Abstract
Thyroiditis and autoantibodies to thyroglobulin (TgAb) and thyroid peroxidase (TPOAb) develop spontaneously in NOD.H2h4 mice, a phenotype enhanced by dietary iodine. NOD.H2h4 mice were derived by introducing the major histocompatibility class (MHC) molecule I-Ak from B10.A(4R) mice to nonobese diabetic (NOD) mice. Apart from I-Ak, the genes responsible for the NOD.H2h4 phenotype are unknown. Extending serendipitous observations from crossing BALB/c to NOD.H2h4 mice, thyroid autoimmunity was investigated in both genders of the F1, F2, and the second-generation backcross of F1 to NOD.H2h4 (N2). Medium-density linkage analysis was performed on thyroid autoimmunity traits in F2 and N2 progeny. TgAb develop before TPOAb and were measured after 8 and 16 weeks of iodide exposure; TPOAb and thyroiditis were studied at 16 weeks. TgAb, TPOAb, and thyroiditis, absent in BALB/c and F1 mice, developed in most NOD.H2h4 and in more N2 than F2 progeny. No linkages were observed in F2 progeny, probably because of the small number of autoantibody-positive mice. In N2 progeny (equal numbers of males and females), a chromosome 17 locus is linked to thyroiditis and TgAb and is suggestively linked to TPOAb. This locus includes MHC region genes from B10.A(4R) mice (such as I-Ak and Tnf, the latter involved in thyrocyte apoptosis) and genes from NOD mice such as Satb1, which most likely plays a role in immune tolerance. In conclusion, MHC and non-MHC genes, encoded within the chromosome 17 locus from both B10.A(4R) and NOD strains, are most likely responsible for the Hashimoto disease–like phenotype of NOD.H2h4 mice.
Linkage analysis of thyroid autoantibodies and thyroiditis demonstrated that MHC and non-MHC genes on chromosome 17 are likely responsible for the Hashimoto disease–like phenotype of NOD.H2h4 mice.
Thyroiditis and autoantibodies to the autoantigens thyroglobulin (TgAb) and thyroid peroxidase (TPOAb) develop spontaneously in NOD.H2h4 mice (1–4). The phenotype of this model of Hashimoto disease is enhanced by exposure to iodine in the drinking water. The NOD.H2h4 strain was derived by crossing nonobese diabetic (NOD) mice with the nonautoimmune B10.A(4R) strain as part of a study that demonstrated the importance of the NOD major histocompatibility (MHC) genes in determining the incidence of autoimmune diabetes (5).
Susceptibility to thyroiditis induced experimentally by immunization with mouse thyroglobulin (Tg) is associated with genes in the MHC region [for example (6–9)]. In particular, induced thyroiditis usually requires the MHC class II molecule I-Ak, which is present in NOD.H2h4 mice (10). The Ceat1 locus, tightly linked to, but distinct from MHC, controlled chronic induced thyroiditis in NOD mice (11). Thyroiditis develops spontaneously in transgenic mice expressing the CCL21 (C-C motif chemokine ligand 21) in the thyroid (12) and in NOD mice lacking the chemokine receptor CCR7 [chemokine (C-C motif) receptor 7] (13). Non-MHC genes associated with induced murine thyroiditis and TgAb include the susceptible Tg haplotype (14) and the absence of interleukin 10 (15).
A segregation analysis performed shortly after the NOD.H2h4 strain was generated showed that susceptibility to thyroiditis was polygenic (10), but this investigation does not appear to have been followed up. In a comparison of NOD.H2h4 and NOD.H2k strains, both I-Ak positive, we found that development of TPOAb most likely involves the absence of MHC class II I-E (16), which is expressed in NOD.H2k, but not in NOD.H2h4 mice (5). Spontaneous development of TgAb and thyroiditis in NOD.H2h4 mice does not involve the susceptible thyroglobulin haplotype associated with induced thyroiditis (14). Apart from I-Ak, it is not known whether any other genes associated with murine thyroiditis contribute to the spontaneous/iodine-enhanced phenotype of NOD.H2h4 mice.
Recently, we performed a series of backcrosses to introduce a transgene for the thyrotropin receptor (TSHR) A-subunit from BALB/c mice to NOD.H2h4 recipients (17). As a control for antibodies to the transgene, we monitored TgAb and TPOAb in transgenic and nontransgenic progeny in each backcross generation. We observed that TgAb and TPOAb were absent in the first filial (F1) generation, but were present in some progeny from the F1 backcrossed to NOD.H2h4 (N2 generation) (17). The goal of the current study was to extend this finding to determine the genetic basis for the NOD.H2h4 phenotype, namely the development of TgAb and TPOAb and thyroiditis.
Materials and Methods
Crossing NOD.H2h4 and BALB/c mice
NOD.H2h4 (NOD.Cg_H2h4/DilTacUmmJ) and BALB/cJ mice (originally from The Jackson Laboratory, Bar Harbor, ME) were bred at Cedars-Sinai Medical Center. For genetic studies, the following crosses were made (Table 1): (1) male BALB/c were crossed to female NOD.H2h4 mice to generate F1 progeny; (2) F2 mice were derived by intercrossing male F1 to female F1 mice; and (3) N2 mice were generated by backcrossing F1 males to NOD.H2h4 females. Some N2 mice were derived from F1 males bearing the human TSHR A-subunit transgene (Lo or Hi expressor) (18, 19) bred to nontransgenic NOD.H2h4 females. We previously showed that the development of TgAb or TPOAb did not differ between NOD.H2h4 mice with or without the transgene (17).
Table 1.
Crossing NOD.H2h4 and BALB/c To Generate F1, N2, and F2 Offspring
| Mouse Strains Crossed | Nomenclature |
|---|---|
| BALB/c male × NOD.H2h4 female | F1 |
| F1 male × to NOD.H2h4 female | N2 |
| F1 male × F1 female | F2 |
Some N2 mice were derived from F1 males bearing the human TSHR A-subunit transgene (Lo or Hi expressor) (18, 19) bred to nontransgenic NOD.H2h4 females. All F2 mice were derived from nontransgenic F1 mice.
From 8 weeks of age, F1, N2, and F2 progeny as well as parental strains (NOD.H2h4 and BALB/c) received water supplemented with 0.05% sodium iodide (NaI). Blood was drawn after 8 weeks on NaI, and mice were euthanized after 16 weeks (aged 24 weeks) to harvest tails (for DNA analysis), blood, and thyroid tissue. All mouse studies were performed with the highest standards of care in accordance with the guidelines of the Institutional Animal Care and Use Committee at Cedars-Sinai Medical Center.
H2-E haplotype
DNA from ear punch tissue (used for identification) or tail clips (at euthanasia) was extracted using DNeasy (Qiagen, Valencia, CA). H2 haplotypes were determined by polymerase chain reaction using the following primer sequences: Ea5 (5′-AGT CTT CCC AGC CTT CAC ACT CAG AGG TAC-3′), Ea3 (5′-CAT AGC CCC AAA TGT CTG ACC TCT GGA GAG-3′), and K5 (5′-CAT GGG CAT AGA AAG GGC ACT CTT TGA ACT-3′). The polymerase chain reaction yields a 250-bp band for BALB/c (H2-d), a 150-bp band for NOD.H2h4 (H2-k), and both bands (kd) for all F1 mice and for some F2 progeny. H2 haplotypes were confirmed by single-nucleotide polymorphism (SNP) genotype data detailed later.
