Abstract
Premature infants have altered glucose regulation early in life and increased risk for diabetes in adulthood. Although prematurity leads to an increased risk of diabetes and metabolic syndrome in adult life, the role of hepatic glucose regulation and adaptation to an early extrauterine environment in preterm infants remain unknown. The purpose of this study was to investigate developmental differences in glucose metabolism, hepatic protein content, and gene expression of key insulin-signaling/gluconeogenic molecules. Fetal baboons were delivered at 67%, 75%, and term gestational age and euthanized at birth. Neonatal baboons were delivered prematurely (67% gestation), survived for two weeks, and compared with similar postnatal term animals and underwent serial hyperinsulinemic-euglycemic clamp studies. Premature baboons had decreased endogenous glucose production (EGP) compared with term animals. Consistent with these results, the gluconeogenic molecule, phosphoenolpyruvate carboxykinase messenger RNA, was decreased in preterm baboons compared with terms. Hepatic insulin signaling was altered by preterm birth as evidenced by decreased insulin receptor–β, p85 subunit of phosphoinositide 3-kinase, phosphorylated insulin receptor substrate 1, and Akt-1 under insulin-stimulated conditions. Furthermore, preterm baboons failed to have the normal increase in glycogen synthase kinase-α from fetal to postnatal life. The blunted responses in hepatic insulin signaling may contribute to the hyperglycemia of prematurity, while impaired EGP leads to hypoglycemia of prematurity.
Neonatal baboons received serial hyperinsulinemic-euglycemic clamps. When born preterm, altered hepatic insulin signaling, decreased PEPCK mRNA, and decreased endogenous glucose production was found.
The rate of preterm birth ranges from 5% to 18% of all live births worldwide, accounting for an estimated 15 million babies every year. Preterm birth increases the risk for both short- and long-term morbidity and mortality, with survivors having a higher incidence of diabetes and metabolic syndrome (1–3). In the first weeks of life, many premature infants have abnormal glucose metabolism; some develop hypoglycemia, affecting more than 50% of preterm infants (4, 5), while others develop hyperglycemia, affecting up to 80% of infants with very low birth weight (birth weight < 1500 g). The clinical impact of hypoglycemia is significant, as it has been associated with central nervous system damage and decreased IQ scores (6, 7). Elucidating the pathophysiologic mechanisms behind hyperglycemia is equally important because this condition increases the risk of death and other morbidities, including retinopathy of prematurity and intraventricular hemorrhage (8–10). Deranged glucose metabolism may persist long term, as episodes of hyperglycemia have been seen in preterm infants even when they mature to near-term age (11, 12). Further, insulin resistance and increased incidence of type 2 diabetes is seen in preterm babies who survive to adulthood and may be a direct consequence of early alterations in glucose metabolism (1, 3, 13–15).
Little is known about hepatic glucose metabolism in prematurity and whether impaired hepatic insulin signaling contributes to the pathophysiology of hyperglycemia/hypoglycemia in infants with very low birth weight. Peripheral insulin resistance of prematurity has been demonstrated in adipose and muscle tissue of premature baboons (16). The presence of hepatic insulin resistance in preterm infants is suggested by persistent hepatic glucose production despite hyperglycemia and high intravenous glucose infusion rates (GIRs) as well as higher proinsulin levels and higher insulin infusion rates required to achieve euglycemia (17–19). We previously have shown significant developmental differences in hepatic gluconeogenic molecules phosphoenolpyruvate carboxykinase (PEPCK) and forkhead Box O1 (FOXO1) in fetal and neonatal baboons (20), which may impair the response to insulin during the neonatal period. A clearer understanding of the molecular and physiological abnormalities responsible or the disturbances in hepatic glucose metabolism in preterm infants is essential for optimal management during the neonatal period and for prevention of long-term morbidities.
No previous study has quantitated endogenous glucose production (EGP) in preterm and term neonates under insulin-stimulated conditions. To our knowledge, there are no data correlating EGP and hepatic insulin-signaling pathways under insulin-stimulated conditions. We examined the effect of insulin on EGP in preterm and term nonhuman primates utilizing stable isotopes. Premature baboons are a pertinent model to study glucose metabolism, as they experience complications that are similar to those in premature infants, including hyperglycemia of prematurity, hypoglycemia, bronchopulmonary dysplasia, necrotizing enterocolitis, and cardiovascular instability (21, 22).
Materials and Methods
Animal care in fetal group
Eighteen fetal baboons were delivered at 67% gestation [125 days gestational age (GA) or preterm, ± two days; n = 6], 75% gestation (140 days GA or near term, ± two days; n = 6), and 95% gestation (175 days GA or term, ± two days; n = 6) via caesarean section from healthy mothers under general anesthesia and were euthanatized immediately after birth with intravenous pentobarbital [20 mg/kg (Virback, AH, Inc, FW, Texas)]. Fetal sex was not able to be accurately determined when animal selection was performed prenatally. Liver samples were collected and snap frozen for future experiments.
Animal care in postnatal group
Twelve additional baboons were delivered prematurely at 67% gestation (n = 6) or at term (n = 6). Animals received a prime (150 mU/kg/min) plus constant infusion of insulin (15 mU/kg/min) (Novolin; Novo Nordisk Pharmaceuticals, Plainsboro, NJ) for two hours immediately prior to liver collection. Animals were euthanized at two to three weeks of age with 20 mg/kg of IV pentobarbital, followed by exsanguination. Insulin-stimulated liver samples were collected and snap frozen for future experiments.
Term postnatal animals
Term animals were delivered via spontaneous vaginal delivery and were breast fed by their mother for the first 24 hours of life and received term formula by mouth every four to six hours thereafter. They were placed in an incubator (36°C) for thermal support and monitored daily by veterinary staff.
Preterm postnatal animals
Preterm animals were delivered via caesarean section under general anesthesia from healthy mothers. Mothers were given prenatal steroids initiated 48 hours prior to delivery with betamethasone [6 mg intramuscular q24h × two doses (American Regent Inc, Shirley, NY)] to mimic the standard procedure for human patients at risk for premature delivery. Preterm animals were intubated immediately after birth and chronically ventilated. Curosurf (Cornerstone Therapeutics, Cary, NC) was administered through the endotracheal at a dose of 2.5 mL/kg. A standard protocol was followed for sedation and anesthesia. Central intravenous lines were placed shortly after birth for fluid management and parenteral nutrition. Glucose was measured every two hours for the first 48 hours and then every four hours, electrolyte levels were obtained every 12 hours, and blood gas analysis was measured every two hours for the first 48 hours and then every four hours for the rest of the study. Parenteral nutrition was started after birth at a rate of 150 mL/kg/d, with 1.75 g/kg amino acids during the first 24 hours and then 3.5 g/kg for the remainder of the protocol. GIRs were advanced per protocol to keep glucose between 50 and 100 mg/dL. Enteral feeds were started with preterm formula on day of life 4 to 5 and advanced to a max of 40 mL/kg/d.
