Abstract
The nonclassical progesterone receptors progesterone receptor membrane component (PGRMC) 1 and PGRMC2 have been implicated in regulating cell survival of endometrial and ovarian cells in vitro and are abundantly expressed in these cell types. The objective of this study was to determine if Pgrmc1 and Pgrmc2 are essential for normal female reproduction. To accomplish this objective, Pgrmc1 and/or Pgrmc2 floxed mice (Pgrmc2fl/fl and Pgrmc1/2fl/fl) were crossed with Pgr-cre mice, which resulted in the conditional ablation of Pgrmc1 and/or Pgrmc2 from female reproductive tissues (i.e., Pgrmc2d/d and Pgrmc1/2d/d mice). A breeding trial revealed that conditional ablation of Pgrmc2 initially led to subfertility, with Pgrmc2d/d female mice producing 47% fewer pups/litter than Pgrmc2fl/fl mice (P = 0.001). Pgrmc2d/d mice subsequently underwent premature reproductive senescence by parities 2 to 5, producing 37.8% fewer litters overall during the trial compared with Pgrmc2fl/fl mice (P = 0.020). Similar results were observed with Pgrmc1/2d/d mice. Based on ovarian morphology and serum P4, the subfertility/infertility was not due to faulty ovulation or luteal insufficiency. Rather an analysis of midgestation implantation sites revealed that postimplantation embryonic death was the major cause of the subfertility/infertility. As with our previous report of Pgrmc1d/d mice, Pgrmc2d/d and Pgrmc1/2d/d mice developed endometrial cysts consistent with accelerated aging of this tissue. Given the timing of postimplantation embryonic demise, uterine decidualization may be disrupted in mice deficient in PGRMC2 or PGRMC1/2. Overall, this study revealed that Pgrmc1 and/or Pgrmc2 are required for the maintenance of uterine histoarchitecture and normal female reproductive lifespan.
Progesterone (P4) is a female sex steroid that regulates tissue homeostasis and is essential for reproduction in the female. Disrupted P4 signaling is causally coupled to many reproductive diseases that result in subfertility or infertility, including leiomyomas, endometriosis, irregular menstrual bleeding, miscarriage, and preterm labor (1–9). In the United States, over 10% of women have impaired fecundity (10), and another 10% of women of reproductive age are clinically diagnosed with endometriosis (11). Infertility and reproductive diseases like endometriosis are multifaceted and difficult to successfully treat. Understanding the etiology of these diseases and how impaired P4 signaling contributes to their pathogenesis is necessary for developing more effective therapeutic approaches.
Much of what is known about P4 actions is centered on the classical progesterone receptor (PGR). However, several putative nonclassical PGRs have been identified, and these include 3 members of the progestin and adipoQ receptor family (12, 13) and 2 members of the progesterone receptor membrane component (PGRMC) family in PGRMC1 and PGRMC2 (14–17). Importantly, although in vitro studies using both primary and transformed reproductive cell lines that lack expression of PGR have established that PGRMC1 mediates at least some of the actions of P4, parallel findings have yet to be confirmed in vivo. As evaluated in several species, PGRMC1 and PGRMC2 are expressed throughout the female reproductive tract in both the nongravid state and during pregnancy (18–29). Aberrant expression of PGRMC1 and PGRMC2 has been implicated in female reproductive diseases. These cumulative findings conceptually indicate that homeostatic expression of PGRMC1 and PGRMC2 is essential for normal female reproductive physiology and maintenance of reproductive tissue homeostasis. Recent findings from our laboratory demonstrate that conditional ablation of Pgrmc1 from the uterus results in subfertility in female mice (30). These animals are subfertile at the time of sexual maturation, but then also develop endometrial cysts at 4 to 5 months of age. This phenotype is consistent with premature aging of the reproductive tract. Given that, as with PGRMC1, PGRMC2 is highly expressed in the uterus, the objective of this study was to evaluate the functional contribution of PGRMC2 to female reproduction. To accomplish this, we developed mutant mice in which Pgrmc2 alone or in combination with Pgrmc1 were conditionally ablated from the female reproductive tract to assess the function of these 2 genes in female fertility.
Materials and Methods
Floxing the Pgrmc2 allele and genotyping
All procedures involving animals were approved by the Institutional Animal Care and Use Committees at Washington State University or the University of Connecticut Health Center. A Pgrmc2-targeting vector was prepared by recombineering according to Lee et al. (31). Briefly, a 13.6-kb section of the Pgrmc2 genomic sequence containing both exon 2 and exon 3, as well as 4 kb of the 3′-downstream sequences, was retrieved from the bacterial artificial chromosome, RP23-2C23, into pPL253 by gap repair. A 5′ LoxP site was inserted into intron 1 approximately 1.3 Kb upstream of exon 2 followed by insertion of Frt-PGFneo-Frt-LoxP into the 3′-downstream sequence approximately 0.9 kb downstream of exon 3. The vector, which contained approximately 4.2 kb and 3.2 kb of the 5′ and 3′ arms, respectively, was linearized by NotI digestion and purified and electroporated into mouse embryonic stem (ES) cells derived from an F1(129Sv/C57BL6j) blastocyst. The cells were then cultured in the presence of 150 µg/mL of G418 and 2 µm ganciclovir. Drug-resistant colonies were selected and screened by nested long-range polymerase chain reaction (PCR) using primers corresponding to sequences outside the arms and specific to the 5′ and 3′ LoxP sites to identify targeted ES clones. Targeted ES cells were used to generate chimeric mice by aggregation with CD1 morula. Chimeric male animals were then bred with ROSA26-Flpe mice to remove the PGKneo cassette to generate the Pgrmc2 floxed (Pgrmc2fl/fl) founder mice. Conditional Pgrmc2 ablated (Pgrmc2d/d) mice were produced by crossing Pgrmc2fl/fl (control) mice with Pgrcre/+ mice (32). The floxed Pgrmc1 allele was then included in the breeding scheme to generate Pgrmc1/2fl/fl and Pgrmc1/2d/d mice. Following DNA isolation from tail snips, PCR was completed to detect the presence of the floxed Pgrmc1 and/or Pgrmc2 allele(s) and cre recombinase using primer sets shown in Table 1.
Table 1.
PCR Primers
| Gene Identifier | Primer Sequence |
|---|---|
| Pgr-cre P1 | 5′-ATGTTTAGCTGGCCCAAATG-3′ |
| Pgr-cre P2 | 5′-TATACCGATCTCCCTGGACG-3′ |
| Pgr-cre P3 | 5′-CCCAAAGAGACACCAGGAAG-3′ |
| Pgrmc1_lox_gt F | 5′-GGCTCAAGCACCCAGAATAG-3′ |
| Pgrmc1_lox_gt R | 5′-GCTTCCTTGCTTTCAACACC-3′ |
| Pgrmc2_lox_gt F | 5′-ATGGTGGATCATAACCATCTG-3′ |
| Pgrmc2_lox_gt R | 5′-CCTTGATTTCTAAGTGAAA-3′ |
| Pgrmc2_exon2 F | 5′-GGGTCCATATGGCATCTTTG-3′ |
| Pgrmc2_exon2 R | 5′-CTTTAAACTGCATTTCCCATTCT-3′ |
| Pecam1 F | 5′-TGATGTTTCTGGAAATGATGCAGT-3′ |
| Pecam1 R | 5′-GCTCAAGGGAGGACACTTCC-3′ |
| Esr1 F | 5′-CCAAAGCCTCGGGAATG-3′ |
| Esr1 R | 5′-CTTTCTCGTTACTGCTGG-3′ |
| Pgr F | 5′-ATGGTCCTTGGAGGTCGTAA-3′ |
| Pgr R | 5′-CACCATCAGGCTCATCC-3′ |
| Rpl13a F | 5′-TTGCTTACCTGGGGCGTCT-3′ |
| Rpl13a R | 5′-CCTTTTCCTTCCGTTTCTCCTCGC-3′ |
Animals and treatments
Six-month fertility trials were completed for Pgrmc2 and Pgrmc1/2 mouse colonies on a Pgr-cre background in which 6-week-old control (Pgrmc2fl/fl and Pgrmc1/2fl/fl) and conditionally mutant (Pgrmc2d/d and Pgrmc1/2d/d) female mice were placed with males of proven fertility. Six female mice were included in each colony for each genotype. Through the duration of the fertility trials, the following information was recorded for each litter: date of birth, number of pups born, number of pups surviving to weaning, pup weights on postnatal days 5 and 21, number of days between parity, number of litters throughout the breeding trial, and number of surviving male and female pups. Female reproductive tracts were collected from mice at the end of the breeding trials, as well as from young and aged nulliparous female mice, for histological analyses.
