Abstract
Growth hormone (GH) is a determinant of glucose homeostasis and adipose tissue (AT) function. Using 7-month-old transgenic mice expressing the bovine growth hormone (bGH) gene and growth hormone receptor knockout (GHR−/−) mice, we examined whether changes in GH action affect glucose, insulin, and pyruvate tolerance and AT expression of proteins involved in the interrelated signaling pathways of GH, insulinlike growth factor 1 (IGF-1), and insulin. Furthermore, we searched for AT depot–specific differences in control mice. Glycated hemoglobin levels were reduced in bGH and GHR−/− mice, and bGH mice displayed impaired gluconeogenesis as judged by pyruvate tolerance testing. Serum IGF-1 was elevated by 90% in bGH mice, whereas IGF-1 and insulin were reduced by 97% and 61% in GHR−/− mice, respectively. Igf1 RNA was increased in subcutaneous, epididymal, retroperitoneal, and brown adipose tissue (BAT) depots in bGH mice (mean increase ± standard error of the mean in all five depots, 153% ± 27%) and decreased in all depots in GHR−/− mice (mean decrease, 62% ± 4%). IGF-1 receptor expression was decreased in all AT depots of bGH mice (mean decrease, 49% ± 6%) and increased in all AT depots of GHR−/− mice (mean increase, 94% ± 8%). Insulin receptor expression was reduced in retroperitoneal, mesenteric, and BAT depots in bGH mice (mean decrease in all depots, 56% ± 4%) and augmented in subcutaneous, retroperitoneal, mesenteric, and BAT depots in GHR−/− mice (mean increase: 51% ± 1%). Collectively, our findings indicate a role for GH in influencing hormone signaling in AT in a depot-dependent manner.
This article shows that the expressions of GH, IGF-1, and insulin receptors in mouse AT are affected by modified GH action. Furthermore, receptor expressions occur in a depot-dependent manner.
In recent decades, the prevalence of obesity and obesity-related comorbidities has increased dramatically (1, 2). Obesity is caused by excessive accumulation of white adipose tissue (WAT). The mass of visceral adipose tissue (AT), more so than subcutaneous (Sc) AT, is associated with metabolic disorders and poor health outcomes (3). Thus, much research has been devoted to the clarification of factors that control AT deposition and physiology. Growth hormone (GH) is the primary stimulator of insulinlike growth factor 1 (IGF-1), and together with insulin, these three anabolic hormones exert potent effects on AT function (4). For example, obese patients present with markedly suppressed pituitary GH secretion (5). Given that GH directly influences fat mass by promoting lipolysis and preventing lipogenesis, obese patients tend to lack the lipolytic and antilipogenic actions of GH. Aside from its lipolytic effects, GH is also diabetogenic and affects the ability of insulin to maintain normal glucose homeostasis (4–6).
The actions of GH, IGF-1, and insulin are mediated through their respective receptors, the growth hormone receptor (GHR), insulinlike growth factor 1 receptor (IGF-1R), and insulin receptor (IR), which are all abundantly expressed in AT (4, 7). Accordingly, GH, IGF-1, and insulin comprise an intricate regulatory hormonal network with immense adaptability. Thus, it is often challenging to discriminate between the direct effects of GH vs those mediated through IGF-1 and/or insulin.
Mouse strains with genetically altered GH-induced action have provided valuable means of studying the effects of GH on obesity. Mice with GH deficiency as a result of disruption of the GHR gene (GHR−/−) have decreased body size and very low circulating IGF-1 levels (8, 9). Interestingly, the lack of GH-induced signaling is associated with improved insulin sensitivity and extended longevity, despite markedly increased adiposity in select depots (4, 9, 10). Of note, GHR−/− mice show a preferential enlargement of the Sc depot, whereas intra-abdominal fat pads are proportional to their dwarf size (4). The depot-specific alterations in mass are accompanied by differences in cellular composition and function (4). This coexistence of improved insulin sensitivity and obesity suggests a metabolically healthy obese phenotype in these mice. Conversely, transgenic mice expressing the bovine growth hormone (bGH) gene are characterized by increased IGF-1 levels, accelerated growth, increased lean body mass, decreased WAT mass, and a shortened lifespan (11–13). Generally, these mice suffer from a number of pathological changes in glucose and lipid metabolism, including hyperinsulinemia and impaired insulin sensitivity, despite normal fasting blood glucose (14–16). Together, these two mouse lines with their extremes of GH activity illustrate how GH is able to influence the deposition of AT in a depot-specific manner, and how the pattern of fat distribution may have a profound influence on whole-body metabolism (3, 7, 17, 18). However, the mechanisms underlying the depot-specific role of GH in AT modeling/remodeling and function remain poorly understood.
The aim of the current study was to investigate to what extent GH regulates the local expression of proteins and protein receptors that influence adipocyte metabolism in bGH and GHR−/− mice. Thus, we studied glucose homeostasis and examined whether changes in GH action result in alterations in the RNA and protein patterns of IGF-1, IGF-1R, IR, and GHR. Finally, we analyzed the adipose depot–specific differences that may explain why the effects of GH depend on AT location.
Methods
Animals
The bGH and GHR−/− mice were produced on a C57BL/6J background or backcrossed more than 10 generations into a C57BL/6J background, as previously described (8, 18). Seven-month-old male cohorts of bGH mice (n = 10) and wild-type (WT) littermate controls (n = 10), and GHR−/− mice (n = 8) and WT littermate controls (n = 8), were bred at the Edison Biotechnology Institute (Ohio University, Athens, OH). The mice were housed in cages that were kept on a 14-hour light/10-hour dark cycle in a temperature-controlled environment (23°C). Mice were housed up to four per cage with standard chow and water provided ad libitum, unless otherwise noted. The Ohio University’s Institutional Animal Care and Use Committee approved all protocols and procedures.
Body weight and body composition
Body weight and composition measurements were performed 1 week prior to dissection. Fat, lean, and fluid mass were measured using a quantitative Minispec mq benchtop nuclear magnetic resonance analyzer (Bruker Optics, The Woodlands, TX), as previously described (16, 19).
Glucose tolerance test, insulin tolerance test, and pyruvate tolerance test
Four weeks prior to dissection, the mice were subjected to a glucose tolerance test (GTT), insulin tolerance test (ITT), and pyruvate tolerance test (PTT). All tests started at ∼9:00 am to ensure that measurements were not affected by diurnal fluctuations in blood glucose. Blood samples were taken from the tail vein. Glucose was measured using OneTouch Ultra test strips and glucometer (LifeScan, Milpitas, CA) before intraperitoneal (IP) injection of glucose, insulin, or pyruvate and at various time points after injections. After each tolerance test, the mice were allowed to rest for a week prior to the subsequent test.
GTTs were performed on mice that were fasted for 12 hours prior to measurements. Each mouse received an IP injection of glucose (Sigma-Aldrich, St. Louis, MO) diluted in sterile phosphate-buffered saline for a final dose of 1 g/kg body weight. Blood glucose measurements were performed at baseline and after 15, 30, 45, 60, 90, 120, and 150 minutes. ITTs were performed on mice in a fed state. Each mouse received an IP injection of insulin (Humulin R; Eli Lilly, Indianapolis, IN) at 0.75 U/kg body weight, and glucose was measured at baseline and at 20, 40, 60, 80, 100, and 120 minutes after injection. A PTT was performed to assess gluconeogenic capacity. Mice were fasted for 12 hours before commencement of the test and received an IP injection of pyruvate at a bolus at 1.5 g/kg body weight. Measurements were performed at baseline and at 20, 40, 60, 90, 120, and 150 minutes after injection. Area under the curve (AUC) was calculated for all groups after each test.
