Abstract
To influence energy homeostasis and reproduction, 17β-estradiol (E2) controls the arcuate nucleus (ARC) through multiple receptor-mediated mechanisms, but primarily via estrogen receptor (ER) α, which signals through both estrogen response element (ERE)–dependent and –independent mechanisms. To determine ERα-mediated, ERE-dependent, and ERE-independent E2 signaling in the ARC, we examined the differential regulation of the mouse arcuate transcriptome by E2 using three mice genotypes: (1) wild-type, (2) ERα knock-in/knockout (ERE-independent mechanisms), and (3) total ERα knockout (ERα-independent mechanisms). Females were ovariectomized and injected with oil or E2, and RNA sequencing on the ARC was used to identify E2-regulated genes in each genotype. Our results show that E2 regulates numerous genes involved in cell signaling, cytoskeleton structure, inflammation, neurotransmission, neuropeptide production, and transcription. Furthermore, ERE-independent signaling regulates ARC genes expressed in kisspeptin neurons and transcription factors that control the hypothalamic/pituitary/gonadal axis. Interestingly, a few genes involved in mitochondrial oxidative respiration were regulated by E2 through ERα-independent signaling. A comparison within oil- and E2-treated females across the three genotypes suggests that genes involved in cell growth and proliferation, extracellular matrices, neuropeptides, receptors, and transcription are differentially expressed across the genotypes. Comparing with previously published chromatin immunoprecipitation sequencing analysis, we found that ERE-independent regulation in the ARC is mainly mediated by tethering of ERα, which is consistent with previous findings. We conclude that the mouse arcuate estrogen-regulated transcriptome is regulated by multiple receptor-mediated mechanisms to modulate the central control of energy homeostasis and reproduction, including novel E2-responsive pathways.
The hypothalamus is the critical brain center in the control of reproduction, energy homeostasis, stress, temperature, and other homeostatic functions. It is well established that the gonadal steroid 17β-estradiol (E2) modulates these functions through central hypothalamic signaling. Classically, E2 binds to its nuclear receptors, estrogen receptor (ER) α and ERβ, to regulate gene transcription by binding to the estrogen response element (ERE)–binding domain located on DNA. This “ERE-dependent” signaling is critical to many of these hypothalamic functions, with the effects lasting hours to days. In addition to ERE-dependent signaling, ERE-independent signaling regulates cellular signaling and gene expression through the activation of second-messenger signaling cascades and/or protein–protein interactions. ERE-independent signaling regulates second-messenger systems, including phosphoinositide 3-kinase, mitogen-activated protein kinase/extracellular signal-regulated kinase, protein kinase C, and protein kinase A pathways that control gene transcription and protein–protein interaction (1–4). Furthermore, G protein–coupled receptors such as the G protein–coupled receptor 30 (GPR30), now defined as the G protein–coupled estrogen receptor (GPER), and the putative Gq-coupled membrane estrogen receptor (Gq-mER) that regulates rapid responses to E2 in various hypothalamic neurons (5–10) are involved in E2 regulation of gene expression.
Previously, ERα knock-in/knockout (KIKO) models that lack a functional ERE-binding domain have been used to study ERE-independent signaling (11–14). Likewise, total ERα knockout (ERKO) animals are important in identification of ERα-independent signaling. Previous studies in our laboratory demonstrated that ERE-dependent and -independent signaling are important in the regulation of selected genes (e.g., neuropeptides, receptors, cation channels) in the arcuate nucleus (ARC) of the mouse (12). The ARC is a critical nucleus in the regulation of energy balance, through regulation by proopiomelanocortin (POMC) neurons and neurons that coexpress neuropeptide Y (NPY) and agouti-related peptide (AgRP), and of reproduction, through kisspeptin/neurokinin B/dynorphin (KNDy) neurons (8, 10, 15–17). Whereas our previous study used a TaqMan low-density array to determine regulation through these mechanisms, the objective of the present study is to examine the ARC “estrogen-regulated transcriptome” using a standard E2 replacement paradigm in KIKO and ERKO females in comparison with their wild-type (WT) littermates (12).
In addition to neuropeptides, hormone receptors, and cation channels, E2 has previously been shown to regulate ARC expression of signaling molecules and genes involved in cell communication, metabolism, cell growth, transcription, translation, and other cellular functions (18, 19). We hypothesize that distinct canonical signaling pathways will be regulated by E2 through both ERE-dependent and -independent mechanisms. Furthermore, although ERα is the primary receptor involved in E2 signaling in the ARC, we expect to see differential gene expression in ERKO animals, suggesting additional receptor-mediated mechanisms of E2’s action. Indeed, comparison between oil- and E2-treated females among the three genotypes identify a host of genes involved in cell growth and proliferation, extracellular matrices, neuropeptides, receptors, and transcription, indicating that multiple receptor-mediated signaling mechanisms modulate the hypothalamic control of energy homeostasis and reproduction. Defining the estrogen-regulated transcriptome in the mouse ARC is an important step to understanding and distinguishing the signaling mechanisms of E2 that control homeostatic processes.
Methods
Animal care
All animal procedures were performed in accordance with the guidelines based on National Institutes of Health standards and were performed with Institutional Animal Care and Use Committee approval at Rutgers University. Adult C57BL/6 female mice were maintained under a defined photoperiod (12 hours light/12 hours dark cycle) and constant temperature (23°C). Animals were given a low phytoestrogen chow diet (<75 isoflavone ppm; Advanced Protocol 5V75 from LabDiet, St. Louis, MO) and water ad libitum. Females were weaned on postnatal day 21. DNA from ear clippings was used to genotype animals, following previously published protocols (11). Three genotypes of mice were used: WT (C57BL/6), KIKO, and ERKO (provided by Dr. Ken Korach, National Institute on Environmental Health Sciences) (11). Crossing heterozygous WT/knock-in males expressing the nonclassical ERα knock-in with WT/KO heterozygous females produced KIKO and their WT littermates. Crossing heterozygous WT/KO males and females produced ERKO and their WT littermates. The average age of females (weeks) at kill is as follows: WT, 10.9 ± 1.2; KIKO, 16.2 ± 1.0; ERKO, 13.5 ± 1.9.
Ovariectomy
Adult female mice were bilaterally ovariectomized under isoflurane anesthesia 7 days prior to kill using sterile no-touch techniques according to the National Institutes of Health “Guidelines for Survival Rodent Surgery.” Animals were given a dose of analgesic [4 mg/kg carprofen (Rimadyl®)] 1 day after surgery for pain management. Females were monitored daily and allowed to recover for 5 days prior to the first injection of E2 benzoate (E2B) or oil.
Experimental design
Following ovariectomy, WT, KIKO, and ERKO females were separated into two groups: a control group received sesame oil (n = 4 per genotype), and the treated group received E2B (n = 4 per genotype). E2B was purchased from Steraloids (Newport, RI) and dissolved in ethanol (1 mg/mL), then mixed in sesame oil (Sigma-Aldrich, St. Louis, MO). The E2B injection protocol used has been shown to previously produce E2-induced gene expression in the hypothalamus (12, 20). We did not include intact females in our experimental design because ERKO and KIKO females do not have a normal estrous cycle, making it difficult to compare among intact WT, KIKO, and ERKO females (21). Animals were injected at 10:00 am with either 0.25 µg of E2B or oil 5 days after ovariectomy and a 1.5-µg dose of E2B or oil 24 hours later. Food was removed from the cages for 1 hour prior to kill on day 7 after ovariectomy at 10:00 am. Animals were sedated with ketamine (100 µl of 100 mg/ml stock, intraperitoneally; Henry Schein Animal Health, Melville, NY) and decapitated. Brains were removed and rinsed in ice-cold Sorensen’s phosphate buffer (0.2 M sodium phosphate, dibasic; and 0.2 M sodium phosphate, monobasic) for 30 to 60 seconds. The basal hypothalamus was cut using a brain slice matrix (Ted Pella, Redding, CA) into 1-mm-thick coronal rostral and caudal blocks corresponding to plates 42 to 47 and plates 48 to 53, respectively, from The Mouse Brain in Stereotaxic Coordinates (22). The slices were transferred to a 50/50 Pyrogard water/RNAlater® (Life Technologies, Grand Island, NY) solution and fixed overnight at 4°C. The ARC tissue from two slices was microdissected using a dissecting microscope, following standard methods (14, 18, 22, 23). Dissected tissue was stored at −80°C until RNA extraction in 50/50 Pyrogard water/RNAlater®.
Tissue extraction
RNA was extracted from ARC using Ambion RNAqueous® micro kits (Life Technologies, Carlsbad, CA) per the manufacturer’s protocol. The RNA was treated with DNase I to remove contaminating genomic DNA. The quality of the RNA was analyzed on an Agilent 2100 bioanalyzer using the RNA 6000 Nano kit (Agilent Technologies, Santa Clara, CA). We chose three samples within each treatment and genotype that had the highest RNA integrity numbers (>8) to use for RNA sequencing (RNA-seq) analysis (n = 3).
RNA-seq
Library preparation and sequencing was performed at the JP Sulzberger Columbia Genome Center (New York, NY) using a TruSeq RNA sample preparation kit v2 on poly(A)-purified RNA, then sequenced on an Illumina HiSeq 2500. We chose a read depth of 30 million 100-bp single end reads. The overall quality of the RNA-seq was sufficient with an average of ∼81% of the reads mapping to the mouse genome.
Bioinformatics
The RNA-seq data were mapped to the mouse genome (mm10) using TopHat v2.0 to produce mapped reads using the mouse gene transfer format as a guide (24, 25). Differentially expressed genes were identified using Cuffdiff. Subsequent statistical analyses among WT, KIKO, and ERKO were performed using the Bioconductor package cummeRbund (Bioconductor, Boston, MA) (26). To determine whether there were any outliers in biological replicates, principal component analysis (PCA) and scatter matrices were evaluated. Genes that were differentially regulated met the following guidelines: P < 0.05; fragments per kilobase of transcript per million mapped reads (FPKM) values >1; and fold change (FC) >1.5. To evaluate FC >1.5, the cutoff for downregulated genes was 0.667 and 1.5 for upregulated genes. To visualize differences in differential gene expression using FPKM, bar graphs were created in cummeRbund. Ingenuity Pathway Analysis (IPA; Qiagen, Redwood City, CA) was used to determine the top canonical pathways that were differentially regulated.
