Abstract
The acute effects of parathyroid hormone (PTH) on fibroblast growth factor 23 (FGF23) in vivo are not well understood. After a single subcutaneous PTH (1–34) injection (50 nmol/kg) in mice, FGF23 levels were assessed in plasma using assays that measure either intact alone (iFGF23) or intact/C-terminal FGF23 (cFGF23). Furthermore, FGF23 messenger RNA (mRNA) and protein levels were assessed in bone. In addition, we examined the effects of PTH treatment on FGF23 production in vitro using differentiated calvarial osteocyte-like cells. cFGF23 levels increased by three- to fivefold within 2 hours following PTH injection, which returned to baseline by 4 hours. In contrast, iFGF23 levels remained unchanged for the first 2 hours, yet declined to ∼60% by 6 hours and remained suppressed before returning to baseline after 24 hours. Using homozygous mice for an autosomal dominant hypophosphatemic rickets–FGF23 mutation or animals treated with a furin inhibitor, we showed that cFGF23 and iFGF23 levels increased equivalently after PTH injection. These findings are consistent with increased FGF23 production in bone, yet rapid cleavage of the secreted intact protein. Using primary osteocyte-like cell cultures, we showed that PTH increased FGF23 mRNA expression through cyclic adenosine monophosphate/protein kinase A, but not inositol triphosphate/protein kinase C signaling; PTH also increased furin protein levels. In conclusion, PTH injection rapidly increases FGF23 production in bone in vivo and in vitro. However, iFGF23 is rapidly degraded. At later time points through an unidentified mechanism, a sustained decrease in FGF23 production occurs.
Parathyroid hormone injection increases FGF23 production and cleavage in mice.
Fibroblast growth factor 23 (FGF23) is a bone-derived hormone regulating phosphate excretion and vitamin D activation in the kidney. Specifically, FGF23 increases phosphate excretion by reducing expression of the sodium-phosphate cotransporters NaPi2a and NaPi2c, and it lowers 1,25(OH)2 vitamin D levels by reducing expression of the 1α-hydroxylase and increasing the 24-hydroxylase in the renal proximal tubule (1). Work both in animals and humans shows that the FGF23 effects on phosphate levels occur 6 to 8 hours after changes in the circulating hormone (1). Similarly, feedback regulation by phosphate on plasma FGF23 concentration occurs with an 8- to 12-hour delay (2).
FGF23 levels increase with loss of kidney function and are elevated by orders of magnitude in patients with end-stage renal disease (3). Elevated FGF23 was linked epidemiologically to increased morbidity and mortality in chronic kidney disease (CKD) and end-stage renal disease patients (4, 5), and animal work suggests at least one possible mechanism, via direct cardiac toxicity (6, 7). Thus, strategies to reduce or prevent the rise in FGF23 levels or function would have considerable clinical implications.
FGF23 undergoes cleavage at a conserved 176RHT178R179/S180AE site by a process that is reduced when tyrosine residue T178 is O-glycosylated (8). Both the full-length protein and FGF23 cleavage products are present in the circulation, although at physiological concentrations the hormonal fragments do not contribute to mineral ion regulation. Heterozygous mutations at either arginine of the RXXR cleavage site lead to reduced processing of FGF23 and increased levels of the biologically intact hormone in the circulation, which leads to hypophosphatemia, as shown in individuals with autosomal dominant hypophosphatemic rickets (ADHR) (9). Homozygous inactivating mutations affecting the glycosylating enzyme (GALNT3) lead to increased processing of FGF23 and thus reduced levels of the intact hormone in the circulation, resulting in hyperphosphatemia, elevated 1,25OH2 vitamin D levels, and hyperphosphatemic tumoral calcinosis (10). Additional posttranslational modification of FGF23 occurs by phosphorylation of serine residue 180 adjacent to the RXXR cleavage site through FAM20C (11). Phosphorylation of this residue inhibits glycosylation, thus enhancing FGF23 cleavage. In contrast, inactivating mutations in FAM20C that cause Raine’s syndrome (ARHR3) or its murine homolog impair phosphorylation of serine residue 180, thereby enhancing O-glycosylation, stabilizing FGF23, and increasing its biological activity. In patients with CKD, particularly those with advanced stages, FGF23 almost exclusively circulates as intact protein due to failure or inhibition of processing (12, 13).
Parathyroid hormone (PTH) regulates phosphate excretion via the NaPi2a and NaPi2c transporters in a manner similar to FGF23, but it conversely increases vitamin D activation rather than inhibiting it as FGF23 does. PTH can affect FGF23 production, although the exact mechanism and timing have not yet been defined. Specifically, most studies in humans and animals have shown that infusion of PTH (1–34) leads to elevation of C-terminal FGF23 (cFGF23) levels in the circulation after 6 to 8 hours (14, 15). Intermittent, daily treatment with PTH for 2 weeks also causes an increase in intact FGF23 (iFGF23) and cFGF23 levels (16). In contrast, one study reported that infusion of PTH (1–34) resulted in reduced FGF23 (17). Furthermore, treatment of osteocyte-like cells (UMR106) increases FGF23 transcription within 24 hours, but not in other cell lines (18). The transcription factor Nurr1 may mediate these PTH effects on FGF23 transcription (19, 20).
Recently, altered processing of FGF23 was implicated in patients with fibrous dysplasia, where activating Gsα mutations cause increased cyclic adenosine monophosphate (cAMP)–mediated signaling, reducing GALNT3 activity and increasing activity of furin proteases; this led to increased levels of cFGF23 fragments in the circulation (21). We previously showed that acute PTH injection in vivo leads to hypercalcemia and elevated cAMP levels in the circulation and to hypophosphatemia via reduced NaPi2a expression in a predominantly cAMP-dependent mechanism in the kidney (22, 23). We now sought to determine the acute effects of PTH injection on FGF23 levels in mice, focusing on FGF23 production and processing.
Materials and Methods
Peptide
Peptide used was as follows: PTH (1–34) (human sequence: SVSEIQLMHNLGKHLNSMERVEWLRKKLQDVHNF). The peptide was C-terminally amidated and synthesized by the Massachusetts General Hospital Biopolymer Core Facility using solid-phase, N-(9-fluorenyl)methoxycarbonyl–based chemistry and an automated peptide synthesizer. Peptide purity and authenticity were verified by analytical high-performance liquid chromatography and matrix-assisted laser desorption ionization mass spectrometry.
