Abstract
X-linked adrenoleukodystrophy (X-ALD) is a rare, genetic disorder characterized by adrenal insufficiency and central nervous system (CNS) demyelination. All patients with X-ALD have the biochemical abnormality of elevated blood and tissue levels of very long chain fatty acids (VLCFAs), saturated fatty acids with 24 to 26 carbons. X-ALD results from loss of function mutations in the gene encoding the peroxisomal transporter ABCD1, which is responsible for uptake of VLCFAs into peroxisomes for degradation by oxidation. One proposed therapeutic strategy for genetic complementation of ABCD1 is pharmacologic upregulation of ABCD2, a gene encoding a homologous peroxisomal transporter. Here, we show that thyroid hormone or sobetirome, a clinical-stage selective thyroid hormone receptor agonist, increases cerebral Abcd2 and lowers VLCFAs in blood, peripheral organs, and brains of mice with defective Abcd1. These results support an approach to treating X-ALD that involves a thyromimetic agent that reactivates VLCFA disposal both in the periphery and the CNS.
Thyroid hormone and the thyromimetic sobetirome lower CNS levels of very long chain fatty acids in a mouse model of X-linked adrenoleukodystrophy supporting a therapeutic strategy for X-ALD.
X-linked adrenoleukodystrophy (X-ALD) is a genetic disorder affecting 1 in 17,000 people worldwide that causes adrenal gland dysfunction and loss of the protective myelin sheaths that envelop nerve fibers in the central nervous system (CNS). In affected men, there are two main clinical presentations of X-ALD, adrenomyeloneuropathy (AMN) and childhood cerebral adrenoleukodystrophy (CCALD), which occur in 45% and 35% of X-ALD cases, respectively. AMN is an adult-onset disease characterized by spinal cord axonopathy and gradual progression over decades. In contrast, CCALD is a rapid and often fatal disease with inflammatory white matter cerebral demyelination that occurs most frequently in preadolescent boys (1–3). Patients with X-ALD have mutations in ABCD1, which encodes a transporter that shuttles C24 and C26 very long chain fatty acids (VLCFAs) into the peroxisome for degradation by β oxidation (4). Because of the genetic defect, VLCFAs are elevated in serum and tissues of patients with X-ALD, leading to the disease pathogenesis (4–6).
Treatment options for symptomatic patients with CCALD are limited. Lorenzo’s oil (LO) is a dietary supplement that inhibits VLCFA synthesis, thus lowering blood levels of elevated VLCFAs in patients with X-ALD (7, 8). In the largest LO clinical study reported to date, chronic treatment in 89 asymptomatic boys lowered the C26:0 VLCFA levels by ~50% from the mean pretreatment value and may have had a beneficial effect in preventing the onset of CCALD (9). However, LO did not halt the progression of CCALD or ameliorate the neurologic deficits of AMN, perhaps because of its inability to achieve therapeutic exposure levels in the CNS (7–9). A second therapeutic approach for lowering VLCFAs in X-ALD involves ABCD2, a peroxisomal transporter with 66% protein sequence identity to the mutated ABCD1 (10) and overlapping substrate specificity (11). Upregulation of ABCD2 by a pharmacologic agent complements the defect in ABCD1 by increasing ABCD2-mediated transport of VLCFAs into the peroxisome for degradation, and several studies have provided support for this strategy. Upregulation of ABCD2 in fibroblasts from patients with X-ALD lowered cellular VLCFAs (12, 13), and transgenic overexpression of Abcd2 in Abcd1(-/y) mice decreased serum and tissue VLCFAs (14).
Prior work discovered a thyroid hormone response element in the promoter region of ABCD2 and verified that this gene was thyroid hormone regulated (15, 16). It was further shown that selective thyroid hormone agonists including sobetirome can upregulate ABCD2 in fibroblasts of patients with X-ALD (17). However, the ability of these agents to affect VLCFA levels in a mammalian model was not explored. Thyromimetics, exemplified by sobetirome, offer a therapeutic index, whereas endogenous thyroid hormones, thyroxine (T4) and triiodothyronine (T3), in pharmacologic excess demonstrate no separation of beneficial from thyrotoxic actions (18). Sobetirome is a potent thyroid hormone receptor (TR) agonist with selectivity for the TRβ isoform and differential tissue distribution from thyroid hormones (19). Both of these factors likely contribute to the selective actions of sobetirome, which have led to its clinical development for cholesterol lowering (19). Sobetirome is unique among clinical-stage thyromimetics for its ability to cross the blood–brain barrier, which makes it a compelling candidate for a disease with CNS involvement such as X-ALD (20, 21). In this study, we demonstrate that thyroid hormone and sobetirome can act on both peripheral and CNS tissues to lower VLCFA levels in a murine model of X-ALD. Here we report an agent that can lower CNS VLCFAs in a sustained manner, and this result combined with the favorable clinical profile of sobetirome support the investigation of sobetirome as a clinical therapy for X-ALD.
Materials and Methods
Reagents
All drugs were prepared at concentrations suitable for an intraperitoneal (IP) injection of 150 μL per 30-g mouse. Sobetirome was synthesized in the Scanlan Laboratory as previously described (22, 23). Sobetirome drug stocks were prepared by dissolving sobetirome at 0.5 mg/mL or 5 mg/mL in dimethyl sulfoxide (D2650; Sigma-Aldrich). The 0.5 and 5 mg/mL sobetirome stocks were diluted in saline to obtain final solutions of 0.1 and 1 mg/mL in 20% dimethyl sulfoxide (DMSO) (corresponding to 0.1 and 1 mg/kg body weight dose). T3 (T2877; Sigma-Aldrich) drug stocks were prepared at 5.0 mg/mL in 40 mM sodium hydroxide (NaOH) in saline and diluted into saline to obtain final solutions of 1.0 mg/mL T3 (corresponding to 1.0 mg/kg body weight dose). Vehicles containing 20% DMSO in saline or 8 mM NaOH in saline were also prepared. In general, vehicle groups were split evenly between NaOH vehicle and DMSO vehicle. Fatty acid standards (C22:0, C24:0, and C26:0) were obtained from Sigma-Aldrich (216941, L6641, and H0388), and internal standards (d4C22:0, d4C24:0, and d4C26:0) were obtained from C/D/N Isotopes (D-6138, D-6167, and D-6145). C26:0-lysophosphatidylcholine (C26-LPC) (855810P) and d4C26:0-LPC (860389P) were obtained from Avanti Polar Lipids.
Mouse experiments
All experiments were performed with institutionally approved protocols (Institutional Animal Care and Use Committee, Animal Welfare Assurance no. A33040-01) and follow all guidelines recommended by the National Institutes of Health’s Guide for the Care of Use of Laboratory Animals. The Abcd1tm1Kan-targeted mutation mouse strain in C57BL/6 background was obtained through Jackson Laboratory Cryo Recovery (003716) (24). All mice were housed at Oregon Health & Science University (Portland, Oregon) with 12-hour light/12-hour dark cycles. Only male mice hemizygous for the Abcd1 knockout gene [Abcd1(-/y)] were used in the experiments. The experiments were terminated early if the mice experienced >20% body weight loss. Mice were randomly allocated into treatment groups. Investigators were not blinded to animal treatments, but were blinded during mass spectrometry sample preparation and analysis.
For the quantitative polymerase chain reaction (qPCR) experiments, C57BL/6J male mice (Jackson Laboratory, aged 6 to 8 weeks) were treated for 7 days with daily IP injections of vehicle, T3 (1.0 mg/kg body weight) or sobetirome (1.0 mg/kg body weight). Hypothyroid mice received 0.1% (w/v) methimazole and 0.2% (w/v) potassium perchlorate in drinking water for 2 weeks (25). For the last 7 days of treatment, hypothyroid mice also received vehicle IP injections. Mice were euthanized 4 to 6 hours after the last IP injection, and tissues were preserved in RNAlater (ThermoFisher) for RNA transcript analysis. In the brain region experiments, the hippocampus, corpus callosum, and cerebellum were microdissected following published protocols (26).
For the short-term treatment experiments, a cohort of adult male Abcd1(-/y) mice (aged 8 to 16 weeks) were treated for 7 or 28 days with daily IP injections of vehicle, T3 (1.0 mg/kg body weight) or sobetirome (0.1 or 1.0 mg/kg body weight). In an additional cohort of mice, hypothyroidism was induced for 8 weeks using the drinking water treatment previously described. The mice were euthanized 4 to 8 hours after the last IP injection on the final day of treatment, and brain and adrenal glands were collected and stored at −80°C. Serum was isolated by centrifugation of harvested blood at 5000 × g for 15 minutes and stored at −20°C.