Gene-level views were done using Integrative Genomics Viewer (IGV; Broad Institute of Massachusetts Institute of Technology and Harvard University, Cambridge, MA) (27). IGV was used to view alignments. DESeq2 (Bioconductor) analysis was used to determine differences across genotype within steroid treatment, that is, compare WT vs KIKO, WT vs ERKO, and KIKO vs ERKO, within both oil- and E2B-treated females (28). Genes with a FC >1.5 were determined to be differentially regulated. Venn analysis was performed using Venny 2.1 (29) to visualize the degree of overlap of genes in the datasets.
Chromatin immunoprecipitation sequencing
Existing estrogen receptor 1 (ESR1) chromatin immunoprecipitation sequencing (ChIP-seq) performed on WT (GSE36455) (30) and KIKO (GSE56466) (31) mice was downloaded from the Gene Expression Omnibus and mapped to the mouse genome (mm10) using Bowtie2 (32). Further ChIP-seq analysis was done with MACS2 (33) and ChIPseeker (34), and then genome views were achieved with IGV (27).
Availability of datasets
RNA sequencing data can be retrieved from Gene Expression Omnibus (GSE86609) (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE86609).
Results
Regulation of the estrogen-regulated transcriptome in WT, KIKO, and ERKO females
The goal of this study was to demarcate E2-dependent genome-wide expression patterns controlled by ERα through the nuclear ERE-dependent, nuclear ERE-independent, or through ERα-independent signaling (e.g., ERβ, GPER1, Gq-mER) in the ARC of the female mouse using RNA-seq. A flowchart illustrating these different transcriptional pathways can be found in Fig. 1(A). To investigate these mechanisms, we used two transgenic ERα mouse models, total ERKO and ERα KIKO, that express an ERα mutation that eliminates DNA binding through ERE, and their WT littermates (11). By comparing the pattern of E2-induced gene expression in these mutants, we can identify genes and canonical pathways elicited by each type of ERα signaling. Our analysis also identifies potential novel signaling mechanisms and cellular processes that E2 impacts via the ARC to control hypothalamic functions such as energy balance and reproduction.
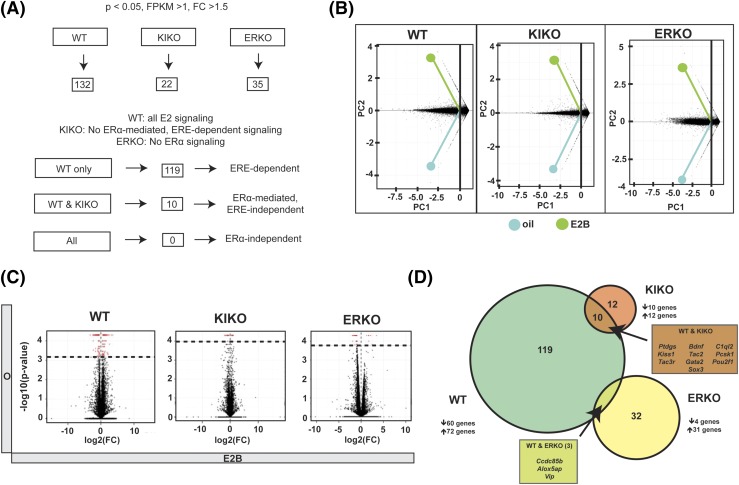
Figure 1.
Number of genes regulated by E2B in WT, KIKO, and ERKO females. (A) Flowchart of the number of genes regulated by E2B in each genotype and overlap of genes. All differentially regulated genes have FPKM > 1 and P < 0.05. (B) PCA of each treatment, showing clustering of replicates. (C) Volcano plots showing number of genes in samples and total genes regulated. Red dots signify genes that are differentially regulated. Black dots represent genes that are not significantly regulated. (D) Venn diagram showing overlap of genes regulated by E2B in WT, KIKO, and ERKO females.
First, we performed PCA on each set of mice individually to examine how global expression correlated between the mice and to gauge the overall effect of E2 [Fig. 1(B)]. In short, the PCA revealed that there was a significant amount of expression variance due to E2, independent of genetic background. We plotted gene expression differences using volcano plots to graphically represent the number of genes that changed expression due to E2B injection in the genotypes [Fig. 1(C)]. The red dots on each plot signify genes differentially expressed (FC > 1.5, P < 0.05, FPKM > 1). WT females displayed the most dramatic response with 132 genes displaying altered expression [Fig. 1(D)], compared with 35 genes in ERKO and 22 genes in KIKO females [Fig. 1(D)]. To gauge the overlap among the 3 groups of mice, we performed Venn analysis. WT and KIKO females had an overlap of 10 genes: prostaglandin D2 synthase (Ptgds), brain-derived neurotrophic factor (Bdnf), complement component 1, q subcomponent–like 2 (C1ql2), kisspeptin (Kiss1), tachykinin 2 (Tac2), proprotein convertase subtilisin/kexin type 1 (Pcsk1), tachykinin 3 receptor (Tac3r), GATA-binding protein 2 (Gata2), POU domain, class 2, transcription factor 1 (Pou2f1), and sex determining region Y-box 3 (Sox3). WT and ERKO females had an overlap of three genes: coiled-coil domain containing 85B (Ccdc85b), arachidonate 5-lipoxygenase activating protein (Alox5ap), and vasoactive intestinal peptide (Vip). There was no overlap between KIKO and ERKO and therefore no overlap among all three genotypes. Taken together, our data indicate that many neuropeptides, transcription factors, and enzymes are regulated by E2 through an ERα-mediated, ERE-independent pathway.
We next performed Gene Ontology analysis on the 132 genes that were E2-responsive in WT, 60 that were decreased and 72 that were increased with E2B (Table 1). In addition to the annotated genes listed in Table 1, additional predicted genes and genes of unknown function were regulated by E2B (data not shown). The annotated genes were grouped into the following functions (number of genes): calcium regulation (2), cell adhesion (3), cell signaling (2), chromosome structure (3), cytoskeleton (6), enzyme (6), extracellular matrix (12), growth factor (3), immune/inflammation (11), ion channel (4), neurodevelopment (5), neuropeptide (12), neurotransmission (7), neurotrophic factors (2), protein modification (3), protein trafficking (2), receptor (7), transcription factor (19), translation (3), and WNT signaling (2).
Table 1.
Genes Regulated by E2B in WT Females
| Gene | FC | P Value | Function | Gene | FC | P Value | Function |
|---|---|---|---|---|---|---|---|
| Mgp | 0.49 | 5.00E-05 | Calcium regulation | Accn4 | 4.10 | 5.00E-05 | Ion channel |
| Stc1 | 1.99 | 0.00035 | Calcium regulation | Fxyd2 | 1.99 | 0.00065 | Ion channel |
| Mfge8 | 0.63 | 5.00E-05 | Cell adhesion | Grik1 | 1.98 | 5.00E-05 | Ion channel |
| Pcdh20 | 1.81 | 5.00E-05 | Cell adhesion | Kcnk10 | 0.47 | 2.00E-04 | Ion channel |
| Pvrl1 | 1.78 | 5.00E-05 | Cell adhesion | C1ql2 | 0.60 | 5.00E-05 | Neurodevelopment |
| Crabp1 | 1.83 | 5.00E-05 | Cell signaling | Cbln2 | 1.74 | 1.00E-04 | Neurodevelopment |
| Dock5 | 0.65 | 0.00055 | Cell signaling | Fam5b | 1.52 | 5.00E-05 | Neurodevelopment |
| Efs | 0.63 | 1.00E-04 | Cell signaling | Prok2 | 2.02 | 5.00E-05 | Neurodevelopment |
| Enpp1 | 0.39 | 1.00E-04 | Cell signaling | Robo1 | 0.63 | 0.00015 | Neurodevelopment |
| Gbp6 | 0.50 | 5.00E-05 | Cell signaling | Agrp | 1.56 | 5.00E-05 | Neuropeptide |
| Grap | 3.67 | 5.00E-05 | Cell signaling | Cartpt | 0.52 | 5.00E-05 | Neuropeptide |
| Jak3 | 0.56 | 5.00E-04 | Cell signaling | Cck | 0.49 | 5.00E-05 | Neuropeptide |
| Map2k1 | 1.55 | 5.00E-05 | Cell signaling | Kiss1 | 0.08 | 5.00E-05 | Neuropeptide |
| Net1 | 1.78 | 5.00E-05 | Cell signaling | Nms | 0.40 | 5.00E-05 | Neuropeptide |
| Pcp4 | 1.85 | 5.00E-05 | Cell signaling | Nts | 2.18 | 5.00E-05 | Neuropeptide |
| Pcp4l1 | 1.58 | 5.00E-05 | Cell signaling | Pdyn | 0.49 | 5.00E-05 | Neuropeptide |
| Pim3 | 1.63 | 5.00E-05 | Cell signaling | Pomc | 0.64 | 5.00E-05 | Neuropeptide |
| Ptprg | 1.51 | 0.00065 | Cell signaling | Scg2 | 1.50 | 5.00E-05 | Neuropeptide |
| Rab37 | 5.51 | 5.00E-05 | Cell signaling | Tac2 | 0.43 | 5.00E-05 | Neuropeptide |
