Abstract
Chronic exposure to high–saturated fat diets (HFDs) increases the prevalence of obesity and contributes to the development of low-grade inflammation and insulin resistance. A possible mediator accounting for obesity-associated inflammation and insulin resistance is Toll-like receptor 4 (TLR4). We investigated the role of adipocyte TLR4 in lipid and glucose homeostasis through an inducible, adipocyte-specific deletion of TLR4 in a mouse model that is referred to as the “Tadipo“ mouse. Consistent with a critical role for inflammation as a positive force for healthy adipose tissue expansion, chronic HFD exposure results in exacerbated whole-body and muscle insulin resistance in the absence of TLR4 in the adipocyte. Elimination of TLR4 in adipocytes affects TLR4 expression in other tissues, with reduced TLR4 expression in peritoneal macrophages and in the liver. In contrast, TLR4 deletion from adipocytes protects whole-body insulin sensitivity after an acute lipid challenge during a hyperinsulinemic euglycemic clamp. Our results therefore demonstrate dichotomous effects of TLR4 on adipose tissue functionality, with an important positive role of TLR4 during a chronic HFD challenge due to the lack of adipose tissue remodeling and a negative role of TLR4 as a mediator of insulin resistance in the adipocyte during an acute challenge with saturated fatty acids.
Lack of adipocyte TLR4 signaling gives rise to a complex metabolic phenotype with improved resistance to acute exposure to saturated fats, whereas it is detrimental upon prolonged HFD exposure.
Obesity is the most common cause of insulin resistance in the Western population. The increasing prevalence of obesity also correlates with increasing risk of type 2 diabetes. Studies within the past 10 years have been heavily focused on identifying the link between obesity and insulin resistance. Among all the underlying mechanisms identified, chronic low-grade inflammation, one of the hallmarks of obesity, is thought to be a major contributor to obesity-induced insulin resistance (1). Activation of systemic Toll-like receptor 4 (TLR4) signaling and the subsequent release of inflammatory cytokines through regulation of several important transcription factors has been postulated to be a critical player in connecting obesity-associated inflammation and insulin resistance (2).
TLR4 was first identified as an endotoxin receptor, recognizing molecules shared among pathogens to initiate host defense innate immune responses (3). A number of studies using TLR4-deficient mice revealed attenuated diet-induced inflammation and insulin resistance (4–6). However, the specific cell types involved in this process most susceptible to TLR4 activation are unclear, because TLR4 is widely expressed throughout the body. Our groups have previously demonstrated the role of hepatic TLR4 in mediating obesity-induced inflammation, insulin resistance, and hepatic steatosis using a liver-specific TLR4 knockout (7). Vila et al. (8) determined that the presence of TLR4 on adipose tissue hematopoietic cells is necessary for the initiation of adipose tissue fibrosis. In this study, we investigated further the specific role of TLR4 in the adipocyte by using an adipocyte-specific inducible knockout system in which we eliminate TLR4 selectively in the mature adipocyte.
In differentiated 3T3-L1 adipocytes, lipopolysaccharide treatment stimulates interleukin (IL)-6 production, demonstrating the effects of the activation of the classic TLR4 endotoxin receptor activity on adipocytes in vitro (9). Later, Shi et al. (10) demonstrated that free fatty acids (FFAs) can also induce the release of proinflammatory cytokines via TLR4 signaling using both the RAW264.7 macrophage cell line and TLR4-deficient mice. The exact mechanism of how FFAs activate TLR4 signaling is still an area under investigation. A recent report suggested that fetuin-A acts as an endogenous ligand for TLR4 and is critical for lipid-induced insulin resistance (11).
It is widely recognized that chronic low-grade inflammation associated with prolonged overnutrition can contribute to the development of insulin resistance. About 15 years ago, we reported that TLR4 is highly expressed on the cell surface of adipocytes (9). The activation of TLR4 on adipocytes can initiate adipose tissue inflammation locally, which we postulated to contribute to the development of insulin resistance. A possible mechanism linking TLR4 activation with insulin resistance is through ceramide biosynthesis. FFAs can trigger TLR4 signaling and stimulate ceramide production, which in turn leads to an inhibition of protein kinase B and directly leads to impaired insulin signaling (12). We have also shown that adipocytes exert potent proinflammatory effects on macrophages in the microenvironment. This leads to an enhanced secretion of cytokines and ultimately causes an elevation in systemic cytokine levels in the obese state (13). We therefore hypothesized that adipocyte TLR4 is a key regulator of obesity-associated inflammatory response, and that adipocyte TLR4 can contribute to the development of insulin resistance through upregulation of cytokines and activation of ceramide biosynthesis. The results obtained from the specific elimination of TLR4 in the mature adipocyte allow us to directly address this hypothesis.
Materials and Methods
Mice
Mice were maintained on a 12-hour dark/12-hour light cycle and housed in groups of three to five with unlimited access to water, chow (LabDiet 5058), or a high-fat (60% kCal from fat) doxycycline (Dox)–containing diet [600 mg/kg; high–saturated fat diet (HFD) Dox; S4107, Bio-Serv] as indicated for the individual experiments. The Institutional Animal Care and Use Committee of the University of Texas Southwestern Medical Center (Dallas, TX) approved all animal experiments. The use of adiponectin P (adnP)–reverse tetracycline–controlled transactivator (rtTA) and cre mice has been previously described (14).
Histology
Adipose and liver tissues were excised and fixed in phosphate-buffered saline–buffered 10% formalin overnight. Following paraffin embedding, the tissue sections were stained with hematoxylin and eosin (H&E) using standard protocols. For immunohistochemistry, sections were deparaffinized. After antigen retrieval and blockage of endogenous peroxidase, sections were stained with primary antibodies against Mac-2 (1:1000; Cedarlane Laboratories, catalog no. CL8942AP; RRID:AB_10060357) and perilipin (1:200; Abcam, catalog no. ab61682; RRID:AB_944751). The secondary antibody used was Alexa Fluor 488 goat antibody to rabbit IgG (H+L) (1:200; Thermo Fisher Scientific, catalog no. A11008; RRID:AB_143165). Slides were counterstained with 4′,6-diamidino-2-phenylindole. Images were acquired using an AxioObserver epifluorescence microscope (Zeiss). For antibodies used, see Table 1.