Animal experiments
Mice (C57BL/6, male; age, 9–12 weeks; Charles River Laboratories) or NaPi2a knockouts, male and female, age 8–10 weeks, were treated in accordance with the ethical guidelines adopted by Massachusetts General Hospital. ADHR mice (male and female; age 8–10 weeks) were treated in accordance with the ethical guidelines adopted by the Institutional Animal Care and Use Committee for Indiana University School of Medicine (24). The mice were injected subcutaneously with either PTH (1–34) (50 nmol/kg) or vehicle (10 mm citric acid, 150 mm NaCl, 0.05% Tween 80, pH 5.0), except in dose-response experiments. All biochemical analysis was done on plasma samples collected either via heparinized capillaries from the tail vein (for time course samples) or into heparin-coated tubes after intracardiac puncture. ADHR mice were bled into heparin-coated tubes from the facial vein. All samples were stored at −20°C or −80°C until analysis. Phosphate levels were measured by the Phospha-C test (Wako, Osaka, Japan). FGF23 was measured using the rodent-specific cFGF23 and iFGF23 enzyme-linked immunosorbent assay (ELISA) kits (Immutopics, San Clemente, CA). The cFGF23 ELISA kit uses two antibodies directed against two epitopes within the C-terminal portion of FGF23 for capture and detection, thereby measuring both the iFGF23 hormone as well as the cFGF23 (iFGF23 plus cFGF23), whereas the iFGF23 ELISA uses a capture antibody directed against cFGF23 and a detection antibody, thereby detecting only the iFGF23. Total calcium was measured using a kit (Stanbio, Boerne, TX). The 1,25 dihydroxyvitamin D (1,25D) was measured using an enzyme-linked immunoassay (IDS, Gaithersburg, MD). In furin experiments, animals were pretreated with an irreversible furin/furin-like proprotein convertase inhibitor 1, 7.5 μg/g (EMD Millipore, Billerica, MA), prior to PTH injections.
FGF23 protein analysis
Femurs from mice 2 hours after PTH or vehicle injection were collected within minutes of kill and processed, as described by Farrow et al. (24). Bone lysates were then subjected to immunoprecipitation experiments using anti-FGF23 antibodies (Immutopics) and subsequently analyzed, as described (see Table 1). Anti-FGF23 antibodies were a gift of J. Lavigne from Immutopics.
Table 1.
List of Antibodies
| Protein Sequence | Antigen Sequence | Antibody | Name of Source | Species | Dilution Used |
|---|---|---|---|---|---|
| FGF23 | aa186–204 mouse | FGF23 | Immutopics | Goat polyclonal | 1 to 1500 |
| Furin | aa780–793 human | Furin | Abcam ab3467 | Rabbit polyclonal | 1 to 2000 WB |
| Fam20c | aa233–512 human | Fam20c | Abcam ab154740 | Rabbit polyclonal | 1 to 1000 WB |
| GALNT3 | Synthetic peptide rat aa93–142 | GALNT3 | Abcam ab113880 | Rabbit polyclonal | 1 to 2000 WB |
Antibody against FGF23 was used as per Farrow et al. (24).
Abbreviations: aa, amino acid; WB, Western blot.
Osteoblast isolation and in vitro experiments
For osteoblast isolation, alvariae were aseptically harvested from 3-day-old wild-type (WT) mice, minced, and incubated with digestion medium [α-minimum essential medium (MEM), 2 mg/mL type II collagenase (Invitrogen, Carlsbad, CA), and 2% penicillin/streptomycin] at 37°C in a water bath for 4 hours with vigorous shaking. Bone fragments were rinsed with cold phosphate-buffered saline (PBS) and cultured in α-MEM supplemented with 2% penicillin/streptomycin and 10% calf serum (PAA/GE Health Care, Piscataway, NJ) for 3 days without changing the medium. Following 3 days, the bone fragments were gently removed from the flasks with α-MEM, and the cells were cultured until reaching confluence in α-MEM supplemented with 2% penicillin/streptomycin and 10% calf serum for ∼6 to 9 days. After 90% confluence, cells were grown in the presence of osteoblastic differentiation medium (50 mg/mL ascorbic acid and 10 mM b-glycerophosphate) for 21 days. After osteocytic differentiation, cells were treated with 100 ng/ml human PTH (1–34) alone or combined with 15 nM protein kinase A (PKA) inhibitor P6062 (Sigma-Aldrich, St. Louis, MO), 5 µM protein kinase C (PKC) inhibitor SCP0214 (Sigma-Aldrich), 10 µg/ml forskolin F3917 (Sigma-Aldrich), and 5 ng/mL extracellular signal-regulated kinase inhibitor PD184352 (Sigma-Aldrich) for 2 and 6 hours. Cell culture medium was collected for cFGF23 ELISAs (Immutopics). For protein samples and for RNA isolation, cells were harvested, collected, shock frozen in liquid nitrogen, and stored at −80°C. For immunohistochemistry, cells were washed twice with cold PBS and fixed in 4% formaldehyde in PBS at 4°C overnight.
Western blotting
Proteins were solubilized in Laemmli sample buffer, fractionated on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (50 mg/well), and transferred to a nitrocellulose membrane (Thermo Scientific). Immunoblots were incubated overnight at 4°C with polyclonal rabbit anti-furin (1:2000; Abcam), anti-Fam20C (1:1000), anti-GALNT3 (1:2000), and monoclonal mouse anti–b-actin (1:5000; Sigma-Aldrich) in 2% (w/v) bovine serum albumin (Sigma-Aldrich) in a TBS-T buffer [150 mM NaCl, 10 mM Tris (pH 7.4/HCl), 0.2% (v/v) Tween 20]. After washing, membranes were incubated with horseradish peroxidase–conjugated secondary antibodies (Amersham Life Sciences/GE Health Care). Specific signal was visualized by enhanced chemiluminescence kit (Amersham Life Sciences/GE Health Care). The protein bands were quantified using Image J software.
Immunohistochemistry
For immunohistochemistry, cells were fixed 10 minutes in 4% paraformaldehyde solution, followed by incubation for 15 minutes in 3% hydrogen peroxide in PBS to block endogenous peroxidase activity, and, after blocking with 10% rabbit serum, incubated with anti-furin, anti-Fam20C, and anti-GALNT3 (Abcam, Cambridge, MA; 1:300) at 4°C overnight. After washing, cells were incubated for 2 hours with biotinylated goat anti-rabbit secondary antibody (1:2000; Vector, Burlingame, CA). Finally, the cells were counterstained with Mayer’s hematoxylin. Negative control was performed by omitting primary antibody. The cells were analyzed using a Zeiss Axioskop 2 microscope.
