Abstract
Most vertebrate organs use adult stem cells to maintain homeostasis and ensure proper repair when damaged. How such organ-specific stem cells are formed during vertebrate development is largely unexplored. We have been using the thyroid hormone (T3)–dependent amphibian metamorphosis to address this issue. Early studies in Xenopus laevis have shown that intestinal remodeling involves complete degeneration of the larval epithelium and de novo formation of adult stem cells through dedifferentiation of some larval epithelial cells. We have further discovered that the histidine ammonia-lyase (HAL; also known as histidase or histidinase)-2 gene is strongly and specifically activated by T3 in the proliferating adult stem cells of the intestine during metamorphosis, implicating a role of histidine catabolism in the development of adult intestinal stem cells. To determine the mechanism by which T3 regulates the HAL2 gene, we have carried out bioinformatics analysis and discovered a putative T3 response element (TRE) in the HAL2 gene. Importantly, we show that this TRE is bound by T3 receptor (TR) in the intestine during metamorphosis. The TRE is capable of binding to the heterodimer of TR and 9-cis retinoic acid receptor (RXR) in vitro and mediate transcriptional activation by liganded TR/RXR in frog oocytes. More importantly, the HAL2 promoter containing the TRE can drive T3-dependent reporter gene expression to mimic endogenous HAL2 expression in transgenic animals. Our results suggest that the TRE mediates the induction of HAL2 gene by T3 in the developing adult intestinal stem cells during metamorphosis.
In vitro and in vivo characterization of Xenopus tropicalis HAL2 promoter that is directly activated by liganded TR specifically in the developing adult intestinal stem cells during metamorphosis.
Intestinal remodeling during Xenopus metamorphosis is an excellent model to study the development of adult organ-specific stem cells (1–4). In the South African clawed frog Xenopus laevis and the highly related species Xenopus tropicalis, the larval intestine is a simple tubular structure consisting of a single layer of primary epithelium and thin layers of connective tissue and muscles. This simple structure is transformed into a complex one consisting of a multifolded adult epithelium surrounded by well-developed connective tissue and muscles (3, 5, 6), which resembles the adult mammalian intestine (5, 7). The remodeling of the intestine during Xenopus metamorphosis involves the degeneration of the larval epithelium through programmed cell death or apoptosis and concurrent de novo formation of the adult intestinal stem cells, which eventually give rise to the adult epithelium.
Similar to other processes during amphibian metamorphosis, intestinal remodeling, including the formation of the adult stem cells, is controlled by thyroid hormone (T3). It can be easily manipulated by controlling the availability of T3 in tadpoles or even in tadpole intestinal organ cultures (5, 8–10). Taking advantages of this model system, we and others have previously demonstrated that some cells in the larval intestinal epithelium dedifferentiate to form the adult stem cells in the presence of T3 through yet unknown mechanism (3, 11–16). Furthermore, recombinant intestinal organ culture studies using wild-type and transgenic tadpoles expressing a dominant-positive T3 receptor (TR) under the control of a heat shock–inducible promoter have revealed that T3 can induce some cells within the larval epithelium to undergo tissue-autonomous dedifferentiation into a precursor form that express sonic hedgehog, which is expressed in intestinal stem cells, but not Musashi-1, a known stem cell marker of adult mammalian intestine (13, 17). The development of the adult stem cells requires T3 signaling in both the epithelium and the rest of the intestinal tissues, most likely the connective tissue underlying the epithelium (11, 13, 17).
T3 is known to regulate metamorphosis by regulating gene expression through TRs, which regulate the expression of T3-inducible genes in a T3-dependent manner as heterodimers with 9-cis retinoic acid receptors (RXRs) (18–20). Thus, we have recently carried out genome-wide microarray analyses to identify genes that are regulated by T3 in the epithelium and the rest of the intestine during metamorphosis (21–25). Of particular interests are those genes that are highly expressed specifically in the intestinal epithelium at the climax of metamorphosis when adult stem cells are forming and proliferating. Such genes are likely to be involved in stem cell development. Among the genes with peak level expression in the intestine at the climax of metamorphosis is a Xenopus homolog of the mammalian histidine ammonia-lyase (HAL) gene (26). The mammalian HAL encodes a cytosolic enzyme known as histidase or histidinase. Histidase catalyzes the nonoxidative deamination of l-histidine to trans-urocanic acid and ammonia (http://www.ncbi.nlm.nih.gov/gene?Db=gene&Cmd=ShowDetailView&TermToSearch=3034), the first step in histidine catabolism (27–30). Histidase deficiency in human leads to histidinemia or histidinuria. Children with histidinemia often have hyperactivity, speech impediment, developmental delay, learning difficulties, and sometimes mental retardation (http://en.wikipedia.org/wiki/Histidinemia) (30–34). The mechanisms underlying the histidase deficiency–mediated pathogenesis and what roles HAL plays during development remain to be investigated.
Whereas only a single HAL gene is found in mammals, two HAL genes are present in X. laevis and X. tropicalis due to a gene duplication event after the separation of amphibians from mammals (26). Our earlier studies have shown that the two HAL genes are regulated in a tissue- and gene-dependent manner in the intestine. At the whole animal level, HAL1 is expressed during embryogenesis whereas HAL2 is expressed during metamorphosis. Importantly, in the intestine, HAL2 has no detectable expression before or after metamorphosis, but its messenger RNA (mRNA) level is highly upregulated at the climax of metamorphosis, specifically in the newly formed proliferating adult stem cells. Furthermore, HAL2 expression can be induced when premetamorphic tadpoles are treated with T3, suggesting that T3 regulates the transcription of HAL2 (26). In this study, we report the identification of a T3 response element (TRE) in the HAL2 gene that can bind to TR/RXR heterodimers in vitro and mediate T3-induced transcription in the frog oocyte transcription system. More importantly, we demonstrate that the HAL2 TRE is bound by TR in the intestine during metamorphosis and that a TRE-containing HAL2 promoter fragment can drive reporter gene expression in a T3-dependent manner in transgenic tadpoles that mimics the endogenous HAL2 expression. Thus, the HAL2 TRE likely mediates the induction of HAL2 gene by T3 in the developing adult intestinal stem cells during development.
Materials and Methods
Animals and treatments
Wild-type X. laevis or X. tropicalis tadpoles were reared in the laboratory or purchased from Nasco (Fort Atkinson, MI) and Xenopus I (Dexter, MI). Premetamorphic tadpoles at stage 54 (35) were treated with 10 nM T3 for 1 to 5 days. At least three tadpoles were analyzed for each stage or day of T3 treatment. All studies were performed as approved by the National Institute of Child Health and Human Development Animal Use and Care Committee.
Cloning of HAL2 promoter
The X. tropicalis HAL2 promoter region was first polymerase chain reaction (PCR) amplified using primers 5′-GTACCCTGCGTCGTGTACGTACACGGAG-3′ and 5′-GCTAACCATTCTCCTCGCACATGCACTG-3′. The PCR product was used for the nested PCR with primers 5′-GTCTGGGTACCCCTGAAATAAACAAACTAAAAGCATAC-3′ (bearing a KpnI site in bold letters) and 5′-GGGGACCGGTTCCAACTGATTCAAAAGATATGGTGCTCTG-3′ (bearing an AgeI site in bold letters). The PCR product was digested with KpnI and AgeI, and cloned into AcGFP1.1 vector (Clontech).
