Abstract
Seasonal reproduction in sheep is primarily due to a dramatic increase in the ability of estradiol (E2) to inhibit the pulsatile secretion of gonadotropin-releasing hormone (GnRH) during the nonbreeding season [anestrus (ANS)]. Recent findings suggest that kisspeptin/neurokinin B/dynorphin (KNDy) neurons of the arcuate nucleus (ARC) play a key role in conveying this negative feedback influence, with dopaminergic projections from the retrochiasmatic area acting upon KNDy cells to decrease kisspeptin release and thus inhibit GnRH pulses. However, several questions remain unanswered: (1) Are the coexpressed KNDy peptides, neurokinin B (NKB) and dynorphin, under seasonal regulation similar to kisspeptin? (2) Are seasonal changes in these peptides and their colocalization of D2 dopamine receptors (D2Rs) steroid dependent? and (3) Do KNDy neurons receive direct input from dopaminergic terminals? We used dual- and triple-label immunofluorescence to analyze brain sections through the ARC of ovariectomized (OVX) and OVX plus E2 ewes perfused during either the breeding season or ANS. Results showed (1) steroid-dependent and steroid-independent seasonal changes in kisspeptin and NKB, but not dynorphin, immunoreactivity; (2) increased D2R coexpression during ANS that was dependent on the presence of E2; and (3) evidence that KNDy cells receive direct contact from dopaminergic terminals and that this input increases during ANS. These results support the hypothesis that dopamine acts to inhibit GnRH secretion in ANS by directly suppressing the activity of ARC KNDy neurons, and implicate NKB as well as kisspeptin in seasonal shifts in E2-negative feedback in the sheep.
Steroids and season regulate peptides, dopamine receptors, and inputs of KNDy (kisspeptin/neurokinin B/dynorphin) neurons in the sheep, supporting their key role in seasonal reproduction.
Seasonal reproduction is a naturally occurring adaptation in many mammalian species that ensures offspring are born into an environment favorable for their subsequent survival (1). Female sheep show distinct cycles of ovarian function during the autumn and winter, termed the breeding season (BRS), and ovarian quiescence and anovulation in spring and summer, termed anestrus (ANS) (2). The primary mechanism responsible for this variation in ovarian function is a significant alteration in the ability of estradiol (E2) to inhibit gonadotropin-releasing hormone (GnRH) secretion (3). Specifically, during ANS, E2 gains the ability to dramatically suppress the frequency of GnRH and luteinizing hormone (LH) pulses (4–6).
A key set of neurons mediating this increased E2-negative influence are the A15 dopaminergic neurons located in the retrochiasmatic area (RCh) of the hypothalamus (2). During ANS, these neurons are stimulated by E2-responsive projections from the RCh (7, 8) and ventromedial preoptic area (POA) (9) and mediate E2-negative feedback via the D2 dopamine receptor (D2R) (10, 11). Tract-tracing studies have confirmed that A15 neurons primarily project caudally toward the mediobasal hypothalamus (MBH) and innervate the arcuate nucleus (ARC) and median eminence (12, 13). However, it remains unclear if these neurons regulate GnRH secretion via direct synapses onto GnRH neurons in the MBH, their terminals in the median eminence, or through an alternate indirect pathway.
Recent evidence suggests that A15-mediated regulation of GnRH secretion may occur via a multisynaptic pathway through the ARC. Kisspeptin neurons in the ARC are an ideal candidate for mediating dopamine (DA) actions on GnRH secretion. Kisspeptin is a key stimulator of GnRH secretion, expression of kisspeptin within the ARC is reduced during ANS (14), and arcuate kisspeptin neurons colocalize D2R (15). ARC kisspeptin cells are unique in that they coexpress two other peptides, neurokinin B (NKB) and dynorphin (Dyn), which have been linked to GnRH pulse initiation and termination, respectively (16). Termed kisspeptin/neurokinin B/dynorphin (KNDy) neurons, this subpopulation has become a prominent area of focus for studies on the neuroendocrine regulation of reproduction (17).
Our current hypothesis is that dopamine, released from A15 afferents, binds to D2R on ARC KNDy cells inhibiting kisspeptin release resulting in the net inhibition of GnRH pulses during ANS (3). However, it remains unclear whether this decrease in kisspeptin release is due to the seasonal increase in DA release or increased responsiveness of KNDy neurons to this signal. Recently it was shown that colocalization of D2R in KNDy cells, using Dyn as a marker for these cells, changes with season, with about 80% of KNDy cells colocalizing D2R in ANS compared to 40% in the BRS (15). However, this study did not determine whether the changes in D2R colocalization were specifically due to the increased responsiveness to E2 during ANS or instead were steroid independent.
Furthermore, although it is clear that kisspeptin expression is seasonally regulated (14), much less is known about the expression of other KNDy peptides. Dyn cell number did not change seasonally in ovariectomized plus estradiol (OVX+E) ewes, but the effects of E2 on this peptide compared to OVX animals were not examined (15). Similarly, circumstantial evidence suggests that NKB, like kisspeptin, may also be under seasonal regulation (18). However, the hypothesis that Dyn and/or NKB expression are seasonally regulated by E2 has yet to be directly tested. Furthermore, it remains to be demonstrated whether ARC KNDy neurons receive direct contact from dopaminergic terminals and whether there is seasonal plasticity in this input.
In the current study, we address several of these unanswered questions. First, we used immunofluorescence to examine the effects of season and E2 on the expression of all three KNDy peptides in the same animals. Second, we extended our previous findings examining the seasonal regulation of D2R colocalization in KNDy neurons to determine whether these changes are steroid dependent or independent. Finally, to determine if arcuate KNDy neurons may potentially receive direct contact from A15 dopamine neurons, we examined appositions of tyrosine hydroxylase (TH) projections upon KNDy cells in the brains of both BRS and ANS ewes.
Materials and Methods
Analysis of KNDy peptides and D2R expression
Animals
Adult mixed-breed blackface ewes were maintained in an open barn with access to water and food daily at West Virginia University. Based on values over four seasons, there is no difference (t test analysis) in weights between anestrous (70.1 ± 2.0 kg, n = 39) and breeding (75.5 ± 2.4 kg, n = 51) seasons in this breed of ewes. Ewes were moved indoors 3 to 7 days before surgeries and remained there for the duration of the experiments. Indoors, ewes were fed alfalfa pellets to maintain weight and given water and supplemental minerals ad libitum. Lights were adjusted every 2 weeks to mimic the duration of natural lighting. Anestrus (May; n = 8) and BRS (November; n = 8) ewes were OVX via midventral laporatomy using isoflurane anesthesia and sterile techniques [see Foradori et al. (19) for detailed methodology].
