Abstract
Groundnut is an economically important N2-fixing legume that can contribute about 100–190 kg N ha−1 to cropping systems. In this study, groundnut-nodulating native rhizobia in South African soils were isolated from root nodules. Genetic analysis of isolates was done using restriction fragment length polymorphism (RFLP)-PCR of the intergenic spacer (IGS) region of 16S-23S rDNA. A total of 26 IGS types were detected with band sizes ranging from 471 to 1415 bp. The rhizobial isolates were grouped into five main clusters with Jaccard's similarity coefficient of 0.00–1.00, and 35 restriction types in a UPGMA dendrogram. Partial sequence analysis of the 16S rDNA, IGS of 16S rDNA-23S rDNA, atpD, gyrB, gltA, glnII and symbiotic nifH and nodC genes obtained for representative isolates of each RFLP-cluster showed that these native groundnut-nodulating rhizobia were phylogenetically diverse, thus confirming the extent of promiscuity of this legume. Concatenated gene sequence analysis showed that most isolates did not align with known type strains, and may represent new species from South Africa. This underscored the high genetic variability associated with groundnut Rhizobium and Bradyrhizobium in South African soils, and the possible presence of a reservoir of novel groundnut-nodulating Bradyrhizobium and Rhizobium in the country.
Keywords: IGS, RFLP, Phylogenetic, Bradyrhizobium, Rhizobium, Promiscuity, Nodulation
Introduction
The use and cultivation of groundnut (Arachis hypogaea L.) dates back to 350 BC in its native South America where it has been used for thousands of years [1]. Traders were responsible for spreading groundnut from South America to Asia and Africa [18]. The Spanish and Portuguese explorers brought groundnut on their voyages to Africa. For example, West Africa Portuguese traders in the 16th century introduced the crop. Groundnut then flourished in many African countries and was incorporated into local traditional food cultures and was revered as a sacred food.
Nitrogen fixation by legume- rhizobia symbiosis plays a major role in sustaining soil health for crop production. However, this process is influenced by many factors, which include geographic location, soil type and host-plant genotypes, as well as the rhizobial symbiont itself [36]. Groundnut is reported to derive about 70–90% of its N requirements from symbiosis and to contribute an estimated amount of 100–190 kg N ha−1 to the cropping system [34]. In Africa, groundnut can obtain approximately 33–67% of its N nutrition from fixation [30] and fix up to 101 kg N ha−1 per cropping season [8].
Groundnut is generally nodulated by slow-growing rhizobia of the genus Bradyrhizobium, although effective fast-growing strains have also been reported to nodulate this legume [19]. Bradyrhizobium is a cosmopolitan and diverse group of microsymbionts capable of nodulating a variety of legumes, as well as the non-legume Parasponia [23]. Species of bradyrhizobia, such as Bradyrhizobium japonicum, Bradyrhizobium elkanii, Bradyrhizobium lablabi, Bradyrhizobium yuanmingense and Bradyrhizobium iriomotense are known to nodulate groundnut [51]. Additionally, fast-growing species of the genus Rhizobium can also nodulate groundnut, and they include Rhizobium giardinii and Rhizobium tropici [45].
The diversity of groundnut-nodulating rhizobia has been widely investigated using molecular techniques, which include the use of PCR-based methods to characterize the genetic relationships between rhizobial species.
Rhizobia are taxonomically diverse [55], and therefore require the use of well-tested, easy, quick techniques to differentiate microsymbionts at the genus, species and even strain level [15]. Restriction fragment length polymorphism (RFLP) analysis of 16S rRNA amplification using the polymerase chain reaction (PCR) provides a simplified method for characterization of rhizobial isolates at the molecular level [24], [32]. However, the use of the 16S rRNA gene alone as a phylogenetic marker in differentiating closely related species and strains within species has run into difficulties because of: (i) its presence as multiple copies in the genome of some bacteria, (ii) its susceptibility to genetic recombination and horizontal gene transfer, and (iii) its low divergence between closely related species [3], [13], [28], [46]. Thus, the IGS and housekeeping genes are currently in use as markers for molecular systematics and for estimation of the phylogenetic relationships among rhizobia. The sequences of 16S-23S rRNA give more coherent results, which are similar to DNA-DNA hybridization rather than 16S rRNA sequence analysis [54].
Based on the 16S-23S rRNA analysis, a higher level of diversity and heterogeneity was observed in groundnut bradyrhizobia in Canada [40], [48], China [57], [59], Cameroon [33], Argentina [34], [45], and other geographic regions [49]. However, despite these studies, the degree of genetic diversity among groundnut-nodulating rhizobia is still not properly understood. Previous studies using only five South African groundnut isolates showed some measure of diversity [41]. Therefore, the aim of this investigation was to obtain a complete understanding of the diversity present in groundnut-nodulating rhizobia in South African soils. To do this, firstly, a wide range of isolates was obtained from groundnut nodules in South Africa and they were analysed using IGS PCR-RFLP. Secondly, the gene sequences were determined for the 16S rDNA, IGS, glnII, gyrB, gltA and atpD genes located in the core genome, as well as the symbiotic genes nifH and nodC from selected isolates, and phylogenetic analysis of these genes was used to identify the bacteria.
Materials and methods
Rhizobial isolation and culture conditions
Root nodules were collected from groundnut plants grown at Klipladrift (26° 56′ 15.58″ S 29° 52′ 29.60″ E) in Mpumalanga Province, and Kwamhlanga (25° 25′ 48.04″ S 28° 42′ 43.85″ E) in Gauteng Province, South Africa. Sampling sites were chosen because groundnut was being introduced into these locations. None of the regions included in this study had any history of inoculation with rhizobial strains. Nodules were collected at 50% flowering of the groundnut crop. Nodules were surface-disinfected, squashed, and the nodule macerate was used to streak plates of yeast mannitol agar (YMA) medium, as described by Somasegaran and Hoben [40]. Pure single-colonies of the bacterial isolates were streaked on YMA agar containing 0.3% CaCO3 in McCartney bottles and preserved at 4 °C for later use.
Nodulation assay
Healthy groundnut seeds were surface-sterilized by treatment with 70% ethyl alcohol for 1.5 min, washed with 3.5% NaOCl for 2 min, and then thoroughly rinsed with sterile distilled water five times. The surface-sterilized seeds were sown in sterile plastic pots containing sterilized sand, and they were watered twice a week with N-free plant nutrient solution [6]. After germination, the groundnut seedlings were thinned to one seedling per pot, and inoculated with 2 mL of a bacterial culture in the log phase (≈107–108 bacterial cells mL−1). Three replicate pots were used per isolate and three pots of uninoculated seedlings that received 2 mL sterile distilled water served as controls. After six weeks, the plants were harvested and visually examined for nodulation.
Isolation of rhizobial DNA and PCR amplification of the IGS (16S-23S rDNA) region
Nodule bacterial genomic DNA was extracted using the GenElute™ Bacterial DNA Isolation Kit (Sigma-Aldrich, USA), according to the manufacturer’s instructions. The integrity of isolated DNA was checked on 1% agarose gel stained with ethidium bromide. The polymerase chain reaction (PCR) was carried out with 60–80 ng DNA in a 25 μL reaction volume containing 5× My Taq PCR buffer, 0.5 U Taq polymerase (Bioline, USA), and 10 pM each of the primers for the IGS region using a standard temperature profile ([2], [25], [27], [29], [35], [42], [43], [52]) in a thermal cycler (T100, Bio-Rad, USA). The amplified product (band) size was estimated from horizontal gel electrophoresis on 2% agarose gel stained with ethidium bromide using a standard DNA marker (GeneDirex, 1 kbp), and photographed using a gel documentation system (Geldoc™ XR+, Bio-Rad, USA).
Restriction fragment length polymorphism (RFLP) of the IGS region
The PCR-amplified IGS region was digested with fast digest restriction endonucleases (HaeII and HindIII), following the manufacturer’s instructions (Thermo Scientific, Lithuania). The digested fragments were separated by horizontal gel electrophoresis on 3% agarose gel containing ethidium bromide. Electrophoresis was performed in tris-acetic acid EDTA (1X TAE) buffer at 85 V for 2.5 h and subsequently photographed under UV light with the Bio-Rad Gel documentation system.
RFLP cluster analysis of the IGS regions
Only distinct, well-resolved, and unambiguous bands were scored, and faint bands were discarded. Bands ≤50 bp in size were not included for cluster analysis. The restriction enzyme-digested fragments were scored as: (1) in the presence of, and (0) in the absence of homologous bands. Thereafter, the similarity of strains tested was evaluated by a simple matching Jaccard similarity coefficient with the help of NTSYSpc 2.1 software [37], and a dendrogram was constructed from the distance matrix using the unweighted pair group method with arithmetic mean algorithm (UPGMA).