Animals were born at the University of Texas Health Science Center at San Antonio (UTHSCSA), Texas Biomedical Research Institute, in San Antonio, Texas, or at the Baboon Research Resources at the University of Oklahoma Health Sciences Center, Oklahoma City, Oklahoma, from 2011 to 2014. All studies were approved by the Institutional Animal Care Committee at the UTHSCSA. Animal experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (23) as well as the Public Health Service Policy on Humane Care and Use of Laboratory Animals. UTHSCSA is accredited with Association for the Assessment and Accreditation of Laboratory Animal Care International.
Insulin clamp
Deuterated glucose (D-[6,6-2H2] glucose) was administered for five hours via umbilical venous catheters or peripherally inserted central catheters at one and two weeks of age (Fig. 1). A hyperinsulinemic-euglycemic clamp was performed during the last two hours of the tracer infusion, and the details have been described elsewhere (16). Animals received a prime (150 mU/kg−1 · minute−1) plus constant infusion of insulin at a rate of 15 mU/kg−1 · minute−1 (Novolin; Novo Nordisk Pharmaceuticals). At the same time, glucose (25% dextrose in water) was infused at a variable rate to clamp blood glucose concentration at 60 to 80 mg/dL. Deuterated glucose was given as a prime (4 mg/kg) plus constant (0.05 mg/kg/min) infusion in 0.45% saline. Blood samples were collected for isotopic enrichment at time –180, 0, +100, and +120 minutes. Isotopic enrichment was measured by gas chromatography/mass spectrometry. Insulin clamps were initiated only if the animal had no evidence of stress (stable vital signs for the last 48 hours, no vasoactive medications, and no signs of sepsis or deterioration). The rate of appearance (Ra) of glucose was calculated by the isotope dilution technique from the [6,6-2H2] enrichment of glucose. Using calculations for steady-state kinetics, Ra equals (Ei/Ep) × I, where Ei and Ep are the [6,6-2H2] enrichments of the infusate and plasma, respectively, and I is the infusion rate of [6,6-2H2] glucose.
Figure 1.
Schematic experimental timelines. Preterm and term postnatal animals received a hyperinsulinemic-euglycemic clamp at one and two weeks of age. Fetal animals completed necropsy immediately after birth.
Western blot
Activation of hepatic insulin-signaling molecules and gluconeogenic enzymes was measured from extracted frozen liver tissues under maximum insulin stimulation. Proteins were extracted from liver tissues, quantified, and measured as previously described (16). Antibodies are specified in Table 1. Glyceraldehyde-3-phosphate dehydrogenase from Santa Cruz was used as loading control to normalize target proteins.
Table 1.
Antibodies Used in This Study
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|
| Akt | AKT | Cell Signaling, #9272 | Rabbit; polyclonal | 1:1000 |
| p85 | Anti-PI3 kinase, p85 | Upstate, 06-497 | Rabbit; polyclonal | 1:800 |
| FBPase | FBPase | Santa Cruz Biotechnology, sc-66946 | Rabbit; polyclonal | 1:1000 |
| FOXO1 | FKHR | Santa Cruz Biotechnology, sc-67140 | Rabbit; polyclonal | 1:200 |
| pFOXO1 | p-FKHR | Santa Cruz Biotechnology, sc-101681 | Rabbit; polyclonal | 1:200 |
| GAPDH | GAPDH | Santa Cruz Biotechnology, sc-25778 | Rabbit; polyclonal | 1:1000 |
| G6Pase | G6Pase-α | Santa Cruz Biotechnology, sc-258400 | Rabbit; polyclonal | 1:1000 |
| GSK-3α | GSK-3α; glycogen synthase kinase-3 | Cell Signaling, #9338 | Rabbit; polyclonal | 1:500 |
| GSK-3β | GSK-3β; glycogen synthase kinase-3 | Cell Signaling, #9315 | Rabbit; monoclonal | 1:500 |
| pGSK-3α/β | Phospho-GSK-3α/β; glycogen synthase kinase-3 | Cell Signaling, #9331 | Rabbit; polyclonal | 1:200 |
| IR-β | Insulin Rβ; insulin receptor | Santa Cruz Biotechnology, sc-711 | Rabbit; polyclonal | 1:200 |
| IRS-1 | IRS-1 | Santa Cruz Biotechnology, sc-559 | Rabbit; polyclonal | 1:500 |
| pIRS-1 | pIRS-1 | Santa Cruz Biotechnology, sc-17200 | Rabbit; polyclonal | 1:500 |
| PEPCK-C | PEPCK-C | Santa Cruz Biotechnology, sc-135278 | Rabbit; polyclonal | 1:1000 |
| PEPCK-M | PEPCK-M | Santa Cruz Biotechnology, sc-130225 | Rabbit; polyclonal | 1:1000 |
| Secondary antibody | Peroxidase-linked, species-specific, whole antibody | GE Healthcare UK Limited, NA934 | Donkey | Varies |
Abbreviations: G6Pase, glucose 6-phosphatase; GAPDH, glyceraldehyde-3-phosphate de-hydrogenase; IR-β, insulin receptor β; IRS-1, insulin substrate receptor 1.
Polymerase chain reaction analysis
Gene expression was measured by extracting total RNA from frozen liver tissues, and relative quantitation of gene expression was performed as previously described (20). Primer sequences for PEPCK-C (PCK1, Hs00159918_m1, RefSeq: NM_002591.3), PEPCK-M (PCK2, Hs00388934_m1, RefSeq: NM_001018073.2), G6Pase (G6PC3, Hs00292720_m1, RefSeq: NM_001319945.1), FBPase (FBP1, Hs00983323_m1, RefSeq: NM_000507.3), FOXO1 (FOXO1, Hs01054576_m1, RefSeq: NM_002015.3), IR (INSR, Hs00961554_m1, RefSeq: NM_000208.3), IRS-1 (IRS1, Hs00178563_m1, RefSeq: NM_005544.2), Akt-1 (AKT1, Hs00178289_m1, RefSeq: NM_001014431.1), and PGC1-α (PPARGC1A, Hs01016719_m1, RefSeq: NM_013261.3) were purchased from Life Technologies (Carlsbad, CA). Iportin 8 gene (IPO8, Hs00183533_m1, RefSeq: NM_001190995.1) was used as an endogenous control.
FOXO1 activation
FOXO1 activity was quantified using the DNA-binding enzyme-linked immunosorbent assay (ELISA) TransAM FKHR (FOXO1) Kit from Active Motif (Carlsbad, CA). Liver samples were homogenized in ice-cold lysis buffer and then centrifuged for supernatant collection. Protein concentrations were measured by Epoch Microplate (BioTek Instruments, Winooskiu, VT). Samples were tested in duplicate wells with 80 µg total protein per sample following the Active Motif instructions. Primary antibodies provided with the kit were used to detect FOXO1 DNA binding activity to the oligonucleotide immobilized in the well. ELISA plate optical density was read on a Biotek spectrophotometer (Winooski, VT) at 450 nm with a reference wavelength of 655 nm.