| Rasd1 | 2.69 | 5.00E-05 | Cell signaling | Vgf | 0.36 | 5.00E-05 | Neuropeptide |
| Rps6ka6 | 1.76 | 5.00E-05 | Cell signaling | Vip | 1.67 | 5.00E-05 | Neuropeptide |
| Rrad | 0.53 | 5.00E-05 | Cell signaling | Chga | 0.66 | 5.00E-05 | Neurotransmission |
| Rybp | 1.64 | 5.00E-05 | Cell signaling | Chgb | 0.63 | 5.00E-05 | Neurotransmission |
| Trp53i11 | 1.82 | 5.00E-05 | Cell signaling | Nxph3 | 0.29 | 5.00E-05 | Neurotransmission |
| Tyro3 | 0.65 | 5.00E-04 | Cell signaling | Slc6a3 | 0.39 | 5.00E-05 | Neurotransmission |
| H2afj | 0.58 | 5.00E-05 | Chromosome structure | Sv2b | 1.68 | 5.00E-05 | Neurotransmission |
| Mad2l1 | 5.19 | 5.00E-05 | Chromosome structure | Syt2 | 2.17 | 5.00E-05 | Neurotransmission |
| Rec8 | 0.26 | 5.00E-05 | Chromosome structure | Syt6 | 2.18 | 5.00E-05 | Neurotransmission |
| Arc | 0.54 | 5.00E-05 | Cytoskeleton | Gfra1 | 2.93 | 5.00E-05 | Neurotrophic factors |
| Ezr | 1.75 | 5.00E-05 | Cytoskeleton | Ret | 1.58 | 5.00E-05 | Neurotrophic factors |
| Gfap | 0.57 | 5.00E-05 | Cytoskeleton | Pcsk1 | 0.45 | 5.00E-05 | Protein modification |
| Inf2 | 0.66 | 2.00E-04 | Cytoskeleton | Pcsk1n | 0.66 | 5.00E-05 | Protein modification |
| Myoc | 0.44 | 5.00E-05 | Cytoskeleton | Pcsk6 | 1.88 | 5.00E-05 | Protein modification |
| Spnb4 | 0.68 | 6.00E-04 | Cytoskeleton | Galnt6 | 2.22 | 5.00E-05 | Protein trafficking |
| Gstm5 | 1.70 | 5.00E-05 | Enzyme | Galntl4 | 1.53 | 1.00E-04 | Protein trafficking |
| Pter | 1.57 | 4.00E-04 | Enzyme | Cckar | 1.80 | 5.00E-05 | Receptor |
| Aldh1a2 | 0.43 | 0.00035 | Enzyme | Ghsr | 3.18 | 5.00E-05 | Receptor |
| Chst11 | 1.54 | 5.00E-05 | Enzyme | Gpr88 | 2.04 | 5.00E-05 | Receptor |
| Ptgds | 0.57 | 5.00E-05 | Enzyme | Oxtr | 3.19 | 5.00E-05 | Receptor |
| Tiparp | 1.54 | 2.00E-04 | Enzyme | Pgr | 2.63 | 5.00E-05 | Receptor |
| Bgn | 0.61 | 2.00E-04 | Extracellular matrix | Tacr3 | 0.20 | 5.00E-05 | Receptor |
| Col1a1 | 0.39 | 5.00E-05 | Extracellular matrix | Unc5b | 1.75 | 5.00E-05 | Receptor |
| Col2a1 | 2.17 | 5.00E-05 | Extracellular matrix | Phf21b | 5.13 | 1.00E-04 | Transcription factor |
| Col3a1 | 0.47 | 5.00E-05 | Extracellular matrix | Ccdc36 | 3.16 | 0.00025 | Transcription factor |
| Fmod | 0.46 | 0.00055 | Extracellular matrix | Ccdc85b | 0.65 | 0.00035 | Transcription factor |
| Lamb1 | 3.32 | 5.00E-05 | Extracellular matrix | Egr4 | 0.32 | 5.00E-05 | Transcription factor |
| Lamb3 | 3.22 | 5.00E-05 | Extracellular matrix | Ets2 | 1.61 | 5.00E-05 | Transcription factor |
| Lor | 0.53 | 0.00015 | Extracellular matrix | Fosl2 | 1.60 | 5.00E-05 | Transcription factor |
| Lum | 0.40 | 5.00E-05 | Extracellular matrix | Gata2 | 0.57 | 0.00055 | Transcription factor |
| Sdc1 | 0.32 | 5.00E-05 | Extracellular matrix | Greb1 | 9.70 | 5.00E-05 | Transcription factor |
| Smoc2 | 4.96 | 5.00E-05 | Extracellular matrix | Lcorl | 1.58 | 5.00E-05 | Transcription factor |
| Tnxb | 3.88 | 5.00E-05 | Extracellular matrix | Mamld1 | 1.62 | 5.00E-05 | Transcription factor |
| Bdnf | 0.29 | 5.00E-05 | Growth factor | Msx1 | 0.50 | 2.00E-04 | Transcription factor |
| Igfbp2 | 0.18 | 5.00E-05 | Growth factor | Myc | 2.47 | 5.00E-05 | Transcription factor |
| Igfbp3 | 0.49 | 5.00E-05 | Growth factor | Mycn | 2.92 | 5.00E-05 | Transcription factor |
| Alox5ap | 0.24 | 0.00045 | Immune/inflammation | Nr4a2 | 5.13 | 5.00E-05 | Transcription factor |
| H2-Aa | 0.33 | 1.00E-04 | Immune/inflammation | Nr5a2 | 1.58 | 0.00055 | Transcription factor |
| H2-Q2 | 1.58 | 5.00E-05 | Immune/inflammation | Olig2 | 0.60 | 5.00E-05 | Transcription factor |
| Hs3st5 | 2.45 | 5.00E-05 | Immune/inflammation | Pou2f1 | 1.95 | 3.00E-04 | Transcription factor |
| Icam5 | 0.59 | 5.00E-05 | Immune/inflammation | Pou3f1 | 2.51 | 5.00E-05 | Transcription factor |
| Islr | 0.64 | 1.00E-04 | Immune/inflammation | Sox3 | 0.36 | 5.00E-05 | Transcription factor |
| Mrc2 | 0.54 | 0.00045 | Immune/inflammation | Nup62cl | 4.89 | 3.00E-04 | Translation |
| Procr | 0.22 | 5.00E-04 | Immune/inflammation | Rbms3 | 1.79 | 5.00E-05 | Translation |
| Spsb1 | 1.84 | 0.00025 | Immune/inflammation | Zfp804a | 1.70 | 2.00E-04 | Translation |
| Tmem90a | 2.23 | 5.00E-05 | Immune/inflammation | Sfrp5 | 0.56 | 5.00E-05 | WNT signaling |
| Tnfrsf11b | 0.33 | 5.00E-05 | Immune/inflammation | Wnt4 | 1.86 | 5.00E-05 | WNT signaling |
Genes listed are significantly regulated by E2B in WT females, which have a P < 0.05 and FPKM values >1. Genes represent increases and decreases in gene expression by a FC of 0.67 < FC < 1.5.
There were 22 genes regulated by E2B in KIKO females (Table 2), grouped by function (Table 1). Of these 22 genes, 10 were decreased and 12 were increased in response to E2B. The E2B-responsive genes in KIKO represented neuropeptides, growth factors, and transcription factors involved in reproduction and energy balance. There were 35 genes regulated by E2B in ERKO females (Table 3). Of these 35 genes, 4 genes were decreased and 31 were increased with E2B. Interestingly, several genes involved in mitochondrial oxidation and respiration were upregulated in response to E2B in ERKO females, possibly suggesting an increase in mitochondrial function and adenosine triphosphate (ATP) generation in ARC neurons. These mitochondrial oxidation genes include: cytochrome c oxidase subunit 5B (Cox5b), NADH:ubiquinone oxidoreductase subunit A11 (Ndufa11), NADH:ubiquinone oxireductase subunit B10 (Ndufb10), NADH:ubiquinone oxidoreductase subunit B7 (Ndufb7), cytochrome c reductase, complex III subunit XI (Uqcr11), and cytochrome c reductase core protein (Uqcr)1. In addition to mitochondrial genes, numerous ribosomal proteins that comprise both the small and large ribosomal subunits were increased with E2B. Many of the genes regulated in ERKO females did not overlap with the genes regulated in WT females and none overlapped in KIKO females.
Table 2.
Genes Regulated by E2B in KIKO Females
| Gene | FC | P Value | Function |
|---|---|---|---|
| Cabp7 | 1.64 | 5.00E-05 | Calcium regulation |
| Cd74 | 1.74 | 5.00E-05 | Cancer |
| Epcam | 2.40 | 5.00E-05 | Cell adhesion |
| Cdkn1c | 1.99 | 5.00E-05 | Cell proliferation |
| Cyp2f2 | 1.90 | 5.00E-05 | Enzyme |
| Inmt | 2.05 | 5.00E-05 | Enzyme |
| Ptgds | 0.46 | 5.00E-05 | Enzyme |
| Bdnf | 0.53 | 5.00E-05 | Growth factor |
| Igf2 | 0.50 | 5.00E-05 | Growth factor |
| Kcnh3 | 2.42 | 5.00E-05 | Ion channel |
| C1ql2 | 0.65 | 5.00E-05 | Neurodevelopment |
| Gldn | 3.93 | 5.00E-05 | Neurodevelopment |
| Kiss1 | 0.53 | 5.00E-05 | Neuropeptide |
| Pnoc | 0.66 | 5.00E-05 | Neuropeptide |
| Tac2 | 0.66 | 5.00E-05 | Neuropeptide |
| Pcsk1 | 0.63 | 5.00E-05 | Protein modification |
| Tacr3 | 0.54 | 5.00E-05 | Receptor |
| Gata2 | 1.80 | 5.00E-05 | Transcription factor |
| Pitx1 | 1.88 | 0.0001 | Transcription factor |
| Pou2f1 | 3.49 | 5.00E-05 | Transcription factor |
| Sox3 | 0.58 | 5.00E-05 | Transcription factor |
| Slc17a7 | 3.39 | 5.00E-05 | Transporter |
Genes listed are significantly regulated by E2B in KIKO females, which have a P value <0.05 and FPKM values >1. Genes represent increases and decreases in gene expression by a FC of 0.67 < FC < 1.5.
Table 3.
