimental autoimmune myocarditis and dilated cardiomyopathy (DCM) in animal models 9, 10, 11. However, a systematic large-scale human study testing the antigenicity of human cMyBP-C domains in patients with AMI is necessary to determine the association of cMyBP-C-AAbs with clinical measurements of cardiac dysfunction and patient outcome.
In the present study, we used serum samples from patients with acute coronary syndrome (ACS) and from patients with DCM, patients with hypertrophic cardiomyopathy (HCM), and donors with no known cardiovascular disease (CVD) for comparison to determine the most antigenic regions of human cMyBP-C and whether cMyBP-C-AAbs are associated with a host of clinical variables. Importantly, our study demonstrated that the presence of serum cMyBP-C-AAbs is directly associated with decreased cardiac function and increased levels of biomarkers of ACS, suggesting that cMyBP-C-AAbs are potential predictive indicators of deteriorating cardiac function and/or outcome for ACS patients.
Methods
Patients
Deidentified human serum samples were gathered from a total of 628 patients with ACS, 73 patients with DCM, 214 patients with HCM, and 141 healthy donors with no known CVD who participated in this study. Samples from Tufts Medical Center were collected from 31 HCM patients and 8 DCM patients (by G.S.G.). Samples from the Department of Genetics at the Harvard Medical School were collected from 22 HCM patients and 3 DCM patients (by C.E.S.). Samples from the Hypertrophic Cardiomyopathy Clinic at the University of Michigan were collected from 106 HCM patients (by S.D.). Samples from the Kerckhoff Heart and Thorax Center were collected from 628 ACS patients upon arrival to the emergency department (ED), 50 DCM patients and 22 HCM patients. Thirteen of the HCM samples were collected at baseline from HCM patients from undergoing transcoronary ablation of septal hypertrophy, and had been previously analyzed to establish protein or microribonucleic acid release kinetics in these patients (by C.L.) 12, 13, 14. Samples from the VU University Medical Center were collected from 33 HCM patients (by J.v.d.V.). Samples from the Outpatient Clinic at Scott and White Hospital were collected from 12 DCM patients (by N.N.). The 141 donor samples were obtained from the University of Texas Health Sciences Center at Houston and the Texas Heart Institute (by A.J.M.). Detailed patient clinical and demographic information is provided in Supplemental Tables 1 to 11. This investigation conforms to the principles outlined in the Declaration of Helsinki. All blood samples were collected under strict guidelines after obtaining consent using institutional review board (IRB) approved consent forms as part of the IRB approved protocol submitted by the respective principal investigator. The IRB at Loyola University Chicago approved the present study using the deidentified samples that were received from the collaborators’ laboratories.
Generation of recombinant proteins using the pET system
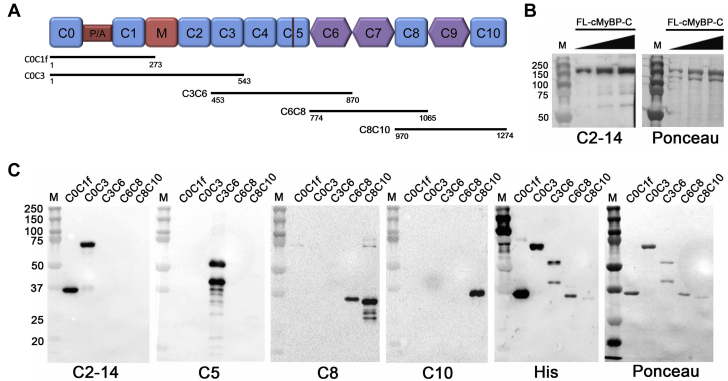
Recombinant, purified fragments of cMyBP-C were generated and used to detect the presence of AAbs recognizing antigenic epitopes within these proteins. The complementary deoxyribonucleic acid coding for amino acid residues 1-273 (C0 to C1f), 1-543 (C0 to C3), 453-870 (C3 to C6), 774-1065 (C6 to C8) or 970-1274 (C8 to C10) of human cMyBP-C was cloned separately into the pET-28a(+) expression vector (Novagen, EMD Millipore, Billerica, Massachusetts). The N-terminus of each protein contained the His epitope. Recombinant clones were confirmed by deoxyribonucleic acid sequencing (ACGT, Inc., Wheeling, Illinois). Plasmids were transformed into the BL21 (DE3) strain of Escherichia coli (E. coli) (Cat. No. C6010-03, Invitrogen, Carlsbad, California), having the gene for the T7 ribonucleic acid polymerase promoter that is induced by isopropyl-β-D-thiogalactopyranoside (Cat. No. 10724815001, Roche, Indianapolis, Indiana). Expression of the recombinant proteins was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analysis. Large-scale preparation