Table 1.
Antibodies Used
| Peptide/Protein Target | Name of Antibody | Source of Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| Mac-2 | CL8942AP | Cedarlane Laboratories | Rabbit; polyclonal | 1:1000 | AB_10060357 |
| Perilipin | ab61682 | Abcam | Rabbit; polyclonal | 1:200 | AB_944751 |
| Rabbit immunoglobulin G | Catalog no. A11008 | Thermo Fisher Scientific | Goat anti-rabbit | 1:200 | AB_143165 |
| Mouse adiponectin | Catalog no. AB3784P | Millipore | Rabbit; polyclonal | 1:1000 | AB_2221646 |
| IRDye 800–conjugated anti-rabbit | Catalog no. 827-08365 | LI-COR Biosciences | Goat | 1:1000 | AB_10796098 |
| Mouse actin | Catalog no. 3700 | Cell Signaling Technology | Monoclonal | 1:1000 | AB_2242334 |
| IRDye 680-conjugated anti-mouse | Catalog no. 827-11080 | LI-COR Biosciences | Monoclonal | 1:1000 | AB_10795014 |
Abbreviation: RRID, Research Resource Identifier.
Hyperinsulinemic euglycemic clamp study
Surgery and experimental setup were similar to those used in previous experiments (12, 15, 16). Briefly, body weight (BW) and age-matched animals were fasted for 3 hours prior to a 90-minute infusion with 3H-glucose (5-mCi bolus followed by 0.05 mCi/min) to measure glucose turnover, and blood samples from the cut tail were taken for measurements of basal insulin and glucose as well as to calculate glucose turnover. The clamp was started at 0 minutes with a continuous insulin infusion (10 mU/kg/min; Humulin, Eli Lilly), and the 3H-glucose was increased to 0.1 mCi/min to minimize changes in specific activity. A variable glucose infusion rate (50% dextrose) was used to maintain blood glucose at 150 mg/dL. Blood samples were taken every 10 minutes during the steady-state period (80 to 120 minutes) to determine hepatic glucose production and glucose disposal rate. For lipid infusion clamp, the mice fed on a Dox-containing chow diet, a Dox-containing HFD for 3 weeks (BW of 24 g), and a Dox-containing HFD for more than 3 weeks (BW of 30 g) were preinfused with lard oil emulsion or glycerol for 3 hours prior to tracer infusion.
Adipocyte and stromal vascular fraction isolation
Subcutaneous white adipose tissue (sWAT) and gonadal white adipose tissue (gWAT) were collected from TLR4fl/fl and adipocyte Toll-like receptor-null (Tadipo) mice fed a HFD for 10 weeks. Tissues were minced and digested using collagenase (Gibco, catalog no. 17703-034) at 37°C for 1.5 hours with shaking. The cell suspension was filtered through a 250-mm mesh, washed with Dulbecco’s modified Eagle medium containing 10% fetal bovine serum, and centrifuged at 800g for 1 minute at room temperature. The floating adipocytes were collected, centrifuged at 500g for 1 minute, and dissolved in TRIzol for RNA isolation. The remaining cell pellets containing stromal vascular fractions were put on ice immediately. The pellets were passed through a 40-mm cell strainer, washed with cold Dulbecco’s modified Eagle medium buffer, and centrifuged at 500g for 5 minutes at 4°C. Then, stromal vascular fractions were collected as pellets and resuspended in TRIzol for RNA isolation.
Western blot analysis
Adipose tissue samples were homogenized on ice in T-PER (Thermo Scientific) supplemented with protease inhibitor cocktail (cOmplete mini, EDTA-free, Roche). This was followed by low-speed centrifugation (3000g at 4°C) to remove the fat cake from the top. The tissue extracts were cleared at 15,000g for 20 minutes at 4°C and total protein concentration was measured with a bicinchoninic acid protein assay kit (Pierce Protein Research Products, Thermo Scientific). Equal amounts of protein from adipose tissue were mixed with 5× Laemmli sample buffer with dithiothreitol and boiled at 95°C for 5 minutes. The samples were resolved on 4% to 15% TGX (Bio-Rad Laboratories), followed by semidry transfer to polyvinylidene difluoride membranes (Bio-Rad Laboratories). Blots were probed with rabbit polyclonal anti-mouse adiponectin antibody (Millipore, catalog no. AB3784P; RRID:AB_2221646). Bound antibodies were detected with IRDye 800–conjugated anti-rabbit secondary antibodies (LI-COR Biosciences, catalog no. 827-08365; RRID:AB_10796098). Membranes were scanned with the LI-COR Odyssey infrared imaging system. Actin controls were visualized with an anti-mouse actin monoclonal antibody from Cell Signaling Technology (catalog no. 3700; RRID:AB_2242334) with IRDye 680–conjugated anti-mouse secondary antibodies (LI-COR Biosciences, catalog no. 827-11080; RRID:AB_10795014).
Blood biochemistry
Insulin levels were measured by commercial ELISA kits from Millipore. Glucose levels were determined with Sigma Diagnostics glucose reagents (Sigma-Aldrich), Triglycerides were measured using Infinity triglycerides reagent (Thermo Scientific), and FFA levels were measured with nonesterified fatty acids (2) (Wako Pure Chemical Industries, product NEFA-HR).
Oral glucose and insulin tolerance tests
An oral glucose tolerance test (OGTT) was performed in mice on HFD Dox for 3 weeks starting at 6 weeks of age. One week later, the same mice were used for insulin tolerance tests (ITTs). For OGTT, mice were fasted for 4 hours during the light phase and blood samples were drawn from the tail vein before and 15, 30, 60, and 120 minutes after oral gavage with 2.5 mg/g BW of glucose in phosphate-buffered saline. For ITT, 4-hour fasted mice were injected intraperitoneally with recombinant human insulin (Novo Nordisk) at 0.75 mU/g BW and blood samples were collected before and 15, 30, 60, and 90 minutes after injections.
