Abstract
The estrogen-synthesizing enzyme aromatase is abundant at the synapse in the zebra finch hippocampus (HP), and its inhibition impairs spatial memory function. To more fully test the role of local estradiol (E2) synthesis in memory, the HP of adult male zebra finches was exposed to either control pellets or those containing the aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD), ATD and E2, ATD and the G protein-coupled estrogen receptor (GPER) agonist G1, or the antagonist G15 alone. Birds were tested for spatial memory acquisition and performance, and HP levels of the postsynaptic protein PSD95 were measured. ATD-treated birds took longer to reach criterion than control birds, whereas acquisition in ATD+E2 and ATD+G1 birds was indistinguishable from control and ATD treatments. Interestingly, all G15 birds failed to acquire the task. Following a retention interval, ATD birds took the longest to reach the (formerly) baited cup and made the most mistakes. ATD+E2 animals displayed the lowest retention latencies and made fewer mistakes than ATD-treated birds, and ATD+G1 birds did not significantly differ from controls in retention latencies. The amount of PSD95 in the HP was lowest in ATD-treated animals compared with birds with silicone-only–implanted craniotomies, ATD+E2, and ATD+G1 birds, who did not differ in this expression. Thus, spatial memory acquisition and performance appear aromatase and E2 dependent, an effect more reliably revealed after consolidation and/or recall compared to acquisition. E2 may exert this effect via GPERs, resulting in an increase in PSD95 levels that may modify receptor activity or intracellular signaling pathways to increase synaptic strength.
Aromatase inhibition plus estradiol replacement or GPER agonism improves or rescues memory performance and increases levels of the postsynaptic protein PSD95 in the male zebra finch hippocampus.
Estrogens have organizational and activating influences on the vertebrate central nervous system via endocrine, paracrine, autocrine, and “synaptocrine” provision. The synthesis and provision of estradiol (E2) at the synapse occurs via the expression of the estrogen-synthesizing enzyme aromatase in presynaptic boutons and postsynaptic dendrites (1–5). Indeed, aromatase is acutely regulated in synaptosomes rather than microsomes (4–7) and can have acute and even rapid behavioral effects (6, 8–10).
The brain of the male zebra finch (Taeniopygia guttata) is likely the sole source of local and circulating E2 (11, 12) due to the abundant expression of aromatase in neurons of the hippocampus (HP), caudomedial nidopallium, nucleus taeniae, lateral nidopallium, and other telencephalic and diencephalic brain regions (3, 4, 13, 14). Aromatase can also be expressed in reactive astrocytes and radial glia (9, 15–19) when the songbird brain is damaged, suggesting the source and mechanisms of estrogen provision may be different in the unperturbed and injured finch brain.
Neuronal aromatase expression is strikingly high in the zebra finch HP and is detectable by the presence of aromatase mRNA, biochemical activity, and immunoexpression (13, 14, 20, 21). Ultrastructural examinations reveal an abundance of aromatase in presynaptic boutons and postsynaptic dendrites at this locus with a surprisingly low level of somal aromatase (3). This characteristic renders the zebra finch HP amenable to understanding the role of synaptic aromatization.
Studies of songbirds have provided compelling evidence for the role of E2 in the learning and expression of singing behavior (22, 23). In addition to song learning, these organisms also demonstrate HP-dependent spatial memory function, which is modulated by circulating steroids, including E2 (24). Given the abundance of presynaptic and postsynaptic aromatase expression in the HP, we and others have hypothesized a critical role for local aromatization, likely restricted to synaptic structures, on the regulation of spatial memory function in the zebra finch (25).
Because the avian HP is located on the dorsal surface of the brain, it is possible to manipulate its biochemical characteristics without damaging the cytoarchitecture of the telencephalon and inducing the expression of aromatase in astroglia. In a previous study, we exploited this neuroanatomical characteristic to infiltrate the HP with the lipophilic aromatase inhibitor 1,4,6-androstatriene-3,17-dione (ATD) and demonstrated an impairment of spatial memory function similar in magnitude to that observed in HP-lesioned birds (26). However, the E2 dependence and mechanism underlying this effect are unknown.
E2 is known to modulate neural function via action on its canonical nuclear estrogen receptors α and β, as well as G protein-coupled estrogen receptor (GPER)-1, an estrogen receptor expressed in the cell membrane and implicated in the rapid effects of E2 on several behaviors, including some aspects of memory function (27–30). However, the influence of GPER-1 expressed specifically within HP circuits on a complex behavior, and the signaling cascades following modulation of these receptors, remains relatively unexplored. A recent study in mice revealed that infusions of a GPER agonist enhances (and an antagonist impairs) object recognition memory, but perhaps through mechanisms that are independent of the effects of E2 on membrane receptors (31).
In this study, we tested the influence of aromatase inhibition, aromatase inhibition plus concurrent E2 replacement, aromatase inhibition and agonism of GPER-1, or antagonism of GPER-1 on spatial memory function in the adult male zebra finch. Levels of the postsynaptic protein PSD95 were measured via Western blot in microdissected HP tissue from animals in each of the treatment conditions. PSD95, a membrane-associated guanylate kinase, is found almost exclusively in dendrites (32, 33) and interacts directly with glutamate receptors (34–36) and a variety of other postsynaptic proteins (33), promoting activity-dependent plasticity. We hypothesized that aromatization and local E2 provision to GPER-1 in the HP supports spatial memory function in the zebra finch via interactions with PSD95.
Methods
Animals and surgery
Adult male zebra finches housed in single-sex aviaries at St. Norbert College (De Pere, WI) were used in the experiment. Birds had ad libitum access to food and water up to the morning of behavioral testing, and the light:dark cycle was 12:12 (lights off at 1900). All procedures were approved by the St. Norbert College Institutional Animal Care and Use Committee.
