Abstract
Background:
For patients with relapsing-remitting multiple sclerosis (RRMS) undergoing continuous immunomodulatory therapy, understanding whether vaccinations can be performed safely and effectively is important. We tested the immune response to inactivated seasonal influenza vaccine during long-term daclizumab beta treatment.
Methods:
In this prospective, open-label, single-arm extension SELECTED study, an optional vaccine substudy was performed on patients with RRMS who had already received daclizumab beta for 1 to 2 years in previous studies. Patients were administered the seasonal vaccine as a single intramuscular dose containing three inactivated influenza virus strains: A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), and B/Massachusetts/2/2012 (B). Endpoints included proportion of patients achieving seroprotection, proportion of patients who seroconverted, geometric mean titer ratio before and after vaccination, and adverse events reported during 28-day follow-up.
Results:
Ninety patients received the influenza vaccine (mean previous daclizumab beta exposure, 49.6 doses). Seroprotection (anti–hemagglutination immunoglobulin G titer ≥40) was detected in 92% (95% confidence interval [CI], 85%–97%) of patients for A/H1N1, 91% (83%–96%) for A/H3N2, and 67% (56%–76%) for B. The proportion of patients who seroconverted was 69% (95% CI, 58%–78%) for A/H1N1, 69% (58%–78%) for A/H3N2, and 44% (34%–55%) for B. The anti–hemagglutination immunoglobulin geometric mean titer ratio was 7.7 for A/H1N1, 9.0 for A/H3N2, and 4.3 for B. There were no significant adverse events considered related to vaccination during 28-day follow-up.
Conclusions:
Patients with RRMS receiving long-term daclizumab beta treatment mounted an immune response to the seasonal influenza vaccine at levels considered to confer protection. No major or new safety issues were identified.
Daclizumab beta is approved as a treatment for relapsing-remitting multiple sclerosis (RRMS) and has shown significant benefits on clinical and magnetic resonance imaging outcomes in placebo-controlled and active-comparator trials.1–3 (Daclizumab beta, formerly daclizumab high-yield process, approved as Zinbryta [Biogen, Cambridge, MA, USA], has a different form and structure than an earlier form of daclizumab.) Daclizumab beta is a humanized monoclonal antibody that binds to the interleukin (IL)-2 receptor α subunit (CD25) and modulates IL-2 signaling.4 CD25 blockade prevents signaling through the high-affinity IL-2 receptor complex on activated T cells and facilitates signaling through the intermediate-affinity IL-2 receptor present on other cell types, such as natural killer cells.4 This results in a targeted reduction in proinflammatory activated T cells and expansion of immunoregulatory CD56bright natural killer cells.4 These cellular effects have been hypothesized to be the key mediators of the beneficial effects of daclizumab beta in MS.4
As part of routine preventive care, patients with RRMS may require common inactivated vaccines, including seasonal influenza,5 yet somewhat reduced response rates to the influenza vaccine have been observed in patients with RRMS treated with some immunomodulatory treatments.6–8 Influenza in patients with MS can lead to hospitalizations and mortality.9,10 Thus, it is important for physicians to have information about whether vaccinations can be performed safely and effectively during immunomodulatory treatment for MS.
The objective of this vaccine substudy was to describe the immune response to inactivated seasonal influenza vaccine during long-term daclizumab beta treatment.
Methods
Study Design
SELECTED is a single-arm, open-label extension study to evaluate the long-term safety and efficacy of daclizumab beta 150 mg subcutaneously every 4 weeks. Patients entering SELECTED had already received 1 to 2 years of treatment with daclizumab beta 150 or 300 mg subcutaneously (with or without a 24-week washout period) in the previous studies, SELECT and SELECTION.1,2
All eligible patients at the subset of sites participating in this substudy were invited to participate in the optional vaccine substudy. Patients entered the vaccine substudy at a visit during the annual influenza vaccination season if they had completed at least 3 months of uninterrupted daclizumab beta treatment. Blood samples were taken before vaccination (day −21 to day 0) and after vaccination (day 28 ±3 days). The vaccination procedure was performed 30 minutes before the administration of study treatment or any assessments scheduled for the visit. Patients were observed for acute vaccine-related adverse reactions for 30 minutes after vaccination. Adverse events (AEs) were monitored throughout the entire SELECTED study, but only events that occurred during the 28-day substudy were assessed herein.