To distinguish between steroid-independent and steroid-dependent changes, ewes were randomly placed into two groups, OVX control and OVX+E (n = 4/group/season). OVX+E ewes received a 3-cm-long SLASTIC brand (Dow Corning Corp., Midland, MI) capsule containing crystalline E2 inserted subcutaneously at the time of OVX. These implants produce E2 concentrations in the range of 2 to 3 pg/mL (20) that do not vary between seasons (21) and are similar to those seen in ovary-intact anestrous ewes (21). Three weeks later, paraformaldehyde-fixed brain tissue was collected (see later) for immunohistochemistry (IHC). The seasonal status of each ewe was determined based on estrous behavior and/or status of the ovaries at the time of OVX or tissue collection. All animal work was approved by the West Virginia University Animal Care and Use Committee and followed National Institutes of Health guidelines for use of animals in research.
Tissue collection
Ewes were heparinized (25,000 U intravenously 10 minutes apart) and euthanized with an overdose of pentobarbital (~7 g intravenously). When breathing ceased, they were rapidly decapitated and immediately perfused via internal carotids with 6 L of 4% paraformaldehyde in 0.1 M phosphate buffer (pH 7.3) containing 0.1% NaNO3. Tissue blocks were stored overnight in fixative at 4°C and then in 30% sucrose at 4°C. After the tissue had been infiltrated with sucrose, a sliding freezing microtome (SM 200R; Leica Biosystems, Walldorf, Germany) was used to cut coronal sections (45μm) in six parallel series. Sections were stored in cryoprotectant solution (30% ethylene glycol, 1% polyvinylpyrrolidone, 30% sucrose in sodium phosphate buffer) at –20°C until needed.
Triple-label immunofluorescent detection of peptide and receptor colocalization
To examine the effects of season and E2 on KNDy peptide immunoreactivity and D2R colocalization, two parallel series of sections containing the ARC were processed for triple-label immunofluorescence and multichannel microscopic analysis. To account for all three KNDy peptides, two separate triple-label protocols were run. Peptide and receptor colocalization was assessed in the middle division of the ARC, with triple-label IHC for D2R [Ms-anti-D2R (B10) 1:100, Santa Cruz Biotechnology Inc., Dallas, TX, Catalog Number: SC-5303, Lot: A2814], NKB (Gp-anti-NKB, 1:1000, gift from Prof. Cioffi, IS-3/63, bleed 210493) and either kisspeptin (Rbt-anti-kisspeptin, 1:5,000, #564, gift from Prof. Caraty, Université Tours) or Dyn (Rbt-Anti-Dyn, 1:250, Phoenix Pharmaceuticals Inc., Burlingame CA, Catalog Number: H-021-03, Lot: 01359-3). Immunoreactive (ir) cells were counted in two to three hemisections and averaged per animal. Two-way analysis of variance (ANOVA) and post hoc analysis was used to determine differences between groups.
IHC was performed as previously reported (15). Briefly, free-floating sections were washed extensively in 0.1 M phosphate-buffered saline (PBS) for 3 hours to remove cryoprotectant. Endogenous peroxidase activity was blocked using a 1% H2O2 in 0.1 M PBS solution for 10 minutes. Sections were then washed for 1 hour in PBS+ (0.1 M PBS with 0.4% Triton-X and 4% normal goat serum) and subsequently incubated for 17 hours with mouse anti-D2-R at 1:100 in PBS+. Following incubation, sections were washed (0.1 M PBS for 5 minutes, four times) and rinsed again after each subsequent step. Next sections were incubated in secondary antibody, biotinylated goat-anti-mouse (Vector Laboratories, Burlingame, CA) at 1:500 in PBS+ for 1 hour. Tissue sections then underwent double amplification via washes in ABC-elite (Vector), 1:500 in PBS for 1 hour, followed by a biotinylated tyramine amplification system (Vector) at 1:250 in PBS containing 0.003% H2O2 for 10 minutes. Sections were then incubated with Alexa 555-conjugated streptavidin 1:100 in PBS for 30 minutes and subsequently incubated with guinea pig anti-NKB (1:1000) and either rabbit anti-kisspeptin (1:5000) or rabbit anti-Dyn (1:250) in PBS+ for 17 hours. After being washed in PBS, sections were incubated in secondary antibody goat-anti-rabbit-Alexa 488 (kisspeptin or Dyn) and Cy5 goat-anti-guinea pig (NKB) 1:100 in PBS for 30 minutes. After a final wash in 1.0 M PB, sections were mounted on Superfrost slides (Fisher Scientific, Ottawa, ON, Canada), stored in dark until dry, and coverslipped with an aqueous mounting medium (Gelvatol) containing an antifading agent [1,4-diazabicyclo(2,2)octane; 50 mg/mL] (Table 1).
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| D2R | MDPLNLSWYDDD LERQNWSRPFNGS DGKADRPHYNYY ATLLTLLIAVIV | D2DR antibody (B-10) | Santa Cruz Biotechnology Inc, Catalog Number: SC-5303 | Mouse (monoclonal) | 1:100 |
| Kisspeptin | Peptide from mouse kisspeptin 10 | Antikisspeptin antibody | Gifted by A. Caraty | Rabbit (polyclonal) | 1:5000 (Experiment 1) 1:2000 (Experiment 2) |
| NKB | Asp - Met - His – Asp - Phe - Phe - Val - Gly - Leu - Met - NH2 | Antineurokinin B; IS-3/63, bleed 210493 | Gifted by P. Cioffi | Guinea pig (polyclonal) | 1:5000 |
| Dyn | Tyr - Gly - Gly – Phe - Leu - Arg - Arg – Ile - Arg - Pro - Lys - Leu - Lys - Trp – Asp - Asn - Gln | Dynorphin A antibody | Phoenix Pharmaceuticals Inc., Catalog Number: H-021-03 | Rabbit (polyclonal) | 1:250 |
| TH | Prokaryotic recombinant protein corresponding to a portion of the carboxyl terminal end of mouse tyrosine hydroxylase | Lyophilized antityrosine hydroxylase | Novocastra, Leica Microsystems Pty Ltd., Catalog Number: NCL-TH | Mouse (monoclonal) | 1:1000 |
Analysis of TH-positive appositions
Animals and tissue collection
Corriedale ewes of similar age (5 to 6 years old) and weight were maintained under ambient photoperiod conditions at the Monash University Sheep Facility, Werribee, Victoria, Australia. Based on data across 5 consecutive years, there is no difference in weights between anestrous (61.5 ± 1.4 kg, n = 34) and BRS (63.2 ± 0.6 kg, n = 83; P = 0.18) ewes of this breed. Experiments were approved by the Monash University, School of Biomedical Sciences Animal Ethics Committee. Ewes were divided into four groups: OVX BRS (March), OVX ANS (December), OVX+E BRS, and OVX+E ANS (n = 4/group). OVX and E treatment was performed as previously described. Ewes were fatally anesthetized with pentobarbital and heads were perfused with 4% paraformaldehyde plus 15% picric acid as previously described (14). Brains were subsequently removed and the hypothalami processed for IHC. Coronal sections (40 um) were cut on a cryostat (extending the rostral POA to the caudal MBH) and placed into cryoprotectant until used for IHC.