To evaluate estrogen receptor (ESR1) and PGR expression, uterine tissues were obtained from Pgrmc1/2fl/fl and Pgrmc1/2d/d (Pgr-cre) synchronized mice under the following regimen. Sexually mature female mice were ovariectomized, rested for 1 week, and then given daily subcutaneous injections of estradiol (E2, 100 ng) diluted in sesame oil for 3 consecutive days. After a 2-day rest, mice were treated with P4 (1 mg) for 2 days and then E2 plus P4 for 1 day. Uterine tissues were collected 24 hours after the last injection and partitioned for RNA isolation and fixation in 4% paraformaldehyde (PFA) in preparation for quantitative polymerase chain reaction (qPCR) and paraffin embedding, respectively. Other mice were treated with 100 ng E2 for 2 days and then, after 4 days, were treated with 50 ng E2 and collected 18 h later. Uterine tissues were collected for RNA isolation in preparation for qPCR and 4% PFA for histology. Expression of the vascular marker PECAM1 was evaluated in estrogen-treated mice. Here, female Pgrmc1fl/fl and Pgrmc1/2d/d female mice were ovariectomized and allowed to rest for 1 week. The mice were then given 3 single daily injections of estradiol (100 ng) diluted in sesame oil. Uteri were collected and processed for PECAM1 immunohistochemistry and reverse transcription PCR.
To evaluate implantation sites and serum hormone levels during early pregnancy, Pgrmc2fl/fl and Pgrmc2d/d females were bred with intact males of proven breeding capacity. Observation of a vaginal plug indicated day of pregnancy (DOP) 0.5. Blood was collected on DOP7.5 and 10.5 for E2 and P4 serum analysis, respectively, using the University of Virginia School of Medicine Ligand Assay and Analysis Core. Reproductive tracts were collected from these same animals, and the number of normal and resorbed implantation sites was counted. Implantation sites were also isolated from Pgrmc2fl/fl and Pgrmc2d/d female mice on DOP7.5 for evaluation of the vascular marker PECAM1. Implantation sites and ovaries were fixed in 4% PFA and processed for histological analysis and DOP6 for evaluation of DBA lectin.
RNA isolation and qPCR
PGRMC1 was previously demonstrated to be conditionally deleted from uteri, but not livers, of Pgrmc1d/d mice when using Pgr-cre driver mice (30). To next confirm conditional deletion of Pgrmc2 from uteri, total cellular RNA was isolated from uteri and livers of E2-synchronized Pgrmc1/2fl/fl and Pgrmc1/2d/d mice using TRI-Reagent (Sigma, St. Louis, MO). RNA samples were subjected to DNAse I digestion (Promega, Madison, WI), and complementary DNA was synthesized with SuperScript II (Life Technologies, Carlsbad, CA) primer reverse transcription and oligo-dT or iScript Reverse Transcription Supermix (BioRad). qPCR was first performed to compare expression of Pgrmc2 in complementary DNA samples generated from uteri and livers of Pgrmc1/2fl/fl and Pgrmc1/2d/d mice. Rpl13a was included for normalization. Primers were designed to amplify a region in exon 2 of Pgrmc2 as listed in Table 1. Esr1 and Pgr were similarly evaluated in the E2+P4–treated tissues. The vascular marker Pecam1 was evaluated in E2-treated nongravid uteri from Pgrmc1/2fl/fl and Pgrmc1/2d/d mice, as well as in implantation sites isolated from Pgrmc2fl/fl and Pgrmc2d/d female mice on DOP7.5. A negative control (no reverse transcription) was included to confirm the absence of genomic DNA.
Histology and immunohistochemistry
All tissues were fixed in 4% paraformaldehyde and stored in 70% ethanol until paraffin embedding. Tissues were processed through an ethanol gradient and xylenes, embedded in paraffin, and sectioned at 5 µm. Tissue sections were deparaffinized in xylenes and rehydrated in a series of decreasing ethanol washes. Picric acid stain or hematoxylin and eosin staining (Scytek HAE-1-IFU kit, Logan, UT) were used for analysis of tissue architecture, including visualization of cytoplasmic vs nuclear morphology and histopathology analysis.
For immunohistochemistry, rehydrated tissue sections underwent quenching (10 minutes in 8% hydrogen peroxide) and antigen retrieval (boiling for 3 minutes in 0.1M sodium citrate followed by incubation in the heated solution for 20 minutes and then cooling to room temperature). Sections were then blocked (0.1% bovine serum albumin, 0.1% normal goat serum, and 1% Triton-X100 in phosphate-buffered saline) for 1 hour at room temperature and incubated overnight at 4°C in blocking solution containing primary antibody as outlined in Table 2. Slides were then washed in phosphate-buffered saline (3 x 10 minutes) and incubated with biotinylated secondary antibody for 45 minutes at room temperature. Slides were washed as before and then incubated with horseradish peroxidase–conjugated streptavidin (Vector Laboratories, Burlingame, CA). After a third series of washes, sections were incubated with 3,3′-diaminobenzidine substrate (BD Biosciences, San Diego, CA). Sections were counterstained with hematoxylin, dehydrated in an ethanol gradient and xylenes, and mounted. The specificity of the secondary antibody was confirmed by incubating some sections with nonimmune immunoglobulin G or omitting the primary antibody. The E2+P4–synchronized uteri were stained for ESR1 and PGR expression using the antibodies listed in Table 2. Uterine natural killer cell recruitment to the uterine mesometrial compartment of Pgrmc2fl/fl and Pgrmc2d/d female mice on DOP6 was evaluated by histochemical staining using biotinylated Dolichos biflorus agglutinin (DBA) lectin (Sigma). Thin sections were prepared as described earlier for immunohistochemistry with the exception that primary and secondary antibodies were replaced by incubation with DBA lectin (10 µg/mL) for 1 hour at room temperature.
Table 2.
Antibodies
| Target | Clone | Manufacturer, Catalog No. (Lot No.) | Species, Clonal Status | Dilution |
|---|---|---|---|---|
| ESR1 | MC-20 | Santa Cruz, SC-542 (I1112) | rabbit, polyclonal | 1:300 |
| PGR | SP2 | Thermo, RM-9102 (9102s1007Z) | rabbit, monoclonal | 1:200 |
| PECAM1 | D8V9E | Cell Signaling, CS-776995 | rabbit, monoclonal | 1:100 |
| Anti-rabbit immunoglobulin G | — | Santa Cruz, SC-2040 | goat, polyclonal | 1:1000 |
| DBA lectin | — | Sigma, L6533 | — | 10 µg/mL |
Transmission Electron Microscopy
For transmission electron microscopy (TEM), tissues were fixed in 2.5% glutaraldehyde in 0.1M phosphate overnight at 4°C, rinsed in 0.1M phosphate, and postfixed in 1% osmium tetroxide overnight. Tissues were then dehydrated with a decreasing gradient of ethanol, infiltrated with acetone:Spurr reagent overnight followed by Spurr overnight, and embedded in resin. Finally, the resin was polymerized and cured by baking at 65°C overnight. Tissues were then sectioned, and TEM images were taken at the Franceschi Microscopy and Imaging Center at Washington State University on a FEI Tecnai G2 20 Twin equipped with a 200-kV LaB6 electron source and a 4K Eagle camera.
Data analyses
Animals were randomly assigned to the various treatment groups. Unless otherwise noted, all data are presented as the mean ± standard error of the mean for n = 3 to 6. Individual animals represent a single experimental replicate within each experiment. Differences between treatment groups were assessed by Student t test, where the mean values of 2 groups were compared. A 2-way analysis of variance was used to identify treatment effects in the breeding trials followed by a Bonferroni posttest. All data were analyzed using GraphPad 5.0 software (San Diego, CA) where P ≤ 0.05 was considered statistically significant.
Results
Conditional ablation of Pgrmc2 or Pgrmc1/2 results in postimplantation embryonic death
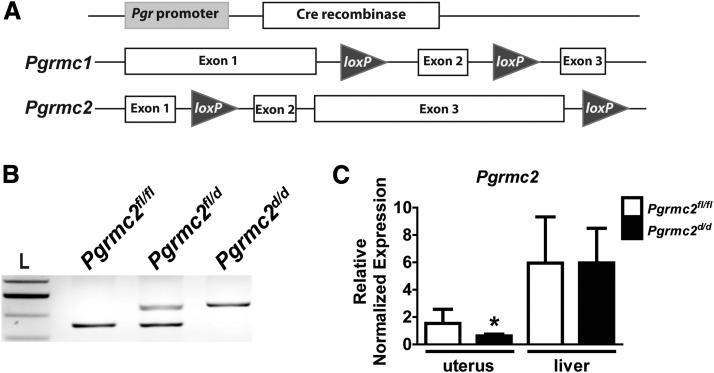
Because PGRMC1 and PGRMC2 are expressed and regulated in the uterus, we hypothesized that they play an essential role in normal pregnancy. To test this hypothesis, the Pgrmc2 allele was floxed by insertion of loxP sites before exon 2 and after exon 3 [Fig. 1(A)]. Pgrmc2 alone or in combination with Pgrmc1 was conditionally ablated from the female reproductive tract using the tissue-specific Pgr-cre transgenic mouse. A PCR-based genotyping protocol was developed to identify control (Pgrmc2fl/fl), heterozygous (Pgrmc2fl/d), and conditional knockout (Pgrmc2d/d) mice [Fig. 1(B); see primer sequences in Table 1]. A similar genotyping strategy was previously reported for identifying the floxed Pgrmc1 allele (30). Conditional ablation of PGRMC1 from the uterus was previously confirmed by Western blot analysis (30). Here, conditional ablation of Pgrmc2 was confirmed by qPCR in the uterus vs the liver of Pgrmc1/2fl/fl and Pgrmc1/2d/d mice [Fig. 1(C)].