Tissue collection
All mice were fasted for 12 hours prior to euthanasia. The mice were anesthetized in a CO2 chamber, after which blood was collected from the orbital sinus. After blood collection, the mice were euthanized by cervical dislocation. Blood was incubated at 20°C for 20 minutes and centrifuged at 7000 × g for 10 minutes at 4°C. Serum was aliquoted and stored at −80°C until further analyses. Five adipose depots were collected and weighed [Sc, epididymal (Epi), retroperitoneal (Ret), mesenteric (Mes), and brown adipose tissue (BAT)], frozen in liquid nitrogen, and stored at −80°C until analysis.
Biochemical blood analyses
Measurements of blood glycated hemoglobin (HbA1c) were performed on whole blood collected 1 week prior to dissection. Blood samples were taken from the tail vein on fasted mice, and HbA1c was measured using a DCA Vantage analyzer and reagent kit (Siemens Medical Solutions, Malvern, PA) with an HbA1c range of 4 to 130 mmol/mol. In serum collected at dissection, levels of insulin were determined using the mouse ultrasensitive insulin enzyme-linked immunosorbent assay kit (ALPCO, Salem, NH), according to the manufacturer's directions. The dynamic range was 0.025 to 1.25 ng/mL. IGF-1 was determined using a mouse IGF-1 enzyme-linked immunosorbent assay kit (ALPCO) with a dynamic range of 0.5 to 18 ng/mL. All measurements were performed in duplicate.
Extraction of RNA and complementary DNA synthesis
Total AT RNA was isolated using TRIzol reagent (Thermo Fisher Scientific, Waltham, MA), following the manufacturer’s protocol. The quality and quantity of total RNA was analyzed by visual inspection of the 18S and 28S RNA on an agarose gel and by measuring absorbance at 260 and 280 nm with a ratio of ≥1.8 using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific). Complementary DNA (cDNA) was synthesized from 2 μg of total RNA using the Maxima First Strand cDNA Synthesis Kit (Thermo Fisher Scientific).
Quantitative polymerase chain reaction
Quantitative polymerase chain reaction (qPCR) was performed using the StepOnePlus real-time PCR machine (Thermo Fisher Scientific/Applied Biosystems, Foster City, CA) with Bullseye EvaGreen qPCR MasterMix Rox (Midsci, Valley Park, MO). Each reaction contained 12.5 μL of MasterMix, 0.3 μM each of backward and forward primer (Sigma-Aldrich) (Table 1), and 5 μL of cDNA. The qPCR reactions were performed in triplicate using the duration-fast protocol: enzyme activation at 95°C for 10 minutes, denaturation at 95°C for 3 seconds, and annealing and extension at 60°C for 30 seconds. A melting curve was produced for each reaction to evaluate the generation of nonspecific products. β-2 microglobulin (B2m), eukaryotic elongation factor 2 (Eef2), and ribosomal protein S3 (Rps3) were used as reference genes (20). Calibrated normalized relative quantities were calculated using the modified Pfaffl equation, taking gene-specific amplification efficiencies and multigene normalization into account (21). The normalization factor was calculated as the geometric mean of the calibrated normalized relative quantities of each reference gene. Expression of the gene of interest in the bGH and GHR−/− mice was compared with that of their WT littermate control group. No significant differences in gene expression levels were observed between the WT controls, and thus, for comparisons between AT depots, all WT mice were analyzed as one group (n = 18).
Table 1.
Primer Sequences for qPCR
| Target Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|
| B2m | GTATGCTATCCAGAAAACCC | CTGAAGGACATATCTGACATC |
| Eef2 | TCGGCGCGCTTCCCTGTTCAC | ATGCCAGCCTTGCACACAAGGG |
| Rps3 | CAAGAAGAGGAAGTTTGTAGC | GTCCTGGTGGCTAAAATAATG |
| Insr | CAAACAGATGCCACTAATCC | CTTTGAGACAATAATCCAGCTC |
| Igf1r | TTGTGTTGTTCGTCCGGTGTG | ATGTGCCCAAGTGTGTGCG |
| Igf1 | GAGACTGGAGATGTACTGTG | CTTCCTCTACTTGTGTTCTTC |
| Ghr/Ghbp | CCACCCAATGCAGATGTTCT | CAAATAGCATCACTGCTACTCCAAATATTC |
| Ghr | CCACCCAATGCAGATGTTCT | CTGGATATCTTCTTCACATGCTTCC |
| Ghbp | CCACCCAATGCAGATGTTCT | GGTGCCCACAAGGCTAGGGAT |
Target genes included Igf1, Igf1r, Ir, and Ghr. In addition, primers were designed to distinguish between transcripts specific for the GHR or the growth hormone–binding protein (GHBP). In rodents, the GHBP is generated from the Ghr gene as an alternatively spliced precursor RNA product (referred to as Ghbp). Exons 8, 9, and 10 of the Ghr gene encode a domain that is specific to the receptor. The soluble GHBP contains a domain, which is encoded by a GHBP-specific exon in the Ghr gene, designated as exon 8A. Differential splicing of exon 7 to either exon 8 or the interposed exon 8A yields the GHR or the GHBP, respectively. Finally, all Ghr gene transcript forms (referred to as Ghr/Ghbp) were quantified using primers that, because of their location, were unable to distinguish between transcripts for GHR or GHBP.
Western blotting
Due to limited amounts of AT, protein was only isolated from Sc AT. Frozen tissue samples were homogenized in radioimmunoprecipitation assay buffer (Sigma-Aldrich) with phenylmethylsulfonyl fluoride protease inhibitor, and protein concentrations were determined using Bradford Protein assay (Bio-Rad, Hercules, CA). Protein (15 μg) was loaded onto a 26-well 4% to 15% stain-free gradient gel (Criterion TGX; Bio-Rad). The gel was imaged using the stain-free application on the ChemiDoc MP imager (Bio-Rad) immediately after protein separation and prior to Western blotting. Protein gels were blotted using the Trans-Blot Turbo transfer apparatus and PVDF Midi transfer packs (Bio-Rad). Membranes were transferred to a blocking solution (Tris-buffered saline, 0.05% Tween 20, 5% bovine serum albumin) and subsequently imaged to verify total protein transfer. Membranes were probed with primary antibodies: rabbit anti-GHR (AL47) (22), rabbit anti-IR-α (sc-710; Santa Cruz Biotechnology, Santa Cruz, CA), rabbit anti–IGF-1R (sc-9038; Santa Cruz Biotechnology), or goat anti–glyceraldehyde 3-phosphate dehydrogenase (sc-20357, Santa Cruz Biotechnology). IGF-1 was not detected using Western blotting. Secondary antibodies coupled to horseradish peroxidase (HRP) were applied [anti-rabbit HRP and anti-goat HRP (R&D Systems, Minneapolis, MN)], and signals were detected by the addition of SuperSignal West Dura Chemiluminescent substrate reagent (Thermo Fisher Scientific). Imaging and densitometric analysis of the blots were performed using the ChemiDoc MP imager (Bio-Rad). The ImageLab software version 5.2 (Bio-Rad) was used to select and determine the background-subtracted density of the bands in all gels and blots. The stain-free total protein quantitation served as loading control. Total protein levels were checked by comparisons with glyceraldehyde 3-phosphate dehydrogenase levels. A liver sample served as control.