Dual-label immunohistochemistry
IHC was performed as described previously for the colocalization of kisspeptin and NKB (16). For kisspeptin and TH IHC, anatomically matching sections representing the rostral, middle, and caudal regions of the ARC were incubated with both a rabbit antikisspeptin (AC566, 1:2000, gift from Caraty) and a mouse monoclonal antibody against TH (1:1000; Catalog Number: NCL-TH, Novocastra, Leica Microsystems Pty Ltd, Wetzlar, Germany) for 48 hours at 4°C. Subsequently, sections were incubated with fluorescent secondary antibodies; goat-anti-rabbit-Alexa 488 (kisspeptin, green) and a goat anti-mouse Alexa 546 (TH, red; both diluted 1:500; Molecular Probes, Inc., Eugene, OR). See Table 1 for detailed information about the primary antibodies named earlier.
Analytical procedures
KNDy peptide and D2R expression
Analyses of single-, double-, and triple-labeled cells were done using a Leica DM5000B fluorescence microscope at ×20 magnifications by an observer blinded to experimental treatments. The total number of single-, double-, and triple-labeled neurons for both IHC protocols (kisspeptin + NKB + D2R; Dyn + NKB + D2R) were counted in sections containing the middle level of the ARC (two to three sections each). Because of the density of KNDy cells in the ARC, Neurolucida software (MBF Biosciences, Williston, VT) was used to append multiple three-channel images captured with the appropriate excitation for Alexa 488, Alexa 555, and Cy5 across the entire extent of the middle ARC in each section. For each ewe, the percentage of total kisspeptin, NKB, and Dyn containing D2R, the percentage of kisspeptin and Dyn neurons containing NKB, and the percentage of NKB neurons containing either kisspeptin or Dyn were calculated. The percentage of each KNDy peptide cell colocalizing D2-R was determined by counting only cells with a clear nucleus present at the focal plane of the image. Data are expressed as means ± standard error of the mean. Seasonal and steroid effects were analyzed using two-way ANOVA with critical level of 0.05. For percentages, data were transformed prior to analysis using two-way ANOVA.
TH-ir appositions
Kisspeptin- and TH-ir cells were identified under fluorescent illumination, with a single observer counting the total number of cells and the number of cells with kisspeptin- or TH-ir terminal appositions. Putative contacts were examined with a Zeiss Apotome microscope (Carl Zeiss, Inc.). Z-stacks of optical sections were captured through neurons and appositions were defined as contacts of terminals with soma or proximal dendrites when no pixels were present between the two components when Z-stacks were rotated and viewed in different planes as previously reported (22). For each animal, the percentage of “contacted” kisspeptin- or TH-ir cells in the ARC was averaged to produce the mean ± standard error of the mean. Data were analyzed using two-way ANOVA (treatment and season).
Results
Steroid-dependent and steroid-independent seasonal changes in the number of kisspeptin- and NKB-positive, but not Dyn-positive, cells in the arcuate nucleus
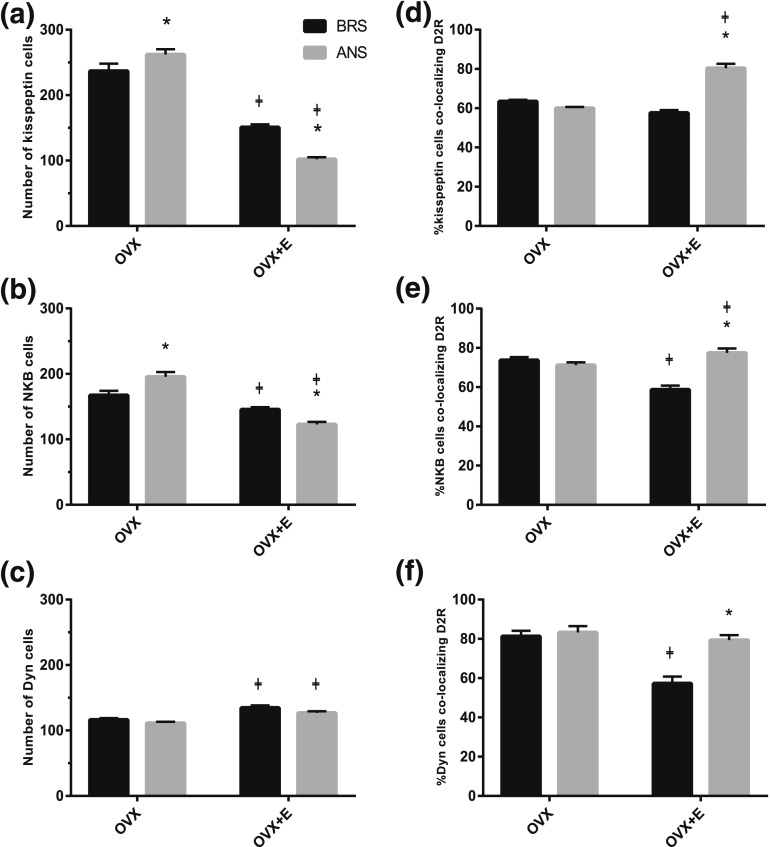
Immunofluorescence microscopy was used to examine the season- and steroid-dependent regulation of ARC KNDy peptides [Fig. 1(a–c)]. Quantitative cell counts of multichannel images (Figs. 2, 3) confirmed previous findings that the number of kisspeptin-positive cells within the ARC are significantly decreased during ANS compared to BRS in OVX+E ewes [P < 0.001; Fig. 1(a)]. Similarly, OVX+E ewes showed a significant decrease in the number of NKB-positive cells during ANS [P < 0.05; Fig. 1(b)]. To ascertain if the seasonal changes in kisspeptin and NKB are steroid dependent, counts were repeated in hypothalamic sections of OVX controls. The ARC of OVX ewes contained significantly higher numbers of both kisspeptin-ir and NKB-ir cells compared to OVX+E animals (P < 0.001); however, unlike OVX+E ewes, OVX controls show significant increases in the total numbers of both kisspeptin (P < 0.05) and NKB cells (P < 0.05) during ANS [Fig. 1(a) and 1(b)].
Figure 1.
(a–c) Mean numbers (±standard error of the mean) of (a) kisspeptin-, (b) NKB-, and (c) Dyn-positive cells in the ARC of OVX and OVX+E BRS (black bars) and anestrous ewes (grey bars). Seasonal differences were seen in kisspeptin, NKB, but not Dyn, cell number in OVX and OVX+E ewes. Additionally, all three peptides showed significant changes in immunoreactivity in the presence of E2 regardless of season. (d–f) Comparison of the mean percentage (±standard error of the mean) of (d) kisspeptin, (e) NKB, and (f) Dyn cells in the ARC that colocalized D2R in OVX and OVX+E BRS (black bars) and anestrous ewes (grey bars). OVX+E ewes showed significant increases in D2R colocalization in all three KNDy peptides during ANS compared to BRS. Significant differences were seen between OVX and OVX+E animals in the percentage of kisspeptin cells that colocalized D2R during ANS, NKB cells that colocalized D2R during both seasons, and Dyn cells that colocalized D2R during the BRS. ‡P < 0.05, OVX vs OVX+E within season; *P < 0.05, BRS vs ANS within treatment group.