RNA isolation and quantitative real-time polymerase chain reaction
Cell culture samples or calvaria were homogenized in TRI Reagent (Molecular Research Center, Cincinnati, OH), and total RNA was extracted, according to the manufacturer’s protocol. Two micrograms of RNA was used for first-strand complementary DNA synthesis (iScript cDNA Synthesis Kit; Bio-Rad, Hercules, CA). Quantitative reverse transcription polymerase chain reaction was performed on a ViiA 7 (Applied Biosystems, Burlington, ON, Canada) using QuantiFast EverGreen polymerase chain reaction kit (Qiagen). Expression of target genes was normalized to the expression of the housekeeping gene ornithine decarboxylase antizyme 1 (OAZ1).
Statistical analysis
Statistics were computed using PASW Statistics 17.0 (SPSS, Chicago, IL). The data were analyzed by two-sided t test (two groups) or one-way analysis of variance, followed by Student-Newman-Keuls multiple comparison test (more than two groups). The p values ˂0.05 were considered significant. Data represent mean values ± standard error of the mean.
Results
PTH injection leads to cFGF23 elevation
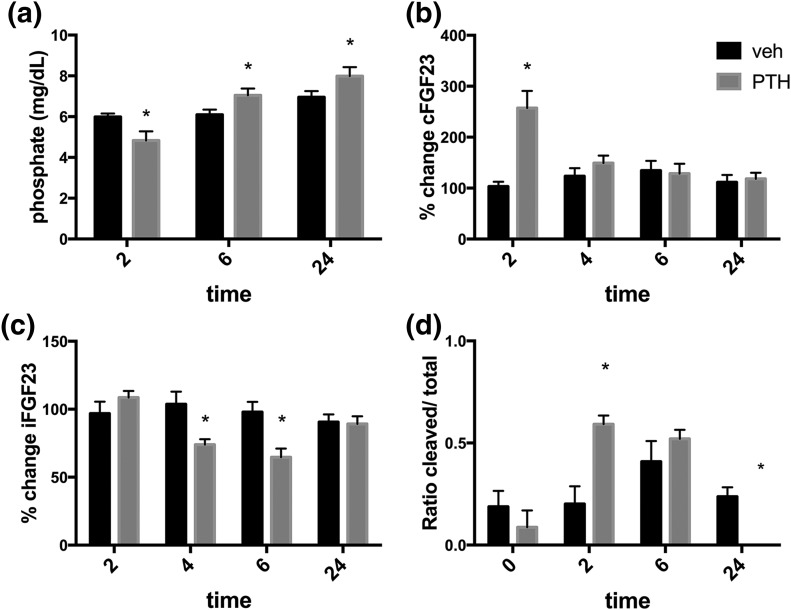
To determine whether PTH can acutely change FGF23 levels, we performed time-course experiments in WT mice, measuring phosphate and cFGF23 levels immediately before and at 2, 4, 6, and 24 hours after PTH injection (50 nmol/kg). As shown in Fig. 1(a), phosphate levels were significantly lower 2 hours after PTH (1–34), but not after vehicle injection; these results are consistent with our previous observations, in which phosphate levels 2 hours after PTH injection decreased by ≥1 mg/dL from baseline following 20 nmol/kg intravenous or 50 nmol/kg subcutaneous delivery (23, 25). Phosphate levels in PTH-injected mice subsequently increased above those encountered in control mice and remained elevated at 6 and 24 hours. Total calcium levels were not different at 6 hours (9.0 ± 0.79 for PTH-injected versus 9.13 ± 0.86 for vehicle-injected mice, n = 6 each) or at 24 hours (9.12 ± 0.24 for PTH-injected versus 9.14 ± 0.23 for vehicle-injected mice, n = 2–4). In addition, because 1,25D production increases after PTH injection, we measured 1,25D levels at 6 and 24 hours in our experiments. As expected, 1,25D levels were higher in PTH-injected mice 6 hours after injection compared with vehicle-injected mice (165 ± 23 versus 83 ± 20 pmol/L, n = 6 each, P < 0.05), and were nonsignificantly different 24 hours after injection (113 ± 9 versus 127 ± 19, n = 5 each, P = 0.53). FGF23 levels as measured by an ELISA that detects both cFGF23 and iFGF23 started to increase by 1 hour (data not shown) and were three- to fivefold elevated by 2 hours after PTH injection; by 4 hours cFGF23 had declined to baseline [Fig. 1(b)]. This acute cFGF23 elevation over baseline ranged between 2.5- and fivefold in different experiments, but was consistently observed in multiple experiments.
Figure 1.
Acute effects of a single PTH injection in WT mice. Baseline cFGF23 levels in different experiments ranged between 218 ± 19 and 345 ± 34 pg/mL, and peak cFGF23 levels ranged between 578 ± 114 and 1244 ± 110 pg/mL; therefore, changes in cFGF23 (and iFGF23) levels are reported as percentage of change from baseline. (a) Plasma phosphate levels 2, 6, and 24 hours after PTH or vehicle injection. (b) Plasma cFGF23 levels, expressed as percentage over preinjection baseline, at 2, 4, 6, and 24 hours after PTH injection. (c) Plasma iFGF23 levels, expressed as percentage over preinjection baseline, at 2, 4, 6, and 24 hours after PTH injection. n = 5 or more mice per group. (d) Ratio of cleaved/total FGF23 was calculated as follows: (value measured by cFGF23 ELISA − value measured by iFGF23 ELISA)/value measured by cFGF23 ELISA. n = 7–8 per group. *P < 0.05.