Figure 1.
Floxing the Pgrmc2 allele. (A) Schematic of conditional mutagenesis approach used to ablate floxed regions of the Pgrmc1 and Pgrmc2 genes. (B) A PCR-based genotyping protocol was developed to identify control (Pgrmc2fl/fl), heterozygous (Pgrmc2fl/d), and conditional knockout (Pgrmc2d/d) mice. (C) qPCR showing that Pgrmc2 expression is reduced in uterine tissue isolated from Pgrmc2d/d female mice compared with tissue obtained from Pgrmc2fl/fl mice. Pgrmc2 expression does not change in the liver regardless of genotype (*P < 0.05, n = 3). L, ladder.
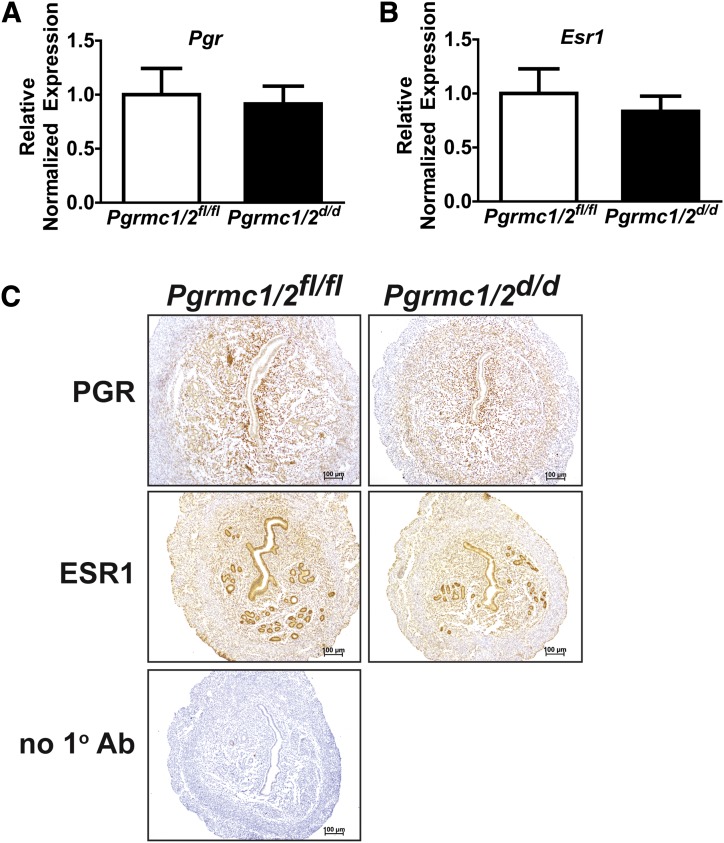
To confirm that any phenotype resulting from the dual ablation of Pgrmc1 and Pgrmc2 was due to depletion of these receptors and not the classical sex steroid hormone receptors, we compared the expression of PGR and ESR1 in uterine tissues isolated from synchronized Pgrmc1/2fl/fl and Pgrmc1/2d/d female mice by qPCR and immunohistochemistry. As shown in Fig. 2, there was no difference in the expression of Pgr and Esr1 messenger RNAs or proteins in uterine tissues isolated from synchronized Pgrmc1/2fl/fl and Pgrmc1/2d/d female mice.
Figure 2.
Conditional ablation of Pgrmc1/2 does not alter uterine expression of the PGR and ESR1. qPCR was used to compare the messenger RNA expression of (A) Pgr and (B) Esr1 in uterine tissues isolated from ovariectomized E2+P4–synchronized Pgrmc1/2fl/fl and Pgrmc1/2d/d female mice (n = 3). (C) Immunohistochemistry shows a comparable expression pattern for PGR and ESR1 protein between Pgrmc1/2fl/fl and Pgrmc1/2d/d ovariectomized E2+P4–synchronized female mice (n = 4). Ab, antibody.
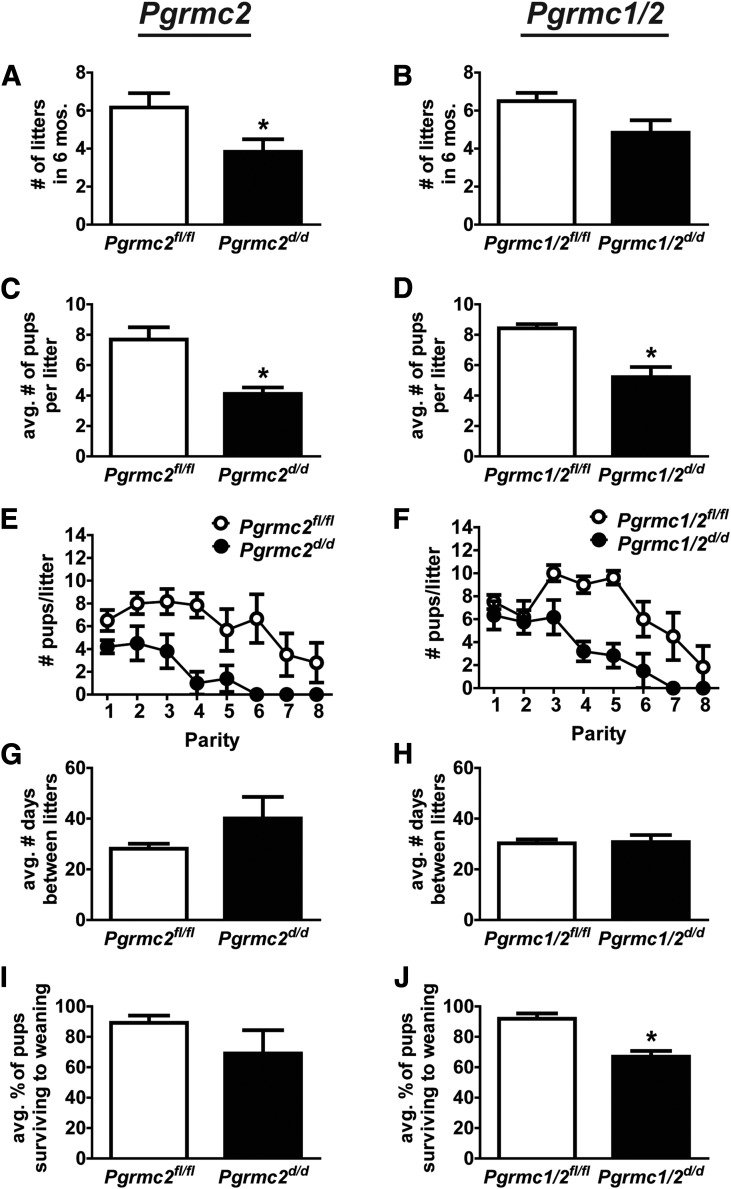
A 6-month breeding trial was conducted in which 6-week-old Pgrmc2fl/fl, Pgrmc2d/d, Pgrmc1/2fl/fl, and Pgrmc1/2d/d female mice (n = 6 per genotype) were individually placed with males of proven breeding capacity. The total number of pups produced during the breeding trial was significantly decreased in Pgrmc2d/d and Pgrmc1/2d/d female mice compared with their control counterparts (Tables 3 and 4). Overall, Pgrmc2fl/fl female mice generated an average of 47.67 ± 8.06 pups during the trial, while Pgrmc2d/d female mice had 13.5 ± 2.705 pups (n = 6, P = 0.0024). Similarly, Pgrmc1/2fl/fl females had 51.33 ± 3.70 pups and Pgrmc1/2d/d females had 22.83 ± 4.51 pups (n = 6, P = 0.0006). Pgrmc2d/d female mice had fewer litters than Pgrmc2fl/fl mice, and this also tended (P = 0.06) to be the case for Pgrmc1/2d/d vs Pgrmc1/2fl/fl mice [Fig. 3(A) and 3(B)]. Pgrmc2d/d mice had fewer pups/litter than Pgrmc2fl/fl mice, and this was also the case for Pgrmc1/2d/d vs Pgrmc1/2fl/fl [Fig. 3(C) and 3(D)]. As shown in Fig. 3(E) and 3(F) where the number of pups/litter is plotted against parity, conditional ablation of Pgrmc2 or Pgrmc1/2 resulted in a steady decline in fecundity to the point of premature reproductive senescence. Although all Pgrmc2d/d female mice experienced a significant reduction in lifetime fecundity, 50% of the Pgrmc2d/d mice became infertile after just the third parity. The decreased number of litters in the conditional mutant mice likely stems from faulty uterine function rather than ovarian function given that the parturition interval between control and conditional mutant mice was not different [Fig. 3(G) and 3(H)]. Furthermore, conditional ablation of Pgrmc2 or Pgrmc1/2 likely did not affect prolactin production by the pituitary as indirectly assessed by 2 measures. First, an evaluation of pup weight at the time of weaning, an index of lactation efficiency, demonstrated that there was no difference (P = 0.449) in weanling weights from Pgrmc1/2fl/fl (8.92 ± 0.621 g) and Pgrmc1/2d/d (8.04 ± 0.892 g) mothers. Second, corpora lutea (CL), which require an intact prolactin:prolactin receptor signaling pathway for P4 production, functioned normally in that serum P4 levels were not decreased during pregnancy (Fig. 4).