For the chronic treatment experiments, Teklad Global 2016 diet (Envigo Research) was compounded with 1 mg/3 mg T3/T4 per kilogram of chow (nominal daily dose 0.2 mg/0.6 mg/ kg body weight), and 0.4 or 2 mg sobetirome per kilogram of chow (nominal daily doses 0.08 or 0.4 mg per kg body weight). Two distinct experimental groups were formed: a juvenile cohort, in which treatment began at weaning (aged 21 days), and an adult cohort (aged 13 to 21 weeks at the start of treatment). Mice were randomly assigned into control, T3/T4, or one of two sobetirome doses and treated for 12 weeks. In the 2 mg/kg sobetirome treatment group, mice exhibited extreme weight loss (up to 20%) and were euthanized early after 11 weeks of treatment. Serum, brain, adrenal glands, and testes were collected from these mice and stored at −80°C.
Fibroblast cell culture
Fibroblast cell lines were obtained from the Coriell Institute for Medical Research from a 6-year-old male patient with CCALD (GM04496), a 22-year-old male patient with AMN (GM07675), and a 30-year-old female heterozygous ALD carrier (GM04903). Fibroblast cells were cultured according to the recommended protocols from the Coriell Institute for Medical Research. Cells were cultured for 3 days in the presence of vehicle (DMSO, 0.03% final), 100 nM T3, or 100 nM sobetirome. Media including drug was changed daily. Cells (~5 × 105 per sample) were harvested for qPCR analysis.
qPCR
RNA was purified from RNAlater-preserved tissue using either RNeasy Mini kits (Qiagen, for cells, liver and microdissected brain tissue) or PureLink RNA Mini kit with TRIzol extraction (Life Technologies, for whole brain). RNA was quantified using a Nanodrop (Thermo Scientific), and complementary DNA was prepared with the QuantiTect Reverse Transcription kit (Qiagen). The qPCR was performed on an Applied Biosciences 7500 Real Time PCR system following the QuantiTect SYBR Green PCR kit protocols (Qiagen). For mouse samples, Gapdh or 36b4 was used as a reference gene (f-Gapdh: 5′-CCGCATCTTCTTGTGCAGTG-3′; r-Gapdh 5′-GAGAAGGCAGCCCTGGTAAC-3′; f-36b4: 5′-ACCCTGAAGTGCTCGACATC-3′; r-36b4: 5′-CCATTGATGATGGAGTGTGG-3′). Transcript levels of Abcd2 were measured using the following primers (f-Abcd2: 5′-GCATCGACGTTGAAGGAAAG-3′; r-Abcd2: 5′-GAAGGCCTGTGTGTTATGGAG-3′). For human fibroblast samples, 36B4 was used as a reference gene (f-36B4: 5′-CTCCTTTGGGCTGGTCATCC-3′; r-36B4 5′-CAGACAGACACTGGCAACATTG-3′) (17). Transcript levels of ABCD2 were measured using the following primers (f-ABCD2: 5′-CTTTGCAGATGGTGAGGATGG-3′; r-ABCD2: 5′-CCAGAGGCCAGTAAATTTCG-3′). Data were analyzed using the 2-ΔΔCT relative comparison method (27).
C26:0-LPC extraction
Serum samples (10 μL) were diluted 1:4 in deionized water and vortexed after the addition of 1 ng of d4C26-LPC internal standard in methanol. The samples were extracted in methanol (450 μL) with vortexing. After incubation [10 minutes at room temperature (RT)], debris was pelleted, and supernatant was analyzed via liquid chromatography-tandem mass spectrometry (LC-MS/MS) (28–30). Whole brain was homogenized at 200 mg tissue/mL in water. Internal standard (20 ng of d4C26-LPC) was added to 1 mL of diluted brain homogenate (4 mg/mL) and thoroughly vortexed. The samples were extracted in 2 mL of 1:1 butanol/0.5 M hydrochloric acid with vortexing and incubation (15 minutes, RT). Samples were separated by centrifugation (3000 × g, 10 minutes), and the butanol layer was removed and dried under vacuum. The final dried sample was dissolved in 150 µL of 10% dimethylformamide in methanol and analyzed by LC-MS/MS.
LC-MS/MS analysis of C26-LPC
An Applied Biosystems 5500 QTRAP hybrid/triple quadrupole linear ion trap mass spectrometer was used to detect C26:0-LPC samples in positive mode with electrospray ionization using multiple reaction monitoring. Chromatographic separation was achieved over an 8-minute analysis period using a Thermo Fisher Scientific BetaBasic C8 or C18 column (20 × 2.1 mm, 5-μm particle size). The solvent system comprised mobile phase A (0.028% ammonium hydroxide in water) and mobile phase B (0.028% ammonium hydroxide in isopropanol). The working gradient began with 40% mobile phase B start increasing to 98% mobile phase B over 5 minutes, was held until 6 minutes, and then returned to 30% organic by 6.1 minutes and held until the end of the analysis. The injection volume was 10 µL, and the solvent flow rate was 0.5 mL/min. The column temperature was held at 40°C throughout the experiment. The instrument parameters for the multiple reaction monitoring transitions in positive mode were optimized by direct infusion for the following transitions: C26-LPC, m/z 636 → 184 and m/z 636 → 104; d4C26-LPC, m/z 640 → 184, and m/z 640 → 104. Peaks were analyzed with Analyst 1.6.2 (Sciex) software. A linear standard curve was prepared by spiking standard and internal standard into the appropriate matrix from wild-type mice. For serum, the standard curve ranged from 2.5 to 500 ng/mL, and for brain, the standard curve ranged from 50 to 2500 ng/mL. Brain levels were normalized by total protein content of the homogenate as determined by the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) or by total C22.
Total VLCFA extraction and derivatization
Sample preparation was performed following published protocols (31). Brain was homogenized at 200 mg tissue/mL water and testes at 100 mg tissue/mL water. Adrenal glands (two per mouse) were homogenized in 1 mL of water per adrenal gland. In brief, 25 μL of homogenized brain, 400 μL of homogenized adrenal glands, or 200 μL of homogenized testes were extracted in the presence of internal standards (d4C22:0, d4C24:0, and d4C26:0) in 2 mL of 2:3 isopropanol/hexane for 1 hour at RT with shaking. For brain, 1.00 μg of d4C22:0, 2.00 μg of d4C24:0, and 0.16 μg of d4C26:0 were added to each sample. For testes, 0.25 μg of d4C22:0, 0.50 μg of d4C24:0, and 0.05 μg of d4C26:0 were added to each sample. For adrenal glands, 0.05 μg of d4C22:0, 0.15 μg of d4C24:0, and 0.05 μg of d4C26:0 were added to each sample. The extracted samples underwent acid hydrolysis, followed by base hydrolysis each for 45 minutes at 100°C. After reacidification, samples were extracted with hexanes and dried under vacuum. Dried samples underwent derivatization with pentafluorobenzyl bromide in the presence of triethylamine for 1 hour at RT, and were further extracted in hexanes and dried under vacuum. The final dried sample was dissolved in 50 µL of hexanes and analyzed by gas chromatography–mass spectrometry (GC-MS).
GC-MS analysis of total VLCFAs
Sample analysis was performed using an Agilent-7890B/5977A GC-MS operating in negative chemical ionization mode with methane as the reagent gas. Peaks were obtained over a 20-minute analysis period for the pentafluorobenzyl bromide esterified fatty acids using an Agilent HP-5ms column (30 m × 0.25 mm; film 0.25 µm), with helium as the carrier gas. The working temperatures of the source and transfer line were 250°C and 325°C, respectively. The split/splitless injector was held at 275°C and was operated with a 1:25 split. The sample injection volume was 1 µL. The initial oven temperature was 150°C, with a ramp rate of 15°C/min, and a final temperature of 325°C, held for 7 minutes. Acquisition was performed in the selected ion-monitoring mode, with a dwell time of 25 ms. The ion m/z values for the endogenous standards were 339.3, 367.4, and 395.4 for C22:0, C24:0, and C26:0, respectively, whereas the deuterated internal standards had m/z values of 343.4, 371.4, and 399.4 for d4C22:0, d4C24:0, and d4C26:0, respectively. Peaks were analyzed with Masshunter (Agilent) software. Standard calibration curves were generated based on the peak area ratios of each fatty acid matched to the deuterated internal standard. For brains, the standard curves ranged from 10 to 250 μg/mL for C22:0, 20 to 500 μg/mL for C24:0, and 1.2 to 30 μg/mL for C26:0. For adrenal glands, the standard curves ranged from 0.01 to 0.31 μg/mL for C22:0 and C26:0, and 0.04 to 0.94 μg/mL for C24:0. For testes, the standard curves ranged from 0.05 to 1.25 μg/mL for C22:0, 0.13 to 3.13 μg/mL for C24:0, and 0.03 to 0.63 μg/mL for C26:0. All values were reported as C26/C22 ratios to normalize for differences in tissue preparation.