Patient Inclusion/Exclusion Criteria
To be included in the substudy, SELECTED patients must have provided informed consent for the 2013–2014 influenza vaccine substudy and completed at least 3 months of uninterrupted daclizumab beta treatment before entering the substudy. Key exclusion criteria for the substudy included severe allergic reaction to any component of the trivalent seasonal influenza vaccine (including eggs and egg proteins), known latex allergy, previous vaccination with 2013–2014 influenza season strain, respiratory illness in the 2 weeks preceding planned vaccination, any ongoing AE or laboratory abnormality in SELECTED that resulted in suspension of daclizumab beta treatment at the time of enrollment in the substudy, any vaccination in the previous 4 weeks, and receiving any of the following in the previous 3 months: blood products, oral or intravenous corticosteroids, or intravenous immunoglobulins. The key inclusion and exclusion criteria for SELECTED and eligibility criteria for SELECT and SELECTION have been reported previously.1,2,11
Vaccine
The trivalent subunit seasonal vaccine (INFLUVAC/IMUVAC; Mylan, formerly Abbott Healthcare, Hatfield, England) was administered as a single intramuscular dose containing three inactivated influenza virus strains selected by the World Health Organization for the 2013–2014 season: A/California/7/2009 (A/H1N1), A/Texas/50/2012 (A/H3N2), and B/Massachusetts/2/2012 (B).
Vaccine Response Assessment and Endpoint
Immune response to vaccination was defined by anti–hemagglutination immunoglobulin G (HA) titer. Serum samples were obtained before vaccination with the trivalent seasonal influenza vaccine (≤21 days previously) and 28±3 days after vaccination. Samples were frozen and HA assays were performed at a central laboratory. The primary endpoint was the proportion of evaluable patients with a postvaccination HA titer of 40 or higher for each strain, termed seroprotection. Other endpoints included the proportion of patients achieving seroconversion, which was defined as a change from negative (HA titer <10 at baseline) to seroprotected after vaccination, or at least a fourfold increase in titer if the titer was 10 or higher at baseline, and the geometric mean titer ratio (GMTR) before and after vaccination.
Safety Assessment
During the 28-day substudy, safety and tolerability were measured as in the SELECTED main study. In SELECTED, safety was measured by AE monitoring, physical and neurologic examinations, vital signs, electrocardiograms, and clinical laboratory evaluations (hematology, blood chemistry, liver function panel, and urinalysis). Investigators rated the severity of each AE based on guidance in the protocol and determined whether it also met the criteria for a serious AE (SAE). All reported AEs were coded using the Medical Dictionary for Regulatory Affairs, version 16.1.
Statistical Analysis
All patients with prevaccination and postvaccination HA titers were considered eligible for the evaluation of vaccine response and were included in the analysis. Prevaccination and postvaccination HA titers were summarized, and the GMTR was computed for each strain. The proportions of patients achieving seroprotection and seroconversion were calculated, together with 95% confidence intervals (CIs). The analysis of seroconversion was repeated, stratifying patients based on baseline titer (<10 vs. ≥10).
The safety analysis included all patients who received the influenza vaccine in the substudy. Because the scheduled safety laboratory assessments in SELECTED may or may not have overlapped with a patient's participation in the substudy, safety analyses for the substudy were limited to the incidence of AEs and SAEs. All treatment-emergent AEs that started or worsened in severity or seriousness during the 28-day vaccine substudy were included in the evaluation. In SELECTED, treatment-emergent AEs were defined as any event that either occurred or worsened in severity after the first dose of study treatment in SELECTED up to 180 days after the last dose of daclizumab beta.
The sample size for the substudy was based on the number of eligible patients in SELECTED and was not designed to provide statistical power for hypothesis testing. However, the accrued sample size of 90 patients allows for proportions to be estimated with 95% exact CIs of width no more than 21.5%.
