Abstract
Background:
Monitoring patients with multiple sclerosis (MS) for “no evidence of disease activity” (NEDA) may help guide disease-modifying therapy (DMT) management decisions. Whereas surveillance brain magnetic resonance imaging (MRI) is common, the role of spinal cord monitoring for NEDA is unknown.
Objective:
To evaluate the role of brain and spinal cord 3T MRI in the 1-year evaluation of NEDA.
Methods:
Of 61 study patients (3 clinically isolated syndrome, 56 relapsing-remitting, 2 secondary progressive), 56 (91.8%) were receiving DMT. The MRI included brain fluid-attenuated inversion recovery and cervical/thoracic T2-weighted fast spin echo images. On MRI, NEDA was defined as the absence of new or enlarging T2 lesions at 1 year.
Results:
Thirty-nine patients (63.9%) achieved NEDA by brain MRI, only one of whom had spinal cord activity. This translates to a false-positive rate for NEDA based on the brain of 2.6% (95% CI, 0.1%–13.5%). Thirty-eight patients (62.3%) had NEDA by brain and spinal cord MRI. Fifty-five patients (90.2%) had NEDA by spinal cord MRI, 17 of whom had brain activity. Of the 22 patients (36.1%) with brain changes, 5 had spinal cord changes. No evidence of disease activity was sustained in 48.3% of patients at 1 year and was the same with the addition of spinal cord MRI. Patients with MRI activity in either the brain or the spinal cord only were more likely to have activity in the brain (P = .0001).
Conclusions:
Spinal cord MRI had a low diagnostic yield as an adjunct to brain MRI at 3T in monitoring patients with MS for NEDA over 1 year. Studies with larger data sets are needed to confirm these findings.
No evidence of disease activity (NEDA) is a new proposed outcome to monitor the risk of disease progression and the effectiveness of disease-modifying therapy (DMT) in patients with multiple sclerosis (MS). The term NEDA is also known as disease activity–free status, freedom from disease activity, and disease-free status.1–5 This definition typically relies on clinical and cerebral imaging data, namely, the absence of new or enlarging T2 lesions or gadolinium-enhancing lesions and no progression of neurologic disability or clinical relapses.6
Magnetic resonance imaging (MRI) plays a critical role in the diagnosis and monitoring of MS.7,8 The introduction of higher-field (eg, 3T) MRI scanners has shown a higher yield in the detection of MS lesions compared with 1.5T.9,10 Furthermore, brain MRI at 3T has also provided higher correlations between lesion load and clinical status, including neurologic disability and cognitive function, than at 1.5T.10 A growing body of evidence has determined that spinal cord MRI involvement shows a particularly close association with MS-related disability.11–24 In addition, spinal cord involvement manifests early in the disease course; such lesions in presymptomatic at-risk individuals predict conversion to overt MS.25 Adding more relevance to the need to consider spinal cord involvement in MS is the observation that such involvement may progress independently from the brain.14 Given the time burden on the patient and health-care costs associated with spinal cord imaging, it is important to assess its utility in the evaluation of NEDA.
Previously, a 7-year longitudinal study evaluating NEDA in a real-world cohort using clinically obtained low-resolution 1.5T MRIs showed that 7% to 11% of patients with MS who developed MRI-defined disease activity in each of the years had disease activity on the spinal cord only.6 Therefore, the goal of this study was to evaluate the diagnostic yield of combined brain and spinal cord 3T MRI in the evaluation of NEDA over a 1-year period.
