Abstract
Background:
Exercise is safe and beneficial for people with multiple sclerosis (MS). Functional electrical stimulation (FES) cycling offers people with significant weakness and mobility challenges an option for exercise. We sought to evaluate the safety of FES cycling and its potential to improve fatigue, pain, spasticity, and quality of life in people with moderate-to-severe MS.
Methods:
Sixteen participants with MS who were nonambulatory cycled for 30 minutes two to three times a week for 1 month. Outcomes assessed included MS Quality of Life Inventory (MSQLI) subscales, Modified Ashworth Scale (MAS), and manual muscle test (MMT).
Results:
Fourteen participants (six women and eight men) with MS completed the training. All were able to maintain or increase their cycle time; half increased the resistance while cycling. Participants demonstrated a significant decrease in the Physical (P = .02) and Psychosocial (P < .01) subscales of the Modified Fatigue Impact Scale. There was no significant change in the other MSQLI subscale scores. There was no change in MAS and MMT scores. Type of MS and the use of antispasticity medications, disease-modifying therapies, or dalfampridine did not seem to influence response to training. There were no adverse events.
Conclusions:
Functional electrical stimulation cycling may be a viable and effective exercise option for people with moderate-to-severe MS. Further study is required to examine the parameters of FES cycling that are most effective for people with different MS symptoms and to fully explore the potential benefits of optimizing function and improving health in people with MS.
People with multiple sclerosis (MS) who are nonambulatory are at the greatest risk of reduced physical activity levels.1–4 Low levels of physical activity can lead to other health conditions, such as cardiovascular disease, obesity, and diabetes,5–7 that will only further impair mobility and function in individuals who use wheeled mobility. Structured exercise is one way to increase physical activity; however, exercise options for people who use wheeled mobility are limited.
Functional electrical stimulation (FES) cycling provides an avenue for exercise in people with lower-limb weakness and mobility restrictions.8–19 Using surface electrical stimulation to muscles of the lower extremities, the FES cycle can provide for passive, active with volitional control, or FES-assisted cycling. The newest FES devices allow the individual to remain seated in a supported position in front of the cycle ergometer, minimizing the need for wheelchair transfers.
Studies of FES cycling in people with weakness or paralysis in the lower limbs due to stroke or spinal cord injury have demonstrated improvements in exercise capacity,8–10 muscle mass11,12 and strength,13–15 and spasticity.15,16 People with moderate-to-severe MS, who have weakness or paralysis in their lower extremities, may benefit in similar ways. Four recent studies have explored the potential of FES cycling in people with MS.17–20 Ratchford et al.19 reported that participants who were ambulatory and trained three times a week for 6 months on the FES cycle demonstrated improvements in strength of stimulated muscles and in walking endurance and speed. Physical and mental health scores and quality of life also improved. These findings suggest that FES cycling is safe for people with MS who have Expanded Disability Status Scale (EDSS) scores less than 6.5 and may provide some benefit for people who have ambulatory capacity. Those with moderate-to-severe MS, with EDSS scores of 6.5 or greater, may also benefit from FES cycling but have not been adequately studied. The three studies that included people with EDSS scores greater than 6.5, one of which was a single case study, demonstrated that FES cycling in this population with more severe MS is safe (ie, they report no adverse events), but they did not explore the effect on impairments and function.17,18,20 In addition, Szecsi et al.18 used a 2-week intervention, so it is unclear what the impact would be of a longer intervention on people with moderate-to-severe MS.
The goals of this study were to evaluate the safety of an FES cycle training program and to determine whether people with severe weakness or paralysis due to MS can improve performance on the FES cycle, indicating an ability to improve leg function. In addition, we evaluated the potential for FES cycling to improve symptoms of MS, including fatigue, spasticity, and pain, as well as quality of life.
Methods
Participants
All the study procedures were approved by the Shepherd Center research review board, and all the participants provided written informed consent before proceeding with study activities. Participants were recruited from the local MS program and community via word of mouth and online. To be included in this study, individuals had to be older than 18 years with physician-diagnosed MS and cleared by a physician to participate in the study activities. We did not establish an upper age limit for this study because inclusion was based on impairment and safety. Participants had to be unable to ambulate more than 70 feet and not ambulating outside the home. Individuals were excluded if they had experienced a diagnosed relapse in the past 6 months; had known cardiovascular disease (ie, previous myocardial infarct, unstable angina, congestive heart failure, history of arrhythmia, or stroke) or uncontrolled hypertension; had a history of epileptic seizures; had lower motor neuron disease or peripheral neuropathy in the lower limb; had an existing pacemaker, defibrillator, or other implanted electronic or metallic device (other than a baclofen pump); had unstable long bone fractures of the lower limb or trunk; had allergy to surface electrodes or conductive gel; or were unable to tolerate sitting for at least 1 hour. These exclusion criteria were verified using medical records and through physician clearance for participation. Each physician reviewed the inclusion and exclusion criteria before providing medical clearance. Participants were also excluded if the investigators could not elicit a contraction from the quadriceps or hamstring muscles during prescreening. Each participant underwent testing to determine whether his or her muscles would respond to the electrical stimulation provided by the FES cycle. If there was at least a visible muscle contraction, the individual was enrolled; if not, the individual was not enrolled in the study.