was conducted for each clone, giving optimal expression of each recombinant protein. A total of 500 ml of LB medium were inoculated with 1% E. coli BL21 cells, which were grown for 12 h at 37°C to an optical density (OD) of 0.6. After induction with 1 mmol/l isopropyl-β-D-thiogalactopyranoside, the cultures were then incubated for an additional 5 h, followed by centrifugation at 4,000 g for 30 min at 4°C to harvest the cells. In accordance with the manufacturer’s suggestion, overexpressed proteins were purified with Ni-NTA agarose chromatography (Cat. No. 1018244, Qiagen, Germantown, Maryland). The proteins were dialyzed against 5 l of 1× phosphate buffered saline (Cat. No. IB70166, MidSci, Valley Park, Missouri). Buffers were changed twice using 15 kDa MWCO of membrane (Cat. No. 132124, Spectrum Laboratories, Rancho Dominguez, California). Protein concentrations were determined following dialysis using the Bradford Assay (Cat No. 500-0205, Bio-Rad Laboratories, Hercules, California). The quality of the recombinant cMyBP-C fragments was determined by Ponceau S stain (Ponceau S solution, Cat. No. P7170, Sigma-Aldrich, St. Louis, Missouri) and Western blot analysis using rabbit polyclonal antibodies against cMyBP-C residues 2 to 14 (cMyBP-C2–14) (15) to detect C0 to C1f and C0 to C3, domain C5 (cMyBP-CC5) (16) to detect C3 to C6, domain C8 (cMyBP-CC8) to detect C6 to C8 and C8 to C10, and domain C10 (cMyBP-CC10) (17) to detect C8 to C10 alone (Figure 1). The N-terminal 6× histidine epitope was detected using the mouse anti-His6 monoclonal antibody (Cat. No. 11922416001, Sigma-Aldrich).
Figure 1.
Structure and Purification of cMyBP-C Domains
(A) cMyBP-C domain structure. Recombinant human cMyBP-C fragments used in this study were C0 to C1f (amino acids 1-273), C0 to C3 (1-543), C3 to C6 (453-870), C6 to C8 (774-1065), and C8 to C10 (970-1274). (B) Western blot analysis and Ponceau stain to detect partially purified FL-cMyBP-C. (C) Western blot analysis and Ponceau S stain to detect the cMyBP-C fragments listed in A. cMyBP-C = cardiac myosin binding protein-C; FL = full-length.
Generation of recombinant FL-cMyBP-C using the Sf9-Baculovirus expression system
To detect the presence of AAbs recognizing antigenic epitopes within FL-cMyBP-C, FL-cMyBP-C was generated using the Sf9-Baculovirus expression system as previously described (18). The quality of recombinant FL-cMyBP-C was determined by Ponceau S stain (Ponceau S solution, Cat. No. P7170, Sigma-Aldrich) and Western blot analysis using the cMyBP-C2-14 antibody.
Enzyme-linked immunosorbant assay procedure
Deidentified serum samples from ACS, DCM, and HCM patients as well as human donors were evaluated for the presence of AAbs that could bind C0 to C1f, C0 to C3, C3 to C6, C6 to C8, and C8 to C10 as well as FL-cMyBP-C by indirect enzyme-linked immunosorbent assay (ELISA). The 96-well MediSorp plates (Cat. No. 467320, Nunc-Immuno, Sigma-Aldrich) were coated overnight at 4°C with 100 ng recombinant cMyBP-C fragments or FL-cMyBP-C for capture. The plates were then washed 3× with a solution containing 0.05% (v/v) tween 20 in phosphate-buffered saline (PBST), and aspecific binding to the plate was blocked by incubating the plate with 5% (w/v) bovine serum albumin (BSA) in PBST for 1 h at room temperature while shaking at 200 rpm. Following another series of washes, serum samples were diluted 1:160 in 1% (w/v) BSA in PBST and plated in duplicate. Plates were incubated for 1 h while shaking, followed by another series of washings. Horseradish peroxidase–conjugated rabbit antihuman immunoglobulin G (Cat. No. sc-2923, Santa Cruz Biotechnology, Dallas, Texas) was diluted 1:2,000 in PBS and added to the plates, followed by incubation for 1 h with shaking. After a final series of washings, ABTS substrate (Cat. No. 37615, Thermo Fisher Scientific, Waltham, Massachusetts) was added to the plates and incubated for 30 min with shaking at 300 rpm at room temperature. Absorbance was read at 405 nm in a 96-well plate ELISA reader (VersaMax ELISA Microplate Reader, Molecular Devices, Sunnyvale, California).