Methods
Patients
We prospectively studied 61 consecutive patients with MS from the Partners Multiple Sclerosis Center at Brigham and Women's Hospital (Boston, MA) who underwent MRI at baseline and 1 year later. Patient demographic and clinical data are summarized in Table 1. All the patients meet the International Panel criteria for either MS or a clinically isolated demyelinating syndrome.26 Progression of Expanded Disability Status Scale (EDSS) score27 was defined as an increase of 1.0 point or more at 6-month follow-up, which was required to be sustained at a clinic visit 6 months later (with the exception that if the EDSS score was 0 at baseline, a 1.5-point increase was required). At each clinic visit, patients had an evaluation of EDSS scores and anteceding relapses. During the observation period, 56 patients (91.8%) were already receiving DMT (monotherapy with intramuscular interferon beta [IFNβ]-1a [n = 20], subcutaneous IFNβ-1a [n = 11], IFNβ-1b [n = 1], glatiramer acetate [n = 20], natalizumab [n = 2], or mycophenolate mofetil [n = 1] or dual therapy with IFNβ-1a and mycophenolate mofetil [n = 1]). Of the 56 patients receiving DMT, 48 (85.7%) were receiving treatment for a mean ± SD of 4.8 ± 4.4 years (range, 0.1–14.1 years) at study entry. The other eight patients were newly started on DMT at the time of baseline MRI. There were three patients who had imaging at follow-up but were lost to clinical follow-up. All the patients signed an informed consent form; this study was approved by the Brigham and Women's Hospital research ethics committee.
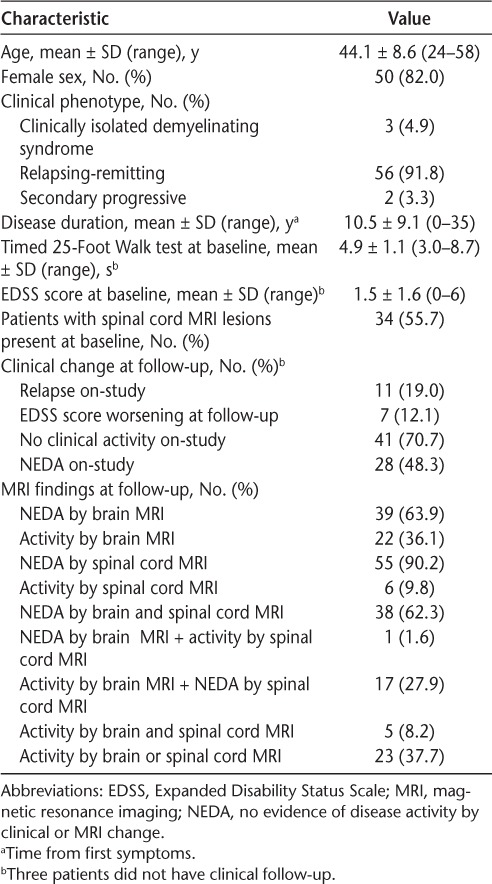
Table 1.
Demographic, clinical, and MRI data for the 61 study participants at baseline and 1-year follow-up
MRI Acquisition
All the patients underwent brain and spinal cord MRI at baseline and follow-up using a 3T scanner (GE Signa; GE Healthcare, Milwaukee, WI). Follow-up MRIs were obtained a mean ± SD of 12.4 ± 1.3 months (range, 9.7–15.2 months) after baseline. Brain and cervical images were obtained at both time points. Fifty patients also underwent thoracic spine imaging at both time points. The following imaging parameters were relevant to the present study: axial T2-weighted fluid-attenuated inversion recovery images of the brain (repetition time [TR] = 9000 milliseconds, echo time [TE] = 151 milliseconds, inversion time = 2250 milliseconds, pixel size = 0.976 × 0.976 × 2 mm; no interslice gaps), axial T2-weighted fast spin echo (FSE) images of the spinal cord (TR = 6166.66 milliseconds, TE = 110.24 milliseconds, voxel size = 0.937 × 0.937 × 3 mm; no interslice gaps), and sagittal T2-weighted FSE images of the spinal cord (TR = 3000 milliseconds, TE = 145.66 milliseconds, voxel size = 0.859 × 0.859 × 3 mm; no interslice gaps). Sample images are shown in Figure 1. Due to scan time limitations and the fact that these were research-related scans, intravenous gadolinium was not administered.
Figure 1.
