Abstract
We previously demonstrated that Fst expression is highest in brown adipose tissue (BAT) and skeletal muscle, but is also present at substantial levels in epididymal and subcutaneous white adipose tissues (WATs). Fst promotes mouse brown preadipocyte differentiation and promotes browning during differentiation of mouse embryonic fibroblasts. Fst-transgenic (Fst-Tg) mice show substantial increases in circulating Fst levels and increased brown adipose mass. BAT of Fst-Tg mice had increased expression of brown adipose-associated markers including uncoupling protein 1 (UCP1), PRDM16, PGC-1α, and Glut4. WATs from Fst-Tg mice show upregulation of brown/beige adipose markers and significantly increased levels of phosphorylated p38 MAPK/ERK1/2 proteins compared with the wild-type (WT) mice. Pharmacological inhibition of pp38 MAPK/pERK1/2 pathway of recombinant mouse Fst (rFst) treated differentiating 3T3-L1 cells led to significant blockade of Fst-induced UCP1 protein expression. On the other hand, BAT from Fst-Tg mice or differentiating mouse BAT cells treated with rFst show dramatic increase in Myf5 protein levels as well as upregulation of Zic1 and Lhx8 gene expression. Myf5 levels were significantly downregulated in Fst knock-out embryos and small inhibitory RNA–mediated inhibition of Myf5 led to significant inhibition of UCP1, Lhx8, and Zic1 gene expression and significant blockade of Fst-induced induction of UCP1 protein expression in mouse BAT cells. Both interscapular BAT and WAT tissues from Fst-Tg mice display enhanced response to CL316,243 treatment and decreased expression of pSmad3 compared with the WT mice. Therefore, our results indicate that Fst promotes brown adipocyte characteristics in both WAT and BAT depots in vivo through distinct mechanisms.
Follistatin promotes brown adipocyte characteristics by targeting distinct pathways in white and brown adipose tissues.
Obesity is a global health concern and represents a major risk factor for development of type 2 diabetes, dyslipidemia, cardiovascular disease, and other related metabolic conditions (1, 2). According to latest global estimates, 2.16 billion individuals will be overweight and 1.12 billion individuals will be obese by the year 2030 (3). Obesity develops from perturbations in overall cellular bioenergetics when energy uptake chronically exceeds total energy expenditure (4). Understanding the cellular processes that contribute to the expansion of adipose tissues is critical for developing therapeutic strategies to combat obesity and related metabolic conditions. Only limited success has been achieved through lifestyle modification, suggesting a pressing need for novel therapeutic approaches.
White adipose tissue (WAT) and brown adipose tissue (BAT) represent two types of adipose tissue present in mammals (5, 6). WAT stores energy in the form of triglycerides in periods of excess energy intake and mobilizes these stores to provide fatty acids to other tissues during energy demand. BAT metabolizes stored energy to generate heat and represents a key site for energy dissipation, triglyceride clearance, and glucose disposal (7–9). White and brown adipocytes are derived from distinct precursor cell lineages. BATs, like skeletal muscle cells, are derived from Myf5+ precursor cells, whereas white adipocytes and the beige or “brown-like” cells that may be present in WAT are derived primarily from non-Myf5 (Myf5–) precursors (10, 11), although recent data support the notion that subsets of white adipocytes may also originate from both Myf5+ lineages (12). Beige cells are biochemically and morphologically similar to BAT and express common markers such as uncoupling protein (UCP)1 and have a multilocular phenotype. However, beige adipocytes express some markers not present in brown or white adipocytes, such as CD137 (5, 13–15). On the other hand, brown adipocytes express markers that are specifically expressed in classical BAT including Eva1, Lhx8, and Zic1 (5, 13, 14), although a stringent validation with mouse adipose tissues only identified Zic1 as a clear brown fat marker (15).
The list of transcriptional regulators, pharmacological, and nutritional agents that induce browning of white adipocytes is growing rapidly (16, 17). Genetic or pharmacological manipulations to induce more brown or beige fat are associated with significant antiobesity and antidiabetic actions (13, 18, 19). Many agents are able to induce browning by activation of the cAMP/PKA/p38 MAPK/PGC-1α pathway in WAT at different levels through the activation of β-adrenergic receptors (20–22). More recently, irisin, a secreted myokine shown to increase with exercise, has been shown to stimulate browning through phosphorylation of p38 MAPK and ERK1/2 (23). Interestingly, overexpression of COX-2, a downstream effector of β-adrenergic signaling promoted de novo brown adipocyte recruitment in WAT depots, increased systemic energy expenditure, and protected mice against high-fat diet–induced obesity (24). Furthermore, inhibition of TGF-β/Smad signaling has been demonstrated to provide protection from diet-induced obesity and insulin resistance, an effect that was associated with the transition from white to brown adipocytes, induction of mitochondrial biogenesis, and enhanced expression of a BAT-related gene signature (25).
We have previously reported that Fst, an inhibitor of TGF-β signaling in a variety of cell lines (26, 27), promoted adipocyte differentiation and browning to regulate overall energy metabolism (28). This enhanced acquisition of brown adipocyte characteristics was associated with induction of a BAT-related gene and protein expression profile and increased cellular oxygen consumption (28). Using Fst-transgenic (Fst-Tg) mice, we now report the in vivo effect of elevated circulating Fst levels on key beige and brown-specific markers in two WAT depots as well as in interscapular BAT (iBAT), and identify possible molecular targets of Fst action in these adipose tissues.
Materials and Methods
Animal model
Fst-Tg mice that express Fst under the myosin light chain promoter are described previously (29). Male and female Fst heterozygous (Fst+/−) mice were received from Dr. Martin Matzuk (Baylor College of Medicine, Houston, TX). Epididymal (Epi) and subcutaneous (SC) white adipose and iBATs were harvested from 6-week-old Fst-Tg and C57BL/6J wild-type (WT) male mice. All mice were housed at constant temperature [20°C (68°F)] under artificial light/dark cycle (12 hour/12 hour) and allowed free access to food and water. Animal experiments were approved by the Institutional Animal Care and Use and Committee at Charles R. Drew University of Medicine and Science. In some experiments, mice were injected (intraperitoneal) with a single dose of CL316,243 (1 mg/kg body weight) for 6 hours (24) and tissues were harvested for analysis of UCP1 expression.
Mouse embryonic fibroblast culture
Male and female Fst heterozygous (Fst+/−) mice were allowed to breed and embryos from 13.5-days-pregnant female mice were genotyped to identify WT (Fst +/+) and Fst knockout (KO; Fst −/−) embryos for isolation of mouse embryonic fibroblast (MEF) as described before (28). Briefly, embryos were harvested, the head, limbs, and the internal organs were removed, and the carcasses were rinsed in 1× PBS and minced. Minced carcasses were suspended with 3 mL 0.025% trypsin/EDTA (Invitrogen, Carlsbad, CA) and incubated at 37°C for 20 minutes. The trypsin was neutralized, after two trypsinization cycles, by adding an equal volume of cell culture media. Cells were centrifuged, suspended with cell culture media, and plated. Early passage MEFs (P ≤ 3) was used for each experiment to avoid senescence.