Pre-adsorption ELISA
Binding of cMyBP-C-AAbs to the C0 to C1f, C0 to C3, C3 to C6, C6 to C8, and C8 to C10 regions of cMyBP-C and to FL-cMyBP-C was confirmed using a pre-adsorption ELISA procedure. Sera from 3 patients positive for cMyBP-C-AAbs to each of the cMyBP-C fragments and to FL-cMyBP-C from the DCM, HCM, and ACS groups were pre-incubated with 0, 1, 2, 4, and 8 ng/ul of C0 to C1f, C0 to C3, C3 to C6, C6 to C8, or C8 to C10 regions of cMyBP-C or with FL-cMyBP-C. Sera were prepared in 1% BSA in PBST at a dilution of 1:160. All samples were incubated at 37°C for 30 min to allow for pre-adsorption before analysis by indirect ELISA. Following pre-adsorption, for indirect ELISA, 100 μl of each diluted serum sample were incubated on 96-well MediSorp plates (Cat. No. 467320, Nunc-Immuno) that had been pre-coated overnight at 4°C with 100 μl of 1 μg/ml of the respective protein. Indirect ELISA was performed as described in the previous text.
Cutoff determination for AAb positivity
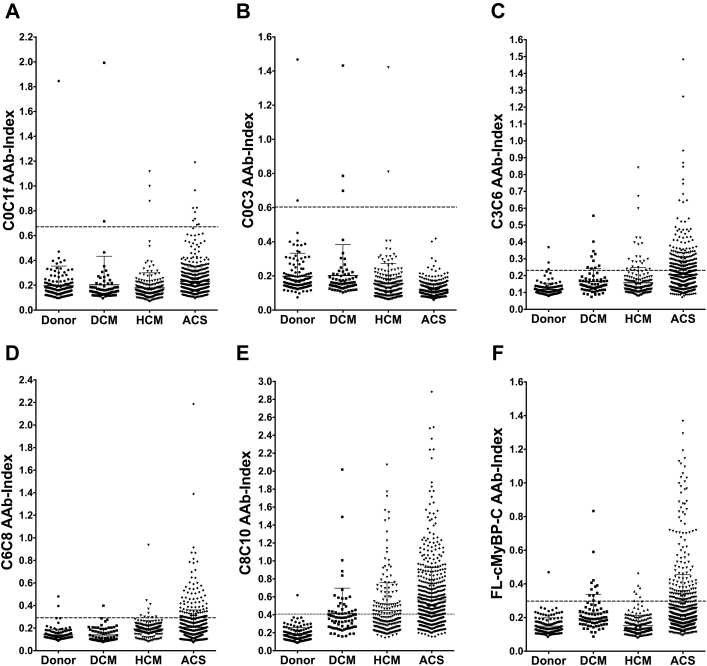
The cutoff above which samples were scored as AAb-positive was defined as the signal of the mean + 3× the SD of the human donor group’s OD value obtained for each protein. For each plate coated with each respective protein, a subset of donor sera was incubated in duplicate, along with ACS, DCM, and HCM patient sera. Given the semiquantitative nature of the ELISA, the signals of the patient samples were normalized per plate to the average of the control group. This normalized value is referred to hereinafter as the “autoantibody index” (AAb index) (Figure 2).
Figure 2.
AAbs Targeting cMyBP-C Domains Are Present in Sera of Patients With ACS and Cardiomyopathy
Sera from 141 donors and 73 DCM, 214 HCM, and 628 ACS patients were assayed at a dilution of 1:160 for the presence of cMyBP-C AAbs to (A) C0 to C1f, (B) C0 to C3, (C) C3 to C6, (D) C6 to C8, and (E) C8 to C10 regions and (F) FL-cMyBP-C by ELISA. A mean + 3 SD of the average OD of donors’ sera diluted 1:160 was used as a cutoff for AAb positivity (indicated by broken line). Values above the broken line are positive for AAbs. AAb = autoantibody; ACS = acute coronary syndrome; DCM = dilated cardiomyopathy; ELISA = enzyme-linked immunosorbent assay; HCM = hypertrophic cardiomyopathy; OD = optical density; other abbreviations as in Figure 1.