Generation of HAL2 promoter construct for transgenesis
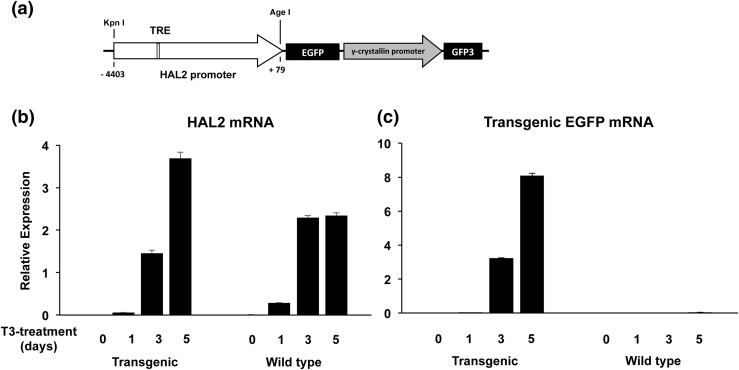
The 4.4-kb HAL2 promoter in AcGFP1.1 above was subcloned into the KpnI-AgeI digested double-promoter construct (pDPCG-SceI, a gift from Dr. D.R. Buchholz) (36, 37) to produce a transgenic construct with the HAL2 promoter driving enhanced green fluorescent protein (EGFP) expression and the γ-crystallin promoter driving GFP3 in the eyes of the animals, which allows easy identification of transgenic tadpoles [see Fig. 2(b)] (38, 39).
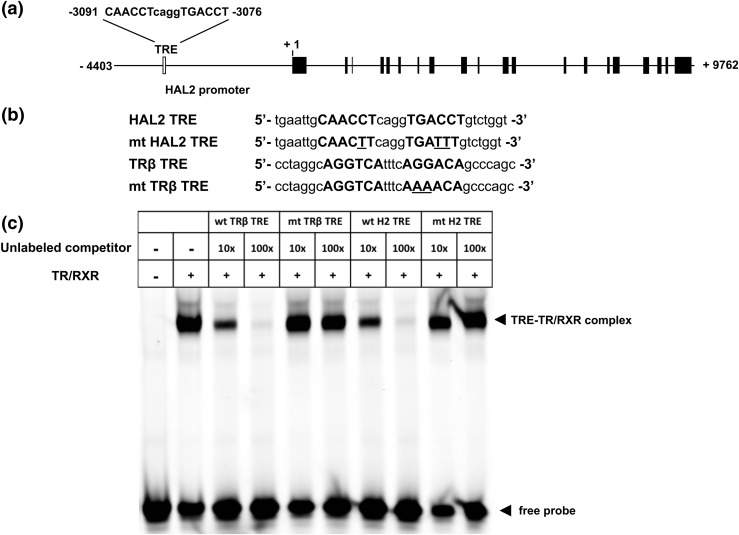
Figure 2.
The HAL2 TRE binds to TR/RXR in vitro. (a) Intron–exon organization of the HAL2 gene. The +1 indicates putative transcription start site based on reported cDNA sequence. A putative TRE is present at −3091 to −3076 with the half TRE repeat shown in capital letters. The exons are shown in filled boxes whereas the introns and upstream sequences are shown as lines. The open box represents the HAL2 TRE. (b) Sequences of wild-type and mutant (mt) TRE of X. tropicalis HAL2 and X. laevis TRβ genes. The sequences of the TRE half sites are shown in capital letters with the mutated residues in the mutant TREs underlined. (c) Wild-type (wt) but not mutant (mt) HAL2 TRE (H2 TRE) competes effectively for binding to TR/RXR in vitro. Gel mobility shift assay was carried out with labeled wild-type X. laevis TRβ TRE and in vitro–translated TR/RXR in the presence or absence of 10 to 100× excess unlabeled wild-type or mutant TREs as indicated. Note that as expected, the complex formed between the labeled, well-characterized X. laevis TRβ TRE and TR/RXR was competed effectively by the unlabeled wild-type but not the mutant TRβ TRE. Similarly, the wild-type but not the mutant HAL2 TRE was able to compete effectively, indicating that HAL2 TRE binds to TR/RXR with similar affinity as the TRβ TRE.
Generation of luciferase report constructs for transcription assay
The promoter constructs harboring pHAL2-TRE-luc or pHAL2-mtTRE-luc were generated through PCR-mediated mutagenesis on a firefly luciferase reporter construct containing X. tropicalis Dot1L promoter with mutant TREs [pmTRE(Dot1L)-luc] (40, 41). The pHAL2-TRE-luc construct was made with primer set 1, 5′-TGTTGGATGCTCATACTCGTCC-3′ (LZ631) and 5′-GCGGGAGGTCACCTGAGGTTGCTTAGCCTGAAGTCTGAGG-3′ (HAL2TRE_R), and primer set 2, 5′-CTAAGCAACCTCAGGTGACCTCCCGCGGGATTATTTATTTTATTC-3′ (HAL2TRE_F) and 5′-GGTAATGTCCACCTCGATATGTGC-3′ (LZ632), in two different PCR reactions to produce 1034-bp and 510-bp fragments, respectively. The two PCR fragments were gel purified and then mixed together as templates in a second round of PCR with primers LZ631 and LZ632 to produce an ∼1.5-kb fragment. The 1.5-kb fragment was then subjected to restriction enzyme digestion with KpnI and HindIII. The 1048-bp restricted fragment was then subcloned into KpnI-HindIII digested firefly luciferase reporter construct pmTRE(Dot1L)-luc, whereby the Dot1L mTRE in pmTRE(Dot1L)-luc was replaced with HAL2 TRE. The pHAL2-mtTRE-luc construct was made similarly with the primers 5′-GCGGGAAATCACCTGAAGTTGCTTAGCCTGAAGTCTGAGG-3′ (HAL2-mtTRE_R) and 5′-CTAAGCAACTTCAGGTGATTTCCCGCGGGATTATTTATTTTATTC-3′ (HAL2-mtTRE_F), which were used to replace the HAL2TRE_R and HAL2TRE_F, respectively, in the initial two PCR reactions.
Gel mobility shift assay
This was done as described (41). Briefly, X. laevis TRα and RXRα proteins were made using a TNT SP6 quick coupled transcription/translation system (Promega, Madison, WI). The proteins were mixed with the probe [an infrared dye IR700 (LI-COR Biosciences, Lincoln, NE)–labeled TRE of the X. laevis TRβ gene] in the in vitro binding reaction in the presence or absence of unlabeled competitors. Sequences of the sense strand of unlabeled competitor oligonucleotides are as follows: 5′-tgaattgCAACCTcaggTGACCTgtctggt-3′ for HAL2 TRE, 5′-tgaattgCAACTTcaggTGATTTgtctggt-3′ for mutant HAL2 TRE, 5′-cctaggcAGGTCAtttcAGGACAgcccagc-3′ for TRβ TRE, and 5′-cctaggcAGGTCAtttcAAAACAgcccagc-3′ for mutant TRβ TRE (the TRE half sites are in bolded uppercase letters, and the mutated nucleotides are underlined). Each binding reaction included 1μL (100 fmol) of IR700-labeled probe, 1 μL of each in vitro–translated TR and RXR protein, and 1 μL at 1 pmol/μL or 10 pmol/μL of indicated unlabeled competitors to obtain 10× or 100× unlabeled competitor oligonucleotides, in a total volume of 20 μL. The mixtures were incubated at room temperature for 20 minutes, electrophoresed on a 6% DNA retardation gel (Invitrogen), and then scanned by using an Odyssey infrared scanner (LI-COR Biosciences).
Transcription assay in the X. laevis oocyte system
The plasmids containing X. laevis TRα and RXRα were linearized and transcribed in vitro using an mMESSAGE mMACHINE Sp6 transcription kit (Ambion, Austin, TX). The cytoplasm of stage VI oocytes from X. laevis was injected with 46 pg per oocyte of the GFP mRNA or TR and RXR mRNAs. Two hours later, the firefly luciferase reporter (345 pg per oocyte), either pTRE(HAL2)-luc or pTRE(TRβ)-luc, and the control phRG-TK Renilla luciferase (Promega) (34.5 pg per oocyte) were coinjected into the oocyte nucleus. After overnight incubation at 18°C in the presence or absence of 100 nM T3, the injected oocytes were prepared for luciferase assay by using the Dual-Luciferase reporter assay system according to the manufacturer’s protocol (Promega). Three oocytes per sample were lysed in 45 μL of 1× passive buffer (Promega), and 10 μL of lysate was used for the luciferase assays. Three independent samples were done for each injection at the same time. The relative expression of firefly luciferase to Renilla luciferase was determined. Each data point represents the average of the five samples with the standard error.