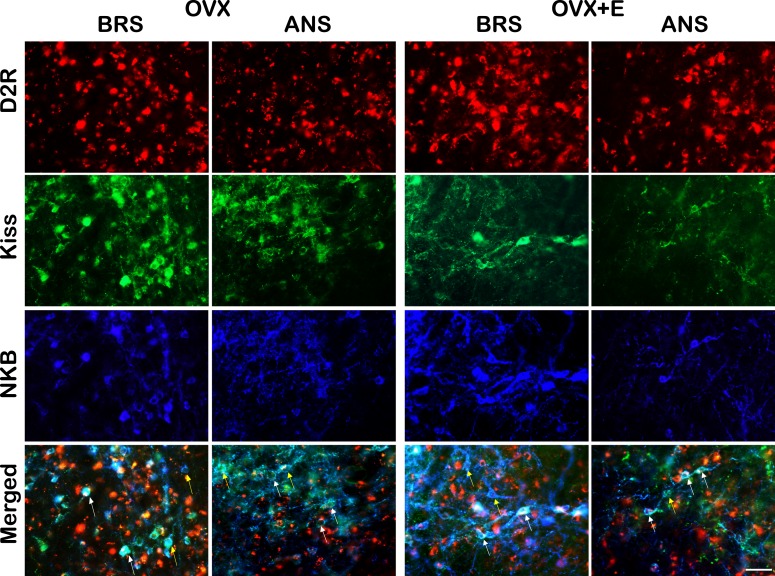
Figure 2.
Multichannel images of ARC D2R- (red), kisspeptin- (green), and NKB- (blue) positive cells in OVX (left) and OVX+E (right) during BRS and ANS. Bottom panels are merged images showing kisspeptin/NKB cells that either colocalize D2R (e.g., white arrows) or do not (e.g., yellow arrows). Scale bar, 50 µm.
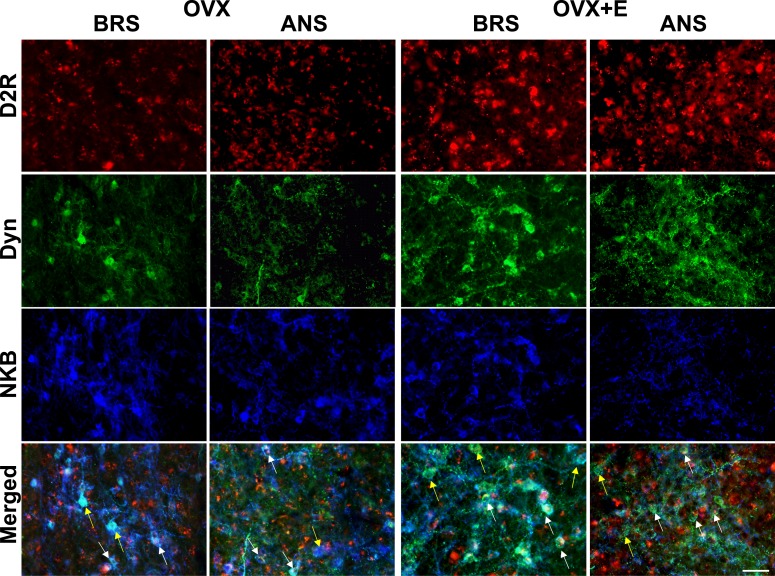
Figure 3.
Multichannel fluorescent images of ARC D2R- (red), Dyn- (green), and NKB- (blue) positive cells in OVX (left) and OVX+E (right) during BRS and ANS. Bottom panels are merged images showing Dyn/NKB cells that either colocalize D2R (e.g., white arrows) or do not (e.g., yellow arrows). Scale bar, 50 µm.
To ascertain if changes in peptide immunoreactivity were occurring primarily in the KNDy population, quantitative analysis of the percentages of kisspeptin and NKB colocalization was also performed. The percentage of kisspeptin cells colocalizing NKB did not change (P = 0.414) between seasons regardless of steroid treatment; however, OVX+E ewes showed a significant decrease in the percentage of NKB cells colocalizing kisspeptin during ANS (P < 0.001; Table 2).
Table 2.
Seasonal Alterations in KNDy Peptide Colocalization in OVX+E Ewes
| Kiss/NKB | NKB/Kiss | Dyn/NKB | NKB/Dyn | |
|---|---|---|---|---|
| BRS | 90.51 ± 0.49 | 94.53 ± 0.94 | 86.54 ± 0.75 | 91.18 ± 0.96 |
| ANS | 91.82 ± 0.41 | 78.40 ± 1.97a | 77.92 ± 0.94a | 89.69 ± 0.35 |
Mean percentage (±standard error of the mean) of ARC cells colocalizing KNDy peptides in OVX+E BRS (top row) and ANS (bottom row) ewes. No significant change was found in either Kiss/NKB or NKB/Dyn between seasons. However, analysis of both NKB/Kiss and Dyn/NKB revealed significant reductions during ANS compared to BRS.
Abbreviations: Kiss/NKB, kisspeptin cells coexpressing neurokinin B; NKB/Kiss, NKB cells coexpressing kisspeptin; Dyn/NKB, dynorphin cells coexpressing NKB; NKB/Dyn, NKB cells coexpressing Dyn.
P < 0.05, BRS vs ANS.
Neither OVX nor OVX+E ewes showed significant changes in the number of Dyn-positive cells between seasons [P = 0.246 and P = 0.079; Fig. 1(c)]. However, Dyn was found to be regulated by E2, independent of season. Interestingly, unlike kisspeptin- and NKB-ir, OVX+E ewes showed small but significant increases in Dyn-ir cell numbers compared to OVX animals [Fig. 1(c)] regardless of season (BRS P < 0.001; ANS P = 0.003). Analysis of the percentage of Dyn cells colocalizing NKB revealed a seasonal effect of E2 with OVX+E (Table 2) ewes showing a significant decrease during ANS (P < 0.001), likely due to the selective inhibition of NKB expression by E2 [Fig. 1(b)]. No effect of season was observed in the percentage of NKB cell colocalizing Dyn in OVX+E ewes (P = 0.297; Table 2).
