Abstract
Mutations in the dentin matrix protein 1 (DMP1) gene cause autosomal recessive hypophosphatemic rickets (ARHR). Hypophosphatemia in ARHR results from increased circulating levels of the phosphaturic hormone, fibroblast growth factor 23 (FGF23). Similarly, elevated FGF23, caused by mutations in the PHEX gene, is responsible for the hypophosphatemia in X-linked hypophosphatemic rickets (XLH). Previously, we demonstrated that a Phex mutation in mice creates a lower set point for extracellular phosphate, where an increment in phosphorus further stimulates Fgf23 production to maintain low serum phosphorus levels. To test the presence of the similar set point defect in ARHR, we generated 4- and 12-week-old Dmp1/Galnt3 double knockout mice and controls, including Dmp1 knockout mice (a murine model of ARHR), Galnt3 knockout mice (a murine model of familial tumoral calcinosis), and phenotypically normal double heterozygous mice. Galnt3 knockout mice had increased proteolytic cleavage of Fgf23, leading to low circulating intact Fgf23 levels with consequent hyperphosphatemia. In contrast, Dmp1 knockout mice had little Fgf23 cleavage and increased femoral Fgf23 expression, resulting in hypophosphatemia and low femoral bone mineral density (BMD). However, introduction of the Galnt3 null allele to Dmp1 knockout mice resulted in a significant increase in serum phosphorus and normalization of BMD. This increased serum phosphorus was accompanied by markedly elevated Fgf23 expression and circulating Fgf23 levels, an attempt to reduce serum phosphorus in the face of improving phosphorus levels. These data indicate that a Dmp1 mutation creates a lower set point for extracellular phosphate and maintains it through the regulation of Fgf23 cleavage and expression.
Autosomal recessive hypophosphatemic rickets (ARHR) is a genetic disorder of phosphate homeostasis and bone mineralization caused by loss-of-function mutations in the dentin matrix protein 1 (DMP1) gene. Lack of DMP1 proteins in both humans and mice increases expression of the fibroblast growth factor 23 (FGF23) gene in osteoblasts and osteocytes (1). FGF23 is a phosphaturic hormone that inhibits renal phosphate reabsorption and 1,25-dihydroxyvitamin D synthesis (2). Thus, the increment in circulating FGF23 levels causes hypophosphatemia and inappropriately low or normal 1,25-dihydroxyvitamin D, resulting in rickets and osteomalacia.
Similar to ARHR, elevated FGF23 is the primary cause of the most common genetic disorder of phosphate homeostasis, X-linked hypophosphatemic rickets (XLH) (3–5). The disease is caused by inactivating mutations in the PHEX gene (6–9), which encodes an endopeptidase homolog (9, 10). Several lines of evidence suggest that patients with XLH and mouse models of XLH have abnormal phosphate sensing. First, the current standard therapy for XLH—high-dose oral phosphate and vitamin D supplementation (11)—raises serum FGF23 concentrations in these patients (12, 13). Second, long-term treatment of a murine model, Hyp mice, with pan-specific fibroblast growth factor receptor 1 inhibitor raises Fgf23 levels (14). Third, in another mouse model of XLH, PhexK496X mutant, increased serum phosphorus by genetic manipulation significantly stimulated Fgf23 production in the bone (15). Lastly, hemizygous male PhexK496X mice, as well as heterozygous and homozygous female mice, all had similarly elevated Fgf23 levels to maintain the low phosphorus levels (16). These observations suggest that FGF23-producing cells (mainly osteoblasts and osteocytes) in XLH sense the phosphate level to be too high when it nears normal because they have a lower set point for phosphate. This abnormal response to extracellular phosphate may underlie the increased FGF23 production in XLH.
Serum FGF23 levels are increased in both ARHR and XLH, and patients with these disorders have similar biochemical and skeletal features. However, it is unknown whether DMP1 mutations also create a lower set point for extracellular phosphate, as observed in XLH. In this study, we tested the presence of the altered response to extracellular phosphate in a murine model of ARHR, Dmp1 knockout mice.