PCR amplification, sequencing and phylogenetic analysis of 16S rDNA, IGS, housekeeping (atpD, glnII, gyrB and gltA) and symbiotic (nodC and nifH) genes
PCR amplification of 16S rDNA, atpD, glnII, gyrB, gltA and the symbiotic nodC and nifH genes of the rhizobial genome was carried out as described above for IGS-PCR amplification. The primers and thermal cycle conditions used are listed in [2], [25], [27], [29], [35], [42], [43], [52]. The PCR-amplified products of IGS, 16S rDNA, atpD, glnII, gyrB, gltA and symbiotic nodC and nifH were purified by the FavorPrep™ PCR Purification Kit (FAVORGEN, Sigma, USA). The purified samples were sequenced (Macrogen, Netherlands), and the quality of all sequences was checked using BioEdit 7.0.0 software [17]. NCBI GenBank databases were used to identify species closely related to the test isolates using the BLASTn program. The sequences were deposited in the GenBank database in order to obtain accession numbers after confirmation of the 3′ and 5′ direction (Table S2). Reference type sequences were selected in order to align the sequences of the test isolates using MUSCLE [9] for the construction of a phylogenetic tree created with the MEGA 6.0 program [44]. Phylogenetic trees were generated by Kimura’s 2-parameter model and the neighbor-joining algorithm [22], [38] with 1000 bootstrap support [12]. Nucleotide information was obtained from conserved, variable, parsimony-informative, and singleton regions using consensus sequences.
Results
A total of 71 bacterial isolates were obtained from the root nodules of groundnut planted in South African soils, and 46 of the isolates elicited nodulation in groundnut (the homologous host) under glasshouse conditions. These authenticated rhizobial isolates were then genetically analyzed using various molecular tools.
IGS PCR amplification
The IGS PCR-amplified product yielded polymorphic bands in the rhizobial isolates tested from groundnut. All the isolates revealed the presence of single bands, except TUTAHSA158 that produced more than one band in the 2% agarose gel. The IGS band lengths across the bacterial population varied from 471 to 1415 bp (Fig. S1A). The polymorphic bands obtained in this study successfully distributed the test rhizobial isolates into 24 groups, denoted by Roman numerals as IGS types I to XXIV (Table 1). IGS type X had the largest number (7) of isolates among the different polymorphic bands (Table 1).
Table 1.
IGS type and restriction pattern of the PCR-amplified IGS (16S-23S rDNA) region of groundnut nodulating rhizobial strains.
| Strains | Site of origin | Size (bp) | Restriction pattern type |
||
|---|---|---|---|---|---|
| IGS type | HindIII | HaeII | |||
| TUTAHSA10 | Klipladrift | 1415 | I | E | O |
| TUTAHSA41 | Klipladrift | 1261 | II | I | C |
| TUTAHSA45 | Klipladrift | 1261 | II | J | M |
| TUTAHSA114 | Kwamhlanga | 1250 | III | C | I |
| TUTAHSA116 | Kwamhlanga | 1250 | III | L | I |
| TUTAHSA80 | Klipladrift | 1225 | IV | E | I |
| TUTAHSA87 | Klipladrift | 1200 | V | I | C |
| TUTAHSA31 | Klipladrift | 1060 | VI | D | B |
| TUTAHSA84 | Klipladrift | 1041 | VII | B | J |
| TUTAHSA19 | Klipladrift | 1039 | VIII | G | B |
| TUTAHSA40 | Klipladrift | 1039 | VIII | H | B |
| TUTAHSA51 | Klipladrift | 1039 | VIII | H | N |
| TUTAHSA156 | Kwamhlanga | 1020 | XVII | N | G |
| TUTAHSA157 | Kwamhlanga | 1200 | V | G | P |
| TUTAHSA27 | Klipladrift | 980 | IX | N | B |
| TUTAHSA7 | Klipladrift | 960 | X | B | B |
| TUTAHSA155 | Kwamhlanga | 960 | X | P | E |
| TUTAHSA61 | Klipladrift | 960 | X | H | H |
| TUTAHSA75 | Klipladrift | 960 | X | B | A |
| TUTAHSA97 | Klipladrift | 960 | X | A | A |
| TUTAHSA159 | Kwamhlanga | 960 | X | F | E |
| TUTAHSA160 | Kwamhlanga | 960 | X | F | E |
| TUTAHSA158 | Kwamhlanga | 942 | XI | – | E |
| TUTAHSA67 | Klipladrift | 936 | XII | B | A |
| TUTAHSA73 | Klipladrift | 915 | XIII | B | A |
| TUTAHSA154 | Kwamhlanga | 895 | XIV | D | A |
| TUTAHSA115 | Kwamhlanga | 876 | XV | A | H |
| TUTAHSA4 | Klipladrift | 866 | XVI | K | H |
| TUTAHSA151 | Kwamhlanga | 856 | XVII | A | A |
| TUTAHSA153 | Kwamhlanga | 856 | XVII | A | G |
| TUTAHSA140 | Kwamhlanga | 838 | XVIII | A | C |
| TUTAHSA143 | Kwamhlanga | 838 | XVIII | F | L |
| TUTAHSA144 | Kwamhlanga | 819 | XIX | A | F |
| TUTAHSA145 | Kwamhlanga | 819 | XIX | A | F |
| TUTAHSA147 | Kwamhlanga | 819 | XIX | D | F |
| TUTAHSA148 | Kwamhlanga | 819 | XIX | A | A |
| TUTAHSA150 | Kwamhlanga | 819 | XIX | D | A |
| TUTAHSA58 | Klipladrift | 819 | XIX | K | D |
| TUTAHSA20 | Klipladrift | 781 | XX | C | D |
| TUTAHSA118 | Kwamhlanga | 750 | XXI | M | D |
| TUTAHSA17 | Klipladrift | 612 | XXII | C | K |
| TUTAHSA152 | Kwamhlanga | 500 | XXIII | C | A |
| TUTAHSA126 | Kwamhlanga | 471 | XXIV | O | L |
IGS PCR-RFLP
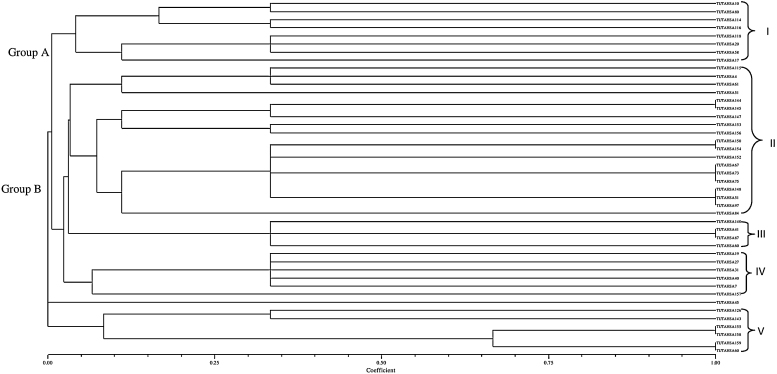
A much greater variation was found among the tested isolates using PCR-RFLP analysis of the 16S-23S rRNA intergenic spacer regions (Fig. S1B). IGS PCR-amplified products were digested with two 6-base cutting restriction endonucleases (HindII and HaeII), and they revealed the presence of 35 IGS PCR-RFLP patterns (Fig. 1). The number of bands generated on 3% agarose gel stained with ethidium bromide ranged from 1 to 5 for HindIII, and 1 to 4 for HaeII (Fig. S1B). Test restriction enzymes HindIII and HaeII yielded same 15 (A-P) restriction banding pattern types. The HindIII restriction type A contained the highest number (8) of bacterial isolates, while HaeII restriction type A had nine isolates (Table 1). As a result of this analysis, a dendrogram was generated from the combined restriction profiles of HindIII and HaeII endonucleases using a binary matrix scoring 0/1 (0 in the absence and 1 in the presence of the restriction type), and by depicted similarity in the IGS region of the isolates (Fig. 1). From the IGS PCR-RFLP analysis, and subsequent UPGMA clustering, all isolates were grouped into five main clusters at Jaccard similarity coefficients of 0.00–1.00 (Fig. 1). Isolated strains were joined at the final Jaccard similarity coefficient level of 0.0. Cluster II contained the largest number (15) of isolates, while cluster III had the lowest number (4) of isolates (Fig. 1). Strain TUTAHSA45 was highly diverse compared to all the other tested isolates, since it stood independently (Fig. 1). Clusters I, II, III and IV formed major Group A joined together at a 0.01 similarity coefficient, while Cluster V and isolate TUTAHSA45 formed major Group B, which was highly diverse. Cluster I contained two sub-clusters joined at a similarity coefficient of 0.07, while Cluster II had three sub-clusters that joined together at a 0.03 similarity coefficient.
Fig. 1.
Dendrogram generated from HindIII and HaeII restriction enzyme digested IGS (16S-23 rDNA) RFLP restriction banding pattern of groundnut nodulating rhizobial isolates.
Sequencing and phylogenetic analysis of the 16S rDNA region
For direct sequencing of 16S rDNA and IGS gene-amplified products, representative isolates from each cluster were randomly selected based on restriction fragment length polymorphism. The BLASTn analysis showed that the test isolates grouped with Bradyrhizobium and Rhizobium species groups.