Ethical Considerations
The study was performed in accordance with the International Conference on Harmonization guidelines on Good Clinical Practice, the European Union Clinical Trial Directive 2001/20/EC, and the ethical principles outlined in the Declaration of Helsinki. Institutional review board/ethics committee approval was obtained from the central and local ethics committees for each site, which are listed in Supplementary Table 1 (87KB, pdf) , which is published in the online version of this article at ijmsc.org. All the patients provided written informed consent to participate in SELECTED and the vaccine substudy.
Results
Patients
Of the 410 patients in SELECTED, 90 at participating sites received the influenza vaccine as part of the substudy (Table 1). Patients who participated in the vaccine substudy had a mean (SD) age of 39 (9) years, and their demographic and baseline MS characteristics were largely similar to those of the entire group of patients in the SELECTED study. There was a slightly higher percentage of male patients in the vaccine substudy versus the SELECTED population (44% vs. 38%). Before the influenza vaccination, patients enrolled in the vaccine substudy had participated in SELECTED for a mean of 27.8 months (range, 18–42 months) and had received a mean of 49.6 doses (range, 26–68 doses) of daclizumab beta across the SELECT trilogy of studies.
Table 1.
Patient demographic and baseline multiple sclerosis characteristics
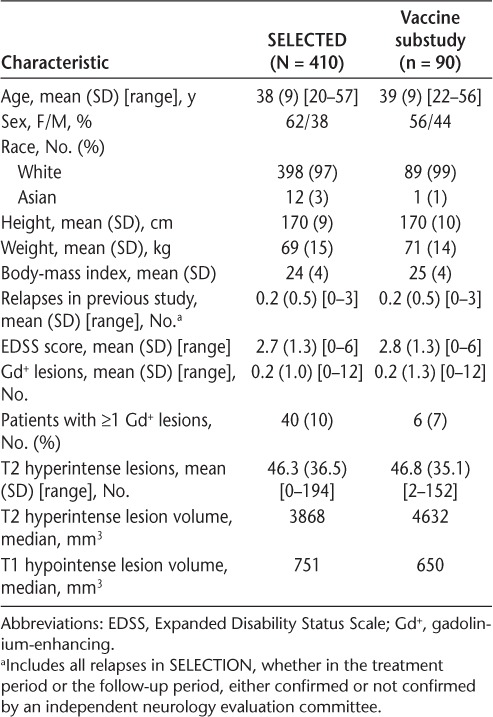
Of the 90 patients who received influenza vaccine in the substudy, 56% were taking at least one concomitant medication at the time of vaccination. Baclofen was the most common concomitant medication, which was taken by 12% of patients. The other concomitant medications taken by at least 5% of patients were ibuprofen (7%), oxybutynin (7%), cod-liver oil (6%), and paracetamol (6%).
Vaccine Seroprotection and Seroconversion Rates
Seroprotection, defined as HA titers of 40 or higher, was detected for most patients for all three strains. Specifically, seroprotection was detected in 92% (95% CI, 85%–97%) of patients for A/H1N1, 91% (95% CI, 83%–96%) for A/H3N2, and 67% (95% CI, 56%–76%) for B (Figure 1).
Figure 1.
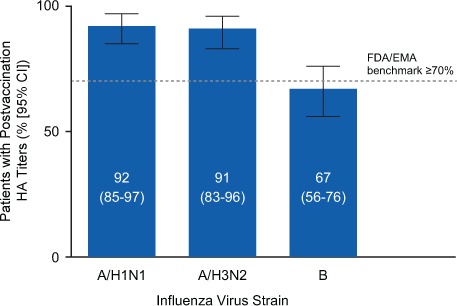
Proportion of the 90 substudy patients achieving seroprotection
Seroprotection was defined as a postvaccination anti–hemagglutination immunoglobulin G titer of 40 or higher according to US Food and Drug Administration (FDA) and European Medicines Agency (EMA) seroprotection benchmark guidelines (≥70%).12,13 A/H1N1, A/California/7/2009; A/H3N2, A/Texas/50/2012; B, B/Massachusetts/2/2012; CI, confidence interval.