Cell culture and treatment
3T3-L1 cells were obtained from American Type Culture Collection (Manassas, VA) and cultured in growth medium containing Dulbecco's modified Eagle medium with 10% fetal bovine serum and 100 U/mL penicillin/streptomycin (Invitrogen). Two days after reaching confluence (day 0), the cells were allowed to differentiate in differentiation medium containing growth medium supplemented with dexamethasone (0.25 μM), isobutylmethylxanthine (0.5 mM), insulin (10 μg/mL), and rosiglitazone (1 μM) for 2 days, followed by culture in maintenance medium supplemented with 10 μg/mL insulin and rosiglitazone (1 μM) for next 6 days to promote browning of white adipocytes as described previously (30). Immortalized mouse brown preadipocyte (BAT) cells were received as a gift from Dr. Bruce Spiegelman (Harvard Medical Center, Boston, MA) (31). Confluent cells seeded on 6-well plates were exposed to an adipogenic cocktail containing 0.5 μM dexamethasone, 20 nM insulin, 0.5 mM isobutylmethylxanthine, 1 nM T3, and 125 μM indomethacin (Sigma Chemicals, St. Louis, MO) in the presence or absence of 0.5 μg/mL recombinant mouse Fst (rFst) protein (R&D Systems, Minneapolis, MN) (28). Forty-eight hours after adipogenic induction, culture medium was replaced with maintenance medium containing 20 nM insulin and 1 nM T3 with or without rFst for an additional 6 days. Cells were harvested and analyzed for adipocyte-specific markers by western blot and real-time quantitative polymerase chain reaction (PCR) analysis.
Real-time quantitative PCR analysis
Total RNA was extracted by using Trizol reagent, and equal amounts (2 µg) of RNA were reverse transcribed using RNA High Capacity complementary DNA kit (Applied Biosystems, Foster City, CA). The Power Sybr Green PCR master mix was used with 7500 fast real-time PCR system (Applied Biosystems). The primer pairs used are shown in Table 1. Samples of 25 ng complementary DNA were analyzed in quadruplicate in parallel with Gapdh controls. The experimental messenger RNA (mRNA) starting quantities were calculated from the standard curves and averaged using 7500 software v1.4 (28, 32).
Table 1.
Primer Sequences Used for Quantitative Real-Time PCR
| Primer | Forward (5′-3′) | Reverse (5′-3′) | GenBank Accession # |
|---|---|---|---|
| Ucp1 | GTGAACCCGACAACTTCCGAA | TGAAACTCCGGCTGAGAAGAT | NM_009463 |
| Prdm16 | CCACCAGCGAGGACTTCAC | GGAGGACTCTCGTAGCTCGAA | NM_001291029 |
| Pgc1a | TATGGAGTGACATAGAGTGTGCT | CTGGGCAAAGAGGCTGGTC | NM_008904 |
| Acsl1 | CTGATTGACATTCGGCAGTACG | CCCCATGAGGGTGTTGGTTG | NM_007981 |
| Fabp3 | ACCAAGCCTACTACCATCATCG | CCTCGTCGAACTCTATTCCCAG | NM_010174 |
| Cidea | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT | NM_007702 |
| Elovl3 | TTCTCACGCGGGTTAAAAATGG | GGCCAACAACGATGAGCAAC | NM_007703 |
| Cox7a1 | GCTCTGGTCCGGTCTTTTAGC | GTACTGGGAGGTCATTGTCGG | NM_009944 |
| Cox8b | TGTGGGGATCTCAGCCATAGT | AGTGGGCTAAGACCCATCCTG | NM_007751 |
| Zic1 | CAGTATCCCGCGATTGGTGT | GCGAACTGGGGTTGAGCTT | NM_009573 |
| Ppara | AACATCGAGTGTCGAATATGTGG | CCGAATAGTTCGCCGAAAGAA | NM_011144 |
| Myf5 | GCCTTCGGAGCACACAAAG | TGACCTTCTTCAGGCGTCTAC | NM_008656 |
| Eva1 | CCACTTCTCCTGAGTTTACAGC | GCATTTTAACCGAACATCTGTCC | AF030454 |
| Lhx8 | ACACGAGCTGCTACATTAAGGA | CCAGTCAGTCGAGTGGATGTG | NM_010713 |
| Glut4 | TGGCCATCTTCTCTGTGGGT | ATTGGCTAGGCCCATGAGG | AB008453 |
| Cd137 | CGTGCAGAACTCCTGTGATAAC | GTCCACCTATGCTGGAGAAGG | DQ832278 |
| Tbx1 | GTCAAGGCTCCGGTGAAGAAG | GCTGATTGAACTCGTCCCACA | NM_011532 |
| Gapdh | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA | NM_008084 |
Western blot analysis
Samples were lysed and protein was quantified using Pierce BCA reagent (Thermo Scientific, Rockford, IL). Equal amounts of proteins were separated by SDS-PAGE and transferred to polyvinyl difluoride membrane, blotted with primary and appropriate HRP-linked secondary antibodies. The primary antibodies dilutions used in this study are provided in Table 2. After the treatment with enhanced chemiluminescence reagent (Pierce; Thermo Scientific), the membranes were exposed to an X-ray film (Kodak; Sigma Aldrich, St. Louis, MO) and Image by Image Quant (GE Lifescience, Pittsburgh, PA) software for densitometric analysis (28, 32).
Table 2.
List of Antibodies
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog Number | Antibody RRID | Species | Dilution Used |
|---|---|---|---|---|---|
| UCP1 | Uncoupling protein1 antibody | Abcam ab10983 | AB_2241462 | Rabbit polyclonal | 1:1000 |
| UCP2 | Uncoupling protein2 antibody | Novus Biologicals NB100-78377 | AB_1085929 | Rabbit polyclonal | 1:1000 |
| UCP3 | Uncoupling protein3 antibody | Abcam ab3477 | AB_2304253 | Rabbit polyclonal | 1:1000 |
| PRDM16 | PRDM16 antibody (N16) | Santa Cruz Biotech sc-130243 | AB_2284344 | Rabbit polyclonal | 1:500 |
| PGC-1α | PGC-1α antibody (H-300) | Santa Cruz Biotech sc-13067 | AB_2166218 | Rabbit polyclonal | 1:1000 |
| Adiponectin | Adiponectin antibody | R & D Systems MAB1119 | AB_2305045 | Rat monoclonal | 1:1000 |
| AMPKα | AMPKα antibody | Cell Signaling 2532 | AB_330331 | Rabbit monoclonal | 1:1000 |
| pAMPKα | pAMPKα antibody | Cell Signaling 2535 | AB_331250 | Rabbit monoclonal | 1:1000 |
| Glut4 | Glucose transporter 4 antibody | Abcam ab65267 | AB_1140009 | Mouse monoclonal | 1:1000 |
| p38MAPK | p38MAPK antibody | Cell Signaling 9212S | AB_330713 | Rabbit polyclonal | 1:1000 |
| pp38MAPK | pp38MAPK antibody | Cell Signaling 9211S | AB_331641 | Rabbit polyclonal | 1:1000 |
| ERK1/2 | ERK1/2 antibody | Cell Signaling 9102S | AB_330744 | Rabbit polyclonal | 1:1000 |
| pERK1/2 | pERK1/2 antibody | Cell Signaling 9101S | AB_331646 | Rabbit polyclonal | 1:1000 |
| Myf5 | Myf5 antibody | Santa Cruz Biotech sc-302 | AB_631994 | Rabbit polyclonal | 1:1000 |
| Cd137 | Cd137 antibody | Abcam ab3169 | AB_303572 | Mouse monoclonal | 1:1000 |
| BMP7 | BMP7 antibody | Abcam ab54904 | AB_940598 | Mouse monoclonal | 1:1000 |
| Eva1 | Eva1 antibody | Novus Biologicals NBP1-88937 | AB_11016118 | Rabbit polyclonal | 1:1000 |
| β-actin | Beta actin antibody | Santa Cruz Biotech sc-81178 | AB_2223230 | Mouse monoclonal | 1:5000 |
| GAPDH | GAPDH antibody | Millipore MAB374 | AB_2107445 | Mouse monoclonal | 1:5000 |
| pSmad2/3 | pSmad2/3 antibody | Cell Signaling 8828 | AB_2631089 | Rabbit monoclonal | 1:1000 |
| AcvRIIB | AcvRIIB antibody | Abcam ab76940 | AB_1565807 | Mouse monoclonal | 1:1000 |
| Smad3 | Smad3 antibody | Cell Signaling 9513 | AB_2286450 | Rabbit polyclonal | 1:1000 |
Immunohistochemical analysis
Embryo sections obtained from WT and Fst-Tg mice were fixed overnight in formalin, embedded with paraffin blocks and sectioned. Tissue sections were stained with either with anti-UCP1, anti-pp38 MAP Kinase, anti-CD137, or anti-Myf5 antibody following standard procedures. Quantitative image analysis was performed by computerized densitometry using the ImagePro Plus 7 program (Media Cybernetics, Silver Spring, MD), coupled to an Leica B microscope equipped with an Spot RT digital camera (Diagnostic Instruments, Portland, OR) (28).