TgAb
Tg was isolated from murine thyroid glands, as described (4). Enzyme-linked immunosorbent assay (ELISA) wells (Immulon 4HBX; Thermo Fisher Scientific, Rochester, NY) were coated with mouse Tg (1.5 μg/mL) and incubated with test sera (duplicate aliquots, 1:100 dilution). Antibody binding was detected with horseradish peroxidase–conjugated goat anti-mouse immunoglobulin G (IgG; A3673; Sigma-Aldrich, St. Louis, MO) (Table 2), the signal developed with o-phenylenediamine, and the reaction terminated using 20% H2SO4. The negative control was serum from 8-week-old NOD.H2h4 mice; the positive control was serum from BALB/c mice immunized with mouse Tg and complete Freund’s adjuvant (20). TgAb values are presented as the optical density at 490 nm, normalized to the TgAb positive standard.
Table 2.
Antibodies Used To Detect Mouse IgG Class Antibodies Binding in ELISA or in Flow Cytometry
| Peptide/Protein Target | Antigen Sequence (If Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| Mouse IgG | Horseradish peroxidase–conjugated anti-mouse IgG | A3673; Sigma-Aldrich | Goat; polyclonal | 1 in 1000 | AB_258099 | |
| Mouse IgG | Fluorescein isothiocyanate–conjugated anti-mouse IgG | M30101; Invitrogen | Goat; polyclonal; affinity purified | 10 μg/mL | AB_10563447 |
Abbreviation: RRID, Research Resource Identifier.
TPOAb
TPOAb were measured using Chinese hamster ovary (CHO) cells stably expressing mouse thyroid peroxidase (TPO) (4). Sera (1:50 dilution) were incubated with mouse TPO-CHO cells, and binding was detected with fluorescein isothyocyanate–conjugated affinity-purified goat anti-mouse IgG (M30101; Invitrogen, Carlsbad, CA) (Table 2). Cells staining with propidium iodide (1 μg/mL) were excluded from the analysis. The negative control for IgG class antibody binding to mouse TPO-CHO cells was serum from 8-week-old NOD.H2h4 mice. Positive controls were mouse monoclonal antibodies 15 and 64 generated to human TPO (21), provided to us by J. Ruf (INSERM-URA, Faculté de Médecine, Marseille, France), which recognize mouse TPO (4, 21). Flow cytometry was performed (10,000 events) using a FACScanto II with CELLQUEST Software (BD Biosciences, San Jose, CA). Data are reported as the geometric mean.
Thyroid histology
Thyroid glands were preserved in zinc fixative (BD Pharmingen, San Diego, CA) and paraffin embedded, and serial sections were stained with hematoxylin and eosin (IDEXX BioResearch Laboratory Animal and Biological Materials Diagnostic Testing, Columbia, MO). Thyroid histology was examined by two researchers (S.M.M. and B.B.) and later compared for consistency. Scoring of thyroid lymphocytic infiltration was expressed as a percentage; thyroid sections with no infiltration were assigned the value 1%.
Statistics
Significant differences between responses in different groups were determined by nonparametric tests (Mann–Whitney rank sum test or U statistic) or, when normally distributed, by Student t test. Multiple comparisons were made using analysis of variance. Tests were performed using SigmaStat (Jandel Scientific Software, San Rafael, CA).
Linkage analysis
The F2 linkage analysis excluded mice homozygous for the BALB/c MHC haplotype and involved 89 F2 mice (41 males, 48 females). Genomic DNA was isolated from the tails of these 89 F2 mice and from all of the 81 N2 mice (40 males, 41 females). SNP were determined using the Illumina mouse medium-density linkage panel by the Centre for Applied Genomics, Hospital for Sick Children (Ontario, Canada). The marker locations were determined using the National Center for Biotechnology Information build m37. As controls, both SNP screens included DNA from NOD.H2h4, BALB/cJ, and NOD.H2h4 × BALB/cJ F1 mice.
Following our previous approach (22), the logarithm of the odds (LOD) scores were obtained through a single quantitative trait locus model using the R/qtl package (23) for the R software (version 3.0.0). To increase SNP resolution, we applied the Haley–Knott algorithm (24). For the N2 linkage analysis, LOD scores of 2.77 were significant for single-dimensional analysis according to permutation tests (n = 10,000, P = 0.05), and LOD scores of >1.51 were considered suggestive. A Pearson’s C2 for allele frequencies was applied, and SNP distributions that deviate from the Hardy–Weinberg equilibrium (P ≤ 0.05) were removed. Two additional markers, D19Mit91 and D19Mit123, were added on chromosome 19 for the high-resolution map. For significant LOD scores on chromosome 17, estimation of the interval coordinates was obtained using a 95% Bayes interval test.
Some N2 mice expressed the transgene for the low- or the high-expressor human TSHR A-subunit (18). The transgene locations were determined to be on chromosome 1 (22.9 to 39.3 Mb) for the low expressor and on chromosome 2 (127 to 147 Mb) for the high expressor (25). The presence of the transgene did not bias the Hardy–Weinberg equilibrium, and no suggestive or significant LOD scores were observed in these genetic intervals.
