Abstract
Bone metastasis is a deadly consequence of cancers, in which osteoclast forms a vicious cycle with tumor cells. Bone metastasis attenuation by clinical usage of osteoclast inhibitors and in our osteopetrotic mouse genetic models with β-catenin constitutive activation or peroxisome proliferator-activated receptor γ deficiency fully support the important role of osteoclast in driving the bone metastatic niche. However, the mechanisms for this “partnership in crime” are underexplored. Here we show that osteoclasts reprogram their lipid secretion to support cancer cells. Metabolomic profiling reveals elevated prometastatic arachidonic acid (AA) but reduced antimetastatic lysophosphatidylcholines (LPCs). This shift in lipid osteoclastokines synergistically stimulates tumor cell proliferation, migration, survival, and expression of prometastatic genes. Pharmacologically, combined treatment with LPCs and BW-755C, an inhibitor of AA signaling via blocking lipoxygenase and cyclooxygenase, impedes breast cancer bone metastasis. Our findings elucidate key paracrine mechanisms for the osteoclast-cancer vicious cycle and uncover important therapeutic targets for bone metastasis.
Bone metastasis is a frequent, debilitating, and essentially incurable cancer complication that accounts for substantial cancer morbidity and mortality. Yet, the regulation of this complex process remains poorly understood. A bevy of tumors exhibit a strong tendency to metastasize to the bone, including breast, prostate, lung, skin, colon, stomach, bladder, uterus, rectum, thyroid, and kidney cancers (1). Bone metastasis occurs in up to 70% of patients with advanced breast or prostate cancer (2) and in 15% to 30% of patients with many other types of cancer. It is estimated that in the United States alone, at least 350,000 people die with bone metastases annually (3). Once tumors metastasize to bone, the patients are usually incurable: only 20% of women with breast cancer are still alive 5 years after the discovery of bone metastasis (4). The consequences of bone metastases are often devastating: severe bone pain, pathologic fractures, life-threatening hypercalcemia, anemia, spinal cord compression, limited mobility, impaired quality of life, and many others. For all these reasons, it is of paramount urgency and importance to develop new cancer remedies that target bone metastasis, which requires further understanding of mechanisms controlling both the tumor cells and the bone metastatic niche.
In breast cancer bone metastasis particularly, a vicious cycle has been recognized in the osseous environment, whereby bidirectional interactions between tumor cells and osteoclasts lead to both bone loss and tumor growth (5–8). It has been proposed that tumor cells produce factors that directly or indirectly induce osteoclastogenesis; in turn, excessive bone resorption by osteoclasts not only causes bone destruction but also releases growth factors from the bone matrix that stimulate tumor seeding and proliferation (5–8). Indeed, clinical application of osteoclast inhibitors, such as bisphosphonates and receptor activator of nuclear factor-κB ligand (RANKL) neutralizing antibody (denosumab), can interrupt this vicious cycle, thereby reducing bone lesions and tumor burden (3, 9, 10).
Our recent discoveries of novel regulators of osteoclastogenesis and establishment of new mouse genetic models have paved the road to further elucidate the roles of osteoclasts in cancer bone metastasis. We have identified the nuclear receptor peroxisome proliferator-activated receptor γ (PPARγ) as a pro-osteoclastogenic transcription factor (11), and osteoclastic PPARγ knockout mice (Oc-PPARg-KO) exhibit osteopetrosis with decreased osteoclasts and bone resorption (11). We have also identified β-catenin as a key biphasic and dosage-dependent regulator of osteoclastogenesis (12). Our mouse genetic models show that osteoclast differentiation and bone resorption are impaired by osteoclastic β-catenin constitutive activation (Oc-bCat-CA) or osteoclastic β-catenin deletion (Oc-bCat-KO) but enhanced by osteoclastic β-catenin heterozygosity (Oc-bCat-Het) (12). This finding was confirmed by subsequent studies (13). Moreover, we have uncovered the microRNA miR-34a as a powerful suppressor of osteoclastogenesis, bone resorption, and cancer bone metastasis by using osteoclastic miR-34a transgenic and knockout mouse genetic models as well as nanoparticle miR-34a delivery mouse pharmacological models (14).
In this study, we have found that osteoclast also exhibits an ability to directly stimulate breast cancer bone metastasis by reprograming osteoclast cytokines (osteoclastokines) and identified a critical paracrine-signaling pathway in which osteoclast-secreted lipid factors promote tumor cell proliferation, migration, survival, and expression of prometastatic genes. These findings uncover new mechanisms underlying the tumor-osteoclast vicious cycle and reveal lipids as key mediators and therapeutic targets of cancer bone metastasis.
Materials and Methods
Mice
As we previously described (11), Oc-PPARg-KO mice driven by Tie2Cre exhibit impaired osteoclastogenesis. As we previously described (12), osteoclastic β-catenin constitutive active (Oc-bCA) mice driven by PPARγ-tTA; TRE-cre (PT-Cre) (15) or Lysozyme-Cre exhibit similarly blunted osteoclastogenesis; osteoclastic β-catenin knockout (Oc-bKO) mice and osteoclastic β-catenin heterozygous (Oc-bHet) mice driven by PT-Cre exhibit decreased and increased osteoclastogenesis, respectively. These mice were bred with nude (athymic, nu/nu) mice to generate immunodeficient mutant and control mice for human breast cancer cell xenograft. All experiments were conducted using littermates. Sample size estimate was based on power analyses performed using the SAS 9.3 (SAS Institute, Cary, NC) TS X64_7PRO platform. All protocols for mouse experiments were approved by the Institutional Animal Care and Use Committee of the University of Texas Southwestern (UTSW).
Reagents and cell lines
Arachidonic acid was from Sigma (St. Louis, MO). Lysophosphatidylcholine 18:0 and 16:0 were from Avanti Polar Lipids (Alabaster, AL). BW-755C was from Cayman Chemicals (Ann Arbor, MI). Luciferase-labeled MDA-MB-231 parental line and BoM-1833 bone metastasis–prone subline of human breast cancer cells were previously described (14). Luciferase-labeled Py8119 bone metastasis–prone mouse mammary tumor cell line was originally derived from spontaneous mammary tumors in C57BL/6 MMTV-PyMT female transgenic mice (16). RAW264.7 mouse macrophage cell line was from ATCC (TIB-71; Manassas, VA).