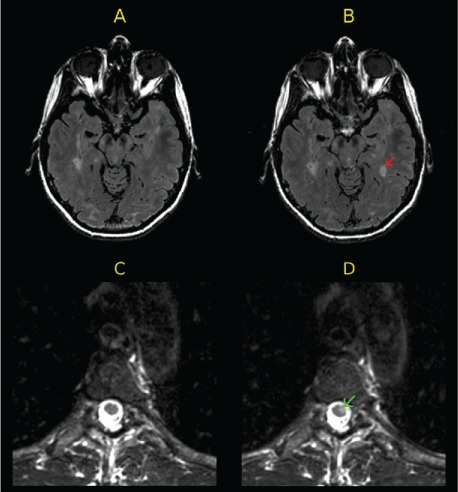
Examples of active magnetic resonance images (MRIs) at follow-up
A and B, Baseline (A) and 1-year (B) axial fluid-attenuated inversion recovery (FLAIR) brain MRIs of a patient. The follow-up FLAIR MRI (B) shows a new T2 hyperintense brain lesion (red arrow) compared with baseline. C and D, Baseline (C) and 1-year (D) axial T2-weighted fast spin echo (FSE) thoracic spinal cord MRIs of another patient. The follow-up T2-weighted FSE MRI (D) shows a new T2 hyperintense lesion in the thoracic spinal cord (green arrow) compared with baseline.
MRI Analysis
An experienced observer (ST) analyzed baseline and follow-up images concurrently using Jim software, version 7 (Xinapse Systems, West Bergholt, UK). Uncertain cases were reviewed by a senior observer (RB). Image window width and level were adjusted by the observer to ensure a consistent comparison between the two time points. The follow-up images were categorized qualitatively as active by the presence of either new or enlarging T2 hyperintense lesions. Thus, achieving NEDA by MRI was defined as no new or enlarging T2 hyperintense lesions. Examples of disease activity in the brain and spinal cord are shown in Figure 1.
Statistical Analysis
The proportions of patients who achieved NEDA in terms of lesion accumulation on brain MRI, lesion accumulation on spinal cord MRI, EDSS score progression, and relapse were estimated, and 95% confidence intervals (CIs) for each proportion were estimated using the exact binomial distribution. For the identification of disease activity using brain versus spinal cord lesions, the paired measurements were compared using the McNemar test. Furthermore, to estimate the additional diagnostic value of including spinal cord imaging, the proportion of patients who achieved NEDA in terms of lesion accumulation on brain MRI, EDSS score progression, or relapse but had disease activity on the spinal cord was calculated.
Results
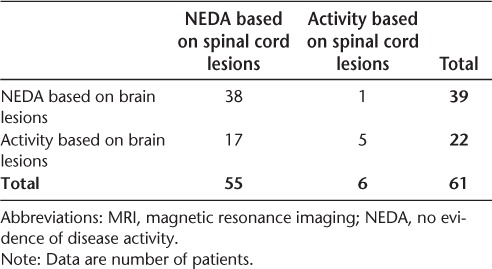
Comparisons of patients achieving NEDA based on brain and spinal cord lesions are shown in Tables 1 and 2. Based on this sample of 61 patients, 63.9% (n = 39; 95% CI, 50.6%–75.8%) maintained NEDA in terms of brain MRI, whereas 90.2% (n = 55; 95% CI, 79.8%–96.3%) maintained NEDA based on spinal cord MRI. Thirty-eight patients achieved NEDA based on both brain and spinal cord MRI, and five patients had disease activity on both measures. Six patients had new spinal cord activity with new lesions at follow-up in the cervical only (n = 2), thoracic only (n = 3), or both (n = 1) regions of the spinal cord. The following spinal cord locations were involved with these new lesions: C2, C7, T6, T6–T7, T8–T9, and C4–C6, T4–T5, and T6 (Figure 1). Patients with disease activity in either the brain or spinal cord only were significantly more likely to have disease activity in the brain (P = .0001). In addition, only 1 of 39 patients who had NEDA based on brain MRI was identified as having disease activity based on the spinal cord (2.6%; 95% CI, 0.1%–13.5%), which can be considered the false-positive rate for NEDA based on the brain. However, this patient also had a clinical relapse that coincided with the new spinal cord activity. Thus, there was no case in which failure of NEDA was shown by spinal cord MRI alone. In total, there were seven patients with enlarging lesions, six of whom also had new lesions. At baseline, 34 patients (55.7%) had spinal cord lesions; 1 additional patient developed spinal cord involvement at follow-up.