Statistical methods
Statistics were performed using a two-tailed Student t test, and statistical significance was determined as P ≤ 0.05 as compared with vehicle treated– or control chow–fed mice. The exact P values are reported in Supplemental Tables 1–5 (201.6KB, pdf) . The data were also analyzed by one-way analysis of variance with the Dunnett posttest to determine significance, and this analysis is reported in Supplemental Tables 1–5 (201.6KB, pdf) . For the short-term 7-day and 28-day experiments, the control mice were grouped together for statistical analysis because of the high variability in C26-LPC levels in the control cohorts. The means of the two control groups were identical (106.5 ± 12.5 for 7 day and 104.5 ± 16.3 for 28 day). The number of mice in each experimental group (N) is indicated both in the figure legends and Supplemental Tables 1–5 (201.6KB, pdf) . The sample sizes were determined based on other similar published studies and our preliminary data, and all samples obtained were included in the analysis.
Results
Abcd2 upregulation by sobetirome in vivo
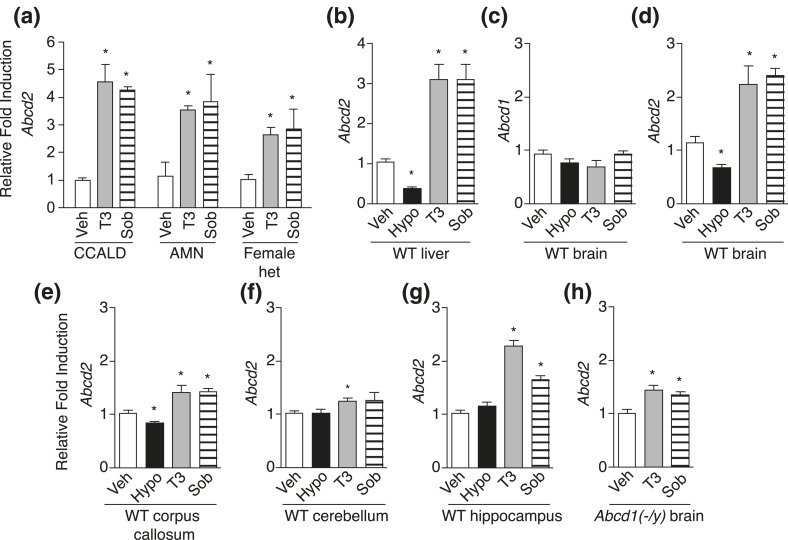
At the outset of our studies, we confirmed previous findings showing that T3 and the thyromimetic sobetirome upregulated ABCD2 in fibroblasts isolated from patients with X-ALD [Fig. 1(a)] (17). We further examined Abcd2 regulation in vivo by treating wild-type and Abcd1(-/y) mice (a knockout mouse model of X-ALD) with T3, sobetirome, or vehicle once-daily for 7 days via IP injection. In addition, hypothyroidism was induced with drinking water containing methimazole (0.1%) and potassium perchlorate (0.2%) for 14 days (25). Tissue messenger RNA levels of Abcd1 and Abcd2 were measured by qPCR. As expected, liver Abcd2 was decreased by hypothyroidism and increased with hyperthyroidism or sobetirome treatment [Fig. 1(b)]. In the brain, Abcd1 was unaffected by thyroid status [Fig. 1(c)]; however, Abcd2 was downregulated by hypothyroidism and upregulated by T3 or sobetirome treatment in the whole brain [Fig. 1(d)] and in the corpus callosum, cerebellum, and hippocampus [Fig. 1(e–g)]. Similarly, brain Abcd2 was upregulated when Abcd1(-/y) mice were treated systemically with T3 or sobetirome for a similar duration [Fig. 1(h)].
Figure 1.
Relative transcript levels of Abcd2 increased in X-ALD fibroblasts and in mice liver and brain after administration of T3 or sobetirome. (a) ABCD2 transcript levels in human fibroblasts were measured after treatment with vehicle (Veh), 100 nM T3, or 100 nM sobetirome (Sob) for 3 days. The fibroblasts were derived from patients with X-ALD [CCALD, AMN, and, female patient heterozygous for X-linked ABCD1 (Female het)]. qPCR was performed with technical duplicates. (b–g) C57BL/6J [wild-type (WT)] mice were treated with once-daily IP injections of vehicle (Veh), T3 (1 mg/kg), or sobetirome (Sob, 1 mg/kg), or hypothyroidism-inducing drinking water for 2 weeks (Hypo). Animal numbers are indicated and qPCR was performed with technical duplicates. (b) Abcd2 in liver: (n = 5 for all groups). (c) Abcd1 in whole brain homogenate (n = 5 for all groups). (d) Abcd2 in whole brain homogenate: Veh (n = 5), Hypo (n = 5), T3 (n = 4), or Sob (n = 3). (e) Abcd2 in corpus callosum (n = 5 for all groups). (f) Abcd2 in cerebellum: Veh (n = 5), T3 (n = 4), Sob (n = 5), and Hypo (n = 5). (g) Abcd2 in hippocampus: Veh (n = 5), T3 (n = 5), Sob (n = 5), and Hypo (n = 4). (h) Abcd1(-/y) mice were treated with once-daily IP injections of vehicle, T3 (1 mg/kg), or Sob (1 mg/kg) for 7 days (n = 6 for all groups). Whole brain from Abcd1(-/y) mice was analyzed for Abcd2 transcript levels. All data represent mean ± standard error of mean. Statistical analyses were performed with a two-tailed t test with comparisons with vehicle group; *P ≤ 0.05.
Thyroid status modulation of VLCFAs
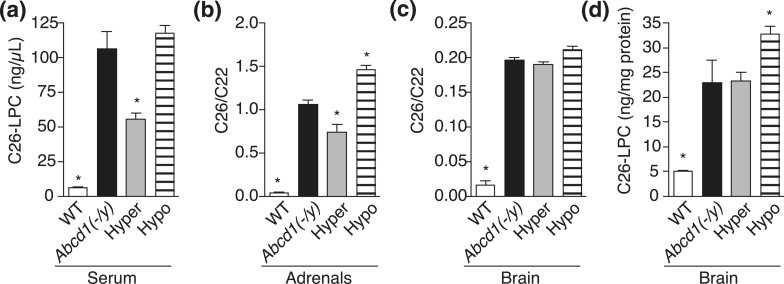
The effects of thyroid status on VLCFA levels were evaluated in Abcd1(-/y) mice. Like patients with X-ALD, Abcd1 knockout mice have elevated circulating and tissue VLCFA levels (24, 32, 33). Hyperthyroidism was induced for 7 days, and hypothyroidism was induced for 8 weeks using the methods described previously. Serum, adrenal glands, and brain were harvested after treatment and quantitatively analyzed for total VLCFA levels and C26-LPC, a validated VLCFA plasma biomarker of X-ALD used for diagnosis and newborn screening (30). The data demonstrated that VLCFAs in serum and adrenal glands were dependent on thyroid status in a manner consistent with T3-positive regulation of Abcd2 transcription and rescue of peroxisomal VLCFA disposal [Fig. 2(a–c); Supplemental Tables 1 and 2 (201.6KB, pdf) ]. In serum, C26-LPC was reduced in hyperthyroid mice and was elevated with hypothyroidism. Adrenal glands showed the same trend with C26/C22 levels decreasing in hyperthyroidism and increasing in hypothyroidism. In brain tissue, the most relevant for CCALD, 7 days of hyperthyroidism did not lower VLCFA levels significantly, but 8 weeks of exposure to agents that induce hypothyroidism was sufficient to elevate C26-LPC levels, although not overall C26/C22 levels [Fig. 2(c) and 2(d); Supplemental Tables 3 and 4 (201.6KB, pdf) ]. C26-LPC represents 5% to 10% of the total C26 population.
Figure 2.
VLCFA levels are dependent on thyroid status in Abcd1(-/y) mice. Abcd1(-/y) mice were treated for 7 days with once-daily IP injections of vehicle [Abcd1(-/y)] or T3 (Hyper, 1 mg/kg), or received 8 weeks of hypothyroidism-inducing drinking water (Hypo). VLCFA levels for C57BL/6J mice [wild-type (WT)] were also determined. (a) C26-LPC in serum: WT (n = 4), Abcd1(-/y) (n = 6), Hyper (n = 6), and Hypo (n = 12). (b) C26/C22 in adrenal glands: WT (n = 6), Abcd1(-/y) (n = 6), Hyper (n = 5), and Hypo (n = 11). (c and d) C26/C22 and C26-LPC in brain: WT (n = 4), Abcd1(-/y) (n = 6), Hyper (n = 6), and Hypo (n = 13). Data represent mean ± standard error of mean. Statistical analyses were performed with a two-tailed t test with comparisons with Abcd1(-/y) mice; *P ≤ 0.05.