Statistical analyses
Unadjusted means or medians were compared between patients who were AAb positive and negative for each protein fragment and between patients who were AAb positive and negative for FL-cMyBP-C in the ACS, DCM, and HCM patient groups using an independent samples Student t test or Wilcoxon rank sum test when appropriate. Proportions were analyzed using a chi-square test or Fisher exact test when appropriate. The effect of variables on the number of protein fragments positive for AAbs on ELISA was tested using ordinal logistic regression or multinomial logistic regression when the proportional odds assumption was violated. Two-sided p values were provided for significance in either direction; p values <0.05 were considered statistically significant. The p values presented in the tables were not adjusted for multiple comparisons, but this was considered in the results section. For the p trend analysis, we divided each of the concentrations for each peptide into low, medium, high, and very-high categories. Stratifying by ACS, HCM, and DCM, a generalized linear model with “OD/OD at 0 ng/μl (405 nm)” as the outcome, and ordinal peptide concentration as the independent variable was used to assess if a simple linear trend existed. The p trend is the p value of the beta coefficient of the independent variable. SAS version 9.4 (SAS Institute, Cary, North Carolina) was used for all statistical analyses conducted. The software package GraphPad Prism 6.0 (GraphPad Software, San Diego, California) was also used for plotting.
Results
cMyBP-C-AAbs were detected in patients with ACS, DCM, and HCM
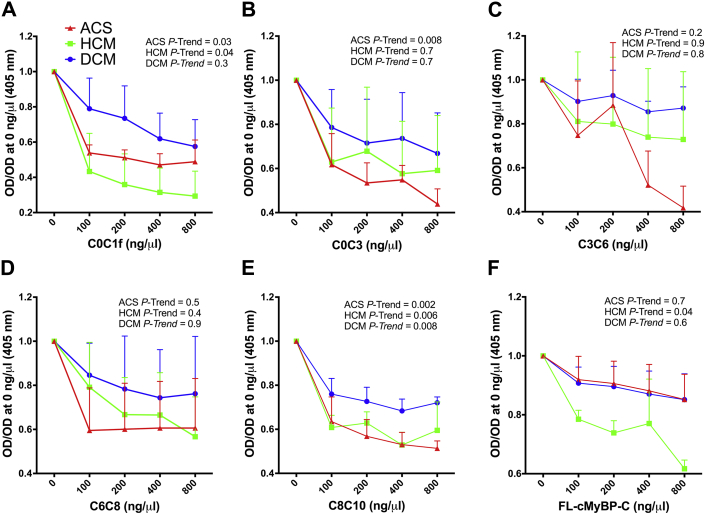
To determine the antigenic region(s) of cMyBP-C, recombinant fragments of cMyBP-C spanning the C0 to C10 domains as well as full-length (FL) cMyBP-C (Figure 1) were incubated with sera collected from 628 myocardial infarction, 73 DCM, and 214 HCM patients in an indirect ELISA. Sera from 141 donors with no known CVD were used to establish the cutoff for AAb positivity, which was defined as the signal of the mean + 3× the SD of the OD value obtained for each protein region in the human donor group. Supplemental Table 1 provides clinical and demographic characteristics of this donor group. Sera from all patient groups and from the donor group were diluted 1:160 for all assays, and OD values read at this dilution were compared and used to generate scatter plots for each protein fragment (Figure 2). Across all disease groups, more patients reported positive for AAbs targeting the C3 to C10 domains of cMyBP-C compared with the more N-terminal domains (Figure 2). Protein-fragment–specific results are available in Supplemental Tables 2 to 11. In total, across all cMyBP-C fragments including FL-cMyBP-C, 454 (72.3%) ACS, 33 (45.2%) DCM, and 94 (43.9%) HCM patients, as well as 8 (5.7%) donors, reported positive for cMyBP-C-AAbs (Supplemental Tables 12 to 14). To confirm that the AAbs targeting cMyBP-C fragments and FL-cMyBP-C identified here were specific to their respective targets on cMyBP-C, we performed a pre-adsorption ELISA. The reactivity of cMyBP-C-AAbs across protein fragments in sera from the ACS, DCM, and HCM patient groups exhibited a dose-dependent decrease upon increasing pre-adsorption concentrations of their target protein with several significant p trends across patient groups and protein fragments (Figure 3).
Figure 3.
cMyBP-C-AAb Reactivity in Cardiomyopathy and ACS Patient AAb-Positive Sera Decrease Upon Pre-Adsorption With cMyBP-C
Sera from 3 AAb-positive patient samples from the DCM, HCM, and ACS patient groups for each cMyBP-C fragment and for FL-cMyBP-C were pre-adsorbed with 0, 1, 2, 4 or 8 ng/μl of their respective target protein. Following pre-adsorption, serum AAb reactivity was tested by ELISA for AAbs to (A) C0 to C1f, (B) C0 to C3, (C) C3 to C6, (D) C6 to C8, (E) C8 to C10, and (F) FL-cMyBP-C. A p trend <0.05 was considered statistically significant. Abbreviations as in Figures 1 and 2.