Table 3.
Fertility Trial From Pgrmc2 Colony
| Genotype | Females (n) | Litters (n) | Pups (n) | Average Pups/Litter | Average Litters/Female |
|---|---|---|---|---|---|
| Pgrmc2fl/fl | 6 | 37 | 286 | 7.69 ± 0.80 | 6.16 ± 0.75 |
| Pgrmc2d/d | 6 | 23 | 81 | 4.11 ± 0.43a | 3.83 ± 0.65b |
P = 0.002 vs Pgrmc2fl/fl.
P < 0.05 vs Pgrmc2fl/fl.
Table 4.
Fertility Trial From Pgrmc1/2 Colony
| Genotype | Females (n) | Litters (n) | Pups (n) | Average Pups/Litter | Average Litters/Female |
|---|---|---|---|---|---|
| Pgrmc1/2fl/fl | 6 | 39 | 308 | 8.43 ± 0.28 | 6.5 ± 0.42 |
| Pgrmc1/2d/d | 6 | 29 | 137 | 5.21 ± 0.67a | 4.83 ± 0.65b |
P = 0.0007 vs Pgrmc1/2fl/fl.
P < 0.05 vs Pgrmc1/2fl/fl.
Figure 3.
Ablation of Pgrmc2 or Pgrmc1/2 causes subfertility that prematurely progresses to infertility. In a 6-month breeding trial, the (A, B) number of pups/litter and (C, D) total number of litters during the 6-month trial were determined in Pgrmc2fl/fl, Pgrmc2d/d, Pgrmc1/2fl/fl, and Pgrmc1/2d/d female mice (n = 6 per genotype). (E, F) The number of pups/litter was plotted against parity to highlight the premature transition from subfertility to infertility. (G, H) The parturition interval did not differ between floxed control and corresponding conditional mutant mice (*P < 0.05). (I, J) The percentage of pups surviving to weaning.
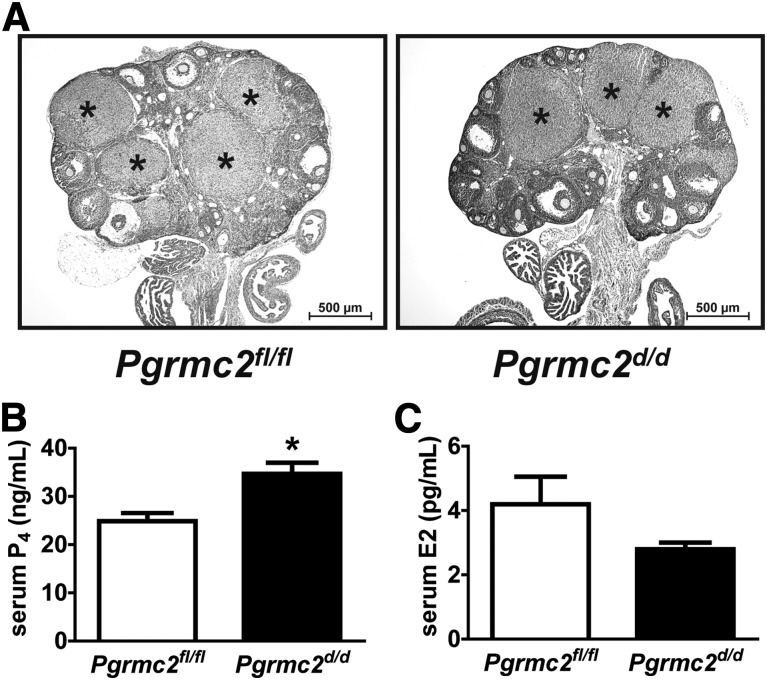
Figure 4.
Ovarian function is not compromised in Pgrmc2d/d mice. (A) Ovaries from both Pgrmc2fl/fl and Pgrmc2d/d females display CL (*) on DOP10.5. (B) Serum P4 levels were not decreased in Pgrmc2d/d female mice on DOP10.5, indicating an absence of luteal insufficiency (*P < 0.05, n = 5 to 6). (C) Serum E2 was not different between Pgrmc2fl/fl and Pgrmc2d/d female mice on DOP7.5 (n = 3).
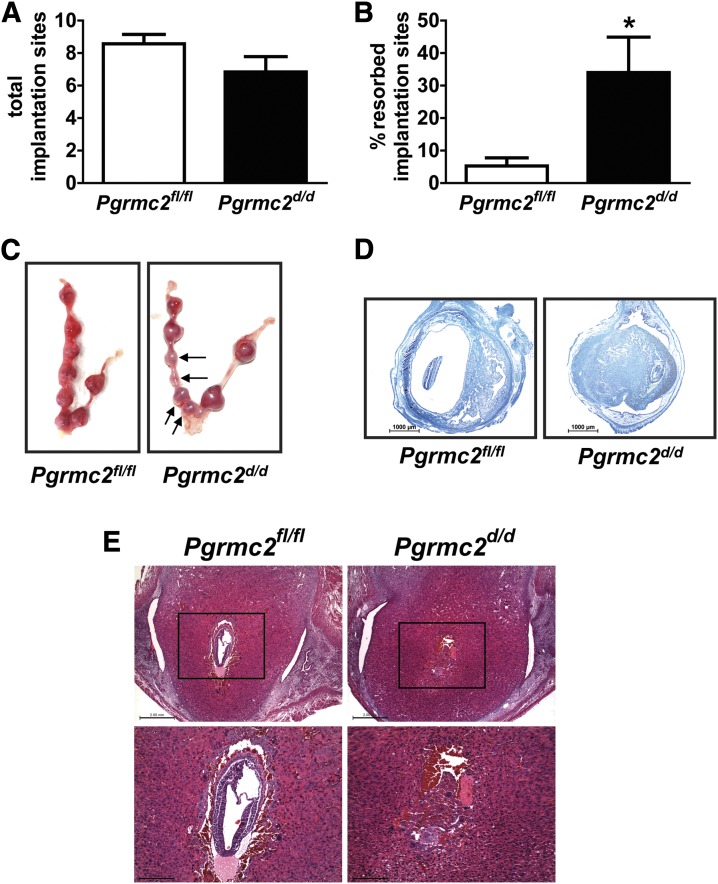
To further assess the relationship between the observed subfertility in Pgrmc2d/d mice and implantation, uterine tracts were examined on DOP10.5. There was no significant difference between Pgrmc2fl/fl and Pgrmc2d/d females in the total number of implantation sites at DOP10.5 [Fig. 5(A)], suggesting that ovulation remained intact in Pgrmc2d/d females, that fertilization occurred, and that the embryos implanted normally. However, there was a significant increase in the percentage of postimplantation resorption sites in Pgrmc2d/d female mice [Fig. 5(B) and 5(C)]. Histological examination of DOP10.5 embryos in Pgrmc2d/d females shows heavy necrosis of decidual and embryonic tissues [Fig. 5(D)]. Because the embryos had died and the sites began to resorb by DOP10.5, we evaluated implantation sites on DOP7.5. Upon histological examination, some of the embryos within sites from Pgrmc2d/d mice were not viable [Fig. 5(E)]. The embryonic demise likely does not derive from altered uterine vasculature. As shown in Supplemental Fig. 1 (23.1MB, tif) , protein and messenger RNA expression of the vascular marker PECAM1 was not different between Pgrmc1/2fl/fl and Pgrmc1/2d/d female mice in response to estradiol treatment. Likewise, there was no difference in the expression of PECAM1 between female mice on DOP7. However, a reduction in the number of uterine natural killer cells was observed in Pgrmc2d/d female mice compared with Pgrmc2fl/fl mice on DOP6, consistent with disruption in the decidualization program [Supplemental Fig. 1(B) (23.1MB, tif) ].