Small inhibitory RNA–mediated knockdown of Myf5 expression
Transient knockdown of Myf5 in BAT cells was done using Trilencer-27 small inhibitory RNA (siRNA) knockdown duplexes (10 nM) against mouse Myf5 (SR407227) and a universal scrambled negative control (SR30004) from OriGene Technologies (Rockville, MD) using Lipofectamine 3000 (Invitrogen). Following transfection, target gene knockdown was quantified by western blot analysis for all three Myf5 siRNA duplexes (siRNA A to siRNA C) along with control (scrambled siRNA) after 3 days following transfection. Two of these siRNAs effectively knocked down (∼75% to 85%) Myf5 expression in BAT cells. Parallel set of control (scrambled siRNA) and siRNA (siRNA B) treated BAT cells were allowed to differentiate for 4 days, harvested, and gene expression for Myf5, Ucp1, LhX8, and Zic1 were analyzed by quantitative real-time PCR. Effect of recombinant Fst protein (0.5 µg/mL) in control and siRNA treated cells on UCP1 protein expression was also analyzed by western blot analysis.
Serum Fst analysis
Serum levels of Fst were analyzed using human follistatin enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN) following manufacturer’s protocol.
Statistical analysis
Data are presented as mean ± standard deviation, and group differences were analyzed by one-way analysis of variance using Graph Pad Prism Version 5.3 (Graph Pad Software, San Diego, CA). If the overall analysis of variance revealed significant differences, then pairwise comparisons between groups were performed by a Newman–Keuls multiple-group test. All comparisons were two-tailed and P values ≤0.05 were considered statistically significant. The experiments were repeated at least three times, and data from representative experiments are shown.
Results
Fst-Tg mice have elevated serum Fst levels and increased iBAT mass
To test the hypothesis that elevated circulating Fst levels may enhance the expression of brown and beige adipocyte functions in vivo, we initially compared serum Fst levels in WT and Fst-Tg mice. Mouse Fst gene in Fst-Tg mice was expressed under the control of a muscle-specific promoter (29). Fst concentrations in serum from 6-week-old Fst-Tg male mice were increased by ∼50% compared with those in WT male mice [294 ± 17 vs 188 ± 21 pg/mL; P ≤ 0.05; Fig. 1(a)]. iBAT mass (as a proportion of body mass) was ∼70% higher in Fst-Tg compared with WT mice [Fig. 1(b)]. Fst-Tg mice had increased protein levels of UCP1 (but not UCP2 and UCP3), PRDM16, PGC-1α, adiponectin, and Glut4 in iBAT tissue compared with WT mice [Fig. 1(c)]. Additionally, there were increased mRNA levels for several genes involved in BAT differentiation (Ucp1, Prdm16, Zic1, Myf5, Lhx8), fatty acid oxidation (Acsl1, Fabp3, Cidea), and mitochondrial biogenesis and function [Pgc1a, Cox7a1, Cox8; Fig. 1(d)]. Thus, increased levels of circulating Fst in Fst-Tg mice are associated with increased iBAT mass and upregulation of brown adipocyte-associated mRNAs and proteins.
Figure 1.
Analysis of serum follistatin levels, BAT weight and expression of BAT-specific markers in iBAT obtained from WT and Fst-Tg mice. (a) Analysis of serum samples from 6-week-old Fst-Tg and WT male mice by enzyme-linked immunosorbent assay (n = 4). Data are shown as mean ± standard deviation. (b) Comparison of iBAT weights from WT and Fst-Tg mice (n = 4). Data are shown as mean ± standard deviation. (c) Western blot analysis of iBAT tissue lysates obtained from 6-week-old WT and Fst-Tg mice. A representative sample is shown. (d) Real-time quantitative PCR analysis of iBAT tissue samples (n = 4). *P ≤ 0.05; **P ≤ 0.01. Data are shown as mean ± standard deviation.