Clinically relevant associations of cMyBP-C-AAb status with clinical parameters for patients with ACS, DCM, and HCM
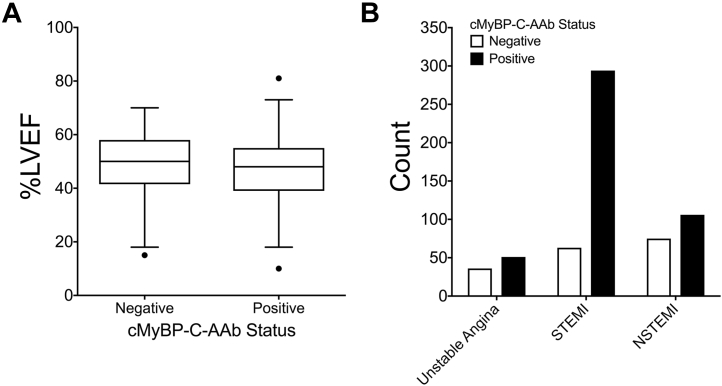
After classifying ACS, HCM, and DCM into those who reported positive or negative for cMyBP-C-AAbs, irrespective of the cMyBP-C protein fragment, no significant associations could be established between AAb status (positive or negative) for patients with DCM or HCM and clinical variables (Supplemental Tables 12 and 13). However, for ACS patients, several clinically relevant associations were found based upon cMyBP-C-AAb status and different clinical variables (Supplemental Table 14, Figure 4). Intriguingly, within the ACS patient group, the average left ventricular ejection fraction (LVEF) measured within the first 3 days following percutaneous intervention/ACS was significantly lower for patients positive for cMyBP-C-AAbs (p = 0.02) (Figure 4). Furthermore, a higher percentage of ACS patients positive for cMyBP-C-AAbs were classified as having STEMI than NSTEMI or unstable angina (UA). In contrast, a greater percentage of ACS patients negative for cMyBP-C-AAbs were classified as having NSTEMI than STEMI or UA (Figure 4). The average level of myoglobin was significantly higher for patients positive for cMyBP-C-AAbs at 1-day post-MI (p < 0.02) compared with patients who were negative for cMyBP-C-AAbs. The average level of N-terminal pro-brain natriuretic peptide (NT-proBNP) was significantly higher for patients positive for cMyBP-C-AAbs at baseline (p = 0.04), 1-day post-ACS (p = 0.002), and 6 months post-ACS (p = 0.03) compared with patients who were negative for cMyBP-C-AAbs. In general, although not significant in all cases, cMyBP-C-AAb–positive patients had increased levels of all serum biomarkers analyzed compared with cMyBP-C-AAb–negative patients.
Figure 4.
Cardiac Function and ACS Category for ACS Patients Based on cMyBP-C-AAb Status
(A) %LVEF in patients positive or negative for cMyBP-C-AAbs irrespective of the cMyBP-C protein fragment. (B) Number of patients who were classified as NSTEMI, STEMI, or UA on the basis of being positive or negative for cMyBP-C-AAbs irrespective of the cMyBP-C protein fragment. AAb = autoantibody; cMyBP-C = cardiac myosin binding protein-C; LVEF = left ventricular ejection fraction; NSTEMI = non–ST-segment elevation myocardial infarction; STEMI = ST-segment elevation myocardial infarction.
cMyBP-C protein fragment-specific analysis indicated several more associations linking cMyBP-C-AAb status to numerous clinical variables for ACS patients (Supplemental Tables 6 to 11). Interestingly, LVEF was significantly reduced in ACS patients who were positive for cMyBP-C-AAbs targeting the C3 to C6 (p = 0.05), C6 to C8 (p = 0.0003), and C8 to C10 (p = 0.01) domains compared with patients who were negative for cMyBP-C-AAbs to these regions. Furthermore, the levels of cardiac troponin-I (cTnI) 1-day post-ACS were significantly increased in ACS patients who were positive for AAbs targeting the C3 to C6 (p < 0.0001) and C6 to C8 (p = 0.004) domains compared with patients who were negative for AAbs to these regions. Additionally, a significantly higher percentage of patients positive for AAbs against the C3 to C6 (p < 0.0001), C6 to C8 (p < 0.0001), and C8 to C10 (p < 0.002) domains of cMyBP-C as well as FL-cMyBP-C (p < 0.0001) had STEMI and a smaller percentage had NSTEMI or had UA than expected compared with patients who were negative for AAbs to these regions.