Statistics
Values are reported as mean ± standard error of the mean. Assumptions of normal distributions were investigated by histograms and quantile-quantile plots. AUC for blood glucose concentrations during GTT, ITT, and PTT was determined using the trapezoidal method, and both total AUC and incremental AUC (i.e., percentage change from baseline values) were analyzed. Student t test was used to compare baseline characteristics, serum measurements, AUC, gene expressions, and protein levels between genotypes (bGH and WT littermates or GHR−/− and WT littermates). Body composition was also analyzed by linear regression with total body weight as a covariate. Pairwise comparisons of means between depots in the WT control mice were performed using one-way analysis of variance with correction for multiple comparisons using Tukey’s post hoc test. Differences were considered statistically significant at P < 0.05. Statistical analyses were performed using Stata 13 (StataCorp, College Station, TX).
Results
Body composition and AT depot weights
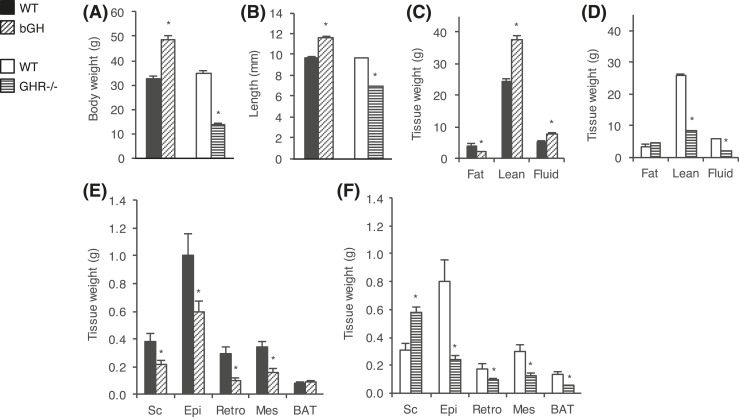
Body compositions and depot weights are shown in Fig. 1. As expected, 7-month-old bGH mice had higher body weight (P < 0.001) [Fig. 1(A)] and longer body length (P < 0.001) [Fig. 1(B)] than WT controls. The excess body weight was due primarily to an increase in lean mass, which was proportional to the larger body size (bGH, 77.7% ± 0.9% vs WT, 75.1% ± 1.8%; P = 0.198). However, as conventional normalization to total body weight tends to underestimate or exaggerate differences, adjustments for body weight were also performed by including this term as a covariate rather than using proportions. Accordingly, lean body mass was significantly increased in the bGH mice (P = 0.020). Total fat mass was significantly decreased, both as absolute weight and percentage body weight (bGH, 4.2% ± 0.5% vs WT, 12.1% ± 1.6%; P < 0.001). The decrease was also significant in a regression analysis adjusted for total body weight (P < 0.001) [Fig. 1(C)].
Figure 1.
Body weight and composition of bGH and GHR−/− mice. Body and tissue weights were determined in bGH mice (diagonal-hatched bars) and WT control mice (black bars) (n = 10 per group) and in GHR−/− mice (horizontal-hatched bars) and WT control mice (white bars) (n = 8 per group). (A) Total body weight was determined 1 week prior to dissection and (B) body length was measured at dissection. Body composition (fat, lean, and fluid mass) is shown for (C) bGH and WT control mice and (D) GHR−/− and WT control mice. Sc, Epi, Ret, and Mes white adipose depots as well as BAT were weighed in (E) bGH and WT mice and (F) GHR−/− and WT mice. Values are expressed as mean ± standard error of the mean; *P < 0.05.
The GHR−/− mice were significantly smaller (P < 0.001) and shorter (P < 0.001) than littermate controls [Fig. 1(A) and Fig. 1(B)]. Total lean mass was significantly reduced, but the decrease was not fully explained by the reduced body size. GHR−/− mice had significantly lower lean mass both when normalized to total body weight (GHR−/−, 61.0% ± 1.1% vs WT, 74.5% ± 1.7%; P < 0.001) and when adjusting for total body weight (P < 0.001). Similar to what has been previously shown (10, 13, 15), absolute fat mass in GHR−/− was comparable to that in WT control mice (P = 0.188), but when normalized to total body weight, GHR−/− mice had a significantly higher percentage of fat mass (GHR−/−, 32.1% ± 0.6% vs WT, 9.5% ± 1.5%; P < 0.001) [Fig. 1(D)].
The weight of all WAT depots was decreased in the bGH mice, with reductions of 43% in Sc (P = 0.027), 41% in Epi (P = 0.027), 66% in Ret (P = 0.003), and 54% in Mes (P < 0.001), as compared with WT littermates [Fig. 1(E)]. BAT depot weights were similar in WT and bGH mice. Although total fat mass was similar in GHR−/− and WT, the weights of the various adipose depots were not uniformly distributed [Fig. 1(F)]. Most notably, the Sc depot was substantially enlarged (P = 0.001), whereas fat loss was evident in all other ATs examined. When normalized to total body weight, only the Sc depot was significantly different in GHR−/− vs WT mice. This preferential Sc depot enlargement has been demonstrated in GHR−/− mice of various ages (4). Total BAT depot weight was significantly reduced in GHR−/− mice (P < 0.001). However, when adjusting for total body weight, BAT depot sizes were comparable in all genotypes.
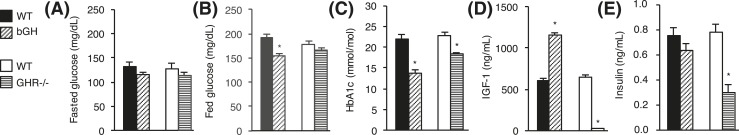
Serum IGF-1 levels were elevated by 90% in bGH mice (P < 0.001) and reduced by 97% in GHR−/− mice (P < 0.001) compared with controls [Fig. 2(D)]. Insulin levels were similar in bGH and control mice, whereas they were decreased by 61% in GHR−/− mice (P < 0.001) [Fig. 2(E)].
Figure 2.
Blood parameters in bGH and GHR−/− mice. (A) Fasting blood glucose, (B) fed blood glucose, (C), HbA1c, (D) IGF-1, and (E) insulin levels are shown for bGH mice (diagonal-hatched bars) and WT control mice (black bars) (n = 10 per group) and for GHR−/− mice (horizontal-hatched bars) and WT control mice (white bars) (n = 8 per group). Values are expressed as mean ± standard error of the mean; *P < 0.05.
Serum hormone levels and glucose homeostasis
Fasting blood glucose levels were unchanged in bGH and GHR−/− mice compared with littermate controls [Fig. 2(A)], whereas fed blood glucose levels were significantly reduced in bGH mice (P < 0.001) [Fig. 2(B)]. HbA1c levels were reduced in both bGH and GHR−/− mice (P < 0.001) [Fig. 2(C)]. Serum IGF-1 levels were elevated by 90% in bGH mice (P < 0.001) and reduced by 97% in GHR−/− mice (P < 0.001) compared with controls [Fig. 2(D)]. Insulin levels were similar in bGH and control mice, whereas levels were decreased by 61% in GHR−/− mice (P < 0.001) [Fig. 2(E)].