Quantitative reverse transcription PCRs
Total RNA was isolated from the tadpole small intestine by using the Direct-zol RNA MiniPrep kit (Zymo Research, Irvine, CA) according to the manufacturer’s protocol. The cDNA was synthesized with 1 μg of total RNA in 20 μL of reaction using the QuantiTect reverse transcription kit (Qiagen, Germantown, MD) according to the manufacturer’s protocol. Diluted cDNA (2 μL) was subjected to quantitative reverse transcription PCR (qRT-PCR) by using SYBR Green PCR master mix (Applied Biosystems, Foster City, CA) in a 20-μL reaction. The qRT-PCR was done by using the StepOnePlus real-time PCR system (Applied Biosystems), according to the manufacturer’s protocol. The primers used were forward 5′-AGCTGCTCACAGGTTGCTAGTT-3′ and reverse 5′-AAGAGTCCAATGAAAAAGATGTA-3′ for X. laevis HAL2, forward 5′-TGCATTCTGCCACCTAGTCG-3′ and reverse 5′-GGCAAAGGTAACCACCATGC-3′ for X. laevis elongation factor-1α (EF1α), forward 5′-GCCAACACTTGTCACTACTTTTACG-3′ and reverse 5′-ACGTGTCTTGTAGTTCCCGTCATCT-3′ for transgenic EGFP, forward 5′-TGAGACCCCATCCTGGACAAGTG-3′ and reverse 5′-GAACAGCACCGTAGTGTATAAGCA-3′ for X. tropicalis HAL2, and forward 5′-CGGAACTACCCTGCTGGAAG-3′ and reverse 5′-GGCAAAGGTAACCACCATGC-3′ for X. tropicalis EF1α. The level of HAL2 and transgenic EGFP mRNA was normalized against the level of EF1α mRNA for each sample. Each data point represents the average of the three samples with the standard error. Each experiment was repeated at least twice.
Chromatin immunoprecipitation assay
A chromatin immunoprecipitation (ChIP) assay on tadpole intestine was performed as descried with anti-TR antibody (new PB) (Table 1) (24, 42). A polyclonal antibody against Id14, an extracellular protein, was used as a negative control (Table 1) (24, 43). Each treatment or control group had three replicas, and each replica consisted of six to eight tadpoles. The chromatins for ChIP assay were fragmented to be ∼500 bp by sonication. The immunoprecipitated DNA was analyzed by quantitative PCR using the following primers: 5′-CCTGTGGCAGTGCGGGTCAG-3′ (KF403) and 5′-ACAGCCCAACCAGACAGGTCAC-3′ (KF404) for the TRE region of X. tropicalis HAL2 gene, and 5′-CAGCAGGTCTACAACCACTCTG-3′ (KF395) and 5′-TGAATCTTACCACTTCCCAGGT-3′ (KF396) for the exon 5 region of X. tropicalis Dot1L gene (41).
Table 1.
Antibodies Used
| Peptide/ Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| ID14 | DKVTPKKDDGATS-KLH, ETKCRCNMDGDVE-MAP | Anti-ID14 | Buchholz et al. (43) | Rabbit polyclonal | 2:125 |
| TR | Recombinat xlTRb protein | Anti-TR new PB | Wang et al. (42) | Rabbit polyclonal | 2:125 |
In situ hybridization
A partial cDNA encoding EGFP was PCR amplified and cloned into pCRII-TOPO (Invitrogen, Carlsbad, CA) as previously described (44). To synthesize the antisense RNA probe, the plasmid was linearized with BamHI and transcribed with T7 RNA polymerase (Roche Applied Science, Indianapolis, IN); to synthesize the sense RNA probe, the plasmid was linearized with XbaI and transcribed with SP6 RNA polymerase (Roche Applied Science). In situ hybridization on frozen cross-sections of intestines from tadpoles at the climax of metamorphosis was performed as described previously with probe concentrations at 2 μg/mL (26, 38). Both the sense and the antisense RNA probes were labeled with digoxin. The hybridized probe was detected with alkaline phosphatase-coupled anti-digoxin antibody and visualized with color development of 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium on alkaline phosphatase activity (GE Health, Pittsburgh, PA). Images were taken by using a digital CCD color camera (Retiga EXi; QImaging, British Columbia, Canada) attached to an optical microscope (BX60; Olympus, Waltham, MA).
Results
The X. tropicalis HAL2 is highly upregulated by T3 during intestinal metamorphosis
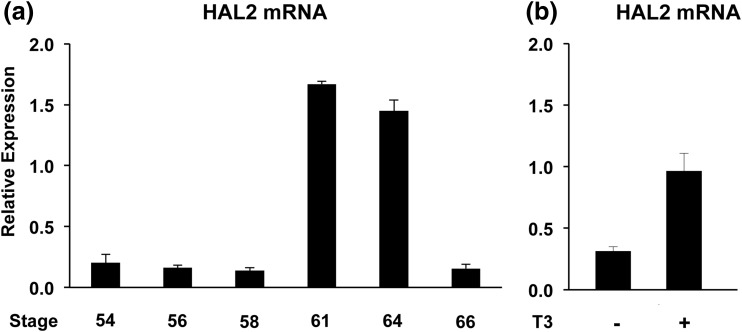
We first investigated whether the X. tropicalis HAL2 gene is regulated similarly as the X. laevis HAL2 gene during intestinal metamorphosis. Total RNA was isolated from whole intestine of tadpoles at premetamorphosis (stage 54), prometamorphosis (stages 56, 58), climax (stages 61, 64), and the end of metamorphosis (stage 66) and subjected to qRT-PCR analysis for HAL2 expression. Exactly as in X. laevis, the HAL2 mRNA levels were low in premetamorphic and prometamorphic tadpole intestine (stages 54 to 58) but were strongly upregulated at climax (stages 61 to 64) when adult stem cells are forming and proliferating in the intestine (1, 5) [Fig. 1(a)]. By the end of the metamorphosis (stage 66) when adult intestine is formed, the HAL2 mRNA level was reduced again. Additionally, when premetamorphic stage 54 tadpoles were treated with 10 nM T3 for 2 days, the HAL2 mRNA level in the intestine was found to be highly upregulated [Fig. 1(b)], again mimicking the pattern in X. laevis. Thus, HAL2 regulation during intestinal metamorphosis is conserved between X. laevis and X. tropicalis.
Figure 1.
X. tropicalis HAL2 is strongly upregulated by T3 at the climax of intestinal metamorphosis when adult stem cells are forming and proliferating. (a) HAL2 expression peaks at the climax of natural metamorphosis. Total RNA was isolated from whole intestine of tadpoles at premetamorphosis (stage 54), prometamorphosis (stages 56, 58), climax (stages 61, 64), and at the end of metamorphosis (stage 66) and subjected to qRT-PCR analysis for HAL2 expression. Note that HAL2 levels were high at the climax. (b) HAL2 expression is induced by T3 treatment of premetamorphic tadpoles. Premetamorphic stage 54 tadpoles were treated with 10 nM T3 for 2 days. Total RNA was isolated from the whole intestine and subjected to qRT-PCR analysis for HAL2 expression.
The X. tropicalis HAL2 gene contains a functional TRE
The induction of HAL2 gene by T3 treatment of premetamorphic tadpoles suggests that the HAL2 gene may be a stem cell–specific gene induced by T3 directly at the transcriptional level. To investigate this possibility, we took advantage of the availability of the sequenced X. tropicalis genome to search for potential TREs around predicted promoter region of the HAL2 gene. This is possible because of the conservation between X. laevis and X. tropicalis, where all known T3-regulated genes that have been studied in both species have conserved spatiotemporal expression patterns (6, 24, 41, 42, 45–49). Bioinformatics analysis (NHR SCAN: http://www.cisreg.ca/cgi-bin/NHR-scan/nhr_scan.cgi) revealed the existence of a single putative TRE located ∼3.1 kb upstream of the putative transcription start site [Fig. 2(a)]. The TRE is highly homologous to the consensus TRE made of two direct repeats of AGGTCA separated by 4 bp. To investigate the function of the putative TRE, we first carried out a gel mobility shift assay by using in vitro–translated TR/RXR and labeled X. laevis TRβ TRE [Fig. 2(b)], a well-characterized, strong TRE (50, 51). As shown in Fig. 2(c), the labeled TRβ TRE formed a complex with TR/RXR, and this complex was effectively competed away by the unlabeled TRβ TRE but not a mutant TRβ TRE. Importantly, the HAL2 TRE was also able to compete effectively, with similar affinity as the TRβ TRE [Fig. 2(c)]. Alternatively, introducing three base substitutions in the HAL2 TRE (mtTRE) abolished its ability to compete [Fig. 2(c)]. Thus, the HAL2 TRE binds to TR/RXR heterodimers with similar affinity as the well-known TRβ TRE.