Cardiomyopathy and ACS patients were further categorized per the number of patients positive for AAbs to 0, 1, 2, or 3+ cMyBP-C protein fragments to determine whether increased AAb-fragment positivity associated with clinical variables. There were no significant associations detected between the number of fragments for which HCM or DCM patients were AAb positive and clinical variables (Supplemental Tables 15 to 18). However, for ACS patients, several significant associations were detected (Supplemental Tables 19 and 20). Strikingly, there was a very significant association between a reduction in LVEF and an elevation in the number of fragments for which patients were AAb positive (p = 0.0008). Additionally, there was a significant association among increased levels of serum brain natriuretic peptide 1-day post-ACS (p = 0.03), myoglobin at baseline (p = 0.007), NT-proBNP 1-day post-ACS (p = 0.0007), and cTnI 1-day post-ACS (p < 0.0001) and an elevation in the number of fragments for which patients were AAb positive. Notably, serum levels of cTnI 1-day post-ACS were significantly increased in patients who were positive for cMyBP-C-AAbs to either 2 (p = 0.006) or 3+ (p = 0.003) fragments of cMyBP-C compared with cMyBP-C-AAb–negative patients. Furthermore, although not significant in all cases, there appeared to be an association between elevated levels of serum biomarkers and being AAb positive for multiple cMyBP-C fragments or the FL protein. Together, these data suggest that cMyBP-C-AAbs are associated with cardiac damage and worsened cardiac function in ACS patients upon arrival to the ED.
Discussion
We previously determined that myocardial damage releases cMyBP-C (6), which may be an immunogenic trigger for cMyBP-C-AAb production in the circulation. Here, we determined the antigenicity of human cMyBP-C in various CVD patients and demonstrated that AAbs targeting regions of cMyBP-C are present in patients with ACS, DCM, and HCM. Importantly, we have elucidated that the increased fragment positivity of cMyBP-C-AAbs is significantly positively associated with clinical biomarkers of cardiac damage and significantly negatively associated with heart function in ACS patients. Previously, the cMyBP-C-AAbs detected by Kasahara et al. (11) were found to react strongly with residues 205-916 and weakly with residues 945-1270 of murine cMyBP-C. It was further shown that cMyBP-C-reactive AAbs were present in 2 of 16 DCM patient sera (11). Similarly, here, we identified cMyBP-C as most highly antigenic across domains C3 to C10 in ACS, DCM, and HCM patients, and we have extended the findings of Kasahara et al. (11) to other CVDs with an increased patient number. Importantly, we have shown that pre-adsorption of cMyBP-C-AAbs reduced AAb titers in AAb-positive patients from all CVD groups, indicating specificity of these AAbs for cMyBP-C.
The novelty of the present study is that within the ACS patient group, we have demonstrated significant associations among AAb status and ACS classification, biomarker levels, and LVEF. For instance, a greater percentage of C3 to C6, C6 to C8, C8 to C10, and FL-cMyBP-C-AAb–positive patients could be classified as STEMI patients than were NSTEMI or UA, which was also the trend observed when comparing AAb-positive patients irrespective of the protein fragment. Interestingly, an elevated percentage of patients who were negative for AAbs to these regions could also be classified as having STEMI than NSTEMI or UA. However, a greater percentage of cMyBP-C-AAb-negative patients were classified as having NSTEMI than STEMI or UA when compared irrespective of the protein fragment. Given the differences in infarct severity between STEMI and NSTEMI and their respective association with cMyBP-C-AAb status, as detailed in the previous text, we propose that cMyBP-C-AAbs may serve as valuable early indicators of cardiac function in ACS patients. Furthermore, patients who were positive for AAbs spanning the C3 to C10 regions of cMyBP-C had a significant elevation in biomarkers of cardiac injury, including brain natriuretic peptide, myoglobin, creatine kinase-myocardial band, NT-proBNP, and cTnI. This could indicate more severe cardiac damage in these individuals that may stimulate an inflammatory response leading to increased AAb production (2). Therefore, as discussed further in the following text, we propose that increased myocardial damage in ACS patients, possibly due to a partially blocked artery, would limit the perfusion of oxygen-rich blood to distal cells. This could potentiate cellular stress and death leading to early myocardial damage and cMyBP-C degradation, release into the circulation, and the production of AAbs. Our data support this theory, as the number of fragments for which ACS patients were positive associated with increased levels of cTnI and with patients having STEMI. This could indicate increased myocardial damage that may begin before complete artery occlusion and thrombus formation.