After anesthesia with isoflurane, birds received 0.8-mm craniotomies above the HP (anteroposterior length, +1.5 mm; mediolateral = ±0.5). Animals were then given silicone implants that fit inside the craniotomies and made to rest on the surface of the brain, such that each implant was roughly 0.25 mm3. In one group (n = 17), only silicone was put into the craniotomies (SIL). In another, the aromatase inhibitor ATD (n = 16; Steraloids) was mixed with the silicone as described previously (26). To determine the effects of aromatase inhibition concurrent with E2 replacement, pellets were prepared with both ATD and E2 (5:1 ratio of E2 to ATD/pellet; n = 10; Steraloids). Finally, to determine the role of GPERs in HP memory function, pellets were made with ATD plus the GPER agonist G1 (n = 7) or only the antagonist G15 (n = 8; both Azano Scientific), such that implants each contained roughly 0.5 mg (approximately 2.5:1 of G1 to ATD per pellet). After the implant procedure and a period of recovery on a warming pad, animals were returned to the colony but housed in an individual cage with ad libitum access to food and water.
Behavioral testing
Seventy-two hours after the surgical procedures, spatial memory was tested in 2 stages (26, 37) after approximately 5 hours of food deprivation that began when lights were turned on. Briefly, birds had to learn the location of a cup that contained seed within a T-shaped apparatus. An hour after reaching a predetermined criterion level (i.e., when, after first eating from the seed-baited cup, birds entered that arm of the apparatus within 30 seconds in three consecutive trials), memory for the seed-containing cup was tested in 4 “probe” trials by examining the latency to reach the cup that contained seed in the acquisition trials as well as the number of mistakes (if any) before contacting it.
Tissue collection and Western blotting
Immediately after spatial memory testing, animals were rapidly decapitated and the HP was microdissected as described previously (26), flash frozen in microcentrifuge tubes on dry ice, and stored at −80°C until preparation for gel electrophoresis.
Tissue samples were thawed on ice, treated with radioimmunoprecipitation assay lysis buffer [90 µL per sample; 50 mM Tris, pH 8.0; 150 mM sodium chloride; 1% IGEPAL CA-630; 0.5% sodium deoxycholate; 0.1% sodium dodecyl sulfate; 3 mM EDTA; 50 mM sodium fluoride; 0.2 mM sodium orthovanadate; 4 µL/mL protease inhibitor mix (Sigma); and 1 mM phenylmethanesulfonyl fluoride], homogenized with sonication, then centrifuged for 10 minutes at 10,000g. After supernatant collection, the protein concentration of each sample was determined by a bicinchoninic acid assay. Samples were electrophoresed on a 12% polyacrylamide gel with 30 µg of protein per lane, and the proteins were transferred from the gels to low-fluorescence polyvinylidene difluoride membranes (Thermo Scientific). Membranes were blocked with 3% bovine serum albumin (BSA) in Tris-buffered saline for 1 hour at room temperature, then incubated overnight at 4°C in primary antibodies [PSD95, 2 µg/mL, Abcam; glyceraldehyde 3-phosphate dehydrogenase (GAPDH), 1:5,000, EMD Millipore] diluted in 3% BSA in Tris-buffered saline with Tween 20 (TBST). After washes in TBST, membranes were incubated with secondary antibodies (donkey anti-rabbit DyLight 650 and donkey anti-mouse DyLight 488; Life Technologies) diluted at 1:10,000 in 3% BSA in TBST for 1 hour at room temperature. After rinses, fluorescence was captured with a Typhoon imager (GE Healthcare Lifesciences) using a 670-nm emission filter and red laser (633 nm), and a 520-nm emission filter and blue laser (488 nm). Individual band intensities and PSD95/GAPDH ratios were quantified using ImageJ software by raters unaware of treatment condition.
Data analysis
Homogeneity of variance was examined in the data sets using the Bartlett test; when appropriate, data (i.e., latencies to contact the baited cup, mistakes, and PSD95/GAPDH levels) were log transformed. Regarding mistakes during the probe trials, given the unequal variance combined with the number of zeroes (no mistakes) in the data set, values were log transformed following an “x+1” conversion. All data presented in the figures are not log transformed.
Acquisition (i.e., trials to criterion) was analyzed via a 1-way analysis of variance (ANOVA) to determine the effect of treatment, and Newman-Keuls post hoc tests (critical value of P < 0.05) were used to compare the performance between individual groups. Retention (i.e., latency to contact the baited cup and mistakes, if any, before doing so) was analyzed with a repeated measures ANOVA to determine effects of treatment, trial, and any interactions of these dependent variables. When appropriate, and given the importance of the first probe trial after the 1-hour interval following acquisition, a 1-way ANOVA was used to determine performance in this trial. Newman-Keuls post hoc tests were used to compare the performance between individual groups.
Band intensities (PSD95/GAPDH) also were analyzed using a 1-way ANOVA, with Newman-Keuls used post hoc. Agreement between raters about Western blot quantification was determined using the Cohen κ statistic. Although PSD95 levels were analyzed in tissue collected from G15 birds after their failed attempt at acquisition, the group was not included in the analysis. Other birds that met the criterion went on to ≥1 hour of probe trials after a 1-hour interval. Thus, we felt comparisons of PSD95 between groups that failed acquisition (G15) and those that did not and went through the additional testing (all others) would be confounded. For this same reason, protein levels were not included from birds in other groups that failed to acquire the task.