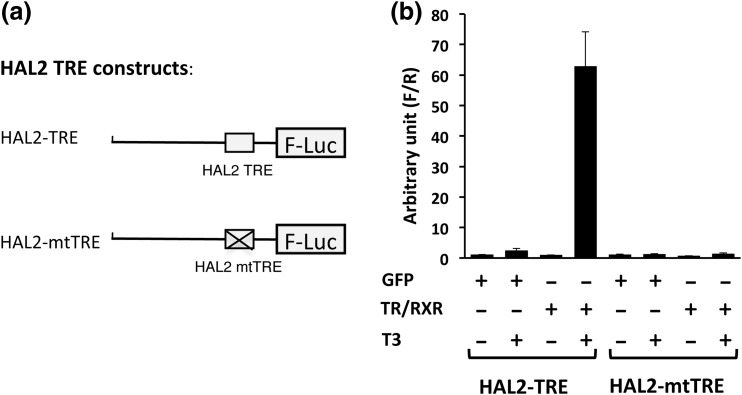
We next investigated whether the HAL2 TRE could mediate transcriptional activation of a reporter gene by liganded TR/RXR in the reconstituted X. laevis oocyte transcription system (42, 51), where the reporter plasmid injected into the oocyte nucleus is chromatinized. We have shown earlier that a TRE located ∼1 kb or more from the transcription start site has little effect on the promoter activity in this in vivo system (52). Because the HAL2 TRE is >3 kb away from the putative start site, we could not study the function of the TRE in its native promoter. Thus, we chose to investigate its function in a reporter promoter. We have shown previously that the X. tropicalis Dot1L promoter is regulated by TR/RXR in a T3-dependent manner in the reconstituted frog oocyte system (40, 41). Furthermore, this regulation depends on the presence of a Dot1L TRE (40, 41). Thus, we replaced the Dot1L TRE with HAL2 TRE or mtTRE in the Dot1L reporter construct to obtain the HAL2 reporter constructs pHAL2-TRE-luc and pHAL2-mtTRE-luc, respectively [Fig. 3(a)]. When the reporter construct pHAL2-TRE-luc was injected into the frog oocyte nucleus, it was regulated by T3 when TR/RXR were introduced into the oocyte via injection of their mRNAs into the oocyte cytoplasm [Fig. 3(b)]. However, when the reporter construct pHAL2-mtTRE-luc was injected to the oocyte nucleus, it was not regulated by T3 even in the presence of TR/RXR, indicating that the HAL2 TRE is capable of, and required for, mediating transcriptional regulation by TR/RXR in a T3-dependent manner.
Figure 3.
The HAL2 TRE mediates transcriptional activation by T3 in frog oocytes. (a) Schematic diagram of promoter constructs pHAL2-TRE-luc (HAL2-TRE) and pHAL2-mtTRE-luc (HAL2-mtTRE) containing the wild-type and mutated HAL2 TRE, respectively. (b) The luciferase reporter construct pHAL2-TRE-luc (HAL2-TRE) or pHAL2-mtTRE-luc (HAL2-mtTRE) was coinjected with the control Renilla luciferase construct phRG-tk into the nuclei of Xenopus oocytes with or without prior cytoplasmic injection of X. laevis TRα and RXRα mRNAs. The oocytes were incubated at 18°C overnight in the presence or absence of 100 nM T3 and then used for Dual-Luciferase assays. The relative activities of the firefly luciferase to Renilla luciferase were plotted. Note that the reporter with the wild-type but not the mutated HAL2 TRE responded to T3 in the presence of TR/RXR.
The X. tropicalis HAL2 TRE is bound by TR in tadpole intestine
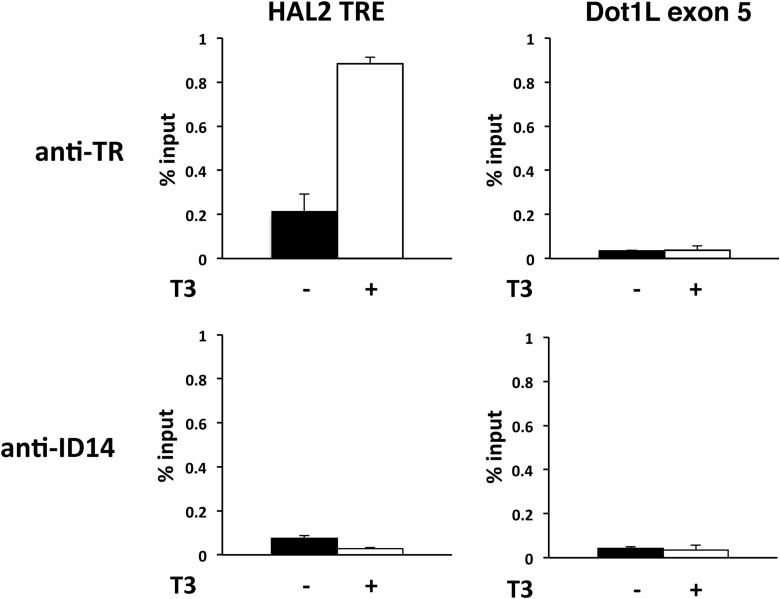
To determine whether the HAL2 TRE is responsible for the regulation of the HAL2 gene by T3 during intestinal metamorphosis, we used a ChIP assay to investigate the binding of the TR to the HAL2 TRE in the intestine of premetamorphic X. tropicalis tadpoles treated with or without T3. As shown in Fig. 4, TR was found to be present at the HAL2 TRE in premetamorphic intestine, and its association was increased upon T3 treatment of premetamorphic tadpoles. In contrast, there were only very low, background signals at the exon 5 of the Dot1L gene, which lacks any TRE (41). Additionally, only background signals were observed at either the HAL2 TRE and Dot1L exon 5 when ChIP assay was done with an antibody against ID14, an extracellular protein (43) (Fig. 4), confirming the specificity of the TR ChIP signal at the HAL2 TRE. Thus, the HAL2 TRE is bound by TR in tadpole intestine and likely mediates the induction of the HAL2 gene by T3 in the intestinal epithelium during metamorphosis.
Figure 4.
The HAL2 TRE is bound by TR in tadpole intestine. Stage 54 premetamorphic tadpoles were treated with or without T3 for 2 days, and the intestine was isolated for ChIP assay with the anti-TR antibody (top) or anti-ID14 antibody (bottom), which served as a negative control for antibody specificity. The immunoprecipitated DNA was analyzed by qPCR for the presence of the TRE region of HAL2 promoter. A region of the exon 5 in the Dot1L gene with no TRE (41) was analyzed as a negative control for binding specificity. Note that TR binding to the HAL2 TRE was increased upon T3 treatment. There was no TR binding to Dot1L exon 5 in the presence or absence of T3 and only background signals were observed with the anti-ID14 antibody.