Liver glycogen content
Liver tissues were hydrolyzed in 2 N HCl at 100°C for two hours followed by neutralization with 2 N NaOH. Glycogen content was then measured by the hexokinase method using the glucose HK reagent (Sigma, St. Louis, MO) and expressed per milligram of tissue.
Blood chemistry
Total serum insulin (ALPCO, Salem, NH) and glucagon (ALPCO, Salem, NH) concentrations were assessed via enzymatic methods using commercial kits. Serum glucose samples were analyzed using the Analox GM9 Glucose Analyzer (Analox Technologies, Atlanta, GA).
Statistical analysis
Statistical calculations and demographic distributions were performed with SPSS for Microsoft Windows (version 17.0; SPSS, Inc., Chicago, IL). Differences between groups were determined utilizing a two-way analysis of variance, followed by the Bonferroni and Tukey test, and paired and unpaired Student t test, and repeated measures were performed when appropriate. A P < 0.05 was considered to be statistically significant. χ2s were performed for categorical data such as sex differences.
Results
Animal characteristics
The clinical and laboratory data of the preterm and term postnatal baboons are summarized in Table 2. Similar to preterm human infants, preterm baboons required constant intravenous dextrose to maintain euglycemia, while term baboons were euglycemic with oral formula feeds. Serum glucose was collected every two to four hours per protocol in preterm animals and ranged from 62 to 75 mg/dL during the first week and 68 to 92 mg/dL during the second week of life (see Table 2 for mean fasting glucose values). Serum glucose ranged from 51 to 76 mg/dL during the first week and 43 to 84 mg/dL during the second week of life in term animals. The basal Ra was similar in preterm and term baboons during the first and second weeks of life. However, preterm baboons required a basal GIR > 7 mg/kg/min to prevent hypoglycemia, while term baboons did not require basal exogenous glucose infusions. Furthermore, clamped Ra was significantly lower in preterm baboons during the first and second week of life, and clamped GIR was significantly lower in preterm baboons during the first week of life. Basal and clamped plasma glucagon was significantly lower at two weeks of age in preterm baboons. Although the mean insulin:glucagon ratio was 99% higher in preterm baboons compared with terms at both weeks 1 and 2 (week 1, 17.5 ± 12.8 vs 0.2 ± 0.1, P = 0.09; week 2, 21.3 ± 12.6 vs 0.2 ± 0.1, P = 0.2), this did not meet statistical significance due to significant variations in the preterm baboons. Epinephrine remained stable throughout the clamp (change in epinephrine level from baseline to end of clamp was similar; P = 0.870 and P = 0.441 during clamp 1 and clamp 2, respectively). The gluconeogenic precursors glycerol and lactate were similar in preterm and term baboons (0.6 ± 0.2 nmol/µL vs 0.8 ± 0.2 nmol/µL, P = 0.2; 0.7 nmol/µL vs 0.7 nmol/µL, P = 1.0, respectively) at the end of week 2. These were not measured on week 1 because of the limited amount of blood available for draws. As previously reported (16), fasting plasma insulin levels tended to be higher in preterm baboons compared with term at both one and two weeks of age prior to insulin administration, and peak insulin levels were similar (>1400 µIU/mL) between groups at the end of the insulin clamp, immediately prior to liver sample collection.
Table 2.
Animal Characteristics
| Preterm Baboons | Term Baboons | P value | ||
|---|---|---|---|---|
| Fetal animals | 67% GA | 75% GA | 95% GA | |
| Female/male ratio | 2/4 | 5/1 | 1/5 | 0.815 |
| Birth weight (g) | 413 ± 14 | 552 ± 24 | 985 ± 53 | <0.001 |
| Postnatal animals | 67% GA | 95% GA | ||
| Female/male ratio | 3/3 | 3/3 | 1.0 | |
| Birth weight (g) | 380 ± 20 | 933 ± 65 | <0.001 | |
| Week 1 | ||||
| Basal glucose (mg/dL) | 68 ± 6 | 64 ± 3 | 0.534 | |
| Basal insulin (µIU/mL) | 7.2 ± 3.3 | 0.9 ± 0.1 | 0.120 | |
| HE clamp insulin (µIU/mL) | 1678 ± 214 | 1599 ± 226 | 0.812 | |
| HE clamp glucose (mg/dL) | 66 ± 5 | 53 ± 2 | 0.075 | |
| Basal Ra (mg/kg/min) | 5.04 ± 1.37 | 7.59 ± 2.15 | 0.321 | |
| Basal GIR (mg/kg/min) | 7.55 ± 1.13 | 0 | 0.001 | |
| HE clamp Ra (mg/kg/min) | 11.67 ± 1.52 | 27.35 ± 2.88 | 0.001 | |
| HE clamp GIR (mg/kg/min) | 12.86 ± 1.07 | 18.24 ± 1.87 | 0.027 | |
| Basal glucagon (pg/mL) | 28 ± 9 | 130 ± 40 | 0.083 | |
| HE clamp glucagon (pg/mL) | 27 ± 9 | 37 ± 14 | 0.562 | |
| M value | 12.82 ± 0.72 | 18.20 ± 2.58 | 0.004 | |
| Week 2 | ||||
| Basal glucose (mg/dL) | 68 ± 6 | 72 ± 7 | 0.708 | |
| Basal insulin (µIU/mL) | 9.0 ± 3.4 | 1.1 ± 0.3 | 0.070 | |
| HE clamp insulin (µIU/mL) | 1584 ± 235 | 1995 ± 759 | 0.623 | |
| HE clamp glucose (mg/dL) | 78 ± 8 | 71 ± 3 | 0.405 | |
| Basal Ra (mg/kg/min) | 6.69 ± 2.14 | 6.79 ± 1.85 | 0.971 | |
| Basal GIR (mg/kg/min) | 7.17 ± 1.17 | 0 | 0.002 | |
| HE clamp Ra (mg/kg/min) | 11.67 ± 1.49 | 21.80 ± 3.87 | 0.057 | |
| HE clamp GIR (mg/kg/min) | 13.43 ± 1.09 | 15.29 ± 1.70 | 0.364 | |
| Basal glucagon (pg/mL) | 34 ± 13 | 208 ± 52 | 0.019 | |
| HE clamp glucagon (pg/mL) | 9 ± 8 | 116 ± 46 | 0.065 | |
| M value | 12.86 ± 0.96 | 15.92 ± 1.86 | 0.180 | |
Means ± standard error of the mean are shown.
Abbreviation: HE, hyperinsulinemic-euglycemic.
Fetal animals were also evaluated, and their experimental time line and characteristics are described in Fig. 1 and Table 2.