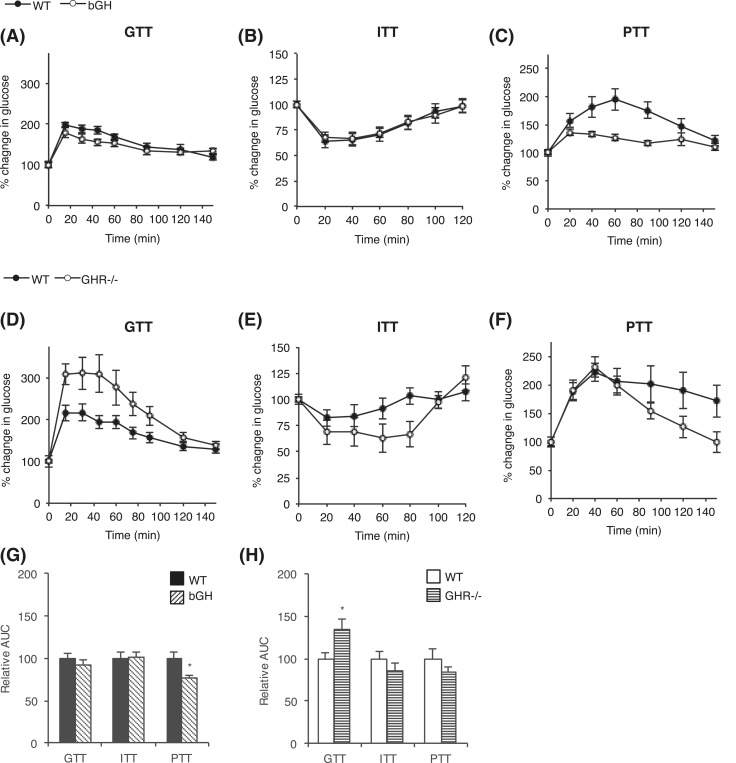
Glucose homeostasis was assessed by a GTT, ITT, and PTT in bGH [Fig. 3(A–C)] and GHR−/− [Fig. 3(D–F)] mice. Interestingly, bGH mice had significantly improved glucose clearance compared with WT controls, with reduced absolute GTT AUC of 26% (P = 0.001), and enhanced insulin sensitivity with a reduced AUC of 19% (P = 0.039) in total ITT. However, when the GTT and ITT were evaluated as relative AUCs (percentage change in glucose from baseline values), no differences were seen [Fig. 3(G)]. During the PTT, a pyruvate bolus elicits a glycemic excursion that reflects hepatic gluconeogenesis. The bGH mice showed significantly lower blood glucose levels upon pyruvate injection, with a total AUC reduction of 34% (P < 0.001). When blood glucose concentrations were expressed as percentage change from baseline, PTT AUC remained significantly decreased in the bGH mice (24%; P = 0.011) [Fig. 3(G)].
Figure 3.
Glucose homeostasis in bGH and GHR−/− mice. (A) GTTs, (B) ITTs, and (C) PTTs were performed in bGH mice (white circles) and WT control mice (black circles) (n = 10 per group). Similarly, (D) GTTs, (E) ITTs, and (F) PTTs were performed in GHR−/− mice (white circles) and WT control mice (black circles) (n = 8 per group). Mice received an IP injection of glucose at 1 g/kg body weight, insulin at 0.75 U/kg body weight, or pyruvate at 1.5 g/kg body weight, respectively. Blood glucose levels are reported as percentage change in glucose from baseline (100%). Relative AUC was calculated for each test in (G) bGH and (H) GHR−/− mice. Values are expressed as mean ± standard error of the mean.
GHR−/− mice showed no difference in total AUC in response to the glucose, insulin, or pyruvate challenge. However, when adjusting for initial blood glucose level, glucose disposal was significantly hampered in the GHR−/− mice during the GTT, with an increase in relative AUC of 35% (P = 0.021) [Fig. 3(H)].
Adipose depot–specific differences in RNA of various receptors in WT mice
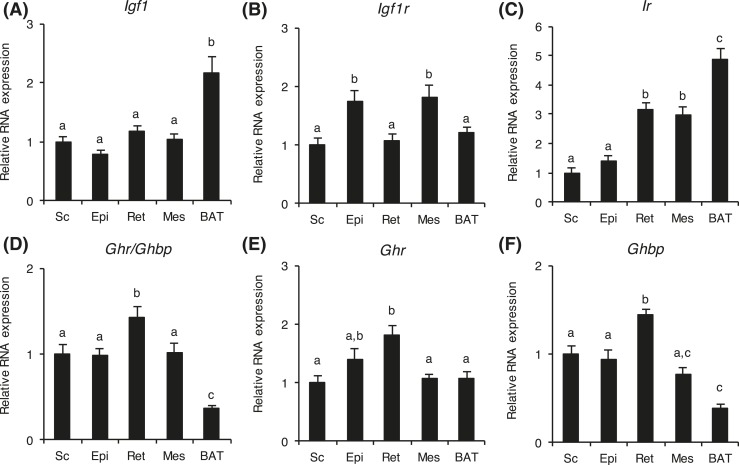
Because AT depots did not respond to the altered GH activity in a uniform manner in bGH or GHR−/− mice, we investigated if the GH- and IGF-related genes were differentially expressed in an AT depot–dependent manner. Because no significant differences in gene expression levels for Igf1, Igf1r, Ir, and Ghr were observed between WT control mice of bGH and GHR−/− litters, the WT control mice were analyzed as one group (n = 18) (Fig. 4). In these WT mice, Igf1 RNA levels were similar in all WAT depots, but significantly higher in BAT (P < 0.001). Igf1r RNA were slightly, but significantly, higher in Epi and Mes depots than in Sc, Ret, and BAT depots. Ir RNA levels were lowest in the Sc and Epi depots and significantly higher in Ret and Mes depots. Levels in BAT were more than 300% higher than in Sc and Epi (P < 0.001) and 60% higher than in Ret and Mes (P < 0.001).
Figure 4.
Adipose depot–specific differences in RNA expressions in WT mice. Relative RNA levels of (A) Igf1, (B) Igf1r, (C) Ir, (D) Ghr/Ghbp, (E) Ghr, and (F) Ghbp were determined in Sc, Epi, Ret, Mes, and BAT depots from WT mice (n = 18). For each gene, RNA levels are relative to the level in the subcutaneous depot. Values are expressed as mean ± standard error of the mean. Means with differing lowercase letters a, b, or c in the same gene are significantly different (P < 0.05).
Primers were designed to distinguish between transcripts specific for Ghr or Ghbp. In addition, all Ghr gene transcript forms (Ghr/Ghbp) were quantified using primers that were unable to distinguish between transcripts for Ghr or Ghbp. The RNA levels of Ghr/Ghbp, Ghr, and Ghbp varied, with the highest RNA levels found in Ret depots. Collectively, expression levels of Ghr and Ghbp were quite similar, and Ghr/Ghbp expression levels appeared to be an average of Ghr and Ghbp RNA levels. Ghr/Ghbp and Ghbp levels were lower in BAT compared with WAT, whereas Ghr levels were similar in WAT and BAT.
AT RNA expressions and protein levels in bGH and GHR−/− mice
The relative RNA levels were determined in Sc, Epi, Ret, Mes, and BAT depots from bGH and GHR−/− mice as well as their WT littermate controls (Fig. 5). Igf1 RNA levels were significantly increased in all depots from bGH mice and decreased in all depots in GHR−/− mice [Fig. 5(A)]. On average, Igf1 RNA levels in the five depots were elevated by 153% ± 27% (P = 0.005) in the bGH and decreased by 62% ± 4.3% (P < 0.001) in the GHR−/− mice. Igf1r RNA levels were significantly decreased in all depots in bGH mice (average decrease, 49% ± 5.6%; P < 0.001), and increased in all depots in GHR−/− mice (average increase, 94% ± 8.2%; P < 0.001) [Fig. 5(B)]. The same pattern was observed for Ir RNA levels, with significant reductions in Ret, Mes, and BAT in bGH mice, and augmented levels in Sc, Ret, Mes, and BAT in GHR−/− mice [Fig. 5(C)]. On average, Ir RNA levels in the five depots were decreased by 56% ± 4.4% (P < 0.001) in the bGH mice and increased by 51% ± 12% (P = 0.013) in the GHR−/− mice.
Figure 5.