Bradyrhizobium group
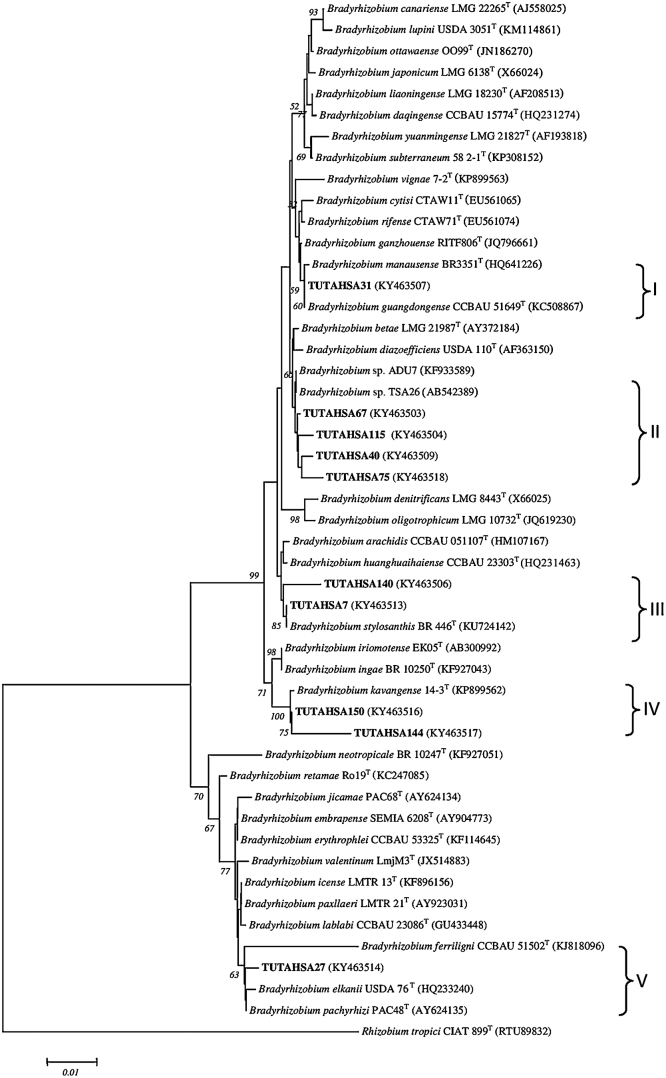
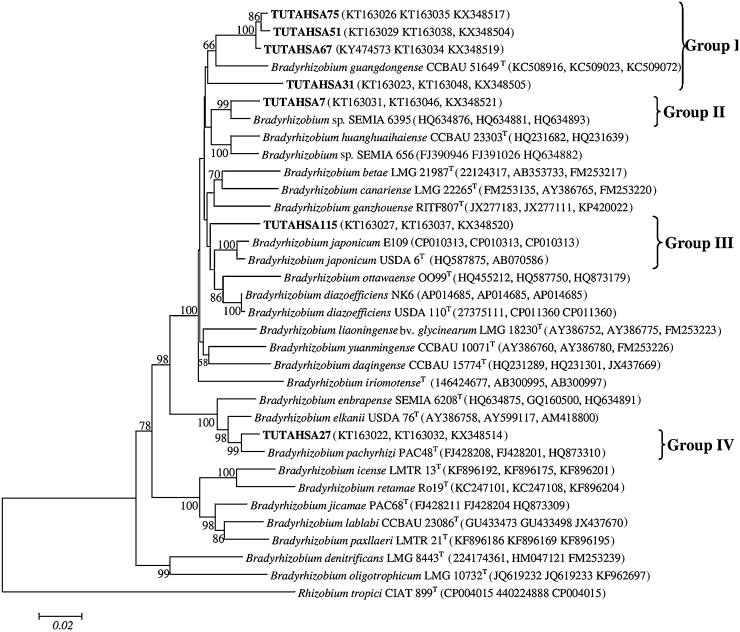
Aligned 1110 bp sequences of 16S rDNA contained 911 conserved, 194 variable, 71 parsimony-informative and 123 singleton sites (Table S3). The phylogenetic tree constructed from the 16S rDNA sequences placed all isolates into five groups (Groups I–V) (Fig. 2a). Group I was formed by isolate TUTAHSA31 and type strains Bradyrhizobium manausense BR3352T and Bradyrhizobium guangdongense CCBAU 51649T with 60 bootstrap support. Isolates TUTAHSA67, TUTAHSA40, TUTAHSA115 and TUTAHSA75 showed their close relationship with Bradyrhizobium sp. ADU7 isolated from groundnut in China with 99.4–99.9% sequence identity in Group II. In Group III, isolates TUTAHSA140 and TUTAHSA7 clustered with Bradyrhizobium stylosanthis BR 446T with 99.1–100% sequence identity. Isolates TUTAHSA150 and TUTAHSA144 showed a close relationship with Bradyrhizobium kavangense 14-3T with high 100 bootstrap support in Group IV. In Group V, isolate TUTAHSA27 was aligned with B. elkanii USDA76T, Bradyrhizobium pachyrhizi PAC 48T and Bradyrhizobium ferriligni CCBAU 51502T with 63 bootstrap support.
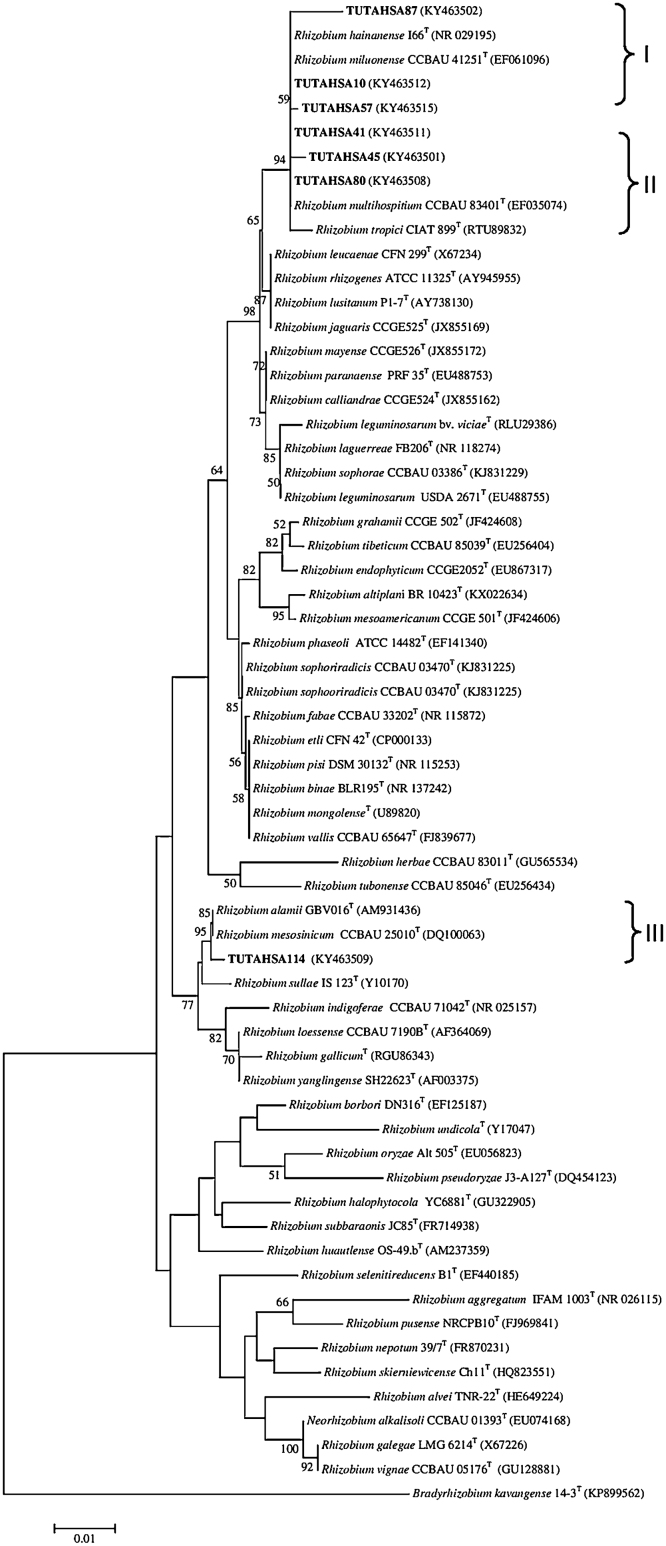
Fig. 2.