Procedures
All the study procedures were performed in an outpatient clinical space of a nonprofit long-term acute-care facility. All the assessments were performed by a trained physical therapist (CM), and training on the FES cycle was overseen by an exercise specialist (LH, BB).
Apparatus
The FES cycle training was performed on the RT300 developed by Restorative Therapies, Inc (Baltimore, MD) (Figure 1). The RT300 is a standalone device designed for easy access by individuals with mobility limitations. Participants remained in their wheelchair and positioned themselves in front of the ergometer to cycle. A trained exercise specialist assisted each participant in applying the surface electrodes and safely positioning the participant's lower limbs on the pedals. Once set up, participants were able to cycle independently, with assistance from the electrical stimulation as needed.
Figure 1.
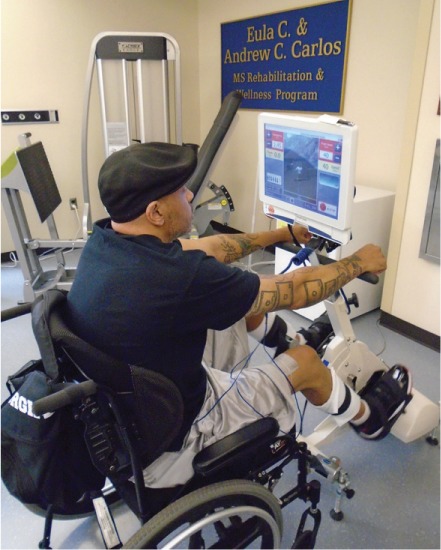
Participant seated at the RT300 device developed by Restorative Therapies, Inc.
FES Cycle Training Protocol
Participants trained three times a week for 4 weeks, for a total of 12 sessions. Four weeks was chosen based on the assumption that this was long enough to test the safety of FES cycling and that if the participant had the capacity for any change in performance or function, this should be evident in this period. This duration was not expected to elicit the greatest change possible but rather information that would be useful for future, larger-scale studies. The stimulation parameters for this study were predetermined based on previous studies of FES cycling in people with complete spinal cord injury and were set at a pulse width of 200 μs21 and a frequency of 50 Hz.22 We used surface electrodes to stimulate the participants' quadriceps, hamstrings, and gluteal muscles. The stimulation intensity varied based on the participant's tolerance and the amount of stimulation required to achieve the target cycling speed of 35 to 50 rpm.21,22
Each session began with a 2-minute warm-up of passive cycling at 35 rpm (no voluntary cycling or electrical stimulation). During this phase, the motor of the ergometer propelled the pedals. This passive phase was followed by a 30-minute active (voluntary or assisted with electrical stimulation) phase, during which the electrical stimulation ramped on to stimulate the muscles to assist active cycling. The session then ended with a 2-minute cool-down of passive cycling.
The goal of each active phase was to achieve a sustained pedal rate of 35 to 50 rpm for 30 minutes. The trainer instructed each participant at the beginning of each session and encouraged him or her throughout the session to maintain the target speed for the duration of the session. If a participant could not maintain at least 35 rpm, even with electrical stimulation, the stimulation automatically turned off and the FES cycle went into passive mode whereby the motor of the ergometer continued to cycle the legs for a full 30-minute session. This was done based on the assumption that if the muscle could no longer respond adequately to electrical stimulation, it was fatigued and, therefore, should no longer be stimulated. However, we wanted to continue the session with the passive cycling for a full 30 minutes of cycling. Once a participant could pedal for 30 minutes at 35 to 50 rpm for three consecutive sessions without defaulting to the passive mode (no active movement with or without stimulation), resistance was added. Resistance was increased in 0.14-Nm increments, and the process was repeated. Each participant progressed at his or her own rate and was monitored for reports of fatigue, pain, and spasticity during and after each session.
Participants were questioned before the start of each FES cycle training session regarding their experience after the previous training session and again immediately after the training session to determine whether they had any significant pain or fatigue or limitations in function due to the cycling. Participants were also monitored throughout each training session.