Results
Acquisition
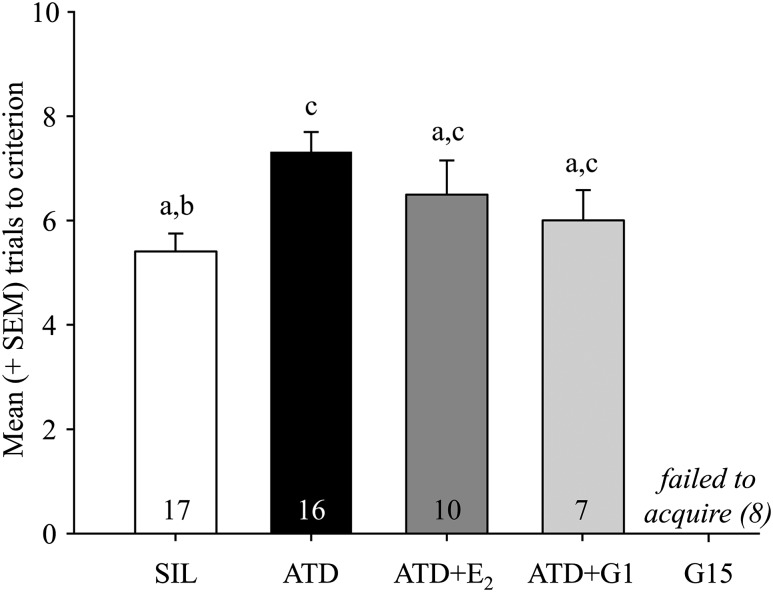
As previously published, the spatial memory task used in these experiments is HP dependent and amenable to testing the acquisition and accuracy of spatial memory function (26, 37). In our study, the task was acquired by most of the birds (all 17 SIL, 16 of 17 ATD, all 10 ATD+E2, and 7 of 11 ATD+G1 birds). In stark contrast, none of the G15 birds acquired the task. Across the 4 groups that demonstrated acquisition, the number of trials to reach criterion was significantly affected by treatment (F3,46 = 3.81; P = 0.016; Fig. 1). Birds treated with ATD took significantly more trials than those in the SIL group; no other comparisons reached significance. The G15-treated birds were excluded from this analysis and all further analyses in this set of studies, because they did not acquire the task.
Figure 1.
Mean (+SEM) number of trials to reach criterion for birds in each of the treatment conditions. Note that none of the birds in the G15 group acquired the task. Groups that do not share a letter are statistically different (P < 0.05), and number of animals per group is indicated. SEM, standard error of the mean.
Mistakes made during probe trials
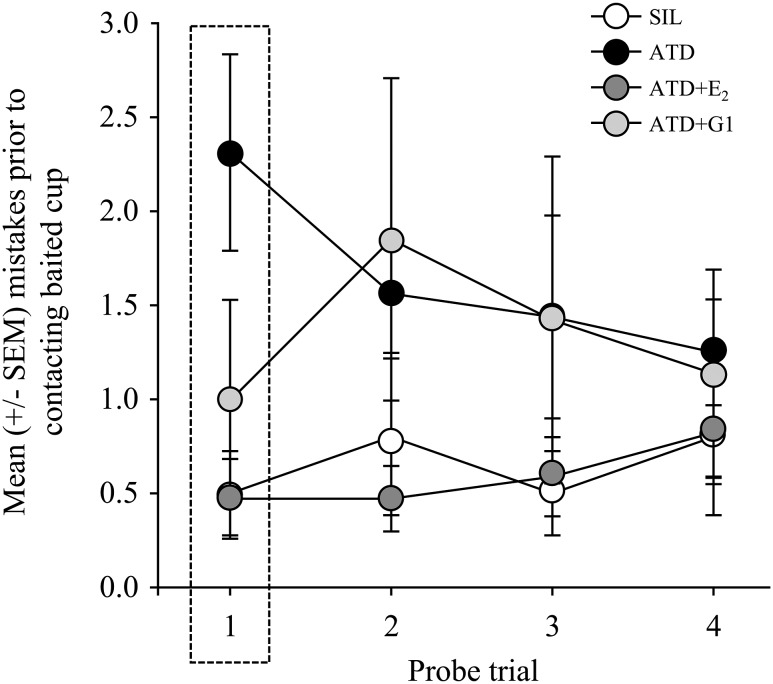
The number of mistakes made during the first probe trial was strongly affected by treatment (F3,46 = 5.959; P = 0.0016; Fig. 2). This effect reflects the fact that ATD birds made significantly more mistakes than SIL, ATD+E2, and ATD+G1 birds. There were no significant differences in the number of mistakes made in the first trial between birds treated with ATD+E2 as compared with SIL or ATD+G1.
Figure 2.
Mean (±SEM) number of mistakes before making contact with the cup that contained seed during acquisition trials. In the first trial, the memory performance of ATD birds was poor relative to animals in the other treatment conditions, which did not significantly differ from each other. SEM, standard error of the mean.
Over the course of the probe trials, the number of mistakes made was also affected by treatment (F3,46 = 5.075, P = 0.004; Fig. 2). Animals treated with ATD made significantly more mistakes than SIL and ATD+E2 birds. Birds treated with ATD+G1 did not significantly differ from any of the other groups in this measure. The number of mistakes did not change significantly over time (F3,138 = 0.329; P = 0.8047), nor was there an interaction between this variable and type of implant (F9,138 = 0.860; P = 0.5629).