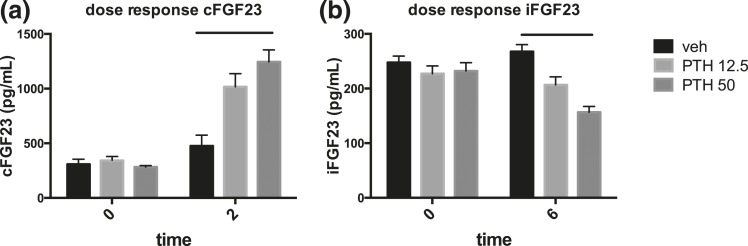
Because increased cAMP signaling had been shown in vitro to increase the generation of cFGF23 fragments, we sought to determine whether the observed increase in FGF23 levels after PTH injection (and the resulting cAMP generation) represented an increase in cFGF23 and/or iFGF23. Although cFGF23 levels were increased considerably 2 hours after PTH injection compared with vehicle [Fig. 1(b)], FGF23 levels measured by an ELISA that only detects iFGF23 did not show an increase over baseline at 2 hours, suggesting that the increase in circulation was due to increased cFGF23 fragments [Fig. 1(c)]. This increase of cFGF23 levels at 2 hours post-PTH injection was dose dependent [Fig. 2(a)]. We calculated the ratio of C-terminal fragments to total FGF23. The ratio increased significantly after 2 hours in PTH-injected, compared with the ratio in vehicle-injected mice [Fig. 1(d)]. Although the ratio in the PTH-injected mice remained elevated at 6 hours similar to the values obtained at 2 hours, suggesting continued augmented degradation of FGF23, no elevation was observed by 24 hours post-PTH injection.
Figure 2.
Dose-response effects of cFGF23 and iFGF23 after PTH injection. PTH doses of 12.5 and 50 nmol/kg were injected, and (a) peak cFGF23 levels (at 2 hours) and (b) nadir iFGF23 levels (at 6 hours) are shown. Absolute values are shown from an individual experiment. n = 5 or more mice per group. Bars show significant response by analysis of variance.
iFGF23 levels decreased after PTH injection
Although iFGF23 levels remained indistinguishable in PTH- and vehicle-injected animals at 2 hours, they decreased significantly by 30% to 50% from baseline at 4 and 6 hours after injection [Fig. 1(c)]. This decline in iFGF23 in the setting of stable total FGF23 levels is consistent with persistent increased cleavage of the intact protein, as shown in Fig. 1(d), at the 4-hour time point. The decline in iFGF23 persisted for up to 9 hours after PTH injection (data not shown) before returning to baseline by 24 hours after PTH injection. The decrease of iFGF23 occurred despite a concurrent elevation of plasma phosphate levels [Fig. 1(a)]. The decrease of iFGF23 levels at 6 hours, which appears to be the nadir of the effect, occurred in a dose-dependent manner, similar to the effect on cFGF23 levels at 2 hours [Fig. 2(b)].
iFGF23 increases after PTH injection in the ADHR mutant and following furin inhibition
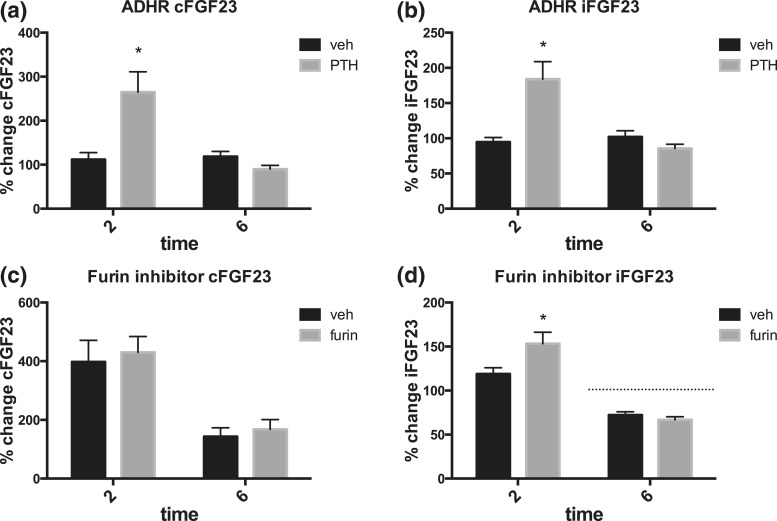
We next sought to determine whether the increase in cFGF23 after PTH injection, which occurs despite unchanged iFGF23 levels, is due to increased cellular FGF23 synthesis that is accompanied by increased cleavage of FGF23 at the conserved RXXR site. We therefore injected PTH (1–34) or vehicle into mice homozygous for a R176Q Fgf23 ADHR mutation (24). Phosphate levels decreased to 81 ± 5% of baseline in ADHR mice 2 hours after injection with PTH compared with 102 ± 6% in vehicle-injected ADHR mice (P < 0.05), indicating preserved PTH-dependent phosphate-lowering effect. Two hours after PTH injection, the ADHR mutants had a similar increase in cFGF23 as WT mice [Fig. 3(a)]. However, unlike the WT animals, iFGF23 levels increased at 2 hours also in the ADHR mutants [Fig. 3(b)]. This suggested that the observed PTH-induced increase in cFGF23 levels in WT mice is caused by increased FGF23 protein expression, which is, however, accompanied by rapid cleavage of iFGF23 protein.
Figure 3.
Effects of PTH injection in ADHR mice or in animals pretreated with the furin inhibitor 537076 (at concentration 7.5 μg/g) or PBS vehicle. (a) cFGF23 and (b) iFGF23 changes after PTH injection (50 nmol/kg), or vehicle (10 mm citric acid, 150 mm NaCl, 0.05% Tween 80, pH 5.0) in ADHR mutant mice. n = 6–8 per group. Baseline cFGF23 levels in ADHR mutant mice in different experiments ranged between 190 ± 13 and 281 ± 19 pg/mL, and peak cFGF23 levels ranged between 485 ± 78 and 1242 ± 239 pg/mL, whereas baseline iFGF23 levels in ADHR mutant mice in different experiments ranged between 262 ± 23 and 345±18 pg/mL, and peak iFGF23 levels ranged between 450 ± 25 and 975 ± 149 pg/mL. Changes in cFGF23 and iFGF23 levels are reported as percentage of change from baseline. (c) cFGF23 and (d) iFGF23 levels in WT mice all injected with PTH; furin refers to mice pretreated with furin inhibitor, and vehicle refers to mice pretreated with vehicle as described. n > 4 mice per group. *P < 0.05. Hashed line shows 100% of baseline.
Similarly, pretreatment of WT animals with a furin inhibitor prior to PTH injection resulted in a significant increase of iFGF23 over baseline at 2 hours, compared with WT mice pretreated with vehicle before PTH [Fig. 3(d)]. All animals in this set of experiments were injected with PTH, but only half of them were injected with the furin inhibitor, whereas the other half received vehicle. Furin inhibitor pretreatment did not change the peak cFGF23 levels 2 hours after PTH injection [Fig. 3(c)]. Interestingly, furin inhibition had no effect on the decrease of iFGF23 6 hours after PTH injection [Fig. 3(d)], whereas no such decrease of iFGF23 was observed in the ADHR mutants [Fig. 3(b)].