Seroconversion, defined as a change from negative (HA titer <10) to seroprotected after vaccination or at least a fourfold increase in titer if the titer was 10 or higher at baseline, was detected for all three strains. Seroconversion was observed in 69% (95% CI, 58%–78%) of patients for A/H1N1, 69% (95% CI, 58%–78%) for A/H3N2, and 44% (95% CI, 34%–55%) for B (Figure 2). The observed rates of seroconversion were higher than the seroconversion benchmark of 40% or more for all three strains, as defined by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA).
Figure 2.
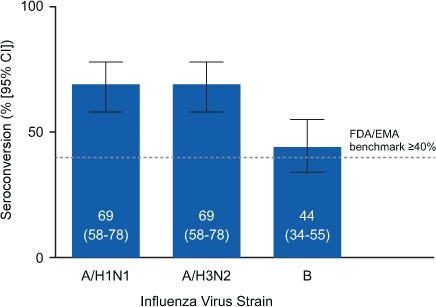
Proportion of the 90 substudy patients who seroconverted
Seroconversion was defined as a change from negative (anti–hemagglutination immunoglobulin G titer <10) to seroprotected after vaccination or a fourfold increase in titer if 10 or higher at baseline according to US Food and Drug Administration (FDA) and European Medicines Agency (EMA) seroconversion benchmark guidelines (≥40%).12,13 A/H1N1, A/California/7/2009; A/H3N2, A/Texas/50/2012; B, B/Massachusetts/2/2012; CI, confidence interval.
GMTR and Stratification by Prevaccination Titer
The HA GMTR provides the fold change from before to after vaccination in HA titers. The HA GMTR was 7.7 for A/H1N1, 9.0 for A/H3N2, and 4.3 for B (Figure 3A). Geometric mean (coefficient of variation) prevaccination HA titers were 22.7 (178.4) for A/H1N1, 20.4 (191.1) for A/H3N2, and 12.2 (196.6) for B (Figure 3B).
Figure 3.
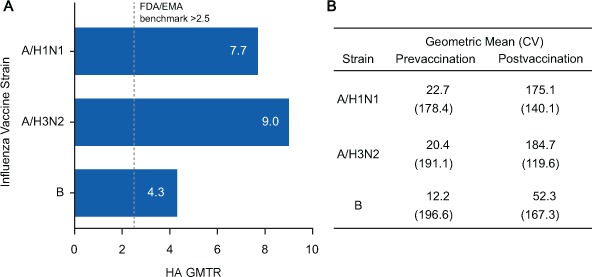
(A) Anti–hemagglutination immunoglobulin G (HA) geometric mean titer ratio (GMTR) and (B) prevaccination and postvaccination geometric mean HA titers in the 90 substudy patients
A, The prevaccination and postvaccination GMTR of HA antibodies by vaccine strain are presented. Mean group-level change in titer prevaccination to postvaccination of greater than 2.5 is the benchmark used by the European Medicines Agency (EMA) and the US Food and Drug Administration (FDA).12,13 B, The geometric mean and coefficient of variation (CV) for each strain before and after vaccination are presented. These values were used to calculate the GMTR. A/H1N1, A/California/7/2009; A/H3N2, A/Texas/50/2012; B, B/Massachusetts/2/2012.
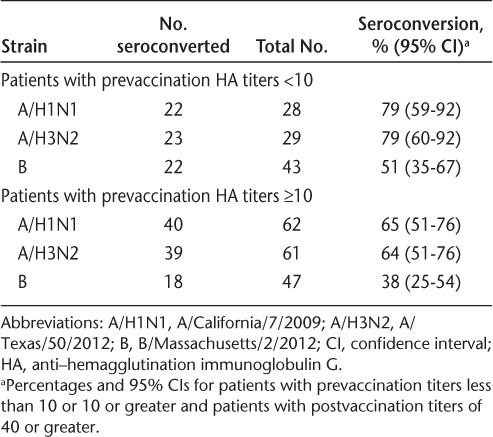
In an additional analysis of seroconversion, patients were grouped by prevaccination titer into those negative at baseline (defined as a prevaccination HA titer <10) and those with a prevaccination HA titer of 10 or higher. Nearly one-third of the patients had prevaccination titers of less than10 for A/H1N1 and A/H3N2, and nearly half of the patients had prevaccination titers of less than10 for B (Table 2). Seroconversion was observed in patients with prevaccination titers of less than 10 and in patients with prevaccination titers of 10 or higher (Table 2).