Latency to find the appropriate location during probe trials
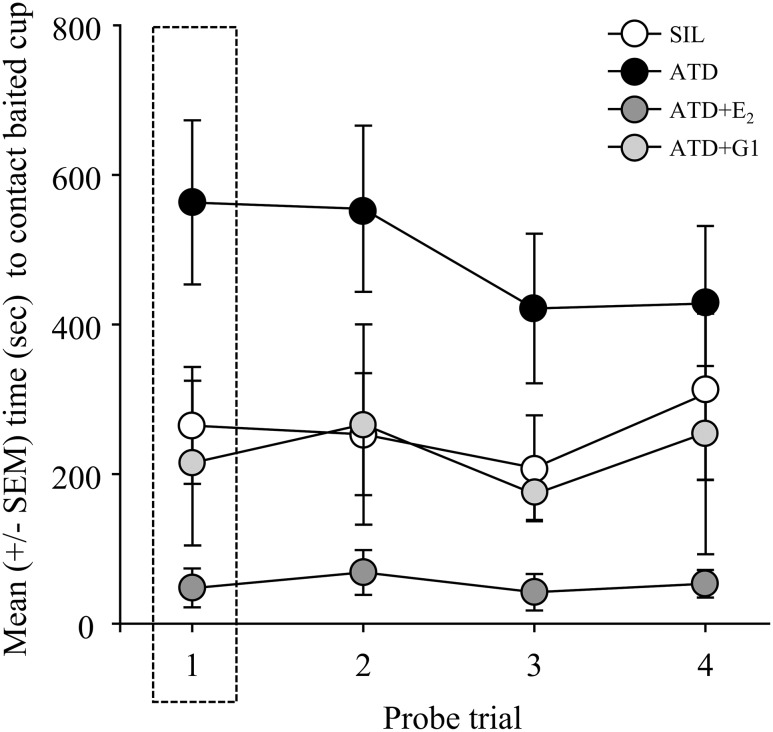
On the very first probe trial (F3,46 = 4.448; P = 0.008; Fig. 3), ATD birds took the longest to reach the (previously) baited cup than those in all the other treatment conditions. This impairment was significantly different from that of ATD+E2 birds. None of the other comparisons reached significance.
Figure 3.
Mean (±SEM) latency to contact the baited cup over the course of the probe trials. Note the highlighted first trial, in which ATD+E2 birds took significantly less time to locate the baited cup than ATD-treated animals. Over the course of the probe trials, animals treated with ATD took significantly more time to reach the baited cup than SIL and ATD+E2 birds, and ATD+E2-treated animals performed significantly better than those given ATD+G1. SEM, standard error of the mean.
Across all 4 probe trials, latency to contact the baited cup was also significantly affected by type of implant (F3,46 = 7.151; P = 0.0005; Fig. 3). Latencies did not differ over time (F3,138 = 0.005; P = 0.9916), and there was no significant interaction of time and treatment (F9,46 = 0.611; P = 0.786). Similar to the pattern of differences when examining the first probe trial alone, there was a significant effect of aromatase inhibition and a rescue of this impairment by concurrent treatment with E2 across all probe trials. Specifically, animals given implants of ATD took significantly more time to reach the baited cup over the course of the probe trials than SIL and ATD+E2 birds. ATD+E2-treated animals performed significantly better than ATD+G1 birds. None of the other comparisons reached significance.
Protein expression
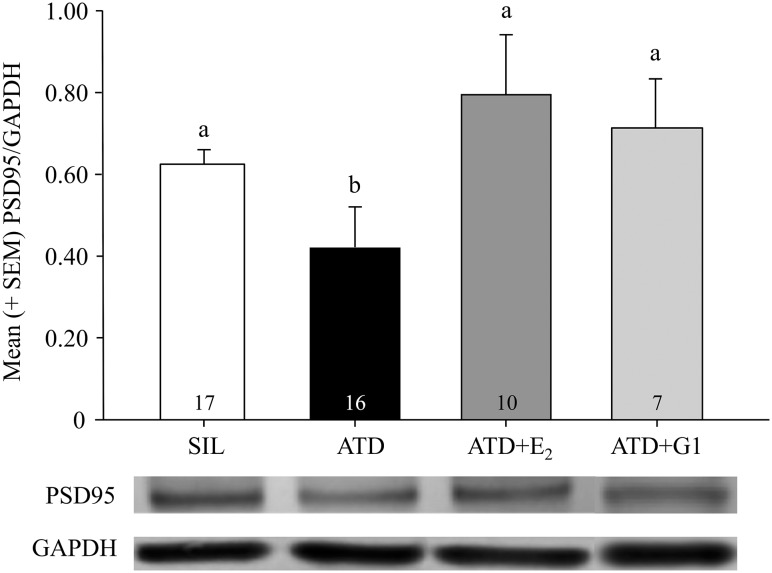
Analysis of protein expression with Western blots demonstrated a striking resemblance to the pattern of effects in spatial memory function. Specifically, the intensity of the bands for PSD95 relative to that of GAPDH (the quotient) differed depending on treatment condition (F3,46 = 4.124; P = 0.0113; Fig. 4). Levels of PSD95/GAPDH were significantly lower in animals treated with ATD relative to SIL, ATD+E2, and ATD+G1 birds. Importantly, there was no systematic difference in PSD95 expression across the latter 3 groups. The Cohen κ comparing calculations of band intensities by 2 raters was 0.82, indicating strong agreement.
Figure 4.
Mean (+SEM) PSD95/GAPDH band intensity from microdissected hippocampal tissue of birds in each of the treatment conditions, as well as representative bands (PSD95 80 kD, GAPDH 36 kD) from the membranes. PSD95/GAPDH levels were significantly lower in animals treated with ATD relative to SIL, ATD+E2, and ATD+G1 birds, and the levels in these latter 3 groups did not significantly differ from each other. Groups that do not share a letter are statistically different (P < 0.05), and number of animals per group is indicated. SEM, standard error of the mean.