Hypophosphatemia abolishes the decrease of iFGF23 levels after PTH injection
To dissect the effect of PTH-induced hypophosphatemia on the changes in FGF23 levels, we injected PTH or vehicle into adult NaPi2a-null mice. These animals had previously been shown to lack a hypophosphatemic effect within 2 hours after PTH injection, while retaining cAMP signaling in the kidney (26). Similarly, we saw no difference in plasma phosphate levels 2 hours after PTH injection (3.26 ± 0.29 mg/dL) or vehicle injection (3.47 ± 0.42 mg/dL, n = 5–6, p value not significant). In comparison, plasma phosphate levels in WT mice were 4.83 ± 0.46 mg/dL at the same time point after PTH injection and 5.99 ± 0.16 mg/dL in vehicle-injected mice [Fig. 1(a), n = 9–10, P < 0.05]. However, cFGF23 levels were approximately threefold higher in PTH-injected than in vehicle-injected Npt2a-null mice (1015 ± 250 versus 298 ± 69 pg/mL, P < 0.05, n = 5), which is similar to the effect observed in WT mice. Interestingly, iFGF23 levels 6 hours after PTH or vehicle injection were not different between the groups (46 ± 4 pg/ml for PTH and 43 ± 3 pg/ml for vehicle injection, n = 5).
PTH injection increases FGF23 transcription and processing in bone
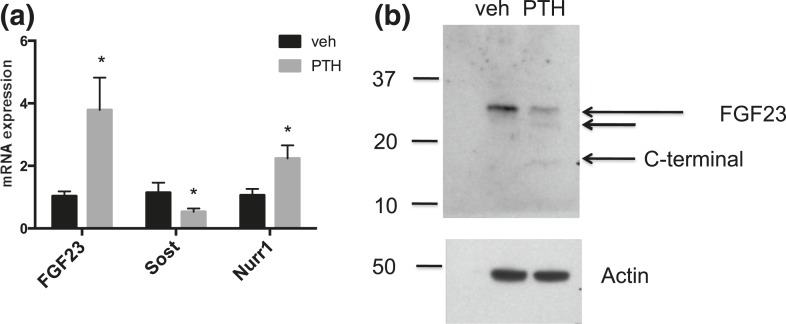
We next sought to determine whether FGF23 production in bone is increased with PTH administration. To this end, we isolated femur messenger RNA (mRNA) from mice 2 hours after PTH or vehicle treatment. FGF23 mRNA levels from mouse femurs were increased 2 hours after PTH, but not after vehicle treatment [Fig. 4(a)]. In contrast, sclerostin levels were decreased, as expected. Expression levels of the transcription factor Nurr1, which has been linked to PTH-dependent FGF23 transcription, were also increased in PTH-treated mice. No significant differences were observed for expression of mRNAs encoding furin, FAM20c, or GALNT3 (data not shown).
Figure 4.
mRNA and protein levels in bone from mice WT after PTH or vehicle injection. (a) mRNA expression in femurs from WT adult mice treated with PTH or vehicle. n = 3–4. Sost = sclerostin. (b) Western blot probed with biotin-conjugated anti-FGF23 antibody (Immutopics), upper panel, or actin, lower panel, in bone lysates from mice injected with vehicle or PTH. *P < 0.05.
We furthermore sought to determine whether the C-terminal fragments seen in the circulation were generated in bone, a known site for PTH action. We therefore isolated femur lysates from long bones using published protocols and then immunoprecipitated FGF23 protein using antibodies directed against the C-terminal portion of FGF23. When the immunoprecipitated samples were probed by immune blotting using an antibody directed against the C-terminal portion of FGF23, a low molecular weight fragment was observed in the PTH-treated, but not the vehicle-treated samples [Fig. 4(b)]. Actin was used as loading control.
PTH treatment increases FGF23 transcription in vitro through a PKA-dependent mechanism
To further dissect the mechanisms underlying the increase of FGF23 after PTH treatment, we treated differentiated mouse calvarial osteocyte-like cells with PTH or vehicle and collected media for FGF23 level measurements and cell lysates for mRNA analysis 2 hours later. cFGF23 levels in the media increased 2 hours after PTH treatment, similar to our in vivo observations (vehicle: 120 pg/mL; after PTH at 100 ng/mL: 1900 ng/mL).
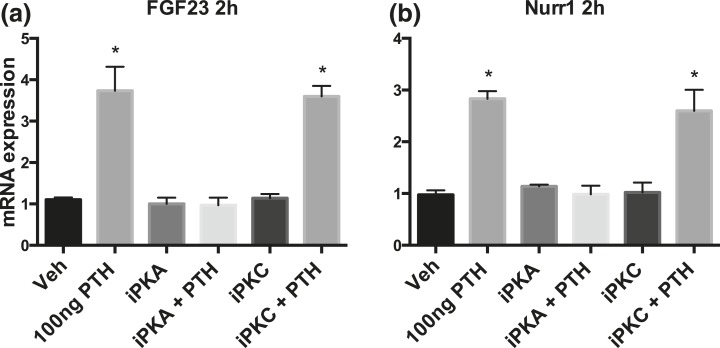
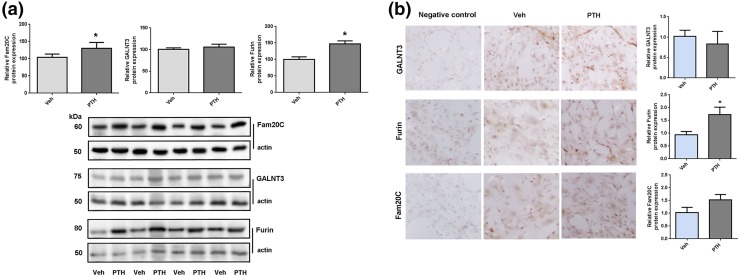
In the same cells, FGF23 mRNA levels increased 2 hours after PTH treatment [Fig. 5(a)]. Furin, Fam20c, and GALNT3 mRNA levels did not change (data not shown). Nurr1 mRNA levels increased at 2 hours after PTH treatment as well [Fig. 5(b)]. When assessed by Western blot, furin and Fam20c protein levels showed a small increase after 2 hours of PTH treatment [Fig. 6(a)], which was not observed in vehicle-treated cells. The furin increase after 2 hours of PTH treatment was confirmed by immunohistochemistry, a less quantitative technique [Fig. 6(b)]. When cells were treated with inhibitors of PKA (P6062) or PKC (SCP0214) signaling in addition to PTH or vehicle, the PTH-dependent increase in FGF23 and Nurr1 mRNA was blunted after PKA inhibition, whereas a PKC inhibitor had no effect.