Materials and Methods
Generation of experimental animals
Two mouse models used in this study have biochemical mirror images of human disorders of phosphate homeostasis. A murine model of ARHR, Dmp1 knockout mice, has a deletion in exon 6 of the Dmp1 gene, and its phenotype is described elsewhere (1, 17). Creation and initial characterization of Galnt3 knockout mice, a murine model of hyperphosphatemic familial tumoral calcinosis, was described previously (18). In brief, the Galnt3 gene encodes a Golgi-associated glycosyltransferase enzyme, UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3 (ppGalNAc-T3). O-glycosylation protects FGF23 from proteolytic cleavage and allows intact FGF23 to be secreted (19, 20). Thus, the absence of ppGalNAc-T3 reduces the secretion of intact Fgf23 proteins and causes hyperphosphatemia in Galnt3 knockout mice. To eliminate the effect of strain background, both Dmp1 and Galnt3 mutant mice were backcrossed to C57/BL6J at least 10 generations. Dmp1/Galnt3 double knockout mice and single knockout mice were generated by crossing the 2 congenic lines. Animals were fed Teklad Global 18% Protein Extruded Rodent Diet containing 1.01% calcium, 0.65% phosphorus, and 2.05 IU/g vitamin D3 (2018SX; Harlan, Indianapolis, IN) and tap water ad libitum. All animal studies were approved by the Indiana University School of Medicine Institutional Animal Care and Use Committee.
Serum biochemistry measurements
Mice were anesthetized with a ketamine/xylazine mix to draw blood by cardiac puncture, and serum was stored at −80°C until analysis. Serum alkaline phosphatase, blood urea nitrogen (BUN), calcium, creatinine, and phosphorus were measured using an RX Daytona clinical chemistry analyzer (Randox Laboratories-US, Ltd., Kearneysville, WV). Intact Fgf23 levels were measured using an FGF-23 enzyme-linked immunosorbent assay kit, which detects only intact Fgf23 (Kainos Laboratories, Inc., Tokyo, Japan) (5). Total Fgf23 levels were measured using the Mouse FGF-23 (C-Terminal) ELISA Kit, which detects both intact and C-terminal fragments of Fgf23 (Immutopics International, San Clemente, CA).
Fgf23 messenger RNA quantification
After the blood draw, femurs were collected and stored in RNAlater RNA Stabilization Reagent (QIAGEN Inc., Valencia, CA). Total RNA was extracted from the whole femurs using TRIzol Plus RNA Purification System (Invitrogen, Carlsbad, CA) and was used for first-strand complementary DNA (cDNA) synthesis using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA). The cDNA was subsequently used for quantification of Fgf23 expression by probe-based quantitative polymerase chain reaction using TaqMan® Gene Expression Master Mix in the 7900HT Fast Real-Time PCR System (Applied Biosystems), as described elsewhere (15). The TATA-box binding protein (Tbp) gene was used to normalize gene expression. Relative gene expression was determined by analyzing the data using the relative standard curve method.
Dual-energy x-ray absorptiometry
Femurs were harvested from mice and fixed in 10% neutral-buffered formalin for 2 days. Length of femurs was measured using a digital caliper. Areal bone mineral density (BMD) was determined using a PIXImus2 densitometer (LUNAR Corp, Madison, WI).
Statistical analysis
Means and standard errors of the mean (SEMs) were calculated for all outcome measures by genotype and sex. Analysis of variance was used to test for overall differences among 4 genotypes within the same sex. Because some measures were known to be different between sexes, the between-genotype comparisons were made separately for each sex. When the analysis of variance P values were significant, differences between 2 groups were tested for significance using the unpaired Student t test. P values <0.05 were considered significant for all analyses.