Genes Regulated by E2B in ERKO Females
| Gene | FC | P Value | Function | Gene | FC | P Value | Function |
|---|---|---|---|---|---|---|---|
| C1qtnf4 | 2.19 | 5.00E-05 | Cancer | Tac1 | 1.91 | 5.00E-05 | Neuropeptide |
| Ccdc85b | 2.15 | 5.00E-05 | Cell growth | Vip | 0.42 | 5.00E-05 | Neuropeptide |
| Nenf | 2.02 | 1.00E-04 | Cell growth | Npy6r | 0.31 | 5.00E-05 | Receptor |
| Katnal1 | 2.72 | 5.00E-05 | Cytoskeleton | Mrpl52 | 2.09 | 5.00E-05 | Ribosomes |
| Tmsb10 | 1.91 | 1.00E-04 | Cytoskeleton | Rpl18a | 1.91 | 0.00015 | Ribosomes |
| Alox5ap | 0.23 | 5.00E-05 | Immune/inflammation | Rpl36 | 2.06 | 5.00E-05 | Ribosomes |
| Fkbp2 | 1.94 | 1.00E-04 | Immune/inflammation | Rpl36a | 2.22 | 5.00E-05 | Ribosomes |
| Iigp1 | 0.31 | 5.00E-05 | Immune/inflammation | Rpl36al | 1.85 | 1.00E-04 | Ribosomes |
| Atp5e | 2.17 | 5.00E-05 | Mitochondrial oxidation | Rpl37 | 2.00 | 1.00E-04 | Ribosomes |
| Cox5b | 1.86 | 0.00015 | Mitochondrial oxidation | Rpl38 | 1.98 | 1.00E-04 | Ribosomes |
| Ndufa11 | 1.88 | 1.00E-04 | Mitochondrial oxidation | Rpl41 | 2.22 | 1.00E-04 | Ribosomes |
| Ndufb10 | 2.05 | 5.00E-05 | Mitochondrial oxidation | Rps24 | 2.12 | 1.00E-04 | Ribosomes |
| Ndufb7 | 1.95 | 0.00015 | Mitochondrial oxidation | Med29 | 1.96 | 1.00E-04 | Transcript factor |
| Uqcr11 | 1.98 | 0.00015 | Mitochondrial oxidation | Scand1 | 3.15 | 5.00E-05 | Transcript factor |
| Uqcrq | 2.13 | 5.00E-05 | Mitochondrial oxidation | Atox1 | 2.11 | 5.00E-05 | Transporter |
| Avp | 3.64 | 5.00E-05 | Neuropeptide | Hba-a1 | 2.30 | 5.00E-05 | Transporter |
| Oxt | 3.03 | 5.00E-05 | Neuropeptide | Timm13 | 1.96 | 5.00E-05 | Transporter |
Genes listed are significantly regulated by E2B in ERKO females, which have a P value <0.05 and FPKM values >1. Genes represent increases and decreases in gene expression by a FC of 0.67 < FC < 1.5.
Quantitative polymerase chain reaction validation of gene expression regulation by E2B
We have previously published data identifying E2B regulation of ARC genes using quantitative polymerase chain reaction (qPCR) (12). Table 4 includes a comparison between selected genes of interest using qPCR and RNA-seq. qPCR and RNA-seq experiments used the same experimental paradigm, although different animals were used. For full methodology used in qPCR analysis, see our previous publication (12). In brief, many of the genes that were regulated by E2B in qPCR analysis were similarly regulated in our RNA-seq experiments. However, there were a few genes that were differentially regulated by E2B in qPCR and in RNA-seq. This includes Tac2 expression in KIKO females, which was decreased by E2B in RNA-seq but not qPCR, and cocaine- and amphetamine-regulated transcript (Cart) expression, which decreased by E2B across all three genotypes in qPCR, but not in RNA-seq.
Table 4.
Verification Table for qPCR and RNA-seq
| Genes |
WT |
KIKO |
ERKO |
|||
|---|---|---|---|---|---|---|
| qPCR | RNA-seq | qPCR | RNA-seq | qPCR | RNA-seq | |
| Kiss1 | ↓ | ↓ | ↓ | ↓ | — | — |
| Tac2 | ↓ | ↓ | — | ↓ | — | — |
| Pdyn | ↓ | — | — | — | — | — |
| Tac3r | ↓ | — | ↓ | — | — | — |
| Kiss1r | ↑ | — | ↑ | — | ↑ | — |
| Pomc | — | ↓ | — | — | — | — |
| Npy | — | — | — | — | — | — |
| Cart | ↓ | ↓ | ↓ | — | ↑ | — |
| Ghsr | ↑ | ↑ | — | — | — | — |
| Esr1 | ↓ | — | — | — | — | — |
| Esr2 | ↑ | — | ↑ | — | — | — |
| Pgr | ↑ | ↑ | — | — | — | — |
Verification table showing direction of change in gene expression with E2B, compared with oil. The table represents selected genes of interest and compares similarities and differences in gene expression using qPCR and RNA-seq.
Distinct canonical pathways are regulated by E2B across WT, KIKO, and ERKO females
To elucidate the cellular pathways that were regulated by E2B in each of the three genotypes, we used IPA to determine relationships among genes of interest. Table 5 lists the top canonical pathways within each genotype. In WT females, many of the pathways are involved in diseases, including Alzheimer’s disease, atherosclerosis, and endometrial cancer. In KIKO females, many pathways involved in immune/inflammation and energy balance were regulated, including prostanoid biosynthesis, eicosanoid signaling, and glucocorticoid signaling. In ERKO females, many genes involved in oxidative phosphorylation and mitochondrial dysfunction are regulated by E2B.
Table 5.
Top 10 Canonical Pathways Regulated by E2B in WT, KIKO, and ERKO Females
|
Top Canonical Pathways |
P Value |
Genes |
|---|---|---|
| WT | ||
| Intrinsic prothrombin activation pathway | 0.0002 | Col1a1, Col2a1, Col3a1 |
| Dendritic cell maturation | 0.0004 | Col1a1, Col2a1, Hla-Dqa1, Tnfrsf11b, Col3a1 |
| Thyroid cancer signaling | 0.0005 | Myc, Cdh1, Bdnf |
| Hepatic fibrosis/hepatic stellate cell activation | 0.0008 | Col1a1, Igfbp3, Col2a1, Tnfrsf11b, Col3a1 |
| Neuroprotective role of THOP1 in Alzheimer disease | 0.0091 | Pdyn, Nts |
| Atherosclerosis signaling | 0.0107 | Col1a1, Col2a1, Col3a1 |
| Endometrial cancer signaling | 0.0195 | Myc, Cdh1 |
| T helper cell differentiation | 0.0302 | Hla-Dqa1, Tnfrsf11b |
| Wnt/β-catenin signaling | 0.0309 | Myc, Cdh1, Sox3 |
| PDGF signaling | 0.0398 | Myc, Sphk1 |
| KIKO | ||
| Prostanoid biosynthesis | 0.004 | Ptgds |
| Role of JAK1, JAK2, and TYK2 in interferon signaling | 0.0091 | Cga |
| Autoimmune thyroid disease signaling | 0.0158 | Cga |
| Nicotine degradation II | 0.0234 | Inmt |
| Glutamate receptor signaling | 0.0245 | Slc17a7 |
| Eicosanoid signaling | 0.0263 | Ptgds |
| Role of BRCA1 in DNA damage response | 0.0339 | Pou2f1 |
| Protein kinase Cθ signaling in T lymphocytes | 0.0479 | Pou2f1 |
| Ovarian cancer signaling | 0.055 | Cga |
| Glucocorticoid receptor signaling | 0.1102 | Pou2f1 |
| ERKO | ||
| Oxidative phosphorylation | 0.0003 | Uqcrq, Ndufb10, Atp5e |
| Circadian rhythm signaling | 0.0009 | Avp, Vip |
| Mitochondrial dysfunction | 0.0013 | Uqcrq, Ndufb10, Atp5e |
| EIF2 signaling | 0.0015 | Rpl36a, Rpl41, Rps24 |
| Retinoic acid–mediated apoptosis signaling | 0.0603 | Tnfsf10 |
| nNOS signaling in neurons | 0.0603 | Capn11 |
| Amyloid processing | 0.0661 | Capn11 |
| Regulation of cellular mechanics by calpain protease | 0.0724 | Capn11 |
| Eicosanoid signaling | 0.0813 | Alox5p |
| GPCR-mediated integration of enteroendocrine signaling exemplified by an L cell | 0.0891 | Vip |
Top 10 canonical pathways as analyzed using IPA. Genes listed following each canonical pathway represent either an increase or decrease in gene expression.
Abbreviations: nNOS, neuronal nitric oxide synthase; PDGF, platelet-derived growth factor; GPCR, G protein–coupled receptor.
Differential expression of genes across genotypes
Because this was a multivectored experiment, we used DESeq2 to compare across genotypes within treatment groups (oil and E2B). Many of the genes that were different between genotypes (WT vs KIKO and WT vs ERKO) in oil-treated animals were also different in E2B-treated animals. The main changes in gene expression were between WT vs KIKO and WT vs ERKO females, as indicated in Supplemental Tables 1 (24.2KB, docx) and 2 (24.2KB, docx) and illustrated in Fig. 2. As expected, there were many differences in genes involved in reproduction and energy balance across genotypes, including 31 genes that overlapped between all four treatments (1, WT vs KIKO oil; 2, WT vs KIKO E2B; 3, WT vs ERKO oil; 4, WT vs ERKO E2B; Fig. 2). These genes include Pck1, Ptgs2, Tac3r, and Map3k15, among others. Additionally, there were 22 genes that were higher or lower in WT compared to KIKO females in both oil and E2B comparisons, including Tlr4 and Grik1. Finally, there were 74 genes that were differentially expressed between WT and ERKO females in both oil and E2B comparisons, including Vgf, Bdnf, Sox3, Pomc, Tac2, progesterone receptor (Pgr), and growth hormone secretagogue receptor (Ghsr).
Figure 2.
Overlap of differential gene expression in WT, KIKO, and ERKO females. Venn diagram of overlapping genes expressed differentially in WT, KIKO, and ERKO females. Genes were compared with WT females and represent either significantly increased or decreased gene expression. Genes represent FC > 1.5 and P < 0.05. All genes differentially expressed by WT vs KIKO and WT vs ERKO females were different in both oil- and E2B-treated groups, except for protein tyrosine phosphatase, nonreceptor type 20 (Ptpn20), which was different between WT vs ERKO oil, but not E2B females.