Figure 5.
mRNA expression in cultured calvaria osteocytes. (a) and (b) show FGF23 and Nurr1 mRNA expression in calvarial osteocytes treated with PTH, vehicle, PKA inhibitor (15 nM PKA inhibitor P6062), PKC inhibitor (5 µM PKC inhibitor SCP0214), or both. Expression normalized to housekeeping gene ornithine decarboxylase antizyme 1 (see Materials and Methods). Data shown are mean of triplicate. *P < 0.05.
Figure 6.
Protein levels in calvarial osteocyte-like cells. (a) Shows Western blot analysis of Fam20C, GALNT3, and furin protein levels in calvarial osteocyte-like cells 2 hours after PTH or vehicle treatment. The top part shows expression levels normalized to actin using Image J, and the lower part shows the Western blot data. (b) Shows immunohistochemical staining of differentiated calvarial osteocyte-like cells after 21 days in culture treated with PTH or vehicle. The staining is normalized to vehicle. GALNT3, Furin, or Fam20c protein levels are shown. Magnification is 40-fold. *P < 0.05.
Discussion
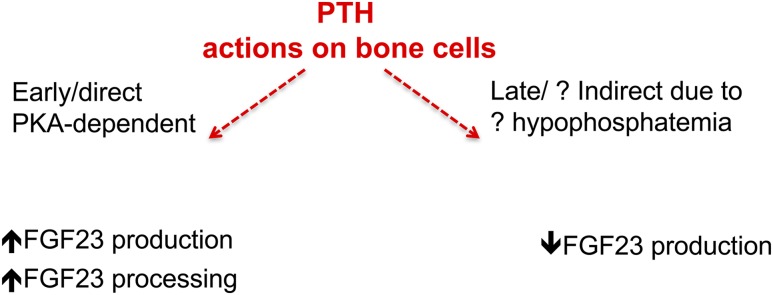
Our study was designed to define the acute effects of PTH on FGF23 production. We revealed two aspects of the regulation of FGF23 secretion by PTH, namely an increase in production and processing within 1 to 2 hours after PTH injection, and a subsequent prolonged lowering of iFGF23 levels. Both observations provide previously unknown insights into FGF23 physiology (Fig. 7).
Figure 7.
Proposed model of PTH action on FGF23 production at early and late time points.
In the initial period after a single PTH injection, circulating cFGF23 levels increased rapidly, indicating more production and/or secretion. Enhanced FGF23 production after PTH treatment was supported by experimental in vivo and in vitro data documenting increased FGF23 mRNA levels in bone and differentiated osteoblasts (Fig. 5). Our data are consistent with the report by Rhee et al. (27), showing that PTH treatment of primary cultures of osteoblasts and osteocytes resulted in increased FGF23 transcription within 1 hour of treatment. These authors also showed that expression of a mutant constitutively active PTH1R under the control of the DMP1 promoter led to higher FGF23 mRNA levels in osteocytes, as well as elevated circulating FGF23 levels. This effect is most likely mediated by PTHR1 via PKA activation because the mutant PTH1Rs show, when tested in vitro, ligand-independent cAMP, but not inositol triphosphate formation. Consistent with this conclusion, we showed that PKA inhibition, but not PKC inhibition, abrogated in our experiments the PTH-induced increase in FGF23 mRNA in osteocyte-like cells. Our data are supportive for FGF23 production being regulated by the cAMP/PKA pathway, but other signaling pathways cannot be excluded. Further dedicated investigation of this pathway in vivo and in vitro is needed to provide additional evidence for the downstream events regulating the acute increase in FGF23 production in response to PTH.
Using Western blotting and immunohistochemistry, we showed that, whereas furin mRNA levels in osteoblasts did not change, furin protein levels increased 2 hours post-PTH injection, which may explain an increase in FGF23 degradation due to increased furin activity. Similarly, although Fam20c mRNA levels did not change after PTH treatment, Fam20c protein levels increased in comparison with vehicle treatment as determined by Western blot analysis, which is again consistent with increased FGF23 degradation. However, the changes were small, and it is therefore not surprising that no major changes were observed by immunohistochemistry. The dual increase in these processing enzymes is in favor of FGF23 cleavage.
The observed acute increase of cFGF23, but not of iFGF23, in WT given PTH mice suggested that increased FGF23 production is tightly coupled to degradation of iFGF23, thereby preventing a rise in iFGF23 and thus excessive urinary phosphate losses. Consistent with this conclusion, our experiments with the ADHR mutant mice revealed similar increases of both cFGF23 and iFGF23 levels; note that all of the FGF23 protein produced in response to PTH carries the R176Q mutation and is thus resistant to cleavage (Fig. 3). Our experiments also suggest that PTH acutely induces FGF23 cleavage at the conserved RXXR site. Supporting this conclusion is our observation that furin inhibition appears to have a similar, albeit smaller effect and leads to an increase in both cFGF23 and iFGF23. The smaller and relatively modest effect of furin inhibition may be due to an insufficient dose used or to an inability of the furin inhibitor, administered intraperitoneally, to reach the furin site of action, in this case bone. The dose we used was the same as that used by David et al. (28), and the reported effect on FGF23 levels was similarly modest. Our data are consistent with findings by Bhattacharyya et al. (21), who showed that Gsα activation, the primary signaling molecule downstream of the activated PTH1R, leads to increased FGF23 cleavage by increasing furin activity and reducing GALNT3 activity.
Additionally, our finding that iFGF23 levels decreased in bone 2 hours after PTH injection (see Fig. 4) along with the appearance of FGF23 fragments could indicate that already synthesized iFGF23 stored within cells can be readily degraded into inactive fragments. This possibility is supported by our observations that cFGF23 levels are increased as early as 1 hour after PTH injection (data not shown). Consistent with this rapid time course, secretory granules have been reported in osteoblasts and osteocytes (suggesting a possible storage compartment for synthesized FGF23 protein) (29).