Triglyceride clearance
Triglyceride clearance was performed in mice on HFD Dox for 5 weeks starting at 6 weeks of age. The mice were fasted for 6 hours during the light phase and blood samples were drawn from the tail vein before and 1, 2, 3, 4, and 6 hours after oral gavage with 15 mL/kg ΒW of 20% intralipid (Fresenius Kabi Clayton, LP).
Liver triglyceride and cholesterol measurements
Frozen liver tissues (100 mg) were pulverized and transferred into 30 mL of chloroform/methanol solution (2:1). Samples were set at room temperature for 20 minutes and 6 mL of 0.05% H2SO4 was added, followed by centrifugation at 2000g for 10 minutes at 4°C. Meanwhile, standards were prepared by serial dilution from 200 μg of Wesson oil (ConAgra Foods)/mL chloroform. For each standard and sample, 2 mL was transferred into glass tubes and mixed with 2 mL of 1% Triton X-100/chloroform. Samples were dried under nitrogen gas and reconstituted with water. Concentrations of triglycerides of final samples were determined by triglycerides/glycerol blanked (Roche Diagnostics, product Trig/GB).
Lipid quantification
Sphingolipids were quantified as described previously by liquid chromatography–electrospray ionization–tandem mass spectrometry using a Shimadzu Nexera X2 ultra–high-performance liquid chromatography system coupled to a Shimadzu LCMS-8050, a triple quadrupole mass spectrometer (15). Lipid species were identified based on exact mass and fragmentation patterns and verified by lipid standards.
Quantitative real-time reverse transcription polymerase chain reaction
Tissues were snap frozen in liquid nitrogen and stored at −80°C. TRIzol reagent (Invitrogen) extraction was followed by RNA purification using an RNeasy mini kit and RNase-free DNase (Qiagen, USA). RNA was reverse transcribed to cDNA by an iScript cDNA synthesis kit (Bio-Rad Laboratories), and SYBR Green (Applied Biosystems, Life Technologies) was used for the quantitative polymerase chain reactions (PCRs). The relative expression level was calculated by the comparative cycle threshold method using β-actin as an endogenous control. Primer sequences can be found in Supplemental Table 1 (675KB, pdf) .
Statistical analysis
All results are presented as means ± standard error of the mean (SEM). Differences between two groups were determined for statistical significance by a standard two-tailed Student t test. Significance was accepted at a value of P < 0.05.
Results
Generation of adipocyte-specific TLR4 deletion (Tadipo mice)
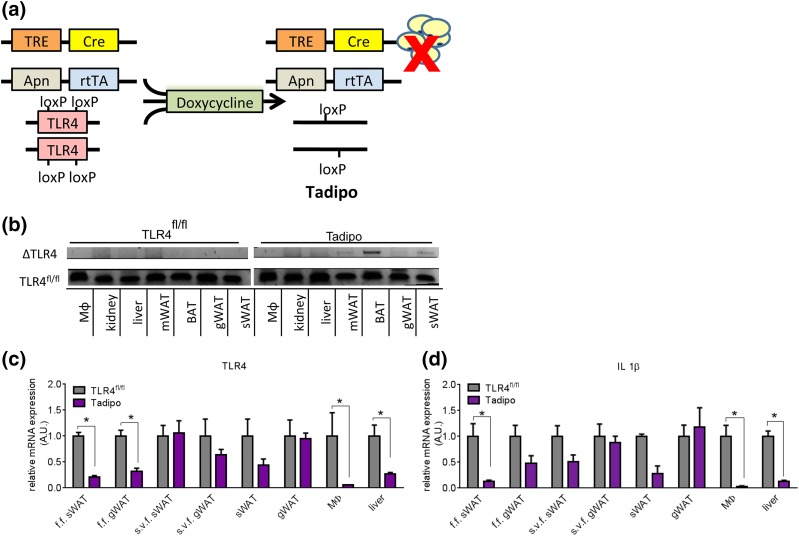
We took advantage of our previously characterized floxed allele of TLR4 that we used for the hepatocyte-specific knockout mouse model (7). In this case, we used an approach with inducible, cell type–specific disruption of the TLR4 gene using a system under tet control. For this purpose, we used an rtTA protein under the control of the mouse adnP promoter (14). Upon binding to Dox, the rtTA will activate the transcription of cre recombinase under the control of a tet-responsive element. Cre will excise exons 2 and exon 3 of TLR4 from mature adipocytes (Tadipo mice) [Fig. 1(a)]. By using the adnP promoter that is only active in mature adipocytes in fully developed mice, we can avoid any potential issues related to the absence of TLR4 during development and adipogenesis. Tadipo mice are systemically still lipopolysaccharide-responsive, because this deletion is highly adipocyte selective. We did not observe gene rearrangements in any other tissues [Fig. 1(b)]. The “floxed-out” gene is seen by focusing on the gene rearrangements in a number of different tissues with a nonquantitative PCR approach, and we observed the rearrangements in brown adipose tissue (BAT), sWAT, gWAT, and mesenteric white adipose tissue (mWAT), but not in other tissues. Because adiponectin is also expressed in stellate cells in the liver and in the kidney at very low levels, there are indications of gene elimination in a subset of cells in these tissues as well with this highly sensitive, nonquantitative approach. However, these are all areas of very low level expression and we do not observe expression in the liver with the adnP-rtTA construct beyond the nonabundant stellate cells. Upon HFD Dox exposure for 10 weeks, we can observe an ∼60% to 80% reduction of TLR4 messenger RNA (mRNA) in the isolated adipocyte fraction (floated fraction) harvested from the sWAT and gWAT [Fig. 1(c)]. Similarly, a reduction in TLR4 mRNA expression is also seen in the adipocyte fraction after only 7 days of Dox chow treatment [Supplemental Fig. 1(a (675KB, pdf) )]. Surprisingly, we observed a significant decrease in TLR4 mRNA in the peritoneal macrophages and in the liver as well with the HFD Dox treatment [Fig. 1(c)]. The reduction in TLR4 mRNA levels in macrophages and liver was not seen when the Tadipo mice were fed for 7 days on a Dox-containing chow diet [Supplemental Fig. 1(a (675KB, pdf) )]. This suggests that the lack of TLR4 signaling in the adipocyte under HFD conditions affects systemic inflammatory readouts. This is further substantiated by the observation that multiple proinflammatory markers, such as IL-1β, were also significantly reduced in the adipocyte fractions, peritoneal macrophages, and in the liver, but only as a function of HFD exposure [Fig. 1(d); Supplemental Fig. 1(b) (675KB, pdf) ]. Importantly, this reduction in TLR4 expression in the peripheral tissues is a purely transcriptional event in response to the TLR4 deletion in the adipocyte, and not a reflection of TLR4 gene rearrangements in these tissues. These secondary effects are therefore a reflection of the cytokine-based crosstalk among the adipocyte, peritoneal macrophages, and the liver.