Sobetirome modulation of VLCFAs
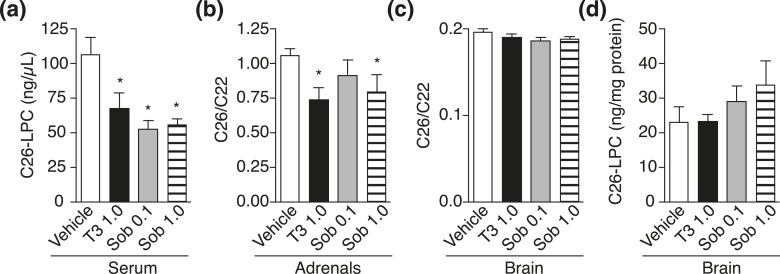
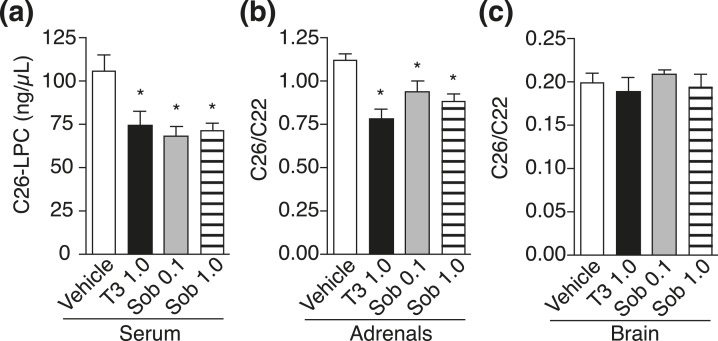
To exploit thyroid hormone action as a clinical therapy to induce VLCFA lowering, use of a selective thyromimetic, such as sobetirome, is essential because unlike T4, sobetirome has a therapeutic index separating beneficial from adverse effects (20). Thus, we next evaluated sobetirome in Abcd1(-/y) mice with daily IP injections of vehicle, T3, or sobetirome (0.1 and 1.0 mg/kg) for 1 week. In serum and adrenal glands, sobetirome lowered C26 levels to a similar extent as T3, indicating that sobetirome mimics T3 action to induce VLCFA lowering, presumably via upregulation of Abcd2 [Fig. 3(a) and 3(b); Supplemental Tables 1 and 2 (201.6KB, pdf) ]. In brain tissue, however, no VLCFA lowering was observed with T3 or sobetirome after 7 days of treatment [Fig. 3(c) and 3(d); Supplemental Tables 3 and 4 (201.6KB, pdf) ]. Abcd1(-/y) mice were also treated with once-daily IP injections for 28 days at the same doses of T3 and sobetirome. Serum and adrenal glands showed reductions in VLCFAs to a similar extent as those observed from the 7-day treatment, but brain VLCFA levels remained unchanged compared with vehicle control (Fig. 4; Supplemental Tables 1–4 (201.6KB, pdf) ).
Figure 3.
Short-term sobetirome treatment lowers VLCFA levels in serum and adrenal glands of Abcd1(-/y) mice. Abcd1(-/y) mice were treated with once-daily IP injections of vehicle, T3 (1.0 mg/kg), or sobetirome (Sob, 0.1 or 1.0 mg/kg) for 7 days. (a) C26-LPC in serum: (n = 6 for all groups). (b) C26/C22 in adrenal glands: vehicle (n = 6), T3 (n = 5), Sob 0.1 (n = 5), and Sob 1.0 (n = 3). (c and d) C26/C22 and C26-LPC in brain: (n = 6 for all groups). Data represent mean ± standard error of mean. Statistical analyses were performed with a two-tailed t test with comparisons with vehicle mice; *P ≤ 0.05.
Figure 4.
Sobetirome treatment for 28 days lowers VLCFA levels in serum and adrenal glands of Abcd1(-/y) mice. Abcd1(-/y) mice were treated for 28 days with once-daily IP injections of vehicle, T3 (1.0 mg/kg), or sobetirome (Sob, 0.1 or 1.0 mg/kg). (a) C26-LPC in serum: vehicle (n = 11), T3 (n = 6), Sob 0.1 (n = 4), and Sob 1.0 (n = 4). (b) C26/C22 in adrenal glands: vehicle (n = 5), T3 (n = 6), Sob 0.1 (n = 5), and Sob 1.0 (n = 3). (c) C26/C22 in brain: vehicle (n = 5), T3 (n = 6), Sob 0.1 (n = 5), and Sob 1.0 (n = 4). Data represent mean ± standard error of mean. Statistical analyses were performed with a two-tailed t test with comparisons with vehicle group; *P ≤ 0.05.
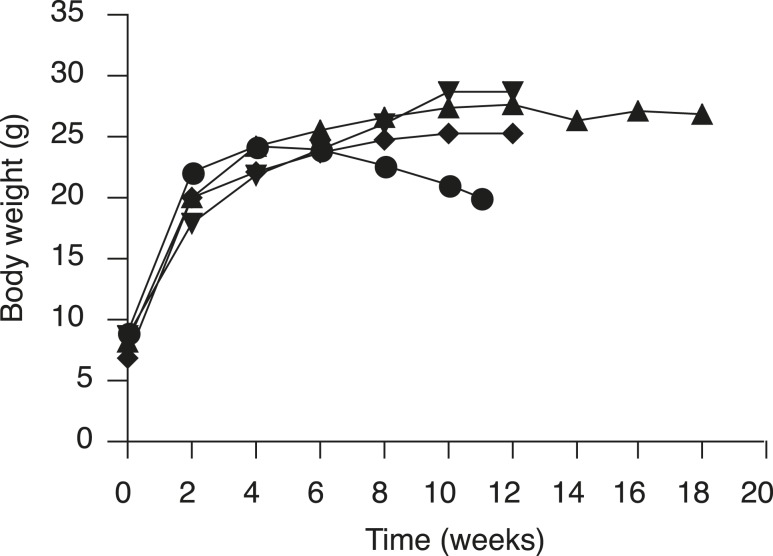
The lack of VLCFA lowering in the brain after 7- and 28-day treatment with T3 or sobetirome was surprising given that both agents upregulate Abcd2 in mouse brain after only 7 days of dosing [Fig. 1(c–h)]. However, the measured turnover rates of murine myelin lipid components that form esters with VLCFAs are quite long (t1/2 = 30 to 360 days depending on lipid) (34). Thus, we hypothesized that the slow kinetics of VLCFA turnover in the mouse brain were responsible for the lack of VLCFA lowering in treatment durations of ≤28 days. Therefore, we initiated a chronic treatment paradigm, in which T3/T4 or sobetirome was compounded into chow for long-term dosing. At 3 weeks of age, mice were weaned and provided ad lib access to T3/T4 or sobetirome chow for 12 weeks (35). Sobetirome is known to cause dose-dependent weight loss in mice (36), and weight loss after 6 to 8 weeks of treatment was observed with the higher 2 mg/kg chow dose of sobetirome (0.4 mg/kg nominal dose) (Fig. 5). Treatment was terminated for the high-dose cohort when weight loss reached 20%, which occurred at 11 weeks for most of these mice. No weight loss was observed at the lower 0.4 mg/kg chow dose of sobetirome (0.08 mg/kg nominal dose), which allowed for 12 weeks of dosing, and a small cohort of animals was treated for an additional 6 weeks (18 weeks total) with no observed weight loss over this period (Fig. 5).
Figure 5.
A higher dose of orally administered sobetirome is associated with weight loss in mice. The mean body weights from juvenile Abcd1(-/y) mice treated for 11 to 18 weeks with control chow (reverse triangles, n = 6), 0.4 mg/kg sobetirome chow (triangles, n = 8, and for 14 to 18 weeks, n = 3), 2.0 mg/kg sobetirome chow (circles, n = 8), and T3/T4 chow (diamonds, n = 5).