Calculating the ratio of cleaved over total FGF23 is a common way to try to understand the generation of cFGF23 fragments, which is done by subtracting the values obtained from measuring FGF23 using the ELISA that detects only the intact level from the values obtained from measuring FGF23 using the ELISA that detects total FGF23 (i.e., both iFGF23 and cFGF23) and then dividing by the values of total FGF23. In our experiments, the ratio of cFGF23 over total FGF23 levels increased after PTH injection both at 2 and 6 hours [Fig. 1(d)], but not after 24 hours.
Compared with vehicle injection, PTH injection led to a delayed, dose-dependent decrease in iFGF23 levels, which was prolonged, lasting from 4 to at least 9 hours postinjection. The only other instance in which FGF23 levels have been shown to decrease after PTH treatment was in human volunteers by 6 hours following PTH infusion (17). However, in this study, cFGF23 levels were measured; moreover, the approximate decrease in cFGF23 levels was at most by 20%. Thus, our study reports a substantial decrease of functionally active iFGF23 levels in vivo following PTH injection. The decrease in iFGF23 in our studies may have been triggered by the PTH-induced hypophosphatemia and the need to limit any FGF23-dependent phosphaturic effect. Reduction of iFGF23 levels through this mechanism may explain why serum phosphate levels were still elevated 24 hours after PTH injection.
To clarify the role of hypophosphatemia in the regulation of FGF23 levels after PTH injection, we used Npt2a-null mice lacking the main transporter by which PTH exerts its rapid/early phosphaturic action (26). PTH injection in Npt2a-null mice failed to induce a further decline by 2 hours of the already reduced plasma phosphate levels. However, these animals showed 2 hours after PTH injection a similar increase in cFGF23 levels as WT animals, suggesting that the acute PTH effect on the cFGF23 elevation occurs independent of plasma phosphate levels. The earliest serum phosphate changes reported after PTH injection are within 1 hour (23). Thus, the timing of iFGF23 decrease in the circulation (within 3 hours of low phosphate, or 4 hours after PTH injection) is inconsistent with prior observations on the effects of phosphate on FGF23 levels. For example, after oral or intravenous phosphate loading was stopped in human volunteers, phosphate levels decreased below baseline due to (phosphate-induced) elevated FGF23 levels. This hypophosphatemia led to a reduction in FGF23 levels with a 12-hour delay (2, 30). Thus, nonphosphate-mediated PTH-dependent mechanisms may be involved in this process. Our observation that the iFGF23 changes after PTH injection did not occur in ADHR mice either, which lowered their phosphate level in response to PTH, supports this conclusion. One explanation is that increased FGF23 cleavage may be coupled to a decrease in iFGF23 acutely; however, furin inhibition did not affect the decrease in iFGF23, making this hypothesis less likely. Alternatively, other molecules may be altered at baseline in ADHR (and Napi2a-null) mice, and this may affect FGF23 regulation.
In summary, our results highlight aspects of FGF23 biology, particularly a delayed and prolonged reduction in circulating iFGF23 levels following PTH injection in WT mice, which may be triggered by PTH-induced lowering of plasma phosphate levels, or may represent a nonphosphate-dependent effect on FGF23 production in intact animals. Recent attempts to lower circulating FGF23 levels by manipulating phosphate levels by phosphate binders in patients with CKD have not yet been proven to be sufficiently efficacious toward controlling FGF23 levels. However, our work represents a potential alternative nonphosphate-mediated pathway to lower FGF23 levels given the high morbidity and mortality associated with increased FGF23 levels.
Acknowledgments
We thank Dr. Thomas J. Gardella (Endocrine Unit, Massachusetts General Hospital) for providing the PTH peptide and for discussions on this project.
Acknowledgments
This work was supported by National Institutes of Health Grants K08-DK093608 (to M.C.), PO1 DK11794 (to H.J.), R01-DK063934 and R01-DK95784 (to K.E.W.), and T32-HL007910 (to J.M.H.). This work was also supported by the National Kidney Foundation Young Investigator Award (to M.C.), the Austrian Marshall Plan Foundation (to V.M.K.), an American Heart Association Postdoctoral Fellowship 16POST-27260108 (to J.M.H.), and the David Weaver Professorship and a Showalter Scholar award through the Ralph W. and Grace M. Showalter Research Trust (to K.E.W.).
Disclosure Summary: K.E.W. receives royalties for licensing the FGF23 gene to Kyowa Hakko Kirin. H.J. is a co-inventor on a patent for measuring FGF23. The remaining authors have nothing to disclose.
Footnotes
- 1,25D
- 1,25 dihydroxyvitamin D
- ADHR
- autosomal dominant hypophosphatemic rickets
- cAMP
- cyclic adenosine monophosphate
- cFGF23
- C-terminal FGF23
- CKD
- chronic kidney disease
- ELISA
- enzyme-linked immunosorbent assay
- FGF23
- fibroblast growth factor 23
- iFGF23
- intact FGF23
- MEM
- minimum essential medium
- mRNA
- messenger RNA
- PBS
- phosphate-buffered saline
- PKA
- protein kinase A
- PKC
- protein kinase C
- PTH
- parathyroid hormone
- WT
- wild-type.