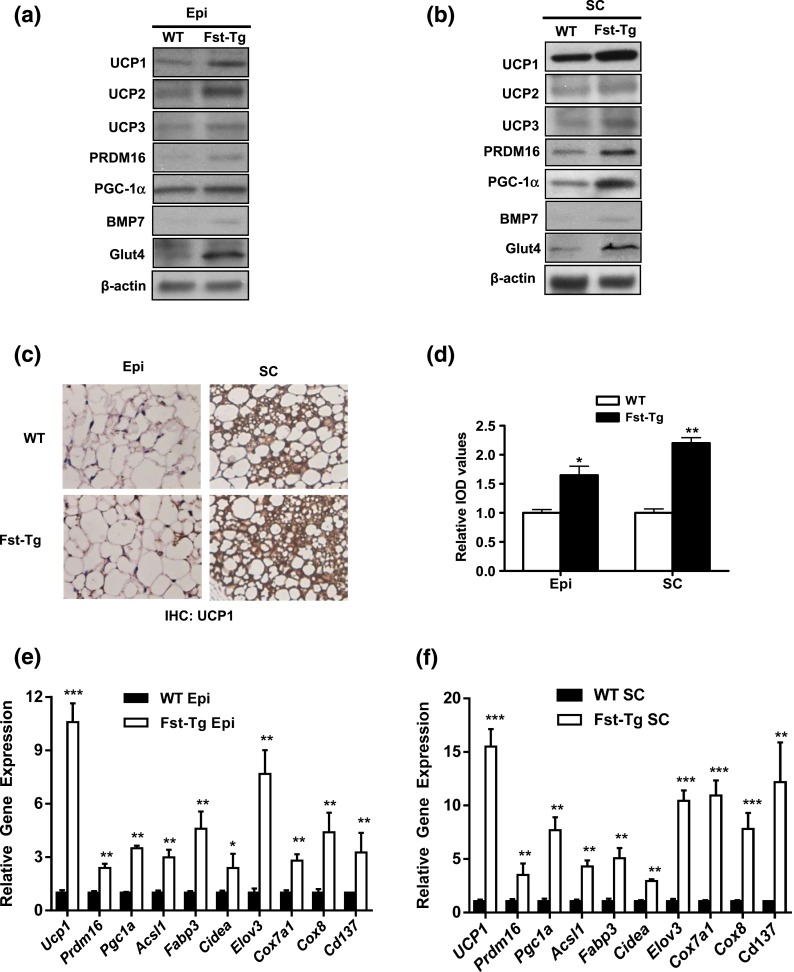
Fst overexpression in Fst-Tg mice promotes browning of Epi and SC white adipose tissues
Based on our previous work showing that Fst enhances browning of cultured white adipocytes (28), we hypothesized that Fst-Tg mice would exhibit enhanced browning of WAT depots. We analyzed both the gonadal Epi and the inguinal SC fat pads in our Fst-Tg and WT mice. In WT mice, the levels of UCP1 and the brown adipocyte transcription factors PRDM16 and BMP7 were low to undetectable in Epi and SC WAT depots [Fig. 2(a) and 2(b)]. The expression levels of brown adipocyte-related proteins were increased in both Epi and SC WAT depots obtained from the Fst-Tg mice compared with the WT mice [Fig. 2(a) and 2(b)]. Immunohistochemical (IHC) analyses using UCP1 antibody showed more multilocular adipocytes in SC compared with Epi fat in both WT and Fst-Tg mice [Fig. 2(c)]. Importantly, UCP1 staining was significantly increased in WAT from Fst-Tg compared with WT mice in both Epi (65% increase) and SC tissue [120% increase; Fig. 2(c) and 2(d)]. Compared with WT mouse tissues, WAT from Fst-Tg mice exhibited elevated expression of key genes involved in thermogenesis, fatty acid oxidation, mitochondrial biogenesis, and Cd137, a beige adipocyte-specific marker [Fig. 2(e)]. Notable among these was the much higher level of Ucp1 expression in Fst-Tg compared with WT WAT, with a 10-fold increase in Epi fat and 15-fold increase in SC fat [Fig. 2(e)]. We found significant induction of Ucp1, Prdm16, Acsl1, Fabp3, Cidea, Elov3, Cox7a1, Cox8, and Cd137 mRNA expression in both Epi and SC adipose tissues in Fst-Tg mice compared with the WT mice [Fig. 2(e) and 2(f)]. Together, our data suggest that Fst overexpression in Fst-Tg mice promotes browning in Epi and SC WATs.
Figure 2.
Analysis of BAT-specific markers in Epi and SC adipose tissues obtained from WT and Fst-Tg mice. A total of 100 µg of tissue lysates were analyzed for expression of various BAT-related genes in (a) Epi and (b) SC adipose tissues by western blot analysis. A representative sample is shown. (c) IHC analysis of paraffin-embedded Epi and SC adipose tissue sections using anti-UCP1 antibody. (d) Quantitative image analysis of UCP1-positive tissue sections using Image-Pro Analysis. Data are presented as mean ± standard deviation from 20 different fields from each group. IOD, integrated optical density (IOD = total area × intensity of staining; n = 4). Real-time quantitative PCR analysis of (e) Epi and (f) SC adipose tissues samples (n = 4). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005. Data are shown as mean ± standard deviation.
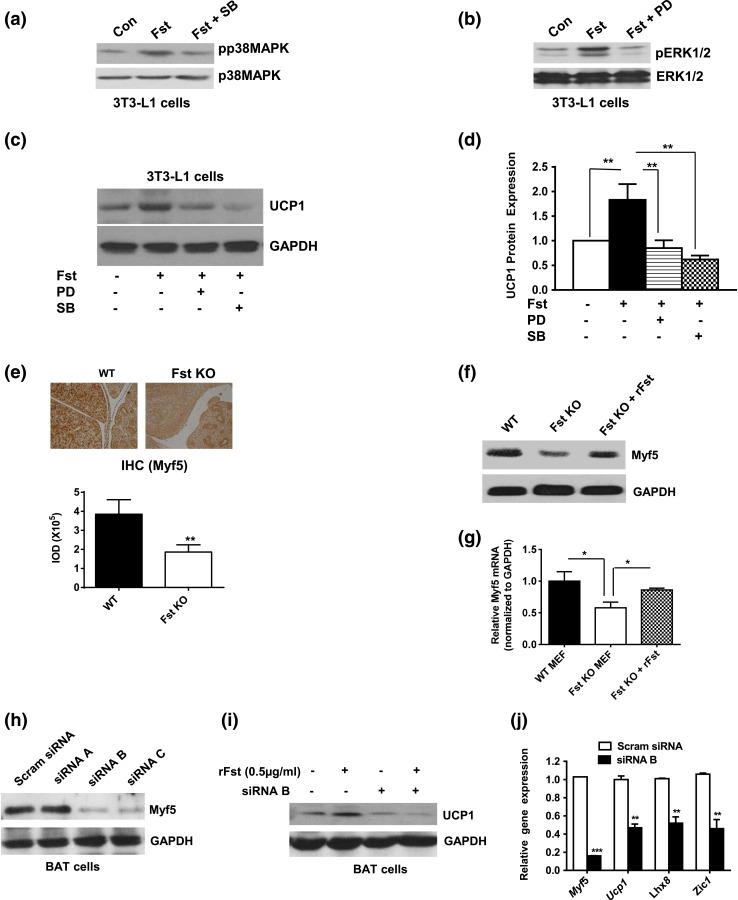
Fst overexpression results in increased phosphorylation of p38 MAP kinase/ERK1/2 in white adipose and upregulation of Myf5 levels in BAT tissue
To explore possible mechanisms responsible for Fst-induced browning of WAT as well as enhanced BAT mass and induced expression of BAT-associated markers in Fst-Tg mice, we analyzed the expression levels of key proteins implicated in these processes. WAT from Fst-Tg mice was characterized by increased phosphorylation of p38 MAPK and ERK1/2 in both Epi and SC adipose tissues, an effect that was observed in WAT but not BAT [Fig. 3(a) and 3(b)]. Beige adipocyte marker CD137 (5, 13) was selectively increased in Fst-Tg WAT, but not in BAT [Fig. 3(a) and 3(b)]. In BAT of Fst-Tg mice, there was an increase in Myf5 protein levels without any significant change in p38MAPK and ERK1/2 phosphorylation. Myf5 protein was not detectable in WAT depots from WT or Fst-Tg mice [Fig. 3(a) and 3(b)]. Quantitative IHC analysis of both WAT tissues further suggested a significant increase in pp38 MAP Kinase (Epi: 1.6-fold; SC: 1.56-fold) and CD137 (Epi: 1.56-fold; SC: 1.34-fold) immunopositive areas in both Epi and SC adipose tissue sections obtained from Fst-Tg mice compared with the WT mice [Fig. 3(c)]. Myf5 immunopositive areas were found to be increased by 1.5- ± 0.2-fold in BAT tissues from Fst-Tg mice [Fig. 3(d)]. Our data, therefore, suggest that Fst may promote browning in WATs by targeting p38MAPK/ERK1/2 pathway. By contrast, elevated expression of Myf5, a key marker for cells of brown adipocyte or skeletal muscle lineage was observed in BAT from Fst-Tg mice compared with the WT BAT. These results suggest that Fst may induce browning in WAT, and promote brown adipocyte characteristics in classical BAT, through distinct mechanisms.