Transgenic EGFP under the control of the X. tropicalis HAL2 promoter is regulated by T3 and mimics the endogenous HAL2 gene
To determine whether the HAL2 promoter is indeed regulated by TR during intestinal metamorphosis, we next used transgenesis to investigate whether the HAL2 promoter can drive transgenic EGFP expression in a T3-dependent manner in vivo. We generated a double promoter transgenic construct, in which the first promoter, a 4.5-kb HAL2 gene fragment spanning −4.4 kb to +79 bp (relative to the putative transcription start site at +1), was cloned in front of the coding region for EGFP [Fig. 5(a)]. The second promoter, the lens-specific γ-crystallin gene promoter, was used to drive the expression of GFP3 (36, 37) [Fig. 5(a)]. Using this double promoter construct, we generated F0 transgenic animals via the sperm-mediated transgenic procedure (53). Sexually mature adult F0 frogs were used to produce homogeneous transgenic (with their eyes having green fluorescence under ultraviolet) and wild-type (with no fluorescent eyes) sibling tadpoles. When the animals reached premetamorphic stage 54, they were treated with 10 nM T3 for 0 to 5 days to induce precocious metamorphosis, including intestinal remodeling. Total intestinal RNA was isolated from the tadpoles and subjected to qRT-PCR analysis of the expression of the endogenous HAL2 as well as the transgenic EGFP. As shown in Fig. 5(b), the HAL2 mRNA was absent in the premetamorphic intestine at stage 54 in both wild-type and transgenic tadpoles but was strongly induced after 3 to 5 days of T3 treatment. The same pattern of expression was observed for the transgenic EGFP in the transgenic tadpoles after T3 treatment [Fig. 5(c)]. As expected, no EGFP mRNA was detected in the wild-type siblings throughout the treatment [Fig. 5(c)]. Thus, the HAL2 promoter was responsive to T3 treatment in the same manner as the endogenous HAL2 gene.
Figure 5.
The transgenic HAL2 promoter, driving EGFP in transgenic tadpoles, respond to T3 similarly as the endogenous HAL2 gene in the intestine. (a) The double promoter transgenic construct used to study the HAL2 promoter in vivo. The HAL2 promoter fragment flanked by the KpnI and AgeI restriction sites [with the numbers indicating the position relative to the transcription start site in Fig. 1(a)] was cloned to drive the expression of EGFP in a double promoter transgenic construct, which also contains an eye-specific promoter, the γ-crystallin promoter, driving the expression of the GFP3 (36, 37). The HAL2 TRE in the promoter is shown as an open box. (b and c) Stage 54 premetamorphic wild-type or transgenic tadpoles were treated with 10 nM T3 for 0 to 5 days and total intestinal RNA was isolated for expression analysis of the endogenous HAL2 mRNA (b) or transgenic EGFP mRNA (c). Note that the endogenous HAL2 expression was upregulated significantly after 3 to 5 days of treatment in both wild-type and transgenic tadpoles. The expression of the transgenic EGFP mimicked that of the endogenous HAL2 in the transgenic tadpoles but was, as expected, absent in the wild-type tadpoles.
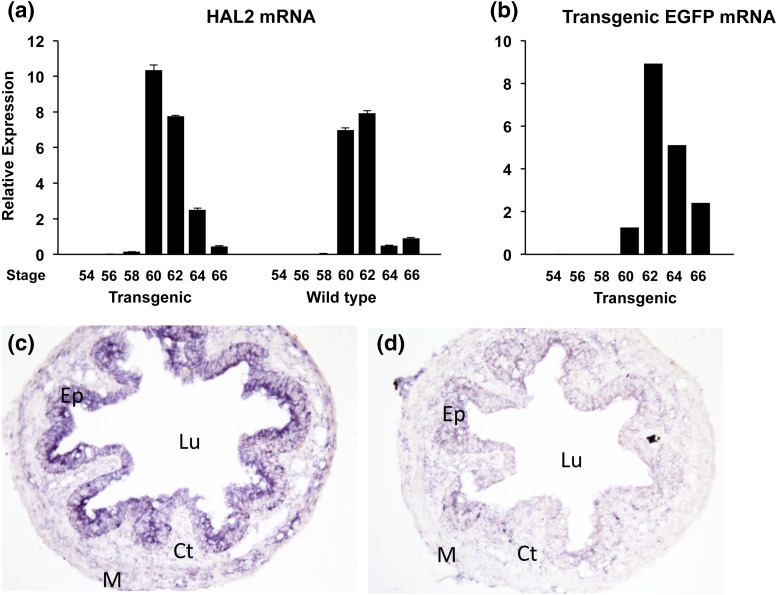
We next investigated the activity of the transgenic HAL2 promoter during natural metamorphosis. As shown previously, in the wild-type tadpoles, the endogenous HAL2 gene was not expressed in premetamorphic intestine and was strongly upregulated at climax of metamorphosis when adult intestinal stem cells are developing and proliferating (stages 60 to 62) (1, 5) [Fig. 6(a)]. The same pattern was observed for the endogenous HAL2 genes in the transgenic animals during intestinal metamorphosis, again as expected [Fig. 6(a)]. Importantly, the expression of the transgenic EGFP under the control of the HAL2 promoter was also found to be regulated in a similar fashion as the endogenous HAL2 mRNA during development in the intestine of the transgenic animals [Fig. 6(b)]. To confirm that the HAL2 promoter is specific to the intestinal epithelium at the climax of metamorphosis, we analyzed the expression of the EGFP in the intestines of transgenic tadpoles at the climax by in situ hybridization and observed strong signals in the intestinal epithelium with the antisense EGFP probe [Fig. 6(c)] but not the sense probe [Fig. 6(c)]. Thus, the 4.4-kb promoter region not only responds to T3 in vivo but also reproduces the spatiotemporal expression pattern during intestinal metamorphosis. These results suggest that the promoter region contains all critical regulatory elements of the HAL2 gene for its expression and regulation in the intestine during development.
Figure 6.
The expression of the transgenic EGFP under the control of the HAL2 promoter mimics that of the endogenous gene in the intestine during natural metamorphosis. (a and b) RT-PCR analysis shows similar temporal expression patterns of the endogenous HAL2 and transgenic EGFP during development. Total intestinal RNA was isolated from wild-type or sibling transgenic tadpoles at indicated stages throughout metamorphosis and subjected to qRT-PCR analysis for expression of the endogenous HAL2 mRNA (a) or transgenic EGFP mRNA (b). Note that a similar pattern was observed for the EGFP and endogenous HAL2 expression in the transgenic tadpoles. (c and d). In situ hybridization confirms the epithelium-specific expression of the transgenic EGFP. The intestinal cross-sections from transgenic tadpoles at the climax of metamorphosis were used for in situ hybridization with an antisense (c) or sense probe (d) for EGFP which was visualized with 5-bromo-4-chloro-3-indolyl-phosphate/nitro blue tetrazolium staining (magnification ×200). Note that the transgenic EGFP was detected in the epithelium with an antisense RNA probe for EGFP (c) but not with the sense probe (d). Ct, connective tissue; Ep, epithelium; Lu, lumen; M, muscle.
Discussion
Adult organ-specific stem cells are essential for the homeostasis of adult organs and tissue repair and regeneration. The life-long self-renewal of the intestinal epithelium through the proliferation of the adult stem cells has made intestine a valuable model for studying the function and properties of adult stem cells. Extensive studies in mammals have revealed several signaling pathways important for adult intestinal stem cells and contributed to our understanding of intestinal homeostasis and neoplasia (54–57). However, the molecular mechanisms regulating the formation of the adult stem cells during development are poorly understood. Intestinal remodeling during Xenopus metamorphosis offers a unique opportunity to investigate adult stem cell development due to the de novo formation of the adult stem cells during a process that is T3-dependent and easily manipulated, especially with the development of gene editing tools for Xenopus studies (3, 58, 59). Furthermore, intestinal remodeling resembles the maturation of the mouse intestine in the first 3 weeks or so after birth (3, 60), the postembryonic developmental period when T3 level peaks (7, 54–57, 61). Additionally, molecular and genetic studies have suggested that the formation of the mammalian adult intestine is dependent on T3 (25, 56, 57, 61–64). Alternatively, little is known about how T3 regulates the formation of adult intestinal stem cells in mammals due to the difficulty to manipulate the mammalian embryos and neonates.