Results
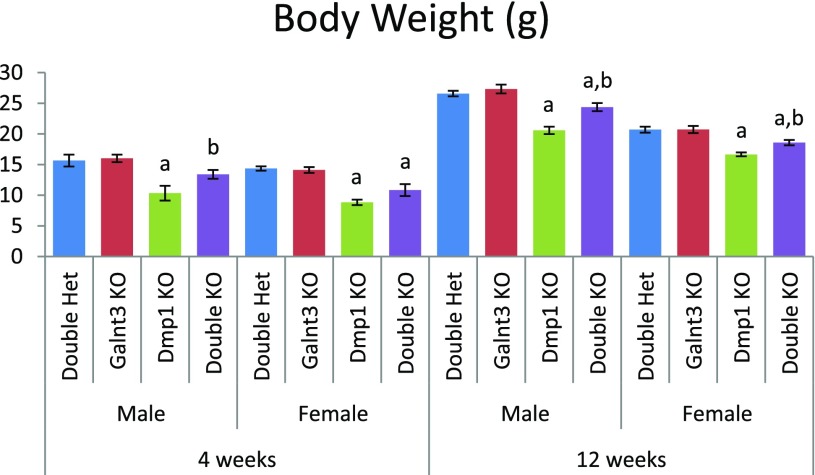
Dmp1 knockout mice were crossed to Galnt3 knockout mice to generate Dmp1/Galnt3 double knockout mice (Dmp1−/−;Galnt3−/−) and control mice. The presence of homozygous Dmp1 and Galnt3 mutations had no effect on survival up to 12 weeks. We compared phenotypes of 4- and 12-week-old double knockout mice with those of controls, including Dmp1 knockout mice (Dmp1−/−;Galnt3+/−), Galnt3 knockout mice (Dmp1+/−;Galnt3−/−), and phenotypically normal double heterozygous mice (Dmp1+/−;Galnt3+/−). Compared with double heterozygotes, male and female Dmp1 knockout mice had 34% and 39% lower body weights at 4 weeks, respectively (Fig. 1). However, body weight reductions in Dmp1 knockout mice were milder at 12 weeks—23% in males and 20% in females. The introduction of Galnt3 null alleles to Dmp1 knockout mice significantly improved their body weights. Although body weights of Dmp1/Galnt3 double knockout mice were not completely normalized, the deficit was reduced to within 10% of double heterozygotes at 12 weeks.
Figure 1.
Body weights of Galnt3/Dmp1 double knockout mice and controls. Data are presented as mean ± SEM. Number of animals = 9 to 12 per group. P value < 0.05: a, compared with same-sex double heterozygous mice; b, compared with same-sex Dmp1 knockout mice (Dmp1 knockout vs double knockout mice only). Het, heterozygote; KO, knockout.
Serum biochemistries
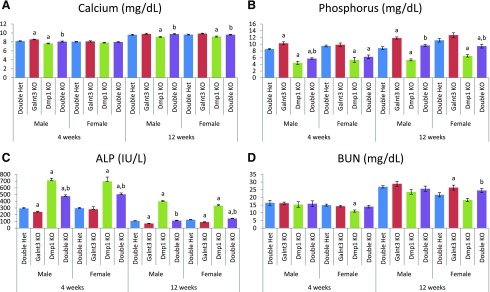
Dmp1 knockout mice, except 4-week-old females, exhibited mild hypocalcemia, but the presence of Galnt3 null alleles completely normalized serum calcium in these mice [Fig. 2(A)].
Figure 2.
Biochemical analysis of mouse serums. (A) Calcium. (B) Phosphorus. (C) Alkaline phosphatase (ALP). ALP levels in 7 mice were higher than the upper detection limit (800 IU/L) of the assay; as a result, 800 IU/L was used in the calculation for these mice. (D) BUN. Data are presented as mean ± SEM. Number of animals = 8 to 12 per group (except BUN = 4 to 12). P value < 0.05: a, compared with same-sex double heterozygous mice; b, compared with same-sex Dmp1 knockout mice (Dmp1 knockout vs double knockout mice only). Het, heterozygote; KO, knockout.
Serum phosphorus was elevated in male Galnt3 knockout mice: +1.7 mg/dL at 4 weeks and +3.0 mg/dL at 12 weeks [Fig. 2(B)]. In contrast, Dmp1 knockout mice had severe hypophosphatemia (3.5 to 4.6 mg/dL lower than that of double heterozygotes) in both sexes and ages. At 4 weeks, the serum phosphorus level in male double knockout mice was slightly higher than the level in Dmp1 knockout mice, but double knockout mice were still severely hypophosphatemic. At 12 weeks, double knockout mice had significant improvement in serum phosphorus, and it was completely normalized in male double knockout mice.
Serum alkaline phosphatase was decreased in Galnt3 knockout mice, except in females at 4 weeks, compared with levels in double heterozygotes [Fig. 2(C)]. Dmp1 knockout mice had notable elevations in alkaline phosphatase, at 2.4- to 3.7-fold. The introduction of the Galnt3 null alleles to Dmp1 knockout mice resulted in significant improvement in alkaline phosphatase levels. Double knockout mice had significantly lower alkaline phosphatase levels than Dmp1 knockout mice with normal Galnt3 alleles. Strikingly, serum alkaline phosphatase level returned to normal in male double knockout mice at 12 weeks; however, it was still 60% to 70% higher than the level in double heterozygotes at 4 weeks.
