The deployment of new parathyroid hormone (PTH)–based potential therapeutic agents has rekindled interest in many less understood actions of the hormone. Unlike the central role of PTH in the control of calcium homeostasis, which has been extensively studied, less is known about the mechanism(s) by which PTH contributes to the regulation of phosphate homeostasis. Indeed, the remarkable actions of the hormone to mediate profound effects on phosphate metabolism via fibroblast growth factor 23 (FGF23) and the osteocyte has led to a revolution in efforts to understand this action (1). FGF23 is now considered part of a previously unrecognized hormonal bone-parathyroid-kidney axis, primarily modulated by PTH with contributions from dietary and serum phosphorus, as well as by 1,25(OH)2vitamin D [1,25(OH)2D] levels (2).
FGF23 is synthesized as a 251-amino acid protein primarily in osteocytes. Cleavage of its signal peptide results in a mature peptide that can be secreted in an intact form [intact FGF23 (iFGF23)] or further processed to N-terminal and C-terminal [C-terminal FGF23 (cFGF23)] fragments (Fig. 1). Hydrolysis occurs within an RXXR domain that is subject to remarkable regulatory controls that can go awry in ways that lead to disordered phosphate homeostasis (3). FGF23 synthesis and secretion is positively regulated by 1,25(OH)2D and serum phosphorus and negatively regulated, via as yet unknown mechanisms, by the phosphate-regulating gene with homologies to endopeptidases on the X chromosome as well as dentin matrix protein 1. In turn, FGF23 inhibits the synthesis of 1,25(OH)2D, which- may negatively regulate the secretion of PTH from the parathyroid glands. However, the details of any acute PTH regulation of FGF23 are largely unknown.
Figure 1.
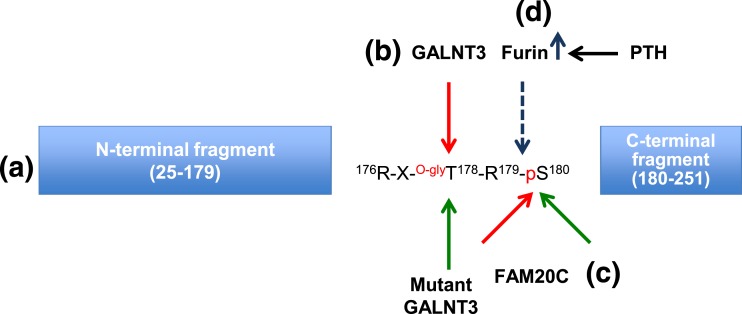
Schematic representation of the processing of FGF23. (a) FGF23 domains (N terminal and C terminal) are produced after specific cleavage. (b) Glycosylation (GALNT3) (red arrow) at T178 stabilizes FGF23, whereas GALNT3 inactivation (green arrow) increases FGF23 cleavage. (c) Phosphorylation by FAM20C (red arrow) increases FGF23 cleavage, whereas inhibition of phosphorylation at S180 [mutant FAM20C (green arrow)] stabilizes iFGF23. (d) Furin/subtilisin-like convertase identified by Knab et al. (4), is upregulated by PTH treatment and stimulates cleavage of FGF23 at R179/S180.
Act 1
In the accompanying article, Knab et al. (4) provide an incisive assessment of the impact of a single subcutaneous PTH(1-34) injection (50 nmol/kg) on plasma FGF23 levels using enzyme-linked immunosorbent assays that measure either iFGF23 or iFGF23 plus cFGF23 in mice. This study provides insight into the acute effects of PTH on FGF23 in vivo and has uncovered several extremely interesting and thought-provoking results. First, Knab et al. independently validated the iFGF23 and cFGF23 assays and demonstrated PTH regulation of FGF23 processing, which should be commended. The cFGF23 assay uses two antibodies directed against different epitopes within the C terminus for capture and detection and thus measures both iFGF23 and cFGF23 fragments. In contrast, the iFGF23 assay detects only the intact molecule. This is important to recognize because the iFGF23 and cFGF23 assays are expressed in different units; therefore, to get specific ratios, the authors must report relative changes. Next, the examination of FGF23 messenger RNA and protein levels in bone supported the assay data and provided evidence of specific in vivo actions. Indeed, in differentiated calvarial-derived osteocyte-like cells (with all their obvious caveats), they demonstrated that PTH treatment increased FGF23 messenger RNA expression through cAMP/PKA, but not IP3/PKC signaling. In addition, in using mice treated with a furin inhibitor in vivo, the authors clearly demonstrated equivalent increases in both cFGF23 and iFGF23 levels after PTH.