RNA expression levels in adipose tissue depots from bGH and GHR−/− mice. Sc, Epi, Ret, and Mes white adipose depots as well as BAT were collected from bGH mice (diagonal-hatched bars) and WT control mice (black bars) (n = 10 per group) and from GHR−/− mice (horizontal-hatched bars) and WT control mice (white bars) (n = 8 per group). Relative RNA levels of (A) Igf1, (B) Igf1r, (C) Ir, (D) Ghr/Ghbp, (E) Ghr, and (F) Ghbp were determined. Ghr and Ghbp RNA levels were only determined in mice from the bGH litter. For each gene, all RNA levels are relative to the level in the Sc depot in the WT control group from the bGH litter. Values are expressed as mean ± standard error of the mean; *P < 0.05.
In bGH mice, the Ghr/Ghbp RNA expressions in Epi, Ret, Mes, and BAT were similar to controls. In Sc, RNA expression was significantly decreased by 37% (P = 0.019). Ghr RNA levels were not significantly different between bGH and WT control mice. However, Ghbp RNA expression was significantly reduced by 45% in Sc (P = 0.006) and increased by 57% (P = 0.010) and 247% (P = 0.011) in Ret and BAT, respectively, vs controls. Levels were unaltered in Epi and Mes (P = 0.109 and P = 0.321, respectively). In GHR−/− mice, Ghr/Ghbp RNA levels were undetectable, as would be expected in a GHR gene-disrupted mouse.
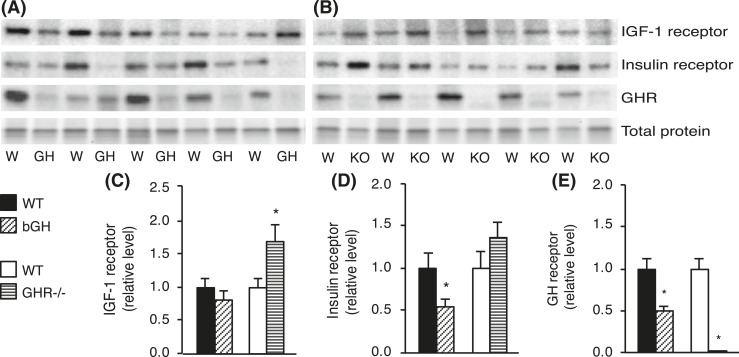
Protein levels of IGF-1R, IR, and GHR were determined in Sc AT from bGH and GHR−/− mice (Fig. 6). Western blot analysis revealed no differences in IGF-1R levels in bGH mice, whereas IR protein was significantly decreased (P = 0.046). IGF-1R levels were increased in GHR−/− mice (P = 0.039), whereas the difference in IR was insignificant. Although not all differences were significant, the direction of change for IGF-1R and IR protein appeared identical to cognate RNA levels. Similar to RNA expressions, GHR protein levels were significantly decreased in Sc AT from bGH mice (P = 0.007), and, as expected, undetectable in GHR−/− mice. Collectively, protein levels appeared to follow the same pattern as RNA levels, although some differences were insignificant at the protein level.
Figure 6.
Protein levels in Sc adipose tissue from bGH and GHR−/− mice. Protein was isolated from Sc white adipose depots collected from bGH mice (diagonal-hatched bars) and WT control mice (black bars) (n = 10 per group) and from GHR−/− mice (horizontal-hatched bars) and WT control mice (white bars) (n = 8 per group). Representative Western blots of IGF-1R, IR, and GHR are shown for (A) bGH (GH) and WT (W) mice and for (B) GHR−/− (KO) and WT (W) mice. Protein levels of (C) IGF-1R, (D) IR, and (E) GHR were quantified and reported as mean ± standard error of the mean; *P < 0.05.
Discussion
GH, IGF-1, and insulin signaling play important roles in the regulation of glucose homeostasis and AT function, as demonstrated in a variety of mammals. In the current study, we used bGH and GHR−/− mice to investigate the effect of modified GH action on the expression of genes and proteins involved in these interrelated signaling pathways in AT. Notably, we found GH to regulate GHR, IGF-1R, and IR expression levels in a depot-dependent manner. Furthermore, the receptors appear to play distinct roles in white and brown fat.
Several studies have assessed body composition in bGH and GHR−/− mice (15, 18, 23, 24). Consistent with prior findings, we report a decreased percentage of fat mass and increased total lean mass in bGH mice as compared with their WT counterparts. The general phenotype of bGH mice is consistent with previous findings (10, 13, 15, 18) and is largely explained by the alterations in metabolic regulation that are induced by increased GH/IGF-1 activity. In particular, the potent lipolytic and antilipogenic effects of GH are likely responsible for the dramatic reduction in WAT mass (15, 18). Simultaneously, the growth-promoting effects increase absolute lean mass, although absolute lean mass appears proportional to the larger body size (15, 16). On the other hand, the GHR−/− mice showed a higher percentage of fat mass and decreased lean mass. Overall, there is little doubt that AT accumulation in GHR−/− mice occurs in a depot-specific manner, with profound enlargement of the Sc depot, and that the GHR−/− mice show improved, rather than impaired, insulin sensitivity (18, 23, 25, 26). It is interesting that the intra-abdominal depots were not increased when normalized to body weight, indicating no additional fat accumulation outside of the Sc depot. Previous studies have shown that lean mass in GHR−/− mice is proportional to body weight (10), whereas other studies report decreased lean mass (23). In the current study, the decrease in lean mass was not fully explained by the reduced body size. The varying results between studies may be explained by age- and sex-specific differences (10, 16, 18, 23). Finally, BAT depot sizes relative to body weights were comparable in mice of all genotypes. Previous studies have demonstrated increased interscapular BAT in GHR−/− mice (27), whereas there are discrepancies in the literature regarding bGH mice, with reports showing both increased (28) and reduced BAT levels (4, 9, 27). Again, the inconsistencies may be explained by differences in age and sex (10, 16, 18, 23).
We observed improved glucose clearance and insulin sensitivity in the bGH mice, whereas fasting blood glucose and insulin levels were similar to WT. Interestingly, HbA1c levels were significantly reduced, suggesting improved long-term glycemic control. This finding may appear paradoxical, given that GH is known to exert diabetogenic effects and oppose insulin actions on glucose metabolism. However, evidence from studies in bGH mice are ambiguous. Previous reports have shown that bGH mice are indeed hyperinsulinemic in early life (14, 24, 28). Unexpectedly, glucose homeostatic control is ameliorated in later life, and the mice are normoinsulinemic by 9 months of age and hypoinsulinemic by 11 months of age (24). Thus, despite high ambient GH concentrations, insulin insensitivity is not observed. Whether this decline in insulin concentration with age results from loss of pancreatic β cells or an actual increase in insulin sensitivity remains unsettled. Blood glucose levels in bGH mice are known to decline with age, and are significantly different from those of WT mice at 9, but not 6, months of age (24). One prior study in bGH mice has demonstrated enhanced glucose tolerance independent of age (29). Furthermore, the mice in that study showed augmented insulin sensitivity throughout life, which counterintuitively suggests an overall beneficial effect of GH transgene expression. The contradictory findings are especially noteworthy, given that increased longevity in GHR−/− mice has been partly attributed to enhanced insulin sensitivity (30). The extended lifespan in GHR−/− mice may, however, relate more to other notable features, such as reduced incidence of diabetes-induced nephropathy and cancers (31).