Alterations in hepatic insulin signaling in preterm baboons
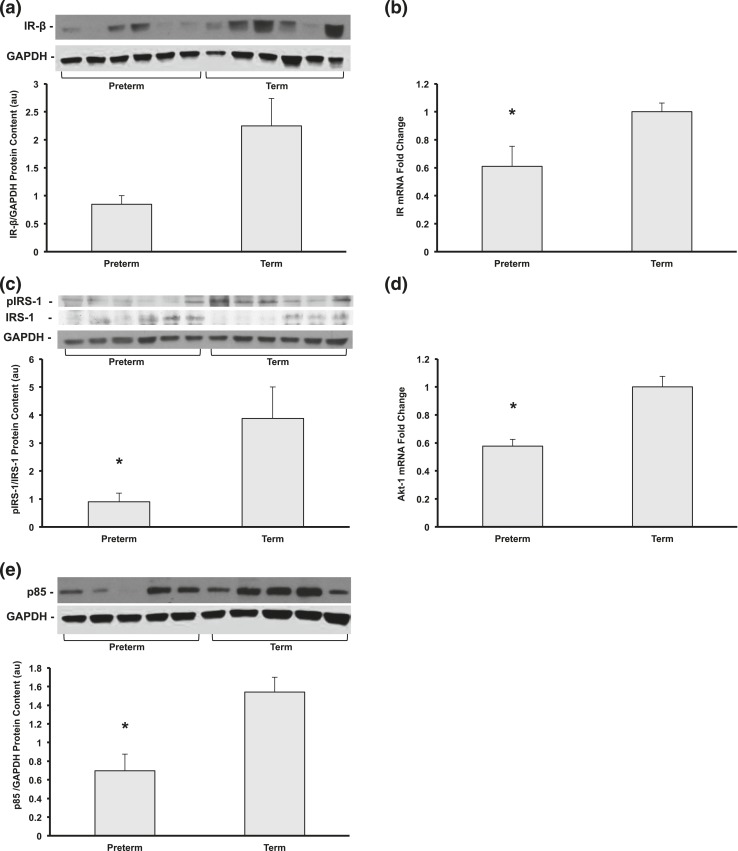
After maximal insulin stimulation, hepatic messenger RNA (mRNA) expression of insulin receptor β (IR-β) was 39% lower in preterm baboons compared with term animals (P < 0.05); the protein content of IR-β also tended to be lower in preterms but did not reach statistical significance [Fig. 2(a) and 2(b)]. Insulin receptor substrate 1 (IRS-1) tyrosine phosphorylation at Tyr612 was 75% lower in preterm compared with term animals (P < 0.05) [Fig. 2(c)]. The phosphoinositide 3-kinase (PI3K) p85 regulatory subunit was 56% lower in preterm baboons compared with terms (P < 0.05) [Fig. 2(e)]. Akt-1 mRNA expression was decreased in preterm vs term animals by 57% (P < 0.001) [Fig. 2(d)]. There were no significant changes in the mRNA expression of IRS-1, protein content of Akt, or levels of phosphorylated Akt at Ser473 (data not shown).
Figure 2.
Preterm baboons are born at 67% completed gestation, compared with term controls at two to three weeks of age after insulin stimulation. (a) Hepatic IR-β protein content, (b) IR mRNA, (c) protein content of phosphorylated Tyr 612 of IRS-1 normalized to IRS-1, (d) Akt-1 gene expression, and (e) protein content of the p85 subunit of PI3K were measured by Western blot or quantitative reverse transcription-PCR (n = 5 to 6 per group, *P < 0.05). Representative blots from each group are shown. All data shown as mean ± standard error of the mean. au, arbitrary units.
Developmental differences in gluconeogenic enzymes and transcription factors in preterm baboons
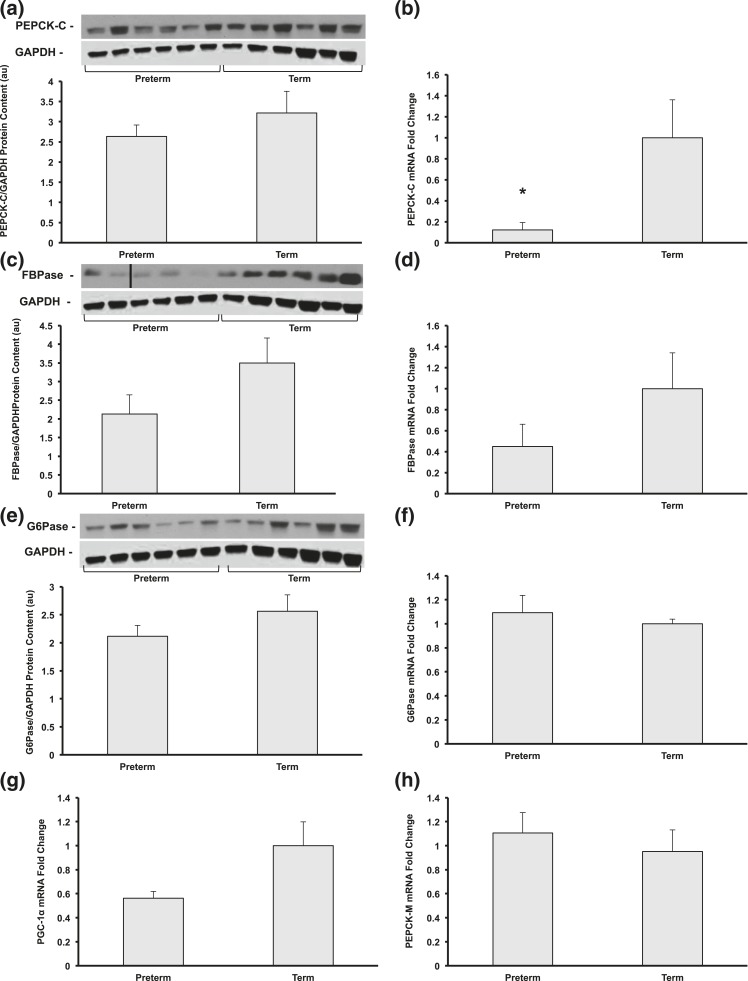
Cytosolic PEPCK (PEPCK-C) mRNA expression was decreased to 91% in preterm vs term baboons (P < 0.001), and protein content was decreased by 20% (not statistically significant) [Fig. 3(a) and 3(b)]. Fructose-1,6-bisphosphatase (FBPase) protein content and gene expression tended to be lower in preterm animals (63% and 45% of term, respectively; P = 0.1) but failed to reach statistical significance [Fig. 3(c) and 3(d)]. There were no differences in gene expression or protein content of glucose 6-phosphatase (G6Pase) or mitochondrial PEPCK (PEPCK-M) [Fig. 3(e), 3(f), and 3(h)].
Figure 3.
(a and b) Protein content and gene expression of PEPCK-C, (c and d) FBPase, and (e and f) and G6Pase and (g) gene expression of gluconeogenic coactivator PGC-1α and (h) gluconeogenic enzyme PEPCK-M were measured by Western blot and quantitative reverse transcription-PCR (n = 5 to 6 per group, *P < 0.05). Representative blots from each group are shown. All data shown as mean ± standard error of the mean.
To investigate the molecular mechanism underlying the reduced expression of PEPCK-C, we quantitated the mRNA expression of peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α), an important amplifier for hepatic gluconeogenesis. PGC-1α mRNA expression tended to be 44% lower in preterm vs term baboons and almost reached statistical significance (P = 0.06) [Fig. 3(g)].