(a) The neighbour-joining phylogenetic relationships of groundnut nodulating Bradyrhizobium based on 16S rDNA sequence analysis. Groundnut nodulating microsymbiont are shown in bold with their nucleotide sequence accession numbers indicated in brackets. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position. Phylogenetic analyses were conducted in MEGA6. (b) The neighbour-joining phylogenetic relationships of groundnut nodulating Rhizobium based on 16S rDNA sequence analysis. Groundnut nodulating microsymbiont are shown in bold with their nucleotide sequence accession numbers indicated in brackets. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
Rhizobium group
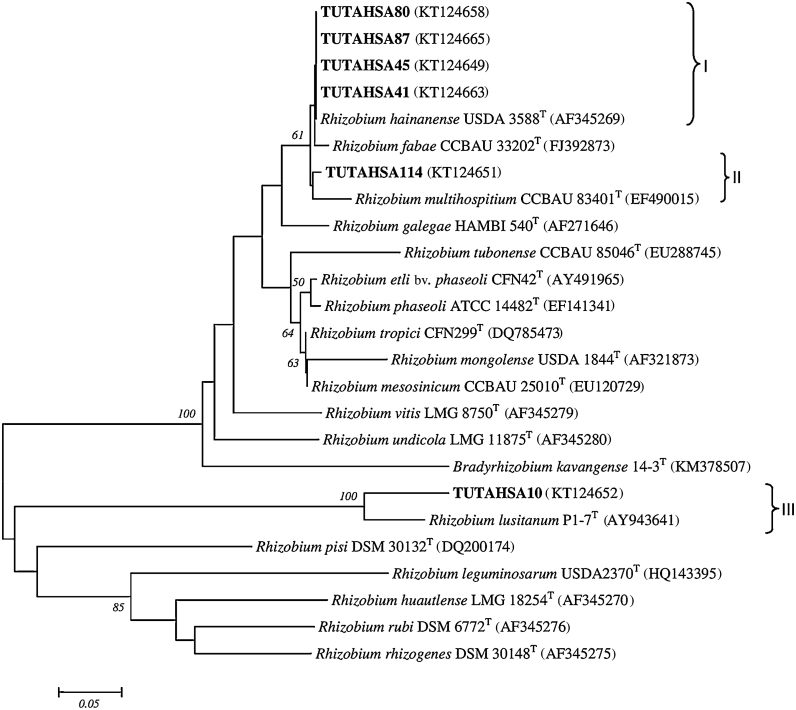
In the Rhizobium group, the 825 aligned nucleotide sequences had 670 conserved, 155 variable, 89 parsimony-informative and 66 singleton sites (Table S3). In the phylogenetic tree, isolates TUTAHSA87, TUTAHSA10, TUTAHSA57, TUTAHSA41, TUTAHSA45 and TUTAHSA80 grouped with R. tropici-related type strains (Rhizobium hainanense I66T, Rhizobium miluonense CCBAU 41251T and Rhizobium multihospitium CCBAU 83401T) in Groups I and II with 94 bootstrap support. In Group III, isolate TUTAHSA114 was closely related to Rhizobium alamii and Rhizobium mesosinicum with 95 bootstrap value (Fig. 2b).
Sequencing and phylogenetic analysis of the IGS region
Due to the divergence of the IGS region among Bradyrhizobium species, phylogenetic studies of this region were considered to be more appropriate. The sequences generated from the IGS region were used to align with type strain IGS sequences selected from GenBank. Based on partial IGS sequence comparisons with the GenBank references, some isolates were identified as Bradyrhizobium sp. and others as Rhizobium sp. The nucleotide sequence analysis results are indicated in Table S3.
As for 16S rDNA, the two phylogenetic trees were constructed from the IGS sequences of test isolates with Bradyrhizobium and Rhizobium species groups (Fig. 3a and b).
Fig. 3.
(a) The neighbour-joining phylogenetic relationships of groundnut nodulating Bradyrhizobium based on IGS (16S-23S rDNA) sequence analysis. Groundnut nodulating microsymbiont are shown in bold with their nucleotide sequence accession numbers indicated in brackets. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position. Phylogenetic analyses were conducted in MEGA6. (b) The neighbour-joining phylogenetic relationships of groundnut nodulating Rhizobium based on IGS (16S-23S rDNA) sequence analysis. Groundnut nodulating microsymbiont are shown in bold with their nucleotide sequence accession numbers indicated in brackets. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position. Phylogenetic analyses were conducted in MEGA6.
Bradyrhizobium group
The topology of the IGS phylogram was similar to the 16S rDNA phylogeny but with a slight variation in the isolate placements in the trees. The test isolates in the Bradyrhizobium tree were further divided into six (I–VI) distinct groups (Fig. 3a). In Group I, isolates TUTAHSA27 and TUTAHSA 110 clustered with B. ferriligni, Bradyrhizobium embrapense, B. pachyrhizi and B. elkanii with 77 bootstrap support and 99.6% sequence similarity. In Group II, isolates TUTAHSA144 and TUTAHSA150 were aligned with Bradyrhizobium subterraneum with 97.8–98.4% sequence similarity. In Group III, TUTAHSA140 aligned with Bradyrhizobium huanghuaihaiense with 59 bootstrap support, while TUTAHSA7 was an outgroup. Isolate TUTAHSA115 was proximally related to the type strains B. japonicum, Bradyrhizobium cytisi and Bradyrhizobium rifense in Group IV. Isolates TUTAHSA51, TUTAHSA67, TUTAHSA75, and TUTAHSA40 were however clustered together and stood alone in Group VI. Isolate TUTAHSA4 also stood alone but formed an outgroup in the phylogram.
Rhizobium group
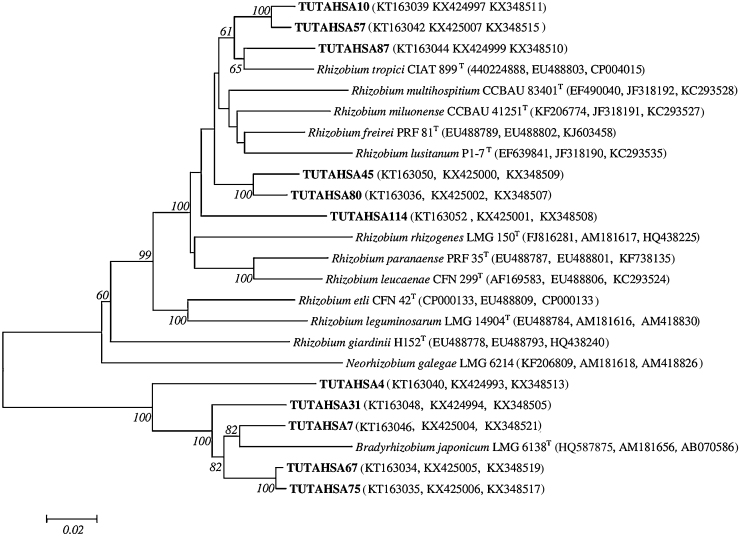
In the Rhizobium group, isolates TUTAHSA41, TUTAHSA87, TUTAHSA10, TUTAHSA114, TUTAHSA45 and TUTAHSA80 clustered with different Rhizobium species in three (I–III) distinct groups. Isolates TUTAHSA80, TUTAHSA87, TUTAHSA45 and TUTAHSA41 were closely related to strain R. hainanense in Group I, while isolate TUTAHSA114 formed a close relationship with R. multihospitium in Group II. Isolate TUTAHSA10 grouped with Rhizobium lusitanum PI-7T with high (100) bootstrap support (Fig. 3b).
Analysis of the housekeeping genes
For a clear resolution of the phylogenetic analysis, the four housekeeping genes gyrB, atpD, glnII and gltA, which are highly conserved among bacteria belonging to the Rhizobiales and encode DNA gyrase subunit B, ATP synthase beta chain, glutamine synthase II and citrate synthase, respectively, were selected for further studies.
Selected representative isolates from RFLP analysis yielded amplified bands of the four genes. Fewer sequences were used in atpD and gltA phylogeny due to difficulties in PCR amplification and/or bad sequence results. Thus, the phylogenetic analysis of these genes was performed individually (Figs. S2–S5). The sequences of glnII, gyrB, atpD and gltA were aligned with local and type strain nucleotide sequences obtained from GenBank. The length of the alignments used was 369 bp for atpD, 424 for glnII, 226 for gltA and 561 bp for gyrB. Of the four gene sequences, gltA was the shortest and the lowest informative with only 56 informative positions. The highest level (314) of parsimony-informative sites was observed in gyrB (Table S3). As in the 16S rDNA and IGS phylograms, Bradyrhizobium and Rhizobium groups were also observed in glnII and gyrB phylogenies. Since not all test isolates were included due to the problem of PCR amplification with the atpD gene, only the Bradyrhizobium group-aligned isolates were observed in the phylogeny. The phylogenetic tree constructed with test isolates and type strains of Bradyrhizobium and Rhizobium species for the four genes did not give consistent or the same topology results in all trees, except for isolate TUTAHSA27 that clustered with B. elkanii and B. pachyrhizi. In all trees, except for gltA, isolates TUAHSA67, TUTAHSA75 and TUTAHSA51 consistently clustered together and shared 99.4-100% sequence similarity with each other while forming a separate branch within the genus Bradyrhizobium. In the gltA phylogram, the test isolates showed some discordance when compared to the phylograms of the other test housekeeping genes. Isolates TUAHSA67, TUTAHSA75 and TUTAHSA51 were grouped with Rhizobium species in the gltA phylogram.