Figure 5.
Subfertility of Pgrmc2d/d mice is uterine in origin. (A) There was no significant difference between Pgrmc2fl/fl and Pgrmc2d/d females in the number of implantation sites at DOP10.5 (n = 6 to 7). (B) There was a sevenfold increase in postimplantation embryonic death in Pgrmc2d/d females compared with Pgrmc2fl/fl females on DOP10.5. *P < 0.05. (C) Representative images of uteri from DOP10.5 Pgrmc2fl/fl and Pgrmc2d/d showing resorption sites, as indicated by black arrows. Picric acid stain showing histology of representative (D) DOP10.5 and (E) DOP7.5 implantation sites from Pgrmc2fl/fl and Pgrmc2d/d mice.
An evaluation of ovaries isolated from DOP10.5 Pgrmc2d/d female mice revealed normal histology with large and abundant CL [Fig. 4(A)]. Pgrmc2d/d females had marginally elevated serum P4 compared with Pgrmc2fl/fl females on DOP10.5 [Fig. 4(B)]. Wild-type mice have between 25 and 40 ng/mL circulating P4 on DOP10.5 (33), so even though the Pgrmc2d/d mice have slightly elevated serum P4 compared with Pgrmc2fl/fl mice, serum P4 levels from Pgrmc2fl/fl and Pgrmc2d/d mice are within the normal range on DOP10.5. This indicates that luteal function remains intact in the Pgrmc2d/d mice. In addition, serum E2 was examined in Pgrmc2fl/fl vs Pgrmc2d/d females during early pregnancy. There was no difference in E2 between Pgrmc2fl/fl and Pgrmc2d/d females [Fig. 4(C)]. Overall, these results demonstrate that ovarian steroidogenesis in Pgrmc2d/d mice during early pregnancy is normal, thus providing further evidence that the subfertility defect stems from faulty uterine function.
Conditional ablation of Pgrmc2 or Pgrmc1/2 results in premature aging of the uterus
Because the subfertility phenotype is due to a uterine defect, we next examined uterine histology. Unlike uteri from nulliparous Pgrmc2fl/fl and Pgrmc1/2fl/fl, which appeared normal, uteri from nulliparous Pgrmc2d/fl, Pgrmc1/2d/fl, Pgrmc2d/d, and Pgrmc1/2d/d mice developed abnormal uterine histoarchitecture with increased glandular content and enlarged amorphic and cystic glands [Fig. 6(A)]. These features are commonly found in endometrium from aged (>8 months) female mice. These mice displayed varying degrees of abnormal histoarchitecture, with Pgrmc2d/d and Pgrmc1/2d/d mice having a higher overall epithelial-stromal ratio and more glandular epithelium than Pgrmc2fl/d and Pgrmc1/2fldl mice. Examination of endometrial cellular ultrastructure using TEM revealed the breakdown of basement membrane and a disrupted epithelial-stromal interface in Pgrmc1/2d/d female mice [Fig. 6(B)]. Glandular epithelial cells contained heavily vacuolated nuclei, accumulation of intracellular inclusion bodies, and excessive, highly convoluted plasma membrane.
Figure 6.
Conditional ablation of Pgrmc2 or Pgrmc1/2 results in formation of endometrial cysts after 3 months of age. (A) Hematoxylin and eosin stain showing histology of representative uterine cross sections from control (fl/fl), heterozygous (d/fl), and conditional knockout (d/d) mice at 5 to 8 months of age (n= 3 to 10 per genotype). (B) High-magnification light microscopy (a, b) and TEM (c, d) show that uteri from Pgrmc1/2d/d mice display abnormal uterine histoarchitecture. Note the (a) breakdown of basement membrane and disrupted epithelial-stromal interface in Pgrmc1/2d/d female mice, (b) heavily vacuolated nuclei in the glandular epithelium, (c) accumulation of intracellular inclusion bodies, and (d) excessive plasma membrane within the epithelial compartments. E, epithelium; L, glandular lumen; S, stroma.
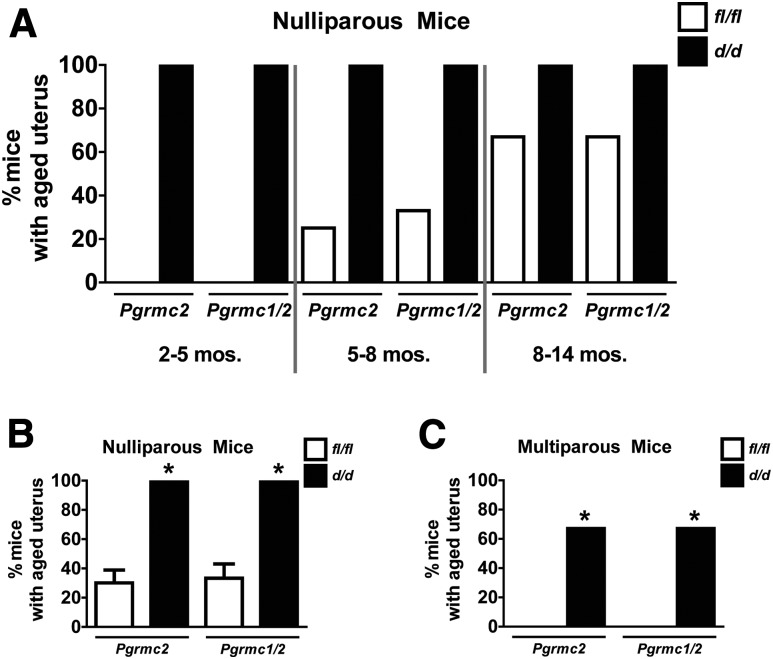
To quantify the prevalence of the cystic phenotype, uteri from nulliparous and multiparous Pgrmc2fl/fl, Pgrmc1/2fl/fl, Pgrmc2d/d, and Pgrmc1/2d/d mice of various ages were histologically examined. Some degree of abnormal uterine histoarchitecture was evident in 100% of nulliparous Pgrmc2d/d and Pgrmc1/2d/d mice by 4 to 5 months [Fig. 7(A)]. This is in contrast to control mice that showed greatly reduced development of endometrial cysts. Here, 20% to 30% of the mice developed cysts at 5 to 8 months of age. The cumulative incidence of cystic gland formation from all ages is shown in Fig. 7(B). Interestingly, parity prevented cyst formation in control mice and greatly reduced the number and size of endometrial cysts in Pgrmc2d/d and Pgrmc1/2d/d mice [Fig. 7(C)].
Figure 7.
Conditional ablation of Pgrmc2 or Pgrmc1/2 accelerates uterine aging. (A) All nulliparous Pgrmc2d/d and Pgrmc1/2d/d mice of young, medium, or older age displayed abnormal uterine histoarchitecture, including development of endometrial cysts, whereas Pgrmc2fl/fl and Pgrmc1/2fl/fl mice developed abnormal phenotype in an age-dependent manner but at a much lower prevalence and severity starting at 5 to 8 months. (B) When combining all age groups, nulliparous Pgrmc2d/d and Pgrmc1/2d/d mice have a much higher prevalence of abnormal uterine histoarchitecture compared with Pgrmc2fl/fl and Pgrmc1/2fl/fl control mice, respectively (*P < 0.0001, n = 3 to 4 per genotype per age group). (C) Sixty-seven percent of multiparous Pgrmc2d/d mice and Pgrmc1/2d/d mice displayed abnormal uterine histoarchitecture, although no multiparous Pgrmc1/2fl/fl and Pgrmc1/2d/d mice displayed abnormal uterine histoarchitecture (n = 3 to 6; *P < 0.05).
Discussion
We previously demonstrated the functional importance of the nonclassical progesterone receptor PGRMC1 in female reproduction, where conditional ablation from the uterus resulted in subfertility and formation of endometrial cysts (30). The objective of the current study was to evaluate the functional contributions of the second member of the PGRMC family, PGRMC2, to female fertility. This was achieved by floxing exons 2 and 3 of the Pgrmc2 allele. The resulting transgenic mice were crossed with Pgr-cre mice to conditionally ablate Pgrmc1 and/or Pgrmc2 from the female reproductive tract. We demonstrate here that conditional ablation of Pgrmc2 alone or in combination with Pgrmc1 from the female reproductive tract initially resulted in subfertility, and this then progressed to premature reproductive senescence. It was interesting to note that Pgrmc2d/d female mice had a more severe reproductive phenotype than Pgrmc1/2d/d female mice, suggesting that PGRMC1 deficiency may provide some protection against reproductive failure when Pgrmc2 alone is ablated. This phenotype was accompanied by development of endometrial cysts starting around 4 months of age. This is, to our knowledge, the first functional evaluation of Pgrmc2 in an in vivo setting.