Figure 3.
Analysis of key proteins involved in the regulation of beige and brown adipocyte markers in white (Epi and SC) and iBATs obtained from WT and Fst-Tg mice. A total of 100 µg of (a) Epi and SC and (b) BAT tissue lysates were analyzed by western blot. A representative sample is shown (n = 4). (c) Quantitative image analysis of pp38 MAPK and CD137 immunostaining in Epi and SC tissues obtained from WT and Fst-Tg (n = 4 mice). (d) Quantitative image analysis of Myf5 immunostaining in iBAT tissues obtained from WT and Fst-Tg mice (n = 4 mice). Data are presented as mean ± standard deviation from 20 different fields from each group. *P ≤ 0.05; **P ≤ 0.01.
rFst induces UCP1 expression in 3T3-L1 and mouse brown preadipocyte cell lines via induction of p38 MAP kinase/ERK1/2 phosphorylation and Myf5 expression, respectively
To further confirm whether Fst promotes browning in WATs and induces Myf5 expression in brown adipose cells, we used 3T3-L1 and mouse brown adipocyte cells. 3T3-L1 cells were grown in modified differentiation medium containing rosiglitazone and T3 that promotes browning of these cells (24) either alone or with rFst (0.5 μg/mL) for 4 days. There was significant increase in UCP1 (1.7-fold) and CD137 (1.8-fold) proteins as well as Ucp1 (2.4-fold), Cd137 (1.43-fold), Tbx1 (1.62-fold), and Tmem26 (1.51-fold) gene expression after Fst treatment [Fig. 4(a), 4(b), and 4(e)]. We also analyzed the expression of Myf5 in differentiated mouse BAT cells treated with or without Fst that promotes UCP1 gene and protein expression as described before (28). We found that Fst significantly induced Myf5 (2.2-fold) and brown adipocyte specific Eva1 (1.45-fold) protein expression [Fig. 4(c) and 4(d)]. Quantitative gene expression analysis further confirmed increased expression of Ucp1 (1.84-fold), as well as other brown adipocyte specific markers Eva1 (1.45-fold), Myf5 (2.7-fold), and Lhx8 (3.2-fold) in Fst-treated BAT cells [Fig. 4(f)]. Thus, our data suggest a role for Fst in browning of WATs and in classical BAT activation through distinct mechanisms.
Figure 4.
Upregulation of beige/brown adipose specific markers in 3T3-L1 and BAT cells after follistatin treatment. (a) 3T3-L1 cells were allowed to differentiate under modified adipogenic differentiation condition for 6 days either in absence or presence of recombinant Fst protein (0.5 µg/mL) as described in materials and methods. Protein expression of CD137 and UCP1 was analyzed by western blot analysis and a representative data are shown. (b) Densitometric analysis showing relative increase in UCP1 and CD137 protein levels (n = 3). **P ≤ 0.01. (c) Mouse BAT cells were allowed to differentiate under adipogenic differentiation conditions 6 days either in absence or presence of recombinant Fst protein (0.5 µg/mL) as described in materials and methods. Protein expression of UCP1, Eva1, and Myf5 was analyzed by western blot analysis and a representative data are shown. (d) Densitometric analysis showing relative increase in UCP1, Eva1, and Myf5 protein levels following Fst treatment (n = 3). *P ≤ 0.05; **P ≤ 0.01. (e) Quantitative gene expression analysis of key beige markers in differentiating 3T3-L1 cells treated with or without recombinant Fst (n = 3). *P ≤ 0.05; **P ≤ 0.01. (f) Quantitative gene expression analysis of key BAT markers in differentiating BAT cells treated with or without recombinant Fst (n = 3). *P ≤ 0.05; **P ≤ 0.01.
To further examine the possible involvements of p38 MAPK and ERK1/2 pathways in Fst-mediated browning, 3T3-L1 cells were treated with recombinant Fst protein (0.5 µg/mL) either in presence or absence of p38 MAPK inhibitor SB203580 or ERK1/2 inhibitor PD98059. Cells were harvested and protein expression of both phosphorylated and total p38 MAPK and ERK1/2 were analyzed. Fst-induced Induction of p38 MAPK and ERK1/2 phosphorylation was significantly blocked by SB203580 (10 µM) or PD98059 (10 µM), respectively [Fig. 5(a) and 5(b)]. Furthermore, Fst-induced UCP1 upregulation was significantly reduced in presence of SB203580 or PD98059, suggesting possible involvements of p38 MAPK and ERK1/2 pathways during Fst-induced browning of 3T3-L1 cells [Fig. 5(c) and 5(d)]. Quantitative image analysis and western blot data showed a significant decrease in Myf5 expression in embryos (51.7 ± 8.7%) and MEF cultures isolated from Fst KO mice compared with the WT mice [Fig. 5(e) and 5(f)]. Simultaneous treatment of Fst KO MEF cultures with rFst (0.5 µg/mL) led to a significant rescue of Myf5 protein and gene expression level [Fig. 5(f) and 5(g)].
Figure 5.
Follistatin stimulates adipose browning via p38MAPK/ERK pathways in 3T3-L1 cells and promotes Myf5-induced upregulation of brown adipogenesis in mouse BAT cells. 3T3-L1 cells were treated with recombinant Fst (0.5 µg/mL) either in presence or absence of (a) SB023580 or (b) PD98059. Total and phosphorylated (a) p38 MAPK and (b) ERK1/2 was analyzed by western blot analysis. 3T3-L1 cells were pretreated with SB (10 µM) or PD (10 µM) for 1 hour and subsequently treated with Fst (0.5 µg/mL) for an additional 5 days. (c) UCP1 protein expression was analyzed by western blot analysis and a representative blot is shown. (d) Densitometric analysis of relative UCP1 protein expression is shown (n = 3). **P ≤ 0.01. (e) Myf5 immunostaining of WT and Fst KO embryo section (top) and quantitative image analysis of immunopositive area (bottom) expressed as IOD. Myf5 (f) protein and (g) gene expression in MEF primary cultures isolated from WT and Fst KO embryos (d14) treated with or without rFst protein (0.5 µg/mL; n = 3). *P ≤ 0.05. (h) Trilencer-27 siRNA-mediated knockdown duplexes (10 nM; siRNA A to C) against mouse Myf5 in BAT cells using standard transfection conditions. Scrambled (Scram) siRNA was used as a control. Cells were harvested after 3 days and Myf5 protein expression was analyzed. (i) Myf5 siRNA B transfected cells were treated with or without rFst protein (0.5µg/mL) and allowed to differentiate for another 4 days. Cells were harvested and UCP1 protein expression was analyzed by western blot analysis. (j) Real-time quantitative PCR analysis of control (Scram siRNA) and Myf5 siRNA B transfected cells that were allowed to differentiate for another 4 days. Experiments were repeated three times and a representative sample is shown (n = 3). **P ≤ 0.01; ***P ≤ 0.001.