The KNDy neuropeptide genes are regulated through differential transcript variants
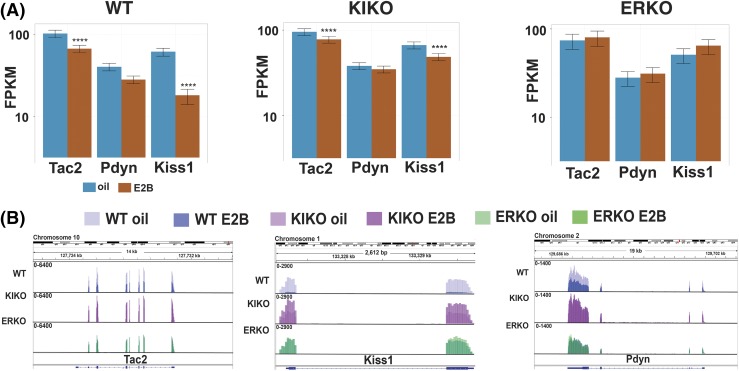
Previous reports have identified E2 regulation of two of the three neuropeptides coexpressed in KNDy neurons. These studies found that E2 regulated Kiss1 through ERE-independent signaling and Dyn (dynorphin) through ERE-dependent signaling (12, 35). We used cummeRbund to generate gene expression graphs of the KNDy neuropeptide genes. In our study, we found that E2B decreased expression of Tac2 (neurokinin B) and Kiss1 in WT and KIKO females [Fig. 3(A)]. Interestingly, we did not see a change in Pdyn or prodynorphin in any genotype [Fig. 3(A)]. To determine whether the transcription across the genotypes and treatments aligned with our previous gene expression data (12), we used IGV (27) to visualize the KNDy neuropeptide transcripts. Figure 3(B) illustrates that Tac2 and Kiss1 transcripts are decreased by E2B in WT and KIKO females, consistent with our gene expression analysis [Fig. 3(A)]. In both Tac2 and Kiss1, there are differences between oil- and E2B-treated females across the entire gene. Conversely, we did visualize a change in Pdyn, in 1 region of the gene (on the 5′ end), contradicting the lack of changes in gene expression in any genotype [Fig. 3(A)], which may be a bias due to the exon length.
Figure 3.
KNDy genes are regulated through differential E2 signaling. (A) Bar graphs of the KNDy neuropeptide genes (Kiss1, Tac2, and Pdyn) in WT, KIKO, and ERKO females. FPKM is a measurement of relative gene expression. Data are expressed as mean ± SEM. (B) Transcript analysis of KNDy neuropeptide genes using IGV.
ERE-independent misregulation occurs via tethering to ERE and HRE sites
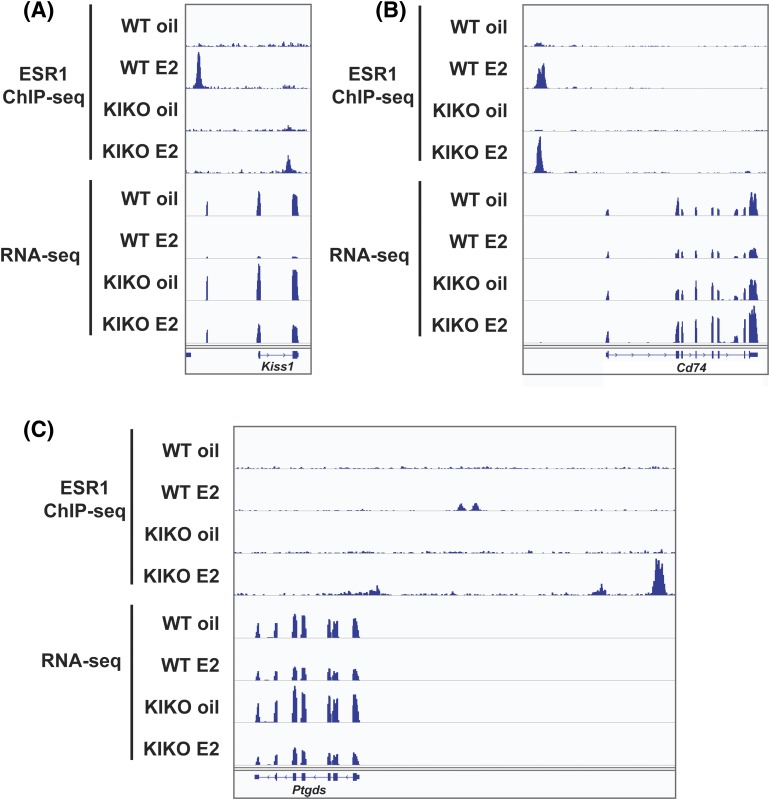
We wanted to understand ERE-independent regulation that occurs in the KIKO mice. ESR1 ChIP-seq performed on KIKO mice revealed that ERα (ESR1) lacking a functional DNA-binding domain can be tethered to both ERE and HRE sites via association with other transcription factors, but this may not contribute to the estrogen response (31). To gain insight into ERE-independent regulation in the ARC, we used existing ESR1 ChIP-seq data on both the WT and KIKO mice (30, 31). In general, we found that E2-repsonsive genes such as Kiss1 were de-repressed in KIKO mice relative to WT because ESR1 was tethered to an HRE site [Fig. 4(A)]. In contrast, other genes such as Cd74 had KIKO ESR1 tethered to the ERE, but the lack of direct DNA binding prevented E2-dependent repression [Fig. 4(B)]. We also observed instances where E2-responsive genes behaved similarly in WT and KIKO mice but regulation in KIKO was mediated by tethering to an HRE. An example of this was found at Ptdgs [Fig. 4(C)]. Collectively, these data reveal that ERE-independent regulation in the ARC is mainly mediated by tethering of ERα, consistent with previous findings (30, 31).
Figure 4.
ERE-independent regulation is mediated by tethering. Gene-specific analysis was done with IGV comparing RNA-seq and ChIP-seq data for (A) Kiss1, (B) Cd74, and (C) Ptgds in WT and KIKO mice treated with E2 or oil.
Discussion
The results of the present study identify the ARC estrogen-regulated transcriptome in WT, KIKO, and ERKO females. We set up a multifactored RNA-seq on three separate genotypes to determine genes that require specific E2B signaling mechanisms. We identify that many genes involved in calcium balance, metabolism, mitochondrial oxidation, transcription, neurotransmission, and inflammation are all regulated by E2. Interestingly, many of the genes expressing transcription factors that control energy balance and reproduction are regulated by ERE-independent mechanisms, present in WT and KIKO females, but not in ERKO females, suggesting that membrane-mediated responses to E2 are important in the ARC estrogen-regulated transcriptome. Furthermore, many mitochondrial oxidation genes were regulated by E2B in ERKO females, but not in WT and KIKO females, suggesting compensatory mechanisms that are ERα-independent may be important for cellular energy production.
The arcuate estrogen-regulated transcriptome regulates homeostatic functions
In WT females, E2 regulates 132 genes. Of these, 119 genes are regulated by E2B in WT females only, indicating that transcription is mediated by ERα interacting with ERE. Although it is not possible to fully discuss each of these genes, we have characterized these genes into different functions. These functions include calcium regulation, cell adhesion, cell signaling, chromosome structure, cytoskeleton, detoxification, enzymes, extracellular matrix genes, growth factors, inflammatory genes, ion channels, neurodevelopment, neuropeptides, neurotransmission, protein modification and tracking, receptors, transcription, and translation.
E2 regulates neuropeptides that are involved in energy balance and reproduction, processes that are controlled centrally in the ARC. Previous studies suggest that E2 functions in the control of expression of a variety of neuropeptides, including POMC, kisspeptin, and dynorphin (15, 36–42). E2 signaling in energy balance includes regulation of POMC/CART and NPY/AgRP neurons that are anorectic and orexigenic, respectively (8, 10, 15, 18, 37, 38, 43). In the present study, Cartpt, CART prepropeptide, and Pomc were decreased by E2B, whereas Agrp was increased by E2B, consistent with our previous findings (44). E2 differentially regulates these orexigenic neurons through multiple receptor-mediated mechanisms. The nonsteroidal selective ligand for the Gq-mER, STX, functions through an ERE-independent and nuclear receptor–independent pathway to enhance the GABAergic postsynaptic response in NPY neurons, leading to a decrease in Npy expression in the ARC (18), which is opposite to the attenuation caused by classical ERα activation.
A number of receptors involved in reproduction (Pgr, Tac3r) and energy balance (Cckar, Ghsr) are regulated by E2B. Pgr and Tac3r are essential for normal functioning of the hypothalamic/pituitary/gonadal (HPG) axis (45) and are increased and decreased with E2B, respectively, consistent with previous studies (12). We previously reported that Ghsr, the ghrelin receptor, is increased by E2B in the ARC but not in NPY neurons, in which GHSR activation inhibits the M-current (44). Ghrelin is a peptide hormone that stimulates food intake through a brain–gut neural connection. The increase in Ghsr by E2B in WT ARC is due to Ghsr augmentation in Tac2 (KNDy) neurons (46). Additional receptors are also regulated in the ARC estrogen-regulated transcriptome.
E2 functions through ERE-independent pathways in the ARC to regulate gene expression
There are many genes that are regulated by E2B in both WT and KIKO females, but not in ERKO females, suggesting that ERα-mediated, ERE-independent signaling is important in the control of gene expression in the ARC. These genes include Bdnf, C1ql2, Gata2, Kiss1, Pcsk1, Pou2f1, Ptgds, Sox3, Tac2, and Tac3r. Of these genes, all were downregulated by E2B in both WT and KIKO animals except for Gata2 (decreased in WT, increased in KIKO) and Pou2f1 (increased in WT and KIKO).