Much of what is known about members of the PGRMC family derives from in vitro studies of PGRMC1. PGRMC1 is expressed in many tissues within and outside the female reproductive system, suggesting that it has both P4-dependent and P4-independent functions as previously described (34, 35). PGRMC1 and/or PGRMC2 are implicated in diverse cellular processes, including mitosis/cell cycle regulation (36, 37), meiosis (23, 38, 39), sterol metabolism (40, 41), cell survival/antiapoptotic action (18, 42), transcription (42, 43), angiogenesis (44, 45), immune regulation (46), autophagy (47), carbon monoxide sensing (48), and heme biosynthesis and transport (49). It is not clear from the current study if the fertility defect observed here is due to faulty P4 signaling or if it derives from disruption of any of the cellular processes with which PGRMC family members associate. The ability to conditionally ablate Pgrmc1 and Pgrmc2 in mice should be helpful in future studies in determining how these proteins function as P4 receptors.
The PGRMC1/2-deficient mice were generated using Pgr-cre to delete Pgrmc1 and/or Pgrmc2 from female reproductive tissues, including the hypothalamus, pituitary gonadotropes, ovarian periovulatory follicles, and the majority of the female reproductive tract. Several lines of evidence suggest that the observed subfertility/infertility phenotype in Pgrmc2d/d and Pgrmc1/2d/d mice stems from faulty uterine function and that other elements of the reproductive system remain intact. First, the parturition interval did not differ between control and mutant mice, suggesting that estrous cyclicity and the hypothalamic-pituitary unit is normal in mutant female mice when in the presence of a male with proven breeding capacity. Second, the number of implantation sites was not different between control and mutant mice, indicating that ovulation, oviductal transport, fertilization, and implantation were normal in mutant mice. Third, a direct assessment of ovarian histology showed no difference in the presence of CL in control and mutant mice. CL from mutant mice were histologically indistinguishable from those of control mice. Fourth, CL steroidogenic functions were consistent between control and mutant mice in that midgestational serum P4 and E2 levels were not different. Finally, despite a normal number of implantation sites, we noted a significant increase in postimplantation resorptions in Pgrmc2d/d and Pgrmc1/2d/d female mice, indicating that embryos are able to implant, begin development, and initiate a decidual response by the mother, but then die prior to development of the placenta around DOP6 to DOP7. With the data presented here, we cannot rule out the possibility that the progression from subfertility to infertility around parities 3 or 4 in mutant mice is caused by defects in other reproductive tissues.
As demonstrated by both qPCR and immunohistochemistry, ablation of Pgrmc1/2 did not alter the expression of the classical estrogen and progesterone receptors, indicating that the observed phenotypes in these mice do not result from disrupted ESR1 or PGR activity. The uterine defect causing embryonic death occurred after the decidualization program was fully engaged, but before placentation. Decidualized stromal cells produce nutrients and growth factors to promote embryonic growth and survival. Concurrently, pregnancy modifies maternal vasculature and the maternal immune system. Modifications of maternal vasculature facilitates the delivery of nutrients to the embryo prior to placentation, while modification of the maternal immune system provides an immune-privileged site that allows the histocompatibly distinct embryo to avoid rejection. PGRMC1/2 could be involved in regulating either of these processes. During decidualization, the uterus produces and/or acquires lipid, protein, and carbohydrate nutrients from the surrounding vasculature for the developing embryo (50), thus performing placenta-like functions. PGRMC1 may be involved in this process given that it interacts with lipid/sterol metabolic enzymes that may be involved in the production of lipid nutrients. For example, PGRMC1 participates in the biosynthesis of cholesterol and other lipids through its interactions with INSIG/Scap (41, 51, 52). Ablation of Pgrmc1/2 may prevent normal nutrient production within the decidua and transport to the embryo, thus attenuating embryonic growth and survival. The decidua produces a variety of growth factors, including vascular endothelial growth factor (VEGF), epidermal growth factor, transforming growth factor β, and others to support the embryo before placentation occurs (53). Aberrant production of such growth factors or uncoupling of their cognate receptors may contribute to the observed postimplantation embryonic loss. Interestingly, PGRMC1 has been implicated in growth factor signaling in several settings. For example, PGRMC1 has now been shown by several laboratories to be necessary for the exteriorization of the epidermal growth factor receptor (EGFR) from intracellular vesicular stores to the plasma membrane (54–56). EGFR plays an essential role in decidualization (57). It is perhaps not surprising that the Pgrmc1/2 phenotype parallels that of the Egfr conditional mutant phenotype. The impact of Pgrmc1/2 ablation on uterine decidualization is currently under investigation.
Progesterone regulates Vegf , which controls uterine decidual angiogenesis and vascular remodeling (58). Disruption of VEGF signaling leads to postimplantation embryonic death (59). PGRMC1 has been shown to mediate the influence of P4 on VEGF in breast cancer cells (45) and retinal glial cells (44), and it is feasible that PGRMC1 and/or PGRMC2 are necessary for mediating P4-dependent vascular remodeling during early pregnancy. However, preliminary evaluation of the vasculature in both the nongravid and gravid uterus suggests that vascular development is not compromised by ablation of Pgrmc1/2. Progesterone is also involved in modifying the action of many components of the maternal immune system during early pregnancy (60), and abnormalities in this process have been implicated in recurrent pregnancy loss (61). Progesterone up-regulates the expression of PGRMC1 in immune cells in pregnant cows (46). Although Pgrmc1 and Pgrmc2 are not ablated in immune cells when using Pgr-cre mice, aberrant P4 signaling in the uterus due to uterine ablation of Pgrmc1/2 may prevent the uterus from properly signaling immune cells to generate an appropriate immune-privileged environment. Of note, there was a reduction in the number of uNK cells recruited to or proliferating in the mesometrial region of the implantation site. However, it is not clear if this reduction in uNK cell numbers is a direct response to PGRMC1/2 deficiency or if it occurs indirectly because of a faulty decidualization program. Further research is needed to understand why Pgrmc2d/d and Pgrmc1/2d/d mice have reduced fecundity and if this is due to a lack of nutrients, impaired vascularization, immune rejection of the embryo, or a combination of these factors.
PGRMC1/2 are implicated in regulating apoptosis and other processes in response to P4. There are multiple mechanisms whereby PGRMC1 could mediate the actions of P4. PGRMC1 is associated with both rapid P4 actions and changes in gene expression (18, 37). Despite lacking a P4-binding domain homologous to that of the classical PGR, PGRMC1 still directly binds P4. This was most recently demonstrated using nuclear magnetic resonance spectroscopy showing that the cytochrome b5 domain indeed binds P4 (62). This study supports initial receptor binding studies conducted over 15 years ago (63, 64). PGRMC1 has also been shown to facilitate the binding of P4 to other purported P4 receptors, such as membrane progestin receptor α (mPRα) (65). PGRMC1 may mediate P4 signaling by any number of cell signaling pathways given that it harbors several SH2 and SH3 domains, tyrosine kinase binding sites, and interaction domains for ERK1, casein kinase 2, and PDK1 (17). Finally, PGRMC1 localizes to the nucleus to regulate gene expression in response to P4 (43). PGRMC1 does not have a DNA-binding domain; as such, PGRMC1 may function as a scaffold protein in a transcriptional complex. Further investigation is needed to understand the mechanisms by which PGRMC1 and PGRMC2 relay P4 signals to exert effects on the cell under normal uterine physiology, and if disruption in these pathways leads to uterine pathology.
Although Pgrmc2d/d and Pgrmc1/2d/d mice do develop endometrial cysts, this does not account for the pregnancy defect because cystic glands do not become evident until around 4 months of age despite delivery of fewer pups/litter from the first parity onward. However, the underlying molecular changes that may occur upon deletion of Pgrmc1 and/or Pgrmc2 may contribute to the initial subfertility phenotype that then later manifests in the development of cysts. The cystic glands are likely a result of faulty mesenchymal-epithelial communication. We previously demonstrated that ablation of Pgrmc1 from the stromal, but not the epithelial compartment, results in a similar phenotype to that described for Pgrmc1 ablation from both compartments (30). Another interesting observation is that conditional ablation of Pgrmc1, Pgrmc2, or Pgrmc1/2 results in a similar, if not identical, histological phenotype. This finding suggests that PGRMC1 and PGRMC2 function distinctly within a similar pathway to maintain normal tissue homeostasis. These glands are indicative of bland endometrial cysts in women commonly associated with atrophic endometria or senile polyps (66). Such cysts, although smaller and less frequent than in Pgrmc1d/d, Pgrmc2d/d, and Pgrmc1/2d/d mice, are generally found in most mouse strains as part of the aging process. As such, the occurrence of endometrial cysts at an early age in Pgrmc1d/d, Pgrmc2d/d, and Pgrmc1/2d/d mice is consistent with a premature aging phenotype. The observed premature reproductive senescence may therefore more aptly be defined as premature uterine senescence.