Concatenated sequence analysis
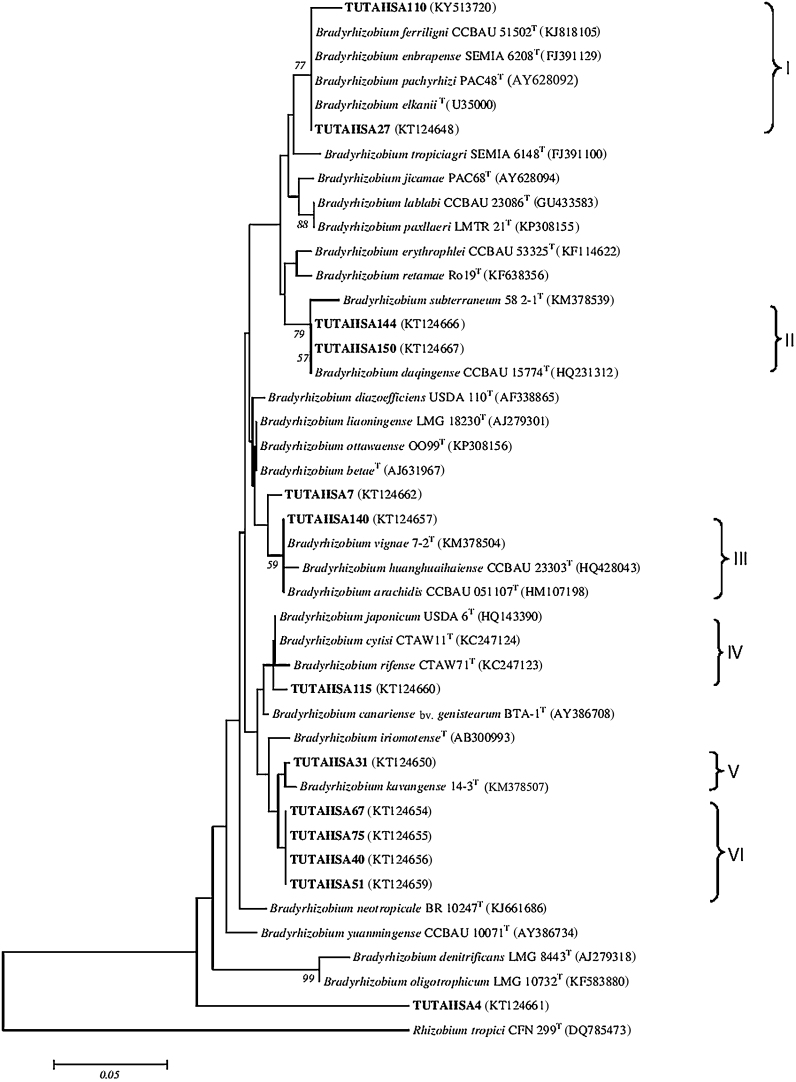
Aligned sequences of glnII, gyrB, gltA and atpD were used to construct the concatenated phylogeny. Due to the unavailability of either PCR-amplified product or nucleotide sequences of atpD and gltA regions of some isolates, two (atpD + glnII + gyrB and glnII + gyrB + gltA) separate concatenated trees were constructed for Bradyrhizobium and Rhizobium species. The concatenated sequences of atpD + glnII + gyrB regions of Bradyrhizobium contained 1355 analyzed sites of which 824 were conserved, 525 were variable, 317 were parsimony-informative and 208 were singletons (Table S3). The tree built with these concatenated sequences resulted in four groups (Fig. 4). In the first group, isolates TUTAHSA67, TUTAHSA51 and TUTAHSA75 were proximally related to B. guangdongense with 95.2–95.4% sequence similarity and 66 bootstrap support. Isolate TUTAHSA115 showed a proximal relationship with B. japonicum and Bradyrhizobium diazoefficiens in Group III, while isolate TUTAHSA 31 stood alone as an outgroup without any type strains in Group I. Isolate TUTAHSA7 in Group II clustered with Bradyrhizobium sp. SEMIA 6395 isolated from the host Calliandra houstoniana in Brazil with high 99 bootstrap support. In Group IV, isolate TUTAHSA27 was closely related to B. pachyrhizi with high 99 bootstrap support and 98% sequence similarity.
Fig. 4.
Phylogenetic relationships of groundnut nodulating Bradyrhizobium based on multilocus concatenated sequence analysis of the atpD, glnII and gyrB genes. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position. Phylogenetic analyses were conducted in MEGA6.
The second phylogenetic tree of glnII + gltA + gyrB concatenated sequences gave a clear view of the test isolates related to Rhizobium (Fig. 5). The concatenated sequences of glnII + gltA + gyrB regions of Rhizobium contained 1211 analyzed sites of which 691 were conserved, 520 were variable, 409 were parsimony-informative and 111 were singletons (Table S3). Isolate TUTAHSA87 clustered with R. tropici with 65 bootstrap support and 94.5% sequence similarity. In this phylogram, most of the isolates stood alone without any type strains. For example, isolates TUTAHSA10 and TUTAHSA57 stood alone and were closely related with 98.2% sequence similarity. Even isolates TUTAHSA45 and TUTAHSA80 stood out, since they clustered together with 97.9% sequence similarity, whereas isolate TUTAHSA114 also stood alone but without any type strains. Isolates TUTAHSA4, TUTAHSA31, TUTAHSA7, TUTAHSS67 and TUTAHSA75 formed an outgroup with B. japonicum.
Fig. 5.
Phylogenetic relationships of groundnut nodulating Rhizobium based on multilocus concatenated sequence analysis of the glnII, gltA and gyrB, genes. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position. Phylogenetic analyses were conducted in MEGA6.
Sequence and phylogenetic analysis of the nifH gene
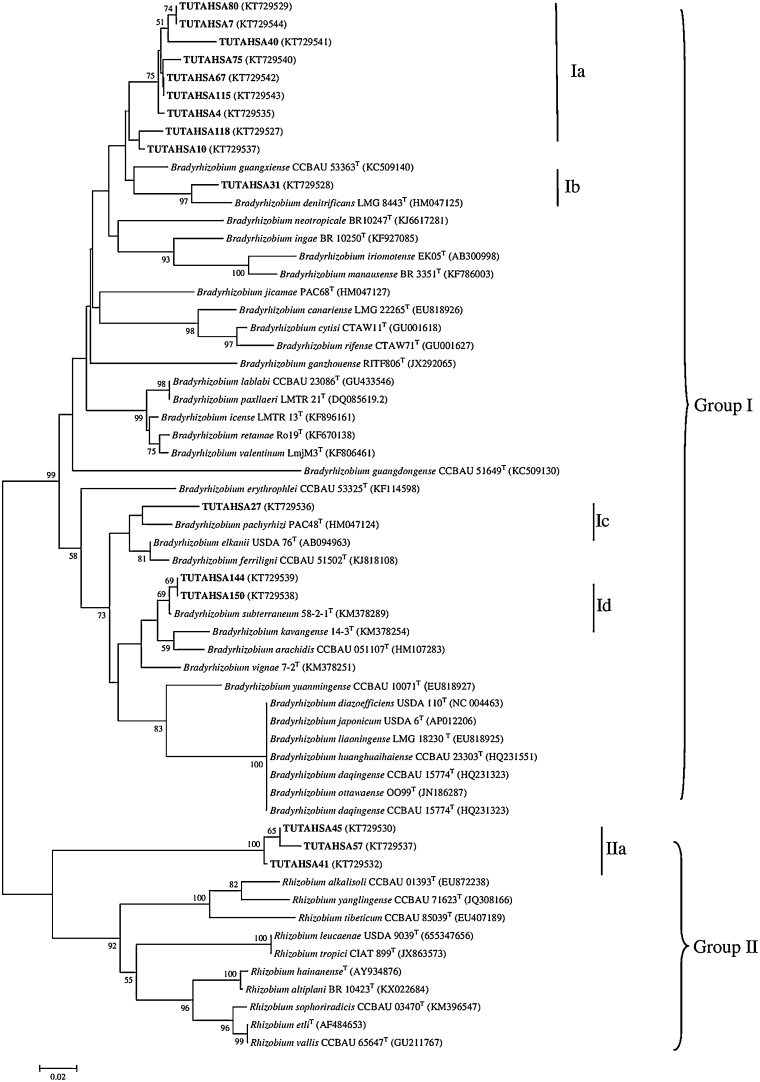
A single band of approximately 800 bp was observed after PCR amplification of the nifH region of each isolate. The nifH sequences of test isolates were aligned with type sequences of Bradyrhizobium and Rhizobium strains and nucleotide sequence information is indicated in Table S3. In the nifH phylogenetic tree, most of the test isolates (TUTAHSA80, TUTAHSA7, TUTAHSA40, TUTAHSA75, TUTAHSA67, TUTAHSA115, TUTAHSA4, TUTAHSA118 and TUTAHSA10) formed a monophyletic group without any type strains in Bradyrhizobium Group I (Subgroup Ia) (Fig. 6). The closest type strain with these isolates was Bradyrhizobium guangxiense with 95.5–96% sequence identity. Isolates TUTAHSA31 and TUTAHSA27 showed a close relationship with Bradyrhizobium denitrificans and B. pachyrhizi in Subgroups Ib and Ic, respectively. In Subgroup Id, isolates TUTAHSA144 and TUTAHSA150 shared their nifH sequences with B. subterraneum with 69 bootstrap support and 96-99.5% sequence similarity. In Rhizobium Group II, a monophyletic group of isolates (TUTAHSA45, TUTAHSA57 and TUTAHSA41) was also observed as Subgroup IIa.