Figure 1.
Generation of adipocyte-specific TLR4 deletion (Tadipo) mice. (a) Schematic model of Dox-inducible adipose tissue–specific deletion (to generate the Tadipo mice). (b) Gene rearrangement in various tissues isolated from the Tadipo mice after Dox induction. (c, d) Quantitative PCR analysis of TLR4 and IL-1β messenger RNA expression in various tissues normalized to TLR4fl/fl. Tissues were collected after 10 weeks of HFD Dox treatment. Data are presented as means ± SEM (n = 4). *P < 0.0 5 compared with WT. Apn, adiponectin; A.U., arbitrary unit; f.f., floated fraction; Mϕ, macrophage; s.v.f., stromal vascular fraction; TRE, tet-responsive element.
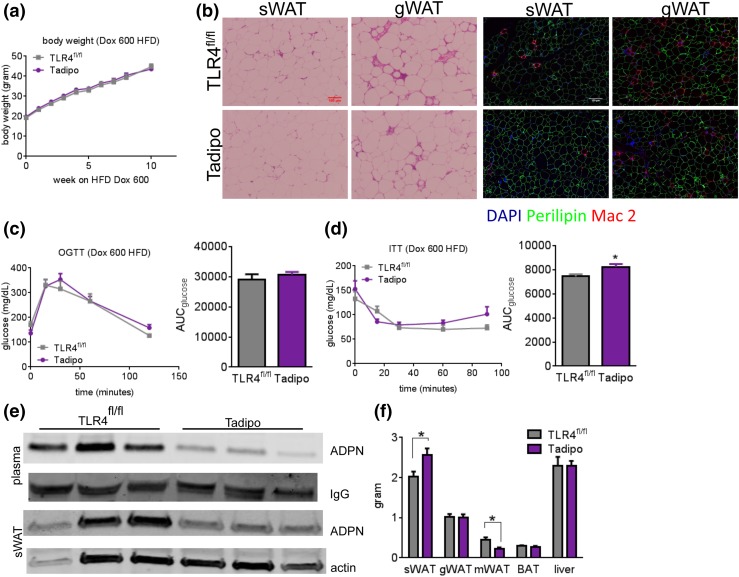
Tadipo mice display decreased white adipose tissue macrophage infiltration, decreased systemic adiponectin expression, and a redistribution of white adipose tissue after chronic HFD
To exert a metabolic stress on the system, we exposed the mice to a HFD. We opted for a HFD with 60% of calories from fat that we routinely use for all of our mouse models to rapidly generate glucose intolerance in our mice. During a 10-week HFD challenge, there were no differences in BWs between the Tadipo mice and the littermate controls (TLR4fl/fl) [Fig. 2(a)]. However, we observed a clear reduction in white adipose tissue (WAT) macrophage infiltration evidenced by reduced crown-like structures in both the sWAT and gWAT after HFD treatment [Fig. 2(b), left panel]. This was also apparent after staining with the inflammatory marker Mac-2 [Fig. 2(b), right panel]. Additional inflammatory markers, such as IL-6 and MCP-1, are also reduced in all fat pads, as well as in macrophages and in the liver (data not shown). Despite the clear reduction in the inflammatory response in WAT, no differences were apparent during OGTTs [Fig. 2(c)], and no differences were seen in the insulin levels during the course of the OGTT (data not shown). Differences seen in insulin sensitivity during ITTs were marginal [Fig. 2(d)]. Furthermore, no significant differences were observed in response to β3 adrenergic receptor stimulation, either on a chow diet or on a chronic HFD [Supplemental Fig. 2(a) and 2(b (675KB, pdf) )]. Interestingly, we did observe a significant reduction in both circulating and intracellular adiponectin levels in the absence of TLR4 [Fig. 2(e)]. These changes in adiponectin levels are associated with a redistribution of adipose tissue. Upon dissecting and weighing each of the WAT pads after the chronic HFD challenge, we noticed that the Tadipo mice had increased sWAT and decreased mWAT, whereas they maintained similar overall BWs compared with the control animals [Fig. 2(f)]. The average adipocyte size does not seem to be altered [Supplemental Fig. 2(c (675KB, pdf) )]. Overall, this suggests a complex phenotype with multiple different processes going on at the same time. Although the decline of adiponectin is generally associated with reduced insulin sensitivity, the overall reduced inflammation and redistribution of adipose tissue observed may drive an overall beneficial metabolic response. The net result seems to balance out without any major systemic effects despite highly significant changes in both directions.
Figure 2.
Tadipo mice display decreased WAT macrophage infiltration, a slight decrease in insulin sensitivity, and decreased systemic adiponectin levels. (a) Ten-week BW follow-up during HFD challenge (n = 10 to 15). (b) H&E staining of sWAT and gWAT after chronic HFD (left). Mac-2 (red) immunostain of sWAT and gWAT (right). Scale bars = 100 μm (left), 110 μm (right). (c) OGTT (2.5 mg/g BW). (d) ITT (0.75 mU/g BW) (n = 6 to 8). (e) Western blot of adiponectin in plasma and the sWAT isolated from Tadipo and control animals after 8 weeks of HFD challenge. Relative intensity is normalized to either IgG or actin signal. (f) Tissue weight after HFD challenge. *P < 0.05 compared with TLR4fl/fl. Data are presented as means ± SEM. AUC, area under the curve.