Table 2.
Patients achieving NEDA at 1 year by brain and spinal cord MRI lesions
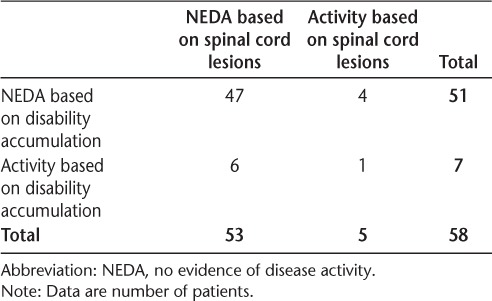
Comparisons of patients achieving NEDA based on spinal cord lesions and relapses are shown in Table 3. Comparisons of patients meeting the definition of NEDA based on spinal cord lesions and accumulation of physical disability (EDSS score) are shown in Table 4. Based on the 58 patients with clinical information, 81.0% (n = 47; 95% CI, 68.6%–90.1%) maintained NEDA in terms of relapses and 87.9% (n = 51; 95% CI, 76.7%–95.0%) maintained NEDA based on disability accumulation. In this slightly reduced sample, 91.4% of patients (n = 53; 95% CI, 81.0%–97.1%) maintained NEDA on spinal cord imaging. Forty-three patients maintained NEDA on both spinal cord imaging and relapses. Interestingly, 80% of the patients who had spinal cord activity at follow-up did not have an accompanying relapse. Four of 47 patients (8.5%; 95% CI, 2.4%–20.4%) who had NEDA based on relapses were identified as having disease activity on spinal cord MRI. When assessing patients who maintained NEDA on both spinal cord imaging and disability accumulation, 47 maintained NEDA on both measures, and only 1 patient had disease activity on both measures. Four of 51 patients (7.8%; 95% CI, 2.2%–18.9%) who achieved NEDA based on disability accumulation were identified as having disease activity in the spinal cord.
Table 3.
Patients achieving NEDA at 1 year by spinal cord MRI lesions and relapses
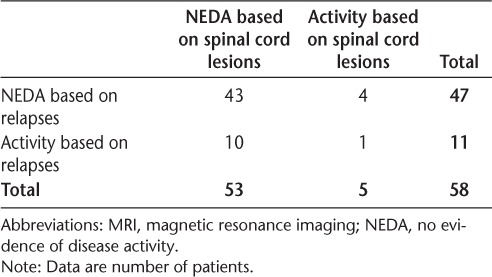
Table 4.
Patients achieving NEDA at 1 year by spinal cord lesions and disability accumulation
Taken together, in decreasing order of sensitivity, the following rates of disease activity over 1 year were shown by the measures used in the present study: brain MRI activity (36.1%), clinical relapse (19.0%), worsening of EDSS score (12.1%), and spinal cord MRI activity (9.8%). More patients maintained NEDA in terms of clinical activity compared with brain and spinal cord imaging (70.7% vs. 62.3%). Overall, NEDA with the combination of clinical relapse, worsening of EDSS score, and brain MRI activity was maintained in 28 patients (48.3%) and remained the same with the addition of the spinal cord MRI findings.
Discussion
The spinal cord is a common site of pathology in MS, occurring early in the disease course12,25 and playing a role in the development of disability11,14–17,19,21–23,28; such involvement includes overt multifocal inflammatory demyelinating lesions and the potential for tissue destruction (axonal loss/atrophy).29 This study evaluated the role of brain and spinal cord 3T MRI in defining NEDA at 1 year. We showed that spinal cord MRI had a low diagnostic yield as an adjunct to brain MRI for defining NEDA. Only one patient had activity on spinal cord imaging while having NEDA on brain imaging. This patient also had an on-study relapse and would not have met NEDA regardless of the spinal cord MRI, further reducing the diagnostic yield of spinal cord imaging.