Although there was some variation, serum BUN, a marker of renal function, was generally comparable among the 4 same-sex genotype groups at the same age [Fig. 2(D)]. Serum creatinine was also variable and did not show consistent genotype-specific patterns (data not shown).
Serum Fgf23 concentrations and femoral Fgf23 expression
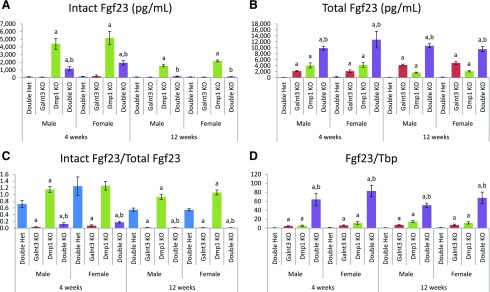
To determine the presence of an altered set point for extracellular phosphate, we measured serum Fgf23 concentrations and femoral Fgf23 messenger RNA (mRNA) expression in these mice. Consistent with their hyperphosphatemia, Galnt3 knockout mice (except 4-week-old females) had, on average, a 33% reduction in intact Fgf23 levels; however, the reduction was not statistically significant [Fig. 3(A)]. Hypophosphatemic Dmp1 knockout mice, on the other hand, had 40- and 35-fold increases in intact Fgf23 levels in 4-week-old males and females, respectively. The increases in 12-week-old mice were smaller, but male and female Dmp1 knockout mice still had 18- and 21-fold higher intact Fgf23 levels than respective double heterozygotes. In Dmp1/Galnt3 knockout mice, the presence of Galnt3 null alleles significantly improved intact Fgf23 levels at 4 weeks and completely normalized them at 12 weeks.
Figure 3.
Femoral Fgf23 mRNA expression and circulating Fgf23 concentrations. (A) Intact Fgf23 levels. (B) Total Fgf23 levels. Total Fgf23 concentrations include intact Fgf23 protein and C-terminal Fgf23 fragments. (C) Intact/total Fgf23 ratio. (D) Femoral Fgf23 expression. Values of Fgf23 mRNA expression are presented by arbitrarily setting wild-type males as 1.0. Data are presented as mean ± SEM. Number of animals = 9 to 12 per group. P value < 0.05: a, compared with same-sex double heterozygous mice; b, compared with same-sex Dmp1 knockout mice (Dmp1 knockout vs double knockout mice only). Het, heterozygote; KO, knockout; Tbp, TATA-box binding protein.
Total Fgf23 levels were determined by the serum assay that measures both biologically active intact Fgf23 protein and inactive C-terminal Fgf23 fragments. In an attempt to lower serum phosphorus and/or maintain normal phosphorus, Galnt3 knockout mice increased total Fgf23 production—14- and 8-fold in 4-week-old males and females and 26- and 25-fold in 12-week-old males and females [Fig. 3(B)]. Dmp1 knockout mice also had higher total Fgf23 levels, which is consistent with their high intact Fgf23 levels and consequent hypophosphatemia. Interestingly, the increase in total Fgf23 level was higher at 4 weeks compared with 12 weeks—26- and 16-fold in males and females at 4 weeks and 11-fold in both males and females at 12 weeks. Double knockout mice had the highest total Fgf23 levels—62- and 48-fold in males and females at 4 weeks and 67- and 48-fold at 12 weeks.
The loss of the Dmp1 function also seems to affect posttranslational processing of Fgf23 protein, as assessed by the proportion of intact Fgf23 in circulation (i.e., the ratio of intact to total Fgf23). In 12-week-old double heterozygous mice, approximately 55% of Fgf23 existed as intact protein [Fig. 3(C)]. Because of the lack of O-glycosylation to protect from proteolytic cleavage, only 2% to 7% of intact Fgf23 was secreted in Galnt3 knockout mice. Interestingly, there was little to no proteolytic cleavage in Dmp1 knockout mice. Of note, although double knockout mice had the same high level of cleavage as Galnt3 knockout mice at 12 weeks, the younger double knockout mice had more intact proteins—12% and 17% in males and females, respectively.