Discussion
This study examined spatial memory function in adult male zebra finches after aromatase inhibition in the HP with or without concurrent E2 replacement, as well as manipulation of GPER-dependent signaling. Aromatase inhibition impaired acquisition and the accuracy of memory function during subsequent probe trials. Replacement with E2 resulted in acquisition rates intermediate between that of birds treated with an aromatase inhibitor or control birds, but successfully restored the accuracy of memory function during probe trials. The presence of a GPER agonist with concurrent aromatase inhibition had no effect on acquisition; however, GPER antagonism resulted in a failure of acquisition in all animals. On the very first probe trial, aromatase inhibition resulted in weaker memory accuracy, as evidenced by more mistakes and longer latencies to find the previously baited cup relative to all other groups. Indeed, agonism of GPER in the presence of an aromatase inhibitor and E2 replacement restored, and even improved, the accuracy of memory function relative to aromatase inhibition alone. In excellent agreement, Western blot analysis for the postsynaptic protein PSD95 on microdissected HP tissue revealed lower expression in birds treated with an aromatase inhibitor relative to that seen in control birds and those replaced with E2 or the GPER agonist G1. Taken together, the data suggest that acquisition and performance of a spatial memory task in zebra finches appear to depend on local aromatase and E2 synthesis, an effect possibly exerted through GPERs. Activation of these receptors by E2 may lead to modifications in PSD95 or its targets to modify membrane receptor stability and availability to promote or enhance synaptic plasticity.
The estrogen-sensitive nature of HP function has been well documented in rodents and humans (38), with fewer studies in songbirds. The results replicate work from a prior study (26) in which treatment of the adult male zebra finch HP with ATD resulted in more mistakes in the acquisition and performance phases of a spatial memory task. In the current study, we queried the E2 dependence of this effect and were surprised that E2 did not restore acquisition to control levels. Moreover, although the addition of G1 in the presence of aromatase inhibition also did not improve acquisition, it did appear to rescue performance (as measured by the number of mistakes) in the first probe trial; however, that effect did not hold for the remainder of the probe trials, and the animals behaved much like those receiving ATD treatment alone. Indeed, prior reports on the effect of E2 on acquisition and retention of HP-dependent spatial memory in zebra finches are unclear. Specifically, although implants of E2 after castration in male zebra finches resulted in an increase in acquisition relative to control and testosterone-treated birds (24), in another study, acquisition was adversely affected when E2 was replaced [and retrieval was impaired in the presence of aromatase inhibition (39)]. Although the results of these studies and the current one may disagree, it should be noted that there are differences between them in dose and route of drug delivery (i.e., pellets directly on the brain’s surface, oral, subcutaneous) and spatial memory testing (i.e., number of trials per day, nature of testing apparatus). These and other differences could have led to the inconsistencies in the pattern of results obtained. Importantly, aromatase inhibition also impaired memory performance in subsequent probe trials, an effect that was completely reversed by replacement with E2. Birds provided with exogenous local E2 made fewer mistakes and took less time to reach the baited cup than ATD-treated and control birds; birds receiving ATD+G1 also made fewer mistakes on the first probe trial but did not differ from control birds in regard to the amount of time needed to reach the baited cup throughout the probe phase. These results suggest a stronger effect of E2 synthesis and action within the HP on the consolidation and/or retrieval of a learned behavior rather than acquisition, an expected result if E2 is perhaps, as described previously (39), produced in “dynamic” and varying levels by the HP depending on learning and memory phase. Additional studies are needed to more precisely determine the contribution of E2 by modifying its levels or activity during different phases of the spatial memory paradigm.
Although one cannot rule out an effect of manipulation of estrogen receptors α and β, the existence and transduction activity of estrogen receptors at extranuclear sites examined relatively recently in rodents (40–45) suggest an additional or perhaps exclusive role for this type of communication. Indeed, post-training infusion of G1 into the dorsal HP of ovariectomized mice resulted in an enhancement of object recognition, whereas G15 impaired this behavior (31). However, there may be indications that E2 itself may not work through GPERs to enhance performance in the spatial memory task used, suggesting that the downstream effects of GPER activity may represent redundant signaling mechanisms that can be activated by more than just E2. Membrane estrogen receptors have also been identified in zebra finches (46), and in high density in the HP. As in the Kim et al. (31) experiment, G15 impaired, but G1 in the presence of the aromatase inhibitor ATD rescued, the learning ability of birds in the current study. Importantly, these manipulations occurred after a route of administration that did not disrupt the neuropil, which is an unavoidable outcome in studies aimed at determining the transient regulation of E2 or its receptor on HP-dependent learning and memory processes in rodents. In birds, the location of the HP on the dorsal surface of the brain permits the modulation of aromatase or delivery of GPER agonists or antagonists without the confound of aromatase induction in nonneuronal cells (18, 19), a process that occurs dramatically in response to disruption of the neuropil in songbirds (9, 15–17) and rodents (47–50). Taken together, these data support a role for GPER expression in neurons in HP-dependent spatial memory function in songbirds.
In addition to facilitating aspects of spatial memory performance, replacement with E2 or the GPER agonist G1 increased levels of PSD95 in HP homogenates relative to ATD-treated birds. Importantly, the latter group demonstrated the lowest levels of PSD95 relative to all groups including SIL-treated control birds. In rodents, PSD95 is known to interact with GPER-1 (51), and levels of it increase in the HP (specifically, field CA3) after administration of G1 (52), an effect that may occur through the Akt/mTOR pathway (53). Although it is estimated that a typical postsynaptic density contains 200 to 300 PSD95 molecules (32), it is not possible to know if, in the current study, more of these were present within each HP synapse or whether there were simply more synapses as a result of the treatment. Also in rodents, PSD95 interacts with the N-methyl-d-aspartate (NMDA) receptor (34) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (54) directly or indirectly, but inhibition of PSD95 via RNAi results in loss of the latter but not NMDA receptors (33) in the postsynaptic membrane. In rodent HP slices, E2 modulates pre- and postsynaptic currents rapidly, an effect strongly influenced by glutamate or glutamate receptor-dependent mechanisms (2, 43, 55, 56). In birds, drugs that modulate glutamate receptor activity affect E2 production, both in the hypothalamus (57) and a region of the auditory forebrain (8, 9, 58). Furthermore, neurons positive for aromatase in the zebra finch HP colocalize with NMDA receptors (59). Collectively, these findings support the idea that activity-dependent synaptic aromatization may be critical to synaptic size and strength, and further demonstrate that E2 may function more like a neurotransmitter, promoting a synaptocrine coupling of hormonal and electrical signaling at the synapse (60, 61).