Results
TgAb in parental strains, F1, F2, and N2 progeny
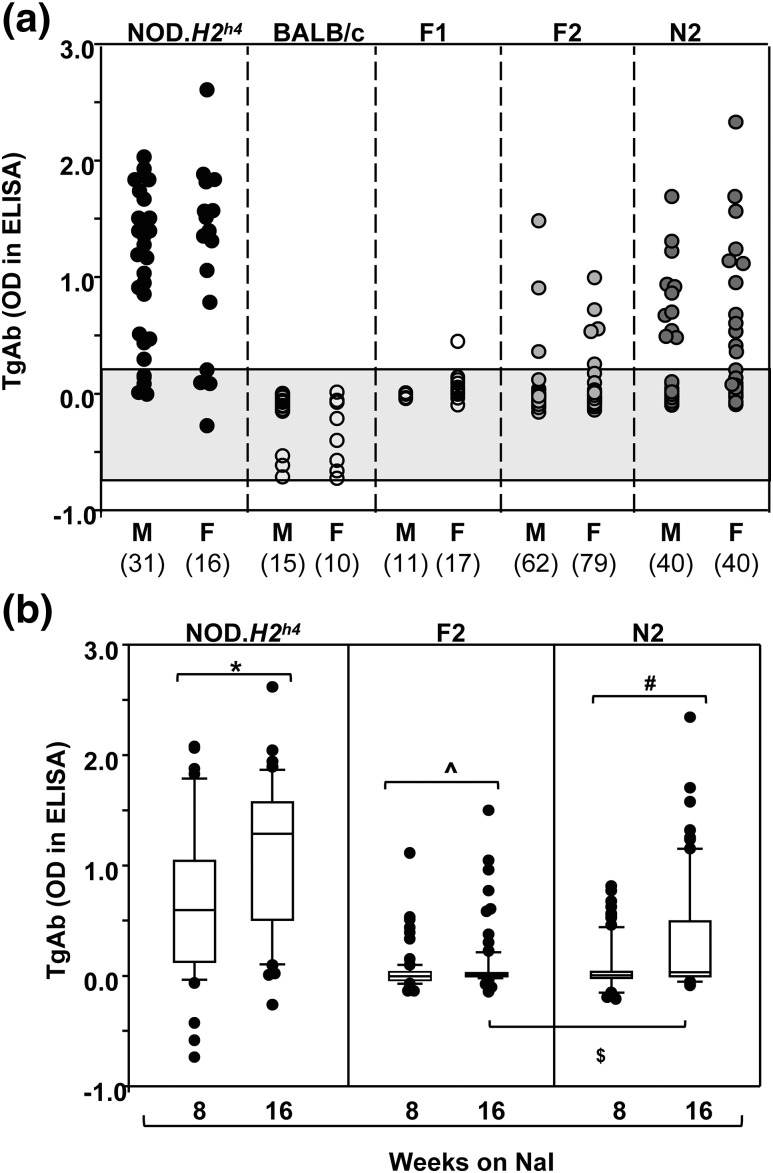
NOD.H2h4, BALB/c, and progeny were exposed to NaI for 16 weeks. Because TgAb develop earlier than TPOAb (4), they were measured after 8 and 16 weeks. TgAb were detectable in the majority of NOD.H2h4 mice, were absent in BALB/c and virtually all F1 pups, and developed in some F2 and N2 progeny [Fig. 1(a)]. Significantly more N2 than F2 offspring were TgAb positive (16 weeks) (χ2 test with Yates correction, P = 0.015). The levels of TgAb were not significantly different between males and females in any group. As expected, after exposure to NaI, TgAb levels increased over time [Fig. 1(b)]. Some N2 offspring were derived by crossing nontransgenic NOD.H2h4 females with transgenic F1 males bearing the human TSHR A-subunit (Lo or Hi expressor) (18, 19). Generation of TgAb and TPOAb developed similarly in nontransgenic and transgenic N2 offspring (Supplemental Fig. 1 (224KB, pdf) ), consistent with our previous observations (17).
Figure 1.
(a) TgAb in male and female NOD.H2h4, BALB/c, and F1, F2, and N2 pups. Values for TgAb (OD 490 nm in ELISA) are shown for individual mice exposed to NaI for 16 weeks. The numbers of mice in each group are provided in parentheses. The shaded area represents the mean ±2 standard deviations for values in BALB/c mice. (b) Comparison of TgAb after 8 and 16 weeks’ exposure to NaI in NOD.H2h4, F2, and N2 mice. The data are shown as box plots, including the median, 10th, 25th, 75th, and 90th percentiles; symbols indicate values for outliers. Significant differences: NOD.H2h4, *P < 0.001 (t test); F2, ^P = 0.017 (rank sum test); and N2, #P = 0.007 (rank sum test). OD, optical density.
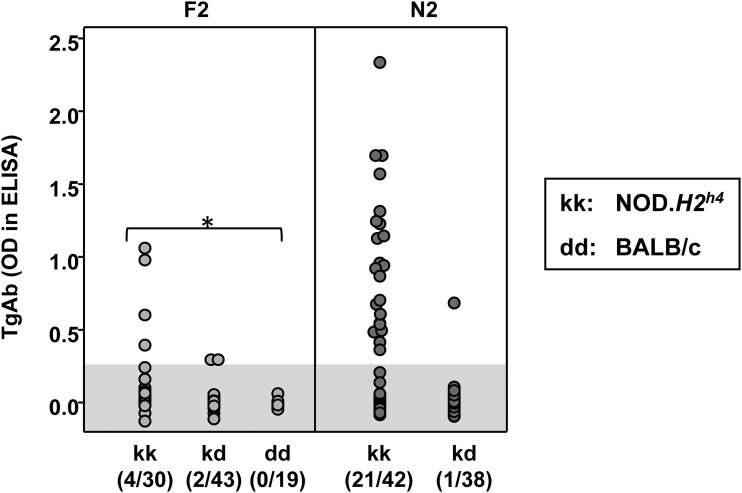
The relationship was determined for TgAb (16 weeks on NaI) and the MHC haplotype (kk in NOD.H2h4 versus dd in BALB/c). TgAb were undetectable in all F2 pups homozygous for the BALB/c MHC haplotype (dd) (Fig. 2, left panel); for this reason, F2 linkage analysis was performed on progeny homozygous or heterozygous for the NOD.H2h4 MHC haplotype. With a few exceptions, TgAb were present only in N2 mice homozygous (kk) for the NOD.H2h4 haplotype (Fig. 2, right panel).
Figure 2.
TgAb in relation to the MHC haplotype: H2 kk, kd, or kd (F2 progeny) and H2 kk or kd (N2 progeny). The haplotypes for NOD.H2h4 and BALB/c are kk and dd, respectively. In parentheses, the number of mice positive for TgAb vs the total number of mice. TgAb levels significantly higher for mice of kk than dd for F2: *analysis of variance, P < 0.05. The shaded area represents the mean ±2 standard deviations for values in BALB/c mice. OD, optical density.
TPOAb and thyroiditis in parental strains, F1, F2, and N2 progeny
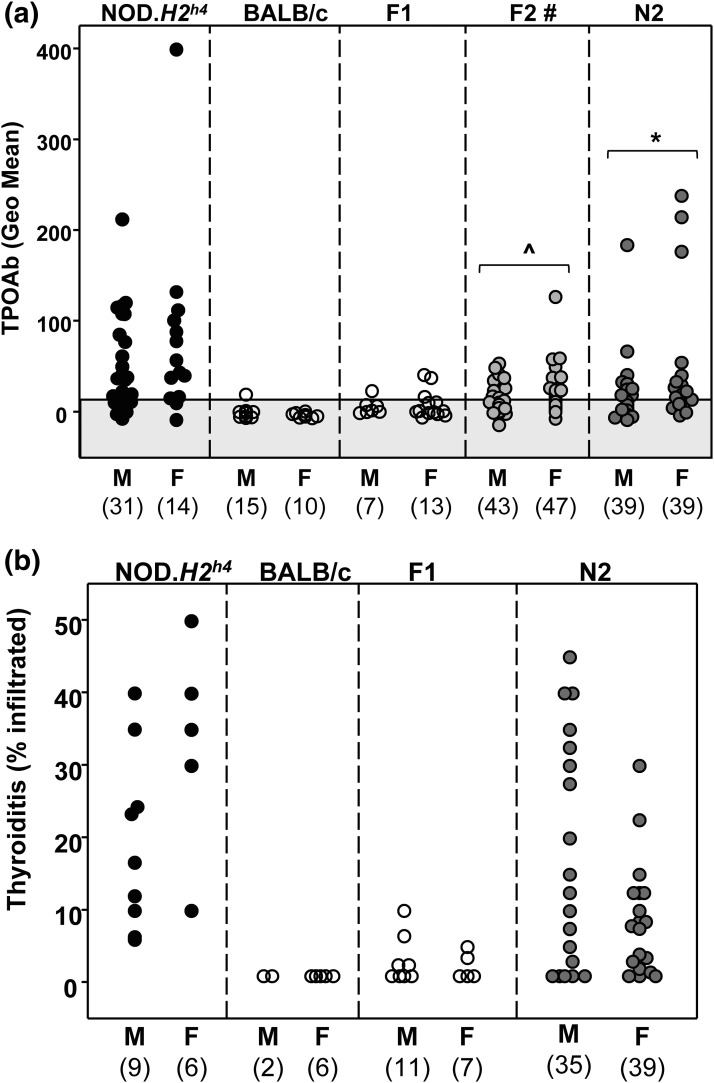
TPOAb (measured at 16 weeks) were present in NOD.H2h4 males and females, were absent in BALB/c and F1 mice, and were detectable in some F2 and N2 mice [Fig. 3(a)]. Unlike the lack of gender differences for TgAb, TPOAb levels were significantly higher in female than male F2 and N2 mice [Fig. 3(a)]. Similar numbers of male and female F2 and N2 mice were included in the linkage analyses.