Fgf23 mRNA expression in the femur corroborated the increased total Fgf23 concentrations in the circulation. In all 3 mutants, femoral Fgf23 expression was significantly increased; however, double mutant mice had particularly high expression [Fig. 3(D)]. Dmp1 and Galnt3 knockout mice had a 5- to 14-fold increase in Fgf23 expression, whereas double knockout mice had a 51- to 83-fold higher expression, compared with sex-matched double heterozygotes.
Skeletal analysis
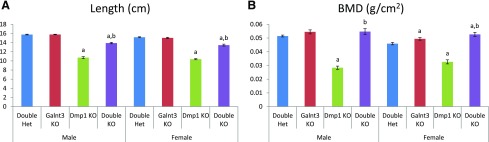
The femurs from 12-week-old mice were also used for skeletal analysis. Compared with double heterozygous mice, Dmp1 knockout mice had shorter femurs—32% reduction in both males and females [Fig. 4(A)]. However, the presence of the Galnt3 null alleles significantly improved femur length in double knockout mice. Femur lengths of double knockout mice were only 11% to 12% shorter than those of double heterozygotes. Similarly, femurs of Dmp1 knockout mice had a lower BMD (45% reduction in males and 29% in females), whereas BMD was essentially normalized in double knockout mice [Fig. 4(B)]. BMD in female double knockout mice was even higher than that of double heterozygous controls.
Figure 4.
Analysis of femurs from 12-week-old mice. (A) Femur length. (B) Areal BMD. Data are presented as mean ± SEM. Number of animals = 8 to 12 per group. P value <0.05: a, compared with same-sex double heterozygous mice; b, compared with same-sex Dmp1 knockout mice (Dmp1 knockout vs double knockout mice only). Het, heterozygote; KO, knockout.
Discussion
Both ARHR and XLH result in elevated FGF23 levels, leading to hypophosphatemia and inappropriately normal or low 1,25-dihydroxyvitamin D level. Our previous study demonstrated that a murine model of XLH, Phex mutant mice, had a lower set point for extracellular phosphate (15). However, it was unknown whether a similar phosphate-sensing abnormality exists in ARHR. To test this, we introduced the Galnt3 null alleles to Dmp1 knockout mice to genetically improve serum phosphorus. At 4 weeks, Dmp1 knockout mice had severe hypophosphatemia with elevated intact Fgf23 levels; however, in the presence of Galnt3 null alleles, double knockout mice had lower intact Fgf23 levels, leading to improved (but still low) serum phosphorus levels. Importantly, despite the persistent hypophosphatemia, double knockout mice had significantly higher Fgf23 mRNA expression and circulating total Fgf23. These data suggest that, similar to bone cells in Phex mutant mice, bone cells in Dmp1 knockout mice have a lower set point for phosphorus and increase Fgf23 production as the serum phosphorus level nears normal.
Although femur BMD was not measured at 4 weeks of age, it is likely that both Dmp1 knockout and Dmp1/Galnt3 double knockout mice had lower BMD, as observed in 5-week-old Dmp1 knockout mice in previous studies (21, 22). Interestingly, the femur BMD was completely normal in the older double knockout mice, whereas it was significantly low in Dmp1 knockout mice. Similarly, both serum phosphorus and Fgf23 levels remained low in 12-week-old Dmp1 knockout mice but were almost completely normalized in Dmp1/Galnt3 knockout mice. These data could be interpreted as correction of the set point defect in the bone or even lack of it at the older age. However, despite normal phosphorus levels, the older double knockout mice were markedly upregulating intact Fgf23 production by increasing gene expression and reducing proteolytic cleavage. Thus, Dmp1 knockout mice likely retain the lower set point for phosphorus at the older age.