Study limitations
One limitation for our study is the nondenaturing nature of the ELISA assay used here. This could explain why FL-cMyBP-C had fewer AAb-positive patients than did smaller fragments of the protein. That is to say, the folding of a larger protein such as the FL protein limits the availability of antibody epitopes for antibody recognition and binding, which could be why a reduced number of AAb-positive patients was observed. Additionally, although blood samples from ACS patients were drawn upon arrival to the ED, it is possible that undiagnosed minor infarcts or partial artery occlusion leading to myocardial damage, as discussed in the previous text, may have occurred before a complete infarction. Therefore, progressive damage could have led to augmented protein degradation and antibody formation and persistence within the circulation of susceptible individuals before the hospitalizing infarction. This would explain why AAbs could be identified in blood sampled upon arrival to the ED. Nonetheless, because these AAbs were present in ACS patients upon arrival to the ED and were associated with increased levels of serum biomarkers and reduced cardiac function, they may serve as early predictive indicators of the degree of cardiac dysfunction and/or patient outcome in terms of the extent of myocardial damage before infarction and percutaneous intervention. This would be valuable to patients with ACS symptoms who seek medical attention before an AMI event. At the same time, however, these clinical associations only serve as indirect evidence that cMyBP-C-AAbs are prognostic indicators of cardiac function and patient outcome. Although proving the relationship is well outside the scope of the present paper, it will be the subject of our future studies. Additionally, the molecular mechanisms underlying the activity of cMyBP-C-AAbs may have no causal relationship with these clinical associations. It should be noted that cMyBP-C is only 1 of several cardiac proteins proteolyzed and released into the circulation upon cardiac damage. Thus, it is possible that proteolysis of other cardiac proteins may lead to AAb formation (2). For example, released cardiac myosin and cTnI can generate their own targeted AAbs and produce myocarditis in animal models (2). As reviewed by Kaya et al. (2), such autoreactive antibodies may act through several mechanisms, including the formation of immune complexes, activation of the complement system, and interaction with cell-surface receptors, to alter cell signaling (2). Therefore, in addition to cMyBP-C and cMyBP-C-AAbs, we can suggest the potential involvement of multiple cardiac proteins and their respective AAbs in mediating the immune responses involved in disease progression following cardiac injury.
Conclusions
We demonstrated that cMyBP-C–targeted AAbs are produced in patients with CVD. We further established a strong clinical link between ACS patients presenting with cMyBP-C-AAb positivity and a host of clinical variables including, for example, classification as NSTEMI or STEMI, LVEF, and levels of serum biomarkers, indicating a potential role for cMyBP-C-AAbs as early predictive indicators of cardiac dysfunction and patient outcome before the infarction.
Perspectives.
COMPETENCE INMEDICAL KNOWLEDGE: The ability to predict whether a patient with ACS symptoms will be STEMI or NSTEMI provides an opportunity to determine infarct severity and patient outcome in terms of cardiovascular function before the infarction. Therefore, clinical treatment and life-style changes before a complete infarction may limit ischemic damage and improve patient survival.
TRANSLATIONAL OUTLOOK: ACS remains a leading cause of morbidity and mortality worldwide. Complete infarction of the coronary blood supply limits perfusion of oxygen and nutrients to distal tissue, promoting cell damage and death. Rapid diagnosis and percutaneous intervention are critical to patient outcome and survival following ischemic injury. Patient diagnosis relies upon electrocardiogram recordings and the measurement of the levels of circulating cardiac biomarkers of ischemic injury. The identification of novel indicators that predict cardiac function and infarct severity before an infarction in individuals who are seeking medical attention for ACS symptoms may steer clinical treatment. Here, we identify cMyBP-C-AAbs as potential early clinical markers of cardiac function and infarct severity in ACS patients. Future studies are required to: 1) examine whether cMyBP-C-AAbs contribute to cardiac dysfunction following the infarction; and 2) develop a commercial assay that could be used clinically for the identification of cMyBP-C-AAbs.
Footnotes
This project was supported in part by the Loyola Clinical Research Office at Loyola University Chicago. The findings presented in this work are based on experimentation and analysis performed by the authors and do not necessarily represent the views of the Loyola Clinical Research Office. This work was supported by the National Institutes of Health (R01 HL130356, R01 HL105826, and K02 HL114749 to Dr. Sadayappan); John T. Babbitt Foundation (to Dr. Huggins); Netherlands Organization for Scientific Research (to Dr. van der Velden); and the American Heart Association Midwest Postdoctoral and Predoctoral Fellowships and the American Heart Association Grant-in-Aid and Cardiovascular Genome-Phenome Study (13POST17220009 to Dr. Kuster, 15PRE22430028 to Dr. Lynch IV, and 14GRNT20490025 and 15CVGPSD27020012 to Dr. Sadayappan). Dr. Seidman is a founder and owns shares in Myokardia Inc., a startup company that is developing therapeutics that target the sarcomere. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
All authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the JACC: Basic to Translational Scienceauthor instructions page.