The formal study of memory and its stages has described the process as involving acquisition, consolidation, retention, and retrieval (62–64). Although the supportive role of E2 provision on memory function has been known for over a decade, we are only beginning to understand the role of E2 on more precise, formal stages of memory function. Specifically, peripheral (65) and central (45) E2 have been shown to increase consolidation in rats and may improve retrieval in zebra finches (39) and rats (66). The hypothesis that central (and mostly synaptically produced) E2 may function identically remains untested but is well supported by recent data showing a dramatic increase in brain E2 following song learning in juvenile finches (67). Preliminary work in the laboratory is examining the before, after, and “online” effects of local estradiol provision on spatial memory function by testing routes of administration, time and duration of drug delivery, and dose, so that a formal examination of the stage(s) of memory function most likely affected by HP aromatization can be performed later.
In summary, the results of this study strongly suggest that local E2 synthesis, via aromatization in the songbird HP, is a critical modulator of spatial memory function in this species. Locally synthesized E2 may exert its memory-enhancing effects via actions on the membrane-bound GPER and its interactions with PSD95. Further work is necessary to determine the precise components of memory function that are sensitive to local aromatization. The abundant expression of aromatase at pre- and postsynaptic loci in the zebra finch HP, combined with its low expression in somata, suggests the possibility that these effects may be driven by synaptic E2 synthesis and action. Experiments that specifically test the role of aromatization on synaptic currents are also necessary to further test this hypothesis.
Acknowledgments
We thank faculty in the Biology and Chemistry disciplines at St. Norbert College for technical assistance (especially Drs. King, Pratt, Feirer, and Garber), and Dominic Schaut and Michaela Sumnicht for bird care.
Acknowledgments
This work was supported by National Institutes of Health grants no. NS042767 and NS080585 (C.J.S.), the McNair Scholars Program (E.R.P. and J.A.G.), the St. Norbert College Office of Faculty Development (D.J.B.), and the St. Norbert Collaborative: Center for Undergraduate Research (E.R.P.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ANOVA
- analysis of variance
- ATD
- 1,4,6-androstatriene-3,17-dione
- HP
- hippocampus
- E2
- estradiol
- GAPDH
- glyceraldehyde 3-phosphate dehydrogenase
- GPER
- G protein-coupled estrogen receptor
- NMDA
- N-methyl-d-aspartate
- SIL
- silicone-only implanted craniotomy.
References
- 1.Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study on quail, rat, monkey and human tissues. Neuroendocrinology. 1996;63(2):149–155. [DOI] [PubMed] [Google Scholar]
- 2.Hojo Y, Hattori TA, Enami T, Furukawa A, Suzuki K, Ishii HT, Mukai H, Morrison JH, Janssen WG, Kominami S, Harada N, Kimoto T, Kawato S. Adult male rat hippocampus synthesizes estradiol from pregnenolone by cytochromes P45017alpha and P450 aromatase localized in neurons. Proc Natl Acad Sci USA. 2004;101(3):865–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc Biol Sci. 2005;272(1576):2089–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rohmann KN, Schlinger BA, Saldanha CJ. Subcellular compartmentalization of aromatase is sexually dimorphic in the adult zebra finch brain. Dev Neurobiol. 2007;67(1):1–9. [DOI] [PubMed] [Google Scholar]
- 5.Cornil CA, Leung CH, Pletcher ER, Naranjo KC, Blauman SJ, Saldanha CJ. Acute and specific modulation of presynaptic aromatization in the vertebrate brain. Endocrinology. 2012;153(6):2562–2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Remage-Healey L, Oyama RK, Schlinger BA. Elevated aromatase activity in forebrain synaptic terminals during song. J Neuroendocrinol. 2009;21(3):191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saldanha CJ, Remage-Healey L, Schlinger BA. Neuroanatomical distribution of aromatase in birds: cellular and subcellular analyses. In: Balthazart J, and Ball GF, eds. Brain Aromatase, Estrogens and Behaviour. New York, NY: Oxford UP; 2012:100–114. [Google Scholar]
- 8.Remage-Healey L, Coleman MJ, Oyama RK, Schlinger BA. Brain estrogens rapidly strengthen auditory encoding and guide song preference in a songbird. Proc Natl Acad Sci USA. 2010;107(8):3852–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11(11):1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Remage-Healey L, Joshi NR. Changing neuroestrogens within the auditory forebrain rapidly transform stimulus selectivity in a downstream sensorimotor nucleus. J Neurosci. 2012;32(24):8231–8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci USA. 1991;88(10):4191–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schlinger BA, Arnold AP. Circulating estrogens in a male songbird originate in the brain. Proc Natl Acad Sci USA. 1992;89(16):7650–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360(1):172–184. [DOI] [PubMed] [Google Scholar]
- 14.Saldanha CJ, Tuerk MJ, Kim Y-H, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423(4):619–630. [DOI] [PubMed] [Google Scholar]