Because the transcription FOXO1 promotes the expression of hepatic gluconeogenic genes, we evaluated the level of FOXO1 in the liver of insulin-stimulated preterm and term baboons using reverse transcription polymerase chain reaction (PCR) and Western blot analysis. FOXO1 mRNA expression and protein content were similar between preterm and term baboons [Fig. 4(a) and 4(b)]. FOXO1 phosphorylation at Ser256 was similar in term and preterm baboons [Fig. 4(a)]. We also evaluated FOXO1 activity using the TransAM transcription factor ELISA (Active Motif); FOXO1 activity was similar in preterm and term animals [Fig. 4(c)].
Figure 4.
Representative blots from each group of phosphorylated FOXO1, normalized to (a) FOXO1, (b) gene expression of FOXO1, and (c) hepatic FOXO1 activity (n = 5 to 6 per group). All data shown as mean ± standard error of the mean. OD, optical density.
Content of hepatic GSK-3 and glycogen after insulin stimulation in preterm baboons
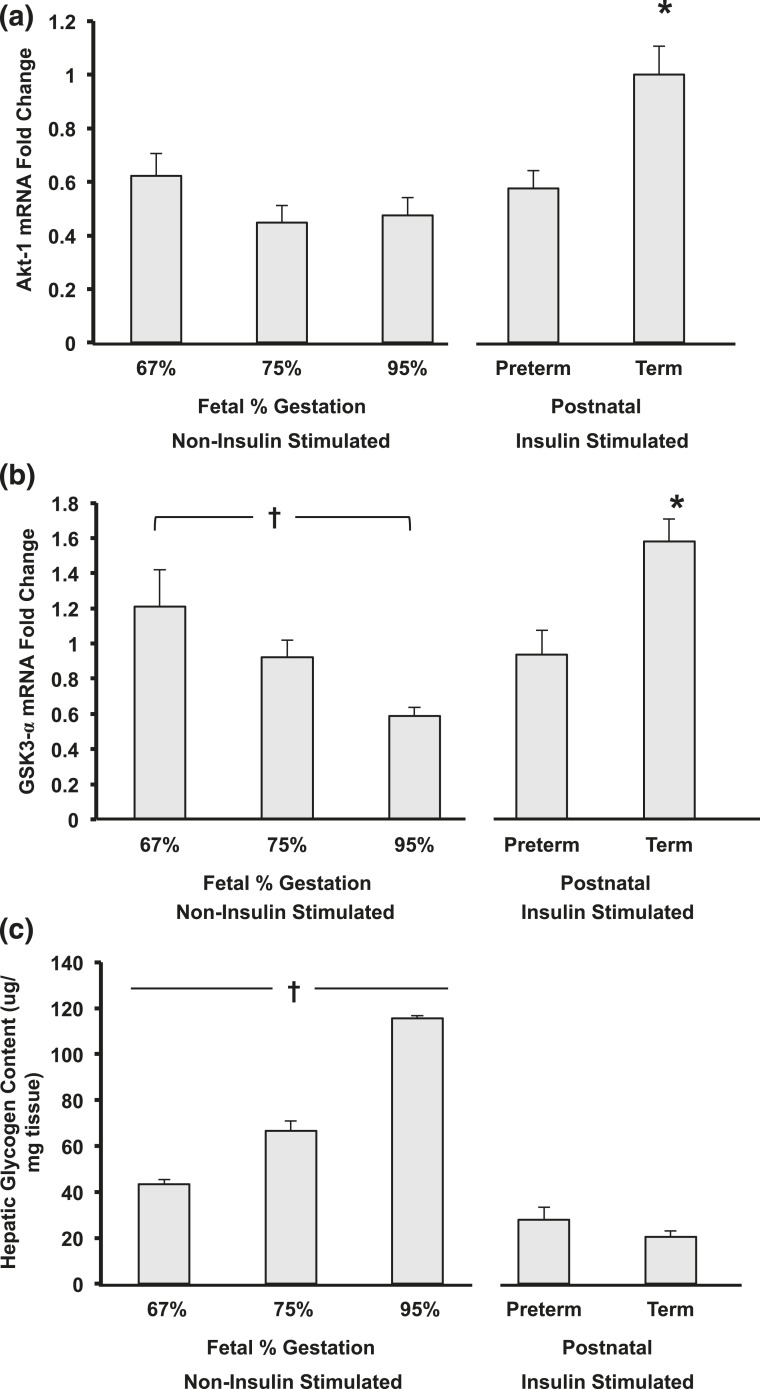
There was a significantly lower gene expression of glycogen synthase kinase-α (GSK-3α) in postnatal preterm baboons when compared with the term counterparts [Fig. 5(b)]. There were no differences in GSK-3α protein expression (data not shown). Glycogen content was similar between postnatal preterm and term baboons during insulin stimulation at two to three weeks of age [Fig. 5(c)].
Figure 5.
Developmental differences of (a) Akt-1, (b) GSK-3α, and (c) glycogen content in hepatic tissue from fetal and postnatal baboons. (a) * term vs all groups (P < 0.05). (b and c) Significant differences were observed in fetal development as indicated by a cross (†P < 0.05) and between postnatal animals (*P < 0.05; n = 5 to 6 per group). All data shown as mean ± standard error of the mean.
Developmental differences from fetal to extrauterine life when born preterm vs term
Similar to what has been previously reported (24), key insulin-signaling molecules were similar across GAs in fetal baboons.
Although Akt-1 gene expression remained similar between fetal (noninsulin stimulated) to extrauterine life (postnatal animals, insulin stimulated) if born preterm, the animals born at term had a greater than twofold increase in Akt-1 gene expression during extrauterine life (P < 0.001) [Fig. 5(a)]. In addition, after birth, term animals had a significantly higher Akt-1 gene expression when compared with their preterm counterparts (P = 0.019) [Fig. 5(a)].
Although there were no differences in glycogen content between postnatal preterm and term baboons, there were marked increases in hepatic glycogen content with advancing GA during fetal life, with a threefold increase from preterm to term fetal animals (noninsulin stimulated; P < 0.001). Consistent with these results, the gene expression of GSK-3α decreased with advancing GA during fetal life [Fig. 5(c)]. There were no differences in the GSK-3α and pGSK-3α protein content between fetal, postnatal preterm, and term animals (data not shown).
Hepatic glycogen content in preterm and term baboons was significantly lower during extrauterine life (postnatal animals, insulin stimulated) as compared with the fetal animals (noninsulin stimulated) of similar gestation (P < 0.001) [Fig. 5(c)], although there was a significant increase in gene expression of GSK-3α in term animals from fetal to extrauterine life (2.7-fold increase, P < 0.05) [Fig. 5(b) and 5(c)].