Earlier studies by us and others on intestinal remodeling during amphibian metamorphosis have revealed that the adult intestinal stem cells are formed de novo via T3-induced dedifferentiation of larval epithelial cells and led to the identification of many candidate stem cell genes, including the HAL2 gene in X. laevis (17, 65). Expression analyses have revealed that X. laevis HAL2 is induced by T3 during intestinal metamorphosis and that its mRNA only expressed during the period of adult stem cell development and proliferation in the intestine during development (26). Specifically, HAL2 mRNA is present at high levels only in the proliferating adult stem cells at the climax of metamorphosis. In this study, we have shown that HAL2 expression during intestinal development is conserved between X. laevis and X. tropicalis. Furthermore, we have taken advantages of both the available genomic sequence information in X. tropicalis and sperm-nucleus–mediated transgenesis to show that this T3-dependent expression of HAL2 during intestinal remodeling is mediated by a TRE located upstream of the coding region and that a 4-kb promoter fragment can faithfully mimic the endogenous HAL2 promoter to drive a reporter gene expression in transgenic tadpoles.
The HAL2 TRE is highly homologous to the consensus TRE made of two direct repeats of AGGTCA separated by 4 bp [note that AGGTCA repeats are on the bottom strand in the HAL2 promoter region, Figs. 2(a) and 2(b)]. Only the last three bases of the second half site, which are less critical for TR binding, were changed from TCA to TTG. Not surprisingly, we observed that the HAL2 TRE binds to TR/RXR heterodimers with strong affinity in vitro, comparable to the well-studied, strong TRE of X. laevis TRβ gene [Fig. 2(c)] (50, 51, 66). The HAL2 TRE also functioned similarly in the frog oocyte in vivo transcription system to mediate the transcriptional regulation by T3 in the presence of TR/RXR [Fig. 3(b)]. Furthermore, the TRE is bound by TR in the intestine during development when HAL2 gene is inducible by T3 treatment. (Note that weak binding of TR to the TRE was observed in the premetamorphic tadpole intestine in the absence of T3 when there were not adult epithelial cells. It is possible that there is weak binding of TR to the TRE in the larval epithelial cells or other cells in the intestine and this weak binding alone is not sufficient for the induction of the gene in these cells.) More importantly, a promoter fragment containing the TRE faithfully reproduced the developmental regulation pattern of the endogenous HAL2 genes in transgenic animals (Fig. 6). These findings strongly argue that the HAL2 gene is a direct target gene of T3 and is regulated via TR binding to the TRE during intestinal metamorphosis.
Although our data indicate that T3 directly activates the HAL2 promoter via TR/RXR binding to the HAL2 TRE, the kinetics of the T3 induction were slow for both the transgenic EGFP and endogenous HAL2 mRNAs, as significant induction was observed only after 3 to 5 days of T3 treatment (Fig. 5). Similarly, during natural metamorphosis, high levels of transgenic EGFP and endogenous HAL2 mRNAs were observed at stage 60 or later, the climax of metamorphosis, whereas only low levels were present up to stage 58, the onset of metamorphic climax (Figs. 1 and 6). There are several possibilities for this delay in T3 induction. First and most likely, HAL2 is only expressed at high levels in the adult stem cell (26). At an early stage of metamorphosis, up to stage 60, or during the first few days of T3 treatment of premetamorphic tadpoles, there are few stem cells present in the tadpole intestine. Thus, the overall HAL2 expression in the whole intestine would be low until the fraction of cells that are adult stem cells increases significantly. In support of this, a similar kinetics was reported for another stem cell–specific, direct T3 target gene, the AMDHD1 gene, which encodes another enzyme in the histidine catabolic pathway (40). Alternatively, the products of one or more T3-induced genes are involved to further enhance HAL2 induction by T3. Thus, in the early phase of T3 treatment or natural metamorphosis, the HAL2 levels would be low but, subsequently, as these products accumulate during development or T3 treatment, HAL2 expression is further increased.
HAL2 is specifically expressed in the developing/proliferating adult intestinal stem cells during metamorphosis. Our findings showing that HAL2 is a direct target gene of TR suggest that HAL2 is induced early during the formation of the adult stem cells. We have shown previously that the adult stem cells are formed from dedifferentiation of some larval epithelial cells (16, 17). It remains to be determined how and why these larval epithelial cells undergo dedifferentiation instead of T3-induced apoptosis, the fate of most of the larval epithelial cells (5, 67). It is possible that the activation of the HAL2 gene in the larval epithelial cells represents one of the early events that help the cells to choose the dedifferentiation fate instead of programmed cell death when T3 becomes available during metamorphosis.
HAL2 encodes histidase, also known as histidinase, the first enzyme in the histidine catabolism pathway, that is, the deamination of histidine to ammonia and urocanic acid (27–30). It is possible that the direct product of the HAL2 catalysis, urocanic acid, or other products of the histidine catabolic pathway are involved in stem cell development. This would resemble the requirement of threonine catabolism for mouse embryonic stem cells (68). Additionally, the ultimate product of the histidine catabolic pathway, glutamate, can affect the activity of mammalian target of rapamycin complex 1 (mTORC1; also known as mechanistic target of rapamycin complex 1) (69, 70). The mTORC1 complex functions as a nutrient/energy/redox sensor and controls protein synthesis, thus affecting cell fate and function (71, 72). Given the lack of feeding during metamorphosis, it is possible that mTORC1 mediates the effect of HAL2 on adult stem cell formation/proliferation.
Our transgenic studies indicate that the HAL2 promoter fragment mimics the endogenous HAL2 promoter to drive the reporter EGFP expression during intestinal remodeling in transgenic animals. This represents the first Xenopus promoter that is active specifically in the developing/proliferating adult epithelial stem cells during metamorphosis. It should be possible in the future to use the promoter to drive the expression of wild-type or mutant genes, such as dominant-negative or dominant-positive TR (73–75), specifically in the stem cells to study their stem cell–specific function during development.
Acknowledgments
We are grateful to Dr. D. R. Buchholz (University of Cincinnati) for providing a double promoter plasmid.
Current affiliation: K. Fujimoto’s current affiliation is the Department of Biology, Nippon Medical School, Tokyo 180-0023, Japan.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Child Health and Human Development, National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- chromatin immunoprecipitation
- EF1α
- elongation factor-1α
- EGFP
- enhanced green fluorescent protein
- GFP
- green fluorescent protein
- HAL
- histidine ammonia-lyase
- mRNA
- messenger RNA
- mTORC1
- mammalian target of rapamycin complex 1
- PCR
- polymerase chain reaction
- qRT-PCR
- quantitative reverse transcription–polymerase chain reaction
- RXR
- 9-cis retinoic acid receptor
- T3
- thyroid hormone
- TR
- thyroid hormone receptor
- TRE
- thyroid hormone response element.