Ex vivo osteoclast differentiation and conditioned medium collection
Osteoclasts were differentiated from mouse bone marrow cells as previously described (14). Briefly, bone marrow cells were purified with a 40-μm cell strainer to remove mesenchymal cells. Osteoclast precursors were expanded with 20 ng/mL macrophage colony-stimulating factor (M-CSF) for 3 days, which were then differentiated with 20 ng/mL M-CSF + 100 ng/mL RANKL for 6 days to derive mature osteoclasts. RAW264.7 mouse macrophage cells were differentiated with 100 ng/mL RANKL for 4 days to derive mature osteoclasts. On the last day of differentiation, culture medium was removed and fresh serum-free minimum essential medium α (α-MEM) without M-CSF or RANKL was added, which was collected 24 hours later as osteoclast conditioned medium. Control conditioned medium was collected in a similar fashion from osteoclast precursors without RANKL stimulation. The conditioned medium was filtered through a 0.45-μm filter, aliquoted, and stored at −20°C until use. For proteinase K treatment, proteinase K was added to the conditioned medium at a final concentration of 200 μg/mL, and the tubes were incubated at 55°C for 2 hours.
Lipid extraction and analyses
Collected conditioned medium was mixed with chloroform and methanol at a 5:2:1 ratio in a glass vial. The sample was centrifuged for 25 minutes at 2000 × g and the bottom organic phase was retrieved as lipid fraction, which was dried under a stream of nitrogen and then stored at −80°C. Metabolomic profiling using untargeted LC-MS and lipid quantification using targeted liquid chromatography–mass spectrometry (LC-MS) were performed as previously described (17, 18). To determine in vivo osteoclast regulation of circulating arachidonic acid (AA) and lysophosphatidylcholines (LPCs), 8-week-old mice were treated via intraperitoneal injections with RANKL (R&D Systems, Minneapolis, MN; 20 μg/mouse once per week for 4 weeks), zoledronate (Sigma 20 μg/mouse once per week for 2 weeks), or phosphate-buffered saline (PBS) control. Serum levels of AA and total LPCs were quantified using enzyme-linked immunosorbent assay (ELISA) (MyBioSource, San Diego, CA).
Cancer cell analyses
To measure cancer cell proliferation, luciferase-labeled cancer cells were seeded in 96-well plates at a density of 2 × 103 cells/well in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS). After cells attached, culture medium was replaced with either 200 μL/well conditioned medium (CM) + 10% FBS or with 200 μL/well fresh Dulbecco’s modified Eagle’s medium + 10% FBS that was supplemented with either 10 μg/well purified Oc/control lipids or AA/LPC at indicated concentration. All medium was refreshed every 2 days. Cells were lysed at the indicated time points. Luciferase output from the cell lysate was quantified to measure cancer cell numbers. The (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay was used as an alternative method to quantify cancer cell proliferation. To measure cancer cell apoptosis, cancer cells were cultured in CM without FBS supplement or serum-free medium with lipids for 48 hours. Serum starvation-induced apoptosis was then quantified by Annexin V: PE Apoptosis Detection Kit I (BD Biosciences, San Jose, CA) followed by flow cytometry. Cancer cell migration was quantified using a Transwell assay. Cells and chambers were prepared following the Cell Migration Protocol from Corning (Tewksbury, MA). Briefly, 5 × 105 cells were seeded in the upper chamber and 600μL CM + 10% FBS or culture medium supplemented with lipids were placed in the bottom chamber. After 48 hours, cells that migrated to the bottom chamber were counted to calculate the percentage of migrating cells. Scratch wound-healing assay was used as an alternative method to assess cell migration.
Gene expression analysis
Cancer cells were seeded in a 12-well plate and cultured in 2 mL CM + 10% FBS or culture medium supplemented with the indicated lipids for 3 days before harvest. Expression of prometastatic genes was quantified using real-time quantitative reverse transcription polymerase chain reaction. RNA was extracted using Trizol (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. RNA was first treated with RNase-free DNase I using the DNA-free kit (Ambion, Austin, TX) to remove all genomic DNA and then reverse-transcribed into complementary DNA using an ABI High Capacity cDNA RT Kit (Invitrogen). The complementary DNA was analyzed using real-time quantitative PCR (SYBR Green; Invitrogen) with an Applied Biosystems (Foster City, CA) 7900 Sequence Detection System. Each reaction was performed in triplicate in a 384-well format. The expression of mouse gene was normalized by mouse L19. The expression of human gene was normalized with human glyceraldehyde 3-phosphate dehydrogenase.
Three-dimensional bone metastasis model
In a 3-dimensional bone metastasis cell culture model, 5 × 105 luciferase-labeled cancer cells were cocultured with a piece of calvarial bone in a roller tube, in the presence of 3 mL low-oxygen (mixture of 5% O2, 5% CO2, and 90% N2) infused osteoclastic CM or control CM. The tubes were sealed and placed horizontally on a rotator that rotated at 5 revolutions/min in 37°C incubator. The medium was changed and flushed with low-oxygen gas every 2 days. After a 7-day incubation, the calvarial bone was carefully washed and homogenized in PBS buffer, and bone-residing cancer cells were quantified by luciferase readout.
Bone metastasis analyses
Bone metastasis in vivo was induced using a human breast cancer xenograft model or a mouse mammary tumor allograft model as previously described (14, 19). Using a VisualSonics (Toronto, Ontario, Canada) Vevo770 small animal ultrasound device, luciferase-labeled cancer cells were injected into the left cardiac ventricle so that they could bypass the lung and efficiently migrate to the bone. Bone metastases were detected and quantified weekly after injection by bioluminescence imaging (BLI) using a Caliper Xenogen Spectrum instrument (PerkinElmer, Waltham, MA) at the UTSW small animal imaging core facility. The luciferase-labeled bone metastasis–prone MDA-MB-231 human breast cancer cell subline (BoM-1833) was generously provided by Joan Massagué (Howard Hughes Medical Institute and Memorial Sloan-Kettering Cancer Center) and Yibin Kang (Princeton University) (19, 20) and injected into 6-week-old female nude mice (NCI-Charles River) at 1 × 105 cells/mouse in 100 μL PBS. The luciferase-labeled C57BL/6-compatible MMTV-PyMT mice-derived metastasis-prone mouse mammary tumor cell line Py8119 was generously provided by Jean Jiang (University of Texas Health Science Center) (21) and injected into 8-week-old female C57BL/6J mice at 2 × 104/mouse in 100 μL PBS. For in vivo treatment with osteoclastic lipids or control lipids, 50 μg of lipids in 5 μL dimethyl sulfoxide + 95 μL PBS was intravenously injected into each mouse twice a week, starting 1 week before Py8119 cancer cell injection and continued for 2 weeks after. Bone metastases were quantified by BLI weekly, and the results for 2 weeks after cancer cell injection are shown.
Statistical analyses
All statistical analyses were performed with the Student t test unless noted as analysis of variance (ANOVA) or log-rank (Mantel-Cox) test. Results are shown as mean ± standard deviation (SD). The P values were designated as *P < 0.05, **P < 0.01, ***P < 0.005, ****P < 0.001, and nonsignificant (P > 0.05).