Discussion
In this study we measured the molecular mechanisms involved in the regulation of EGP under maximal insulin stimulation in newborn nonhuman primates at various stages of postnatal development. We found a significant decrease during fasting in EGP (basal Ra-GIR) in preterm baboons during their first two weeks of life in association with a decrease in PEPCK (and perhaps PGC-1α) in the liver of preterm baboons (Fig. 6). Although the Ra was similar in both preterm and term animals under basal conditions, preterm baboons required a continuous glucose infusion to sustain euglycemia, whereas term animals did not. The low EGP persisted throughout the first two weeks of postnatal life. Interestingly, under maximal insulin stimulation, the EGP in preterm baboons remained low, which is likely due to the extremely low production they begin with (basal conditions). Furthermore, the glucose uptake by peripheral tissues was lower in preterm animals as demonstrated by their lower M values, in particular during week 1 (Table 2). In addition, preterm animals had consistently lower glucagon compared with term, and the insulin:glucagon ratio was 99% higher in preterm baboons compared with terms. Preterm baboons had wide variations in their baseline insulin at both weeks 1 and 2. Others have measured EGP utilizing stable isotopes (D-[6,6-2H2] glucose) in term human newborns, but they had minimal insulin stimulation and were born at term. We therefore attempted to reach maximal insulin stimulation similar to the levels that suppressed glucose production in newborn beagles; these animals did not achieve complete suppression of EGP despite of reaching plasma insulin levels > 1000 (25). Contrary to what is thought to be contributing factors to the etiology of abnormal glucose regulation in neonates, gluconeogenic precursors (glycerol and lactate) were similar between preterm and term animals.
Figure 6.
Preterm birth results in alterations in gluconeogenic gene expression and hepatic insulin signaling impairments.
Preterm baboons had a decreased gene expression of IR-β and Akt, decreased protein content of the PI3K regulatory subunit p85, and decreased phosphorylation of IRS-1 at Tyr612 compared with their term counterparts (Fig. 6). These alterations likely explain the development of hyperglycemia and decreased hepatic responses frequently seen in preterm infants despite elevated serum glucose levels (11, 17, 19, 26). This hepatic insulin resistance and higher insulin levels at fasting found in preterm baboons are consistent with the clinical findings in extremely preterm infants who have higher serum c-peptide levels at fasting (17) and require higher insulin doses to maintain normoglycemia (27). Persistent hepatic insulin impairments, particularly decreased IRS-1 phosphorylation, could explain the emergence of diabetes earlier in life in surviving preterm infants and need to be further studied (1).
In our baboon model, GSK-3 tended to sequentially decrease with advancing fetal age and increased after birth. GSK-3 is involved in numerous intracellular signaling mechanisms, including protein synthesis, gene transcription, apoptosis, and glucose regulation (28, 29). Our results demonstrate that baboons, if born at term, had a significant increase in GSK-3α from intrauterine (fetal) to extrauterine (postnatal life); however, if born preterm, the normal gestational rises were not observed. These differences in GSK-3 surges after birth may be secondary to the upstream insulin-signaling impairments found in preterm baboons (decreased phosphorylation of IRS-1 and decreased gene expression of IR-β and Akt) as insulin acts through IRS-1 and Akt to phosphorylate (and deactivate) GSK-3. This phosphorylation prevents GSK-3 from inhibiting glycogen synthase (30). Although little is known about GSK-3 during neonatal development, it recently has been shown to enhance insulin sensitivity, and GSK-3α has been suggested as a potential target for the treatment of diabetes and insulin resistance in adults (31). The lack of postnatal surge in GSK-3 gene expression after preterm birth in conjunction with impairments in insulin-signaling molecules in the liver are consistent with the insulin-resistant state in prematurity as previously demonstrated by our group (16). Another explanation for the lack in postnatal surge would be due to differences in responses to insulin affecting only preterm baboons. Interestingly, hepatic glycogen content sequentially increased with advancing GA in fetuses and then decreased postnatally regardless of preterm or term birth. The postnatal hepatic glycogen content may not correlate with the postnatal expression of GSK-3 due to differences in glycogen utilization between preterm and term infants and differences in glycogen synthase activity, which we did not measure. A limitation to the study is that sex fetal differences may have played a role in the differences in glycogen content or gene expression of GSK-3 in the fetuses born at 75% and 95% gestation. Sex differences have been previously found in some genes related to gluconeogenesis, glycogen content, and glucose output (32, 33); in this study, we didn’t find statistically significant sex differences in the fetuses born at 75% and 95% gestation and the remainder of the groups had equally distributed sex. Nonetheless, future studies are needed to elucidate the role of sex.
FOXO1, a nuclear transcription factor involved in the regulation of hepatic gluconeogenesis, was not altered by preterm birth. In adult mice, insulin acts on IRS-1 to activate Akt, which in turn promotes phosphorylation and nuclear exclusion of FOXO1 (34–37). Therefore, we expected to find alterations in FOXO1 as a result of downstream effects from a decrease in IRS-1 and Akt found in preterm animals. Although we found significant differences in key insulin-signaling molecules in the liver, we were surprised not to find any differences in FOXO1 activity, protein content, or gene expression. This might be explained by alterations in other transcription factors such as cAMP response element binding protein and hepatocyte nuclear factor 4α or changes in coactivators such as protein arginine methyltransferases. Another possibility could be that FOXO1 may have an abnormal response to IRS-1/Akt phosphorylation during the fetal to neonatal period. This could be explained by the rapid growth that occurs during the fetal and early neonatal period because FOXO1 is an important cofactor acting as a gate keeper for c-MYC signaling, a powerful driver of anabolic metabolism and growth (38).
In contrast to the impaired upstream insulin-signaling molecules, we found significantly decreased PEPCK-C in preterm baboons. Our research group has previously shown that PEPCK-C increases with advancing gestation in fetal baboons (20). In preterm baboons, PGC-1α gene expression was decreased by 44%. PGC-1α induces mRNA for PEPCK, G6Pase, and FBPase in a dose-dependent fashion, and the induction of these gluconeogenic genes results in increased glucose production in rat hepatocytes (39). Because coactivation of FOXO1 by PGC-1α is required for gluconeogenic gene expression, and PGC-1α increases the transcriptional activity of FOXO1 fourfold in mouse hepatocytes (40), the low PEPCK-C activity in preterm baboons may be, in part, secondary to the tendency of PGC1-α to be decreased. Our results indicate that PEPCK-C, and perhaps PGC1-α, undergo significant changes during neonatal development. The decrease in these key hepatic gluconeogenic enzymes, in conjunction with hypoglucagonemia, most likely are responsible for the reduction in glucose production and resultant hypoglycemia that occurs in prematurity.
These findings in extremely premature baboons raise an interesting question of how reduced insulin signaling with decreased levels of IRS-1 phosphorylation, decreased p85 protein content, and decreased Akt mRNA are found along with decreased PGC-1α and PEPCK-C gene expression. This paradox can explain the simultaneous occurrence of hyperglycemia and hypoglycemia frequently reported in premature infants.