Figure 3.
(a) TPOAb develop in male and female parental NOD.H2h4 and some F2 and N2 pups, but not in parental BALB/c or F1 mice. Data are shown for individual male and female mice; the numbers of mice in each group are provided in parentheses. The shaded area represents the mean ±2 standard deviations for values in BALB/c mice. Significant differences between males and females: F2, ^P = 0.01 (Mann–Whitney U statistic); N2, *P = 0.004 (Mann–Whitney U statistic). #TPOAb were measured in F2 offspring that were homozygous or heterozygous for the NOD.H2h4 MHC haplotype (kk or kd). (b) Thyroiditis in male and female parental NOD.H2h4, BALB/c, F1, and N2 pups. Thyroiditis was not determined in F2 offspring. Data are shown for individual mice. The numbers of mice in each group are provided in parentheses. The value 1 was assigned to thyroid glands with no infiltration. Geo, geometric.
Thyroiditis developed in NOD.H2h4 males and females, was absent in BALB/c mice, and developed in some male and female N2 progeny [Fig. 3(b)]. The degree of thyroiditis was not significantly different in males and females. Thyroiditis was not studied in F2 offspring.
Linkage analysis
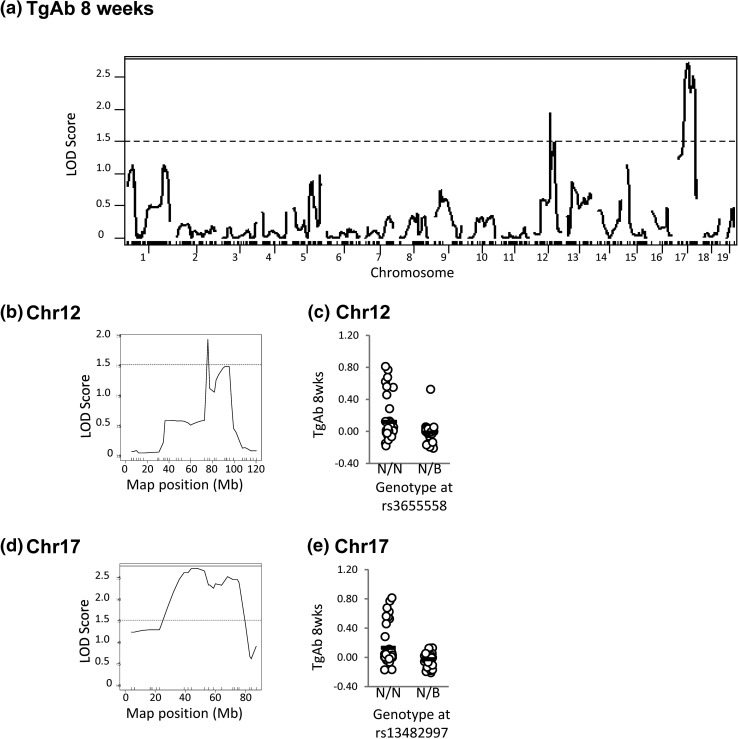
For linkage analysis, LOD scores of 1.51 are suggestive and scores of 2.77 are significant for single-dimensional analysis according to permutation tests (n = 10,000, P = 0.05). The analysis performed for TgAb and TPOAb in F2 progeny showed no suggestive or significant linkages. In contrast, the following linkages were observed for N2 mice:
-
(1)
Loci on chromosomes 12 and 17 are suggestively (LOD >1.51) linked to TgAb after 8 weeks on NaI (Fig. 4).
-
(2)
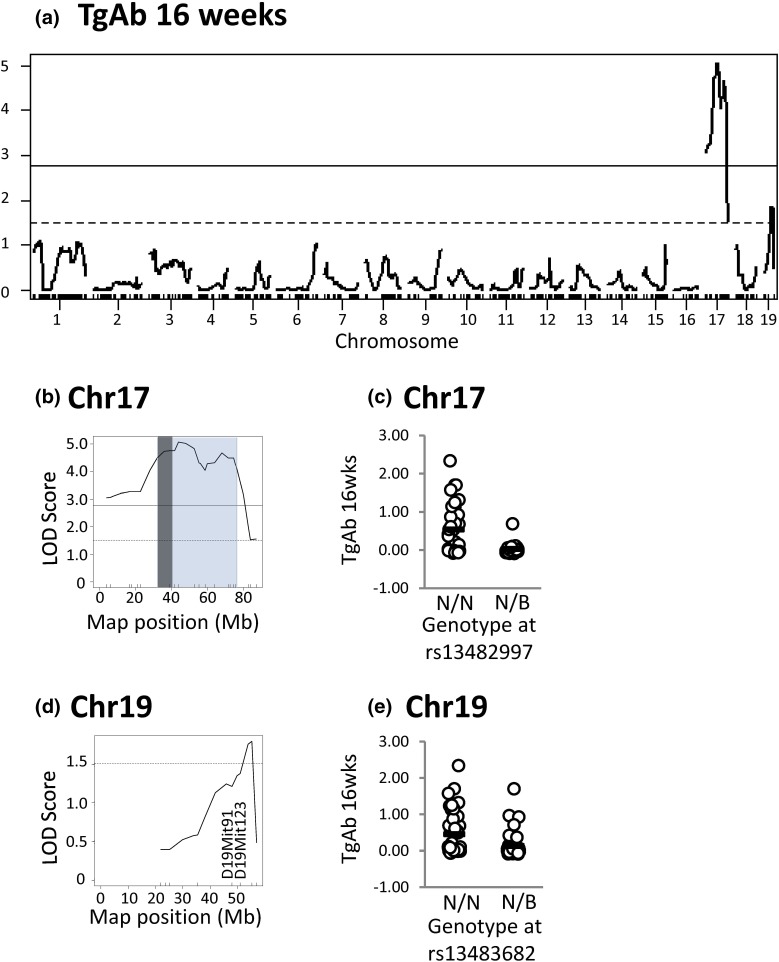
After 16 weeks on NaI, the chromosome 17 locus is highly significantly linked to TgAb (LOD score > 5.0) and a locus on chromosome 19 is suggestively linked to this trait (Fig. 5).