Results
Inhibition of osteoclastogenesis suppresses bone metastasis of cancer
To determine how osteoclast alterations regulate breast cancer bone metastasis, we first examined our previously described osteoclastic β-catenin mouse genetic models (12). As shown in Fig. 1(A), 1(E), and 1(H), bone metastasis from human breast cancer cells developed at 80% to 90% penetrance in wild-type mice 5 weeks after xenograft. In contrast, bone metastasis was significantly dampened in Oc-bCat-CA [Fig. 1(A–G] and Oc-bCat-KO mice [Fig. 1(H–J)] that had fewer osteoclasts but exacerbated in Oc-bCat-Het mice [Fig. 1(H–J)] that had more osteoclasts. Reduced susceptibility of bone metastasis was also observed in our other mouse models with decreased osteoclastogenesis, including Oc-PPARg-KO mice (11) [Fig. 1(F–H)] and osteoclastic miR-34a transgenic mice (14), whereas augmented susceptibility of bone metastasis was observed in our other mouse models with increased osteoclastogenesis such as miR-34a knockout mice (14). These observations using a variety of osteoporotic and osteopetrotic mouse models fully support the crucial bone metastasis–promoting role of osteoclast in vivo.
Figure 1.
Osteoclast promotes breast cancer bone metastasis in vivo. (A–G) Bone metastasis was blocked in Oc-bCA mice. (A, E) Kaplan-Meier plot of time to metastasis over 8 weeks after cardiac injection of BoM-1833 cells. Similar results were obtained from Oc-bCA mice generated with PPARγ-tTA: (A) TRE-cre (PT) or (E) lysozyme-Cre (Ly). Statistical analyses were performed with log-rank (Mantel-Cox) test. (B, F) Quantification of BLI signals. (C, G) Quantification of the number of bone metastatic sites. (D) Representative BLI images showing location and severity of bone metastases. (H) Kaplan-Meier plot of time to metastasis showing that bone metastasis was diminished in Oc-bKO mice but exacerbated in Oc-bHet mice. Log-rank (Mantel-Cox) test showed P = 0.0016 (**) for Oc-bKO vs wild-type (WT); P = 0.0219 (*) for Oc-bHet vs WT; and P < 0.0001 for all 3 groups. (I) Quantification of BLI signals. (J) Quantification of the number of bone metastatic sites. (K–N) Bone metastasis was attenuated in osteoclastic Oc-PPARg-KO mice. (K) Kaplan-Meier plot of time to metastasis. Statistical analyses were performed with log-rank (Mantel-Cox) test. (L) Quantification of BLI signals. (M) Quantification of the number of bone metastatic sites. (N) BLI images. Error bars indicate SD; P values were from Student t test unless noted otherwise. *P < 0.05; **P < 0.01; ***P < 0.005.
Osteoclast-secreted factors promote tumor cell metastatic features
We asked whether osteoclasts directly stimulate cancer cell bone metastasis by providing a hospital tumor microenvironment, in addition to the previously described indirect mechanism via growth factor release from the bone matrix. To analyze the direct effects of osteoclasts on cancer cells, we established an in vitro system to coculture the BoM-1833 cancer cells with CM collected from mature bone marrow–derived osteoclasts. Compared with CM collected from vehicle-treated control cultures with no osteoclast formation, CM from RANKL-induced wild-type osteoclast differentiation cultures significantly increased cancer cell growth, quantified by luciferase output [Fig. 2(A)]. This effect was largely abolished for the CM collected from bCA cultures that failed to form any osteoclasts (12) [Fig. 2(A)]. Consistent with these observations in primary cell cultures, CM collected from osteoclasts differentiated from the RAW267.4 mouse macrophage cell line also enhanced BoM-1833 breast cancer cell growth compared with CM collected from undifferentiated control RAW cells [Fig. 2(B)]. Moreover, osteoclast CM also accelerated cancer cell migration [Fig. 2(C)] and reduced cancer cell apoptosis [Fig. 2(D)].
Figure 2.
Lipid osteoclastokines enhance breast cancer bone metastasis. (A) Cancer cell proliferation was enhanced by CM from wild-type (WT) bone marrow–differentiated osteoclasts (WT Oc CM) but not bCA differentiation cultures that failed to form osteoclast (bCA Oc CM), compared with CM from undifferentiated WT control cultures (WT Ctrl CM). BoM-1833 human breast cancer cell proliferation was quantified by luciferase readout (n = 6). Comparisons with “WT Ctrl CM” at each time point were performed with the Student t test, and P values are indicated in the graph. Two-way ANOVA of all three groups showed P < 0.0001 (***). (B) Cancer cell proliferation was enhanced by CM from RAW264.7 cell line–differentiated osteoclasts (Raw-Oc CM) compared with CM from undifferentiated RAW cells (Raw-Ctrl CM) (n = 6). (C) Cancer cell migration was increased by WT Oc CM but not bCA Oc CM, compared with WT Ctrl CM. In a wound-healing assay, cancer cell cultures were scratch-wounded to form a gap; cell migration was measured by gap closure 24 hours later. Representative images are shown (n = 4). (D) Cancer cell apoptosis was reduced by Oc CM vs Ctrl CM, quantified by Annexin V staining followed by flow cytometry (n = 4). (E) Cancer cell seeding and proliferation on bone was increased by Oc CM vs Ctrl CM in a 3-dimensional bone metastasis model where cancer cells were cocultured with a piece of calvarial bone in a roller tube for 7 days (n = 6). (F) The ability of Oc CM to enhance cancer cell proliferation was intact after proteinase K (Pro.K) treatment and mainly stemmed from lipid factors (n = 6). Comparison between Oc-CM and Ctrl-CM under each treatment condition was performed with the Student t test, and P values are indicated in the graph. One-way ANOVA shows a nonsignificant P value. (G) The proliferation-enhancing effects of osteoclast-secreted lipids (Oc lipids) were specific for breast cancer cells but not for normal mammary epithelial cells, measured by the (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) tetrazolium reduction assay (n = 6). (H) Oc lipids stimulated cancer cell migration equally well as Oc CM, quantified by the Transwell assay (n = 4). (I) Cancer cell expression of prometastatic genes was activated by Oc CM (n = 3). ANOVA P < 0.05. (J) Proliferation (n = 6) and (K) prometastatic gene expression (n = 3) in Py8119 mouse mammary tumor cells were also enhanced by Oc lipids vs Ctrl lipids. (L–N) Treatment with Oc lipids augmented breast cancer bone metastasis. Female C57BL/6J mice (8 weeks old, n = 18) were intravenously injected with 50 μg Oc lipids or Ctrl lipids twice a week, started 1 week before Py8119 cancer cell intracardiac injection, and continued for 2 weeks afterward. Bone metastases were analyzed 2 weeks after tumor cell inoculation. (L) Representative BLI images. (M) Quantification of the number of bone metastatic sites. (N) Quantification of bone metastasis BLI signals. Purified lipids were administered at 2.5 mg/kg in each mouse. Error bars indicate SD; P values were from the Student t test unless noted otherwise. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001; n.s., nonsignificant.