Steroid-dependent seasonal changes in the D2R colocalization in arcuate KNDy cells
Quantitative analysis of ARC kisspeptin-, NKB-, and Dyn-positive cells colocalizing D2R in OVX and OVX+E ewes during BRS and ANS [Figs. 1(d–f), 2, 3] revealed steroid-dependent seasonal changes in the percentage of D2R colocalization. OVX+E ewes showed significant increases of D2R colocalization with all three KNDy peptides during ANS compared to BRS [P < 0.001; Fig. 1(d–f)]. By contrast, OVX controls showed no significant changes between seasons (P = 0.209, 0.357, and 0.151, respectively). Overall, we observed a significant interaction of season with steroid treatment for the percentages of kisspeptin-, NKB-, and Dyn-positive cells containing D2R [P < 0.001, F(1,14) = 38.51 (kisspeptin), F(1,14) = 33.104 (NKB), F(1,15) = 113.626 (Dyn)]. In addition, analysis of the effects of E2 within season revealed significant increases in both the percentage of kisspeptin and NKB cells colocalizing D2R during ANS [Fig. 1(d) and 1(e)] and significant decreases in the percentages of NKB and Dyn cells colocalizing D2R during the BRS [Fig. 1(e) and 1(f)] in OVX+E ewes compared to OVX animals.
Estradiol regulates seasonal plasticity in TH inputs to ARC kisspeptin cells
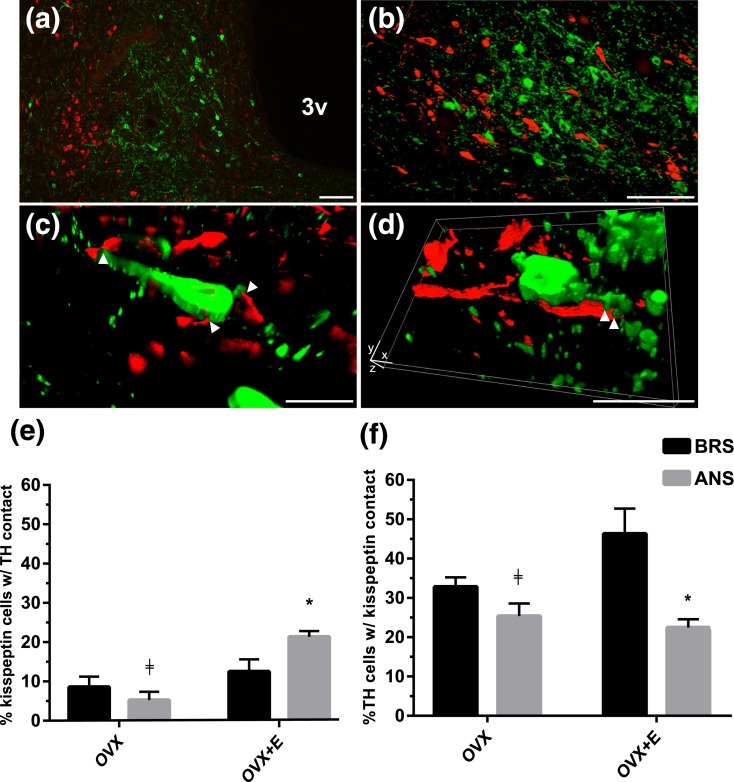
Analysis of confocal Z-stacks revealed TH terminals apposing ARC kisspeptin neurons in both OVX and OVX+E ewes [Fig. 4(a–d)] in both seasons. OVX+E ewes showed a significant increase in the percentage of kisspeptin cells with TH contacts during ANS compared to breading season [P < 0.05; Fig. 4(e)]. Conversely, the percentage of TH-positive cells in the ARC of OVX+E ewes that were in contact with kisspeptin-positive terminals was lower during ANS than the BRS [P < 0.05; Fig. 4(f)]. OVX ewes showed no significant seasonal changes in either TH inputs onto kisspeptin cells (P = 0.975), or kisspeptin inputs onto TH-positive cells (P = 0.203). Additionally, OVX+E ewes showed significant increases in the percentage of kisspeptin cells with TH contacts during ANS (P < 0.001) and the percentage of TH cells with kisspeptin contacts during BRS (P < 0.05) compared to OVX controls. Finally, we saw no significant changes in the total number of TH-positive cells within the MBH of OVX (193.25 ± 59.85, BRS; 168 ± 16.2, ANS) or OVX+E (205.5 ± 29.11, BRS; 117.75 ± 30.61, ANS) ewes due to season (P = 0.622), steroid treatment (P = 0.158) or interactions between season and steroid treatment [F(1,15) = 0.694, P = 0.421].
Figure 4.
(a–d) Low and higher power fluorescent Z-stack-Apotome images showing kisspeptin-positive (green) and TH-positive (red) cells and fibers in the ARC. Arrowheads indicate examples of close contacts between TH-positive fibers and kisspeptin cells. (e, f) Comparison of the mean percentage (±standard error of the mean) of (e) kisspeptin-positive cells receiving TH inputs and (f) TH-positive cells receiving kisspeptin inputs in MBH tissue from OVX and OVX+E BRS (black bars) and anestrous ewes (grey bars). OVX+E ewes showed a significant increase in the percentage of TH inputs to kisspeptin cells during ANS compared to breading season, while there was a significant decrease in the percentage of TH cells with kisspeptin contacts between the same groups. ‡P < 0.05, OVX vs OVX+E within season; *P < 0.05, BRS vs ANS within treatment group. 3v, third ventricle. In (d), xyz notations indicate dimensions in a rotated image. Scale bars in (a, b), 100 µm; (c, d), 20 µm.
Discussion
Seasonal regulation of GnRH secretion is known to be mediated by steroid-independent (1) and steroid-dependent effects of photoperiod (23, 24), with the latter reflecting a seasonal shift in the response to E2-negative feedback evident in OVX+E ewes. Previous studies in the ewe have indicated ARC KNDy neurons as potential mediators of seasonal changes in E2-negative feedback via inhibitory projections from the A15 DA population (3). However, several important questions had yet to be addressed. In the current study, we first demonstrated that kisspeptin and NKB, but not Dyn, undergo steroid-dependent, as well as steroid-independent, seasonal changes in immunoreactivity within the ARC. Secondly we extended previous findings of seasonal changes in D2R colocalization on KNDy neurons (15) and showed that increased D2R expression during ANS is dependent on the presence of E2. Finally, we observed increased numbers of DA contacts onto kisspeptin cells during ANS complementing these changes in D2R expression. These data consistent with the hypothesis implicating ARC KNDy neurons in seasonal changes in E2-negative feedback, and suggest that the influence of dopamine on ARC kisspeptin and NKB peptide immunoreactivity is conveyed directly via afferents to KNDy cells.