-
(3)
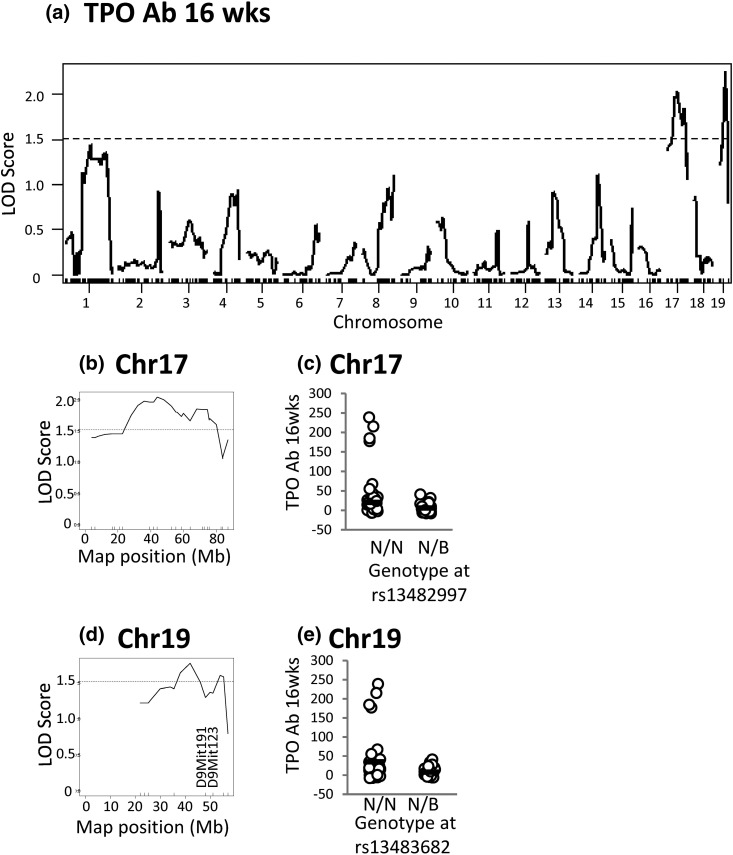
The same loci linked to TgAb on chromosomes 17 and 19 are suggestively linked to TPOAb (16 weeks) (Fig. 6).
-
(4)
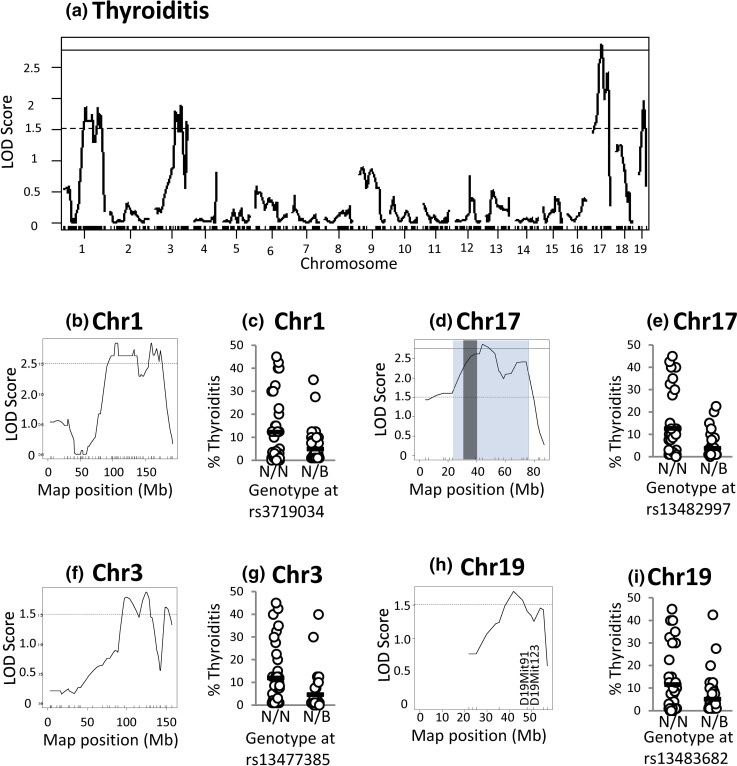
Thyroiditis is a complex trait most likely involving multiple mechanisms. The locus on chromosome 17 linked to three other traits (TgAb 8 weeks, TgAb 16 weeks, and TPOAb) is also significantly linked to thyroiditis. In addition, loci on chromosomes 1, 3, and 19 are suggestively linked to thyroiditis (Fig. 7).
Figure 4.
Linkage analysis in N2 mice for TgAb (8 weeks). (a) Genome-wide LOD scores. (b) High-resolution map for chromosome 12. (c) TgAb levels in mice segregated on their genotype at rs3655558. (d) High-resolution map for chromosome 17. (e) TgAb levels in mice segregated on their genotype at rs13482997. N2 genotypes N/N or N/B: N indicates NOD.H2h4, and B indicates BALB/c. The significant threshold is 2.77 (solid lines), and the suggestive threshold is 1.51 (dotted line).
Figure 5.
Loci on chromosomes 17 and 19 are significantly and suggestively linked to TgAb (16 weeks) in N2 mice. (a) Genome-wide LOD scores. (b) High-resolution map for chromosome 17, in which the Bayes confidence interval is shaded (blue) with the MHC region in dark gray. (c) TgAb levels in mice segregated on their genotype at rs13482997. (d) High-resolution map for chromosome 19. (e) TgAb levels in mice segregated according to their genotype at rs13483682. N2 genotypes N/N or N/B: N indicates NOD.H2h4, and B indicates BALB/c. The significant threshold is 2.77 (solid lines), and suggestive threshold is 1.51 (dotted lines). *P < 0.001 (t test).
Figure 6.
Loci on chromosomes 17 and 19 are suggestively linked to TPOAb levels (16 weeks) in N2 mice. (a) Genome-wide LOD scores. (b) High-resolution map for chromosome 17. (c) TPOAb levels in mice segregated according to their genotype at rs13482997. (d) High-resolution map for chromosome 19. (e) TPOAb levels in mice segregated according to their genotype at rs13483682. For N2 genotypes N/N or N/B, N indicates NOD.H2h4, and B indicates BALB/c. The suggestive threshold is 1.51 (dotted lines).
Figure 7.
Linkage analysis of thyroiditis in N2 mice (16 weeks) reveals a complex trait significantly linked to loci on Chr 17 and suggestively linked to loci on Chr 1, 3, and 19. (a) Genome-wide LOD scores. (b) High-resolution map for chromosome 1. (c) Degree of thyroiditis for mice segregated on their genotype at rs3719034. (d) High-resolution map for chromosome 17, in which the Bayes confidence interval is shaded (blue) with the MHC region in dark gray. (e) Degree of thyroiditis for mice segregated on their genotype at rs13482997. (f) High-resolution map for chromosome 3. (g) Degree of thyroiditis for mice segregated according to their genotype at rs13477485. (h) High-resolution map for chromosome 19. (i) Degree of thyroiditis for mice segregated according to their genotype at rs13483682. N2 genotypes N/N or N/B: N indicates NOD.H2h4, and B indicates BALB/c. The significant threshold is 2.77 (solid lines), and the suggestive threshold is 1.51 (dotted lines).