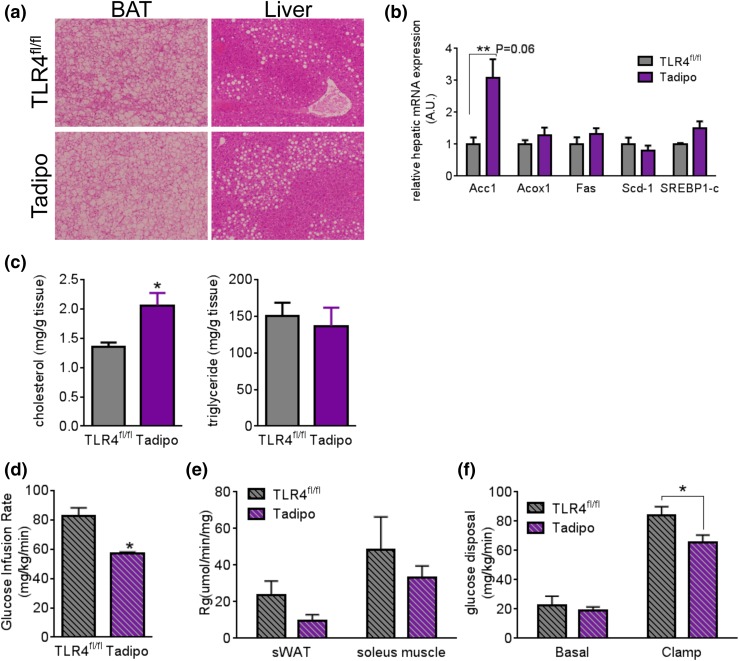
Adipocyte-specific TLR4 deletion leads to increased steatosis and decreased insulin sensitivity after chronic HFD exposure
Increased lipid deposition was observed in the BAT, whereas the liver does not significantly differ after chronic HFD exposure [Fig. 3(a)]. This was accompanied by trends toward increased expression of fatty acid synthesis–related genes in the liver, particularly Acc1 [Fig. 3(b)], although most others remained unchanged. Biochemical assays revealed significant increases in hepatic cholesterol, but not in triglycerides in the Tadipo mice [Fig. 3(c)]. This is a surprising result in light of a large body of work describing systemic elimination of proinflammatory components leading to metabolic improvements on HFDs. However, we have previously argued that reducing inflammation in the local microenvironment of adipocytes can lead to detrimental effects (17). In this context, we demonstrated that adipose tissue inflammation is critical to maintaining healthy adipose tissue expansion and remodeling in response to HFD exposure using several mouse models. The Tadipo mouse is another example of impaired local adipose tissue inflammation. Although we did not observe differences in the OGTTs (Fig. 2), the more sensitive euglycemic clamp technique addresses insulin sensitivity more directly. We therefore decided to embark on a series of hyperinsulinemic euglycemic clamp studies with these mice. Consistent with our previously reported models, upon chronic HFD exposure, Tadipo mice show impaired insulin sensitivity, as judged by significantly lower glucose infusion and glucose disposal rates during the clamp. This was in part explained by the overall trends toward reduced glucose uptake into adipose tissue and muscle [Fig. 3(d–f)].
Figure 3.
Adipocyte TLR4 deletion leads to increased steatosis and decreased insulin sensitivity after chronic HFD exposure. (a) H&E staining of BAT and liver after HFD challenge. Images captured under 10× magnification. (b) Lipogenesis-related gene expression in the liver after HFD challenge. (c) Liver tissue cholesterol (left). Triglyceride content after HFD challenge (right). (d) Glucose infusion rate. (e) Tissue glucose uptake. (f) Glucose disposal during hyperinsulinemic euglycemic clamp after chronic HFD feeding (n = 4). Data are presented as means ± SEM. *P < 0.05 compared with TLR4fl/fl. A.U., arbitrary unit.
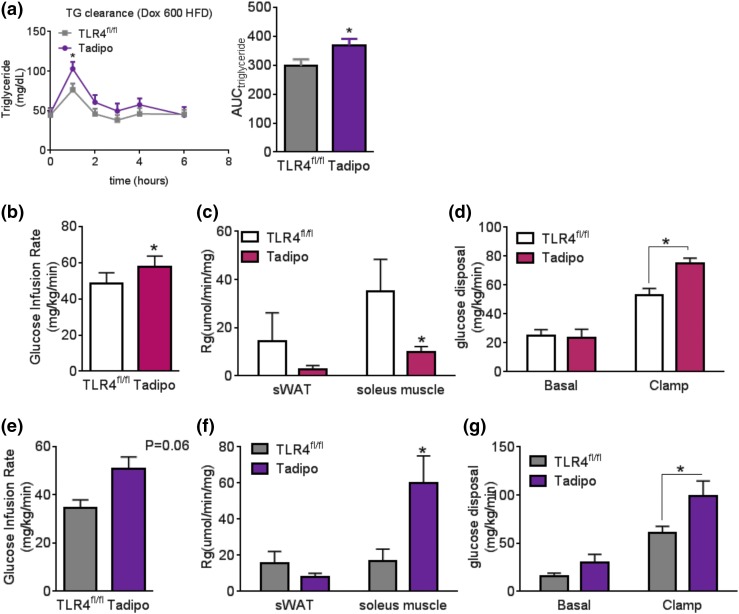
Tadipo mice display increased insulin sensitivity during an acute lard infusion
The increased lipid deposition in the BAT observed earlier suggested that deleting TLR4 from adipocytes might also lead to impaired lipid homeostasis. We orally gavaged the Tadipo and control animals with intralipid to assess their lipid clearance [Fig. 4(a)]. The Tadipo mice show elevated triglycerides upon gavage, confirming that the deletion of TLR4 can lead to impaired lipid clearance. We were interested to determine whether Tadipo mice differentially respond to chronic vs short-term lipid challenges with respect to insulin sensitivity. Given that chronic HFD exposure in Tadipo mice reduces insulin sensitivity compared with control animals [Fig. 3(d) and (e)], we repeated these clamp experiments after a short, 3-week-long HFD exposure, a time when the mice had not yet gained a large amount of weight (BW <30 g). With a shorter time course of HFD exposure, we found that the Tadipo mice were more insulin sensitive as judged by the increased glucose infusion rate and more efficient glucose disposal compared with littermate controls [Fig. 4(b–d)] without statistically significant changes in sWAT but reduced clearance into muscle. To further elaborate and analyze this protective effect of loss of TLR4 in the adipocyte in response to acute lipid exposure, we preinfused the chronic HFD-challenged animals with a lard oil emulsion prior to a standard clamp study to examine the changes associated with acute lipid exposure even after prolonged HFD exposure. This clarified this acute phenotype further. Surprisingly, Tadipo mice showed enhanced insulin sensitivity and improved glucose disposal compared with littermate controls [Fig. 4(e–g)]. During the lipid infusion clamp, lower insulin levels were found in the Tadipo mice at both basal and steady-state, suggesting that the effects on insulin sensitivity were greater than what we observed with the glucose infusion rate (Supplemental Fig. 3 (675KB, pdf) ). Following the same experimental setup and timelines as in the HFD study, we repeated the acute lipid infusion clamp studies with animals on a chow diet and found the same pattern as that observed under chronic HFD conditions (Supplemental Fig. 1 (675KB, pdf) ). Not surprisingly, this protective effect is more profound under chow-fed conditions. After 1 week of Dox chow diet, Tadipo mice receiving lard oil infusions maintain higher insulin sensitivity compared with Tadipo mice receiving control infusions during the clamp [Supplemental Fig. 1(g) and 1(h (675KB, pdf) )].