Acknowledgments
We thank the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core, supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health P50 Grant HD28934, for performing the serum steroid hormone assays. We thank the Franceschi Microscopy and Imaging Center at Washington State University for assisting with the TEM. We are grateful for funding support from the National Institutes of Health through RR030264 and OD016564.
Acknowledgments
This work was supported by the National Institutes of Health Grants RR030264 and OD016564.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CL
- corpora lutea
- DBA
- Dolichos biflorus agglutinin
- DOP
- day of pregnancy
- EGFR
- epidermal growth factor receptor
- ES
- embryonic stem
- PCR
- polymerase chain reaction
- PFA
- paraformaldehyde
- PGR
- progesterone receptor
- PGRMC
- progesterone receptor membrane component
- qPCR
- quantitative polymerase chain reaction
- TEM
- transmission electron microscopy
- VEGF
- vascular endothelial growth factor
References
- 1.Kim JJ, Kurita T, Bulun SE. Progesterone action in endometrial cancer, endometriosis, uterine fibroids, and breast cancer. Endocr Rev. 2013;34(1):130–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab. 2011;22(4):145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szekeres-Bartho J, Barakonyi A, Par G, Polgar B, Palkovics T, Szereday L. Progesterone as an immunomodulatory molecule. Int Immunopharmacol. 2001;1(6):1037–1048. [DOI] [PubMed] [Google Scholar]
- 4.Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform B (PR-B) in endometriosis. Epigenetics. 2006;1(2):106–111. [DOI] [PubMed] [Google Scholar]
- 5.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148(8):3814–3826. [DOI] [PubMed] [Google Scholar]
- 6.Ehn NL, Cooper ME, Orr K, Shi M, Johnson MK, Caprau D, Dagle J, Steffen K, Johnson K, Marazita ML, Merrill D, Murray JC. Evaluation of fetal and maternal genetic variation in the progesterone receptor gene for contributions to preterm birth. Pediatr Res. 2007;62(5):630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ito K, Utsunomiya H, Yaegashi N, Sasano H. Biological roles of estrogen and progesterone in human endometrial carcinoma: new developments in potential endocrine therapy for endometrial cancer. Endocr J. 2007;54(5):667–679. [DOI] [PubMed] [Google Scholar]
- 8.Salazar EL, Calzada L. The role of progesterone in endometrial estradiol- and progesterone-receptor synthesis in women with menstrual disorders and habitual abortion. Gynecol Endocrinol. 2007;23(4):222–225. [DOI] [PubMed] [Google Scholar]
- 9.Boruban MC, Altundag K, Kilic GS, Blankstein J. From endometrial hyperplasia to endometrial cancer: insight into the biology and possible medical preventive measures. Eur J Cancer Prev. 2008;17(2):133–138. [DOI] [PubMed] [Google Scholar]
- 10. Infertility. Available at: www.cdc.gov/nchs/fastats/infertility.htm. Accessed 23 September 2016.
- 11. Endometriosis: a guide for patients. American Society for Reproductive Medicine; 2012. Available at: www.asrm.org/booklet_endometriosis. Accessed 21 September 2016.
- 12.Tang YT, Hu T, Arterburn M, Boyle B, Bright JM, Emtage PC, Funk WD. PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif. J Mol Evol. 2005;61(3):372–380. [DOI] [PubMed] [Google Scholar]
- 13.Thomas P. Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions. Front Neuroendocrinol. 2008;29(2):292–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pru JK, Clark NC. PGRMC1 and PGRMC2 in uterine physiology and disease. Front Neurosci. 2013;7:168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerdes D, Wehling M, Leube B, Falkenstein E. Cloning and tissue expression of two putative steroid membrane receptors. Biol Chem. 1998;379(7):907–911. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Sargent C, Quilter C, Yang Z, Ren J, Affara N, Brenig B, Huang L. Cloning, mapping and molecular characterization of porcine progesterone receptor membrane component 2 (PGRMC2) gene. Genet Mol Biol. 2010;33(3):471–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahill MA. Progesterone receptor membrane component 1: an integrative review. J Steroid Biochem Mol Biol. 2007;105(1-5):16–36. [DOI] [PubMed] [Google Scholar]
- 18.Peluso JJ, Pappalardo A, Losel R, Wehling M. Progesterone membrane receptor component 1 expression in the immature rat ovary and its role in mediating progesterone’s antiapoptotic action. Endocrinology. 2006;147(6):3133–3140. [DOI] [PubMed] [Google Scholar]
- 19.Kowalik MK, Kotwica J. Progesterone receptor membrane component 1 (PGRMC1) gene expression in corpus luteum during the estrous cycle in cows. Reprod Biol. 2008;8(3):291–297. [DOI] [PubMed] [Google Scholar]
- 20.Luciano AM, Corbani D, Lodde V, Tessaro I, Franciosi F, Peluso JJ, Modina S. Expression of progesterone receptor membrane component-1 in bovine reproductive system during estrous cycle. Eur J Histochem. 2011;55(3):e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahir MZ, Reynaud K, Mawa G, Thoumire S, Chastant-Maillard S, Saint-Dizier M. Immunolocalization of progesterone receptors in the canine oviduct around ovulation. Reprod Domest Anim. 2012;47(Suppl 6):35–39. [DOI] [PubMed] [Google Scholar]
- 22.Saint-Dizier M, Sandra O, Ployart S, Chebrout M, Constant F. Expression of nuclear progesterone receptor and progesterone receptor membrane components 1 and 2 in the oviduct of cyclic and pregnant cows during the post-ovulation period. Reprod Biol Endocrinol. 2012;10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luciano AM, Lodde V, Franciosi F, Ceciliani F, Peluso JJ. Progesterone receptor membrane component 1 expression and putative function in bovine oocyte maturation, fertilization, and early embryonic development. Reproduction. 2010;140(5):663–672. [DOI] [PubMed] [Google Scholar]
- 24.Rambags BP, van Tol HT, van den Eng MM, Colenbrander B, Stout TA. Expression of progesterone and oestrogen receptors by early intrauterine equine conceptuses. Theriogenology. 2008;69(3):366–375. [DOI] [PubMed] [Google Scholar]
- 25.Kowalik MK, Slonina D, Rekawiecki R, Kotwica J. Expression of progesterone receptor membrane component (PGRMC) 1 and 2, serpine mRNA binding protein 1 (SERBP1) and nuclear progesterone receptor (PGR) in the bovine endometrium during the estrous cycle and the first trimester of pregnancy. Reprod Biol. 2013;13(1):15–23. [DOI] [PubMed] [Google Scholar]
- 26.Slonina D, Kowalik MK, Kotwica J. Expression of progesterone receptor membrane component 1, serpine mRNA binding protein 1 and nuclear progesterone receptor isoforms A and B in the bovine myometrium during the estrous cycle and early pregnancy. J Reprod Dev. 2012;58(3):288–294. [DOI] [PubMed] [Google Scholar]
- 27.Wu W, Shi SQ, Huang HJ, Balducci J, Garfield RE. Changes in PGRMC1, a potential progesterone receptor, in human myometrium during pregnancy and labour at term and preterm. Mol Hum Reprod. 2011;17(4):233–242. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, Kanda Y, Roberts DJ, Ecker JL, Losel R, Wehling M, Peluso JJ, Pru JK. Expression of progesterone receptor membrane component 1 and its partner serpine 1 mRNA binding protein in uterine and placental tissues of the mouse and human. Mol Cell Endocrinol. 2008;287(1-2):81–89. [DOI] [PubMed] [Google Scholar]
- 29.Feng L, Antczak BC, Lan L, Grotegut CA, Thompson JL, Allen TK, Murtha AP. Progesterone receptor membrane component 1 (PGRMC1) expression in fetal membranes among women with preterm premature rupture of the membranes (PPROM). Placenta. 2014;35(5):331–333. [DOI] [PubMed] [Google Scholar]
- 30.McCallum ML, Pru CA, Niikura Y, Yee SP, Lydon JP, Peluso JJ, Pru JK. Conditional ablation of progesterone receptor membrane component 1 results in subfertility in the female and development of endometrial cysts. Endocrinology. 2016;157(9):3309–3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee EC, Yu D, Martinez de Velasco J, Tessarollo L, Swing DA, Court DL, Jenkins NA, Copeland NG. A highly efficient Escherichia coli-based chromosome engineering system adapted for recombinogenic targeting and subcloning of BAC DNA. Genomics. 2001;73(1):56–65. [DOI] [PubMed] [Google Scholar]
- 32.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. [DOI] [PubMed] [Google Scholar]