Fig. 6.
The neighbour-joining phylogenetic relationships of groundnut nodulating microsymbionts based on nifH sequence analysis. The significance of each branch is indicated by a bootstrap value = >50 are indicated for each node (1000 replicates). The scale bar represents the number of changes per nucleotide position.
PCR amplification of the nodC symbiotic region
The nodC symbiotic region of the isolates was amplified using different primer pairs ([2], [25], [27], [29], [35], [42], [43], [52]). All test bradyrhizobial isolates (TUTAHSA67, TUTAHSA115, TUTAHSA4, TUTAHSA75, TUTAHSA40, TUTAHSA80, TUTAHSA7, TUTAHSA118, TUTAHSA31, TUTAHSA27, TUTAHSA140, TUTAHSA144 and TUTAHSA150) identified by 16S rDNA, IGS and housekeeping genes yielded a single band of approximately 300 bp as a nodC-amplified product using nodCp8 and nodCIf primer pairs. A single band of approximately 1000 bp was obtained as a PCR-amplified product for the rhizobial isolates TUTAHSA114, TUTAHSA45, TUTAHSA80, TUTAHSA41 and TUTAHSA87 using nodCFn and nodCI primer pairs.
Discussion
The N2-fixing ability of groundnut is essential for sustainable yields and economic returns, especially in less developed countries [11]. A common problem encountered by groundnut farmers and growers of other legumes is the presence of low symbiotically efficient native microsymbionts in the soil. Although legumes are generally nodulated by indigenous root-nodule bacteria [33], inoculation of groundnut with selected rhizobial strains has been shown to improve crop yields [5]. In this study, all the test rhizobial isolates were able to form effective nodules with groundnut as their homologous host under glasshouse conditions. The isolated groundnut-nodulating bacterial symbionts consisted of both fast-growers and slow-growers. Approximately 66% of the isolates grew on YMA plates after 6 days incubation, while the colonies of 34% of the isolates took 3 days to appear on YMA plates. These results are in contrast to those of Zhang et al. [59] and Saleena et al. [39] who detected only slow-growers as the sole occupants of groundnut root nodules. However, our results are consistent with those of Taurian et al. [45] who found both fast- and slow-growing, nodule-forming, N2-fixing bacteria in the root nodules of groundnut.
Knowledge of indigenous soil rhizobial populations is essential for selecting potential inoculant strains, as well as prior to the application of foreign inoculant strains [33]. Based on morphological, physiological and 16S rDNA-RFLP analysis of root nodules, a considerably large diversity has been found among rhizobia-nodulating groundnut in different countries [34], [45], [47], [58], [57]. In this study, the analysis of IGS-restriction fragment length polymorphism with two restriction endonucleases revealed the presence of 35 restriction types that showed huge diversity with 24 IGS types of groundnut-nodulating bacteria. This contrasts with the findings of Yang et al. [57], Ngo Nkot et al. [33] and Wang et al. [51], who respectively found only three, eight and two IGS types in their studies of groundnut rhizobia. Furthermore, our data also showed that all the test rhizobial isolates from South Africa had a very low (0.00) final similarity coefficient (Fig. 1) with a variable range (471–1415 bp) for the IGS PCR-amplified products. IGS length variability between and among rhizobial species has been previously reported [4], [21], [24]. The presence of different size bands can be explained by the variation in the conserved block within the IGS region [58], and the presence or absence of tRNA [16], [50], [53], [57].
The fragments produced in this study were smaller than those obtained by Yang et al. [57] and Yang and Zhou [58], and this could be attributed to the different primers used. In this study, some rhizobial isolates showed the same length in the IGS region but differed in restriction sites. However, it is known that the presence of polymorphic bands from the IGS region does not necessarily indicate differences in restriction sites [24]. Oddly, only strain TUTAHSA158 yielded more than one band from IGS amplification. This could possibly be due to the insertion of various tRNA genes in the IGS region [21], the heteroduplex DNA structure of single-stranded DNA [20], [24], or the existence of several copies of the rrs operon.
The IGS PCR-amplified products of some test rhizobial isolates (e.g. TUTAHSA4, TUTAHSA20, TUTAHSA41, TUTAHSA58, TUTAHSA87, TUTAHSA118, TUTAHSA17 and TUTAHSA126) could not be digested with the restriction enzymes HindIII and HaeII. This could have been due to the absence of restriction sites for the endonuclease enzymes used in the IGS sequences [33]. It was also found that cluster analysis of the RFLP data showed no linkage between strain clustering and the location from where groundnut nodules were collected for bacterial isolation. In fact, rhizobial isolates from the same origin could be seen in different clusters, while isolates from different origins were also found in the same group.
The observed variation in the IGS region of isolates in this study was consistent with the findings of Wang et al. [51] and Nievas et al. [34], who similarly found genetic variability among groundnut-nodulating rhizobia in China and Argentina. The results of the current study therefore showed that nodulation of groundnut as a host plant was elicited by an extremely diverse group of microsymbionts, and that two rhizobial genera induced nodulation of groundnut in South African soils.
Phylogenetic analysis of the selected microsymbionts using 16S rDNA, IGS, atpD, gyrB, gltA and glnII gene sequences revealed Bradyrhizobium and Rhizobium as the major predominant symbionts of groundnut. These data confirmed the high level symbiotic promiscuity of this legume. The fact that all the selected test isolates (both Bradyrhizobium and Rhizobium) could, in fulfilment of Koch’s postulates, form effective root nodules on groundnut (the homologous host) is in contrast to the results of Chen et al. [7] who found that only species of Bradyrhizobium nodulated groundnut, whereas Wong et al. [56] reported that groundnut nodules formed by fast-growing rhizobia were ineffective.
To test the robustness of the techniques used in the current study, two separate concatenated trees were constructed for Bradyrhizobium and Rhizobium. With three-gene (atpD + glnII + gyrB) concatenated tree analysis of Bradyrhizobium, the groundnut test isolates fell into four phylogenetic groups (Groups I–IV). Isolate TUTAHSA27 in Group IV shared high identity with B. pachyrhizi and B. elkanii with 97.1–98% sequence similarity, which interestingly was consistent in all individual housekeeping phylogenetic trees. All the test isolates in Group I could be defined as different new lineages. To date, eight defined Bradyrhizobium species (namely, B. japonicum, B. elkanii, B. lablabi, B. yuanmingense, B. iriomotense, B. guangxiense, B. guangdongense and Bradyrhizobium arachidis) and an unidentified Bradyrhizobium sp. have been reported to be capable of nodulating groundnut [7], [26], [31], [33], [39], [48], [49], [51], [59]. In Group I, isolates TUTAHSA67, TUTAHSA51 and TUTAHSA75 were closely related to B. guangdongense with 95.2–95.4% sequence identity. Isolate TUTAHSA115 was closely related to B. japonicum and B. diazoefficiens in the phylogram.
Furthermore, the new lineages were assessed in a second concatenated (glnII + gyrB + gltA) Rhizobium phylogram (Fig. 5), and all the groundnut-nodulating test isolates (namely, TUTAHSA87, TUTAHSA10, TUTAHSA57, TUTAHSA45, TUTAHSA80, and TUTAHSA114) grouped with Rhizobium species. This finding agreed with the results of Taurian et al. [45] and El-Akhal et al. [10] that groundnut rhizobia were phylogenetically related to R. giardinii and R. tropici.
However, the phylogenetic study of individual and concatenated genes revealed that many of these South African isolates were novel species, since they clustered with both Bradyrhizobium and Rhizobium species groups, and some were not even positioned in the tree. Thus, the taxonomic position of rhizobia nodulating groundnut is still not well defined. Therefore, isolates have been named by reference to the host plant as Bradyrhizobium sp. (Arachis) [10], [14], [45], [49].
In this study, the phylogeny of the nifH gene showed consistency with the core (housekeeping) gene phylogenies (Fig. 5). For example, isolates in Groups Ia and IIa formed a monophyletic group without any reference type strains in the nifH phylogeny, which was the same for the core genes. This suggested that they had the same evolutionary history for the chromosomal and symbiotic genes.
Considered together, the results of this study suggested that PCR-RFLP analysis of the 16S-23S rDNA IGS region in rhizobial isolates had sufficient discriminatory power to group chromosomally closely related strains based on the simple, reproducible results of restriction fragments. Combined data analysis from various restriction enzymes enabled the relatedness between 16S-23S rDNA IGS regions to be estimated. Phylogenetic analysis from this study revealed high-level promiscuity of groundnut, since it was nodulated by a diverse group of microsymbionts. The sequence alignment of the isolated strains with a divergent group of rhizobial strains further emphasized that groundnut was a highly promiscuous legume. The results showed the presence of abundant, widely distributed, diverse and novel types of native Rhizobium and Bradyrhizobium species in South African soils. Therefore, identifying indigenous rhizobial populations with high symbiotic efficiency could help increase groundnut yield and quality.