- 15.Peterson RS, Lee DW, Fernando G, Schlinger BA. Radial glia express aromatase in the injured zebra finch brain. J Comp Neurol. 2004;475(2):261–269. [DOI] [PubMed] [Google Scholar]
- 16.Wynne RD, Saldanha CJ. Glial aromatization decreases neural injury in the zebra finch (Taeniopygia guttata): influence on apoptosis. J Neuroendocrinol. 2004;16(8):676–683. [DOI] [PubMed] [Google Scholar]
- 17.Saldanha CJ, Rohmann KN, Coomaralingam L, Wynne RD. Estrogen provision by reactive glia decreases apoptosis in the zebra finch (Taeniopygia guttata). J Neurobiol. 2005;64(2):192–201. [DOI] [PubMed] [Google Scholar]
- 18.Saldanha CJ, Duncan KA, Walters BJ. Neuroprotective actions of brain aromatase. Front Neuroendocrinol. 2009;30(2):106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duncan KA, Walters BJ, Saldanha CJ. Traumatized and inflamed--but resilient: glial aromatization and the avian brain. Horm Behav. 2013;63(2):208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res Mol Brain Res. 1994;24(1-4):227–237. [DOI] [PubMed] [Google Scholar]
- 21.Saldanha CJ, Popper P, Micevych PE, Schlinger BA. The passerine hippocampus is a site of high aromatase: inter- and intraspecies comparisons. Horm Behav. 1998;34(2):85–97. [DOI] [PubMed] [Google Scholar]
- 22.Gurney ME, Konishi M. Hormone-induced sexual differentiation of brain and behavior in zebra finches. Science. 1980;208(4450):1380–1383. [DOI] [PubMed] [Google Scholar]
- 23.Adkins-Regan E, Mansukhani V, Seiwert C, Thompson R. Sexual differentiation of brain and behavior in the zebra finch: critical periods for effects of early estrogen treatment. J Neurobiol. 1994;25(7):865–877. [DOI] [PubMed] [Google Scholar]
- 24.Oberlander JG, Schlinger BA, Clayton NS, Saldanha CJ. Neural aromatization accelerates the acquisition of spatial memory via an influence on the songbird hippocampus. Horm Behav. 2004;45(4):250–258. [DOI] [PubMed] [Google Scholar]
- 25.Bailey DJ, Saldanha CJ. The importance of neural aromatization in the acquisition, recall, and integration of song and spatial memories in passerines. Horm Behav. 2015;74:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bailey DJ, Ma C, Soma KK, Saldanha CJ. Inhibition of hippocampal aromatization impairs spatial memory performance in a male songbird. Endocrinology. 2013;154(12):4707–4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ervin KS, Phan A, Gabor CS, Choleris E. Rapid oestrogenic regulation of social and nonsocial learning. J Neuroendocrinol. 2013;25(11):1116–1132. [DOI] [PubMed] [Google Scholar]
- 28.Ervin KS, Mulvale E, Gallagher N, Roussel V, Choleris E. Activation of the G protein-coupled estrogen receptor, but not estrogen receptor α or β, rapidly enhances social learning. Psychoneuroendocrinology. 2015;58:51–66. [DOI] [PubMed] [Google Scholar]
- 29.Briz V, Liu Y, Zhu G, Bi X, Baudry M. A novel form of synaptic plasticity in field CA3 of hippocampus requires GPER1 activation and BDNF release. J Cell Biol. 2015;210(7):1225–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gabor C, Lymer J, Phan A, Choleris E. Rapid effects of the G-protein coupled oestrogen receptor (GPER) on learning and dorsal hippocampus dendritic spines in female mice. Physiol Behav. 2015;149:53–60. [DOI] [PubMed] [Google Scholar]
- 31.Kim J, Szinte JS, Boulware MI, Frick KM. 17β-Estradiol and agonism of G-protein-coupled estrogen receptor enhance hippocampal memory via different cell-signaling mechanisms. J Neurosci. 2016;36(11):3309–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Vinade L, Leapman RD, Petersen JD, Nakagawa T, Phillips TM, Sheng M, Reese TS. Mass of the postsynaptic density and enumeration of three key molecules. Proc Natl Acad Sci USA. 2005;102(32):11551–11556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Nelson CD, Li X, Winters CA, Azzam R, Sousa AA, Leapman RD, Gainer H, Sheng M, Reese TS. PSD-95 is required to sustain the molecular organization of the postsynaptic density. J Neurosci. 2011;31(17):6329–6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269(5231):1737–1740. [DOI] [PubMed] [Google Scholar]
- 35.El-Husseini AE, Schnell E, Chetkovich DM, Nicoll RA, Bredt DS. PSD-95 involvement in maturation of excitatory synapses. Science. 2000;290(5495):1364–1368. [PubMed] [Google Scholar]
- 36.Schnell E, Sizemore M, Karimzadegan S, Chen L, Bredt DS, Nicoll RA. Direct interactions between PSD-95 and stargazin control synaptic AMPA receptor number. Proc Natl Acad Sci USA. 2002;99(21):13902–13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bailey DJ, Wade J, Saldanha CJ. Hippocampal lesions impair spatial memory performance, but not song--a developmental study of independent memory systems in the zebra finch. Dev Neurobiol. 2009;69(8):491–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luine VN. Estradiol and cognitive function: past, present and future. Horm Behav. 2014;66(4):602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rensel MA, Salwiczek L, Roth J, Schlinger BA. Context-specific effects of estradiol on spatial learning and memory in the zebra finch. Neurobiol Learn Mem. 2013;100:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491(2):81–95. [DOI] [PubMed] [Google Scholar]
- 41.Brailoiu E, Dun SL, Brailoiu GC, Mizuo K, Sklar LA, Oprea TI, Prossnitz ER, Dun NJ. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J Endocrinol. 2007;193(2):311–321. [DOI] [PubMed] [Google Scholar]