Outcome Measures
The primary outcome measure was safety measured by adverse events and any increase in symptoms that remained or worsened between sessions. An adverse event was defined as any increase in MS-related symptoms or injury deemed related to the training session. The exercise specialist (trainer) overseeing each FES cycle training session documented participant self-report of fatigue, pain, and spasticity at the beginning and end of each session using a Likert scale for each assessment (0 being no perception of the symptom and 10 being severe perception of the symptom). The trainer also monitored participant symptoms throughout the session, frequently asking the participant how he or she was feeling and whether there was any increase in symptoms; if there was any indication of a problem, this was documented in the participant records for that day. Participants were specifically questioned at the beginning and end of each session regarding any changes in impairment or functional abilities that may have occurred between the two training sessions.
As a measure of ability to improve during training, data related to FES cycling performance were collected offline for each training session and included the amount of time spent in active cycling, the amount of resistance (if applied), and the distance cycled. All the following measures have been used in people with MS, are validated for use in the MS population, and were collected before training (pretest) and within 1 week after the completion of training (posttest).
After the participant provided informed consent, the study coordinator administered the MS Quality of Life Index (MSQLI)23,24 to assess quality of life. The MSQLI is an MS-specific health-related quality of life instrument consisting of the 36-item Short Form Health Status Survey (SF-36) supplemented by nine symptom-specific measures: fatigue, pain, bladder function, bowel function, emotional status, perceived cognitive function, visual function, sexual satisfaction, and social relationships.
During the next testing session, on a separate day, a trained physical therapist performed measures of spasticity and strength in the lower limbs. The Modified Ashworth Scale (MAS)25 was performed first to assess spasticity. Bilateral hip flexors, knee flexors and extensors, and ankle dorsiflexors were graded separately, and a total score for each limb was obtained. A manual muscle test26 was then performed to assess strength; a grade of 0 to 5 was assigned to each lower-limb muscle. A total score was computed for each leg for data analysis.
Data Analysis
All the data were entered into a database for analyses. Descriptive analyses were performed to determine means, change scores, and percentage change using a statistical software program (IBM SPSS Statistics version 22 for Windows; IBM Corp, Armonk, NY). Paired two-tailed t tests were used to compare the pretraining and posttraining effects of FES cycling, that is, for time, distance, speed, and power output. Statistical significance was set at P < .05. No corrections for multiple comparisons were made because this was a pilot study and exploratory in nature.
Effect size indicates the standardized difference between two dependent means and expresses this relationship in standard deviation (SD) units. We determined the effect size using Cohen's d formula for dependent, single-group, pre-post change.27 The formula takes the difference between pretest and posttest means for the group and divides it by the baseline variance. Baseline variance is the SD for the first period (pre) of measurement. Effect size (Cohen's d) for dependent means differences (matched pairs t tests) is calculated by the following equation27:
To determine the effect size for change in resistance on the FES cycle, Cohen's d was modified because the baseline variance (SD) is zero. Therefore, the paired difference SD was used to calculate effect size. Using Cohen's d, 0.2 is considered a small effect size, 0.5 a medium effect size, and 0.8 a large effect size.27
Results
Participant Characteristics
Fourteen participants (seven women) completed training for this study. Participant demographic characteristics are presented in Table 1. The mean ± SD age was 55.28 ± 10.98 years (range, 31–70 years), and the mean ± SD approximate time since diagnosis was 15.29 ± 7.35 years (range, 3–28 years). Five participants were not receiving any disease-modifying therapy, and three were receiving natalizumab, four interferon beta-1a, and two dimethyl fumarate. Nine participants (64%) had progressive MS, and two of these were physician diagnosed as having primary progressive MS (PPMS).
Table 1.