To further investigate how osteoclast CM affected cancer cell seeding and proliferation on bone, we established an ex vivo 3-dimensional cancer bone metastasis model originally developed by Curtin et al. (22). BoM-1833 breast cancer cells were cultured with osteoclast CM or control CM, in the presence of a piece of mouse calvarial bone in a roller tube. After 7 days, significantly more bone-residing tumor cells were detected when cultured in osteoclast CM [Fig. 2(E)]. These results indicate that osteoclasts secret factors (osteoclastokines) to promote cancer bone metastasis by stimulating tumor cell proliferation, migration, survival, and bone seeding.
The prometastatic osteoclastokines are lipids
To determine the functionally important components in osteoclast CM, we treated the CM with proteinase K to degrade proteins but leaving lipids and inorganic small molecules intact. The ability of osteoclast CM to increase cancer cell growth was intact after proteinase K treatment [Fig. 2(F)], indicating that the key factors are likely nonprotein. Further experiments showed that lipids extracted from osteoclast CM exhibited similar ability to promote cancer cell growth as osteoclast CM [Fig. 2(F)], indicating that osteoclast-secreted lipids are mainly responsible.
Importantly, the proliferation-stimulating effect of osteoclast-secreted lipids was specific to cancer cells, including both the bone metastasis–prone BoM-1833 subline and the parental MDA-MB-231 cancer cells but not the MCF-10a normal mammary epithelial cells [Fig. 2(G)]. Furthermore, Transwell assay showed that osteoclast-secreted lipids also facilitated cancer cell migration equally well as osteoclast CM [Fig. 2(H)]. Collectively, these results indicate that the metastasis-promoting activity of osteoclast secretome resides in its lipid factors.
We next examined how osteoclast CM affects the expressions of well-established prometastatic genes. Osteopontin (20, 23), cyclooxygenase 2 (24, 25), integrin β3 (26, 27), RANKL (28), parathyroid hormone-related protein (29), and interleukin 1β (30) were expressed at much higher levels in the bone metastasis–prone BoM-1833 subline compared with the nonmetastatic MDA-MB-231 parental line [Fig. 2(I)]. Importantly, treatment with osteoclast CM significantly enhanced the expression of these genes in both cell lines [Fig. 2(I)].
To verify that the effects of osteoclast CM were not limited to MDA-MB-231 and BoM-1833 human breast cancer cell lines, we conducted similar experiments using a luciferase-labeled bone metastasis–prone mouse mammary tumor cell line (Py8119) that was derived from spontaneous mammary tumors in C57BL/6 MMTV-PyMT female transgenic mice (16). Osteoclast-secreted lipids also significantly enhanced prometastatic features of Py8119 cells, including increased cell proliferation [Fig. 2(J)] and metastatic gene expression [Fig. 2(K)].
Py8119 tumor cells also allowed us to examine breast cancer bone metastasis in an immunocompetent setting using C57BL/6J mice to take consideration of any potential modulation by the adaptive immune system. Compared with vehicle control, intravenous daily administration of osteoclast-secreted lipids significantly exacerbated Py8119 cell bone metastasis, demonstrated by the increased metastatic sites and BLI signal intensity [Fig. 2(L–N)]. These findings reveal that osteoclasts promote breast cancer bone metastasis by altering its secreted lipids, thereby providing a favorable bone metastatic niche.
Metabolomic profiling detects elevated AA secretion from osteoclasts
To pinpoint the differences between the lipids secreted by mature osteoclasts vs undifferentiated precursors, we performed metabolomics profiling using untargeted LC-MS analyses as we previously described (17, 18, 31, 32). The most significantly altered lipids under negative ionization mode were polyunsaturated fatty acids (PUFAs), including AA (C20:4) and eicosapentaenoic acid (C20:5); both were elevated by >6-fold in osteoclastic lipids compared with control lipids [Fig. 3(A)]. Subsequent targeted LC-MS analysis confirmed this observation and revealed AA as the most upregulated and abundant species [Fig. 3(B)]. We hypothesize that elevated AA may contribute to the prometastatic functions of osteoclast-secreted lipids.
Figure 3.
Osteoclasts increase AA secretion to promote cancer cell metastatic features. (A) Metabolomic profiling revealed increased AA and eicosapentaenoic acid (EPA) secretion from osteoclasts. Untargeted LC-MS analysis was conducted under negative ionization mode to compare lipids isolated from the CM of osteoclasts vs undifferentiated precursors (n = 3). (B) Quantification of AA and EPA in Oc lipids and Ctrl lipids by targeted LC-MS analysis (n = 3). (C) AA treatment increased cancer cell migration. In a Transwell assay, AA was added in the bottom chamber at the indicated concentration, and BoM-1833 cell migration from top chamber to bottom chamber was quantified by cell counting (n = 4). (D) AA treatment decreased cancer cell apoptosis, quantified by Annexin V staining followed by flow cytometry (n = 4). (E) AA treatment enhanced cancer cell expression of prometastatic genes (n = 3). ANOVA P < 0.05. (F) AA treatment did not alter cancer cell growth, for both MDA-MB231 (left) and BoM-1833 (right) cells, measured by luciferase output (n = 6). Two-way ANOVA indicated a nonsignificant P value. (G) Py8119 cancer cell growth was unaltered by AA treatment but significantly reduced by the treatment of BW-755C (1.5 μg/mL). Py8119 cell growth was measured by luciferase output (n = 6). Comparisons with Ctrl at each time point were performed with the Student t test, and P values are indicated in the graph. Two-way ANOVA shows P = 0.0003 (***). (H) BW-755C significantly inhibited the growth of MDA-MB231 (left) and BoM-1833 (right) human breast cancer cells in a dose-dependent manner (n = 6). Comparisons with 0 μg/mL control were performed with the Student t test, and P values are indicated in the graph. Two-way ANOVA shows P < 0.0001 (****).BW-755C treatment abolished the effects of (I) Oc lipids and (J) AA to increase cancer cell migration (n = 4). BW-755C treatment was at 1.5 μg/mL; AA treatment was at 10 μM. Error bars indicate SD; P values were from the Student t test unless noted otherwise. *P < 0.05; **P < 0.01; ***P < 0.005; ****P < 0.001; n.s., nonsignificant.