It should be emphasized that transgenic and nontransgenic N2 mice have a similar phenotype (Supplemental Fig. 1 (224KB, pdf) ). Removing the N2 transgenic mice from the linkage analysis does not alter the results. However, because the linkage is performed on fewer mice, the statistical power is reduced and the linkage would only reach the suggestive threshold.
All data are shown as genome-wide linkage [Figs. 4(a), 5(a), 6(a), and 7(a)] and as high-resolution maps for suggestive and significant linkages: chromosomes 12 and 17 [Fig. 4(b) and 4(d)]; chromosomes 17 and 19 [Fig. 5(b) and 5(d) and Fig. 6(b) and 6(d)]; and chromosomes 1, 17, 3, and 19 [Fig. 7(b), 7(d), 7(f), and 7(h)]. In addition, adjacent to the high-resolution maps, we plot the genotypes of N2 mice at the SNP closest to the highest LOD score that are homozygous or heterozygous for the NOD.H2h4 allele (NN or NB, respectively, in which N and B represent the NOD.H2h4 and BALB/c alleles, respectively). For thyroid autoantibody traits, N2 mice homozygous for NOD.H2h4 alleles at the SNP nearest to the suggestive or the significant LOD score exhibit higher levels of the relevant autoantibody [Fig. 4(c) and 4(e), Fig. 5(c) and 5(e), and Fig. 6(c) and 6(e)]. This was also true for thyroid infiltration [Fig. 7(c), 7(e), 7(g), and 7(i)]. In agreement with the MHC haplotype data in Fig. 2, the segregation analysis was statistically significant for chromosome 17 and TgAb [Fig. 5(e), P < 0.001] and for chromosome 17 and thyroiditis [Fig. 7(e), P = 0.003, t test].
N2 mice bearing only one allele of the NOD.H2h4 haplotype at these loci have significantly reduced thyroid autoantibody levels and thyroiditis compared with mice homozygous for the NOD.H2h4 haplotype. Together, the linkage analysis and the distribution of mice based on their haplotypes indicate that NOD.H2h4 alleles are not dominant in their contribution to thyroid autoantibodies or thyroiditis.
Based on the Haley–Knott algorithm (24), a locus on chromosome 19 is suggestively linked to three traits, namely TgAb, TPOAb, and thyroiditis (all at 16 weeks). Determining the allelic distribution at two additional genetic markers, D19Mit91 and D19Mit123, did not confirm linkage to these traits. Consequently, finer genetic mapping on a larger cohort of mice would be required to confirm linkage of this chromosome 19 locus to thyroid autoantibody traits.
Genes of potential interest on chromosomes 19 and 17
Apoptosis of thyrocytes exposed to NaI is enhanced in NOD.H2h4 versus CBA/J mice (26). In this context, Fas is of interest as one of the genes involved in cell death by apoptosis [reviewed in (27)] is located on chromosome 19. However, suggestive linkage of a chromosome 19 locus to thyroid autoantibodies and thyroiditis could not be confirmed (see earlier). In any case, the position of Fas at 32.49 Mb is outside the locus (36.03 to 55.01 Mb) suggestively linked to thyroiditis.
Of greater potential relevance are genes in the chromosome 17 locus significantly linked to TgAb and thyroiditis. Within this locus (32.08 to 75.96 Mb), 100 genes are classified as involved in immunological processes (highlighted in Supplemental Table 1 (224KB, pdf) ), namely: MHC class I (K, O, D1-Q; H2-T, H2-L, and -M) and class II (Dm, H2-Ab1, Aa, Eb1, Eb2, and Ea), complement (C), lymphotoxin (Ltn), and tumor necrosis factor (Tnf). One of the two apoptosis-related genes upregulated in NOD.H2h4 versus CBA/J thyrocytes exposed to iodide is Tnf (26). In addition, the genome organizer Satb1 (special AT-rich sequence-binding protein) (28), which is involved in establishing immune tolerance, is included within the linked interval (51.9 Mb).
The position of the MHC locus on chromosome 17 is highlighted within the Bayes confidence interval in Figs. 5 and 7. Genes in the chromosome 17 locus are derived from the B10.A(4R) donor of the MHC region as well as from the NOD parental strain (16) (Supplemental Fig. 2 (224KB, pdf) ).
Discussion
Building on serendipitous findings arising from transferring a transgene from BALB/c mice to the NOD.H2h4 strain (17), we crossed NOD.H2h4 and BALB/c mice to generate F2 and N2 progeny for the purpose of investigating the genetic basis for thyroid autoimmunity by linkage analysis. TgAb, TPOAb, and thyroiditis, which were absent in BALB/c and F1 mice, developed in most mice of the parental strain NOD.H2h4 and in significantly more N2 than F2 progeny. TgAb develop earlier than TPOAb (4), and the levels were higher in mice after 16 than 8 weeks on water supplemented with NaI. The N2 mice included offspring derived from F1 males bearing the human TSHR A-subunit transgene (18, 19). TgAb and TPOAb development was comparable in transgenic and nontrangenic N2 offspring. No F2 offspring homozygous for the BALB/c MHC haplotype were TgAb positive, and virtually all mice that develop TgAb are homozygous for the NOD.H2h4 haplotype. This finding confirms the critical role of I-Ak in generating thyroid autoantibodies. Analysis of TgAb and TPOAb in F2 offspring did not reveal linkage, probably because of the small number of thyroid autoantibody-positive progeny. In contrast, in N2 progeny, we observed suggestive linkage of a locus on chromosome 19 to TgAb, TPOAb, and thyroiditis. More importantly, a locus spanning a broad region on chromosome 17, including MHC genes, is suggestively linked to TPOAb and is significantly linked to TgAb and thyroiditis.
Our major observation, namely chromosome 17 linkage to thyroid autoimmunity traits in NOD.H2h4 mice, confirms and extends other findings. Early studies of experimentally induced thyroiditis demonstrated “genetic interaction between I-region and D-end gene products in the response to a self-antigen, MTg” (7). Moreover, our recent deduction that development of TPOAb in NOD.H2h4 mice is most likely due to the absence of I-E expression (16) is consistent with suggestive MHC linkage to TPOAb. Kolypetri et al. (29) commented that “unknown genes within, or tightly linked to, the H-2 region [the H2h4 haplotype is derived from B10.A(4R) mice] may contribute to susceptibility” to spontaneous autoimmune thyroiditis.
As is well known, the role of MHC in autoimmunity lies in the ability of the MHC class II–binding pocket to accommodate T cell epitopes of particular autoantigens [for example (30, 31)] and thereby provide help to autoreactive B cells to generate autoantibodies. In humans, recombinant HLA-DR3 was used to dissect the interaction between the HLA-binding pocket and immunogenic peptides of human Tg (32). A number of I-Ak–restricted Tg peptides are recognized by T cells in mice immunized with mouse Tg, one of which has recently been shown to be recognized by splenocytes from NOD.H2h4 mice (33). Consequently, a major contribution by the chromosome 17 locus linked to thyroid autoimmunity in NOD.H2h4 mice is likely to involve the genes encoding I-Ak. However, two points should be emphasized, as follows: first, the presence of I-Ak alone is insufficient to permit the development of thyroid autoimmunity, as exemplified by B10.A(4R) mice that lack the thyroid autoimmune phenotype (10); second, this locus also includes Tnf, which is one of only two genes shown by Kolypetri and Carayanniotis (26) to be upregulated in apoptosis of NOD.H2h4 thyrocytes exposed to iodide.