Figure 4.
Tadipo mice display increased insulin sensitivity during hyperinsulinemic euglycemic clamp with acute lard infusion after chronic HFD exposure. (a) Triglyceride clearance test (15 mL/kg BW of 20% intralipid) (n = 6 to 8). (b) Glucose infusion rate. (c) Tissue glucose uptake. (d) Glucose disposal during hyperinsulinemic euglycemic clamp after 3 weeks of HFD feeding (n = 8 to 10). (e) Glucose infusion rate. (f) Tissue glucose uptake. (g) Glucose disposal during hyperinsulinemic euglycemic clamp with lard infusion after chronic HFD feeding (n = 4 to 5). *P < 0.05 compared with TLR4fl/fl. Data are presented as means ± SEM. AUC, area under the curve; TG, triglyceride.
Tadipo mice have altered sphingolipid profiles
Similar to wild-type mice, Tadipo mice developed hepatic steatosis and a worsened metabolic profile after the chronic HFD challenge. Because TLR4 signaling may also serve as a link between obesity and lipid-induced insulin resistance (12), we ran a full sphingolipid profile on tissues collected from chow and chronic HFD induction. Although we did not observe any significant changes in total plasma ceramide levels [Supplemental Fig. 4(a) and 4(b (675KB, pdf) )], several subspecies of ceramides, dihydroceramides, and glycosylceramides were increased in the livers isolated from chow-fed Tadipo mice, whereas the livers from the chronic HFD-exposed animals were unaffected. Similarly, the sWAT from chow-fed animals showed trends toward increased sphingolipids, and sWATs from HFD-exposed mice were significantly lower almost across the entire spectrum of analytes measured [Supplemental Fig. 4(c–f (675KB, pdf) )]. Inflammation, saturated fatty acids, and adiponectin are all known factors modulating ceramide biosynthesis in different directions. The complexity of the adipocyte TLR4 deletion model is reflected in the complex readout of the ceramides. Decreased systemic and intracellular adiponectin levels are seen when inflammation is suppressed in adipocytes. A decreased inflammatory response has also been associated with reduced ceramide levels. However, decreased adiponectin levels have been associated with increased ceramide levels. These two opposing forces together seem to neutralize the effects of adipocyte TLR4 deletion on insulin sensitivity through ceramides. This can also partially explain the relatively mild metabolic phenotype observed during 8 weeks of HFD challenge.
Discussion
When we first examined the Tadipo mice, there were no obvious overall metabolic phenotypes despite a significant reduction in the inflammatory response and a reduction of adiponectin in WAT upon HFD treatment. In our previous hepatocyte TLR4 deletion model, we had reported secondary effects in the adipose tissue caused by the hepatocyte-specific loss of TLR4 upon HFD challenge (7). Hence, we expanded our analysis of the adipocyte-specific TLR4 deletion to other peripheral tissues. We found further evidence for an adipocyte, peritoneal macrophage, and liver crosstalk during the development of obesity. After HFD exposure, the TLR4 deletion in adipocytes leads to decreased TLR4 and proinflammatory gene expressions in peritoneal macrophages and the liver. We repeated the metabolic characterization and gene expression analysis of the Tadipo mice following the same experimental timeline as the HFD-treated cohort with Dox-containing chow diet. TLR4 expression in the adipocyte fraction was markedly reduced as expected as a result of the gene disruption [Supplemental Fig. 1(a (675KB, pdf) )]. However, no other secondary effects were found, indicating that the observed changes in glucose and lipid homeostasis are restricted to HFD conditions [Supplemental Fig. 1(b–f (675KB, pdf) )].