Table 2.
Seroconversion stratified by prevaccination HA titers
AEs During the 28-Day Study
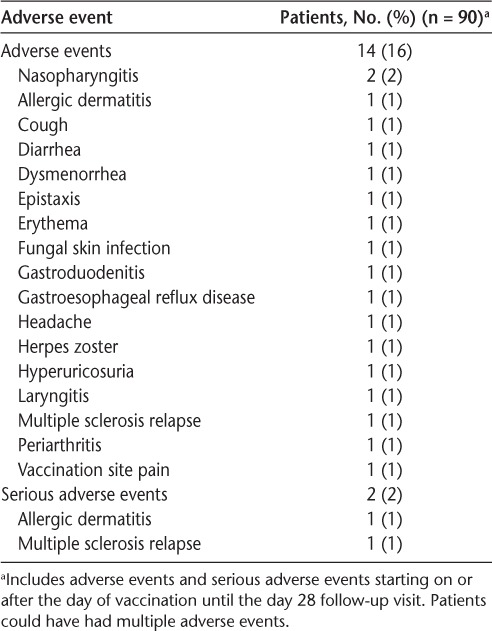
During the 28-day substudy, AEs were reported by 14 patients (16%) (Table 3). Vaccination site pain was reported as an AE by one patient. The only AE that occurred in more than one patient in any Medical Dictionary for Regulatory Affairs Preferred Term was nasopharyngitis in two patients (2%). MS relapse and allergic dermatitis were each reported by one patient as SAEs.
Table 3.
Adverse events in the vaccine substudy
Discussion
These data indicate that patients receiving long-term daclizumab beta treatment mounted an immune response to the seasonal influenza vaccine at levels considered to confer protection against influenza infection. For both A strains, a robust response was observed, with more than 90% of the patients in the vaccine substudy achieving seroprotection. A more modest response was observed for the B strain, although most patients (67%) achieved seroprotection to the B strain.
Response to influenza vaccination is well defined in healthy adults.14 Three benchmarks are used by the EMA and the FDA to evaluate response during licensure or annual update of new influenza vaccines: 1) proportion of individuals achieving seroprotection of 70% or higher, with seroprotection for an individual defined as an HA titer of 40 or higher; 2) proportion of individuals who seroconvert of 40% or more, with seroconversion defined as either a postvaccination change from negative to seroprotected or a fourfold rise in titer; and 3) mean group-level change in titer prevaccination to postvaccination of more than 2.5.12,13 The levels of seroprotection observed in SELECTED are comparable or surpass the EMA- and FDA-established benchmark of 70% or higher for two of the three strains.12,13 The EMA and FDA seroconversion guideline (≥40% of patients) was attained for all strains for the overall patient population. For the subgroups evaluated, the benchmark was met for all strains except patients with a prevaccination titer of 10 or higher and seroconversion for strain B, which approached but did not meet the criterion. All strains achieved mean group-level changes in titer with prevaccination to postvaccination GMTR of more than 2.5.