Participant demographics and summary of performance on the FES cycle and amount of change
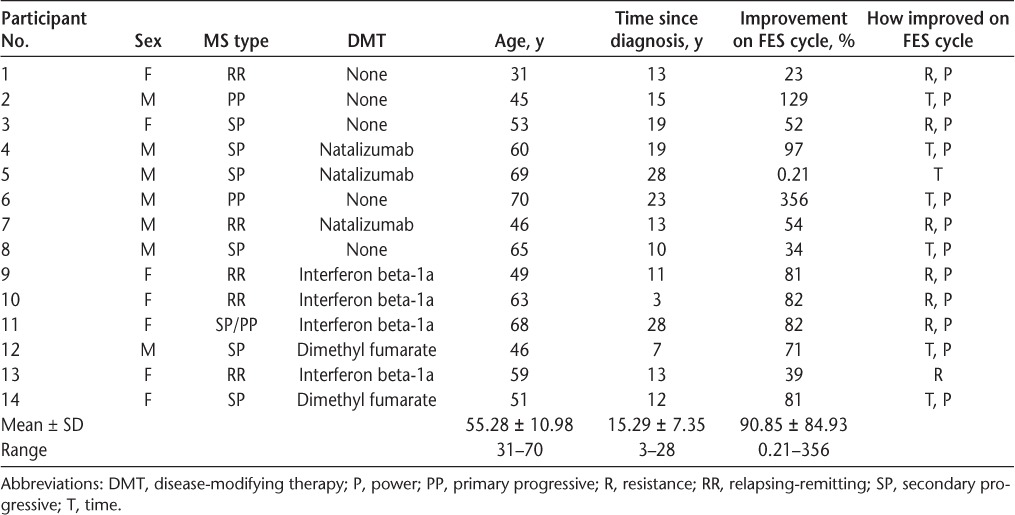
No adverse events or observed increases in MS-related symptoms (ie, fatigue, pain, or spasticity) were experienced by participants within or between sessions for this study. All the participants demonstrated improved performance on the FES cycle, either by increasing the amount of time cycling or by increasing the amount of resistance provided during the session, suggesting that there were no detrimental effects of the training.
FES Cycling Performance
All the participants required at least minimal electrical stimulation during the active cycling time. The mean ± SD percentage of improvement in performance, as measured by resistance and time during the active phase on the FES cycle, was 84% ± 85% (range, 0.21%–356%). One participant demonstrated a less than 1% change between pretest and posttest. When this person is removed from the group, the mean ± SD percentage of improvement is 95% ± 87% (range, 23%–356%). Twelve participants (86%) significantly increased their mean ± SD power output from pretest (2.10 ± 1.01) to posttest (3.17 ± 1.97) (P = .01), and this was a large effect size (Cohen's d = 1.06).
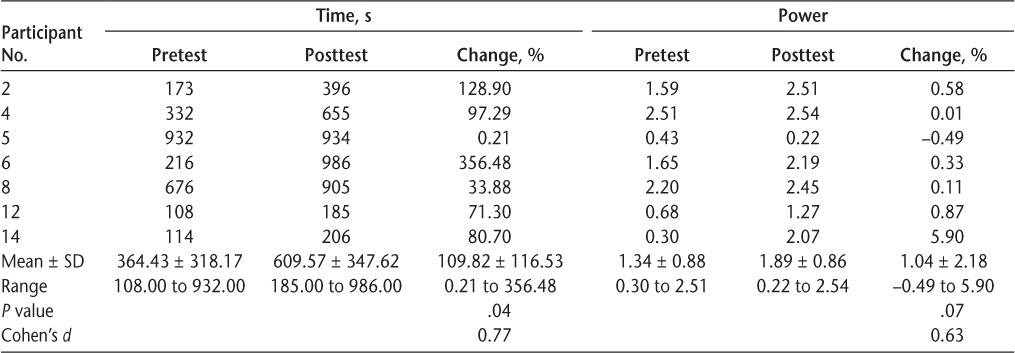
Half of the group (n = 7) was not able to cycle for 30 minutes during the active phase, either voluntarily or with FES. Of these participants, six were men, and all seven had some form of progressive MS (two had PPMS). Table 2 shows that all seven of these participants significantly increased their active cycling time (P = .04), and this improvement was large in magnitude (Cohen's d = 0.77). One participant increased his time cycling by only 0.21%; however, the remainder of the group ranged from 33.88% to 356.48% improvement after 4 weeks of FES cycle training. All but one of these participants were also able to increase their average power output from pretraining to posttraining, and this was of medium magnitude (Cohen's d = 0.63). However, this change was not significant (P = .07). Both the least amount (0.21%) and the greatest amount (356.48%) of improvement were seen in individuals diagnosed as having PPMS. The participant with the least amount of improvement had been diagnosed as having MS approximately 28 years ago (the longest of all participants), whereas the participant with the most improvement had been diagnosed 23 years ago (the second longest of all participants).
Table 2.