AA promotes breast cancer cell migration, survival, and metastatic gene expression
To dissect the effects on cancer cell metastatic features, we performed similar experiments as for osteoclastic CM and osteoclastic lipids. First, Transwell assay showed that AA enhanced cancer cell migration in a dose-dependent manner [Fig. 3(C)]. Among all the dosages tested, 10 μM AA promoted cancer cell migration the most. Thus, 10 μM AA was used for the subsequent experiments. Second, Annexin V staining followed by fluorescence-activated cell sorting analysis revealed that AA inhibited cancer cell apoptosis [Fig. 3(D)]. Third, real-time RT-qPCR illustrated that AA also augmented the expression of prometastatic genes, including osteopontin, cyclooxygenase 2, integrin β3, RANKL, parathyroid hormone-related protein, and interleukin 1β, in both MDA-MB-231 and BoM-231 cancer cells [Fig. 3(E)]. Although cancer cell growth was not significantly affected by AA treatment [Fig. 3(F) and 3(G)], it was significantly reduced by the treatment of a dual inhibitor of cyclooxygenases and lipoxygenases BW-755C for Py8119, MDA-MB-231, and BoM-1833 cells [Fig. 3(G) and 3(H)]. BW-755C treatment also abolished the abilities of osteoclastic lipids and AA to promote cancer cell migration [Fig. 3(I) and 3(J)], further supporting that BW-755C is an effectively pharmacological strategy to block AA signaling. Together, these findings reveal that osteoclasts promote cancer bone metastasis, at least in part, by increasing the abundance of AA in the bone microenvironment. Thus, either reducing AA level in the bone metastatic niche or blocking AA signaling in cancer cells may exert antitumor and antimetastatic effects.
Metabolomic profiling reveals diminished LPC secretion from osteoclasts
Metabolomic profiling using untargeted LC-MS under positive ionization mode also detected differentially regulated lipid species. The most significantly altered class of lipids was LPC, including LPC 18:0 and LPC 16:0, which were decreased by 4- to 7-fold in osteoclastic lipids compared with control lipids [Fig. 4(A)]. Subsequent targeted LC-MS analysis confirmed this observation [Fig. 4(B)]. We hypothesize that diminished LPC may also contribute to the prometastatic functions of osteoclast-secreted lipids.
Figure 4.
Osteoclasts decrease LPC secretion to promote cancer cell metastatic features. (A) Metabolomic profiling revealed decreased LPC 18:0 and LPC 16:0 secretions from osteoclasts. Untargeted LC-MS analysis was conducted under positive ionization mode to compare lipids isolated from the CM of osteoclasts vs undifferentiated precursors (n = 3). (B) Quantification of LPC 18:0 and LPC 16:0 in Oc lipids and Ctrl lipids by targeted LC-MS analysis (n = 3). (C) LPC treatment decreased cancer cell migration. In a Transwell assay, LPC (10 μM) was added in the bottom chamber, and BoM-1833 cell migration from top chamber to bottom chamber was quantified by cell counting (n = 4). (D) LPC treatment (10 μM) increased cancer cell apoptosis, quantified by Annexin V staining followed by flow cytometry (n = 4). (E) LPC treatment increased Bax expression but decreased Bcl2 expression in a dose-dependent manner (n = 3). (F, G) LPC treatment suppressed cancer cell growth for both MDA-MB231 cells (F) and BoM-1833 cells (G), measured by luciferase output (n = 6). (H) LPC treatment did not alter cancer cell expression of prometastatic genes (n = 3). Error bars indicate SD; P values are from the Student t test unless noted otherwise. *P < 0.05; ***P < 0.005; ****P < 0.001; n.s., nonsignificant. (E–G) ANOVA P < 0.05.
LPCs inhibits breast cancer cell growth, migration, and survival
We performed a series of experiments to determine the effects of LPC 18:0 and LPC 16:0 on breast cancer cells. First, both LPCs suppressed cancer cell migration [Fig. 4(C)]. Second, both LPCs enhanced cancer cell apoptosis, demonstrated by the higher Annexin V staining [Fig. 4(D)], as well as the increased expression of proapoptotic genes such as Bax and the decreased expression of antiapoptotic genes such as Bcl2 [Fig. 4(E)]. These results suggest that increased AA and decreased LPCs from osteoclast secretome have overlapping functions to promote cancer cell migration and survival. Interestingly, although cancer cell growth was unaffected by AA treatment [Fig. 3(F–H)], it was significantly inhibited by both LPCs, in both 231 and BoM-1833 cells [Fig. 4(F) and 4(G)]. Moreover, although cancer cell expression of prometastatic genes was enhanced by AA [Fig. 3(E)], it was not significantly altered by either LPC [Fig. 4(H)]. These results suggest that increased AA and decreased LPC from osteoclast secretome also exert distinct yet complementary effects to promote cancer cell migration and survival. For all the effects observed with LPCs, LPC 18:0 showed stronger regulation than LPC 16:0 [Fig. 4(C–G)]. Together, these findings support that osteoclasts promote cancer bone metastasis also by decreasing the abundance of LPCs in the bone microenvironment. Therefore, replenishing LPCs in the bone metastatic niche may exert antitumor and antimetastatic effects.
Combination of AA blockade and LPC administration impedes cancer bone metastasis
It is intriguing how osteoclasts polarize lipid secretion to increase AA but decrease LPCs in the bone environment to synergistically confer advantages for cancer cell metastasis. We found that AA, LPCs, or BW-755C did not significantly alter osteoclast differentiation (Supplemental Fig. 1 (441.5KB, eps) ), indicating that these lipids modulate bone metastasis by mainly acting on cancer cells via paracrine mechanisms rather than directly acting on osteoclasts via autocrine mechanisms.
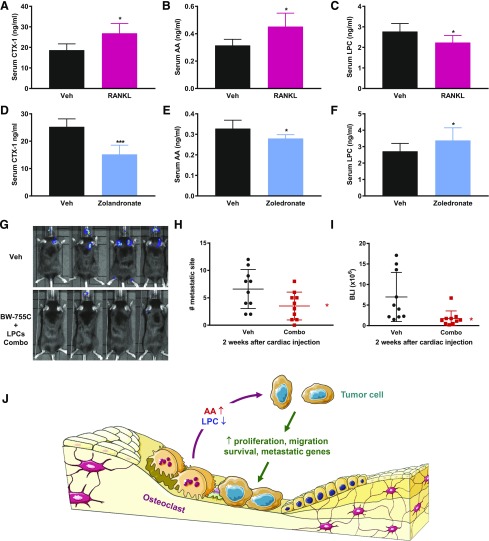
To determine the in vivo significance of osteoclast regulation of AA and LPCs, we treated mice with recombinant RANKL or a bisphosphonate zoledronate to activate or inhibit osteoclasts, respectively, and assessed the effects on serum AA and LPCs. The results showed that osteoclast activation increased AA but decreased LPCs [Fig. 5(A–C)], whereas osteoclast inhibition decreased AA but increased LPCs [Fig. 5(D–F)]. These findings further bolster the notion that osteoclast is a physiologically relevant regulator of circulating AA and LPCs that is sufficient to reprogram the lipid cancer environment.