In conclusion, we found a significant reduction in IRS-1 phosphorylation, PI3K p85 subunit protein content, and reduced IR-β and Akt gene expression along with a blunted response of GSK-3 after preterm birth in the liver of preterm baboons, which contributes to the insulin resistance and hyperglycemia of prematurity. EGP was reduced in conjunction with decreased gene expression of the gluconeogenic enzyme PEPCK (and perhaps cofactor PGC-1α), which can explain the hypoglycemia after short periods of fasting in the newborn period. These findings have important therapeutic implications because we currently treat the hyperglycemia with insulin, to which the preterm infants are resistant (27) due to impairments in the insulin-signaling pathway. Other therapies might be developed to enhance IRS-1 phosphorylation or GSK-3 inhibition to augment hepatic glucose uptake as a treatment of hyperglycemia of prematurity. Further, the infants with persistent/severe hypoglycemia may have significant alterations in glucagon, PEPCK, and PGC-1α, which represent therapeutic targets for neonatal hypoglycemia.
In this study, we demonstrate that baboons serve as an excellent translational model to study glucose metabolism throughout a life span as they develop many metabolic derangements similar to humans if born preterm. Our unique findings in a nonhuman primate model provide insight into the etiology of the glucose dysregulation observed in neonates, in particular if born preterm. Preterm birth affects millions, and as their incidence of diabetes in childhood and young adulthood continues to increase (1, 14, 41), further studies are critical to elucidate how preterm birth and other interventions provided in the neonatal intensive care unit and during early life impact their glucose regulation and insulin sensitivity.
Acknowledgments
We thank the personnel from the Veterinarian Services at UTHSCSA, the Oklahoma Primate Center, and the Texas Biomedical Research Institute for their dedication and support for this project and Michelle Leland, DMV, for her dedication to nonhuman primates.
Acknowledgments
This work was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118 (to L.M.-V.), the American Diabetes Association (to C.B., R.D., and N.M.), The Robert Wood Johnson Foundation (to C.B.), the UTHSCSA Clinical and Translational Science Award [UL1RR025767 (to C.B.)], the University Health System Research Fund (to C.B.), the National Institutes of Health [DK24092 (to R.D.); RO1DK80157, RO1DK089229, P30AG013319, and P30AG044271 (to N.M.)], and the US Department of Veterans Affairs (to R.D.).
Author contributions: L.M.-V., C.B., N.M., R.D., and A.G. designed the study. L.M.-V., H.L., D.A.G., T.J.-P., S.S., D.M., and G.M. performed key experiments. All authors participated in planning of the work, the interpretation of the results, and the writing of the paper. All authors have approved the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- EGP
- endogenous glucose production
- ELISA
- enzyme-linked immunosorbent assay
- FBPase
- fructose-1,6-bisphosphatase
- FOX01
- forkhead Box O1
- G6Pase
- glucose 6-phosphatase
- GA
- gestational age
- GIR
- glucose infusion rate
- IR-β
- insulin receptor β
- IRS-1
- insulin receptor substrate 1
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- PEPCK
- phosphoenolpyruvate carboxykinase
- PGC-1α
- peroxisome proliferator-activated receptor gamma coactivator 1-α
- PI3K
- phosphoinositide 3-kinase
- Ra
- rate of appearance
- UTHSCSA
- University of Texas Health Science Center at San Antonio.
References
- 1.Kajantie E, Osmond C, Barker DJP, Eriksson JG. Preterm birth—a risk factor for type 2 diabetes? The Helsinki birth cohort study. Diabetes Care. 2010;33(12):2623–2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khashan AS, Kenny LC, Lundholm C, Kearney PM, Gong T, McNamee R, Almqvist C. Gestational age and birth weight and the risk of childhood type 1 diabetes: a population-based cohort and sibling design study. Diabetes Care. 2015;38(12):2308–2315. [DOI] [PubMed] [Google Scholar]
- 3.Li S, Zhang M, Tian H, Liu Z, Yin X, Xi B. Preterm birth and risk of type 1 and type 2 diabetes: systematic review and meta-analysis. Obes Rev. 2014;15(10):804–811. [DOI] [PubMed] [Google Scholar]
- 4.Harris DL, Weston PJ, Harding JE. Incidence of neonatal hypoglycemia in babies identified as at risk. J Pediatr. 2012;161(5):787–791. [DOI] [PubMed] [Google Scholar]
- 5.Uettwiller F, Chemin A, Bonnemaison E, Favrais G, Saliba E, Labarthe F. Real-time continuous glucose monitoring reduces the duration of hypoglycemia episodes: a randomized trial in very low birth weight neonates. PLoS One. 2015;10(1):e0116255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burns CM, Rutherford MA, Boardman JP, Cowan FM. Patterns of cerebral injury and neurodevelopmental outcomes after symptomatic neonatal hypoglycemia. Pediatrics. 2008;122(1):65–74. [DOI] [PubMed] [Google Scholar]
- 7.Duvanel CB, Fawer CL, Cotting J, Hohlfeld P, Matthieu JM. Long-term effects of neonatal hypoglycemia on brain growth and psychomotor development in small-for-gestational-age preterm infants. J Pediatr. 1999;134(4):492–498. [DOI] [PubMed] [Google Scholar]
- 8.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118(5):1811–1818. [DOI] [PubMed] [Google Scholar]
- 9.Auerbach A, Eventov-Friedman S, Arad I, Peleg O, Bdolah-Abram T, Bar-Oz B, Zangen DH. Long duration of hyperglycemia in the first 96 hours of life is associated with severe intraventricular hemorrhage in preterm infants. J Pediatr. 2013;163(2):388–393. [DOI] [PubMed] [Google Scholar]
- 10.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26(12):737–741. [DOI] [PubMed] [Google Scholar]
- 11.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, Ong K, vanWeissenbruch M, Midgley P, Thompson M, Thio M, Cornette L, Ossuetta I, Iglesias I, Theyskens C, de Jong M, Gill B, Ahluwalia JS, de Zegher F, Dunger DB. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE study. J Pediatr. 2010;157(5):715–719.e3. [DOI] [PubMed] [Google Scholar]
- 12.Pertierra-Cortada A, Ramon-Krauel M, Iriondo-Sanz M, Iglesias-Platas I. Instability of glucose values in very preterm babies at term postmenstrual age. J Pediatr. 2014;165(6):1146–1153.e2. [DOI] [PubMed] [Google Scholar]
- 13.Kajantie E, Strang-Karlsson S, Hovi P, Wehkalampi K, Lahti J, Kaseva N, Järvenpää AL, Räikkönen K, Eriksson JG, Andersson S. Insulin sensitivity and secretory response in adults born preterm: the Helsinki Study of Very Low Birth Weight Adults. J Clin Endocrinol Metab. 2015;100(1):244–250. [DOI] [PubMed] [Google Scholar]