There are specific aspects of this patient population that may have reduced the yield of spinal cord imaging. Almost half of the patients were free of spinal cord lesions at baseline. This is a lower rate of spinal cord involvement than reported in other studies.12,18 In addition, the present patients had a relatively long disease duration (on average, 10 years), whereas previous studies showing higher rates of spinal cord activity have reported patients with shorter disease duration.12,18 In addition, most of the patients in this study had relapsing forms of MS. Studies have shown that these subtypes are less likely to have spinal cord involvement compared with patients with progressive forms of the disease.30,31 Another important limitation of this study is the possibility that the results would have been different if a higher proportion of the study participants were taking the newer, higher-efficacy DMTs.3,6 The absence of gadolinium administration is a limitation of this study in that the current definition of NEDA includes gadolinium-enhancing lesions.6 The sample size was small, which limited the statistical power of the study. This, combined with the other limitations, urges caution in the interpretation of the results and suggests that further studies with larger data sets are needed to confirm the findings.
Previous studies assessed NEDA in patients with MS using lower-field 1.5T MRI platforms.2,3,6,32–37 Using brain and spinal cord 3T MRI in the present study, we found that nearly half of the patients maintained overall NEDA at 1 year, which was similar to other studies.3,6,30,33,36 Approximately two-thirds of the patients in the present study had no MRI activity at 1 year; this was 50% to 60% in previous studies.3,36,37 When considering only the clinical criteria for achieving NEDA, slightly more than two-thirds of the present patients met this definition; this was slightly higher (75% to 77%) in previous studies using newer, more potent DMTs than the present study.3,36 Note that NEDA is still under investigation for its validity and is not yet considered a standard or primary outcome measure of disease status or therapeutic efficacy.
There are several potential strategies available to extend these findings regarding the utility of spinal cord MRI in defining NEDA. Patients with higher levels of disability, a higher proportion of spinal cord involvement, and a larger proportion with progressive forms of the disease than in the present study may show a greater role of spinal cord MRI. It would also be of interest to determine whether longer observation periods (ie, ≥2 years) would increase such a yield. This study evaluated the spinal cord with T2-weighted FSE, which is the clinical standard but may have a reduced sensitivity to lesions versus newer MRI sequences, such as short time inversion recovery and phase-sensitive inversion recovery.38–43 Furthermore, the concept of NEDA is still evolving, with recent proposals to include brain volume loss and changes in neuropsychological test scores in the definition.5,44 Thus, spinal cord atrophy may provide an additional tool to assess NEDA and complement lesion assessment, particularly given the proposed discordance between lesions and atrophy in a subset of patients with MS.45 In addition, given that a recent study has shown a higher yield in the detection of spinal cord MS lesions at 7T versus 3T,46 a high-resolution ultra-high-field approach may be more sensitive.
Considering that spinal cord imaging is a separate and distinct evaluation from a utilization standpoint, these findings suggest that this might not be necessary for routine monitoring. However, owing to the limitations of this study and general uncertainty about the role of NEDA, these results should not be directly used to change routine clinical practice. We would urge that further research is needed regarding the utility of spinal cord MRI in the routine monitoring of MS.
PracticePoints
We used 3T magnetic resonance imaging (MRI) to evaluate the role of the spinal cord as an adjunct to brain imaging in assessing disease activity in a real-world setting of patients with MS.
Most patients had no activity on either brain (63.9%) or spinal cord (90.2%) MRI at 1 year.
Of the 39 patients who had no activity on brain MRI, only 1 had spinal cord activity. This translates to a false-positive rate for no activity based on brain MRI of 2.6% (95% CI, 0.1%–13.5%).
No evidence of disease activity was sustained in 48.3% of patients at 1 year and was the same with the addition of spinal cord MRI.