siRNA-mediated knockdown of Myf5 expression in BAT cells blunts rFst-induced upregulation of UCP1
To further test whether Fst-induced increase of UCP1 expression was mediated via upregulation of Myf5 expression, we blocked Myf5 expression in brown adipocyte cells by Trilencer-27 siRNA knockdown duplexes against mouse Myf5 as described in Methods section [Fig. 5(h)]. We selected siRNA B (∼80% inhibition) for our next experiment to test the effect of Myf5 inhibition on Fst-induced increase in UCP1 protein expression [Fig. 5(i)]. We found significant increase in UCP1 protein expression in Fst treated BAT cells undergoing brown adipose differentiation, but this effect was abolished in Myf5-knockdown BAT cells, suggesting an intermediate role of Myf5 during Fst-induced brown adipocyte activation [Fig. 5(i)]. Furthermore, gene expression analysis of siRNA treated cells show significant decrease of Myf5 (84%), Ucp1 (53%), Lhx8 (48%), and Zic1 (54%) expression [Fig. 5(j)], suggesting that loss of Myf5 may lead to a decreased pool of BAT precursors resulting in compromised ability of these cells to differentiate and express UCP1 protein.
WAT and BAT from Fst-Tg mice show heightened response to β3-adrenergic agonist compared with the WT tissues
To test whether β3-adrenergic agonist treatment of WT and Fst-Tg mice show differential thermogenic response, we injected (intraperitoneally) these male mice (3 months old) with 1mg/kg CL316,243 (CL) for 6 hours and analyzed the tissue expression of UCP1 in both WAT (EpI and SC) and iBAT. We found that although CL treatment upregulated UCP1 protein expression in all adipose tissues obtained from both Fst-Tg and WT groups [Fig. 6(a)], the relative increases of UCP1 protein and gene (Epi: 1.8-fold; SC: 3.3-fold; BAT: 1.6-fold) was higher in CL treated Fst-Tg group compared with the WT group [Fig. 6(b–d)]. IHC analysis of tissue section further confirmed that the response to CL administration on adipose browning and UCP1 expression was enhanced in Fst-Tg group compared with the WT group [Fig. 6(e–g)].
Figure 6.
Effect of CL316,243 treatment on UCP1 expression in WAT and BAT isolated from Fst-Tg and WT mice. Three-month-old male Fst-Tg and WT mice were subjected to a single dose of intraperitoneal injection of CL316,243 (1 mg/kg body weight) or saline for 6 hours. White (Epi and SC) and iBAT were isolated and UCP1 expression was analyzed by (a) western blot analysis, (b–d) quantitative real-time PCR, and (e–g) IHC analysis. Experiments were repeated three times and a representative sample is shown (n = 3). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001.
Downregulation of TGF-β signaling in adipose tissues isolated from Fst-Tg mice
Because Fst is known to antagonize TGF-β signaling, which has been demonstrated to inhibit brown adipose characteristics and browning, we investigated whether there is differential expression of key TGF-β signaling components Smad3/pSmad3 and activin receptor type IIB in adipose tissues obtained from WT and Fst-Tg mice. Our western blot analysis using Epi [Fig. 7(a)], SC [Fig. 7(b)], and BAT [Fig. 7(c)] tissues obtained from 3-month-old male mice show decreased levels of total Smad3 (Epi: 34%; SC: 43%; BAT: 38%), pSmad3 (Epi: 44%; SC: 38%; BAT: 65%), and activin receptor type IIB (Epi: 29%; SC: 32%; BAT: 32%) protein expression in Fst-Tg mice tissues compared with the tissues obtained from WT mice. Our data, therefore, suggest that TGF-β signaling components may play an intermediate role during regulation of brown adipose characteristics.
Figure 7.
Downregulation of key TGF-β signaling proteins in both WAT and iBAT isolated from Fst-Tg mice compared with the WT mice. Top panel: Western blot analysis of pSmad3/Smad3 and activin receptor type IIB (ActRIIB) proteins isolated from (a) Epi, (b) SC, and (c) iBATs isolated from 3-month-old male mice from WT and Fst-Tg mice. Bottom panel: Densitometric analysis of relative protein expression from Epi, SC, and iBAT are shown (n = 3). *P ≤ 0.05; **P ≤ 0.01.
Discussion
The direct conversion of white adipocytes into brown adipocytes that occurs through physiological genome reprogramming has gained significant attention for developing therapeutic strategies aimed at preventing excessive development of WATs and augmenting the amount of brown adipocytes for improved metabolic health (33–35). Identifying novel pharmacological and nutritional agents and delineating the molecular mechanisms involved in browning of WAT holds great promise for protection against obesity and development of related metabolic diseases. We have previously demonstrated that Fst inhibits TGF-β/Smad3 signaling in mouse mesenchymal pluripotent (26) and satellite cells to promote myogenesis (27) and induce brown adipocyte phenotype in MEF primary culture (28). Fst interacts and antagonizes several members of the TGF-β superfamily to enhance skeletal muscle mass and muscle differentiation (26, 27). The inhibition of TGF-β signaling has shown promise for treatment of muscle disorders (25, 36, 37). However, recent studies have demonstrated that genetic or pharmacological manipulation of TGF-β signaling in vitro or in vivo leads to global alterations in the brown adipogenic program implicated in the control of energy metabolism and pathogenesis of diabetes and obesity (32, 38–40). We have previously demonstrated that Fst gene expression was highest in mouse BAT and skeletal muscle, and was also expressed at substantial levels in WAT depots. In vitro, Fst promotes adipocyte differentiation and energy metabolism in MEF cultures (28). The primary objective of this study was to investigate the role of Fst in regulating brown adipocyte characteristics in both WATs and BATs in vivo and to identify the molecular targets of follistatin responsible for expansion and activation of BAT in Fst-Tg mice.
The expression of Fst from a muscle-specific promoter in transgenic mice led to significant increase in circulating Fst levels. The increased circulating Fst levels were associated with effects in BATs and WATs. Our data suggest that Fst increased the levels of key markers of brown adipocyte function in BAT as well as in Epi and SC WAT depots. Unexpectedly, however, Fst appears to influence brown adipose characteristics in white and brown adipocytes by distinct mechanisms. Using tissues isolated from both brown and white adipocyte depots in WT and Fst-Tg mice, we report for the first time that Fst uniquely targets both pool of adipocyte cells to promote brown adipocyte expansion as well as its activity. Fst specifically induced p38 MAPK/ERK pathways in Myf5– Epi and SC WATs. Previous studies have convincingly demonstrated that p38 MAPK is essential for cyclic AMP-dependent activation of PKA and transcription of UCP1 expression (20–22). Following activation of β-adrenergic receptor (AR) signaling, p38 MAPK phosphorylates transcription factor ATF2 and PGC-1α, which are recruited to the specific motifs (PPRE and CRE2) within the UCP1 enhancer following their interactions with PPARγ and RXRα to trigger expression of brown adipose thermogenic program (41). More recently, phosphorylation of p38 MAPK has also been linked to the stimulation of browning of white adipocytes via induction of irisin, a PGC-1α and exercise inducible myokine (23). Similar to irisin, Fst levels have also been reported to be increased following exercise (42). Also, recombinant Fst or anti-Mst antibody treatment of mouse muscle cells increases irisin encoded Fndc5 gene (43). Although, we did not compared the circulating levels of irisin or Fndc5 gene expression in Epi and SC adipose tissues isolated from WT and Fst-Tg mice, it is possible that irisin/Fndc5 levels are upregulated in Fst-Tg mice, which may have contributed to the observed phosphorylation of p38 MAPK and induction of browning. Our data provide significant insight into the molecular mechanisms responsible for Fst-induced brown adipocyte activation. Fst upregulates classical BAT progenitor markers Myf5, Zic1, and Lhx8 in iBAT, as well as in differentiating BAT cells, whereas it upregulates key beige adipose specific marker CD137 in Epi and SC WAT and 3T3-L1 cells undergoing adipogenic differentiation. Upregulation of additional beige adipose markers Tmem26 and Tbx1 in Fst treated 3T3-L1 cells further confirm that Fst promotes browning in WATs and cells. This effect was abolished by inhibiting p38 MAPK and ERK phosphorylation and prevented Fst-induced UCP1 expression in differentiating 3T3-L1 cells.