Hepatic gluconeogenesis in bGH mice was assessed by measuring blood glucose response to an IP injection of pyruvate. This method can reveal severe gluconeogenic deficit but is highly dependent on glucose-stimulated insulin secretion and insulin sensitivity, and thus, should be evaluated in relation to GTT and ITT results. The response in bGH mice was blunted relative to their WT littermates, suggesting that bGH mice have reduced ability to convert pyruvate into glucose. On the whole, GH is thought to increase gluconeogenesis, probably in a lactate-dependent manner (32); thus, the mechanism responsible for this discrepancy is unknown. To our knowledge, only one other study has assessed pyruvate tolerance in bGH mice, with similar findings (29). In that study, the authors also demonstrated a downregulation of key liver enzymes involved in gluconeogenesis. This supports the hypothesis that bGH mice have reduced gluconeogenic proclivity, and that the lack of blood glucose response is not merely the result of an increased or rapid glucose clearance. There is also the likelihood of a contribution of increased IGF-1, which is known to decrease hepatic phosphoenol pyruvate carboxykinase and thereby inhibit gluconeogenesis (33). Clearly, GH effects on glucose homeostasis remain incompletely understood.
GHR−/− mice displayed no difference in glucose clearance in response to the glucose, insulin, or pyruvate challenge. Fasting glucose levels were normal, whereas fed glucose levels were significantly reduced. Glucose levels are generally lower in young GHR−/− mice, and higher in older mice (25, 34, 35). Circulating insulin levels were reduced, which is in agreement with previous findings and a hallmark of GHR−/− mice of most ages (25, 34, 36). The reduced insulin levels are due, in part, to the removal of the anti-insulin activity of GH and suggest increased insulin sensitivity, which would be consistent with the extended longevity observed in these mice (30). Interestingly, like bGH mice, GHR−/− mice presented with reduced HbA1c levels, suggesting improved long-term glycemic control.
GH, IGF-1, and insulin signaling are essential for AT development and function (26, 37). Thus, we investigated the RNA levels of Igf1, Igf1r, Ir, and Ghr in AT as well as levels of IGF-1R, IR, and GHR protein in Sc in WT, bGH, and GHR−/− mice. First, we examined the interdepot differences in gene expression in WT mice. Igf1 RNA levels were similar in all WAT depots, but significantly higher in BAT. Igf1r levels were similar in Sc, Ret, and BAT, whereas Ir levels were significantly higher in BAT compared with the WAT depots. This suggests that both IGF-1 and insulin signaling are important for adipocyte homeostasis, although the two receptors play distinct roles in white and brown fat. It has recently been shown that mice with adipocyte-specific deletion of Igf1r have normal BAT mass, whereas Ir deletion results in a 50% mass reduction (38–40). Thus, insulin signaling may have a more integrated role in BAT. Surprisingly, Ghr/Ghbp and Ghbp RNA levels were lower in BAT than in most WAT, whereas Ghr RNA levels were not.
Igf1 RNA levels were increased in the bGH mice and, consistent with GH resistance, the levels were greatly reduced in GHR−/− mice. However, whereas serum levels in the GHR−/− were negligible, AT RNA levels of Igf1 were only reduced by ∼62%. Previous studies have shown a lack of liver Igf1 RNA in GHR−/− mice (34, 41). This confirms that circulating liver-derived IGF-1 is highly GH dependent, whereas local AT synthesis of IGF-1 is probably regulated by other factors.
The expression pattern of Igf1r in ATs seemed to be inversely related to Igf1 expression, being twofold lower in bGH mice and twofold higher in GHR−/− mice compared with WT controls. This relationship has been demonstrated previously in muscle tissue in bGH (42) and GHR−/− mice (41), and suggests that the IGF-1R is somehow regulated by negative feedback from IGF-1. The regulation of IGF-1R may also be a result of local counterregulatory mechanisms working to compensate for the high IGF-1 signaling.
Like Igf1r, expression of Ir was decreased in most AT depots of bGH mice and increased in GHR−/− mice. Decreased Ir expression has previously been demonstrated in the livers of bGH mice (43), and increased levels have been established in the livers of GHR−/− mice, but not in skeletal muscles (34, 41). Considering the low circulating insulin levels in GHR−/− mice, this tissue-specific regulation may represent a mechanism that allows GHR−/− mice to maintain efficient insulin-induced signaling and sensitivity at the tissue level, despite low insulin levels. Conversely, bGH mice may downregulate AT Ir expression to counteract the hyperinsulinemia that is usually observed in early life. However, in this study, the mice were normoinsulinemic and had normal glucose and insulin tolerance; thus, it is difficult to reconcile the absence of insulin resistance in bGH mice with the widely accepted association of high GH levels and reduced insulin sensitivity. We believe that there may be several explanations. First, the high ambient GH concentrations, more so than insulin levels, may be responsible for the alterations in Ir levels, and thereby affect local insulin sensitivity. Second, bGH mice may display local tissue-specific insulin resistance that is not reflected by circulating insulin levels or revealed in GTT or ITT results. Hearts of bGH mice are reported to show an increased insulin sensitivity at baseline, but they are resistant to acute insulin stimulation (44). Finally, as discussed previously in regard to the PTT, the disconnect between the presence of normal glucose homeostasis and high GH action in bGH mice may, in part, be explained by the reduced gluconeogenic proclivity, which affects whole-body glucose homeostasis, and the high IGF-1 level, which is known to inhibit hepatic gluconeogenesis (33, 45).
Ghr/Ghbp expression levels were generally similar in WT and bGH mice, with the highest levels in the Ret depot. Interestingly, levels were significantly lower in only the Sc depot in bGH mice, and this reduction was also observed at the protein level. Ghbp was significantly reduced in Sc and increased in Ret AT and BAT. Expression of Ghr followed the same pattern as Ghr/Ghbp, although the reduction in Sc was insignificant. A previous study in humans has shown a higher Ghr expression in Sc than omental WAT from lean, but not obese, subjects (46), whereas a recent study by Gude et al. (47) also demonstrated this interdepot difference in obese subjects. However, these findings were not confirmed in the current study. The differences found in humans may be explained by several mechanisms. Primarily, the decline in GH with obesity may modulate Ghr RNA levels. However, on the basis of our findings in bGH mice, high GH does not appear to substantially regulate Ghr RNA levels. Another explanation may be that GHR reduction is a pathological consequence of obesity. Yet, we did not observe higher Ghr RNA levels in the lean bGH mice. Another potential explanation may be the heterogeneity of Sc in humans, which is indeed location dependent. Taken together, our findings are difficult to reconcile, but give rise to further speculation. Mounting evidence suggests that altered GH action has the most pronounced impact on Sc fat pads as compared with other WAT depots (4). GHR−/− mice that lack the GHR have a preferential enlargement of the Sc in particular, and generally, Sc depots have a greater capability of shrinking or expanding. Given that the Sc depot appears uniquely affected, it is intriguing that Ghbp is differentially regulated in Sc compared with other WAT depots. Accordingly, WAT heterogeneity may partly be due to variations in GHR and GHBP distributions among depots. Whether these observations are linked warrants further investigations.
The GH-induced intracellular signaling pathways have previously been investigated in tissues from bGH and GHR−/− mice of various ages (34, 41–44). In these studies, comparisons were made between age-matched mice. However, at 7 months of age, WT mice have reached ∼35% of their mean lifespan, whereas bGH mice have reached ∼50% and GHR−/− mice have reached ∼19% of their average life expectancy (18, 48, 49). It is well known that GH, IGF-1, and insulin actions change with age, due to a decline in insulin sensitivity, among other factors. Thus, in the current study, it is possible that differences in gene expression levels were caused by differences in physiological ages, despite equivalent chronological ages. To fully understand the discrepancies, it is important to investigate whether the observed differences in expression levels of the examined genes between bGH and GHR−/− mice are present at different ages and perhaps also at equivalent physiological ages (9, 16).