Recently, we reported a number of ARC genes that were regulated by ERE-dependent and -independent mechanisms (12). Kiss1-expressing genes in the ARC are often coexpressed in KNDy neurons that act as an important pulse generator in regulation of GnRH neurons and function in E2 negative feedback on the HPG axis (47, 48). Previous studies, and in our present study using cummeRbund and IGV analysis, found that Kiss1 is regulated, in part, through ERα-mediated, ERE-independent mechanisms (12, 35). Additionally, we have previously shown that Tac2 (neurokinin B) is decreased by E2B only in WT females (12), unlike in the present study where E2B suppressed Tac2 in both WT and KIKO females. Tac3r is also decreased by E2B in WT and KIKO females (12). Thus, KNDy-associated genes are regulated through multiple ERα-mediated pathways. Regulation of ARC gene expression for selected genes was similar, for the most part, between qPCR and RNA-seq analysis such as Kiss1, Ghsr, and Pgr. However, other genes were not, such as Tac2, which was decreased by E2B in WT females in both qPCR and RNA-seq, and decreased by E2B in KIKO females only in RNA-seq analysis. In the RNA-seq, the FC for Tac2 in KIKO is 0.66, which is close to our cut-off at 0.67, whereas in our previous study, EB did not alter ARC Tac2 expression, although Tac2 was differentially expressed between the 3 genotypes (12). One additional example is Cart, which was decreased by E2B in WT and KIKO in the qPCR analysis, but had no change in the RNA-seq in the KIKO. These differences highlight the importance of using multiple techniques to identify changes in gene expression, preferably from the same ARC RNA samples whether amplified or not.
Many genes regulated by E2B in WT and KIKO females are classified as transcription factors, including Gata2, Pou2f1, and Sox3. Gata2 belongs to the GATA family of transcription factors, which regulate gonadotropin gene expression (49). Furthermore, GATA-2 is involved in gonadotrope differentiation (49) and stimulation of gonadotropin-releasing hormone receptor genes, and suppression of GATA-2 in gonadotropes is associated with decreased luteinizing hormone expression (50, 51). In the ARC, Gata2 was decreased by E2B in WT females and increased by E2B in KIKO females. It is possible that Gata2 is negatively regulated by E2 through ERE-dependent mechanisms. Conversely, without ERE-dependent signaling, E2 augments Gata2 expression via ERE-independent mechanisms. Pou2f1, or Oct-1, is a transcription factor in the POU transcription factor family important in numerous neuroendocrine functions, including regulation of GnRH expression (52, 53). We found that E2B increased Pou2f1 twofold in WT females and more than fourfold in KIKO females. Although there have been no studies to date on the role of Pou2f1 in the ARC, in MCF7, an ERα-positive breast cancer cell line, high concentrations of E2 increase genes associated with the Pou2f1 binding regions (54). SOX3 is important for the formation of the hypothalamic/pituitary axis (55, 56) as well as neurogenesis (57), and its expression is decreased by E2B in WT and KIKO females. Interestingly, there are no studies that examine Sox3 in the ARC or its interactions with E2. However, other members of the SOX family of transcription factors, including SOX4 and SOX11, are coexpressed in GnRH neurons and thus are important regulators of GnRH mRNA expression (58). Sox3 transcription is decreased by E2B through ERα-mediated, ERE-independent mechanisms and, combined with the regulation of Gata2 and Pou2f1 expression, suggests that E2 also controls transcription of ARC reproductive genes through the indirect regulation of specific transcription factors.
Pcsk1, or PC1/3, is involved in the differential cleavage of POMC into adrenocorticotropic hormone, and another proprotein convertase, Pcsk2 (PC2), cleaves the POMC protein into α-melanocyte stimulating hormone (59). Pcsk1 expression was decreased with E2B through ERα-mediated, ERE-independent mechanisms, presumably shifting the cleavage of the POMC protein toward α-melanocyte stimulating hormone processing for subsequent release at downstream MC4 receptor-expression neurons to reduce food intake. In ovariectomized guinea pigs, E2B increases Pomc expression and STX did not increase Pomc expression (18), suggesting that Pomc is regulated through nuclear receptor–mediated mechanisms. Consequently, the regulation of Pomc through transcriptional and posttranslational mechanisms is controlled through ERE-dependent and -independent pathways, respectively. Ptgds regulates prostaglandin D2 production, which increases food intake through activation of orexigenic (NPY/AgRP) neurons in the ARC (60). The suppression of Ptgds by E2 through ERE-independent mechanisms potentially reduces prostaglandin D2 synthesis and may be a secondary mechanism activated by E2 to suppress food intake. However, E2 does suppress a gene whose role in energy homeostasis is to reduce food intake and augment energy expenditure, i.e., Bdnf (61). Bdnf primarily acts through the ventromedial nucleus of the hypothalamus and is involved with multiple processes besides the control of food intake, including cell growth, proliferation, and synaptic plasticity (62). In the ARC, E2B reduces Bdnf through ERα-mediated, ERE-independent mechanisms potentially to reduce localized synaptic plasticity in orexigenic neurons (63). Collectively, these data suggest that E2 suppresses and activates multiple pathways to regulate ARC control of energy homeostasis and feeding behavior.
The electron transport chain is regulated by ERα-independent pathways
In ERKO females, 35 genes were regulated by E2B, which suggests a role for ERα-independent pathways. Many of these genes are predicted and are currently not annotated (data not shown). Furthermore, several ribosomal proteins were regulated by E2B in ERKO females. These ribosomal proteins include those that are found in both the small and large ribosomal subunits and all were upregulated by E2B. We found that many genes regulated by E2B in ERKO females were those involved in mitochondrial respiration and the electron transport chain (ETC). These genes include the following: Atp5e, Cox5b, Ndufa11, Ndufb10, Ndufb7, Timm13, Uqcr11, and Uqcrq. Interestingly, all these genes were upregulated in ERKO females and were not regulated by E2B in either WT or KIKO females, suggesting that in the absence of ERα, there are compensatory mechanisms that regulate the ETC.
The enzymes that transfer electrons through the ETC are grouped into several complexes in the mitochondria that span the matrix, inner mitochondrial membrane, and intermembrane space. Components of these complexes are regulated by E2B in ERKO females. Complex I, or the NADH dehydrogenase complex, transfers electrons from NADH and is made up of peripheral and membrane portions (64). Within the peripheral portion, there are four subunits: α, β, γ, and δ (64). Ndufa11 is part of the α subunit and is regulated by E2B in ERKO (64). Additionally, Ndufb10 and Ndufb7 were upregulated by E2B, and they represent part of the NADH dehydrogenase β subunit. To date, there are no studies suggesting that regulation of these genes is controlled by E2, regardless of receptor subtype or signaling mechanisms.
Complex IV includes cytochrome c oxidase (CcO), which is critical for transferring electrons to the final electron acceptor, O2. In ERKO, 3 cytochrome c–associated genes were increased by E2B: Cox5b, Uqcr11, and Uqcrq. Cox5b is a peripheral subunit of CcO, and previous studies indicate that it is critical for CcO activity (65). Complex V of the ETC includes ATP synthase, which uses the H+ ion gradient produced during complexes I to IV to power ATP synthase. In ERKO, Atp5e was increased by E2. Atep5e is critical to a functional F1 epsilon subunit in ATP synthase, which represents the catalytic portion of ATP synthase and spans into the mitochondrial matrix (66). In addition to these genes, Timm13, a translocase of the inner mitochondrial membrane, was increased by E2B in ERKO females. Although it is unclear whether there is a role of Timm13 in the ETC, it functions in import of metabolites from the cytoplasm.
There are no studies that examine E2 regulation of mitochondrial genes in the hypothalamus and especially in the ARC. A recent study found that P4 and E2 regulate mitochondrial oxidative metabolism in the rat brain by increasing expression and activity of CcO (67). Our studies suggest that ERα-independent signaling is critical for maintaining metabolism, and the bioenergetics of the ARC and may be critical to the neuroprotective effects of E2. The ERKO ARC estrogen-regulated transcriptome functions in ERα-independent signaling through multiple mechanisms and signaling pathways. Although ERβ is not as highly expressed in the ARC as ERα, it is possible that E2 functions to control genes through this classical receptor (68). Furthermore, E2 may signal through additional estrogen receptors, including GPER1/GPR30 and the putative membrane estrogen receptor, Gq-mER (8–10, 69). Thus, although we have identified the ARC estrogen-regulated transcriptome in ERKO females, the results of our study require further investigation to determine which E2 signaling pathway is activated.
Comparing gene expression across genotypes using DESeq
Using DESeq, we analyzed the transcriptome between genotypes within the same steroid condition. There were few differences present between oil- and E2B-treated ERKO and KIKO females. However, striking differences were found between WT vs KIKO and WT vs ERKO. Among the genes differentially expressed between WT vs KIKO and WT vs ERKO, similar genes were found in oil-treated females and in E2B-treated females. Many of these genes overlapped with the previously discussed genes of the ARC estrogen-regulated transcriptome. All genes that were different between WT vs KIKO females were different in both oil- and E2B- treated females (total of 53 genes). Two of these genes were the enzymes Pck1 and Ptgs2, discussed earlier. In oil-treated females, Pck1 and Ptgs2 expression was lower in KIKO compared with WT and, in contrast, was higher in KIKO compared with WT in E2B-treated females.
Furthermore, many of the genes differentially regulated between WT and KIKO were also differentially expressed between WT and ERKO (31 genes). In the comparison of WT vs ERKO females, there was only one gene that was significantly different in WT vs ERKO oil-treated females, and not in WT vs KIKO females. This gene is Ptpn20, which to date has an unknown function. Of the remainder of the genes (105 genes) significantly different between WT vs ERKO females, 31 genes overlap with WT vs KIKO females. There are 74 genes differently expressed between oil- and E2B-treated WT and ERKO females, including the KNDy neuropeptides. In KNDy neurons, Pdyn and Tac2 are lower in oil-treated ERKO females than WT and higher in E2B-treated ERKO. Using quantitative real-time PCR, we found that the expression of Pdyn is higher in KIKO than in WT or ERKO females (12). Although not consistent, these data do illustrate that the KNDy genes are differentially expressed in females lacking a fully functional ERα, the consequence of which is dysregulation of negative feedback of E2 onto the HPG axis. Comparisons between the genotypes within steroid treatments provide an important framework to future studies identifying central signaling of the estrogen-regulated transcriptome.