An overall biochemical phenotype of Dmp1 knockout mice was similar to that of Phex mutant mice in our previous study (15). The increased intact Fgf23 levels in the 2 mouse models were a result of upregulation of Fgf23 expression, as well as reduced proteolytic cleavage of Fgf23 protein. These observations are consistent with the presence of similar biochemical and skeletal phenotypes in Phex and Dmp1 knockout mice and a lack of additive effects in Dmp1/Phex double mutant mice in another study (22). These data suggest that both Phex and Dmp1 most likely act in the same pathway and that loss of either gene leads to increased intact Fgf23 by the same mechanism. However, we observed a few critical differences between the 2 models. Most notably, the 2 had different responses to the presence of Galnt3 null alleles at the older age. Dmp1/Galnt3 double knockout mice had severe hypophosphatemia at 4 weeks, but it was mostly normalized at 12 weeks. Similarly, Phex/Galnt3 double mutant mice were hypophosphatemic at the younger age; phosphate levels were closer to normal but still remained low at 12 weeks (15). Intact Fgf23 levels were also significantly higher in the younger Dmp1 and double knockout mice, whereas their Phex counterparts had largely similar Fgf23 levels at 4 and 12 weeks. Thus, it appears that Dmp1 function is more important in the younger mice. Because the higher intact Fgf23 level occurred with higher intact-to-total Fgf23 ratio in the younger mice, glycosylation of Fgf23 protein by ppGalNAc-T3 may play a role in this difference between Phex and Dmp1 mutant mice.
In summary, Dmp1 knockout mice had markedly elevated Fgf23 levels with consequent hypophosphatemia due to increased expression of the Fgf23 gene and decreased proteolytic processing of intact Fgf23 proteins. Lack of O-glycosylation by Galnt3 significantly improved serum phosphorus and femoral BMD levels, particularly in the older Dmp1/Galnt3 double knockout mice. However, the increase in serum phosphorus level resulted in a further increase in Fgf23 production in the double knockouts. Therefore, as in Phex mutant mice, a mutation in the Dmp1 gene likely creates a lower set point for extracellular phosphate.
Acknowledgments
Support for this study was provided by National Institutes of Health Grant R01 AR042228 (to M.J.E.) and R01 DE025014 (to J.Q.F.) and Indiana University School of Medicine Life-Health Sciences Internship (to I.E.S. and P.C.W.).
Disclosure Summary: M.J.E. receives royalties from and has been a consultant for Kyowa Hakko Kirin Co., Ltd. The remaining authors have nothing to disclose.
Footnotes
- ARHR
- autosomal recessive hypophosphatemic rickets
- BMD
- bone mineral density
- BUN
- blood urea nitrogen
- cDNA
- complementary DNA
- DMP1
- dentin matrix protein 1
- FGF23
- fibroblast growth factor 23
- mRNA
- messenger RNA
- ppGalNAc-T3
- UDP-N-acetyl-alpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 3
- SEM
- standard error of the mean
- XLH
- X-linked hypophosphatemic rickets
References
- 1.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38(11):1310–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. [DOI] [PubMed] [Google Scholar]
- 3.Endo I, Fukumoto S, Ozono K, Namba N, Tanaka H, Inoue D, Minagawa M, Sugimoto T, Yamauchi M, Michigami T, Matsumoto T. Clinical usefulness of measurement of fibroblast growth factor 23 (FGF23) in hypophosphatemic patients: proposal of diagnostic criteria using FGF23 measurement. Bone. 2008;42(6):1235–1239. [DOI] [PubMed] [Google Scholar]
- 4.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348(17):1656–1663. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, Takeuchi Y, Fujita T, Nakahara K, Yamashita T, Fukumoto S. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87(11):4957–4960. [DOI] [PubMed] [Google Scholar]
- 6.Francis F, Strom TM, Hennig S, Böddrich A, Lorenz B, Brandau O, Mohnike KL, Cagnoli M, Steffens C, Klages S, Borzym K, Pohl T, Oudet C, Econs MJ, Rowe PS, Reinhardt R, Meitinger T, Lehrach H. Genomic organization of the human PEX gene mutated in X-linked dominant hypophosphatemic rickets. Genome Res. 1997;7(6):573–585. [DOI] [PubMed] [Google Scholar]
- 7.Holm IA, Huang X, Kunkel LM. Mutational analysis of the PEX gene in patients with X-linked hypophosphatemic rickets. Am J Hum Genet. 1997;60(4):790–797. [PMC free article] [PubMed] [Google Scholar]