Figure 5.
Combination of BW-755C and LPCs suppresses bone metastasis in vivo. (A–C) Osteoclast activation increased AA but decreased LPCs in vivo. Mice were treated with RANKL (20 μg/mouse) or PBS control once per week for 4 weeks, and then serum levels of CTX-1 (A), AA (B), and total LPC (C) were quantified by ELISA (n = 5). (D–F) Osteoclast inhibition decreased AA but increased LPCs in vivo. Mice were treated with zoledronate (20 μg/mouse) or PBS control once per week for 2 weeks, and then serum levels of CTX-1 (D), AA (E), and total LPC (F) were quantified by ELISA (n = 5). (G–I) Female C57BL/6J mice (8 weeks old, n = 10) were treated with either BW-755C + LPC Combo (300 μg BW-755C in 10% EtOH and 5 μg LPC 16:0 + 5 μg LPC18:0 in 10% dimethyl sulfoxide) or vehicle placebo (10% EtOH + 10% dimethyl sulfoxide). The treatment was performed intravenously twice per week for 1 week before cardiac injection of Py8119 cancer cells and then continued for 3 more weeks. Bone-met quantifications at week 2 are shown as some mice died by week 3. (G) Representative BLI images from each experimental group. (H) Quantification of the number of bone metastatic sites. (I) Quantification of bone metastasis BLI signals. Error bars indicate SD; P values are from the Student t test. *P < 0.05; ***P < 0.005. (J) A working model for how osteoclasts alter the secretion of lipids by increasing prometastatic AA and decreasing antimetastatic LPCs to promote cancer cell metastasis to bone.
With our identification of AA as a potential pro–bone-metastasis (met) lipid and LPCs as potential anti–bone-met lipids, we next investigated whether the simultaneous AA signaling inhibition by BW-755C and LPC administration could attenuate breast cancer bone metastasis in vivo. Female C57BL/6J mice were pretreated with 2 doses of combo (BW-755C + LPC 18:0 + LPC 16:0) or vehicle placebo control before cardiac injection of Py8119 cells. The combo or vehicle treatment was continued for 3 more weeks at 2 doses per week. Remarkably, combo treatment significantly reduced both the incidence and severity of bone metastasis, demonstrated by the decreased number of metastatic sites and BLI signal intensity [Fig. 5(G–I)]. LPC treatment alone and, to a lesser extent, BW-755C treatment alone also exhibited anti–bone-met effects (Supplemental Fig. S2 (491KB, eps) ). These findings highlight the exciting potential of lipid-based therapies as a new strategy to alleviate cancer bone metastasis.
Discussion
It has been recognized for decades that breast cancer cells and osteoclasts form a “vicious cycle,” in which breast cancer cells stimulate osteoclastogenesis, and osteoclasts in turn promote cancer cell seeding and growth in bone (5–8). The molecular underpinnings for how osteoclasts support cancer cells described to date have been mainly attributed to the release and activation of bone matrix–embedded growth factors as the result of bone resorption (5–8). Our present study reveals an important yet previously unrecognized additional mechanism in which osteoclasts also enhance cancer cell growth and metastatic features by reprograming lipid secretion to create a favorable bone metastatic niche [Fig. 5(J)]. The significance and novelty of our findings reside in the following aspects. First, we uncover that osteoclasts directly stimulate cancer cell metastatic behavior in the absence of bone matrix via the secretion of lipid osteoclastokines. Second, our global metabolomic analyses reveal that osteoclasts shift lipid secretion from their undifferentiated precursors to create an AA-rich and LPC-poor microenvironment. Third, we identify AA as a prometastatic lipid and LPCs as antimetastatic lipids, which act in a synergistic and complementary fashion to enhance tumor cell growth, migration, survival, and metastatic gene expression. Fourth, we provide exciting preclinical evidence that the combination treatment with LPCs and an AA signaling inhibitor BW-755C can significantly alleviate breast cancer bone metastasis. Fifth, our mouse genetic models reveal that bone metastasis can be markedly suppressed by β-catenin activation or PPARγ deficiency in the osteoclast lineage.
Lipid overabundance in the body has been shown to aggravate cancer metastasis (33). Intracellular lipid accumulation has been observed in many types of cancers, including breast, brain, adrenal, and others (34, 35). Highly proliferative cancer cells show a strong lipid avidity, which they satisfy by either increasing the uptake of exogenous lipids and lipoproteins or overactivating their endogenous biosynthesis (36), both of which can be heavily influenced by niche cell types in the tumor microenvironment. However, it has been largely overlooked how niche cells and tumor cells perform crosstalk using lipid-based language. What are the unique lipidomic profiles in the cancer-promoting niche cells and their secretome? What are the key lipid species from the niche cells that confer their protumor and prometastatic functions? What are the functions of these lipids, metabolic or signaling? Using osteoclast niche cells and breast cancer cells, we begin to address these questions to understand how lipid osteoclastokines modulate cancer behavior and malignancy during bone metastasis.
AA and its metabolites generated by cyclooxygenases and lipoxygenases, such as prostaglandins and leukotrienes, have been reported to regulate a variety of biological processes, including chemotaxis, cancer, and rheumatoid arthritis (37, 38). In accordance with previous studies showing a protumor role of AA signaling, here we report that AA also exerts a prometastatic role to enhance cancer cell migration, survival, and metastatic gene expression. Moreover, we show that the levels of AA are elevated in the osteoclastic bone metastatic niche, as well as in circulation upon osteoclast activation of bone resorption, revealing AA as a key player in the osteoclast–cancer cell vicious cycle. A potential explanation for why cancer cell proliferation was inhibited by BW-755C but unaltered by AA is that BW-755C may exert a broader effect to suppress the signaling of several unsaturated fatty acids, including AA, by inhibiting both cyclooxygenases and lipoxygenases.
LPCs have been implicated to exhibit anticancer effects. Reduced plasma LPC levels have been observed in patients with advanced cancer (39). In vitro studies indicated that LPCs can reduce cancer cell adhesion, viability, and invasion (40, 41). Their anticancer abilities are unique because LPCs do not target DNA but insert into the plasma membrane to affect cellular processes such as adhesion and apoptosis through influencing several signaling pathways (40, 41). Our study supports and extends these previous findings by showing 1) the levels of LPCs are downregulated in the osteoclastic bone metastatic niche, as well as in circulation upon osteoclast activation of bone resorption; 2) LPCs inhibit breast cancer cell growth, migration, and survival in vitro; and 3) treatment with LPCs alone, and more so in combination of an AA signaling inhibitor BW-755C, is sufficient to impede breast cancer bone metastasis in vivo. These new results further support LPCs as effective anticancer and antimetastatic therapies.