- 42.Hammes SR, Levin ER. Minireview: recent advances in extranuclear steroid receptor actions. Endocrinology. 2011;152(12):4489–4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Woolley CS. Acute effects of estrogen on neuronal physiology. Annu Rev Pharmacol Toxicol. 2007;47:657–680. [DOI] [PubMed] [Google Scholar]
- 44.Frick KM. Molecular mechanisms underlying the memory-enhancing effects of estradiol. Horm Behav. 2015;74:4–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tuscher JJ, Szinte JS, Starrett JR, Krentzel AA, Fortress AM, Remage-Healey L, Frick KM. Inhibition of local estrogen synthesis in the hippocampus impairs hippocampal memory consolidation in ovariectomized female mice. Horm Behav. 2016;83:60–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Acharya KD, Veney SL. Characterization of the G-protein-coupled membrane-bound estrogen receptor GPR30 in the zebra finch brain reveals a sex difference in gene and protein expression. Dev Neurobiol. 2012;72(11):1433–1446. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Segura LM, Wozniak A, Azcoitia I, Rodriguez JR, Hutchison RE, Hutchison JB. Aromatase expression by astrocytes after brain injury: implications for local estrogen formation in brain repair. Neuroscience. 1999;89(2):567–578. [DOI] [PubMed] [Google Scholar]
- 48.Garcia-Segura LM, Azcoitia I, DonCarlos LL. Neuroprotection by estradiol. Prog Neurobiol. 2001;63(1):29–60. [DOI] [PubMed] [Google Scholar]
- 49.Azcoitia I, Sierra A, Veiga S, Garcia-Segura LM. Aromatase expression by reactive astroglia is neuroprotective. Ann N Y Acad Sci. 2003;1007:298–305. [DOI] [PubMed] [Google Scholar]
- 50.Azcoitia I, Santos-Galindo M, Arevalo MA, Garcia-Segura LM. Role of astroglia in the neuroplastic and neuroprotective actions of estradiol. Eur J Neurosci. 2010;32(12):1995–2002. [DOI] [PubMed] [Google Scholar]
- 51.Akama KT, Thompson LI, Milner TA, McEwen BS. Post-synaptic density-95 (PSD-95) binding capacity of G-protein-coupled receptor 30 (GPR30), an estrogen receptor that can be identified in hippocampal dendritic spines. J Biol Chem. 2013;288(9):6438–6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waters EM, Thompson LI, Patel P, Gonzales AD, Ye HZ, Filardo EJ, Clegg DJ, Gorecka J, Akama KT, McEwen BS, Milner TA. G-protein-coupled estrogen receptor 1 is anatomically positioned to modulate synaptic plasticity in the mouse hippocampus. J Neurosci. 2015;35(6):2384–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Akama KT, McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synthesis via the Akt/protein kinase B pathway. J Neurosci. 2003;23(6):2333–2339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Elias GM, Funke L, Stein V, Grant SG, Bredt DS, Nicoll RA. Synapse-specific and developmentally regulated targeting of AMPA receptors by a family of MAGUK scaffolding proteins. Neuron. 2006;52(2):307–320. [DOI] [PubMed] [Google Scholar]
- 55.Smejkalova T, Woolley CS. Estradiol acutely potentiates hippocampal excitatory synaptic transmission through a presynaptic mechanism. J Neurosci. 2010;30(48):16137–16148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74(5):801–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Balthazart J, Baillien M, Ball GF. Rapid control of brain aromatase activity by glutamatergic inputs. Endocrinology. 2006;147(1):359–366. [DOI] [PubMed] [Google Scholar]
- 58.Jeong JK, Burrows K, Tremere LA, Pinaud R. Neurochemical organization and experience-dependent activation of estrogen-associated circuits in the songbird auditory forebrain. Eur J Neurosci. 2011;34(2):283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Saldanha CJ, Schlinger BA, Micevych PE, Horvath TL. Presynaptic N-methyl-D-aspartate receptor expression is increased by estrogen in an aromatase-rich area of the songbird hippocampus. J Comp Neurol. 2004;469(4):522–534. [DOI] [PubMed] [Google Scholar]
- 60.Schlinger BA, Saldanha CJ. Songbirds: a novel perspective on estrogens and the aging brain. Age (Dordr). 2005;27(4):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saldanha CJ, Remage-Healey L, Schlinger BA. Synaptocrine signaling: steroid synthesis and action at the synapse. Endocr Rev. 2011;32(4):532–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGaugh JL. Memory--a century of consolidation. Science. 2000;287(5451):248–251. [DOI] [PubMed] [Google Scholar]
- 63.Cahill L, McGaugh JL, Weinberger NM. The neurobiology of learning and memory: some reminders to remember. Trends Neurosci. 2001;24(10):578–581. [DOI] [PubMed] [Google Scholar]
- 64.Allen TA, Fortin NJ. The evolution of episodic memory. Proc Natl Acad Sci USA. 2013;110(Suppl 2):10379–10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fortress AM, Fan L, Orr PT, Zhao Z, Frick KM. Estradiol-induced object recognition memory consolidation is dependent on activation of mTOR signaling in the dorsal hippocampus. Learn Mem. 2013;20(3):147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lynch JF III, Dejanovic D, Winiecki P, Mulvany J, Ortiz S, Riccio DC, Jasnow AM. Activation of ERβ modulates fear generalization through an effect on memory retrieval. Horm Behav. 2014;66(2):421–429. [DOI] [PubMed] [Google Scholar]
- 67.Chao A, Paon A, Remage-Healey L. Dynamic variation in forebrain estradiol levels during song learning. Dev Neurobiol. 2015;75(3):271–286. [DOI] [PMC free article] [PubMed] [Google Scholar]