Conclusion
The current study showed that expression of several proteins involved in GH, IGF-1, and insulin signaling in AT are regulated by GH. In addition, bGH mice responded to a pyruvate challenge to a lesser extent than WT and GHR−/− mice, suggesting a suppressed gluconeogenic capacity in these mice. Collectively, our findings support a role for GH in regulating whole-body glucose metabolism and energy balance, and point to a role for GH in regulating the GH/IGF-1/insulin signaling pathways in AT depots. Thus, the results provide insights into molecular mechanisms that may either delay or attenuate AT dysfunction, and translate into the onset and progression of systemic metabolic alterations.
Acknowledgments
Acknowledgments
R.H. was supported by funding from the Danish Diabetes Academy supported by the Novo Nordisk Foundation and by Aarhus University. J.J.K. was supported by The Diabetes Institute and the Edison Biotechnology Institute at Ohio University, and The State of Ohio’s Eminent Scholar Program, including a gift from Milton and Lawrence Goll; and S.J.F. was supported by a Veterans Affairs Merit Review Award and a grant from the National Institutes of Health (NIH R01 DK107441). Funding sources had no influence on the design or conduct of the study.
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| GHR | Human GHR residues 271–620 | AL47 | Stuart J. Frank | Rabbit; polyclonal | 1000 | |
| IGF-1R | sc-9038 | Santa Cruz Biotechnology | Rabbit; monoclonal | 400 | AB_671793 | |
| Insulin receptor A | sc-710 | Santa Cruz Biotechnology | Rabbit; polyclonal | 400 | AB_631106 | |
| GAPDH | sc-20357 | Santa Cruz Biotechnology | Goat; polyclonal | 2000 | AB_641107 |
Abbreviations: GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RRID, Research Resource Identifier.
Footnotes
- AT
- adipose tissue
- AUC
- area under the curve
- BAT
- brown adipose tissue
- bGH
- bovine growth hormone
- cDNA
- complementary DNA
- Epi
- epididymal
- GH
- growth hormone
- GHBP
- growth hormone–binding protein
- GHR
- growth hormone receptor
- GHR−/−
- growth hormone receptor knockout
- GTT
- glucose tolerance test
- HbA1c
- glycated hemoglobin
- IGF-1
- insulinlike growth factor 1
- IGF-1R
- insulinlike growth factor 1 receptor
- IP
- intraperitoneal
- IR
- insulin receptor
- ITT
- insulin tolerance test
- Mes
- mesenteric
- PTT
- pyruvate tolerance test
- qPCR
- quantitative polymerase chain reaction
- Ret
- retroperitoneal
- Sc
- subcutaneous
- WAT
- white adipose tissue
- WT
- wild-type.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J-P, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB Heart disease and stroke statistics—2016 update. A report from the American Heart Association. Circulation. 2016;133 (4):e38–e360. [DOI] [PubMed] [Google Scholar]
- 2.American Diabetes Association 6. Obesity management for the treatment of type 2 diabetes. Diabetes Care. 2016;39(Suppl 1):S47–S51. [DOI] [PubMed] [Google Scholar]
- 3.Lee M-J, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berryman DE, List EO, Sackmann-Sala L, Lubbers E, Munn R, Kopchick JJ. Growth hormone and adipose tissue: beyond the adipocyte. Growth Horm IGF Res. 2011;21(3):113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjortebjerg R, Flyvbjerg A, Frystyk J. Insulin growth factor binding proteins as therapeutic targets in type 2 diabetes. Expert Opin Ther Targets. 2014;18(2):209–224. [DOI] [PubMed] [Google Scholar]
- 6.Clemmons DR. Metabolic actions of insulin-like growth factor-I in normal physiology and diabetes. Endocrinol Metab Clin North Am. 2012;41(2):425–443, vii–viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berryman DE, Glad CAM, List EO, Johannsson G. The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol. 2013;9(6):346–356. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, Xu BC, Maheshwari HG, He L, Reed M, Lozykowski M, Okada S, Cataldo L, Coschigamo K, Wagner TE, Baumann G, Kopchick JJ. A mammalian model for Laron syndrome produced by targeted disruption of the mouse growth hormone receptor/binding protein gene (the Laron mouse). Proc Natl Acad Sci USA. 1997;94(24):13215–13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piotrowska K, Borkowska SJ, Wiszniewska B, Laszczyńska M, Słuczanowska-Głabowska S, Havens AM, Kopchick JJ, Bartke A, Taichman RS, Kucia M, Ratajczak MZ. The effect of low and high plasma levels of insulin-like growth factor-1 (IGF-1) on the morphology of major organs: studies of Laron dwarf and bovine growth hormone transgenic (bGHTg) mice. Histol Histopathol. 2013;28(10):1325–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berryman DE, List EO, Palmer AJ, Chung M-Y, Wright-Piekarski J, Lubbers E, O’Connor P, Okada S, Kopchick JJ. Two-year body composition analyses of long-lived GHR null mice. J Gerontol A Biol Sci Med Sci. 2010;65(1):31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen WY, Wight DC, Wagner TE, Kopchick JJ. Expression of a mutated bovine growth hormone gene suppresses growth of transgenic mice. Proc Natl Acad Sci USA. 1990;87(13):5061–5065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Knapp JR, Chen WY, Turner ND, Byers FM, Kopchick JJ. Growth patterns and body composition of transgenic mice expressing mutated bovine somatotropin genes. J Anim Sci. 1994;72(11):2812–2819. [DOI] [PubMed] [Google Scholar]
- 13.Brooks NE, Hjortebjerg R, Henry BE, List EO, Kopchick JJ, Berryman DE. Fibroblast growth factor 21, fibroblast growth factor receptor 1, and β-Klotho expression in bovine growth hormone transgenic and growth hormone receptor knockout mice. Growth Horm IGF Res. 2016;30-31:22–30. [DOI] [PubMed] [Google Scholar]
- 14.Ding J, Berryman DE, Kopchick JJ. Plasma proteomic profiles of bovine growth hormone transgenic mice as they age. Transgenic Res. 2011;20(6):1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benencia F, Harshman S, Duran-Ortiz S, Lubbers ER, List EO, Householder L, Al-Naeeli M, Liang X, Welch L, Kopchick JJ, Berryman DE. Male bovine GH transgenic mice have decreased adiposity with an adipose depot-specific increase in immune cell populations. Endocrinology. 2015;156(5):1794–1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palmer AJ, Chung M-Y, List EO, Walker J, Okada S, Kopchick JJ, Berryman DE. Age-related changes in body composition of bovine growth hormone transgenic mice. Endocrinology. 2009;150(3):1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berryman DE, List EO, Kohn DT, Coschigano KT, Seeley RJ, Kopchick JJ. Effect of growth hormone on susceptibility to diet-induced obesity. Endocrinology. 2006;147(6):2801–2808. [DOI] [PubMed] [Google Scholar]
- 18.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm IGF Res. 2004;14(4):309–318. [DOI] [PubMed] [Google Scholar]
- 19.List EO, Palmer AJ, Berryman DE, Bower B, Kelder B, Kopchick JJ. Growth hormone improves body composition, fasting blood glucose, glucose tolerance and liver triacylglycerol in a mouse model of diet-induced obesity and type 2 diabetes. Diabetologia. 2009;52(8):1647–1655. [DOI] [PubMed] [Google Scholar]