Acknowledgements
This work was supported with grants from the Bill and Melinda Gates Foundation Project on Capacity Building in Legume Sciences in Africa, the South African Department of Science and Technology, the Tshwane University of Technology, the National Research Foundation in Pretoria, and the South African Research Chair in Agrochemurgy and Plant Symbioses. LAM is grateful for a competitive fellowship from the Bill and Melinda Gates Foundation Project on Capacity Building in Legume Sciences in Africa.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.syapm.2017.02.002.
Contributor Information
Sanjay K. Jaiswal, Email: sanjay_siswa@rediffmail.com.
Felix D. Dakora, Email: dakorafd@tut.ac.za.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Akhtar S., Khalid N., Ahmed I., Shahzad A., Suleria H.A.R. Physicochemical characteristics, functional properties, and nutritional benefits of groundnut oil. Crit. Rev. Food. Sci. Nutr. 2014;54:1562–1575. doi: 10.1080/10408398.2011.644353. [DOI] [PubMed] [Google Scholar]
- 2.Appunu C., Sasirekha N., Prabavathy R.V., Nair S. A significant proportion of indigenous rhizobia from India associated with soybean (Glycine max L.) distinctly belong to Bradyrhizobium and Ensifer genera. Biol. Fertil. Soils. 2009;46:57–63. [Google Scholar]
- 3.Aserse A.A., Räsänen L.A., Assefa F., Hailemariam A., Lindström K. Phylogeny and genetic diversity of native rhizobia nodulating common bean (Phaseolus vulgaris L.) in Ethiopia. Syst. Appl. Microbiol. 2012;35:120–131. doi: 10.1016/j.syapm.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 4.Barry T., Colleran G., Glennon M., Dunican L.K., Gannon F. The 16s/23s ribosomal spacer region as a target for DNA probes to identify eubacteria. PCR Methods Appl. 1991;1:51–56. doi: 10.1101/gr.1.1.51. [DOI] [PubMed] [Google Scholar]
- 5.Bogino P., Banchio E., Rinaudi L., Cerioni G., Bonfiglio C., Giordano W. Peanut (Arachis hypogaea) response to inoculation with Bradyrhizobium sp. in soils of Argentina. Ann. Appl. Biol. 2006;148:207–212. [Google Scholar]
- 6.Broughton W.J., Dilworth M.J. Control of leghaemoglobin synthesis in snake beans. Biochem. J. 1971;125:1075–1080. doi: 10.1042/bj1251075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen J.Y., Gu J., Wang E.T., Ma X.X., Kang S.T., Huang L.Z., Wu Y.L. Wild peanut Arachis duranensis are nodulated by diverse and novel Bradyrhizobium species in acid soils. Syst. Appl. Microbiol. 2014;37:525–532. doi: 10.1016/j.syapm.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Dakora F.D., Aboyinga R.A., Mahama Y., Apaseku J. Assessment of N2 fixation in groundnut (Arachis hypogaea L.) and cowpea (Vigna unguiculata L. Walp) and their relative N contribution to a succeeding maize crop in Northern Ghana. MIRCEN J. Appl. Microbiol. Biotechnol. 1987;3:389–399. [Google Scholar]
- 9.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucl. Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.El-Akhal M.R., Rincón A., Arenal F., Lucas M.M., El Mourabit N., Barrijal S., Pueyo J.J. Genetic diversity and symbiotic efficiency of rhizobial isolates obtained from nodules of Arachis hypogaea in northwestern Morocco. Soil Biol. Biochem. 2008;40:2911–2914. [Google Scholar]
- 11.El-Akhal M.R., Rincón A., Coba de la Peña T., Lucas M.M., El Mourabit N., Barrijal S., Pueyo J.J. Effects of salt stress and rhizobial inoculation on growth and nitrogen fixation of three peanut cultivars. Plant Biol. 2013;15:415–421. doi: 10.1111/j.1438-8677.2012.00634.x. [DOI] [PubMed] [Google Scholar]
- 12.Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 13.Germano M.G., Menna P., Mostasso F.L., Hungria M. RFLP analysis of the rRNA operon of a Brazilian collection of bradyrhizobial strains from 33 legume species. Int. J. Syst. Evol. Microbiol. 2006;56:217–229. doi: 10.1099/ijs.0.02917-0. [DOI] [PubMed] [Google Scholar]
- 14.Gillette W.K., Elkan G.H. Bradyrhizobium (Arachis) sp. strain NC92 contains two nodD genes involved in the repression of nodA and a nolA gene required for the efficient nodulation of host plants. J. Bacteriol. 1996;178:2757–2766. doi: 10.1128/jb.178.10.2757-2766.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giongo A., Ambrosini A., Vargas L.K., Freire J.R.J., Bodanese-Zanettini M.H., Passaglia L.M.P. Evaluation of genetic diversity of bradyrhizobia strains nodulating soybean [Glycine max (L.) Merrill] isolated from South Brazilian fields. Appl. Soil Ecol. 2008;38:261–269. [Google Scholar]
- 16.Gurtler V., Stanisich V.A. New approaches to typing and identification of bacteria using the 16S–23S rDNA spacer region. Microbiol. 1996;142:3–16. doi: 10.1099/13500872-142-1-3. [DOI] [PubMed] [Google Scholar]
- 17.Hall T. 2004. BioEdit version 7.0. 0. Distributed by the author. website: www.mbio.ncsu.edu/BioEdit/bioedit.html. [Google Scholar]
- 18.Hammons R.O. Origin and early history of peanut. In: Pattee H.E., Young C.T., editors. Peanut Science and Technology. American Peanut Research and Education Society; Yoakum, Texas: 1982. pp. 1–20. [Google Scholar]
- 19.Hu Z.Y., Huang H.Q. Collection of a Thesis on Peanut Rhizobia Research. Sichuan Agriculture University Publication; Yaan, Sichuan: 1991. Investigation of the effectiveness and competitiveness of fast-growing peanut rhizobial strain 85-7. [Google Scholar]
- 20.Jensen M.A., Straus N. Effects of PCR conditions on the formation of heteroduplex and single-stranded DNA products in the amplification of bacterial ribosomal DNA spacer regions. PCR Methods Appl. 1993;3:186–194. doi: 10.1101/gr.3.3.186. [DOI] [PubMed] [Google Scholar]
- 21.Jensen M.A., Webster J.A., Straus N. Rapid identification of bacteria on the basis of polymerase chain reaction-amplified ribosomal DNA spacer polymorphisms. Appl. Environ. Microbiol. 1993;59:945–952. doi: 10.1128/aem.59.4.945-952.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura M. A simple method for estimating evolutionary rate of base substitution through comparative studies of nucleotide sequences. J. Mol. Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 23.Lafay B., Bullier E., Burdon J.J. Bradyrhizobia isolated from root nodules of Parasponia (Ulmaceae) do not constitute a separate coherent lineage. Int. J. Syst. Evol. Microbiol. 2006;56:1013–1018. doi: 10.1099/ijs.0.63897-0. [DOI] [PubMed] [Google Scholar]
- 24.Laguerre G., Mavingui P., Allard M.R., Charnay M.P., Louvrier P., Mazurier S.I., Amarger N. Typing of rhizobia by PCR DNA fingerprinting and PCR-restriction fragment length polymorphism analysis of chromosomal and symbiotic gene regions: application to Rhizobium leguminosarum and its different biovars. Appl. Environ. Microbiol. 1996;62:2029–2036. doi: 10.1128/aem.62.6.2029-2036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laguerre G., Nour S.M., Macheret V., Sanjuan J., Drouin P., Amarger N. Classification of rhizobia based on nodC and nifH gene analysis reveals a close phylogenetic relationship among Phaseolus vulgaris symbionts. Microbiology. 2001;147:981–993. doi: 10.1099/00221287-147-4-981. [DOI] [PubMed] [Google Scholar]
- 26.Li Y.H., Wang R., Zhang X.X., Young J.P.W., Wang E.T., Sui X.H., Chen W.X. Bradyrhizobium guangdongense sp. nov. and Bradyrhizobium guangxiense sp. nov., isolated from effective nodules of peanut. IJSEM. 2015;65:4655–4661. doi: 10.1099/ijsem.0.000629. [DOI] [PubMed] [Google Scholar]
- 27.Marek-Kozaczuk M., Leszcz A., Wielbo J., Wdowiak-Wróbel S., Skorupska A. Rhizobium pisi sv. trifolii K3.22 harboring nod genes of the Rhizobium leguminosarum sv. trifolii cluster. Syst. Appl. Microbiol. 2013;36:252–258. doi: 10.1016/j.syapm.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Martens M., Dawyndt P., Coopman R., Gillis M., De Vos P., Willems A. Advantages of multilocus sequence analysis for taxonomic studies: a case study using 10 housekeeping genes in the genus Ensifer (including former Sinorhizobium) Int. J. Syst. Evol. Microbiol. 2008;58:200–214. doi: 10.1099/ijs.0.65392-0. [DOI] [PubMed] [Google Scholar]