Our observation of decreased kisspeptin-ir during ANS in OVX+E ewes is consistent with previous studies in the sheep (14) which showed ~50% reduction in cell number during the non-BRS. Previous work has also indirectly indicated that NKB is under seasonal control (18) with ewes showing similar proportions of dual-labeled NKB and kisspeptin cells in the ARC in both seasons suggesting that NKB-ir must parallel seasonal changes in kisspeptin. In the present study we show that similar to kisspeptin, NKB-ir is significantly decreased during ANS in the ARC of OVX+E ewes. This finding supports the hypothesis that during ANS the increased responsiveness to E2-negative feedback is mediated via increased inhibition of the stimulatory KNDy peptides, NKB and kisspeptin, ultimately resulting in decreased GnRH secretion. The data demonstrating a lack of seasonal changes in Dyn-ir cell number in both OVX and OVX+E ewes confirm and extend our previous work (15) and are consistent with other data demonstrating the selective suppression of some, but not all, KNDy peptides under physiological conditions (25, 26). Finally, we showed that although both kisspeptin and NKB are reduced during ANS in OVX+E ewes, neither the percentages of kisspeptin cells colocalizing NKB, nor NKB cells colocalizing Dyn, were altered suggesting that the seasonal change is targeted to the KNDy population and not single-labeled kisspeptin or NKB cells.
While much of the results from the analysis of KNDy peptide expression reported here are consistent with previous work, two unexpected observations were made. First, the number of both Kiss-ir and NKB-ir cell numbers were higher in OVX ewes during ANS then the BRS. Because this occurred in OVX animals it most likely relates to the steroid-independent effects of photoperiod that produce a small decrease in LH pulse frequency and a corresponding increase in pulse amplitude (20, 27). Given the stimulatory actions of NKB and kisspeptin on GnRH and LH secretion, it is tempting to speculate that these changes contribute to the increased LH pulse amplitude in OVX anestrous ewes. Serotonergic, not dopaminergic, systems have been implicated in the steroid-independent actions of photoperiod (11, 28) so it is possible that the modest inhibition of LH pulse frequency occurs independent of kisspeptin and NKB.
Second, we found that OVX+E animals show increased Dyn-ir compared to OVX ewes in both seasons. The lack of seasonal changes in Dyn in OVX+E ewes suggests a stimulatory role of E2 on Dyn in both seasons consistent with previous findings showing decreased Dyn mRNA following the removal of E2 and progesterone via ovariectomy during the BRS, which was not restored to intact levels by progesterone alone (29). However, the stimulatory effects of E2 on Dyn expression in sheep stand in contrast to evidence of inhibitory effects in rodents, with E2 replacement decreasing Dyn mRNA in the ARC of OVX mice (29). This distinction between rodents and sheep may reflect several physiological differences in the role of Dyn. First, in sheep, Dyn plays a key role as a mediator of progesterone negative feedback during the luteal phase of the estrous cycle (30). The rodent estrous cycle, unlike that of sheep and primates, lacks a true luteal phase, and the major role of progesterone during the rodent estrous cycle is in the generation of the GnRH/LH surge (31). Second, evidence in ruminants (sheep and goats) suggests that Dyn, acting via kappa opioid receptors, serves as the stop signal terminating individual GnRH pulses (32, 33). By contrast, although exogenous Dyn inhibits KNDy cells in mice, kappa antagonists have no effect upon KNDy cell activity or LH pulses in mice and rats respectively [reviewed in Goodman et al. (30)]. Thus, while Dyn plays an obligatory role for GnRH pulse termination in sheep, other, perhaps redundant, signals are required in rodents (34). Additionally, the inhibitory effects of E2 on Dyn expression are mediated via the classical estrogen response element (35), which is not required for negative feedback inhibition of LH secretion by E2 in mice (35).
The stimulatory influence of E2 on Dyn in BRS ewes raises the possibility that Dyn mediates the inhibitory actions of E2 on LH pulse amplitude at this time of year. This hypothesis is consistent with a recent report that the kappa-opioid receptor antagonist, norbinaltorphimine, increased LH secretion in OVX+E, but not OVX, female rats (36), and several earlier studies showing similar effects with nonspecific opioid receptor antagonists in ewes (37, 38). There is one report that naloxone increased GnRH pulse amplitude in both OVX+E and OVX ewes, data that are not consistent with this hypothesis (39). However, there were subtle differences in the effects of naloxone in these two groups that led the authors to point out that the data do not rule out a role for endogenous opioids in E2-negative feedback. If Dyn does mediate E2 inhibition of pulse amplitude, the increase in Dyn-ir in both seasons indicates that this inhibition also occurs in ANS, but is masked by the dramatic inhibition of pulse frequency at this time of year. This would not be surprising because other feedback actions of E2 (21) and progesterone (20) occur in both the breeding and anestrous seasons.
Because D2-R has been identified as the specific receptor subtype holding GnRH pulse frequency in check in ovary-intact anestrous ewes (10), any neural population that plays a role in this inhibition must contain this receptor. Our lab has previously shown a twofold increase in the percentage of Dyn neurons containing D2R during ANS in OVX+E ewes (15). Here, we replicated this finding and extended it to show that, as expected, all three KNDy peptides show increased D2R colocalization during ANS. Moreover, we show that this seasonal effect is steroid dependent with no seasonal differences found in OVX ewes. Furthermore, by demonstrating that Dyn-ir is indeed unaltered between seasons, our results indicate that increased proportion of D2R colocalization is not simply due to decreased KNDy cell numbers. The seasonal changes in D2R suggests that KNDy neurons may have increased sensitivity to dopaminergic inhibition during ANS. It should be noted that an early study was unable to detect changes in response to an exogenous DA agonist between seasons (11), but this did not test for changes in sensitivity because only one dose of agonist was used. Thus further investigation into potential alterations in dopamine responsiveness may be necessary. Together, these findings further support our hypothesis that E2 acts directly on the ARC KNDy population to increase D2R during ANS.
Providing further support for the role of KNDy neurons in the seasonal regulation of GnRH is the observation that kisspeptin neurons show increased DA contacts during ANS consistent with the seasonal changes in D2R. Moreover, this finding provides an alternative explanation to a previous study that reported no change in the number of dopaminergic synapses onto GnRH neurons between seasons (40) despite increased dendritic length and branching of A15 neurons during ANS (41). While the percentage of KNDy cells found to receive TH-ir contacts in our study was relatively low (10% to 20%), this should to be viewed in light of the limitation of kisspeptin immunocytochemistry to reveal the entire dendritic array of these neurons. Thus, the percentage of KNDy cells with TH-ir inputs reported here is likely an underestimate. In addition, the proportion of KNDy cells colocalizing D2R was fairly high (60% to 80%), suggesting that a majority of KNDy neurons have the potential to be targets of dopamine action, whether by synaptic release or by diffusion from nearby dopamine fibers (e.g., volume transmission). Regardless of the exact proportion of KNDy cells involved, kisspeptin signaling appears to play a critical role in the seasonal ability of dopamine to inhibit LH pulses (15). Finally, it should be noted that our current results do not identify the precise area(s) from which the TH-ir contacts onto KNDy neurons originated. The two most likely sources of input are retrochiasmatic area (RCh) A15 DA and ARC A12 DA neurons. Previous tract tracing studies in the sheep showed that DA fibers arising from the A15 cell group innervate the ARC (13), although the precise phenotype of ARC neurons contacted was not determined. More recent work has shown that KNDy neurons do, in fact, receive direct projections from the RCh (42), but that study did not determine whether those projections were dopaminergic or arose from some other neuronal subpopulation in the RCh. The lack of any seasonal change in the number of TH-positive cells in the ARC is consistent with input from the A15 being the source of the increased TH-ir contacts, but further work is needed to definitively distinguish between these two possibilities.