The extent of the chromosome 17 locus linked to the development of TgAb, TPOAb, and thyroiditis in NOD.H2h4 mice deserves further consideration. This region spans 32.08 to 75.96 Mb, extending far beyond the portion of Chr 17 (31.76 to 39.32 Mb), that is focused on MHC class I, II, and III genes that are linked (for example) to the generation of induced TSHR antibodies in recombinant inbred mice (34). Previously, we found that the chromosome 17 region (including I-Ak) in NOD.H2h4 mice, which was derived from the B10.A(4R) strain, only extends from 27.3 to 44.8 Mb (16). Consequently, the adjacent larger portion on chromosome 17, from 44.8 to 75.96 Mb, is derived from the NOD parent. It should be emphasized ∼3 SNPs per 5 Mb are evenly distributed across all chromosomes in the Illumina Medium Density Linkage panel used for linkage analysis. The NOD-derived portion contains fewer immune-associated genes than the MHC-associated region from the B10.A(4R) strain. However, this region includes the genome organizer Satb1 (special AT-rich sequence-binding protein) (28), which is involved in establishing immune tolerance. Consequently, our data clearly point to the pivotal role of genes in the region from the nonautoimmune-prone B10.A(4R) strain (including but not restricted to genes responsible for I-Ak) and genes (such as Satb1) on chromosome 17 from the autoimmune-prone NOD parent in the development of thyroid autoimmunity in NOD.H2h4 mice.
Which chromosomes or genes (other than MHC) that we might have expected to be involved in the NOD.H2h4 thyroid autoimmunity phenotype were not revealed by our linkage studies? First, we obtained no evidence to support a role for the extra DNA carried over to NOD.H2h4 mice from B10.A(4R) mice (chromosomes 7 and 15; amounting to 88.6 megabase) (16) in the development of thyroid autoantibodies or thyroiditis. Second, we found no evidence of a role for any of the non-MHC genes associated with thyroiditis in humans [reviewed in (35)]. Third, linkage analysis did not reveal genes responsible for spontaneous thyroiditis as occurs with intrathyroidal expression of the CC chemokine ligand 21 (12) or the absence of CC chemokine receptor 7 (13); nor was there linkage with interleukin 10, which influences experimentally induced thyroiditis (15). In contrast, the absence of linkage with chromosome 10 (on which the gene for Tg is located) is consistent with our previous demonstration that NOD.H2h4 mice do not have the Tg haplotype associated with susceptibility to induced thyroiditis in some mouse strains (14). It should be emphasized that our data do not preclude contributions from other genes because, as for all linkage studies, this approach reveals the role of major genetic variants from two different strains, namely NOD.H2h4 and BALB/c, in determining the phenotype.
In conclusion, a locus on chromosome 17 is linked to the development of TgAb, TPOAb, and thyroiditis in the NOD.H2h4 strain. Importantly, the presence of chromosome 17 genes derived from both the B10.A(4R) strain (MHC genes) and the NOD parent (possibly including Satb1) appears to be responsible for the Hashimoto disease–like phenotype of NOD.H2h4 mice.
Acknowledgments
We are grateful to Dr. Jean Ruf (INSERM-URA, Faculté de Médecine, Marseille, France) for generously providing us with mouse monoclonal antibodies to human TPO.
Acknowledgments
This work was supported by National Institutes of Health Grants DK 82390 and DK 54684 (to S.M.M.) and DK 19289 (to B.R.) and by Canadian Diabetes Association Grant OG-3-13-4018 (to S.L.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHO
- Chinese hamster ovary
- ELISA
- enzyme-linked immunosorbent assay
- IgG
- immunoglobulin G
- LOD
- logarithm of the odds
- MHC
- major histocompatibility class
- NaI
- sodium iodide
- NOD
- nonobese diabetic
- SNP
- single-nucleotide polymorphism
- Tg
- thyroglobulin
- TgAb
- autoantibodies to thyroglobulin
- TPO
- thyroid peroxidase
- TPOAb
- autoantibodies to thyroid peroxidase
- TSHR
- thyrotropin receptor.
References
- 1.Rasooly L, Burek CL, Rose NR. Iodine-induced autoimmune thyroiditis in NOD-H-2h4 mice. Clin Immunol Immunopathol. 1996;81(3):287–292. [DOI] [PubMed] [Google Scholar]
- 2.Hutchings PR, Verma S, Phillips JM, Harach SZ, Howlett S, Cooke A. Both CD4(+) T cells and CD8(+) T cells are required for iodine accelerated thyroiditis in NOD mice. Cell Immunol. 1999;192(2):113–121. [DOI] [PubMed] [Google Scholar]
- 3.Braley-Mullen H, Sharp GC, Medling B, Tang H. Spontaneous autoimmune thyroiditis in NOD.H-2h4 mice. J Autoimmun. 1999;12(3):157–165. [DOI] [PubMed] [Google Scholar]
- 4.Chen CR, Hamidi S, Braley-Mullen H, Nagayama Y, Bresee C, Aliesky HA, Rapoport B, McLachlan SM. Antibodies to thyroid peroxidase arise spontaneously with age in NOD.H-2h4 mice and appear after thyroglobulin antibodies. Endocrinology. 2010;151(9):4583–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Podolin PL, Pressey A, DeLarato NH, Fischer PA, Peterson LB, Wicker LSI-E. I-E+ nonobese diabetic mice develop insulitis and diabetes. J Exp Med. 1993;178(3):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vladutiu AO, Rose NR. Autoimmune murine thyroiditis relation to histocompatibility (H-2) type. Science. 1971;174(4014):1137–1139. [DOI] [PubMed] [Google Scholar]
- 7.Kong Y, David CS, Giraldo AA, Elrehewy M, Rose NR. Regulation of autoimmune response to mouse thyroglobulin: influence of H-2D-end genes. J Immunol. 1979;123(1):15–18. [PubMed] [Google Scholar]
- 8.Tomazic V, Rose NR, Shreffler DC. Autoimmune murine thyroiditis. IV. Localization of genetic control of the immune response. J Immunol. 1974;112(3):965–969. [PubMed] [Google Scholar]
- 9.Beisel KW, David CS, Giraldo AA, Kong YM, Rose NR. Regulation of experimental autoimmune thyroiditis: mapping of susceptibility to the I-A subregion of the mouse H-2. Immunogenetics. 1982;15(4):427–430. [DOI] [PubMed] [Google Scholar]
- 10.Wicker LS, Todd JA, Peterson LB. Genetic control of autoimmune diabetes in the NOD mouse. Annu Rev Immunol. 1995;13:179–200. [DOI] [PubMed] [Google Scholar]
- 11.Boulard O, Damotte D, Deruytter N, Fluteau G, Carnaud C, Garchon HJ. An interval tightly linked to but distinct from the H2 complex controls both overt diabetes (Idd16) and chronic experimental autoimmune thyroiditis (Ceat1) in nonobese diabetic mice. Diabetes. 2002;51(7):2141–2147. [DOI] [PubMed] [Google Scholar]