To confirm that mice fed ad libitum with chow containing sobetirome results in systemic sobetirome exposure comparable with once-daily IP dosing, the concentration of sobetirome in both serum and brain tissues was measured at the terminus of the 28-day IP treatment and the 12-week chow treatment (Supplemental Table 6 (201.6KB, pdf) ). Comparable blood and brain levels of sobetirome are observed with 0.1 mg/kg daily IP and 0.4 mg/kg in chow (0.08 mg/kg nominal daily dose) and with 1 mg/kg daily IP and 2 mg/kg in chow (0.4 mg/kg nominal daily dose). In addition, the levels of sobetirome in serum and brain are dose dependent for both experiments, and the sobetirome brain/serum concentration ratios (0.02 to 0.09) are similar to those measured in previous studies (20, 37, 38).
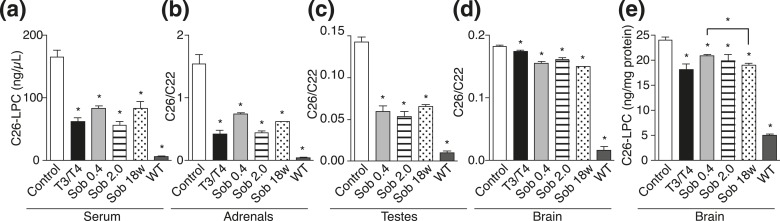
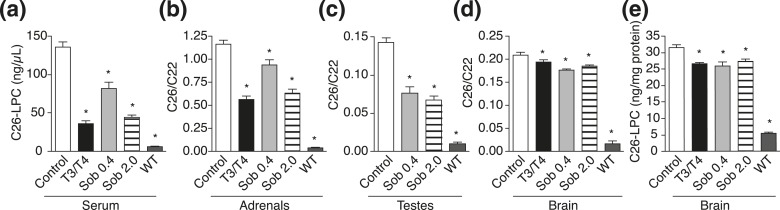
After chronic chow-based treatment, lowering of C26-LPC and total C26/C22 was observed in all treatment groups in serum, adrenal glands, testes, and brain (Fig. 6; Supplemental Tables 1–5 (201.6KB, pdf) ). The VLCFAs in the brain were lowered in the T3/T4 group (24%) and with both doses of sobetirome (0.4 mg/kg chow, 13%; 2 mg/kg chow, 17%) as compared with control. Moreover, further substantial lowering of C26-LPC was observed in brain at 18 weeks (21%) compared with 12 weeks with the lower sobetirome dose (13%) [Fig. 6(e); Supplemental Table 4 (201.6KB, pdf) ], supporting the hypothesis that peroxisomal disposal of cerebral VLCFAs is dependent on the slow kinetics of cerebral lipid turnover. An additional cohort of adult mice (13 to 21 weeks old) was treated for 12 weeks with T3/T4 chow or sobetirome chow (0.4 and 2 mg/kg chow), and the drug-induced VLCFA lowering in brain, blood, and peripheral organs was similar in the adult mice as compared with the juvenile mice (Fig. 7; Supplemental Tables 1–5 (201.6KB, pdf) ).
Figure 6.
Chronic sobetirome treatment lowers VLCFA levels in juvenile Abcd1(-/y) mice. At weaning Abcd1(-/y) mice were treated for 11 to 12 weeks with control chow, T3/T4 chow, or sobetirome chow (Sob 0.4 or 2.0 mg/kg chow). A small cohort (n = 2) was treated for 18 weeks with Sob 0.4 chow (Sob 18w). (a) C26-LPC in serum: control (n = 7), T3/T4 (n = 4), Sob 0.4 (n = 3), Sob 2.0 (n = 5), and C57BL/6J mice on control chow [wild-type (WT), n = 4]. (b) C26/C22 in adrenal glands: control (n = 9), T3/T4 (n = 5), Sob 0.4 (n = 5), Sob 2.0 (n = 5), and WT (n = 6). (c) C26/C22 in testes: control (n = 5), Sob 0.4 (n = 5), Sob 2.0 (n = 4), and WT (n = 4). (d and e) C26/C22 and C26-LPC in brain: control (n = 9), T3/T4 (n = 5), Sob 0.4 (n = 5), Sob 2.0 (n = 5), and WT (n = 4). Data represent mean ± standard error of mean. Statistical analyses were performed with a two-tailed t test with comparisons with Abcd1(-/y) mice receiving control chow; *P ≤ 0.05.
Figure 7.
Chronic oral dosing with T3 or sobetirome lowers VLCFA levels in adult Abcd1(-/y) mice. Adult Abcd1(-/y) mice were treated for 11 to 12 weeks with control chow, T3/T4 chow, or sobetirome chow (Sob 0.4 or 2.0 mg/kg chow). (a) C26-LPC in serum: control (n = 4), T3/T4 (n = 6), Sob 0.4 (n = 5), Sob 2.0 (n = 7), and C57BL/6J mice treated with control chow [wild-type (WT), n = 4]. (b) C26/C22 in adrenal glands: control (n = 4), T3/T4 (n = 6), Sob 0.4 (n = 5), Sob 2.0 (n = 7), and WT, (n = 6). (c) C26/C22 in testes: control (n = 5), Sob 0.4 (n = 5), Sob 2.0 (n = 7), and WT (n = 4). The control testes group plotted here is from the juvenile cohort because testes were not collected from the control chow group in the adult cohort. (d and e) C26-LPC and C26/C22 in brain: control (n = 4), T3/T4 (n = 6), Sob 0.4 (n = 5), Sob 2.0 (n = 7), and WT (n = 4). Data represent mean ± standard error of mean. Statistical analyses were performed with a two-tailed t test with comparisons with Abcd1(-/y) mice receiving control chow; *P ≤ 0.05.
Discussion
Because clinical studies revealed that LO had no clinical benefit in treating patients with X-ALD presenting with neurologic involvement, there has been great interest in discovering effective therapeutic agents for this devastating disease (10). If cerebral involvement is detected in patients at risk for CCALD before the onset of neurological symptoms, hematopoietic stem cell transplant (HSCT) is performed. HSCT is effective in halting the progression of inflammatory demyelination, but this occurs after a delay of up to a year or more following the procedure, and during this time irreversible neurological deficits can accumulate (39). Although HSCT does result in lower plasma VLCFAs, the postprocedure levels decrease to levels similar to those of heterozygous carriers of ABCD1 deficiency and higher than unaffected individuals or patients with X-ALD taking LO (40). This suggests that in addition to halting progression of CCALD, correcting the ABCD1 mutation in myeloid lineage cells corrects a portion—but not all—of the X-ALD biochemical abnormality assessed by measuring plasma VLCFA levels. Moreover, HSCT is only successful in presymptomatic CCALD and is not effective in symptomatic CCALD, adult-onset cerebral ALD, or AMN. For patients with cerebral ALD and symptomatic CCALD, no treatments are available that halt the neurological disease progression that typically ends in a vegetative state or death within a few years of the onset of symptoms. Thus, there is substantial interest in developing effective treatments for X-ALD (41).
It is generally accepted that increased levels of VLCFAs lead to adrenal gland dysfunction, cerebral demyelination, and other tissue pathogenesis that manifests in X-ALD (1, 6, 42). The exact mechanisms of VLCFA toxicity are unknown, but VLCFAs are speculated to act through multiple mechanisms, including increased cellular membrane instability and elevated oxidative stress (5, 43). Support for the pathogenicity of increased VLCFAs in X-ALD comes from several in vitro studies showing that VLCFAs in culture caused mitochondrial dysfunction, oxidative stress, and cell death (41, 44). Direct injection of C24-LPC into the corpus callosum of mouse brains caused both microglial activation and apoptosis (45). Further, in the patient population with X-ALD, a correlation is observed between VLCFA levels and disease severity in female heterozygous patients, who have one defective ABCD1 allele and one correct copy. Female patients have intermediate levels of VLCFA elevation, and 50% to 60% of patients develop mild AMN symptoms in the fifth and sixth decade of life, which is a later onset than male patients with AMN who develop symptoms in the second and third decade (46, 47). Thus, although the mechanisms of how elevated levels of VLCFAs cause disease in CCALD and AMN remain unclear, there is substantial support for the toxicity of accumulating VLCFAs.