Time and power data for participants who could not cycle 30 minutes at any point during the trial
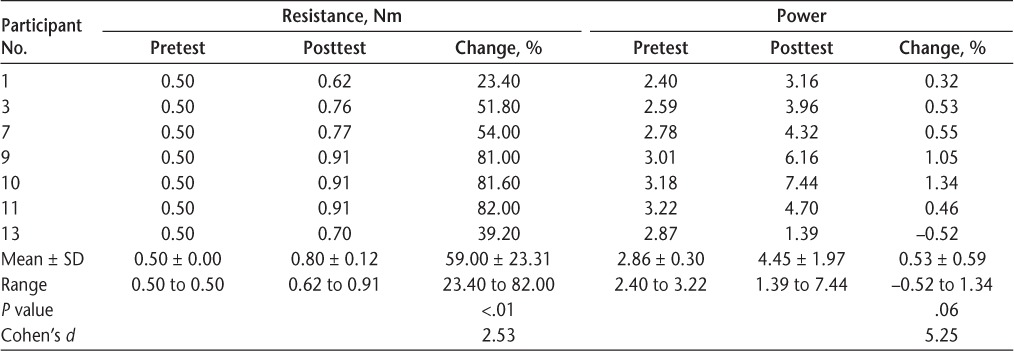
The other half of the group (n = 7) could actively cycle 30 minutes by the end of the second training session. Six of these participants (86%) were women, and five (71%) had relapsing-remitting MS. Table 3 shows that this group significantly increased resistance during cycling (P < .01) between pretest and posttest, ranging from 23.40% to 82.00% improvement, and this change was of large magnitude (Cohen's d = 2.53). A Cohen's d greater than 2 indicates a difference between means that is greater than 2 SD. All but one of the participants were able to increase their power over the course of training, and this change was of a large magnitude (Cohen's d = 5.25). This change was not significant (P = .06). The least and greatest amounts of improvement (ie, change in resistance) were seen in participants with relapsing-remitting MS. The greatest amount of improvement was found in the participant diagnosed as having MS only 3 years ago (the shortest time since diagnosis of all 14 study participants). The participant with the least amount of improvement was diagnosed 13 years ago.
Table 3.
Resistance and power for participants who could cycle 30 minutes per session while training
Impairment Outcomes
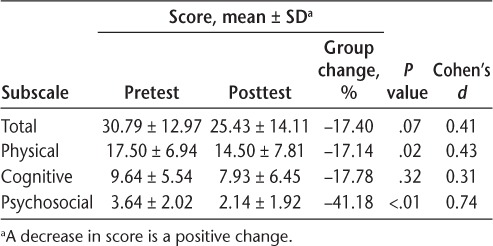
There was no significant increase or decrease in muscle strength or spasticity in either lower limb as indicated by manual muscle test (left P = .68, right P = .30) and MAS (left P = .11, right P = .79) scores (Table 4). Ten of 14 participants (71%) demonstrated a nonsignificant decrease in the total Modified Fatigue Impact Scale (fatigue) scores from 30.79 ± 12.97 to 25.43 ± 14.11 (P = .07). However, there was a significant decrease in the Physical (P = .02) and Psychosocial (P < .01) subscales of the Modified Fatigue Impact Scale (Table 5). The magnitude of this change ranged from small to large (Cohen's d = 0.31–0.74), with the largest effect size for the Psychosocial subscale (Cohen's d = 0.74).
Table 4.
MMT and MAS scores (n = 13)
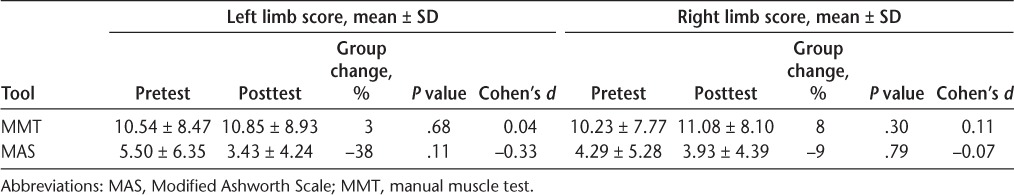
Table 5.
Modified Fatigue Impact Scale scores for the 14 study participants
Seven of the 13 participants (54%) who completed the Medical Outcomes Study (MOS) Pain Effects Scale reported a decrease in pain after completion of the 4 weeks of FES cycle training. The group MOS Pain Effects Scale mean ± SD total score decreased from 14.62 ± 5.56 to 11.54 ± 4.63 after training, and this change was not significant (P = .08), with a medium effect size (Cohen's d = −0.55).
There was no significant change in the Mental Health Inventory (MHI) score after training (P = .18), but there was a significant change in the Positive Affect subscale score of the MHI (P < .01). Most participants (79%) reported a decrease in their Positive Affect score.
The only significant and meaningful change in the SF-36 was in the Social subscale (P < .01; Cohen's d = 0.90), and 11 participants (79%) demonstrated an improvement in this subscale score (Table 6).
Table 6.