References
- 1.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429–435. [DOI] [PubMed] [Google Scholar]
- 2.Scanni R, vonRotz M, Jehle S, Hulter HN, Krapf R. The human response to acute enteral and parenteral phosphate loads. J Am Soc Nephrol. 2014;25(12):2730–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Larsson T, Nisbeth U, Ljunggren O, Jüppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64(6):2272–2279. [DOI] [PubMed] [Google Scholar]
- 4.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M; Chronic Renal Insufficiency Cohort (CRIC) Study Group . Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Jüppner H, Wolf M. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutiérrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro-O M, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121(11):4393–4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grabner A, Amaral AP, Schramm K, Singh S, Sloan A, Yanucil C, Li J, Shehadeh LA, Hare JM, David V, Martin A, Fornoni A, Di Marco GS, Kentrup D, Reuter S, Mayer AB, Pavenstädt H, Stypmann J, Kuhn C, Hille S, Frey N, Leifheit-Nestler M, Richter B, Haffner D, Abraham R, Bange J, Sperl B, Ullrich A, Brand M, Wolf M, Faul C. Activation of cardiac fibroblast growth factor receptor 4 causes left ventricular hypertrophy. Cell Metab. 2015;22(6):1020–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kato K, Jeanneau C, Tarp MA, Benet-Pagès A, Lorenz-Depiereux B, Bennett EP, Mandel U, Strom TM, Clausen H. Polypeptide GalNAc-transferase T3 and familial tumoral calcinosis: secretion of fibroblast growth factor 23 requires O-glycosylation. J Biol Chem. 2006;281(27):18370–18377. [DOI] [PubMed] [Google Scholar]
- 9.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ. Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int. 2001;60(6):2079–2086. [DOI] [PubMed] [Google Scholar]
- 10.Topaz O, Shurman DL, Bergman R, Indelman M, Ratajczak P, Mizrachi M, Khamaysi Z, Behar D, Petronius D, Friedman V, Zelikovic I, Raimer S, Metzker A, Richard G, Sprecher E. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–581. [DOI] [PubMed] [Google Scholar]
- 11.Tagliabracci VS, Engel JL, Wiley SE, Xiao J, Gonzalez DJ, Nidumanda Appaiah H, Koller A, Nizet V, White KE, Dixon JE. Dynamic regulation of FGF23 by Fam20C phosphorylation, GalNAc-T3 glycosylation, and furin proteolysis. Proc Natl Acad Sci USA. 2014;111(15):5520–5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith ER, Cai MM, McMahon LP, Holt SG. Biological variability of plasma intact and C-terminal FGF23 measurements. J Clin Endocrinol Metab. 2012;97(9):3357–3365. [DOI] [PubMed] [Google Scholar]
- 13.Shimada T, Urakawa I, Isakova T, Yamazaki Y, Epstein M, Wesseling-Perry K, Wolf M, Salusky IB, Jüppner H. Circulating fibroblast growth factor 23 in patients with end-stage renal disease treated by peritoneal dialysis is intact and biologically active. J Clin Endocrinol Metab. 2010;95(2):578–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wesseling-Perry K, Harkins GC, Wang HJ, Elashoff R, Gales B, Horwitz MJ, Stewart AF, Jüppner H, Salusky IB. The calcemic response to continuous parathyroid hormone (PTH)(1-34) infusion in end-stage kidney disease varies according to bone turnover: a potential role for PTH(7-84). J Clin Endocrinol Metab. 2010;95(6):2772–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burnett-Bowie SM, Henao MP, Dere ME, Lee H, Leder BZ. Effects of hPTH(1-34) infusion on circulating serum phosphate, 1,25-dihydroxyvitamin D, and FGF23 levels in healthy men. J Bone Miner Res. 2009;24(10):1681–1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yuan Q, Sato T, Densmore M, Saito H, Schüler C, Erben RG, Lanske B. FGF-23/Klotho signaling is not essential for the phosphaturic and anabolic functions of PTH. J Bone Miner Res. 2011;26(9):2026–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutiérrez OM, Smith KT, Barchi-Chung A, Patel NM, Isakova T, Wolf M. (1-34) Parathyroid hormone infusion acutely lowers fibroblast growth factor 23 concentrations in adult volunteers. Clin J Am Soc Nephrol. 2012;7(1):139–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lavi-Moshayoff V, Wasserman G, Meir T, Silver J, Naveh-Many T. PTH increases FGF23 gene expression and mediates the high-FGF23 levels of experimental kidney failure: a bone parathyroid feedback loop. Am J Physiol Renal Physiol. 2010;299(4):F882–F889. [DOI] [PubMed] [Google Scholar]
- 19.Fan Y, Bi R, Densmore MJ, Sato T, Kobayashi T, Yuan Q, Zhou X, Erben RG, Lanske B. Parathyroid hormone 1 receptor is essential to induce FGF23 production and maintain systemic mineral ion homeostasis. FASEB J. 2016;30(1):428–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meir T, Durlacher K, Pan Z, Amir G, Richards WG, Silver J, Naveh-Many T. Parathyroid hormone activates the orphan nuclear receptor Nurr1 to induce FGF23 transcription. Kidney Int. 2014;86(6):1106–1115. [DOI] [PubMed] [Google Scholar]
- 21.Bhattacharyya N, Wiench M, Dumitrescu C, Connolly BM, Bugge TH, Patel HV, Gafni RI, Cherman N, Cho M, Hager GL, Collins MT. Mechanism of FGF23 processing in fibrous dysplasia. J Bone Miner Res. 2012;27(5):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okazaki M, Ferrandon S, Vilardaga JP, Bouxsein ML, Potts JT Jr, Gardella TJ. Prolonged signaling at the parathyroid hormone receptor by peptide ligands targeted to a specific receptor conformation. Proc Natl Acad Sci USA. 2008;105(43):16525–16530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nagai S, Okazaki M, Segawa H, Bergwitz C, Dean T, Potts JT Jr, Mahon MJ, Gardella TJ, Jüppner H. Acute down-regulation of sodium-dependent phosphate transporter NPT2a involves predominantly the cAMP/PKA pathway as revealed by signaling-selective parathyroid hormone analogs. J Biol Chem. 2011;286(2):1618–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, Garringer HJ, Vidal R, Chan RJ, Goodwin CB, Hui SL, Peacock M, White KE. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci USA. 2011;108(46):E1146–E1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeda A, Okazaki M, Baron DM, Dean T, Khatri A, Mahon M, Segawa H, Abou-Samra AB, Jüppner H, Bloch KD, Potts JT Jr, Gardella TJ. Critical role of parathyroid hormone (PTH) receptor-1 phosphorylation in regulating acute responses to PTH. Proc Natl Acad Sci USA. 2013;110(15):5864–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao N, Tenenhouse HS. Npt2 gene disruption confers resistance to the inhibitory action of parathyroid hormone on renal sodium-phosphate cotransport. Endocrinology. 2000;141(6):2159–2165. [DOI] [PubMed] [Google Scholar]
- 27.Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T. Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone. 2011;49(4):636–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.David V, Martin A, Isakova T, Spaulding C, Qi L, Ramirez V, Zumbrennen-Bullough KB, Sun CC, Lin HY, Babitt JL, Wolf M. Inflammation and functional iron deficiency regulate fibroblast growth factor 23 production. Kidney Int. 2016;89(1):135–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solberg LB, Stang E, Brorson SH, Andersson G, Reinholt FP. Tartrate-resistant acid phosphatase (TRAP) co-localizes with receptor activator of NF-KB ligand (RANKL) and osteoprotegerin (OPG) in lysosomal-associated membrane protein 1 (LAMP1)-positive vesicles in rat osteoblasts and osteocytes. Histochem Cell Biol. 2015;143(2):195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burnett SM, Gunawardene SC, Bringhurst FR, Jüppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res. 2006;21(8):1187–1196. [DOI] [PubMed] [Google Scholar]