Our data show that osteoclast activation or inhibition is sufficient to modulate circulating levels of AA and LPCs in vivo [Fig. 5(A–F)], supporting the physiological significance of our in vitro findings. Nonetheless, it is possible that these changes in AA and LPCs not only originate from osteoclasts but also contributed by other AA- and LPC-producing cells/tissues, such as brain, muscle, and liver. We found that AA, LPCs, and BW-755C do not directly alter osteoclast differentiation (Supplemental Fig. S1 (491KB, eps) ), suggesting that these lipids mainly exert effects on tumor cells in the bone metastatic niche. However, they may indirectly alter bone resorption in vivo via the cancer cell–osteoclast vicious cycle such that their effects on cancer cells can relay to osteoclasts. Moreover, AA and LPCs may also modulate other cell types in the local and systemic cancer environment such as osteoblast and endothelial cells.
Previous studies show that factors released from bone matrix upon bone resorption play important roles in bone metastasis; our current work provides evidence for an additional and complementary mechanism in which osteoclast-derived lipids also polarize the bone metastatic niche to facilitate the cancer-osteoclast vicious cycle. Our examination of the specific roles of AA and LPCs in osteoclasts during bone metastasis in vivo is limited by the availability of AA-specific inhibitors and osteoclast-specific pharmacological targeting. In future studies, genetic strategies such as osteoclast-specific deletion or overexpression of AA- and LPC-specific metabolic enzymes may further elucidate the in vivo significance of this regulation.
It remains to be determined whether combo treatment with BW-755C and LPCs confers any therapeutic advantages compared with conventional osteoclast inhibitors, such as bisphosphonates or denosumab. However, in light of the limitations of these current drugs such as lack of survival benefit as well as side effects, including osteonecrosis of the jaw and renal toxicity, it is important to identify new signaling mechanisms and develop better therapeutic strategies. To this end, our studies using preclinical mouse models have demonstrated the functional significance of lipid osteoclastokines in bone metastasis and the therapeutic efficacy of their pharmacological targeting. Moreover, the levels of these osteoclast-regulated lipids in serum or biopsy, quantified by LC-MS or ELISA kits, may be used as prognostic indicators for cancer bone metastasis.
In conclusion, here we uncover, for the first time, to our knowledge, a direct and lipid-mediated mechanism for how osteoclast enhances breast cancer cell metastatic features to promote the vicious cycle in bone metastasis. Provocatively, it is plausible that the unique profiles of lipid storage and lipid secretion in individual metastatic niche cell type may contribute to cancer metastasis organotropism. Our work suggests that the reprogramming of lipid composition by osteoclasts in the osseous milieu may cater to bone-seeking cancer cells and function as an attractant to facilitate cancer cell seeding and thriving in bone. Our findings reveal the exciting potential of lipids as future prognostic and therapeutic targets for breast cancer bone metastasis.
Acknowledgments
We thank UTSW Small Animal Imaging Core for the support on ultrasound-assisted intracardiac injection and bioluminescence imaging. Y. Wan is Lawrence Raisz Professor in Bone Cell Metabolism and a Virginia Murchison Linthicum Scholar in Medical Research.
Acknowledgments
This work was supported in part by Cancer Prevention and Research Institute of Texas Grant RP130145 (to Y.W.), US Department of Defense Grant W81XWH-13-1-0318 (to Y.W.), Mary Kay Foundation Grant 073.14 (to Y.W.), National Institutes of Health Grants R01 DK089113 (to Y.W.) and UH2 TR000943 (to A.S.), The Welch Foundation Grant I-1751 (to Y.W.), March of Dimes Grant 6-FY13-137 (to Y.W.), and National Cancer Institute Cancer Center Support Grant 5P30CA142543. Additional funding came from the Simmons Cancer Center and the UTSW Endowed Scholar Startup Fund (both to Y.W.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AA
- arachidonic acid
- ANOVA
- analysis of variance
- bCA
- β-catenin constitutive active
- bKO
- β-catenin knockout
- BLI
- bioluminescence imaging
- CM
- conditioned medium
- ELISA
- enzyme-linked immunosorbent assay
- FBS
- fetal bovine serum
- KO
- knockout
- LC-MS
- liquid chromatography–mass spectrometry
- LPC
- lysophosphatidylcholine
- M-CSF
- macrophage colony-stimulating factor
- MEM
- minimum essential medium; met, metastasis; Oc, osteoclast-secreted
- Oc-bCA
- osteoclastic β-catenin constitutive activation
- Oc-bCat-CA
- osteoclastic β-catenin constitutive activation
- Oc-bCat-Het
- osteoclastic β-catenin heterozygosity
- Oc-bCat-KO
- osteoclastic β-catenin deletion
- Oc-bHet
- osteoclastic β-catenin heterozygous
- Oc-bKO
- osteoclastic β-catenin knockout
- Oc-PPARg-KO
- osteoclastic PPARγ knockout
- PBS
- phosphate-buffered saline
- PPARγ
- peroxisome proliferator-activated receptor γ
- RANKL
- receptor activator of nuclear factor-κB ligand
- SD
- standard deviation
References
- 1.Coleman RE. Skeletal complications of malignancy. Cancer. 1997;80(8, Suppl):1588–1594. [DOI] [PubMed] [Google Scholar]
- 2.Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987;55(1):61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–593. [DOI] [PubMed] [Google Scholar]
- 4.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27(3):165–176. [DOI] [PubMed] [Google Scholar]
- 5.Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Expr. 2000;10(2):159–178. [PubMed] [Google Scholar]
- 6.Ell B, Kang Y.. SnapShot: bone metastasis. Cell. 2012;151(3):690.e1. [DOI] [PubMed] [Google Scholar]
- 7.Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2(6):570–572. [PubMed] [Google Scholar]
- 8.Mundy GR. Mechanisms of bone metastasis. Cancer. 1997;80(8, Suppl):1546–1556. [DOI] [PubMed] [Google Scholar]
- 9.Coleman RE. Bone cancer in 2011: prevention and treatment of bone metastases. Nat Rev Clin Oncol. 2011;9(2):76–78. [DOI] [PubMed] [Google Scholar]
- 10.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–1664. [DOI] [PubMed] [Google Scholar]