The magnitude of the immune response was consistent with norms defined for healthy volunteer populations.14 A meta-analysis of published vaccination studies found seroprotection mean response rates of 78% for A/H1N1, 81% for A/H3N2, and 75% for B, whereas the present study observed seroprotection of 92% for A/H1N1, 91% for A/H3N2, and 67% for B. For seroconversion, the meta-analysis reported mean response rates of 58% for A/H1N1, 61% for A/H3N2, and 51% for B compared with 69% for A/H1N1, 69% for A/H3N2, and 44% for B in the present study.14 Patients with RRMS treated with daclizumab beta experienced significant expansions of adaptive immune cells and attained the FDA criteria for seroconversion and seroprotection, although the response was slightly reduced compared with that of controls.15 Positive responses to influenza vaccine also have been observed with other treatments for RRMS.6,16–19 A similar proportion of patients with MS who were or were not treated with interferon beta-1a mounted an appropriate immune response to inactivate the influenza vaccine, and no new safety concerns were noted.18 In an exploratory study of immunomodulatory treatments, rates of protection were lower in patients treated with glatiramer acetate, natalizumab, and mitoxantrone versus controls, but rates of protection in patients treated with interferon beta were similar to those in controls 10 to 12 months after vaccination.8 In contrast to the substudy reported herein, patients with MS treated with natalizumab or controls experienced a significant increase in anti–influenza B immunoglobulin G titers but a smaller and nonsignificant increase in A strain responses.17
There are several potential limitations to this study. The study design was not sufficient to rule out whether there was some attenuation of vaccine response in daclizumab beta–treated patients. Response to the B strain was present but did not always achieve recommended benchmarks for seroconversion and seroprotection. For strain B, 51% of patients with a prevaccination HA titer of less than 10 seroconverted (ie, achieved a postvaccination titer ≥40), whereas fewer patients (38%) with a prevaccination HA titer of 10 or higher seroconverted. Baseline antibody titers have been inversely associated with seroconversion previously,14 and this same phenomenon may have occurred in this study.
This was not a placebo-controlled study; a large randomized trial is required to definitely establish whether there is some attenuation of vaccine response. Also, the response to influenza vaccine reflects a mixture of recall and neoantigen response but may not predict response to other vaccines. The vaccine-induced humoral immune response was assessed, but the vaccine-specific T-cell responses were not assessed, although they also may be clinically relevant. A strength of the present study is the long-term use of daclizumab beta before vaccination such that the data capture the full effect of daclizumab beta's immunomodulatory effect on the vaccine response.
The results of this vaccine substudy provide clinically relevant information regarding vaccine response during long-term daclizumab beta treatment and indicate that the immune response generally seems intact. Immune responses were consistent with expectations and at levels considered to confer protection against influenza infection for strains A/H1N1 and A/H3N2, although a small reduction in vaccine response was noted for strain B. Furthermore, there were no major or new safety issues identified after administration of the vaccine in patients undergoing treatment with daclizumab beta.
PracticePoints
Patients with relapsing-remitting MS treated with daclizumab beta responded to a seasonal inactivated influenza virus vaccine at levels consistent with normal responses to influenza vaccination in this prospective, open-label, single-arm study.
The immune responses of patients seemed to be generally intact during daclizumab beta treatment, and no major or new safety concerns were observed.
Supplementary Material
Acknowledgments
We thank Rebecca Priestley and Marianne Sweetser for their contributions to the vaccine substudy. Karen Spach from Excel Scientific Solutions wrote the first draft of the manuscript based on input from the authors, and Elizabeth Cassell from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements.
Footnotes
Financial Disclosures: Dr. Elkins is an employee of and holds stock in Biogen. Dr. Umans was an employee of Biogen at the time of this analysis and holds stock in Biogen, and a family member holds stock in Sinovac Biotech Ltd. Dr. Robinson is an employee of and holds stock in AbbVie Biotherapeutics Inc. Drs. Mehta and Ozen were employees of Biogen at the time of this analysis and hold stock in Biogen.
Funding/Support: Biogen and AbbVie Biotherapeutics Inc provided funding for medical writing support in the development of this article and reviewed and provided feedback on the article to the authors. The authors had full editorial control and provided their final approval of all content.