References
- 1.Ishizuya-Oka A, Shi YB. Thyroid hormone regulation of stem cell development during intestinal remodeling. Mol Cell Endocrinol. 2008;288(1–2):71–78. [DOI] [PubMed] [Google Scholar]
- 2.Sun G, Fu L, Shi Y-B. Epigenetic regulation of thyroid hormone-induced adult intestinal stem cell development during anuran metamorphosis. Cell Biosci. 2014;4:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi YB, Hasebe T, Fu L, Fujimoto K, Ishizuya-Oka A. The development of the adult intestinal stem cells: insights from studies on thyroid hormone-dependent amphibian metamorphosis. Cell Biosci. 2011;1(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Okada M, Wen L, Miller TC, Su D, Shi YB. Molecular and cytological analyses reveal distinct transformations of intestinal epithelial cells during Xenopus metamorphosis. Cell Biosci. 2015;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogenesis and remodeling during metamorphosis. Curr Top Dev Biol. 1996;32:205–235. [DOI] [PubMed] [Google Scholar]
- 6.Sterling J, Fu L, Matsuura K, Shi Y-B. Cytological and morphological analyses reveal distinct features of intestinal development during Xenopus tropicalis metamorphosis. PLoS One. 2012;7(10):e47407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun G, Shi Y-B. Thyroid hormone regulation of adult intestinal stem cell development: mechanisms and evolutionary conservations. Int J Biol Sci. 2012;8(8):1217–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ishizuya-Oka A, Shimozawa A. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol. 1991;27A(11):853–857. [DOI] [PubMed] [Google Scholar]
- 9.Shi Y-B, Ishizuya-Oka A. Thyroid hormone regulation of apoptotic tissue remodeling: implications from molecular analysis of amphibian metamorphosis. Prog Nucleic Acid Res Mol Biol. 2001;65:53–100. [DOI] [PubMed] [Google Scholar]
- 10.Su Y, Shi Y, Stolow MA, Shi Y-B. Thyroid hormone induces apoptosis in primary cell cultures of tadpole intestine: cell type specificity and effects of extracellular matrix. J Cell Biol. 1997;139(6):1533–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Rouxs Arch Dev Biol. 1992;201:322–329. [DOI] [PubMed] [Google Scholar]
- 12.Ishizuya-Oka A, Shimozawa A. Inductive action of epithelium on differentiation of intestinal connective tissue of Xenopus laevis tadpoles during metamorphosis in vitro. Cell Tissue Res. 1994;277(3):427–436. [DOI] [PubMed] [Google Scholar]
- 13.Hasebe T, Fu L, Miller TC, Zhang Y, Shi YB, Ishizuya-Oka A. Thyroid hormone-induced cell-cell interactions are required for the development of adult intestinal stem cells. Cell Biosci. 2013;3(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schreiber AM, Mukhi S, Brown DD. Cell–cell interactions during remodeling of the intestine at metamorphosis in Xenopus laevis. Dev Biol. 2009;331(1):89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreiber AM, Cai L, Brown DD. Remodeling of the intestine during metamorphosis of Xenopus laevis. Proc Natl Acad Sci USA. 2005;102(10):3720–3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishizuya-Oka A, Hasebe T, Buchholz DR, Kajita M, Fu L, Shi YB. Origin of the adult intestinal stem cells induced by thyroid hormone in Xenopus laevis. FASEB J. 2009;23(8):2568–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hasebe T, Buchholz DR, Shi YB, Ishizuya-Oka A. Epithelial-connective tissue interactions induced by thyroid hormone receptor are essential for adult stem cell development in the Xenopus laevis intestine. Stem Cells. 2011;29(1):154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchholz DR, Paul BD, Fu L, Shi YB. Molecular and developmental analyses of thyroid hormone receptor function in Xenopus laevis, the African clawed frog. Gen Comp Endocrinol. 2006;145(1):1–19. [DOI] [PubMed] [Google Scholar]
- 19.Yen PM. Unliganded TRs regulate growth and developmental timing during early embryogenesis: evidence for a dual function mechanism of TR action. Cell Biosci. 2015;5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. doi: 10.1016/j.bbagen.2012.04.020. Grimaldi A, Buisine N, Miller T, Shi YB, Sachs LM. Mechanisms of thyroid hormone receptor action during development: lessons from amphibian studies. Biochim Biophys Acta. 2013;1830(7):3882–3892. [DOI] [PubMed] [Google Scholar]
- 21.Sun G, Heimeier RA, Fu L, Hasebe T, Das B, Ishizuya-Oka A, Shi Y-B. Expression profiling of intestinal tissues implicates tissue-specific genes and pathways essential for thyroid hormone-induced adult stem cell development. Endocrinology. 2013;154(11):4396–4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchholz DR, Heimeier RA, Das B, Washington T, Shi Y-B. Pairing morphology with gene expression in thyroid hormone-induced intestinal remodeling and identification of a core set of TH-induced genes across tadpole tissues. Dev Biol. 2007;303(2):576–590. [DOI] [PubMed] [Google Scholar]
- 23.Heimeier RA, Das B, Buchholz DR, Shi YB. The xenoestrogen bisphenol A inhibits postembryonic vertebrate development by antagonizing gene regulation by thyroid hormone. Endocrinology. 2009;150(6):2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Das B, Heimeier RA, Buchholz DR, Shi YB. Identification of direct thyroid hormone response genes reveals the earliest gene regulation programs during frog metamorphosis. J Biol Chem. 2009;284(49):34167–34178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heimeier RA, Das B, Buchholz DR, Fiorentino M, Shi YB. Studies on Xenopus laevis intestine reveal biological pathways underlying vertebrate gut adaptation from embryo to adult. Genome Biol. 2010;11(5):R55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luu N, Wen L, Fu L, Fujimoto K, Shi YB, Sun G. Differential regulation of two histidine ammonia-lyase genes during Xenopus development implicates distinct functions during thyroid hormone-induced formation of adult stem cells. Cell Biosci. 2013;3(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawai Y, Moriyama A, Asai K, Coleman-Campbell CM, Sumi S, Morishita H, Suchi M. Molecular characterization of histidinemia: identification of four missense mutations in the histidase gene. Hum Genet. 2005;116(5):340–346. [DOI] [PubMed] [Google Scholar]
- 28.Suchi M, Harada N, Wada Y, Takagi Y. Molecular cloning of a cDNA encoding human histidase. Biochim Biophys Acta. 1993;1216(2):293–295. [DOI] [PubMed] [Google Scholar]
- 29.Suchi M, Sano H, Mizuno H, Wada Y. Molecular cloning and structural characterization of the human histidase gene (HAL). Genomics. 1995;29(1):98–104. [DOI] [PubMed] [Google Scholar]
- 30.Taylor RG, Levy HL, McInnes RR. Histidase and histidinemia. Clinical and molecular considerations. Mol Biol Med. 1991;8(1):101–116. [PubMed] [Google Scholar]
- 31.Bulfield G, Kacser H. Histidinaemia in mouse and man. Arch Dis Child. 1974;49(7):545–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuroda Y, Ogawa T, Ito M, Watanabe T, Takeda E, Toshima K, Miyao M. Relationship between skin histidase activity and blood histidine response to histidine intake in patients with histidinemia. J Pediatr. 1980;97(2):269–272. [DOI] [PubMed] [Google Scholar]
- 33.Auerbach VH, Digeorge AM, Baldridge RC, Tourtellotte CD, Brigham MP. Histidinemia. A deficiency in histidase resulting in the urinary excretion of histidine and of imidazolepyruvic acid. J Pediatr. 1962;60:487–497. [DOI] [PubMed] [Google Scholar]
- 34.Kacser H, Mya KM, Duncker M, Wright AF, Bulfield G, McLaren A, Lyon MF. Maternal histidine metabolism and its effect on foetal development in the mouse. Nature. 1977;265(5591):262–266. [DOI] [PubMed] [Google Scholar]
- 35.Nieuwkoop PD, Faber J, eds. Normal Table of Xenopus laevis (Daudin): A Systematical and ChronologicalSurvey of the Development from the Fertilized Egg Till the End of Metamorphosis. 1st ed. Amsterdam, The Netherlands: North Holland Publishing; 1965. [Google Scholar]
- 36.Rankin SA, Hasebe T, Zorn AM, Buchholz DR. Improved cre reporter transgenic Xenopus. Dev Dyn. 2009;238(9):2401–2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rankin SA, Zorn AM, Buchholz DR. New doxycycline-inducible transgenic lines in Xenopus. Dev Dyn. 2011;240(6):1467–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu L, Ishizuya-Oka A, Buchholz DR, Amano T, Matsuda H, Shi YB. A causative role of stromelysin-3 in extracellular matrix remodeling and epithelial apoptosis during intestinal metamorphosis in Xenopus laevis. J Biol Chem. 2005;280(30):27856–27865. [DOI] [PubMed] [Google Scholar]