- 20.Lubbers ER, List EO, Jara A, Sackman-Sala L, Cordoba-Chacon J, Gahete MD, Kineman RD, Boparai R, Bartke A, Kopchick JJ, Berryman DE. Adiponectin in mice with altered GH action: links to insulin sensitivity and longevity? J Endocrinol. 2013;216(3):363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2):R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang J, Liang L, Kim SO, Zhang Y, Mandler R, Frank SJ. Growth hormone-dependent tyrosine phosphorylation of a GH receptor-associated high molecular WEIGHT protein immunologically related to JAK2. Biochem Biophys Res Commun. 1998;253(3):774–779. [DOI] [PubMed] [Google Scholar]
- 23.Sackmann-Sala L, Berryman DE, Lubbers ER, Zhang H, Vesel CB, Troike KM, Gosney ES, List EO, Kopchick JJ. Age-related and depot-specific changes in white adipose tissue of growth hormone receptor-null mice. J Gerontol A Biol Sci Med Sci. 2014;69(1):34–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jara A, Benner CM, Sim D, Liu X, List EO, Householder LA, Berryman DE, Kopchick JJ. Elevated systolic blood pressure in male GH transgenic mice is age dependent. Endocrinology. 2014;155(3):975–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu J-L, Coschigano KT, Robertson K, Lipsett M, Guo Y, Kopchick JJ, Kumar U, Liu YL. Disruption of growth hormone receptor gene causes diminished pancreatic islet size and increased insulin sensitivity in mice. Am J Physiol Endocrinol Metab. 2004;287(3):E405–E413. [DOI] [PubMed] [Google Scholar]
- 26.Darcy J, McFadden S, Fang Y, Huber JA, Zhang C, Sun LY, Bartke A Brown adipose tissue function is enhanced in long-lived, male Ames dwarf mice. Endocrinology.157(12):4744–4753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Knapp JR, Kopchick JJ. Enlargement of interscapular brown adipose tissue in growth hormone antagonist transgenic and in growth hormone receptor gene-disrupted dwarf mice. Exp Biol Med (Maywood). 2003;228(2):207–215. [DOI] [PubMed] [Google Scholar]
- 28.Olsson B, Bohlooly-Y M, Fitzgerald SM, Frick F, Ljungberg A, Ahrén B, Törnell J, Bergström G, Oscarsson J. Bovine growth hormone transgenic mice are resistant to diet-induced obesity but develop hyperphagia, dyslipidemia, and diabetes on a high-fat diet. Endocrinology. 2005;146(2):920–930. [DOI] [PubMed] [Google Scholar]
- 29.Boparai RK, Arum O, Khardori R, Bartke A. Glucose homeostasis and insulin sensitivity in growth hormone-transgenic mice: a cross-sectional analysis. Biol Chem. 2010;391(10):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bartke A. Insulin and aging. Cell Cycle. 2008;7(21):3338–3343. [DOI] [PubMed] [Google Scholar]
- 31.Berryman DE, Christiansen JS, Johannsson G, Thorner MO, Kopchick JJ. Role of the GH/IGF-1 axis in lifespan and healthspan: lessons from animal models. Growth Horm IGF Res. 2008;18(6):455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Møller N, Jørgensen JOL. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr Rev. 2009;30(2):152–177. [DOI] [PubMed] [Google Scholar]
- 33.Wang J, Zhu X, Chen C, Li X, Gao Y, Li P, Zhang Y, Long M, Wang Z, Liu G. Effect of insulin-like growth factor-1 (IGF-1) on the gluconeogenesis in calf hepatocytes cultured in vitro. Mol Cell Biochem. 2012;362(1-2):87–91. [DOI] [PubMed] [Google Scholar]
- 34.Panici JA, Wang F, Bonkowski MS, Spong A, Bartke A, Pawlikowska L, Kwok P-Y, Masternak MM. Is altered expression of hepatic insulin-related genes in growth hormone receptor knockout mice due to GH resistance or a difference in biological life spans? J Gerontol A Biol Sci Med Sci. 2009;64(11):1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Regaiey KA, Masternak MM, Bonkowski M, Sun L, Bartke A. Long-lived growth hormone receptor knockout mice: interaction of reduced insulin-like growth factor i/insulin signaling and caloric restriction. Endocrinology. 2005;146(2):851–860. [DOI] [PubMed] [Google Scholar]
- 36.List EO, Sackmann-Sala L, Berryman DE, Funk K, Kelder B, Gosney ES, Okada S, Ding J, Cruz-Topete D, Kopchick JJ. Endocrine parameters and phenotypes of the growth hormone receptor gene disrupted (GHR-/-) mouse. Endocr Rev. 2011;32(3):356–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berryman DE, Henry BE, Hjortebjerg R, List EO, Kopchick JJ. Developments in our understanding of the effects of growth hormone on white adipose tissue from mice: implications to the clinic. Expert Rev Endocrinol Metab. 2016;11(2):197–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boucher J, Softic S, El Ouaamari A, Krumpoch MT, Kleinridders A, Kulkarni RN, O’Neill BT, Kahn CR. Differential roles of insulin and IGF-1 receptors in adipose tissue development and function. Diabetes. 2016;65(8):2201–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boucher J, Mori MA, Lee KY, Smyth G, Liew CW, Macotela Y, Rourk M, Bluher M, Russell SJ, Kahn CR. Impaired thermogenesis and adipose tissue development in mice with fat-specific disruption of insulin and IGF-1 signalling. Nat Commun. 2012;3:902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blüher M, Michael MD, Peroni OD, Ueki K, Carter N, Kahn BB, Kahn CR. Adipose tissue selective insulin receptor knockout protects against obesity and obesity-related glucose intolerance. Dev Cell. 2002;3(1):25–38. [DOI] [PubMed] [Google Scholar]
- 41.Masternak MM, Al-Regaiey KA, Del Rosario Lim MM, Jimenez-Ortega V, Panici JA, Bonkowski MS, Bartke A. Effects of caloric restriction on insulin pathway gene expression in the skeletal muscle and liver of normal and long-lived GHR-KO mice. Exp Gerontol. 2005;40(8-9):679–684. [DOI] [PubMed] [Google Scholar]
- 42.Nielsen RH, Clausen NM, Schjerling P, Larsen JO, Martinussen T, List EO, Kopchick JJ, Kjaer M, Heinemeier KM. Chronic alterations in growth hormone/insulin-like growth factor-I signaling lead to changes in mouse tendon structure. Matrix Biol. 2014;34:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gesing A, Bartke A, Masternak MM. Pioglitazone does not improve insulin signaling in mice with GH over-expression. J Endocrinol. 2013;219(2):109–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miquet JG, Giani JF, Martinez CS, Muñoz MC, González L, Sotelo AI, Boparai RK, Masternak MM, Bartke A, Dominici FP, Turyn D. Prolonged exposure to GH impairs insulin signaling in the heart. J Mol Endocrinol. 2011;47(2):167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Cola G, Cool MH, Accili D. Hypoglycemic effect of insulin-like growth factor-1 in mice lacking insulin receptors. J Clin Invest. 1997;99(10):2538–2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Erman A, Veilleux A, Tchernof A, Goodyer CG. Human growth hormone receptor (GHR) expression in obesity: I. GHR mRNA expression in omental and subcutaneous adipose tissues of obese women. Int J Obes. 2011;35(12):1511–1519. [DOI] [PubMed] [Google Scholar]
- 47.Gude MF, Hjortebjerg R, Oxvig C, Thyø AA, Magnusson NE, Bjerre M, Pedersen SB, Frystyk J. PAPP-A, IGFBP-4 and IGF-II are secreted by human adipose tissue cultures in a depot-specific manner. Eur J Endocrinol. 2016;175(6):509–519. [DOI] [PubMed] [Google Scholar]
- 48.Bonkowski MS, Rocha JS, Masternak MM, Al Regaiey KA, Bartke A. Targeted disruption of growth hormone receptor interferes with the beneficial actions of calorie restriction. Proc Natl Acad Sci USA. 2006;103(20):7901–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding J, Sackmann-Sala L, Kopchick JJ. Mouse models of growth hormone action and aging: a proteomic perspective. Proteomics. 2013;13(3-4):674–685. [DOI] [PMC free article] [PubMed] [Google Scholar]