36-item Short Form Health Status Survey scores for the 14 study participants
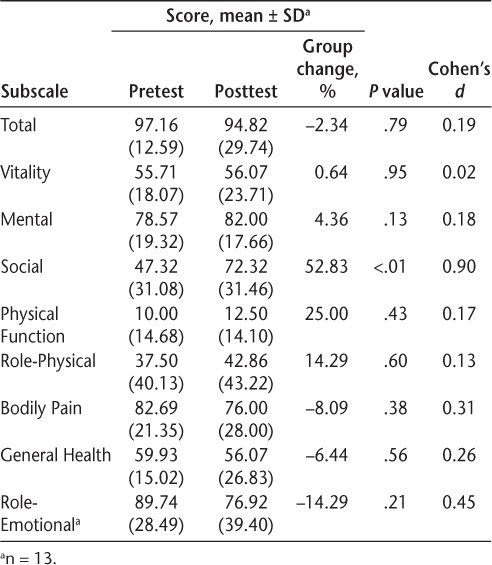
There was no change in scores on the Sexual Satisfaction Scale, Bladder Control Scale, Bowel Control Scale, Impact of Visual Impairment Scale, Perceived Deficits Questionnaire, and MOS Modified Social Support Survey of the MSQLI.
Discussion
This study extends earlier findings that FES cycling is a safe intervention for people with MS, including those with moderate-to-severe MS, who are generally nonambulatory. In addition, we found that people who are nonambulatory not only are able to exercise but also may demonstrate training effects as noted through improvements in the ability to cycle. Finally, this was the first study to demonstrate that people with moderate-to-severe MS who are nonambulatory can achieve physical benefit from exercise with the FES cycle, as well as improved quality of life.
Ratchford et al.19 found that FES cycling is safe for people with EDSS scores of 6.0 to 6.5 based on the lack of negative effects in cerebrospinal fluid cytokine markers and no reports of serious adverse events. Although we did not collect data related to cytokine markers, the present data extend these findings by including people who were nonambulatory and support the earlier findings related to safety in these people with moderate-to-severe MS.
Also of interest is the fact that some people could cycle for only a short duration, whereas others were able to cycle for the full 30-minute protocol and increase in resistance over the duration of training. This demonstrates the variability between individuals. Further study is required to determine which parameters will work for each specific individual to optimize outcomes.
Although participants progressed in their ability to cycle on the FES cycle, they did not demonstrate a measurable change in spasticity via the MAS after 4 weeks of training. This is different from what is reported by Krause et al.,17 who reported that the participant in their case study experienced decreased spasticity. Because that was a case study, it is possible that this individual was different from those in the present study. However, similar to Szecsi et al.,18 the present participants who had spasticity reported decreased spasticity on the Likert scale immediately after the FES cycling session.
Also similar to Szecsi et al.,18 we did not find significant changes in muscle strength in any of the muscles stimulated in the participants, whereas Ratchford et al.19 reported an increase in strength of the muscles stimulated in their study. This may be because participants in the study by Ratchford et al.19 had less disability than those in the present study and may have had more capacity for improvements in muscle strength with this type of training than did those in the present study. Furthermore, the participants in the study by Ratchford et al. cycled for 1 hour each session, on average 3.8 times a week for 6 months, receiving a much greater dosage of FES cycling than those in the present study. Finally, limitations related to the manual muscle test may also account for the lack of change. Quantitative assessment of strength would likely provide more insight into changes in muscle strength and should be considered for future studies. Further research is also warranted to determine whether there would be greater effects on muscle strength in people with moderate-to-severe MS, as in the present study, if a greater volume of training was performed. The present participants did demonstrate improvements in power output on the FES cycle. Although not statistically significant, the effect was medium to large, suggesting that participants were able to improve in their output on the FES cycle. Although not a direct measure of muscle strength, this does demonstrate the capacity for the muscle to improve in function and requires further investigation.
Improvement in the Social subscale of the SF-36 is a positive outcome and may be explained by the fact that the participants in this study are generally underserved and often do not receive much attention or rehabilitation related to their MS. Being enrolled in the study alone may have induced the Hawthorne effect and affected their scores on this subscale.28 The decrease in the Positive Affect subscale score of the MHI in most participants is of concern. However, one explanation could be that participants in the present study reported that they did not want to discontinue their FES cycle training, and this discontent may have been reflected in the scores on the Positive Affect subscale. Further research is required to explore the effect of exercise on social and physical outcomes in people with moderate-to-severe MS.