Acknowledgments
This work was presented in preliminary form at the 2015 annual meeting of the European Committee on Treatment and Research in Multiple Sclerosis (ECTRIMS), Barcelona, Spain; and at the 2016 annual meeting of the American Academy of Neurology, Vancouver, British Columbia, Canada.
Footnotes
Financial Disclosures: Dr. Healy has received research support from Genzyme, Merck Serono, Novartis, and Verily Life Sciences. Dr. Bakshi has received consulting fees from AbbVie, Alkermes, Biogen, Novartis, and Questcor and has received research support from Biogen, Genzyme, Merck Serono, Novartis, and Teva. Dr. Bakshi's spouse owns stock in Biogen. The other authors have no conflicts of interest to disclose.
Funding/Support: Dr. Bakshi was supported in part by the National Multiple Sclerosis Society (research grant RG3705A1).
References
- 1. Bevan CJ, Cree BA.. Disease activity free status: a new end point for a new era in multiple sclerosis clinical research? JAMA Neurol. 2014; 71: 269– 270. [DOI] [PubMed] [Google Scholar]
- 2. Giovannoni G, Cook S, Rammohan K, . et al. Sustained disease-activity-free status in patients with relapsing-remitting multiple sclerosis treated with cladribine tablets in the CLARITY study: a post-hoc and subgroup analysis. Lancet Neurol. 2011; 10: 329– 337. [DOI] [PubMed] [Google Scholar]
- 3. Havrdova E, Galetta S, Hutchinson M, . et al. Effect of natalizumab on clinical and radiological disease activity in multiple sclerosis: a retrospective analysis of the Natalizumab Safety and Efficacy in Relapsing-Remitting Multiple Sclerosis (AFFIRM) study. Lancet Neurol. 2009; 8: 254– 260. [DOI] [PubMed] [Google Scholar]
- 4. Havrdova E, Galetta S, Stefoski D, Comi G.. Freedom from disease activity in multiple sclerosis. Neurology 2010; 74( suppl 3): S3– S7. [DOI] [PubMed] [Google Scholar]
- 5. Stangel M, Penner IK, Kallmann BA, Lukas C, Kieseier BC.. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: the multiple sclerosis decision model. Ther Adv Neurol Disord. 2015; 8: 3– 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL.. Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol. 2015; 72: 152– 158. [DOI] [PubMed] [Google Scholar]
- 7. Bakshi R, Thompson AJ, Rocca MA, . et al. MRI in multiple sclerosis: current status and future prospects. Lancet Neurol. 2008; 7: 615– 625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Filippi M, Rocca MA, Arnold DL, . et al. EFNS guidelines on the use of neuroimaging in the management of multiple sclerosis. Eur J Neurol. 2006; 13: 313– 325. [DOI] [PubMed] [Google Scholar]
- 9. Sicotte NL, Voskuhl RR, Bouvier S, Klutch R, Cohen MS, Mazziotta JC.. Comparison of multiple sclerosis lesions at 1.5 and 3.0 Tesla. Invest Radiol. 2003; 38: 423– 427. [DOI] [PubMed] [Google Scholar]
- 10. Stankiewicz JM, Glanz BI, Healy BC, . et al. Brain MRI lesion load at 1.5T and 3T versus clinical status in multiple sclerosis. J Neuroimaging. 2011; 21: e50– e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bakshi R, Neema M, Tauhid S, . et al. An expanded composite scale of MRI-defined disease severity in multiple sclerosis: MRDSS2. Neuroreport. 2014; 25: 1156– 1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bot JC, Barkhof F, Polman CH, . et al. Spinal cord abnormalities in recently diagnosed MS patients added value of spinal MRI examination. Neurology. 2004; 62: 226– 233. [DOI] [PubMed] [Google Scholar]