We also observed significant increases in the percentage of ARC TH cells receiving kisspeptin contacts in OVX+E BRS ewes compared to OVX controls. Because ARC TH neurons are important regulators of prolactin secretion (43), this finding is consistent with previous rodent data showing that the ability of kisspeptin to increase prolactin secretion via A12 DA neuron inhibition is dependent on the presence of E2 (44). Interestingly this effect of E2 was not seen in anestrous ewes, which showed a slight decrease in kisspeptin contacts onto TH cells. However, the decrease during ANS could simply be due to the decreased kisspeptin expression at that time of year. Furthermore the increased kisspeptin inputs observed in BRS OVX+E ewes could be arising from the POA kisspeptin population because E2 increases kisspeptin expression in this population (45). Previous findings have indicated that many prolactin pulses occur in synchrony with LH pulses (46, 47), thus kisspeptin-TH connections may represent a potential circuitry mediating the synchronized secretion of the two hormones. However, further investigation is clearly needed to test this hypothesis.
Taken together, our findings provide further evidence that the ARC KNDy population plays a key role in the neural circuitry regulating seasonal breeding in sheep. The increased D2R colocalization and dopaminergic input onto the KNDy population support the hypothesis that the negative feedback actions of E2 during ANS are conveyed via A15 dopaminergic neurons that suppress kisspeptin release from ARC neurons and thereby inhibit GnRH pulse frequency. Steroid-dependent changes in NKB, as well as kisspeptin, suggest that seasonal regulation of NKB may also contribute to increased E2-negative feedback during ANS. Finally, the unexpected observations of (1) steroid-independent increases in the stimulatory KNDy peptides kisspeptin and NKB during ANS and (2) the ability of E2 to increase the inhibitory KNDy peptide Dyn during both seasons, provide novel insights into the complex mechanisms regulating this critical neural population in seasonal breeders.
Acknowledgments
J.T.S. thanks Bruce Doughton, Lynda Morrish, and Elaine Chase for their technical assistance.
Acknowledgments
J.S. was supported by the Australian Research Council (Grants FT0990986 and DP120100521). I.J.C. was supported by the National Health and Medical Research Council of Australia. R.L.G. and M.N.L. were supported by National Institutes of Health Grant RO1 HD17864.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- ANS
- anestrus
- ARC
- arcuate nucleus
- BRS
- breeding season
- D2R
- D2 dopamine receptor
- DA
- dopamine
- Dyn
- dynorphin
- E2
- estradiol
- GnRH
- gonadotropin-releasing hormone
- IHC
- immunohistochemistry
- ir
- immunoreactive
- KNDy
- kisspeptin/neurokinin B/dynorphin
- LH
- luteinizing hormone
- MBH
- mediobasal hypothalamus
- NKB
- neurokinin B
- OVX
- ovariectomized
- PBS
- phosphate-buffered saline
- RCh
- retrochiasmatic area
- TH
- tyrosine hydroxylase.
References
- 1.Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res. 1984;40:185–232. [DOI] [PubMed] [Google Scholar]
- 2.Lehman MN, Ladha Z, Coolen LM, Hileman SM, Connors JM, Goodman RL. Neuronal plasticity and seasonal reproduction in sheep. Eur J Neurosci. 2010;32(12):2152–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weems PW, Goodman RL, Lehman MN. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front Neuroendocrinol. 2015;37:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Legan SJ, Karsch FJ. Neuroendocrine regulation of the estrous cycle and seasonal breeding in the ewe. Biol Reprod. 1979;20(1):74–85. [DOI] [PubMed] [Google Scholar]
- 5.Malpaux B, Wayne NL, Karsch FJ. Termination of the breeding season in the Suffolk ewe: involvement of an endogenous rhythm of reproduction. Biol Reprod. 1988;39(2):254–263. [DOI] [PubMed] [Google Scholar]
- 6.Karsch FJ, Dahl GE, Evans NP, Manning JM, Mayfield KP, Moenter SM, Foster DL. Seasonal changes in gonadotropin-releasing hormone secretion in the ewe: alteration in response to the negative feedback action of estradiol. Biol Reprod. 1993;49(6):1377–1383. [DOI] [PubMed] [Google Scholar]
- 7.Gallegos-Sánchez J, Delaleu B, Caraty A, Malpaux B, Thiéry JC. Estradiol acts locally within the retrochiasmatic area to inhibit pulsatile luteinizing-hormone release in the female sheep during anestrus. Biol Reprod. 1997;56(6):1544–1549. [DOI] [PubMed] [Google Scholar]
- 8.Hardy SL, Anderson GM, Valent M, Connors JM, Goodman RL. Evidence that estrogen receptor alpha, but not beta, mediates seasonal changes in the response of the ovine retrochiasmatic area to estradiol. Biol Reprod. 2003;68(3):846–852. [DOI] [PubMed] [Google Scholar]
- 9.Anderson GM, Connors JM, Hardy SL, Valent M, Goodman RL. Oestradiol microimplants in the ventromedial preoptic area inhibit secretion of luteinizing hormone via dopamine neurones in anoestrous ewes. J Neuroendocrinol. 2001;13(12):1051–1058. [DOI] [PubMed] [Google Scholar]
- 10.Meyer SL, Goodman RL. Neurotransmitters involved in mediating the steroid-dependent suppression of pulsatile luteinizing hormone secretion in anestrous ewes: effects of receptor antagonists. Endocrinology. 1985;116(5):2054–2061. [DOI] [PubMed] [Google Scholar]
- 11.Meyer SL, Goodman RL. Separate neural systems mediate the steroid-dependent and steroid-independent suppression of tonic luteinizing hormone secretion in the anestrous ewe. Biol Reprod. 1986;35(3):562–571. [DOI] [PubMed] [Google Scholar]
- 12.Gayrard V, Thiéry JC, Thibault J, Tillet Y. Efferent projections from the retrochiasmatic area to the median eminence and to the pars nervosa of the hypophysis with special reference to the A15 dopaminergic cell group in the sheep. Cell Tissue Res. 1995;281(3):561–567. [DOI] [PubMed] [Google Scholar]
- 13.Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. J Neuroendocrinol. 2010;22(7):674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goodman RL, Maltby MJ, Millar RP, Hileman SM, Nestor CC, Whited B, Tseng AS, Coolen LM, Lehman MN. Evidence that dopamine acts via kisspeptin to hold GnRH pulse frequency in check in anestrous ewes. Endocrinology. 2012;153(12):5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–5760. [DOI] [PubMed] [Google Scholar]