Conclusions
Although numerous studies have examined role of E2 in controlling many hypothalamic homeostatic processes described in this study, the receptor-mediated mechanisms are largely unexplored. We show that ERα-mediated, ERE-independent mechanisms regulate expression of transcription factors that are involved in reproduction, primarily through the control of GnRH expression and the downstream HPG axis. These results enable the design of future studies exploring these transcription factors, especially GATA2, POU2F1, and SOX3 pathways. In addition to reproduction, many of the genes and pathways impacted by E2, through both ERE-dependent and -independent pathways, are involved in the control of energy expenditure and feeding behavior. Finally, regulation of mitochondrial oxidation genes in ERKO females, but not in WT and in KIKO females, suggests that ERα-independent pathways may compensate to maintain cellular energy production when ERα is lacking. These results suggest that ERα-independent pathways, such as ERβ, GPR30, or the putative Gq-mER, are important in regulating mitochondrial functions in the arcuate nucleus. These pathways may be important not only in distinct processes and pathways regulating reproduction and energy balance, but also the interaction of the two. It is critical to understand these interactions of reproduction and energy balance, as reproduction is negatively impacted by both overnutrition (obesity) and undernutrition (anorexia) (70–73). Our investigation of the ARC estrogen-regulated transcriptome is an important step in this understanding of the molecular and cellular pathways impacted by E2 and provides novel cellular targets for future studies.
Acknowledgments
We thank Dr. Tracy Anthony and Ashley George for guidance and input on RNA sequencing and bioinformatics analyses and interpretation.
Current affiliation: J.A. Yang’s current affiliation is the Department of Reproductive Medicine, University of California, San Diego, San Diego, CA 92103. H. Stires’s current affiliation is the Lombardi Comprehensive Cancer Center, Georgetown University, Washington, DC 20007.
Acknowledgments
This work was supported by National Institutes of Health Grants R00DK083457 and R00DK083457-S1 (to T.A.R.) and GM101378 and AA024330 (to W.J.B.). T.A.R. and W.J.B. are members of the National Institute of Environmental Health Sciences Center for Environmental Exposures and Disease (Grant ES005022). Additional support came from US Department of Agriculture, National Institute of Food and Agriculture Grants NJ0610 (to T.A.R.) and NE1439 (to W.J.B.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AgRP
- agouti-related peptide
- Alox5ap
- arachidonate 5-lipoxygenase activating protein
- ARC
- arcuate nucleus
- ATP
- adenosine triphosphate
- Bdnf
- brain-derived neurotrophic factor
- Cart
- cocaine- and amphetamine-regulated transcript
- Ccdc85b
- coiled-coil domain containing 85B
- CcO
- cytochrome c oxidase
- ChIP-seq
- chromatin immunoprecipitation sequencing
- Cox5b
- cytochrome c oxidase subunit 5B
- C1ql2
- complement component 1, q subcomponent-like 2
- E2
- 17β-estradiol
- E2B
- 17β-estradiol benzoate
- ER
- estrogen receptor
- ERE
- estrogen response element
- ERKO
- ERα knockout
- ESR1
- estrogen receptor 1
- ETC
- electron transport chain
- FC
- fold change
- FPKM
- fragments per kilobase of transcript per million mapped reads
- Gata2
- GATA-binding protein 2
- Ghsr
- growth hormone secretagogue receptor
- GPER
- G protein–coupled estrogen receptor
- GPR30
- G protein–coupled receptor 30
- Gq-MER
- Gq-coupled membrane estrogen receptor
- HPG
- hypothalamic/pituitary/gonadal
- HRE
- hormone response element
- IGV
- Integrative Genomics Viewer
- IPA
- Ingenuity Pathway Analysis
- KIKO
- knock-in/knockout
- Kiss1
- kisspeptin
- KNDy
- kisspeptin/neurokinin B/dynorphin
- Ndufa11
- NADH:ubiquinone oxidoreductase subunit A11
- Ndufb7
- NADH:ubiquinone oxidoreductase subunit B7
- Ndufb10
- NADH:ubiquinone oxireductase subunit B10
- NPY
- neuropeptide Y
- PCA
- principal component analysis
- Pcsk1
- proprotein convertase subtilisin/kexin type 1
- Pgr
- progesterone receptor
- POMC
- proopiomelanocortin
- Pou2f1
- POU domain, class 2, transcription factor 1
- Ptgds
- prostaglandin D2 synthase
- qPCR
- quantitative polymerase chain reaction
- RNA-seq
- RNA sequencing
- Sox3
- sex-determining region Y-box 3
- Tac2
- tachykinin 2
- Tac3r
- tachykinin 3 receptor
- Uqcr
- cytochrome c reductase core protein
- Uqcr11
- cytochrome c reductase, complex III subunit XI
- Vip
- vasoactive intestinal peptide
- WT
- wild-type
References
- 1.Lee YR, Park J, Yu HN, Kim JS, Youn HJ, Jung SH. Up-regulation of PI3K/Akt signaling by 17β-estradiol through activation of estrogen receptor-α, but not estrogen receptor-beta, and stimulates cell growth in breast cancer cells. Biochem Biophys Res Commun. 2005;336(4):1221–1226. [DOI] [PubMed] [Google Scholar]
- 2.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16(2–3):140–153. [DOI] [PubMed] [Google Scholar]
- 3.Dos Santos EG, Dieudonne MN, Pecquery R, Le Moal V, Giudicelli Y, Lacasa D. Rapid nongenomic E2 effects on p42/p44 MAPK, activator protein-1, and cAMP response element binding protein in rat white adipocytes. Endocrinology. 2002;143(3):930–940. [DOI] [PubMed] [Google Scholar]
- 4.Zhang C, Kelly MJ, Rønnekleiv OK. 17β-Estradiol rapidly increases ATP-sensitive potassium channel activity in gonadotropin-releasing hormone neurons [corrected] via a protein kinase signaling pathway. Endocrinology. 2010;151(9):4477–4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45(3):607–617. [DOI] [PubMed] [Google Scholar]
- 6.Prossnitz ER, Barton M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol. 2011;7(12):715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filardo EJ, Quinn JA, Frackelton AR Jr, Bland KI. Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Mol Endocrinol. 2002;16(1):70–84. [DOI] [PubMed] [Google Scholar]
- 8.Smith AW, Bosch MA, Wagner EJ, Rønnekleiv OK, Kelly MJ. The membrane estrogen receptor ligand STX rapidly enhances GABAergic signaling in NPY/AgRP neurons: role in mediating the anorexigenic effects of 17β-estradiol. Am J Physiol Endocrinol Metab. 2013;305(5):E632–E640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roepke TA, Qiu J, Bosch MA, Rønnekleiv OK, Kelly MJ. Cross-talk between membrane-initiated and nuclear-initiated oestrogen signalling in the hypothalamus. J Neuroendocrinol. 2009;21(4):263–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roepke TA, Bosch MA, Rick EA, Lee B, Wagner EJ, Seidlova-Wuttke D, Wuttke W, Scanlan TS, Rønnekleiv OK, Kelly MJ. Contribution of a membrane estrogen receptor to the estrogenic regulation of body temperature and energy homeostasis. Endocrinology. 2010;151(10):4926–4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hewitt SC, O’Brien JE, Jameson JL, Kissling GE, Korach KS. Selective disruption of ERα DNA-binding activity alters uterine responsiveness to estradiol. Mol Endocrinol. 2009;23(12):2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang JA, Mamounis KJ, Yasrebi A, Roepke TA. Regulation of gene expression by 17β-estradiol in the arcuate nucleus of the mouse through ERE-dependent and ERE-independent mechanisms. Steroids. 2016;107:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hewitt SC, Korach KS. Estrogenic activity of bisphenol A and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane (HPTE) demonstrated in mouse uterine gene profiles. Environ Health Perspect. 2011;119(1):63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mamounis KJ, Yang JA, Yasrebi A, Roepke TA. Estrogen response element-independent signaling partially restores post-ovariectomy body weight gain but is not sufficient for 17β-estradiol’s control of energy homeostasis. Steroids. 2014;81:88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pelletier G, Li S, Luu-The V, Labrie F. Oestrogenic regulation of pro-opiomelanocortin, neuropeptide Y and corticotrophin-releasing hormone mRNAs in mouse hypothalamus. J Neuroendocrinol. 2007;19(6):426–431. [DOI] [PubMed] [Google Scholar]
- 16.Kelly MJ, Rønnekleiv OK. Membrane-initiated actions of estradiol that regulate reproduction, energy balance and body temperature. Front Neuroendocrinol. 2012;33(4):376–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittelman-Smith MA, Williams H, Krajewski-Hall SJ, Lai J, Ciofi P, McMullen NT, Rance NE. Arcuate kisspeptin/neurokinin B/dynorphin (KNDy) neurons mediate the estrogen suppression of gonadotropin secretion and body weight. Endocrinology. 2012;153(6):2800–2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roepke TA, Xue C, Bosch MA, Scanlan TS, Kelly MJ, Rønnekleiv OK. Genes associated with membrane-initiated signaling of estrogen and energy homeostasis. Endocrinology. 2008;149(12):6113–6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roepke TA, Malyala A, Bosch MA, Kelly MJ, Rønnekleiv OK. Estrogen regulation of genes important for K+ channel signaling in the arcuate nucleus. Endocrinology. 2007;148(10):4937–4951. [DOI] [PubMed] [Google Scholar]
- 20.Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-estradiol. Mol Cell Endocrinol. 2013;367(1-2):85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park CJ, Zhao Z, Glidewell-Kenney C, Lazic M, Chambon P, Krust A, Weiss J, Clegg DJ, Dunaif A, Jameson JL, Levine JE. Genetic rescue of nonclassical ERα signaling normalizes energy balance in obese Erα-null mutant mice. J Clin Invest. 2011;121(2):604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed. San Diego, CA: Academic Press; 2008. [Google Scholar]
- 23.Bosch MA, Hou J, Fang Y, Kelly MJ, Rønnekleiv OK. 17β-Estradiol regulation of the mRNA expression of T-type calcium channel subunits: role of estrogen receptor alpha and estrogen receptor β. J Comp Neurol. 2009;512(3):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, Pimentel H, Salzberg SL, Rinn JL, Pachter L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7(3):562–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Pertea G, Trapnell C, Pimentel H, Kelley R, Salzberg SL. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14(4):R36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goff L, Trapnell C, Kelley D. cummeRbund: analysis, exploration, manipulation, and visualization of Cufflinks high-throughput sequencing data. R Package version 2.14.0; 2013.