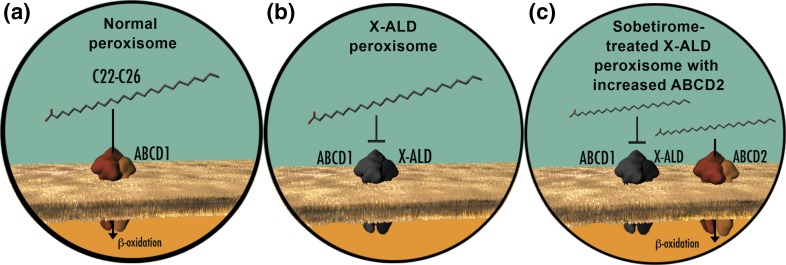
A strategy for lowering VLCFAs based on targeted upregulation of ABCD2 has been validated mechanistically in both cellular and mouse models (Fig. 8) (10, 12, 14). Several different classes of drugs have been investigated for the ability to induce ABCD2 and lower VLCFAs, including peroxisome proliferator–activated receptor α agonists (48–50), histone deacetylase inhibitors (6, 51–53), liver X receptor antagonists (54, 55), retinoid X receptor agonists (56), and most recently, an AMP-activated protein kinase α1 activator (57). Of these, only the histone deacetylase inhibitor 4–phenylbutyrate has been shown to lower brain VLCFAs in Abcd1(-/y) mice, albeit at very high doses (~2800 mg/kg body weight/d) over 4 to 6 weeks of treatment (6). However, follow-up studies indicated that tachyphylaxis occurred on repeat, long-term dosing that diminished histone deacetylase inhibitor 4-phenylbutyrate–induced VLCFA lowering (58). In contrast, we found that brain levels of total C26 or C26-LPC in Abcd1(-/y) mice were lowered by ~15% to 20% only after oral dosing for 12 weeks with T3/T4 or sobetirome (~0.08 and 0.4 mg/kg body weight/d) and that longer dosing periods >12 weeks resulted in additional VLCFA lowering. Therefore, the results with thyromimetics reported here show sustained lowering of VLCFAs in brain tissue after prolonged treatment. The lipid-lowering activity of thyromimetics such as sobetirome has been previously established with lowering of elevated cholesterol through increased hepatic clearance; however, VLCFA degradation occurs exclusively in peroxisomes in all tissues of the body, indicating that the observed sobetirome-induced VLCFA lowering in serum and tissues occurs via a mechanism involving peroxisome reactivation in Abcd1(-/y) mice (Fig. 8).
Figure 8.
Sobetirome increases ABCD2, a peroxisomal transporter, which leads to VLCFA lowering in cells lacking ABCD1. (a) In healthy cells, ABCD1 is a peroxisomal transporter that is responsible for uptake of VLCFAs into the peroxisome (yellow) for degradation via β oxidation. (b) In cells from patients with X-ALD, ABCD1 (black) is not functional, which prevents VLCFAs from being transported from the cytoplasm (blue) to the peroxisome (yellow). VLCFAs accumulate leading to disease pathology. (c) ABCD2 is a close homolog that has overlapping substrate specificity with ABCD1. Sobetirome-induced overexpression of ABCD2 can compensate for the defect in ABCD1 by transporting VLCFAs into the peroxisome for degradation by β oxidation. Figure by O'Reilly Science Art, LLC.
The finding that thyromimetics can induce lowering of cerebral VLCFAs after 12 weeks of dosing raises two important questions: First, why is chronic treatment required to observe an effect in brain and not peripheral tissue? Second, will lowering brain VLCFAs by ~20%, which is the extent of lowering shown in this study, be sufficient for therapeutic benefit? To address the first question, most lipids in the brain reside in myelin, and myelin lipid turnover is slow with half-lives between 1 and 12 months depending on the lipid head group (34). This suggests that the chronic exposure required for reduction in brain VLCFAs may be caused by the slower kinetics of VLCFA transport to peroxisomes for disposal in the CNS. If this hypothesis is correct, then the slow kinetics of brain VLCFA lowering is independent of thyromimetic action and should be observed with other agents that lower VLCFAs by peroxisomal reactivation. Indeed, sobetirome and T3/T4 show essentially the same levels of brain VLCFA lowering after 12 weeks of treatment, suggesting that the longer time required for VLCFA lowering in the brain compared with peripheral organs and plasma results from the biology of VLCFA turnover in the brain and not thyromimetic pharmacology.
Regarding the second question, the limitations of the X-ALD mouse model prevent a direct assessment of how much VLCFA lowering is required to prevent or treat cerebral deficits. Abcd1(-/y) mice do not show any cerebral demyelination phenotype (24, 32, 33). Abcd1(-/y) mice aged to 15 to 20 months develop a condition that has been described as AMN-like with spinal cord axonopathy and mild motor deficits (59), but there is no mouse model of the cerebral inflammatory demyelinating phenotype recapitulating the clinical symptoms of CCALD. Thus, the question of how much VLCFA lowering in the brain is required for therapeutic benefit in CCALD can only be addressed with human clinical data. However, one line of evidence suggests that the moderate decreases observed in this study may be sufficient to have a beneficial effect on arresting disease progression. In patients with CCALD treated with HSCT, which can arrest progression of the neurological symptoms, the only myeloid lineage cell type that can be replaced in the brain is the microglia/macrophage population (39). In a study with two patients with CCALD that underwent HSCT with lentiviral-mediated gene therapy, only 15% of the peripheral CD14+ monocytes had the corrected ABCD1 gene, but this was sufficient to arrest cerebral demyelination and disease progression (60). This suggests that even a partial correction of the genetic defect with a presumably moderate reduction in total brain VLCFAs may be sufficient to halt accumulation of neurological deficits.
In addition, we show that treatment with sobetirome at a nominal dose of ~0.08 mg/kg body weight/d for an extended period of 18 weeks results in additional decreases in C26-LPC compared with the 12-week treatment values (Fig. 6; Supplemental Table 4 (201.6KB, pdf) ). Thus, daily oral treatment with sobetirome over time leads to continued and greater lowering of VLCFAs in affected CNS and peripheral tissues, suggesting that chronic sobetirome therapy over months and years may lower levels beyond the 20% reductions reported here.
Our data indicate that thyromimetic treatment rapidly induces transcription of Abcd2, the compensating VLCFA transporter in the peroxisome, which is accompanied by the expected rapid reduction in elevated VLCFAs in the periphery (Fig. 8). In the brain, daily thyromimetic treatment for 12 weeks is required to observe a reduction in VLCFAs, and we predict further brain VLCFA decreases will occur with longer treatment regimes. This agent has the sustained ability to induce CNS Abcd2 transcription and lower cerebral VLCFAs, and demonstrates that the use of a blood–brain barrier permeable thyromimetic such as sobetirome may represent a promising therapeutic strategy for treatment of X-ALD.
Acknowledgments
We thank the Bioanalytical Shared Resource/Pharmacokinetics Core Facility, which is part of the University Shared Resource Program at Oregon Health & Science University, for analytical support. We also thank Lisa Bleyle for her assistance with both GC-MS and LC-MS/MS analysis.
Acknowledgments
The research was supported by the National Institutes of Health (DK52798) and the OHSU Laura Fund for Innovation in Multiple Sclerosis. M.D.H. received postdoctoral support from the National Institutes of Health (2T32DK007680-21) and the National Multiple Sclerosis Society (FG 2023A 1/2).
Disclosure Summary: T.S.S. and M.D.H. are inventors on a pending patent application for the use of sobetirome in X-linked adrenoleukodystrophy. T.S.S. is a founder and director of NeuroVia, Inc. The remaining authors have nothing to disclose.
Footnotes
- AMN
- adrenomyeloneuropathy
- C26-LPC
- C26:0-lysophosphatidylcholine
- CCALD
- childhood cerebral adrenoleukodystrophy
- CNS
- central nervous system
- DMSO
- dimethyl sulfoxide
- GC-MS
- gas chromatography–mass spectrometry
- HSCT
- hematopoietic stem cell transplant
- IP
- intraperitoneal(ly)
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- LO
- Lorenzo’s oil
- NaOH
- sodium hydroxide
- qPCR
- quantitative polymerase chain reaction
- RT
- room temperature
- T3
- triiodothyronine
- T4
- thyroxine
- TR
- thyroid hormone receptor
- VLCFA
- very long chain fatty acid
- X-ALD
- X-linked adrenoleukodystrophy.