- 8.Rowe PS, Oudet CL, Francis F, Sinding C, Pannetier S, Econs MJ, Strom TM, Meitinger T, Garabedian M, David A, Macher MA, Questiaux E, Popowska E, Pronicka E, Read AP, Mokrzycki A, Glorieux FH, Drezner MK, Hanauer A, Lehrach H, Goulding JN, O’Riordan JL. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP). Hum Mol Genet. 1997;6(4):539–549. [DOI] [PubMed] [Google Scholar]
- 9.Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, Lehrach H, Rowe PSN, Goulding JN, Summerfield T, Mountford R, Read AP, Popowska E, Pronicka E, Davies KE, O’Riordan JLH, Econs MJ, Nesbitt T, Drezner MK, Oudet C, Pannetier S, Hanauer A, Strom TM, Meindl A, Lorenz B, Cagnoli B, Mohnike KL, Murken J, Meitinger T. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet . 1995;11(2):130–136. [Google Scholar]
- 10.Du L, Desbarats M, Viel J, Glorieux FH, Cawthorn C, Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36(1):22–28. [DOI] [PubMed] [Google Scholar]
- 11.Harrell RM, Lyles KW, Harrelson JM, Friedman NE, Drezner MK. Healing of bone disease in X-linked hypophosphatemic rickets/osteomalacia: induction and maintenance with phosphorus and calcitriol. J Clin Invest. 1985;75(6):1858–1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alon US, Levy-Olomucki R, Moore WV, Stubbs J, Liu S, Quarles LD. Calcimimetics as an adjuvant treatment for familial hypophosphatemic rickets. Clin J Am Soc Nephrol. 2008;3(3):658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imel EA, DiMeglio LA, Hui SL, Carpenter TO, Econs MJ. Treatment of X-linked hypophosphatemia with calcitriol and phosphate increases circulating fibroblast growth factor 23 concentrations. J Clin Endocrinol Metab. 2010;95(4):1846–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wöhrle S, Henninger C, Bonny O, Thuery A, Beluch N, Hynes NE, Guagnano V, Sellers WR, Hofmann F, Kneissel M, Graus Porta D. Pharmacological inhibition of fibroblast growth factor (FGF) receptor signaling ameliorates FGF23-mediated hypophosphatemic rickets. J Bone Miner Res . 2013;28(4):899–911. [DOI] [PubMed] [Google Scholar]
- 15.Ichikawa S, Austin AM, Gray AK, Econs MJ. A Phex mutation in a murine model of X-linked hypophosphatemia alters phosphate responsiveness of bone cells. J Bone Miner Res. 2012;27(2):453–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichikawa S, Gray AK, Bikorimana E, Econs MJ. Dosage effect of a Phex mutation in a murine model of X-linked hypophosphatemia. Calcif Tissue Int. 2013;93(2):155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng JQ, Huang H, Lu Y, Ye L, Xie Y, Tsutsui TW, Kunieda T, Castranio T, Scott G, Bonewald LB, Mishina Y. The Dentin matrix protein 1 (Dmp1) is specifically expressed in mineralized, but not soft, tissues during development. J Dent Res. 2003;82(10):776–780. [DOI] [PubMed] [Google Scholar]
- 18.Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ. Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology. 2009;150(6):2543–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frishberg Y, Ito N, Rinat C, Yamazaki Y, Feinstein S, Urakawa I, Navon-Elkan P, Becker-Cohen R, Yamashita T, Araya K, Igarashi T, Fujita T, Fukumoto S. Hyperostosis-hyperphosphatemia syndrome: a congenital disorder of O-glycosylation associated with augmented processing of fibroblast growth factor 23. J Bone Miner Res. 2007;22(2):235–242. [DOI] [PubMed] [Google Scholar]
- 20.Kato K, Jeanneau C, Tarp MA, Benet-Pagès A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis: secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem . 2006;281(27):18370–18377. [DOI] [PubMed] [Google Scholar]
- 21.Martin A, David V, Li H, Dai B, Feng JQ, Quarles LD. Overexpression of the DMP1 C-terminal fragment stimulates FGF23 and exacerbates the hypophosphatemic rickets phenotype in Hyp mice. Mol Endocrinol. 2012;26(11):1883–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martin A, Liu S, David V, Li H, Karydis A, Feng JQ, Quarles LD. Bone proteins PHEX and DMP1 regulate fibroblastic growth factor Fgf23 expression in osteocytes through a common pathway involving FGF receptor (FGFR) signaling. FASEB J. 2011;25(8):2551–2562. [DOI] [PMC free article] [PubMed] [Google Scholar]