Several features of Tadipo mice share a strong resemblance to our previously reported adipocyte anti-inflammatory models. Upon chronic HFD exposure, a redistribution of adipose tissue is observed in the Tadipo mice, along with increased hepatic steatosis and impaired insulin sensitivity, a striking resemblance to the mice expressing a dominant-negative form of tumor necrosis factor α (aP2-dnTNF), which suppresses tumor necrosis factor signaling in the adipocyte (17). As we analyzed the phenotypes associated with the lack of adipose tissue remodeling due to the lack of adipose tissue inflammation, we observed a time dependency with respect to the phenotypes. Inflammation is critical to propagate healthy adipose tissue expansion and remodeling to accommodate excess lipid storage. However, elevated glucose and lipid levels can also induce acute inflammatory responses and interfere with insulin signaling. To assess the effects of adipose tissue inflammation in response to different levels of lipid exposure, we divided the Tadipo mice and littermate controls into three groups, that is, low lipid (chow), intermediate lipid (3 weeks HFD), and high lipid (chronic HFD), and measured their insulin sensitivity via hyperinsulinemic euglycemic clamps. The low- and high-lipid groups were further subdivided into acute lipid infusions to analyze the effects of acute lipid exposure. Tadipo mice maintained similar glucose infusion rates under these conditions despite exposure to different lipid backgrounds, whereas the insulin sensitivity for control mice with intact adipose tissue inflammation fluctuates with changes in dietary lipid intake. Our data suggests that the lack of adipocyte TLR4 can protect whole-body insulin sensitivity, including insulin sensitivity in muscle, during acute lipid exposure, regardless of the previous lipid exposure background. It seems to us that the mechanisms for this metabolic benefit are the reduced FFA-mediated effects on insulin signaling via TLR4 signaling due to the ablation of receptor (12).
In contrast, during chronic HFD exposure, deleting TLR4 from adipocytes creates a disadvantageous microenvironment in the adipose tissue. Although the Tadipo mice maintained similar glucose infusion rates between chow and HFD cohorts, littermate controls were able to better maintain their insulin sensitivity on high-lipid backgrounds. Contrary to the acute exposure, intact adipose inflammation protects insulin sensitivity through promoting healthy adipose tissue remodeling and expansion. Despite the observed redistribution of white adipose tissue toward the more metabolically beneficial sWAT, a significant reduction in systemic adiponectin can be viewed as a sign of unhealthy adipose tissue expansion. Because adipocyte inflammation is critical to maintain a healthy expansion potential during nutrient oversupply, the lack of TLR4-mediated inflammatory responses exacerbates whole-body insulin resistance after chronic HFD (Fig. 5).
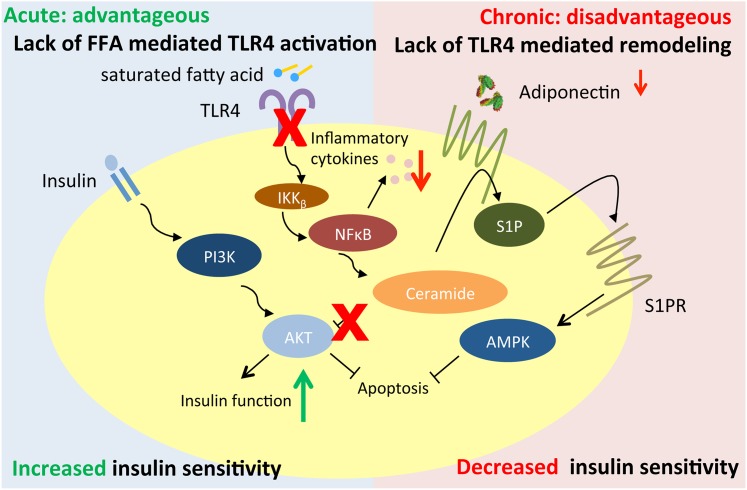
Figure 5.
Working model of adipocyte TLR4 deletion. During acute lipid exposure, the lack of adipocyte TLR4 is advantageous and can increase insulin sensitivity due to lack of FFA-mediated TLR4 activation. However, during chronic HFD exposure, lack of adipocyte TLR4 is disadvantageous and results in decreased insulin sensitivity due to the lack of TLR4-mediated adipose remodeling and healthy expansion.
When we deleted TLR4 from adipocytes, we observed both beneficial and disadvantageous phenotypes, affecting both adipocytes and peripheral tissues. We also observed an additional temporal element in systemic insulin sensitivity in response to lipid exposure. The complexity of the phenotypes displayed also highlights the importance of TLR4 in adipocytes. Our studies with the adipocyte TLR4 deletion add new insights into the contribution of TLR4 in obesity-associated inflammation and insulin resistance. It will be interesting to know whether we can promote healthy adipose tissue expansion and observe the opposite trends when we overexpress TLR4 in adipocytes during chronic HFD exposure. Insights from these TLR4 overexpression and reactivation models will further validate this working model and extend our understanding of the role of adipose inflammation triggered by both FFAs and endotoxin in obesity-induced insulin resistance.
Several elegant studies previously looked at the impact of TLR4 signaling in adipose tissue, although not specifically in the adipocyte. Coenen et al. (18) examined the role of macrophage-derived TLR4 on insulin resistance using bone marrow transplants and reached the conclusion that specific dietary conditions lead to a TLR4-dependent accumulation of macrophages in adipose tissue. The same laboratory examined macrophage polarization in adipose tissue of systemic TLR4-null mice and bone marrow transplants. TLR4 deficiency introduced by both methods generated a shift in the polarization of adipose tissue macrophages, with more macrophages seen in the alternatively activated state, associated with reduced adipose tissue inflammation. Consistent with the complex phenotype we report in this study, neither the systemic nor the macrophage-specific knockout had an impact on insulin sensitivity (19).
We think that the adipocyte-specific TLR4-null mouse is a typical example of a complex metabolic phenotype commonly observed during manipulation of inflammatory pathways in adipose tissue. The complexity is apparent when different approaches are compared. We appreciate that classical anti-inflammatory treatment regimens do not lead to an improvement in insulin sensitivity. However, there are many examples of whole-body loss of proinflammatory components, including members of the nuclear factor κB cascade and TLR4 itself that lead to an improved metabolic profile (20), yet supplying anti-inflammatory components locally at the level of adipocytes leads to increased systemic inflammation and metabolic dysfunction (17). That different readouts can be observed between short-term and long-term exposure to metabolic challenges further highlights that not only spatial (i.e., tissue-specific) effects but also temporal (acute vs chronic) elements need to be factored into the analysis of these complex inflammatory phenotypes.
Acknowledgments
We thank Dr. Bob Hammer and the Transgenic Core Facility at the University of Texas Southwestern Medical Center for generation of the transgenic lines.