References
- 1.Ferrer I, Aubourg P, Pujol A. General aspects and neuropathology of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):817–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moser HW, Mahmood A, Raymond GV. X-linked adrenoleukodystrophy. Nat Clin Pract Neurol. 2007;3(3):140–151. [DOI] [PubMed] [Google Scholar]
- 3.Kemp S, Huffnagel IC, Linthorst GE, Wanders RJ, Engelen M. Adrenoleukodystrophy - neuroendocrine pathogenesis and redefinition of natural history. Nat Rev Endocrinol. 2016;12(10):606–615. [DOI] [PubMed] [Google Scholar]
- 4.Kemp S, Wanders R. Biochemical aspects of X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singh I, Pujol A. Pathomechanisms underlying X-adrenoleukodystrophy: a three-hit hypothesis. Brain Pathol. 2010;20(4):838–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemp S, Wei HM, Lu JF, Braiterman LT, McGuinness MC, Moser AB, Watkins PA, Smith KD. Gene redundancy and pharmacological gene therapy: implications for X-linked adrenoleukodystrophy. Nat Med. 1998;4(11):1261–1268. [DOI] [PubMed] [Google Scholar]
- 7.Moser HW, Moser AB, Hollandsworth K, Brereton NH, Raymond GV. “Lorenzo’s oil” therapy for X-linked adrenoleukodystrophy: rationale and current assessment of efficacy. J Mol Neurosci. 2007;33(1):105–113. [DOI] [PubMed] [Google Scholar]
- 8.Aubourg P, Adamsbaum C, Lavallard-Rousseau MC, Rocchiccioli F, Cartier N, Jambaqué I, Jakobezak C, Lemaitre A, Boureau F, Wolf C, Bougneres Pierre-Francois. A two-year trial of oleic and erucic acids (“Lorenzo’s oil”) as treatment for adrenomyeloneuropathy. N Engl J Med. 1993;329(11):745–752. [DOI] [PubMed] [Google Scholar]
- 9.Moser HW, Raymond GV, Lu SE, Muenz LR, Moser AB, Xu J, Jones RO, Loes DJ, Melhem ER, Dubey P, Bezman L, Brereton NH, Odone A. Follow-up of 89 asymptomatic patients with adrenoleukodystrophy treated with Lorenzo’s oil. Arch Neurol. 2005;62(7):1073–1080. [DOI] [PubMed] [Google Scholar]
- 10.Berger J, Pujol A, Aubourg P, Forss-Petter S. Current and future pharmacological treatment strategies in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):845–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Genin EC, Geillon F, Gondcaille C, Athias A, Gambert P, Trompier D, Savary S. Substrate specificity overlap and interaction between adrenoleukodystrophy protein (ALDP/ABCD1) and adrenoleukodystrophy-related protein (ALDRP/ABCD2). J Biol Chem. 2011;286(10):8075–8084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flavigny E, Sanhaj A, Aubourg P, Cartier N. Retroviral-mediated adrenoleukodystrophy-related gene transfer corrects very long chain fatty acid metabolism in adrenoleukodystrophy fibroblasts: implications for therapy. FEBS Lett. 1999;448(2-3):261–264. [DOI] [PubMed] [Google Scholar]
- 13.Netik A, Forss-Petter S, Holzinger A, Molzer B, Unterrainer G, Berger J. Adrenoleukodystrophy-related protein can compensate functionally for adrenoleukodystrophy protein deficiency (X-ALD): implications for therapy. Hum Mol Genet. 1999;8(5):907–913. [DOI] [PubMed] [Google Scholar]
- 14.Pujol A, Ferrer I, Camps C, Metzger E, Hindelang C, Callizot N, Ruiz M, Pàmpols T, Giròs M, Mandel JL. Functional overlap between ABCD1 (ALD) and ABCD2 (ALDR) transporters: a therapeutic target for X-adrenoleukodystrophy. Hum Mol Genet. 2004;13(23):2997–3006. [DOI] [PubMed] [Google Scholar]
- 15.Weinhofer I, Kunze M, Rampler H, Forss-Petter S, Samarut J, Plateroti M, Berger J. Distinct modulatory roles for thyroid hormone receptors TRalpha and TRbeta in SREBP1-activated ABCD2 expression. Eur J Cell Biol. 2008;87(12):933–945. [DOI] [PubMed] [Google Scholar]
- 16.Fourcade S, Savary S, Gondcaille C, Berger J, Netik A, Cadepond F, El Etr M, Molzer B, Bugaut M. Thyroid hormone induction of the adrenoleukodystrophy-related gene (ABCD2). Mol Pharmacol. 2003;63(6):1296–1303. [DOI] [PubMed] [Google Scholar]
- 17.Genin EC, Gondcaille C, Trompier D, Savary S. Induction of the adrenoleukodystrophy-related gene (ABCD2) by thyromimetics. J Steroid Biochem Mol Biol. 2009;116(1-2):37–43. [DOI] [PubMed] [Google Scholar]
- 18.Baxter JD, Webb P. Thyroid hormone mimetics: potential applications in atherosclerosis, obesity and type 2 diabetes. Nat Rev Drug Discov. 2009;8(4):308–320. [DOI] [PubMed] [Google Scholar]
- 19.Scanlan TS. Sobetirome: a case history of bench-to-clinic drug discovery and development. Heart Fail Rev. 2010;15(2):177–182. [DOI] [PubMed] [Google Scholar]
- 20.Trost SU, Swanson E, Gloss B, Wang-Iverson DB, Zhang H, Volodarsky T, Grover GJ, Baxter JD, Chiellini G, Scanlan TS, Dillmann WH. The thyroid hormone receptor-beta-selective agonist GC-1 differentially affects plasma lipids and cardiac activity. Endocrinology. 2000;141(9):3057–3064. [DOI] [PubMed] [Google Scholar]
- 21.Manzano J, Morte B, Scanlan TS, Bernal J. Differential effects of triiodothyronine and the thyroid hormone receptor beta-specific agonist GC-1 on thyroid hormone target genes in the b ain. Endocrinology. 2003;144(12):5480–5487. [DOI] [PubMed] [Google Scholar]
- 22.Placzek AT, Scanlan TS. New synthetic routes to thyroid hormone analogs: d6-sobetirome, 3H-sobetirome, and the antagonist NH-3. Tetrahedron. 2015;71(35):5946–5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiellini G, Apriletti JW, Yoshihara HA, Baxter JD, Ribeiro RC, Scanlan TS. A high-affinity subtype-selective agonist ligand for the thyroid hormone receptor. Chem Biol. 1998;5(6):299–306. [DOI] [PubMed] [Google Scholar]
- 24.Forss-Petter S, Werner H, Berger J, Lassmann H, Molzer B, Schwab MH, Bernheimer H, Zimmermann F, Nave KA. Targeted inactivation of the X-linked adrenoleukodystrophy gene in mice. J Neurosci Res. 1997;50(5):829–843. [DOI] [PubMed] [Google Scholar]
- 25.Hackenmueller SA, Marchini M, Saba A, Zucchi R, Scanlan TS. Biosynthesis of 3-iodothyronamine (T1AM) is dependent on the sodium-iodide symporter and thyroperoxidase but does not involve extrathyroidal metabolism of T4. Endocrinology. 2012;153(11):5659–5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spijker S. Dissection of Rodent Brain Regions. Neuroproteomics. 2011;57:13–26. [Google Scholar]
- 27.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hubbard WC, Moser AB, Liu AC, Jones RO, Steinberg SJ, Lorey F, Panny SR, Vogt RF Jr, Macaya D, Turgeon CT, Tortorelli S, Raymond GV. Newborn screening for X-linked adrenoleukodystrophy (X-ALD): validation of a combined liquid chromatography-tandem mass spectrometric (LC-MS/MS) method. Mol Genet Metab. 2009;97(3):212–220. [DOI] [PubMed] [Google Scholar]
- 29.Hubbard WC, Moser AB, Tortorelli S, Liu A, Jones D, Moser H. Combined liquid chromatography-tandem mass spectrometry as an analytical method for high throughput screening for X-linked adrenoleukodystrophy and other peroxisomal disorders: preliminary findings. Mol Genet Metab. 2006;89(1-2):185–187. [DOI] [PubMed] [Google Scholar]
- 30.Sandlers Y, Moser AB, Hubbard WC, Kratz LE, Jones RO, Raymond GV. Combined extraction of acyl carnitines and 26:0 lysophosphatidylcholine from dried blood spots: prospective newborn screening for X-linked adrenoleukodystrophy. Mol Genet Metab. 2012;105(3):416–420. [DOI] [PubMed] [Google Scholar]
- 31.Lagerstedt SA, Hinrichs DR, Batt SM, Magera MJ, Rinaldo P, McConnell JP. Quantitative determination of plasma c8-c26 total fatty acids for the biochemical diagnosis of nutritional and metabolic disorders. Mol Genet Metab. 2001;73(1):38–45. [DOI] [PubMed] [Google Scholar]
- 32.Lu JF, Lawler AM, Watkins PA, Powers JM, Moser AB, Moser HW, Smith KD. A mouse model for X-linked adrenoleukodystrophy. Proc Natl Acad Sci USA. 1997;94(17):9366–9371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi T, Shinnoh N, Kondo A, Yamada T. Adrenoleukodystrophy protein-deficient mice represent abnormality of very long chain fatty acid metabolism. Biochem Biophys Res Commun. 1997;232(3):631–636. [DOI] [PubMed] [Google Scholar]