- 17.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakamoto K, Murata K, Wakabayashi Y, Yayou K, Ohkura S, Takeuchi Y, Mori Y, Okamura H. Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. J Reprod Dev. 2012;58(6):700–706. [DOI] [PubMed] [Google Scholar]
- 19.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 20.Goodman RL, Bittman EL, Foster DL, Karsch FJ. Alterations in the control of luteinizing hormone pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biol Reprod. 1982;27(3):580–589. [DOI] [PubMed] [Google Scholar]
- 21.Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol. 1981;89(2):229–240. [DOI] [PubMed] [Google Scholar]
- 22.Backholer K, Smith JT, Rao A, Pereira A, Iqbal J, Ogawa S, Li Q, Clarke IJ. Kisspeptin cells in the ewe brain respond to leptin and communicate with neuropeptide Y and proopiomelanocortin cells. Endocrinology. 2010;151(5):2233–2243. [DOI] [PubMed] [Google Scholar]
- 23.Bittman EL, Karsch FJ, Hopkins JW. Role of the pineal gland in ovine photoperiodism: regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology. 1983;113(1):329–336. [DOI] [PubMed] [Google Scholar]
- 24.Woodfill CJ, Wayne NL, Moenter SM, Karsch FJ. Photoperiodic synchronization of a circannual reproductive rhythm in sheep: identification of season-specific time cues. Biol Reprod. 1994;50(4):965–976. [DOI] [PubMed] [Google Scholar]
- 25.Cheng G, Coolen LM, Padmanabhan V, Goodman RL, Lehman MN. The kisspeptin/neurokinin B/dynorphin (KNDy) cell population of the arcuate nucleus: sex differences and effects of prenatal testosterone in sheep. Endocrinology. 2010;151(1):301–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Overgaard A, Ruiz-Pino F, Castellano JM, Tena-Sempere M, Mikkelsen JD. Disparate changes in kisspeptin and neurokinin B expression in the arcuate nucleus after sex steroid manipulation reveal differential regulation of the two KNDy peptides in rats. Endocrinology. 2014;155(10):3945–3955. [DOI] [PubMed] [Google Scholar]
- 27.Thomas GB, Pearce DT, Oldham CM, Martin GB, Lindsay DR. Effects of breed, ovarian steroids and season on the pulsatile secretion of LH in ovariectomized ewes. J Reprod Fertil. 1988;84(1):313–324. [DOI] [PubMed] [Google Scholar]
- 28.Whisnant CS, Goodman RL. Further evidence that serotonin mediates the steroid-independent inhibition of luteinizing hormone secretion in anestrous ewes. Biol Reprod. 1990;42(4):656–661. [DOI] [PubMed] [Google Scholar]
- 29.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, Clifton DK, Tena-Sempere M, Steiner RA. Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. Am J Physiol Endocrinol Metab. 2010;300(1):E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 31.Goodman RL, Lehman MN. Kisspeptin neurons from mice to men: similarities and differences. Endocrinology. 2012;153(11):5105–5118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, Millar RP, Cernea M, Coolen LM, Lehman MN. Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology. 2013;154(11):4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodman RL, Coolen LM, Lehman MN. Unraveling the mechanism of action of the GnRH pulse generator: a possible role for kisspeptin/neurokinin B/dynorphin (KNDy) neurons. In: Ulloa-Aguirre A, Conn E, eds. Cellular Endocrinology in Health and Disease. London, United Kingdom: Academic Press; 2014:133–152. [Google Scholar]
- 35.Gottsch ML, Navarro VM, Zhao Z, Glidewell-Kenney C, Weiss J, Jameson JL, Clifton DK, Levine JE, Steiner RA. Regulation of Kiss1 and dynorphin gene expression in the murine brain by classical and nonclassical estrogen receptor pathways. J Neurosci. 2009;29(29):9390–9395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mostari P, Ieda N, Deura C, Minabe S, Yamada S, Uenoyama Y, Maeda K, Tsukamura H. Dynorphin-kappa opioid receptor signaling partly mediates estrogen negative feedback effect on LH pulses in female rats. J Reprod Dev. 2013;59(3):266–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang K, Haynes NB, Lamming GE, Brooks AN. Ovarian steroid hormone involvement in endogenous opioid modulation of LH secretion in mature ewes during the breeding and non-breeding seasons. J Reprod Fertil. 1988;83(1):129–139. [DOI] [PubMed] [Google Scholar]
- 38.Whisnant CS, Goodman RL. Effects of an opioid antagonist on pulsatile luteinizing hormone secretion in the ewe vary with changes in steroid negative feedback. Biol Reprod. 1988;39(5):1032–1038. [DOI] [PubMed] [Google Scholar]
- 39.Goodman RL, Robinson JE, Kendrick KM, Dyer RG. Is the inhibitory action of estradiol on luteinizing hormone pulse frequency in anestrous ewes mediated by noradrenergic neurons in the preoptic area? Neuroendocrinology. 1995;61(3):284–292. [DOI] [PubMed] [Google Scholar]
- 40.Jansen HT, Cutter C, Hardy S, Lehman MN, Goodman RL. Seasonal plasticity within the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in identified GnRH inputs and glial association. Endocrinology. 2003;144(8):3663–3676. [DOI] [PubMed] [Google Scholar]
- 41.Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147(10):4843–4851. [DOI] [PubMed] [Google Scholar]
- 42.Grachev P, Porter KL, Coolen LM, McCosh RB, Connors JM, Hileman SM, Lehman MN, Goodman RL. Surge-like luteinising hormone secretion induced by retrochiasmatic area NK3R activation is mediated primarily by arcuate kisspeptin neurones in the ewe. J Neuroendocrinol. 2016;28(6):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ben-Jonathan N, Hnasko R. Dopamine as a prolactin (PRL) inhibitor. Endocr Rev. 2001;22(6):724–763. [DOI] [PubMed] [Google Scholar]
- 44.Ribeiro AB, Leite CM, Kalil B, Franci CR, Anselmo-Franci JA, Szawka RE. Kisspeptin regulates tuberoinfundibular dopaminergic neurones and prolactin secretion in an oestradiol-dependent manner in male and female rats. J Neuroendocrinol. 2015;27(2):88–99. [DOI] [PubMed] [Google Scholar]
- 45.Smith JT. Sex steroid control of hypothalamic Kiss1 expression in sheep and rodents: comparative aspects. Peptides. 2009;30(1):94–102. [DOI] [PubMed] [Google Scholar]
- 46.Masaoka K, Kitazawa M, Kumasaka T. Pulsatile secretion of prolactin and luteinizing hormone and their synchronous relationship during the human menstrual cycle. Gynecol Endocrinol. 1988;2(4):293–303. [DOI] [PubMed] [Google Scholar]
- 47.Graves GR, Gotelli NJ. Assembly of avian mixed-species flocks in Amazonia. Proc Natl Acad Sci USA. 1993;90(4):1388–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]