Although promising, findings from this study should be viewed with some caution given the small number of participants and the lack of a control group. Furthermore, given the nature of the study, we had to choose a protocol that may or may not have been the most beneficial for all people in the study. A different method of delivery, that is, frequency and duration of training, may have yielded different results. Given that the FES cycle defaults to start with 0.5 Nm of resistance, we are not truly clear about each participant's starting capacity. Because the study was only 4 weeks in duration, we also do not know participants' full potential for change. Further study is required to explore the efficacy of this protocol in more individuals with moderate-to-severe MS and to compare this with other potentially effective protocols. We also did not have a measure of function. Although participants reported that they experienced increased ease with transfers and household ambulation, we did not objectively collect or measure these changes. The fact that there were improvements in the speed and quality of walking in the study by Ratchford et al.19 suggests that including functional outcome measures may be beneficial for future studies to determine the effect of FES cycling on function in people with moderate-to-severe MS. Finally, although we collected information related to the disease-modifying therapies that the participants were using, the type of MS they had, their age, sex, and time since diagnosis, we did not control for these variables and did not have enough participants to evaluate whether they affected performance in this protocol. Future studies should explore the effect of these variables, as well as other medications, on FES cycle training.
Conclusion
Although this was a pilot study, it provides meaningful information. First, FES cycling may be a safe, accessible, and effective option for people with moderate-to-severe MS who are nonambulatory. That people with moderate-to-severe MS demonstrate improvements in performance while cycling, as well as in physical symptoms, after only 4 weeks of intervention suggests that they respond favorably to this intervention. Although further research is warranted to fully understand the potential for FES cycle training to decrease disability and improve health outcomes, clinicians working with people with MS should consider exploring this and other exercise options for their clients with moderate-to-severe MS who are nonambulatory.
PracticePoints
Functional electrical stimulation (FES) cycle training may be a safe and viable option for exercise for individuals with MS who are nonambulatory.
Individuals with MS who are nonambulatory are able to improve performance on the FES cycle after training for 30 minutes three times a week for 4 weeks.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
References
- 1. Beckerman H, de Groot V, Scholten MA, Kempen JC, Lankhorst GJ.. Physical activity behavior of people with multiple sclerosis: understanding how they can become more physically active. Phys Ther. 2010; 90: 1001– 1013. [DOI] [PubMed] [Google Scholar]
- 2. Motl RW. Physical activity and irreversible disability in multiple sclerosis. Exerc Sport Sci Rev. 2010; 38: 186– 191. [DOI] [PubMed] [Google Scholar]
- 3. Vanner EA, Block P, Christodoulou CC, Horowitz BP, Krupp LB.. Pilot study exploring quality of life and barriers to leisure-time physical activity in persons with moderate to severe multiple sclerosis. Disabil Health J. 2008; 1: 58– 65. [DOI] [PubMed] [Google Scholar]
- 4. Gulick EE, Goodman S.. Physical activity among people with multiple sclerosis. Int J MS Care. 2006; 8: 121– 129. [Google Scholar]
- 5. Thijssen DH, Maiorana AJ, O'Driscoll G, Cable NT, Hopman MT, Green DJ.. Impact of inactivity and exercise on the vasculature in humans. Eur J Appl Physiol. 2010; 108: 845– 875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ranadive SM, Yan H, Weikert M, . et al. Vascular dysfunction and physical activity in multiple sclerosis. Med Sci Sports Exerc. 2012; 44: 238– 243. [DOI] [PubMed] [Google Scholar]
- 7. Motl RW, McAuley E, Snook EM.. Physical activity and multiple sclerosis: a meta-analysis. Mult Scler. 2005; 11: 459– 463. [DOI] [PubMed] [Google Scholar]
- 8. Ferrante S, Pedrocchi A, Ferrigno G, Molteni F.. FES cycling treatment on hemiplegic patients: preliminary results. : Proceedings of the 11th Annual Conference of the International Functional Electrical Stimulation Society. Milan, Italy: International Functional Electrical Stimulation Society; 2006; 12– 15. [Google Scholar]