References
- 1. Giovannoni G, Gold R, Selmaj K, . et al. SELECTION Study Investigators Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECTION): a multicentre, randomised, double-blind extension trial. Lancet Neurol. 2014; 13: 472– 481. [DOI] [PubMed] [Google Scholar]
- 2. Gold R, Giovannoni G, Selmaj K, . et al. SELECT Study Investigators Daclizumab high-yield process in relapsing-remitting multiple sclerosis (SELECT): a randomised, double-blind, placebo-controlled trial. Lancet. 2013; 381: 2167– 2175. [DOI] [PubMed] [Google Scholar]
- 3. Kappos L, Wiendl H, Selmaj K, . et al. Daclizumab HYP versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2015; 373: 1418– 1428. [DOI] [PubMed] [Google Scholar]
- 4. Wiendl H, Gross CC.. Modulation of IL-2Rα with daclizumab for treatment of multiple sclerosis. Nat Rev Neurol. 2013; 9: 394– 404. [DOI] [PubMed] [Google Scholar]
- 5. Loebermann M, Winkelmann A, Hartung HP, Hengel H, Reisinger EC, Zettl UK.. Vaccination against infection in patients with multiple sclerosis. Nat Rev Neurol. 2011; 8: 143– 151. [DOI] [PubMed] [Google Scholar]
- 6. Kappos L, Mehling M, Arroyo R, . et al. Randomized trial of vaccination in fingolimod-treated patients with multiple sclerosis. Neurology. 2015; 84: 872– 879. [DOI] [PubMed] [Google Scholar]
- 7. Pellegrino P, Carnovale C, Perrone V, . et al. Efficacy of vaccination against influenza in patients with multiple sclerosis: the role of concomitant therapies. Vaccine. 2014; 32: 4730– 4735. [DOI] [PubMed] [Google Scholar]
- 8. Olberg HK, Cox RJ, Nostbakken JK, Aarseth JH, Vedeler CA, Myhr KM.. Immunotherapies influence the influenza vaccination response in multiple sclerosis patients: an explorative study. Mult Scler. 2014; 20: 1074– 1080. [DOI] [PubMed] [Google Scholar]
- 9. Redelings MD, McCoy L, Sorvillo F.. Multiple sclerosis mortality and patterns of comorbidity in the United States from 1990 to 2001. Neuroepidemiology. 2006; 26: 102– 107. [DOI] [PubMed] [Google Scholar]
- 10. Marrie RA, Elliott L, Marriott J, . et al. Dramatically changing rates and reasons for hospitalization in multiple sclerosis. Neurology. 2014; 83: 929– 937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gold R, Radue EW, Giovannoni G, . et al. Safety and efficacy of daclizumab in relapsing-remitting multiple sclerosis: 3-year results from the SELECTED open-label extension study. BMC Neurol. 2016; 16: 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. The European Agency for the Evaluation of Medical Products, Human Medicines Evaluation Unit. . Note for guidance on harmonisation of requirement for influenza vaccines. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003945.pdf. Published March 12, 1997. Accessed December 1, 2015.
- 13. US Department of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research. . Guidance for industry: clinical data needed to support the licensure of seasonal inactivated influenza vaccines. http://www.fda.gov/cber/gdlns/trifluvac.htm. Published May 2007. Accessed December 1, 2015.
- 14. Seidman JC, Richard SA, Viboud C, Miller MA.. Quantitative review of antibody response to inactivated seasonal influenza vaccines. Influenza Other Respir Viruses. 2012; 6: 52– 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lin YC, Winokur P, Blake A, . et al. Patients with MS under daclizumab therapy mount normal immune responses to influenza vaccination. Neurol Neuroimmunol Neuroinflamm. 2016; 3: e196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bar-Or A, Freedman MS, Kremenchutzky M, . et al. Teriflunomide effect on immune response to influenza vaccine in patients with multiple sclerosis. Neurology. 2013; 81: 552– 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vagberg M, Kumlin U, Svenningsson A.. Humoral immune response to influenza vaccine in natalizumab-treated MS patients. Neurol Res. 2012; 34: 730– 733. [DOI] [PubMed] [Google Scholar]
- 18. Schwid SR, Decker MD, Lopez-Bresnahan M; Rebif-Influenza Vaccine Study Investigators. . Immune response to influenza vaccine is maintained in patients with multiple sclerosis receiving interferon beta-1a. Neurology. 2005; 65: 1964– 1966. [DOI] [PubMed] [Google Scholar]
- 19. Mehling M, Fritz S, Hafner P, . et al. Preserved antigen-specific immune response in patients with multiple sclerosis responding to IFNβ-therapy. PLoS One. 2013; 8: e78532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