Because both skeletal muscle and classical BAT are thought to originate from an Myf5+ precursor pool and express very high basal levels of Myf5 (44), it is possible that Fst may directly target these Myf5-expressing cells to influence both skeletal muscle and BAT mass. Comparative analyses of Myf5 expression further suggest significantly decreased levels of Myf5 expression in Fst KO embryos and MEF primary cultures compared with the WT group. Also, rFst treatment induces Myf5 expression in differentiating mouse brown preadipocyte cultures. Moreover, siRNA-mediated inhibition of Myf5 expression in these cells significantly abolished Fst-induced increase in UCP1 protein. These observations suggest that Myf5 could be a potential target of Fst action in Myf5+ classical BATs and cells and thereby may promote brown adipose characteristics.
We have previously demonstrated that Fst is a downstream mediator of androgen action that promotes myogenesis and increases skeletal muscle mass both in vitro and in vivo (27) without promoting unwanted increased levels of prostate-specific antigen (45) and, therefore, has the potential of being used as a safe anabolic steroid for treatment of sarcopenia and aging related decrease in muscle mass and muscle strength. Our current findings provide further evidence that Fst could act as a novel regulator of both classical brown adipocyte precursors as well as inducer of key signaling pathways involved in the browning of WAT through distinct mechanisms. Thus, unlike several other pharmacological agents and regulators of endogenous signaling reported to promote browning, our findings suggest that Fst targets both pools of adipocytes to promote brown adipose expansion and activity. We acknowledge that Fst is driven by muscle specific promoter in the Fst-Tg mice used for this study and have significantly higher skeletal muscle mass and body weight (29). As circulating Fst levels in these Fst-Tg mice were found to be significantly higher compared with WT mice, we proposed to assess the systemic effects of Fst in regulating brown adipose characteristics in both WATs and BATs. Our findings suggest that irrespective of the source of Fst production, both WATs and BATs respond to increased levels of systemic Fst production by significantly upregulating key beige and brown adipose specific markers using distinct pathways. Future studies are in progress to directly assess the role of Fst in regulating brown adipose characteristics using transgenic mice where Fst is driven by adiponectin or UCP1 regulatory elements to study the local effects of Fst in WATs and BATs. In summary, our studies provide reasonable evidence that Fst could not only be useful for the treatment of aging-associated loss of muscle mass and function, but it could also provide unique therapeutic advantage for the treatment of obesity and related metabolic diseases by regulating overall energy metabolism (Fig. 8).
Figure 8.
A hypothetical model of Fst regulation of browning of WAT and induction of classical BAT via targeting Myf5 negative and positive pools, respectively.
Acknowledgments
We acknowledge Dr. Martin Matzuk (Baylor College of Medicine, Houston, TX) for a generous gift of male and female Fst heterozygous (Fst+/−) mice. We also acknowledge Dr. Bruce Spiegelman (Harvard Medical Center, Boston, MA) for his generous help with immortalized mouse brown preadipocyte (BAT) cells.
Acknowledgments
This work was supported by National Institutes of Health Grants SC1AG049682 (R.S.) and SC1CA1658650 (S.P.) and in part by Accelerating Excellence in Translational Science (AXIS) U54MD007598 and National Institute on Minority Health and Health Disparities grant S21MD000103.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- Epi
- epididymal
- Fst-Tg
- Fst-transgenic
- iBAT
- interscapular brown adipose tissue
- IHC
- immunohistochemical
- KO
- knockout
- MEF
- mouse embryonic fibroblast
- mRNA
- messenger RNA
- PCR
- polymerase chain reaction
- rFst
- recombinant mouse Fst
- SC
- subcutaneous
- siRNA
- small inhibitory RNA
- UCP
- uncoupling protein
- WAT
- white adipose tissue
- WT
- wild-type.
References
- 1.Haslam DW, James WP. Obesity. Lancet. 2005;366(9492):1197–1209. [DOI] [PubMed] [Google Scholar]
- 2.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9(6):465–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tam CS, Lecoultre V, Ravussin E. Brown adipose tissue: mechanisms and potential therapeutic targets. Circulation. 2012;125(22):2782–2791. [DOI] [PubMed] [Google Scholar]
- 4.Speakman JR. FTO effect on energy demand versus food intake. Nature 2010;464(7289):E1–E2. [DOI] [PubMed] [Google Scholar]
- 5.Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–1263. [DOI] [PubMed] [Google Scholar]
- 6.Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170(5):R159–R171. [DOI] [PubMed] [Google Scholar]
- 7.Nedergaard J, Bengtsson T, Cannon B. New powers of brown fat: fighting the metabolic syndrome. Cell Metab. 2011;13(3):238–240. [DOI] [PubMed] [Google Scholar]
- 8.Bartelt A, Bruns OT, Reimer R, Hohenberg H, Ittrich H, Peldschus K, Kaul MG, Tromsdorf UI, Weller H, Waurisch C, Eychmüller A, Gordts PL, Rinninger F, Bruegelmann K, Freund B, Nielsen P, Merkel M, Heeren J. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17(2):200–205. [DOI] [PubMed] [Google Scholar]
- 9.Wang GX, Zhao XY, Lin JD. The brown fat secretome: metabolic functions beyond thermogenesis. Trends Endocrinol Metab. 2015;26(5):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peirce V, Carobbio S, Vidal-Puig A. The different shades of fat. Nature. 2014;510(7503):76–83. [DOI] [PubMed] [Google Scholar]
- 12.Rosenwald M, Perdikari A, Rülicke T, Wolfrum C. Bi-directional interconversion of brite and white adipocytes. Nat Cell Biol. 2013;15(6):659–667. [DOI] [PubMed] [Google Scholar]
- 13.Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, Huang K, Tu H, van Marken Lichtenbelt WD, Hoeks J, Enerbäck S, Schrauwen P, Spiegelman BM. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell. 2012;150(2):366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jespersen NZ, Larsen TJ, Peijs L, Daugaard S, Homøe P, Loft A, de Jong J, Mathur N, Cannon B, Nedergaard J, Pedersen BK, Møller K, Scheele C. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 2013;17(5):798–805. [DOI] [PubMed] [Google Scholar]
- 15.de Jong JM, Larsson O, Cannon B, Nedergaard J. A stringent validation of mouse adipose tissue identity markers. Am J Physiol Endocrinol Metab. 2015;308(12):E1085–E1105. [DOI] [PubMed] [Google Scholar]
- 16.Bonet ML, Oliver P, Palou A Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim Biophys Acta 2013;1831:969–985. [DOI] [PubMed] [Google Scholar]