- 9. Ambrosini E, Ferrante S, Ferrigno G, Molteni F, Pedrocchi A.. Cycling induced by electrical stimulation improves muscle activation and symmetry during pedaling in hemiparetic patients. IEEE Trans Neural Syst Rehabil Eng. 2012; 20: 320– 330. [DOI] [PubMed] [Google Scholar]
- 10. Thrasher TA, Ward JS, Fisher S.. Strength and endurance adaptations to functional electrical stimulation leg cycle ergometry in spinal cord injury. NeuroRehabilitation. 2013; 33: 133– 138. [DOI] [PubMed] [Google Scholar]
- 11. Castro MJ, Apple DF Jr, Staron RS, Campos GE, Dudley GA.. Influence of complete spinal cord injury on skeletal muscle within 6 mo of injury. J Appl Physiol. 1999; 86: 350– 358. [DOI] [PubMed] [Google Scholar]
- 12. Sköld C, Lönn L, Harms-Ringdahl K, . et al. Effects of functional electrical stimulation training for six months on body composition and spasticity in motor complete tetraplegic spinal cord-injured individuals. J Rehabil Med. 2002; 34: 25– 32. [DOI] [PubMed] [Google Scholar]
- 13. Ferrante S, Pedrocchi A, Ferrigno G, Molteni F.. Cycling induced by functional electrical stimulation improves the muscular strength and the motor control of individuals with post-acute stroke: Europa Medicophysica-SIMFER 2007 Award Winner. Eur J Phys Rehabil Med. 2008; 44: 159– 167. [PubMed] [Google Scholar]
- 14. Bélanger M, Stein RB, Wheeler GD, Gordon T, Leduc B.. Electrical stimulation: can it increase muscle strength and reverse osteopenia in spinal cord injured individuals? Arch Phys Med Rehabil. 2000; 81: 1090– 1098. [DOI] [PubMed] [Google Scholar]
- 15. Ambrosini E, Ferrante S, Pedrocchi A, Ferrigno G, Molteni F.. Cycling induced by electrical stimulation improves motor recovery in post-acute hemiparetic patients: a randomized controlled trial. Stroke. 2011; 42: 1068– 1073. [DOI] [PubMed] [Google Scholar]
- 16. Chen CC, He ZC, Hsueh YH.. An EMG feedback control functional electrical stimulation cycling system. J Signal Process Sys. 2011; 64: 195– 203. [Google Scholar]
- 17. Krause P, Szecsi J, Straube A.. FES cycling reduces spastic muscle tone in a patient with multiple sclerosis. NeuroRehabilitation. 2007; 22: 335– 337. [PubMed] [Google Scholar]
- 18. Szecsi J, Schlick C, Schiller M, Pöllmann W, Koenig N, Straube A.. Functional electrical stimulation-assisted cycling of patients with multiple sclerosis: biomechanical and functional outcome: a pilot study. J Rehabil Med. 2009; 41: 674– 680. [DOI] [PubMed] [Google Scholar]
- 19. Ratchford JN, Shore W, Hammond ER, . et al. A pilot study of functional electrical stimulation cycling in progressive multiple sclerosis. NeuroRehabilitation. 2010; 27: 121– 128. [DOI] [PubMed] [Google Scholar]
- 20. Fornusek C, Hoang P.. Neuromuscular electrical stimulation cycling exercise for persons with advanced multiple sclerosis. J Rehabil Med. 2014; 46: 698– 702. [DOI] [PubMed] [Google Scholar]
- 21. Duffell LD, de N Donaldson N, Newham DJ.. Why is the metabolic efficiency of FES cycling low? IEEE Trans Neural Syst Rehabil Eng. 2009; 17: 263– 269. [DOI] [PubMed] [Google Scholar]
- 22. Eser PC, Donaldson NN, Knecht H, Stussi E.. Influence of different stimulation frequencies on power output and fatigue during FES–cycling in recently injured SCI people. IEEE Trans Neural Syst Rehabil Eng. 2003; 11: 236– 240. [DOI] [PubMed] [Google Scholar]
- 23. Rudick R, Antel J, Confavreux C, . et al. Recommendations from the National Multiple Sclerosis Society clinical outcomes assessment task force. Ann Neurol. 1997; 42: 379– 382. [DOI] [PubMed] [Google Scholar]
- 24. Ritvo P, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG.. MSQLI—Multiple Sclerosis Quality of Life Inventory: A User's Manual. New York, NY: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 25. Bohannon RW, Smith MB.. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. 1987; 67: 206– 207. [DOI] [PubMed] [Google Scholar]
- 26. Kendall FP, McCreary EK, Provance PG, Rodgers MM, Romani WA.. Lower extremity. : Muscles: Testing and Function, with Posture and Pain. Baltimore, MD: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 27. Parmenter BA, Weinstock-Guttman B, Garg N, Munschauer F, Benedict RH.. Screening for cognitive impairment in multiple sclerosis using the Symbol Digit Modalities Test. Mult Scler. 2007; 13: 52– 57. [DOI] [PubMed] [Google Scholar]
- 28. McCarney R, Warner J, Iliffe S, van Haselen R, Griffin M, Fisher P.. The Hawthorne Effect: a randomised, controlled trial. BMC Med Res Methodol. 2007; 7: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]