Acknowledgments
This work was supported by National Institutes of Health Grants P01-DK088761 (to J.K.E. and P.E.S.) and R01-DK55758 and R01-DK099110 (to P.E.S.). Q.A.W. is supported by National Institutes of Health Grant K01DK107788. L.J. was supported by a Ruth L. Kirschstein National Research Service Award (Postdoctoral Training Grant RL9-DK081180). W.L.H is supported by National Institutes of Health Grant R00-DK094973 and by Juvenile Diabetes Research Foundation Award 5-CDA-2014-185-A-N.
Acknowledgments
Author contributions: C.T., J.K.E., and P.E.S. designed the experiments and wrote the manuscript. L.J., K.S., and Y.-c.K. generated mouse models. M.S assisted with immunohistochemistry staining. C.T., X.L., J.A.J., and W.L.H. performed clamp studies. R.G. performed sphingolipid analysis. C.T., Q.A.W., and W.L.H. performed animal experiments and analyzed data.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- adnP
- adiponectin P
- BAT
- brown adipose tissue
- BW
- body weight
- Dox
- doxycycline
- FFA
- free fatty acid
- gWAT
- gonadal white adipose tissue
- H&E
- hematoxylin and eosin
- HFD
- high–saturated fat diet
- IL
- interleukin
- ITT
- insulin tolerance test
- mRNA
- messenger RNA
- mWAT, mesenteric white adipose tissue; OGTT
- oral glucose tolerance test
- PCR
- polymerase chain reaction
- rtTA
- reverse tetracycline–controlled transactivator
- SEM
- standard error of the mean
- sWAT
- subcutaneous white adipose tissue
- Tadipo, adipocyte Toll-like receptor-null; TLR4
- Toll-like receptor 4
- WAT
- white adipose tissue.
References
- 1.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148(5):852–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dasu MR, Ramirez S, Isseroff RR. Toll-like receptors and diabetes: a therapeutic perspective. Clin Sci (Lond). 2012;122(5):203–214. [DOI] [PubMed] [Google Scholar]
- 3.Beutler BA. TLRs and innate immunity. Blood. 2009;113(7):1399–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res. 2007;100(11):1589–1596. [DOI] [PubMed] [Google Scholar]
- 5.Poggi M, Bastelica D, Gual P, Iglesias MA, Gremeaux T, Knauf C, Peiretti F, Verdier M, Juhan-Vague I, Tanti JF, Burcelin R, Alessi MC. C3H/HeJ mice carrying a Toll-like receptor 4 mutation are protected against the development of insulin resistance in white adipose tissue in response to a high-fat diet. Diabetologia. 2007;50(6):1267–1276. [DOI] [PubMed] [Google Scholar]
- 6.Tsukumo DML, Carvalho-Filho MA, Carvalheira JBC, Prada PO, Hirabara SM, Schenka AA, Araújo EP, Vassallo J, Curi R, Velloso LA, Saad MJA. Loss-of-function mutation in Toll-like receptor 4 prevents diet-induced obesity and insulin resistance. [Retracted in: Diabetes. 2016;65(4):1126–1127]. Diabetes. 2007;56(8):1986–1998. [DOI] [PubMed] [Google Scholar]
- 7.Jia L, Vianna CR, Fukuda M, Berglund ED, Liu C, Tao C, Sun K, Liu T, Harper MJ, Lee CE, Lee S, Scherer PE, Elmquist JK. Hepatocyte Toll-like receptor 4 regulates obesity-induced inflammation and insulin resistance. Nat Commun. 2014;5:3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vila IK, Badin P-M, Marques M-A, Monbrun L, Lefort C, Mir L, Louche K, Bourlier V, Roussel B, Gui P, Grober J, Štich V, Rossmeislová L, Zakaroff-Girard A, Bouloumié A, Viguerie N, Moro C, Tavernier G, Langin D. Immune cell Toll-like receptor 4 mediates the development of obesity- and endotoxemia-associated adipose tissue fibrosis. Cell Reports. 2014;7(4):1116–1129. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y, Lee H, Berg AH, Lisanti MP, Shapiro L, Scherer PE. The lipopolysaccharide-activated Toll-like receptor (TLR)-4 induces synthesis of the closely related receptor TLR-2 in adipocytes. J Biol Chem. 2000;275(32):24255–24263. [DOI] [PubMed] [Google Scholar]
- 10.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116(11):3015–3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18(8):1279–1285. [DOI] [PubMed] [Google Scholar]
- 12.Holland WL, Bikman BT, Wang L-P, Yuguang G, Sargent KM, Bulchand S, Knotts TA, Shui G, Clegg DJ, Wenk MR, Pagliassotti MJ, Scherer PE, Summers SA. Lipid-induced insulin resistance mediated by the proinflammatory receptor TLR4 requires saturated fatty acid-induced ceramide biosynthesis in mice. J Clin Invest. 2011;121(5):1858–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11(7):797–803. [DOI] [PubMed] [Google Scholar]
- 14.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, Wade MR, Tenorio VM, Kuo M-S, Brozinick JT, Zhang BB, Birnbaum MJ, Summers SA, Scherer PE. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17(1):55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270(45):26746–26749. [DOI] [PubMed] [Google Scholar]
- 17.Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coenen KR, Gruen ML, Lee-Young RS, Puglisi MJ, Wasserman DH, Hasty AH. Impact of macrophage Toll-like receptor 4 deficiency on macrophage infiltration into adipose tissue and the artery wall in mice. Diabetologia. 2009;52(2):318–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Orr JS, Puglisi MJ, Ellacott KL, Lumeng CN, Wasserman DH, Hasty AH. Toll-like receptor 4 deficiency promotes the alternative activation of adipose tissue macrophages. Diabetes. 2012;61(11):2718–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davis JE, Gabler NK, Walker-Daniels J, Spurlock ME. Tlr-4 deficiency selectively protects against obesity induced by diets high in saturated fat. Obesity (Silver Spring). 2008;16(6):1248–1255. [DOI] [PubMed] [Google Scholar]