- 17.Bartelt A, Heeren J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol. 2014;10(1):24–36. [DOI] [PubMed] [Google Scholar]
- 18.Singh R, Braga M, Pervin S. Regulation of brown adipocyte metabolism by myostatin/follistatin signaling. Front Cell Dev Biol. 2014;2:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao L, Choi EY, Liu X, Martin A, Wang C, Xu X, During MJ. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic-adipocyte axis. Cell Metab. 2011;14(3):324–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robidoux J, Cao W, Quan H, Daniel KW, Moukdar F, Bai X, Floering LM, Collins S. Selective activation of mitogen-activated protein (MAP) kinase kinase 3 and p38alpha MAP kinase is essential for cyclic AMP-dependent UCP1 expression in adipocytes. Mol Cell Biol. 2005;25(13):5466–5479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao W, Medvedev AV, Daniel KW, Collins S. Beta-adrenergic activation of p38 MAP kinase in adipocytes: cAMP induction of the uncoupling protein 1 (UCP1) gene requires p38 MAP kinase. J Biol Chem. 2001;276(29):27077–27082. [DOI] [PubMed] [Google Scholar]
- 22.Cao W, Daniel KW, Robidoux J, Puigserver P, Medvedev AV, Bai X, Floering LM, Spiegelman BM, Collins S. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24(7):3057–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, Qi L, Zhang M, Wang X, Cui T, Yang LJ, Tang D. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63(2):514–525. [DOI] [PubMed] [Google Scholar]
- 24.Vegiopoulos A, Müller-Decker K, Strzoda D, Schmitt I, Chichelnitskiy E, Ostertag A, Berriel Diaz M, Rozman J, Hrabe de Angelis M, Nüsing RM, Meyer CW, Wahli W, Klingenspor M, Herzig S. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328(5982):1158–1161. [DOI] [PubMed] [Google Scholar]
- 25.Yadav H, Quijano C, Kamaraju AK, Gavrilova O, Malek R, Chen W, Zerfas P, Zhigang D, Wright EC, Stuelten C, Sun P, Lonning S, Skarulis M, Sumner AE, Finkel T, Rane SG. Protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling. Cell Metab. 2011;14(1):67–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braga M, Bhasin S, Jasuja R, Pervin S, Singh R. Testosterone inhibits transforming growth factor-β signaling during myogenic differentiation and proliferation of mouse satellite cells: potential role of follistatin in mediating testosterone action. Mol Cell Endocrinol. 2012;350(1):39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh R, Bhasin S, Braga M, Artaza JN, Pervin S, Taylor WE, Krishnan V, Sinha SK, Rajavashisth TB, Jasuja R. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150(3):1259–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Braga M, Reddy ST, Vergnes L, Pervin S, Grijalva V, Stout D, David J, Li X, Tomasian V, Reid CB, Norris KC, Devaskar SU, Reue K, Singh R. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014;55(3):375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee SJ, McPherron AC. Regulation of myostatin activity and muscle growth. Proc Natl Acad Sci USA. 2001;98(16):9306–9311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Asano H, Kanamori Y, Higurashi S, Nara T, Kato K, Matsui T, Funaba M. Induction of beige-like adipocytes in 3T3-L1 cells. J Vet Med Sci. 2014;76(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Uldry M, Yang W, St-Pierre J, Lin J, Seale P, Spiegelman BM. Complementary action of the PGC-1 coactivators in mitochondrial biogenesis and brown fat differentiation. Cell Metab. 2006;3(5):333–341. [DOI] [PubMed] [Google Scholar]
- 32.Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity (Silver Spring). 2013;21(6):1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elsen M, Raschke S, Eckel J. Browning of white fat: does irisin play a role in humans? J Endocrinol. 2014;222(1):R25–R38. [DOI] [PubMed] [Google Scholar]
- 34.Lee YH, Jung YS, Choi D. Recent advance in brown adipose physiology and its therapeutic potential. Exp Mol Med. 2014;46:e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kajimura S, Saito M. A new era in brown adipose tissue biology: molecular control of brown fat development and energy homeostasis. Annu Rev Physiol. 2014;76:225–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogdanovich S, Krag TO, Barton ER, Morris LD, Whittemore LA, Ahima RS, Khurana TS. Functional improvement of dystrophic muscle by myostatin blockade. Nature. 2002;420(6914):418–421. [DOI] [PubMed] [Google Scholar]
- 37.Acuña MJ, Pessina P, Olguin H, Cabrera D, Vio CP, Bader M, Muñoz-Canoves P, Santos RA, Cabello-Verrugio C, Brandan E. Restoration of muscle strength in dystrophic muscle by angiotensin-1-7 through inhibition of TGF-β signalling. Hum Mol Genet. 2014;23(5):1237–1249. [DOI] [PubMed] [Google Scholar]
- 38.Fournier B, Murray B, Gutzwiller S, Marcaletti S, Marcellin D, Bergling S, Brachat S, Persohn E, Pierrel E, Bombard F, Hatakeyama S, Trendelenburg AU, Morvan F, Richardson B, Glass DJ, Lach-Trifilieff E, Feige JN. Blockade of the activin receptor IIb activates functional brown adipogenesis and thermogenesis by inducing mitochondrial oxidative metabolism. Mol Cell Biol. 2012;32(14):2871–2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koncarevic A, Kajimura S, Cornwall-Brady M, Andreucci A, Pullen A, Sako D, Kumar R, Grinberg AV, Liharska K, Ucran JA, Howard E, Spiegelman BM, Seehra J, Lachey J. A novel therapeutic approach to treating obesity through modulation of TGFβ signaling. Endocrinology. 2012;153(7):3133–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan CK, Chong HC, Tan EH, Tan NS. Getting “Smad” about obesity and diabetes. Nutr Diabetes. 2012;2:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bordicchia M, Liu D, Amri EZ, Ailhaud G, Dessì-Fulgheri P, Zhang C, Takahashi N, Sarzani R, Collins S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansen J, Brandt C, Nielsen AR, Hojman P, Whitham M, Febbraio MA, Pedersen BK, Plomgaard P. Exercise induces a marked increase in plasma follistatin: evidence that follistatin is a contraction-induced hepatokine. Endocrinology. 2011;152(1):164–171. [DOI] [PubMed] [Google Scholar]
- 43.Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27(5):1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Seale P, Kajimura S, Spiegelman BM. Transcriptional control of brown adipocyte development and physiological function: of mice and men. Genes Dev. 2009;23(7):788–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jasuja R, Costello JC, Singh R, Gupta V, Spina CS, Toraldo G, Jang H, Li H, Serra C, Guo W, Chauhan P, Narula NS, Guarneri T, Ergun A, Travison TG, Collins JJ, Bhasin S. Combined administration of testosterone plus an ornithine decarboxylase inhibitor as a selective prostate-sparing anabolic therapy. Aging Cell. 2014;13(2):303–310. [DOI] [PMC free article] [PubMed] [Google Scholar]