- 13. Chen M, Carass A, Oh J, . et al. Automatic magnetic resonance spinal cord segmentation with topology constraints for variable fields of view. Neuroimage. 2013; 83: 1051– 1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cohen AB, Neema M, Arora A, . et al. The relationships among MRI-defined spinal cord involvement, brain involvement, and disability in multiple sclerosis. J Neuroimaging. 2012; 22: 122– 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gass A, Rocca MA, Agosta F, . et al. MRI monitoring of pathological changes in the spinal cord in patients with multiple sclerosis. Lancet Neurol. 2015; 14: 443– 454. [DOI] [PubMed] [Google Scholar]
- 16. Horsfield MA, Sala S, Neema M, . et al. Rapid semi-automatic segmentation of the spinal cord from magnetic resonance images: application in multiple sclerosis. Neuroimage. 2010; 50: 446– 455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kearney H, Rocca MA, Valsasina P, . et al. Magnetic resonance imaging correlates of physical disability in relapse onset multiple sclerosis of long disease duration. Mult Scler. 2014; 20: 72– 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kidd D, Thorpe JW, Thompson AJ, . et al. Spinal cord MRI using multi-array coils and fast spin echo, II: findings in multiple sclerosis. Neurology. 1993; 43: 2632– 2637. [DOI] [PubMed] [Google Scholar]
- 19. Kim G, Khalid F, Oommen VV, . et al. T1- vs. T2-based MRI measures of spinal cord volume in healthy subjects and patients with multiple sclerosis. BMC Neurol. 2015; 15: 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Papinutto N, Schlaeger R, Panara V, . et al. Age, gender and normalization covariates for spinal cord gray matter and total cross-sectional areas at cervical and thoracic levels: a 2D phase sensitive inversion recovery imaging study. PLoS One. 2015; 10: e0118576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rocca MA, Horsfield MA, Sala S, . et al. A multicenter assessment of cervical cord atrophy among MS clinical phenotypes. Neurology. 2011; 76: 2096– 2102. [DOI] [PubMed] [Google Scholar]
- 22. Schlaeger R, Papinutto N, Panara V, . et al. Spinal cord gray matter atrophy correlates with multiple sclerosis disability. Ann Neurol. 2014; 76: 568– 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stevenson VL, Leary SM, Losseff NA, . et al. Spinal cord atrophy and disability in MS: a longitudinal study. Neurology. 1998; 51: 234– 238. [DOI] [PubMed] [Google Scholar]
- 24. Yiannakas MC, Mustafa AM, De Leener B, . et al. Fully automated segmentation of the cervical cord from T1-weighted MRI using PropSeg: application to multiple sclerosis. Neuroimage Clin. 2016; 10: 71– 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Okuda DT, Siva A, Kantarci O, . et al. Radiologically isolated syndrome: 5-year risk for an initial clinical event. PLoS One. 2014; 9: e90509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Polman CH, Reingold SC, Edan G, . et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria.” Ann Neurol. 2005; 58: 840– 846. [DOI] [PubMed] [Google Scholar]
- 27. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an Expanded Disability Status Scale (EDSS). Neurology. 1983; 33: 1444– 1452. [DOI] [PubMed] [Google Scholar]
- 28. Nijeholt GJ, van Walderveen MA, Castelijns JA, . et al. Brain and spinal cord abnormalities in multiple sclerosis: correlation between MRI parameters, clinical subtypes and symptoms. Brain. 1998; 121: 687– 697. [DOI] [PubMed] [Google Scholar]
- 29. Lovas G, Szilagyi N, Majtenyi K, Palkovits M, Komoly S.. Axonal changes in chronic demyelinated cervical spinal cord plaques. Brain. 2000; 123: 308– 317. [DOI] [PubMed] [Google Scholar]
- 30. Filippi M, Bozzali M, Horsfield MA, . et al. A conventional and magnetization transfer MRI study of the cervical cord in patients with MS. Neurology. 2000; 54: 207– 213. [DOI] [PubMed] [Google Scholar]