- 39.Fu L, Buchholz D, Shi YB. Novel double promoter approach for identification of transgenic animals: a tool for in vivo analysis of gene function and development of gene-based therapies. Mol Reprod Dev. 2002;62(4):470–476. [DOI] [PubMed] [Google Scholar]
- 40.Okada M, Miller TC, Fu L, Shi YB. Direct activation of amidohydrolase domain-containing 1 gene by thyroid hormone implicates a role in the formation of adult intestinal stem cells during Xenopus metamorphosis. Endocrinology. 2015;156(9):3381–3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Matsuura K, Fujimoto K, Das B, Fu L, Lu CD, Shi YB. Histone H3K79 methyltransferase Dot1L is directly activated by thyroid hormone receptor during Xenopus metamorphosis. Cell Biosci. 2012;2(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang X, Matsuda H, Shi Y-B. Developmental regulation and function of thyroid hormone receptors and 9-cis retinoic acid receptors during Xenopus tropicalis metamorphosis. Endocrinology. 2008;149(11):5610–5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buchholz DR, Ishizuya-Oka A, Shi YB. Spatial and temporal expression pattern of a novel gene in the frog Xenopus laevis: correlations with adult intestinal epithelial differentiation during metamorphosis. Gene Expr Patterns. 2004;4(3):321–328. [DOI] [PubMed] [Google Scholar]
- 44.Mathew S, Fu L, Fiorentino M, Matsuda H, Das B, Shi Y-B. Differential regulation of cell type-specific apoptosis by stromelysin-3: a potential mechanism via the cleavage of the laminin receptor during tail resorption in Xenopus laevis. J Biol Chem. 2009;284(27):18545–18556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujimoto K, Matsuura K, Das B, Fu L, Shi YB. Direct activation of Xenopus iodotyrosine deiodinase by thyroid hormone receptor in the remodeling intestine during amphibian metamorphosis. Endocrinology. 2012;153(10):5082–5089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fu L, Das B, Mathew S, Shi YB. Genome-wide identification of Xenopus matrix metalloproteinases: conservation and unique duplications in amphibians. BMC Genomics. 2009;10:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Amaya E, Offield MF, Grainger RM. Frog genetics: Xenopus tropicalis jumps into the future. Trends Genet. 1998;14(7):253–255. [DOI] [PubMed] [Google Scholar]
- 48.Bilesimo P, Jolivet P, Alfama G, Buisine N, Le Mevel S, Havis E, Demeneix BA, Sachs LM. Specific histone lysine 4 methylation patterns define TR-binding capacity and differentiate direct T3 responses. Mol Endocrinol. 2011;25(2):225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bagamasbad PD, Bonett RM, Sachs L, Buisine N, Raj S, Knoedler JR, Kyono Y, Ruan Y, Ruan X, Denver RJ. Deciphering the regulatory logic of an ancient, ultraconserved nuclear receptor enhancer module. Mol Endocrinol. 2015;29(6):856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ranjan M, Wong J, Shi YB. Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269(40):24699–24705. [PubMed] [Google Scholar]
- 51.Wong J, Shi Y-B. Coordinated regulation of and transcriptional activation by Xenopus thyroid hormone and retinoid X receptors. J Biol Chem. 1995;270(31):18479–18483. [DOI] [PubMed] [Google Scholar]
- 52.Wong J, Shi Y-B, Wolffe AP. Determinants of chromatin disruption and transcriptional regulation instigated by the thyroid hormone receptor: hormone-regulated chromatin disruption is not sufficient for transcriptional activation. EMBO J. 1997;16(11):3158–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kroll KL, Amaya E. Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development. 1996;122(10):3173–3183. [DOI] [PubMed] [Google Scholar]
- 54.van der Flier LG, Clevers H. Stem cells, self-renewal, and differentiation in the intestinal epithelium. Annu Rev Physiol. 2009;71:241–260. [DOI] [PubMed] [Google Scholar]
- 55.Sancho E, Batlle E, Clevers H. Signaling pathways in intestinal development and cancer. Annu Rev Cell Dev Biol. 2004;20:695–723. [DOI] [PubMed] [Google Scholar]
- 56.Harper J, Mould A, Andrews RM, Bikoff EK, Robertson EJ. The transcriptional repressor Blimp1/Prdm1 regulates postnatal reprogramming of intestinal enterocytes. Proc Natl Acad Sci USA. 2011;108(26):10585–10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kress E, Rezza A, Nadjar J, Samarut J, Plateroti M. The frizzled-related sFRP2 gene is a target of thyroid hormone receptor alpha1 and activates β-catenin signaling in mouse intestine. J Biol Chem. 2009;284(2):1234–1241. [DOI] [PubMed] [Google Scholar]
- 58.Wang F, Shi Z, Cui Y, Guo X, Shi YB, Chen Y. Targeted gene disruption in Xenopus laevis using CRISPR/Cas9. Cell Biosci. 2015;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lei Y, Guo X, Deng Y, Chen Y, Zhao H. Generation of gene disruptions by transcription activator-like effector nucleases (TALENs) in Xenopus tropicalis embryos. Cell Biosci. 2013;3(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ishizuya-Oka A, Shi YB. Evolutionary insights into postembryonic development of adult intestinal stem cells. Cell Biosci. 2011;1:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muncan V, Heijmans J, Krasinski SD, Büller NV, Wildenberg ME, Meisner S, Radonjic M, Stapleton KA, Lamers WH, Biemond I, van den Bergh Weerman MA, O’Carroll D, Hardwick JC, Hommes DW, van den Brink GR. Blimp1 regulates the transition of neonatal to adult intestinal epithelium. Nat Commun. 2011;2:452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Plateroti M, Gauthier K, Domon-Dell C, Freund JN, Samarut J, Chassande O. Functional interference between thyroid hormone receptor α (TRα) and natural truncated TRΔα isoforms in the control of intestine development. Mol Cell Biol. 2001;21(14):4761–4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Flamant F, Poguet AL, Plateroti M, Chassande O, Gauthier K, Streichenberger N, Mansouri A, Samarut J. Congenital hypothyroid Pax8−/− mutant mice can be rescued by inactivating the TRα gene. Mol Endocrinol. 2002;16(1):24–32. [DOI] [PubMed] [Google Scholar]
- 64.Plateroti M, Chassande O, Fraichard A, Gauthier K, Freund JN, Samarut J, Kedinger M. Involvement of T3Rα- and β-receptor subtypes in mediation of T3 functions during postnatal murine intestinal development. Gastroenterology. 1999;116(6):1367–1378. [DOI] [PubMed] [Google Scholar]
- 65.Ishizuya-Oka A, Hasebe T. Establishment of intestinal stem cell niche during amphibian metamorphosis. Curr Top Dev Biol. 2013;103:305–327. [DOI] [PubMed] [Google Scholar]
- 66.Wong J, Shi YB, Wolffe AP. A role for nucleosome assembly in both silencing and activation of the Xenopus TR beta A gene by the thyroid hormone receptor. Genes Dev. 1995;9(21):2696–2711. [DOI] [PubMed] [Google Scholar]
- 67.Ishizuya-Oka A, Hasebe T, Shi YB. Apoptosis in amphibian organs during metamorphosis. Apoptosis. 2010;15(3):350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang J, Alexander P, Wu L, Hammer R, Cleaver O, McKnight SL. Dependence of mouse embryonic stem cells on threonine catabolism. Science. 2009;325(5939):435–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014;24(7):400–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shimobayashi M, Hall MN. Multiple amino acid sensing inputs to mTORC1. Cell Res. 2016;26(1):7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim DH, Sarbassov DD, Ali SM, King JE, Latek RR, Erdjument-Bromage H, Tempst P, Sabatini DM. mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell. 2002;110(2):163–175. [DOI] [PubMed] [Google Scholar]
- 72.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18(16):1926–1945. [DOI] [PubMed] [Google Scholar]
- 73.Buchholz DR, Tomita A, Fu L, Paul BD, Shi Y-B. Transgenic analysis reveals that thyroid hormone receptor is sufficient to mediate the thyroid hormone signal in frog metamorphosis. Mol Cell Biol. 2004;24(20):9026–9037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Buchholz DR, Hsia SC, Fu L, Shi Y-B. A dominant-negative thyroid hormone receptor blocks amphibian metamorphosis by retaining corepressors at target genes. Mol Cell Biol. 2003;23(19):6750–6758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schreiber AM, Das B, Huang H, Marsh-Armstrong N, Brown DD. Diverse developmental programs of Xenopus laevis metamorphosis are inhibited by a dominant negative thyroid hormone receptor. Proc Natl Acad Sci USA. 2001;98(19):10739–10744. [DOI] [PMC free article] [PubMed] [Google Scholar]