- 12.Martin AP, Coronel EC, Sano G, Chen SC, Vassileva G, Canasto-Chibuque C, Sedgwick JD, Frenette PS, Lipp M, Furtado GC, Lira SA. A novel model for lymphocytic infiltration of the thyroid gland generated by transgenic expression of the CC chemokine CCL21. J Immunol. 2004;173(8):4791–4798. [DOI] [PubMed] [Google Scholar]
- 13.Martin AP, Marinkovic T, Canasto-Chibuque C, Latif R, Unkeless JC, Davies TF, Takahama Y, Furtado GC, Lira SA. CCR7 deficiency in NOD mice leads to thyroiditis and primary hypothyroidism. J Immunol. 2009;183(5):3073–3080. [DOI] [PubMed] [Google Scholar]
- 14.Ban Y, Greenberg DA, Concepcion E, Skrabanek L, Villanueva R, Tomer Y. Amino acid substitutions in the thyroglobulin gene are associated with susceptibility to human and murine autoimmune thyroid disease. Proc Natl Acad Sci USA. 2003;100(25):15119–15124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu Z, Liu T, Liu S, Zou H, Sun X, Shi X, Li Y, Shan Z, Teng W. Interleukin-10 influences susceptibility to experimental autoimmune thyroiditis independently of the H-2 gene. Int J Mol Med. 2015;35(2):413–424. [DOI] [PubMed] [Google Scholar]
- 16.Pelletier AN, Aliesky HA, Banuelos B, Chabot-Roy G, Rapoport B, Lesage S, McLachlan SM. Evidence that MHC I-E dampens thyroid autoantibodies and prevents spreading to a second thyroid autoantigen in I-A(k) NOD mice. Genes Immun. 2015;16(4):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rapoport B, Aliesky HA, Banuelos B, Chen CR, McLachlan SM. A unique mouse strain that develops spontaneous, iodine-accelerated, pathogenic antibodies to the human thyrotrophin receptor. J Immunol. 2015;194(9):4154–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLachlan SM, Nagayama Y, Pichurin PN, Mizutori Y, Chen CR, Misharin A, Aliesky HA, Rapoport B. The link between Graves’ disease and Hashimoto’s thyroiditis: a role for regulatory T cells. Endocrinology. 2007;148(12):5724–5733. [DOI] [PubMed] [Google Scholar]
- 19.Misharin AV, Nagayama Y, Aliesky HA, Rapoport B, McLachlan SM. Studies in mice deficient for the autoimmune regulator (Aire) and transgenic for the thyrotropin receptor reveal a role for Aire in tolerance for thyroid autoantigens. Endocrinology. 2009;150(6):2948–2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McLachlan SM, Aliesky HA, Chen CR, Chong G, Rapoport B. Breaking tolerance in transgenic mice expressing the human TSH receptor A-subunit: thyroiditis, epitope spreading and adjuvant as a ‘double edged sword.’ PLoS One. 2012;7(9):e43517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruf J, Toubert ME, Czarnocka B, Durand-Gorde JM, Ferrand M, Carayon P. Relationship between immunological structure and biochemical properties of human thyroid peroxidase. Endocrinology. 1989;125(3):1211–1218. [DOI] [PubMed] [Google Scholar]
- 22.Collin R, Dugas V, Pelletier AN, Chabot-Roy G, Lesage S. The mouse idd2 locus is linked to the proportion of immunoregulatory double-negative T cells, a trait associated with autoimmune diabetes resistance. J Immunol. 2014;193(7):3503–3512. [DOI] [PubMed] [Google Scholar]
- 23.Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19(7):889–890. [DOI] [PubMed] [Google Scholar]
- 24.Haley CS, Knott SA.. A simple regression method for mapping quantitative trait loci in line crosses using flanking markers. Heredity (Edinb) 1992;69(4):315–324. [DOI] [PubMed] [Google Scholar]
- 25.McLachlan SM, Aliesky HA, Banuelos B, Lesage S, Collin R, Rapoport B. High level intrathymic thyrotropin receptor expression in thyroiditis-prone mice protects against the spontaneous generation of pathogenic thyrotropin receptor autoantibodies. Clin Exp Immunol (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kolypetri P, Carayanniotis G. Apoptosis of NOD.H2 h4 thyrocytes by low concentrations of iodide is associated with impaired control of oxidative stress. Thyroid. 2014;24(7):1170–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waring P, Müllbacher A. Cell death induced by the Fas/Fas ligand pathway and its role in pathology. Immunol Cell Biol. 1999;77(4):312–317. [DOI] [PubMed] [Google Scholar]
- 28.Kondo M, Tanaka Y, Kuwabara T, Naito T, Kohwi-Shigematsu T, Watanabe A. SATB1 plays a critical role in establishment of immune tolerance. J Immunol. 2016;196(2):563–572. [DOI] [PubMed] [Google Scholar]
- 29.Kolypetri P, King J, Larijani M, Carayanniotis G. Genes and environment as predisposing factors in autoimmunity: acceleration of spontaneous thyroiditis by dietary iodide in NOD.H2(h4) mice. Int Rev Immunol. 2015;34:542–556. [DOI] [PubMed] [Google Scholar]
- 30.Todd JA, Acha-Orbea H, Bell JI, Chao N, Fronek Z, Jacob CO, McDermott M, Sinha AA, Timmerman L, Steinman L, McDevitt HO. A molecular basis for MHC class II--associated autoimmunity. Science. 1988;240(4855):1003–1009. [DOI] [PubMed] [Google Scholar]
- 31.Wucherpfennig KW, Yu B, Bhol K, Monos DS, Argyris E, Karr RW, Ahmed AR, Strominger JL. Structural basis for major histocompatibility complex (MHC)-linked susceptibility to autoimmunity: charged residues of a single MHC binding pocket confer selective presentation of self-peptides in pemphigus vulgaris. Proc Natl Acad Sci USA. 1995;92(25):11935–11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobson EM, Yang H, Menconi F, Wang R, Osman R, Skrabanek L, Li CW, Fadlalla M, Gandhi A, Chaturvedi V, Smith EP, Schwemberger S, Osterburg A, Babcock GF, Tomer Y. Employing a recombinant HLA-DR3 expression system to dissect major histocompatibility complex II-thyroglobulin peptide dynamism: a genetic, biochemical, and reverse immunological perspective. J Biol Chem. 2009;284(49):34231–34243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kolypetri P, Carayanniotis K, Rahman S, Georghiou PE, Magafa V, Cordopatis P, Carayanniotis G. The thyroxine-containing thyroglobulin peptide (aa 2549-2560) is a target epitope in iodide-accelerated spontaneous autoimmune thyroiditis. J Immunol. 2014;193(1):96–101. [DOI] [PubMed] [Google Scholar]
- 34.McLachlan SM, Aliesky H, Banuelos B, Magana J, Williams RW, Rapoport B. Immunoglobulin heavy chain variable region and major histocompatibility region genes are linked to induced Graves’ disease in females from two very large families of recombinant inbred mice. Endocrinology. 2014;155(10):4094–4103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomer Y. Mechanisms of autoimmune thyroid diseases: from genetics to epigenetics. Annu Rev Pathol. 2014;9:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]