- 31. Lycklama à Nijeholt GJ, Barkhof F, Scheltens P, . et al. MR of the spinal cord in multiple sclerosis: relation to clinical subtype and disability. AJNR Am J Neuroradiol. 1997; 18: 1041– 1048. [PMC free article] [PubMed] [Google Scholar]
- 32. Arnold DL, Calabresi PA, Kieseier BC, . et al. Effect of peginterferon beta-1a on MRI measures and achieving no evidence of disease activity: results from a randomized controlled trial in relapsing-remitting multiple sclerosis. BMC Neurol. 2014; 14: 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cohen JA, Coles AJ, Arnold DL, . et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. 2012; 380: 1819– 1828. [DOI] [PubMed] [Google Scholar]
- 34. Coles AJ, Twyman CL, Arnold DL, . et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet. 2012; 380: 1829– 1839. [DOI] [PubMed] [Google Scholar]
- 35. De Stefano N, Stromillo ML, Giorgio A, . et al. Long-term assessment of no evidence of disease activity in relapsing-remitting MS. Neurology. 2015; 85: 1722– 1723. [DOI] [PubMed] [Google Scholar]
- 36. Havrdova E, Giovannoni G, Stefoski D, . et al. Disease-activity-free status in patients with relapsing–remitting multiple sclerosis treated with daclizumab high-yield process in the SELECT study. Mult Scler. 2014; 10: 464– 470. [DOI] [PubMed] [Google Scholar]
- 37. Lublin FD, Cofield SS, Cutter GR, . et al. Randomized study combining interferon and glatiramer acetate in multiple sclerosis. Ann Neurol. 2013; 73: 327– 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Campi A, Pontesilli S, Gerevini S, Scotti G.. Comparison of MRI pulse sequences for investigation of lesions of the cervical spinal cord. Neuroradiology. 2000; 42: 669– 675. [DOI] [PubMed] [Google Scholar]
- 39. Hittmair K, Mallek R, Prayer D, Schindler EG, Kollegger H.. Spinal cord lesions in patients with multiple sclerosis: comparison of MR pulse sequences. Am J Neuroradiol. 1996; 17: 1555– 1565. [PMC free article] [PubMed] [Google Scholar]
- 40. Hou P, Hasan KM, Sitton CW, Wolinsky JS, Narayana PA.. Phase-sensitive T1 inversion recovery imaging: a time-efficient interleaved technique for improved tissue contrast in neuroimaging. Am J Neuroradiol. 2005; 26: 1432– 1438. [PMC free article] [PubMed] [Google Scholar]
- 41. Philpott C, Brotchie P.. Comparison of MRI sequences for evaluation of multiple sclerosis of the cervical spinal cord at 3T. Eur J Radiol. 2011; 80: 780– 785. [DOI] [PubMed] [Google Scholar]
- 42. Poonawalla AH, Hou P, Nelson FA, Wolinsky JS, Narayana PA.. Cervical spinal cord lesions in multiple sclerosis: T1-weighted inversion-recovery MR imaging with phase-sensitive reconstruction. Radiology. 2008; 246: 258– 264. [DOI] [PubMed] [Google Scholar]
- 43. Rocca MA, Mastronardo G, Horsfield MA, . et al. Comparison of three MR sequences for the detection of cervical cord lesions in patients with multiple sclerosis. Am J Neuroradiol. 1999; 20: 1710– 1716. [PMC free article] [PubMed] [Google Scholar]
- 44. Kappos L, De Stefano N, Freedman MS, . et al. Inclusion of brain volume loss in a revised measure of ‘no evidence of disease activity’ (NEDA-4) in relapsing–remitting multiple sclerosis. Mult Scler. 2016; 22: 1297– 1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tauhid S, Neema M, Healy BC, Weiner HL, Bakshi R.. MRI phenotypes based on cerebral lesions and atrophy in patients with multiple sclerosis. J Neurol Sci. 2014; 346: 250– 254. [DOI] [PubMed] [Google Scholar]
- 46. Dula AN, Pawate S, Dortch RD, . et al. Magnetic resonance imaging of the cervical spinal cord in multiple sclerosis at 7T. Mult Scler. 2016; 22: 320– 328. [DOI] [PMC free article] [PubMed] [Google Scholar]