- 29.Martens M., Delaere M., Coopman R., De Vos P., Gillis M., Willems A. Multilocus sequence analysis of Ensifer and related taxa. Int. J. Syst. Evol. Microbiol. 2007;57:489–503. doi: 10.1099/ijs.0.64344-0. [DOI] [PubMed] [Google Scholar]
- 30.Mokgehle S.N., Dakora F.D., Mathews C. Variation in N2 fixation and N contribution by 25 groundnut (Arachis hypogaea L.) varieties grown in different agro-ecologies, measured using 15 N natural abundance. Agric. Ecosyst. Environ. 2014;195:161–172. [Google Scholar]
- 31.Munoz V., Ibanez F., Tonelli M.L., Valetti L., Anzuay M.S., Fabra A. Phenotypic and phylogenetic characterization of native peanut Bradyrhizobium isolates obtained from Cordoba, Argentina. Syst. Appl. Microbiol. 2011;34:446–452. doi: 10.1016/j.syapm.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Neves M.C.P., Rumjanek N.G. Diversity and adaptability of soybean and cowpea rhizobia in tropical soils. Soil Biol. Biochem. 1997;29:889–895. [Google Scholar]
- 33.Ngo Nkot L., Krasova-Wade T., Etoa F.X., Sylla S.N., Nwaga D. Genetic diversity of rhizobia nodulating Arachis hypogaea L. in diverse land use systems of humid forest zone in Cameroon. Appl. Soil Ecol. 2008;40:411–416. [Google Scholar]
- 34.Nievas F., Bogino P., Nocelli N., Giordano W. Genotypic analysis of isolated groundnut-nodulating rhizobial strains reveals differences among populations obtained from soils with different cropping histories. Appl. Soil Ecol. 2012;53:74–82. [Google Scholar]
- 35.Nzoué A., Miché L., Klonowska A., Laguerre G., de Lajudie P., Moulin L. Multilocus sequence analysis of bradyrhizobia isolated from Aeschynomene species in Senegal. Syst. Appl. Microbiol. 2009;32:400–412. doi: 10.1016/j.syapm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Paffetti D., Daguin F., Fancelli S., Gnocchi S., Lippi F., Scotti C., Bazzicalupo M. Influence of plant genotype on the selection of nodulating Sinorhizobium meliloti strains by Medicago sativa. Antonie Van Leeuwenhoek. 1998;73:3–8. doi: 10.1023/a:1000591719287. [DOI] [PubMed] [Google Scholar]
- 37.Rohlf F.J. Exeter Software; Setauket, New York: 2009. NTSYSpc: Numerical Taxonomy System. Ver. 2.21c. [Google Scholar]
- 38.Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 39.Saleena L.M., Loganathan P., Rangarajan S., Nair S. Genetic diversity of Bradyrhizobium strains isolated from Arachis hypogaea. Can. J. Microbiol. 2001;47:118–122. doi: 10.1139/w00-139. [DOI] [PubMed] [Google Scholar]
- 40.Somasegaran P., Hoben H.J. Springer-Verlag; NewYork: 1994. Handbook for Rhizobia. Methods in Legume-Rhizobium Technology; pp. 332–341. [Google Scholar]
- 41.Steenkamp E.T., Stępkowski T., Przymusiak A., Botha W.J., Law I.J. Cowpea and groundnut in southern Africa are nodulated by diverse Bradyrhizobium strains harboring nodulation genes that belong to the large pantropical clade common in Africa. Mol. Phylogenet. Evol. 2008;48:1131–1144. doi: 10.1016/j.ympev.2008.04.032. [DOI] [PubMed] [Google Scholar]
- 42.Stępkowski T., Żak M., Moulin L., Króliczak J., Golińska B., Narożna D., Mądrzak C.J. Bradyrhizobium canariense and Bradyrhizobium japonicum are the two dominant Rhizobium species in root nodules of lupin and serradella plants growing in Europe. Syst. Appl. Microbiol. 2011;34:368–375. doi: 10.1016/j.syapm.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 43.Sterner J.P., Parker M.A. Diversity and relationships of bradyrhizobia from Amphicarpaea bracteata based on partial nod and ribosomal sequences. Syst. Appl. Microbiol. 1999;22:387–392. doi: 10.1016/S0723-2020(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 44.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taurian T., Ibañez F., Fabra A., Aguilar O.M. Genetic diversity of rhizobia nodulating Arachis hypogaea L. in central Argentinean soils. Plant Soil. 2006;282:41–52. [Google Scholar]
- 46.Thies J.E., Holmes E.M., Vachot E.M. Application of molecular techniques to studies in Rhizobium ecology: a review. Aust. J. Exp. Agric. 2001;41:299–319. [Google Scholar]
- 47.Torres-Júnior C.V., Liete J., de Rosália e Silva Santos C.E., Fernades-Júnior P.I., Zilli J.É., Rumjanek N.G., Xavier G.R. Diversity and symbiotic performance of peanut rhizobia from south east region of Brazil. Afr. J. Microbiol. Res. 2014;8:566–577. [Google Scholar]
- 48.Urtz B.E., Elkan G.H. Genetic diversity among Bradyrhizobium isolates that effectively nodulate peanut (Arachis hypogaea) Can. J. Microbiol. 1996;42:1121–1130. doi: 10.1139/m96-144. [DOI] [PubMed] [Google Scholar]
- 49.Van Rossum D., Schuurmans F.P., Gillis M., Muyotcha A., Van Verseveld H.W., Stouthamer A.H., Boogerd F.C. Genetic and phenetic analyses of Bradyrhizobium strains nodulating peanut (Arachis hypogaea L.) roots. Appl. Environ. Microbiol. 1995;61:1599–1609. doi: 10.1128/aem.61.4.1599-1609.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinuesa P., Rademaker J.L., De Bruijn F.J., Werner D. Genotypic characterization of Bradyrhizobium strains nodulating endemic woody legumes of the Canary Islands by PCR-restriction fragment length polymorphism analysis of genes encoding 16S rRNA (16S rDNA) and 16S-23S rDNA intergenic spacers, repetitive extragenic palindromic PCR genomic fingerprinting, and partial 16S rDNA sequencing. Appl. Environ. Microbiol. 1998;64:2096–2104. doi: 10.1128/aem.64.6.2096-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang R., Chang Y.L., Zheng W.T., Zhang D., Zhang X.X., Sui X.H., Wang E.T., Hu J.Q., Zhang L.Y., Chen W.X. Bradyrhizobium arachidis sp nov., isolated from effective nodules of Arachis hypogaea grown in China. Syst. Appl. Microbiol. 2013;36:101–105. doi: 10.1016/j.syapm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 52.Weisburg W.G., Barns S.M., Pelletier D.A., Lane D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991;173:697–703. doi: 10.1128/jb.173.2.697-703.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Willems A., Coopman R., Gillis M. Comparison of sequence analysis of 16S-23S rDNA spacer regions, AFLP analysis and DNA–DNA hybridizations in Bradyrhizobium. Syst. Appl. Microbiol. 2001;51:623–632. doi: 10.1099/00207713-51-2-623. [DOI] [PubMed] [Google Scholar]
- 54.Willems A., Munive A., De Lajudie P., Gillis M. In most Bradyrhizobium groups sequence comparison of 16S-23S rDNA internal transcribed spacer regions corroborates DNA-DNA hybridizations. Syst. Appl. Microbiol. 2003;26:203–210. doi: 10.1078/072320203322346056. [DOI] [PubMed] [Google Scholar]
- 55.Wolde-Meskel E., Terefework Z., Lindström K., Frostegård Å. Metabolic and genomic diversity of rhizobia isolated from field standing native and exotic woody legumes in southern Ethiopia. Syst. Appl. Microbiol. 2004;27:603–611. doi: 10.1078/0723202041748145. [DOI] [PubMed] [Google Scholar]
- 56.Wong C.H., Patchamuthu R., Meyer H., Pankhurst C.E. Rhizobia in tropical legumes: ineffective nodulation of Arachis hypogaea L. by fast-growing strains. Soil Biol. Biochem. 1988;20:677–681. [Google Scholar]
- 57.Yang J.K., Xie F.L., Zou J., Zhou Q., Zhou J.C. Polyphasic characteristics of bradyrhizobia isolated from nodules of peanut (Arachishypogaea) in China. Soil Biol. Biochem. 2005;37:141–153. [Google Scholar]
- 58.Yang J.K., Zhou J.C. Diversity, phylogeny and host specificity of soybean and peanut bradyrhizobia. Biol. Fertil. Soils. 2008;44:843–851. [Google Scholar]
- 59.Zhang X., Nick G., Kaijalainen S., Terefework Z., Paulin L., Tighe S.W., Lindström K. Phylogeny and diversity of Bradyrhizobiumstrains isolated from the root nodules of peanut (Arachishypogaea) in Sichuan, China. Syst. Appl. Microbiol. 1999;22:378–386. doi: 10.1016/S0723-2020(99)80046-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.