Appendix
References
- 1.Lynch T.L., Sadayappan S. Surviving the infarct: a profile of cardiac myosin binding protein-C pathogenicity, diagnostic utility, and proteomics in the ischemic myocardium. Proteomics Clin Appl. 2014;8:569–577. doi: 10.1002/prca.201400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaya Z., Leib C., Katus H.A. Autoantibodies in heart failure and cardiac dysfunction. Circ Res. 2012;110:145–158. doi: 10.1161/CIRCRESAHA.111.243360. [DOI] [PubMed] [Google Scholar]
- 3.Caforio A.L. Role of autoimmunity in dilated cardiomyopathy. Br Heart J. 1994;72:S30–S34. doi: 10.1136/hrt.72.6_suppl.s30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caforio A.L., Iliceto S. Genetically determined myocarditis: clinical presentation and immunological characteristics. Curr Opin Cardiol. 2008;23:219–226. doi: 10.1097/HCO.0b013e3282fbf572. [DOI] [PubMed] [Google Scholar]
- 5.Gregor P., Jira M., Raska I. Autoantibodies in hypertrophic cardiomyopathy and their clinical significance. Eur Heart J. 1987;8:773–778. doi: 10.1093/eurheartj/8.7.773. [DOI] [PubMed] [Google Scholar]
- 6.Govindan S., McElligott A., Muthusamy S. Cardiac myosin binding protein-C is a potential diagnostic biomarker for myocardial infarction. J Mol Cell Cardiol. 2012;52:154–164. doi: 10.1016/j.yjmcc.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sadayappan S., de Tombe P.P. Cardiac myosin binding protein-C: redefining its structure and function. Biophys Rev. 2012;4:93–106. doi: 10.1007/s12551-012-0067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baker J.O., Tyther R., Liebetrau C. Cardiac myosin-binding protein C: a potential early biomarker of myocardial injury. Basic Res Cardiol. 2015;110:23. doi: 10.1007/s00395-015-0478-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto Y., Park I.K., Kohyama K. B-cell epitope spreading is a critical step for the switch from C-protein-induced myocarditis to dilated cardiomyopathy. Am J Pathol. 2007;170:43–51. doi: 10.2353/ajpath.2007.060544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto Y., Tsukada Y., Miyakoshi A., Sakuma H., Kohyama K. C protein-induced myocarditis and subsequent dilated cardiomyopathy: rescue from death and prevention of dilated cardiomyopathy by chemokine receptor DNA therapy. J Immunol. 2004;173:3535–3541. doi: 10.4049/jimmunol.173.5.3535. [DOI] [PubMed] [Google Scholar]
- 11.Kasahara H., Itoh M., Sugiyama T. Autoimmune myocarditis induced in mice by cardiac C-protein. Cloning of complementary DNA encoding murine cardiac C-protein and partial characterization of the antigenic peptides. J Clin Invest. 1994;94:1026–1036. doi: 10.1172/JCI117416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liebetrau C., Gaede L., Dorr O. Release kinetics of N-terminal pro-B-type natriuretic peptide in a clinical model of acute myocardial infarction. Clin Chim Acta. 2013;429C:34–37. doi: 10.1016/j.cca.2013.11.017. [DOI] [PubMed] [Google Scholar]
- 13.Liebetrau C., Mollmann H., Dorr O. Release kinetics of circulating muscle-enriched microRNAs in patients undergoing transcoronary ablation of septal hypertrophy. J Am Coll Cardiol. 2013;62:992–998. doi: 10.1016/j.jacc.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 14.Liebetrau C., Nef H., Szardien S. Release kinetics of copeptin in patients undergoing transcoronary ablation of septal hypertrophy. Clin Chem. 2013;59:566–569. doi: 10.1373/clinchem.2012.194001. [DOI] [PubMed] [Google Scholar]
- 15.Copeland O., Sadayappan S., Messer A.E., Steinen G.J., van der Velden J., Marston S.B. Analysis of cardiac myosin binding protein-C phosphorylation in human heart muscle. J Mol Cell Cardiol. 2010;49:1003–1011. doi: 10.1016/j.yjmcc.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Kulikovskaya I., McClellan G., Flavigny J., Carrier L., Winegrad S. Effect of MyBP-C binding to actin on contractility in heart muscle. J Gen Physiol. 2003;122:761–774. doi: 10.1085/jgp.200308941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govindan S., Sarkey J., Ji X. Pathogenic properties of the N-terminal region of cardiac myosin binding protein-C in vitro. J Muscle Res Cell Motil. 2012;33:17–30. doi: 10.1007/s10974-012-9292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karsai A., Kellermayer M.S., Harris S.P. Mechanical unfolding of cardiac myosin binding protein-C by atomic force microscopy. Biophys J. 2011;101:1968–1977. doi: 10.1016/j.bpj.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.