- 33.Piekorz RP, Gingras S, Hoffmeyer A, Ihle JN, Weinstein Y. Regulation of progesterone levels during pregnancy and parturition by signal transducer and activator of transcription 5 and 20alpha-hydroxysteroid dehydrogenase. Mol Endocrinol. 2005;19(2):431–440. [DOI] [PubMed] [Google Scholar]
- 34.Lösel RM, Besong D, Peluso JJ, Wehling M. Progesterone receptor membrane component 1: many tasks for a versatile protein. Steroids. 2008;73(9-10):929–934. [DOI] [PubMed] [Google Scholar]
- 35.Peluso JJ, Pru JK. Non-canonical progesterone signaling in granulosa cell function. Reproduction. 2014;147(5):R169–R178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, Wang J, Zhao L, Nilsen J, McClure K, Wong K, Brinton RD. Progesterone increases rat neural progenitor cell cycle gene expression and proliferation via extracellularly regulated kinase and progesterone receptor membrane components 1 and 2. Endocrinology. 2009;150(7):3186–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Griffin D, Liu X, Pru C, Pru JK, Peluso JJ. Expression of progesterone receptor membrane component-2 within the immature rat ovary and its role in regulating mitosis and apoptosis of spontaneously immortalized granulosa cells. Biol Reprod. 2014;91(2):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terzaghi L, Tessaro I, Raucci F, Merico V, Mazzini G, Garagna S, Zuccotti M, Franciosi F, Lodde V. PGRMC1 participates in late events of bovine granulosa cells mitosis and oocyte meiosis. Cell Cycle. 2016;15(15):2019–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luciano AM, Peluso JJ. PGRMC1 and the faithful progression through mitosis and meiosis. Cell Cycle. 2016;15(17):2239–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura I, Nakayama Y, Konishi M, Terasawa K, Ohta M, Itoh N, Fujimoto M. Functions of MAPR (membrane-associated progesterone receptor) family members as heme/steroid-binding proteins. Curr Protein Pept Sci. 2012;13(7):687–696. [DOI] [PubMed] [Google Scholar]
- 41.Hughes AL, Powell DW, Bard M, Eckstein J, Barbuch R, Link AJ, Espenshade PJ. Dap1/PGRMC1 binds and regulates cytochrome P450 enzymes. Cell Metab. 2007;5(2):143–149. [DOI] [PubMed] [Google Scholar]
- 42.Peluso JJ, Liu X, Gawkowska A, Lodde V, Wu CA. Progesterone inhibits apoptosis in part by PGRMC1-regulated gene expression. Mol Cell Endocrinol. 2010;320(1-2):153–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peluso JJ, Lodde V, Liu X. Progesterone regulation of progesterone receptor membrane component 1 (PGRMC1) sumoylation and transcriptional activity in spontaneously immortalized granulosa cells. Endocrinology. 2012;153(8):3929–3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Swiatek-De Lange M, Stampfl A, Hauck SM, Zischka H, Gloeckner CJ, Deeg CA, Ueffing M. Membrane-initiated effects of progesterone on calcium dependent signaling and activation of VEGF gene expression in retinal glial cells. Glia. 2007;55(10):1061–1073. [DOI] [PubMed] [Google Scholar]
- 45.Neubauer H, Adam G, Seeger H, Mueck AO, Solomayer E, Wallwiener D, Cahill MA, Fehm T. Membrane-initiated effects of progesterone on proliferation and activation of VEGF in breast cancer cells. Climacteric. 2009;12(3):230–239. [DOI] [PubMed] [Google Scholar]
- 46.Maeda Y, Ohtsuka H, Tomioka M, Oikawa M. Effect of progesterone on Th1/Th2/Th17 and regulatory T cell-related genes in peripheral blood mononuclear cells during pregnancy in cows. Vet Res Commun. 2013;37(1):43–49. [DOI] [PubMed] [Google Scholar]
- 47.Mir SU, Schwarze SR, Jin L, Zhang J, Friend W, Miriyala S, St Clair D, Craven RJ. Progesterone receptor membrane component 1/Sigma-2 receptor associates with MAP1LC3B and promotes autophagy. Autophagy. 2013;9(10):1566–1578. [DOI] [PubMed] [Google Scholar]
- 48.Kabe Y, Nakane T, Koike I, Yamamoto T, Sugiura Y, Harada E, Sugase K, Shimamura T, Ohmura M, Muraoka K, Yamamoto A, Uchida T, Iwata S, Yamaguchi Y, Krayukhina E, Noda M, Handa H, Ishimori K, Uchiyama S, Kobayashi T, Suematsu M. Haem-dependent dimerization of PGRMC1/Sigma-2 receptor facilitates cancer proliferation and chemoresistance. Nat Commun. 2016;7:11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piel RB III, Shiferaw MT, Vashisht AA, Marcero JR, Praissman JL, Phillips JD, Wohlschlegel JA, Medlock AE. A novel role for progesterone receptor membrane component 1 (PGRMC1): A partner and regulator of ferrochelatase. Biochemistry. 2016;55(37):5204–5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bazer FW, Wu G, Johnson GA, Kim J, Song G. Uterine histotroph and conceptus development: select nutrients and secreted phosphoprotein 1 affect mechanistic target of rapamycin cell signaling in ewes. Biol Reprod. 2011;85(6):1094–1107. [DOI] [PubMed] [Google Scholar]
- 51.Suchanek M, Radzikowska A, Thiele C. Photo-leucine and photo-methionine allow identification of protein-protein interactions in living cells. Nat Methods. 2005;2(4):261–267. [DOI] [PubMed] [Google Scholar]
- 52.Rohe HJ, Ahmed IS, Twist KE, Craven RJ. PGRMC1 (progesterone receptor membrane component 1): a targetable protein with multiple functions in steroid signaling, P450 activation and drug binding. Pharmacol Ther. 2009;121(1):14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hempstock J, Cindrova-Davies T, Jauniaux E, Burton GJ. Endometrial glands as a source of nutrients, growth factors and cytokines during the first trimester of human pregnancy: a morphological and immunohistochemical study. Reprod Biol Endocrinol. 2004;2:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ahmed IS, Rohe HJ, Twist KE, Craven RJ. Pgrmc1 (progesterone receptor membrane component 1) associates with epidermal growth factor receptor and regulates erlotinib sensitivity. J Biol Chem. 2010;285(32):24775–24782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hampton KK, Craven RJ. Pathways driving the endocytosis of mutant and wild-type EGFR in cancer. Oncoscience. 2014;1(8):504–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Aizen J, Thomas P. Role of Pgrmc1 in estrogen maintenance of meiotic arrest in zebrafish oocytes through Gper/Egfr. J Endocrinol. 2015;225(1):59–68. [DOI] [PubMed] [Google Scholar]
- 57.Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, Jeong JW, DeMayo FJ. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet. 2014;10(6):e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim M, Park HJ, Seol JW, Jang JY, Cho YS, Kim KR, Choi Y, Lydon JP, Demayo FJ, Shibuya M, Ferrara N, Sung HK, Nagy A, Alitalo K, Koh GY. VEGF-A regulated by progesterone governs uterine angiogenesis and vascular remodelling during pregnancy. EMBO Mol Med. 2013;5(9):1415–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.García-Pascual CM, Ferrero H, Zimmermann RC, Simón C, Pellicer A, Gómez R. Inhibition of Delta-like 4 mediated signaling induces abortion in mice due to deregulation of decidual angiogenesis. Placenta. 2014;35(7):501–508. [DOI] [PubMed] [Google Scholar]
- 60.Schumacher A, Costa SD, Zenclussen AC. Endocrine factors modulating immune responses in pregnancy. Front Immunol. 2014;5:196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krieg S, Westphal L. Immune function and recurrent pregnancy loss. Semin Reprod Med. 2015;33(4):305–312. [DOI] [PubMed] [Google Scholar]
- 62.Kaluka D, Batabyal D, Chiang BY, Poulos TL, Yeh SR. Spectroscopic and mutagenesis studies of human PGRMC1. Biochemistry. 2015;54(8):1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Falkenstein E, Heck M, Gerdes D, Grube D, Christ M, Weigel M, Buddhikot M, Meizel S, Wehling M. Specific progesterone binding to a membrane protein and related nongenomic effects on Ca2+-fluxes in sperm. Endocrinology. 1999;140(12):5999–6002. [DOI] [PubMed] [Google Scholar]
- 64.Falkenstein E, Eisen C, Schmieding K, Krautkrämer M, Stein C, Lösel R, Wehling M. Chemical modification and structural analysis of the progesterone membrane binding protein from porcine liver membranes. Mol Cell Biochem. 2001;218(1-2):71–79. [DOI] [PubMed] [Google Scholar]
- 65.Thomas P, Pang Y, Dong J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology. 2014;155(3):1107–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mutter GL. Endometrial hyperplasia without atypia and EIN. In: Mutter GL, Prat J, eds. Pathology of the Female Reproductive Tract. 3rd ed. London, UK: Churchill Livingstone Elsevier; 2014.