- 11.Wan Y, Chong LW, Evans RM. PPAR-gamma regulates osteoclastogenesis in mice. Nat Med. 2007;13(12):1496–1503. [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Zeve D, Suh JM, Wang X, Du Y, Zerwekh JE, Dechow PC, Graff JM, Wan Y. Biphasic and dosage-dependent regulation of osteoclastogenesis by β-catenin. Mol Cell Biol. 2011;31(23):4706–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weivoda MM, Ruan M, Hachfeld CM, Pederson L, Howe A, Davey RA, Zajac JD, Kobayashi Y, Williams BO, Westendorf JJ, Khosla S, Oursler MJ. Wnt signaling inhibits osteoclast differentiation by activating canonical and noncanonical cAMP/PKA pathways. J Bone Miner Res. 2015;31(1):65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, Sood AK, Mendell JT, Wan Y. miR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature. 2014;512(7515):431–435. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Wei W, Zeve D, Wang X, Du Y, Tang W, Dechow PC, Graff JM, Wan Y. Osteoclast progenitors reside in the peroxisome proliferator-activated receptor γ-expressing bone marrow cell population. Mol Cell Biol. 2011;31(23):4692–4705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas T, Gu X, Yang J, Ellies LG, Sun LZ. Attenuation of TGF-β signaling supports tumor progression of a mesenchymal-like mammary tumor cell line in a syngeneic murine model. Cancer Lett. 2014;346(1):129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saghatelian A, Trauger SA, Want EJ, Hawkins EG, Siuzdak G, Cravatt BF. Assignment of endogenous substrates to enzymes by global metabolite profiling. Biochemistry. 2004;43(45):14332–14339. [DOI] [PubMed] [Google Scholar]
- 18.Wan Y, Saghatelian A, Chong LW, Zhang CL, Cravatt BF, Evans RM. Maternal PPAR gamma protects nursing neonates by suppressing the production of inflammatory milk. Genes Dev. 2007;21(15):1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu X, Wang Q, Hu G, Van Poznak C, Fleisher M, Reiss M, Massagué J, Kang Y. ADAMTS1 and MMP1 proteolytically engage EGF-like ligands in an osteolytic signaling cascade for bone metastasis. Genes Dev. 2009;23(16):1882–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordón-Cardo C, Guise TA, Massagué J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3(6):537–549. [DOI] [PubMed] [Google Scholar]
- 21.Zhou JZ, Riquelme MA, Gao X, Ellies LG, Sun LZ, Jiang JX. Differential impact of adenosine nucleotides released by osteocytes on breast cancer growth and bone metastasis. Oncogene. 2014;34(14):1831–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Curtin P, Youm H, Salih E. Three-dimensional cancer-bone metastasis model using ex-vivo co-cultures of live calvarial bones and cancer cells. Biomaterials. 2012;33(4):1065–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kruger TE, Miller AH, Godwin AK, Wang J. Bone sialoprotein and osteopontin in bone metastasis of osteotropic cancers. Crit Rev Oncol Hematol. 2014;89(2):330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karavitis J, Zhang M. COX2 regulation of breast cancer bone metastasis. OncoImmunology. 2014;2(3):e23129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh B, Berry JA, Shoher A, Ayers GD, Wei C, Lucci A. COX-2 involvement in breast cancer metastasis to bone. Oncogene. 2007;26(26):3789–3796. [DOI] [PubMed] [Google Scholar]
- 26.Liapis H, Flath A, Kitazawa S.. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5(2):127–135. [DOI] [PubMed] [Google Scholar]
- 27.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26(42):6238–6243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Azim H, Azim HA Jr. Targeting RANKL in breast cancer: bone metastasis and beyond. Expert Rev Anticancer Ther. 2014;13(2):195–201. [DOI] [PubMed] [Google Scholar]
- 29.Powell GJ, Southby J, Danks JA, Stillwell RG, Hayman JA, Henderson MA, Bennett RC, Martin TJ. Localization of parathyroid hormone-related protein in breast cancer metastases: increased incidence in bone compared with other sites. Cancer Res. 1991;51(11):3059–3061. [PubMed] [Google Scholar]
- 30.Holen I, Lefley DV, Francis SE, Rennicks S, Bradbury S, Coleman RE, Ottewell P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget. 2016;7(48):75571–75584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saghatelian A, Cravatt BF. Assignment of protein function in the postgenomic era. Nat Chem Biol. 2005;1(3):130–142. [DOI] [PubMed] [Google Scholar]
- 32.Wei W, Schwaid AG, Wang X, Wang X, Chen S, Chu Q, Saghatelian A, Wan Y. Ligand activation of ERRα by cholesterol mediates statin and bisphosphonate effects. Cell Metab. 2016;23(3):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Le TT, Huff TB, Cheng JX. Coherent anti-Stokes Raman scattering imaging of lipids in cancer metastasis. BMC Cancer. 2009;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramos CV, Taylor HB. Lipid-rich carcinoma of the breast: a clinicopathologic analysis of 13 examples. Cancer. 1974;33(3):812–819. [DOI] [PubMed] [Google Scholar]
- 35.Sijens PE, Levendag PC, Vecht CJ, van Dijk P, Oudkerk M. 1H MR spectroscopy detection of lipids and lactate in metastatic brain tumors. NMR Biomed. 1996;9(2):65–71. [DOI] [PubMed] [Google Scholar]
- 36.Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5:e189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villegas-Comonfort S, Castillo-Sanchez R, Serna-Marquez N, Cortes-Reynosa P, Salazar EP. Arachidonic acid promotes migration and invasion through a PI3K/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Prostaglandins Leukot Essent Fatty Acids. 2014;90(5):169–177. [DOI] [PubMed] [Google Scholar]
- 38.Hyde CA, Missailidis S. Inhibition of arachidonic acid metabolism and its implication on cell proliferation and tumour-angiogenesis. Int Immunopharmacol. 2009;9(6):701–715. [DOI] [PubMed] [Google Scholar]
- 39.Jantscheff P, Schlesinger M, Fritzsche J, Taylor LA, Graeser R, Kirfel G, Fürst DO, Massing U, Bendas G. Lysophosphatidylcholine pretreatment reduces VLA-4 and P-selectin-mediated b16.f10 melanoma cell adhesion in vitro and inhibits metastasis-like lung invasion in vivo. Mol Cancer Ther. 2011;10(1):186–197. [DOI] [PubMed] [Google Scholar]
- 40.van Blitterswijk WJ, Verheij M. Anticancer alkylphospholipids: mechanisms of action, cellular sensitivity and resistance, and clinical prospects. Curr Pharm Des. 2008;14(21):2061–2074. [DOI] [PubMed] [Google Scholar]
- 41.Raynor A, Jantscheff P, Ross T, Schlesinger M, Wilde M, Haasis S, Dreckmann T, Bendas G, Massing U. Saturated and mono-unsaturated lysophosphatidylcholine metabolism in tumour cells: a potential therapeutic target for preventing metastases. Lipids Health Dis. 2015;14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]