These studies are valuable because the observed significant increases in cFGF23 levels (three- to fivefold within 2 hours) after PTH injection in vivo occur in the face of iFGF23 levels that are unchanged for the first 2 hours, an observation consistent with the idea that FGF23 production in bone increases; however, rapid cleavage of the secreted intact protein is also induced. Mechanistically, this is difficult to reconcile, and it is not directly examined in the study; however, there is the suggestion that PTH stimulates furin (a ubiquitous subtilisin-like proprotein convertase) (Fig. 1) to snip the tail of FGF23 because furin levels are increased by PTH in vitro. These efforts re-address the role of PTH, FGF23, and osteocyte and expand our focus and interest in PTH to include consideration of its substantial contributions to phosphate homeostasis in addition to calcium.
We should thank the authors for reminding us of the importance of both these important mineral ions.
Act 2
As with all high-quality studies, more questions are generated than answered. Do levels of iFGF23 or cFGF23 change in disease states, and if so, is this clinically relevant?
The processing of FGF23 from intact molecule to C-terminal fragments is indeed remarkably reminiscent of the processing of PTH into bioactive (or not) fragments (5, 6) and PTH-related protein (PTHrP) processing (7–9). Such similarities in hormonal regulators of the bone-parathyroid-kidney axis suggest a conservation of function and potentially therefore of the regulators of these processing events. Indeed, the mechanisms by which PTH, PTHrP, and iFGF23 are rapidly degraded/processed warrants further investigation. The implication that furin is involved, although interesting, remains largely speculative. Indeed, how an enzyme that is the major processing enzyme of the secretory pathway and localized in the trans-Golgi network (10) could mediate such rapid and specific events in the skeleton is entirely unknown. The answers to these and many other intriguing and challenging questions will hopefully be addressed in future studies.
In sum, Knab et al. (4) provide the field with information regarding the sequence of events after acute PTH administration. They describe a mechanism where PTH directly affects FGF23 processing, and by reminding us of the important interactions between these two important phosphate regulators, open directions for continued interrogation. It remains to be seen whether these data will be important in humans and if the field will be reinvigorated to explore the considerable intersections between the PTH and FGF23.
Acknowledgments
This work was supported by the National Institutes of Health Grants R01 DK105811 (to P.A.F.) and R01 CA166060 (to L.J.S.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- 1,25(OH)2D
- 1,25(OH)2vitamin D
- cFGF23
- C-terminal FGF23
- FGF23
- fibroblast growth factor 23
- iFGF23
- intact FGF23
- PTH
- parathyroid hormone
- PTHrP
- PTH-related protein.
References
- 1.Gonciulea AR, Jan De Beur SM. Fibroblast Growth Factor 23-mediated bone disease. Endocrinol Metab Clin North Am. 2017;46(1):19–39. [DOI] [PubMed] [Google Scholar]
- 2.Bergwitz C, Jüppner H. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med. 2010;61:91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhattacharyya N, Chong WH, Gafni RI, Collins MT. Fibroblast growth factor 23: state of the field and future directions. Trends Endocrinol Metab. 2012;23(12):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Knab VM, Corbin B, Andrukhova O, Hum JM, Ni P, Rabadi S, Maeda A, White KE, Erben RG, Jüppner H, Christov M. Acute parathyroid hormone injection increases C-terminal but not intact fibroblast growth factor 23 levels. Endocrinology. 2017;158(5):1130–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Erdmann S, Burkhardt H, von der Mark K, Müller W. Mapping of a carboxyl-terminal active site of parathyroid hormone by calcium-imaging. Cell Calcium. 1998;23(6):413–421. [DOI] [PubMed] [Google Scholar]
- 6.Lim SK, Gardella TJ, Baba H, Nussbaum SR, Kronenberg HM. The carboxy-terminus of parathyroid hormone is essential for hormone processing and secretion. Endocrinology. 1992;131(5):2325–2330. [DOI] [PubMed] [Google Scholar]
- 7.Cramer SD, Chen Z, Peehl DM. Prostate specific antigen cleaves parathyroid hormone-related protein in the PTH-like domain: inactivation of PTHrP-stimulated cAMP accumulation in mouse osteoblasts. J Urol. 1996;156(2 Pt 1):526–531. [DOI] [PubMed] [Google Scholar]
- 8.de Miguel F, Fiaschi-Taesch N, Lopez-Talavera JC, Takane KK, Massfelder T, Helwig JJ, Stewart AF. The C-terminal region of PTHrP, in addition to the nuclear localization signal, is essential for the intracrine stimulation of proliferation in vascular smooth muscle cells. Endocrinology. 2001;142(9):4096–4105. [DOI] [PubMed] [Google Scholar]
- 9.Washam CL, Byrum SD, Leitzel K, Ali SM, Tackett AJ, Gaddy D, Sundermann SE, Lipton A, Suva LJ. Identification of PTHrP(12-48) as a plasma biomarker associated with breast cancer bone metastasis. Cancer Epidemiol Biomarkers Prev. 2013;22(5):972–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner DF. The proprotein convertases. Curr Opin Chem Biol. 1998;2(1):31–39. [DOI] [PubMed] [Google Scholar]