- 27.Robinson JT, Thorvaldsdóttir H, Winckler W, Guttman M, Lander ES, Getz G, Mesirov JP. Integrative genomics viewer. Nat Biotechnol. 2011;29(1):24–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collazos JCO. Venny 2.1.0. http://bioinfogp.cnb.csic.es/tools/venny/. Accessed June 1, 2016.
- 30.Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, Li Y, Bushel PR, Fargo D, Korach KS. Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq. Mol Endocrinol. 2012;26(5):887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, DeMayo FJ, Schütz G, Korach KS. Novel DNA motif binding activity observed in vivo with an estrogen receptor α mutant mouse. Mol Endocrinol. 2014;28(6):899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 2009;10(3):R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, Myers RM, Brown M, Li W, Liu XS. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 2008;9(9):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu G, Wang LG, He QY. ChIPseeker: an R/Bioconductor package for ChIP peak annotation, comparison and visualization. Bioinformatics. 2015;31(14):2382–2383. [DOI] [PubMed] [Google Scholar]
- 35.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23(29):9529–9540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao Y, He JR, Kapcala LP. Estrogen inhibits hypothalamic pro-opiomelanocortin gene expression in hypothalamic neuronal cultures. Brain Res Mol Brain Res. 1997;45(2):340–344. [DOI] [PubMed] [Google Scholar]
- 38.Treiser SL, Wardlaw SL. Estradiol regulation of proopiomelanocortin gene expression and peptide content in the hypothalamus. Neuroendocrinology. 1992;55(2):167–173. [DOI] [PubMed] [Google Scholar]
- 39.Brock O, Bakker J. The two kisspeptin neuronal populations are differentially organized and activated by estradiol in mice. Endocrinology. 2013;154(8):2739–2749. [DOI] [PubMed] [Google Scholar]
- 40.Bosch MA, Xue C, Rønnekleiv OK. Kisspeptin expression in guinea pig hypothalamus: effects of 17β-estradiol. J Comp Neurol. 2012;520(10):2143–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2011;300(1):E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eghlidi DH, Urbanski HF. Effects of age and estradiol on gene expression in the rhesus macaque hypothalamus. Neuroendocrinology. 2015;101(3):236–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roepke TA, Qiu J, Smith AW, Rønnekleiv OK, Kelly MJ. Fasting and 17β-estradiol differentially modulate the M-current in neuropeptide Y neurons. J Neurosci. 2011;31(33):11825–11835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yasrebi A, Hsieh A, Mamounis KJ, Krumm EA, Yang JA, Magby J, Hu P, Roepke TA. Differential gene regulation of GHSR signaling pathway in the arcuate nucleus and NPY neurons by fasting, diet-induced obesity, and 17β-estradiol. Mol Cell Endocrinol. 2016;422:42–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gill JC, Navarro VM, Kwong C, Noel SD, Martin C, Xu S, Clifton DK, Carroll RS, Steiner RA, Kaiser UB. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012;153(10):4883–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang JA, Yasrebi A, Snyder M, Roepke TA. The interaction of fasting, caloric restriction, and diet-induced obesity with 17β-estradiol on the expression of KNDy neuropeptides and their receptors in the female mouse. Mol Cell Endocrinol. 2016;437:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eghlidi DH, Haley GE, Noriega NC, Kohama SG, Urbanski HF. Influence of age and 17β-estradiol on kisspeptin, neurokinin B, and prodynorphin gene expression in the arcuate-median eminence of female rhesus macaques. Endocrinology. 2010;151(8):3783–3794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.LaVoie HA. The role of GATA in mammalian reproduction. Exp Biol Med (Maywood). 2003;228(11):1282–1290. [DOI] [PubMed] [Google Scholar]
- 50.Steger DJ, Hecht JH, Mellon PL. GATA-binding proteins regulate the human gonadotropin α-subunit gene in the placenta and pituitary gland. Mol Cell Biol. 1994;14(8):5592–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lo A, Zheng W, Gong Y, Crochet JR, Halvorson LM. GATA transcription factors regulate LHβ gene expression. J Mol Endocrinol. 2011;47(1):45–58. [DOI] [PubMed] [Google Scholar]
- 52.Eraly SA, Nelson SB, Huang KM, Mellon PL. Oct-1 binds promoter elements required for transcription of the GnRH gene. Mol Endocrinol. 1998;12(4):469–481. [DOI] [PubMed] [Google Scholar]
- 53.Clark ME, Mellon PL. The POU homeodomain transcription factor Oct-1 is essential for activity of the gonadotropin-releasing hormone neuron-specific enhancer. Mol Cell Biol. 1995;15(11):6169–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chandrasekharan S, Kandasamy KK, Dayalan P, Ramamurthy V. Estrogen induced concentration dependent differential gene expression in human breast cancer (MCF7) cells: role of transcription factors. Biochem Biophys Res Commun. 2013;437(3):475–481. [DOI] [PubMed] [Google Scholar]
- 55.Rizzoti K, Brunelli S, Carmignac D, Thomas PQ, Robinson IC, Lovell-Badge R. SOX3 is required during the formation of the hypothalamo-pituitary axis. Nat Genet. 2004;36(3):247–255. [DOI] [PubMed] [Google Scholar]
- 56.Alatzoglou KS, Kelberman D, Dattani MT. The role of SOX proteins in normal pituitary development. J Endocrinol. 2009;200(3):245–258. [DOI] [PubMed] [Google Scholar]
- 57.Rogers N, Cheah PS, Szarek E, Banerjee K, Schwartz J, Thomas P. Expression of the murine transcription factor SOX3 during embryonic and adult neurogenesis. Gene Expr Patterns. 2013;13(7):240–248. [DOI] [PubMed] [Google Scholar]
- 58.Kim HD, Choe HK, Chung S, Kim M, Seong JY, Son GH, Kim K. Class-C SOX transcription factors control GnRH gene expression via the intronic transcriptional enhancer. Mol Endocrinol. 2011;25(7):1184–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Benjannet S, Rondeau N, Day R, Chrétien M, Seidah NG. PC1 and PC2 are proprotein convertases capable of cleaving proopiomelanocortin at distinct pairs of basic residues. Proc Natl Acad Sci USA. 1991;88(9):3564–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ohinata K, Takagi K, Biyajima K, Fujiwara Y, Fukumoto S, Eguchi N, Urade Y, Asakawa A, Fujimiya M, Inui A, Yoshikawa M. Central prostaglandin D2 stimulates food intake via the neuropeptide Y system in mice. FEBS Lett. 2008;582(5):679–684. [DOI] [PubMed] [Google Scholar]
- 61.Xu B, Goulding EH, Zang K, Cepoi D, Cone RD, Jones KR, Tecott LH, Reichardt LF. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat Neurosci. 2003;6(7):736–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhu Z, Liu X, Senthil Kumar SP, Zhang J, Shi H. Central expression and anorectic effect of brain-derived neurotrophic factor are regulated by circulating estradiol levels. Horm Behav. 2013;63(3):533–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liao GY, Bouyer K, Kamitakahara A, Sahibzada N, Wang CH, Rutlin M, Simerly RB, Xu B. Brain-derived neurotrophic factor is required for axonal growth of selective groups of neurons in the arcuate nucleus. Mol Metab. 2015;4(6):471–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weiss H, Friedrich T, Hofhaus G, Preis D. The respiratory-chain NADH dehydrogenase (complex I) of mitochondria. Eur J Biochem. 1991;197(3):563–576. [DOI] [PubMed] [Google Scholar]
- 65.Galati D, Srinivasan S, Raza H, Prabu SK, Hardy M, Chandran K, Lopez M, Kalyanaraman B, Avadhani NG. Role of nuclear-encoded subunit Vb in the assembly and stability of cytochrome c oxidase complex: implications in mitochondrial dysfunction and ROS production. Biochem J. 2009;420(3):439–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayr JA, Havlícková V, Zimmermann F, Magler I, Kaplanová V, Jesina P, Pecinová A, Nusková H, Koch J, Sperl W, Houstek J. Mitochondrial ATP synthase deficiency due to a mutation in the ATP5E gene for the F1 ε subunit. Hum Mol Genet. 2010;19(17):3430–3439. [DOI] [PubMed] [Google Scholar]
- 67.Irwin RW, Yao J, Hamilton RT, Cadenas E, Brinton RD, Nilsen J. Progesterone and estrogen regulate oxidative metabolism in brain mitochondria. Endocrinology. 2008;149(6):3167–3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-α and -β mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–525. [DOI] [PubMed] [Google Scholar]
- 69.Mizukami Y. In vivo functions of GPR30/GPER-1, a membrane receptor for estrogen: from discovery to functions in vivo. Endocr J. 2010;57(2):101–107. [DOI] [PubMed] [Google Scholar]
- 70.Helge EW. [High prevalence of eating disorders among elite athletes. Increased risk of amenorrhea and premenopausal osteoporosis]. Ugeskr Laeger. 2001;163(25):3473–3475. [PubMed] [Google Scholar]
- 71.Jacobs HS. Amenorrhea in athletes. Br J Obstet Gynaecol. 1982;89(7):498–500. [DOI] [PubMed] [Google Scholar]
- 72.Moran LJ, Dodd J, Nisenblat V, Norman RJ. Obesity and reproductive dysfunction in women. Endocrinol Metab Clin North Am. 2011;40(4):895–906. [DOI] [PubMed] [Google Scholar]
- 73.Bray GA. Obesity and reproduction. Hum Reprod. 1997;12(Suppl 1):26–32. [DOI] [PubMed] [Google Scholar]