- 34.Ando S, Tanaka Y, Toyoda Y, Kon K. Turnover of myelin lipids in aging brain. Neurochem Res. 2003;28(1):5–13. [DOI] [PubMed] [Google Scholar]
- 35.Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, Refetoff S, Sharlin DS, Simonides WS, Weiss RE, Williams GR; American Thyroid Association Task Force on Approaches and Strategies to Investigate Thyroid Hormone Economy and Action . American Thyroid Association Guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24(1):88–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin JZ, Martagón AJ, Cimini SL, Gonzalez DD, Tinkey DW, Biter A, Baxter JD, Webb P, Gustafsson JA, Hartig SM, Phillips KJ. Pharmacological activation of thyroid hormone receptors elicits a functional conversion of white to brown fat. Cell Reports. 2015;13(8):1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Placzek AT, Ferrara SJ, Hartley MD, Sanford-Crane HS, Meinig JM, Scanlan TS. Sobetirome prodrug esters with enhanced blood-brain barrier permeability. Bioorg Med Chem. 2016;24(22):5842–5854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Takahashi N, Asano Y, Maeda K, Watanabe N. In vivo evaluation of 1-benzyl-4-aminoindole-based thyroid hormone receptor β agonists: importance of liver selectivity in drug discovery. Biol Pharm Bull. 2014;37(7):1103–1108. [DOI] [PubMed] [Google Scholar]
- 39.Cartier N, Aubourg P. Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 2010;20(4):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shapiro E, Krivit W, Lockman L, Jambaqué I, Peters C, Cowan M, Harris R, Blanche S, Bordigoni P, Loes D, Ziegler R, Crittenden M, Ris D, Berg B, Cox C, Moser H, Fischer A, Aubourg P. Long-term effect of bone-marrow transplantation for childhood-onset cerebral X-linked adrenoleukodystrophy. Lancet. 2000;356(9231):713–718. [DOI] [PubMed] [Google Scholar]
- 41.Hein S, Schönfeld P, Kahlert S, Reiser G. Toxic effects of X-linked adrenoleukodystrophy-associated, very long chain fatty acids on glial cells and neurons from rat hippocampus in culture. Hum Mol Genet. 2008;17(12):1750–1761. [DOI] [PubMed] [Google Scholar]
- 42.Schönfeld P, Reiser G. Brain lipotoxicity of phytanic acid and very long-chain fatty acids. Harmful cellular/mitochondrial activities in refsum disease and X-linked Adrenoleukodystrophy. Aging Dis. 2016;7(2):136–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho JK, Moser H, Kishimoto Y, Hamilton JA. Interactions of a very long chain fatty acid with model membranes and serum albumin. Implications for the pathogenesis of adrenoleukodystrophy. J Clin Invest. 1995;96(3):1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fourcade S, López-Erauskin J, Galino J, Duval C, Naudi A, Jove M, Kemp S, Villarroya F, Ferrer I, Pamplona R, Portero-Otin M, Pujol A. Early oxidative damage underlying neurodegeneration in X-adrenoleukodystrophy. Hum Mol Genet. 2008;17(12):1762–1773. [DOI] [PubMed] [Google Scholar]
- 45.Eichler FS, Ren JQ, Cossoy M, Rietsch AM, Nagpal S, Moser AB, Frosch MP, Ransohoff RM. Is microglial apoptosis an early pathogenic change in cerebral X-linked adrenoleukodystrophy? Ann Neurol. 2008;63(6):729–742. [DOI] [PubMed] [Google Scholar]
- 46.Moser AB, Kreiter N, Bezman L, Lu S, Raymond GV, Naidu S, Moser HW. Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol. 1999;45(1):100–110. [DOI] [PubMed] [Google Scholar]
- 47.Engelen M, Kemp S, de Visser M, van Geel BM, Wanders RJ, Aubourg P, Poll-The BT. X-linked adrenoleukodystrophy (X-ALD): clinical presentation and guidelines for diagnosis, follow-up and management. Orphanet J Rare Dis. 2012;7:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Engelen M, Tran L, Ofman R, Brennecke J, Moser AB, Dijkstra IM, Wanders RJ, Poll-The BT, Kemp S. Bezafibrate for X-linked adrenoleukodystrophy. PLoS One. 2012;7(7):e41013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Engelen M, Schackmann MJ, Ofman R, Sanders RJ, Dijkstra IM, Houten SM, Fourcade S, Pujol A, Poll-The BT, Wanders RJ, Kemp S. Bezafibrate lowers very long-chain fatty acids in X-linked adrenoleukodystrophy fibroblasts by inhibiting fatty acid elongation. J Inherit Metab Dis. 2012;35(6):1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rampler H, Weinhofer I, Netik A, Forss-Petter S, Brown PJ, Oplinger JA, Bugaut M, Berger J. Evaluation of the therapeutic potential of PPARalpha agonists for X-linked adrenoleukodystrophy. Mol Genet Metab. 2003;80(4):398–407. [DOI] [PubMed] [Google Scholar]
- 51.Fourcade S, Ruiz M, Guilera C, Hahnen E, Brichta L, Naudi A, Portero-Otín M, Dacremont G, Cartier N, Wanders R, Kemp S, Mandel JL, Wirth B, Pamplona R, Aubourg P, Pujol A. Valproic acid induces antioxidant effects in X-linked adrenoleukodystrophy. Hum Mol Genet. 2010;19(10):2005–2014. [DOI] [PubMed] [Google Scholar]
- 52.Gondcaille C, Depreter M, Fourcade S, Lecca MR, Leclercq S, Martin PG, Pineau T, Cadepond F, ElEtr M, Bertrand N, Beley A, Duclos S, De Craemer D, Roels F, Savary S, Bugaut M. Phenylbutyrate up-regulates the adrenoleukodystrophy-related gene as a nonclassical peroxisome proliferator. J Cell Biol. 2005;169(1):93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh J, Khan M, Singh I. Caffeic acid phenethyl ester induces adrenoleukodystrophy (Abcd2) gene in human X-ALD fibroblasts and inhibits the proinflammatory response in Abcd1/2 silenced mouse primary astrocytes. Biochim Biophys Acta. 2013;1831:747–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gondcaille C, Genin EC, Lopez TE, Dias AM, Geillon F, Andreoletti P, Cherkaoui-Malki M, Nury T, Lizard G, Weinhofer I, Berger J, Kase ET, Trompier D, Savary S. LXR antagonists induce ABCD2 expression. Biochim Biophys Acta. 2014;1841:259–266. [DOI] [PubMed] [Google Scholar]
- 55.Weinhofer I, Kunze M, Rampler H, Bookout AL, Forss-Petter S, Berger J. Liver X receptor alpha interferes with SREBP1c-mediated Abcd2 expression. Novel cross-talk in gene regulation. J Biol Chem. 2005;280(50):41243–41251. [DOI] [PubMed] [Google Scholar]
- 56.Weber FD, Weinhofer I, Einwich A, Forss-Petter S, Muneer Z, Maier H, Weber WH, Berger J. Evaluation of retinoids for induction of the redundant gene ABCD2 as an alternative treatment option in X-linked adrenoleukodystrophy. PLoS One. 2014;9(7):e103742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh J, Olle B, Suhail H, Felicella MM, Giri S. Metformin-induced mitochondrial function and ABCD2 up-regulation in X-linked adrenoleukodystrophy involves AMP-activated protein kinase. J Neurochem. 2016;138(1):86–100. [DOI] [PubMed] [Google Scholar]
- 58.McGuinness MC, Zhang HP, Smith KD. Evaluation of pharmacological induction of fatty acid beta-oxidation in X-linked adrenoleukodystrophy. Mol Genet Metab. 2001;74(1-2):256–263. [DOI] [PubMed] [Google Scholar]
- 59.Pujol A, Hindelang C, Callizot N, Bartsch U, Schachner M, Mandel JL. Late onset neurological phenotype of the X-ALD gene inactivation in mice: a mouse model for adrenomyeloneuropathy. Hum Mol Genet. 2002;11(5):499–505. [DOI] [PubMed] [Google Scholar]
- 60.Cartier N, Hacein-Bey-Abina S, Bartholomae CC, Veres G, Schmidt M, Kutschera I, Vidaud M, Abel U, Dal-Cortivo L, Caccavelli L, Mahlaoui N, Kiermer V, Mittelstaedt D, Bellesme C, Lahlou N, Lefrère F, Blanche S, Audit M, Payen E, Leboulch P, l’Homme B, Bougnères P, Von Kalle C, Fischer A, Cavazzana-Calvo M, Aubourg P. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science. 2009;326(5954):818–823. [DOI] [PubMed] [Google Scholar]