- 14.Finken MJJ, Keijzer-Veen MG, Dekker FW, Frölich M, Hille ETM, Romijn JA, Wit JM; Dutch POPS-19 Collaborative Study Group . Preterm birth and later insulin resistance: effects of birth weight and postnatal growth in a population based longitudinal study from birth into adult life. Diabetologia. 2006;49(3):478–485. [DOI] [PubMed] [Google Scholar]
- 15.Morrison KM, Ramsingh L, Gunn E, Streiner D, Van Lieshout R, Boyle M, Gerstein H, Schmidt L, Saigal S. Cardiometabolic health in adults born premature with extremely low birth weight. Pediatrics. 2016;138(4):138. [DOI] [PubMed] [Google Scholar]
- 16.Blanco CL, McGill-Vargas LL, Gastaldelli A, Seidner SR, McCurnin DC, Leland MM, Anzueto DG, Johnson MC, Liang H, DeFronzo RA, Musi N. Peripheral insulin resistance and impaired insulin signaling contribute to abnormal glucose metabolism in preterm baboons. Endocrinology. 2015;156(3):813–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny JF, Roger M, Voyer M. Both relative insulin resistance and defective islet beta-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113(3):537–541. [DOI] [PubMed] [Google Scholar]
- 18.Chacko SK, Sunehag AL. Gluconeogenesis continues in premature infants receiving total parenteral nutrition. Arch Dis Child Fetal Neonatal Ed. 2010;95(6):F413–F418. [DOI] [PubMed] [Google Scholar]
- 19.Chacko SK, Ordonez J, Sauer PJJ, Sunehag AL. Gluconeogenesis is not regulated by either glucose or insulin in extremely low birth weight infants receiving total parenteral nutrition. J Pediatr. 2011;158(6):891–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGill-Vargas LL, Johnson-Pais T, Johnson MC, Blanco CL. Developmental regulation of key gluconeogenic molecules in nonhuman primates. Physiol Rep. 2014;2(12):e12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanco CL, McGill-Vargas LL, McCurnin D, Quinn AR. Hyperglycemia increases the risk of death in extremely preterm baboons. Pediatr Res. 2012;73(3):337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namachivayam K, Blanco CL, MohanKumar K, Jagadeeswaran R, Vasquez M, McGill-Vargas L, Garzon SA, Jain SK, Gill RK, Freitag NE, Weitkamp JH, Seidner SR, Maheshwari A. Smad7 inhibits autocrine expression of TGF-β2 in intestinal epithelial cells in baboon necrotizing enterocolitis. Am J Physiol Gastrointest Liver Physiol. 2012;304(2):G167–G180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Research Council. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 2011.
- 24.Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The ontogeny of insulin signaling in the preterm baboon model. Endocrinology. 2010;151(5):1990–1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrag HM, Nawrath LM, Healey JE, Dorcus EJ, Rapoza RE, Oh W, Cowett RM. Persistent glucose production and greater peripheral sensitivity to insulin in the neonate vs the adult. Am J Physiol. 1997;272(1 Pt 1):E86–E93. [DOI] [PubMed] [Google Scholar]
- 26.Mitanchez D. Glucose regulation in preterm newborn infants. Horm Res. 2007;68(6):265–271. [DOI] [PubMed] [Google Scholar]
- 27.Ng SM, May JE, Emmerson AJB. Continuous insulin infusion in hyperglycaemic extremely-low- birth-weight neonates. Biol Neonate. 2005;87(4):269–272. [DOI] [PubMed] [Google Scholar]
- 28.Beurel E, Grieco SF, Jope RS. Glycogen synthase kinase-3 (GSK3): regulation, actions, and diseases. Pharmacol Ther. 2015;148:114–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frame S, Cohen P. GSK3 takes centre stage more than 20 years after its discovery. Biochem J. 2001;359(1):1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pugazhenthi S, Khandelwal RL. Regulation of glycogen synthase activation in isolated hepatocytes. Mol Cell Biochem. 1995;149-150:95–101. [DOI] [PubMed] [Google Scholar]
- 31.MacAulay K, Woodgett JR. Targeting glycogen synthase kinase-3 (GSK-3) in the treatment of type 2 diabetes. Expert Opin Ther Targets. 2008;12(10):1265–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gustavsson C, Yassin K, Wahlström E, Cheung L, Lindberg J, Brismar K, Ostenson CG, Norstedt G, Tollet-Egnell P. Sex-different hepaticglycogen content and glucose output in rats. BMC Biochem. 2010;11:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kley S, Hoenig M, Glushka J, Jin ES, Burgess SC, Waldron M, Jordan ET, Prestegard JH, Ferguson DC, Wu S, Olson DE. The impact of obesity, sex, and diet on hepatic glucose production in cats. Am J Physiol Regul Integr Comp Physiol. 2009;296(4):R936–R943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dong X, Park S, Lin X, Copps K, Yi X, White MF. Irs1 and Irs2 signaling is essential for hepatic glucose homeostasis and systemic growth. J Clin Invest. 2006;116(1):101–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dong XC, Copps KD, Guo S, Li Y, Kollipara R, DePinho RA, White MF. Inactivation of hepatic Foxo1 by insulin signaling is required for adaptive nutrient homeostasis and endocrine growth regulation. Cell Metab. 2008;8(1):65–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kubota N, Kubota T, Itoh S, Kumagai H, Kozono H, Takamoto I, Mineyama T, Ogata H, Tokuyama K, Ohsugi M, Sasako T, Moroi M, Sugi K, Kakuta S, Iwakura Y, Noda T, Ohnishi S, Nagai R, Tobe K, Terauchi Y, Ueki K, Kadowaki T. Dynamic functional relay between insulin receptor substrate 1 and 2 in hepatic insulin signaling during fasting and feeding. Cell Metab. 2008;8(1):49–64. [DOI] [PubMed] [Google Scholar]
- 37.Guo S, Copps KD, Dong X, Park S, Cheng Z, Pocai A, Rossetti L, Sajan M, Farese RV, White MF. The Irs1 branch of the insulin signaling cascade plays a dominant role in hepatic nutrient homeostasis. Mol Cell Biol. 2009;29(18):5070–5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wilhelm K, Happel K, Eelen G, Schoors S, Oellerich MF, Lim R, Zimmermann B, Aspalter IM, Franco CA, Boettger T, Braun T, Fruttiger M, Rajewsky K, Keller C, Brüning JC, Gerhardt H, Carmeliet P, Potente M. FOXO1 couples metabolic activity and growth state in the vascular endothelium. Nature. 2016;529(7585):216–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon JC, Puigserver P, Chen G, Donovan J, Wu Z, Rhee J, Adelmant G, Stafford J, Kahn CR, Granner DK, Newgard CB, Spiegelman BM. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413(6852):131–138. [DOI] [PubMed] [Google Scholar]
- 40.Puigserver P, Rhee J, Donovan J, Walkey CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D, Spiegelman BM. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423(6939):550–555. [DOI] [PubMed] [Google Scholar]
- 41.Wang G, Divall S, Radovick S, Paige D, Ning Y, Chen Z, Ji Y, Hong X, Walker SO, Caruso D, Pearson C, Wang MC, Zuckerman B, Cheng TL, Wang X. Preterm birth and random plasma insulin levels at birth and in early childhood. JAMA. 2014;311(6):587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]