Abstract
When the skin is injured, keratinocytes proliferate, migrate, and differentiate to regenerate the epidermis. We recently showed that ablation of the vitamin D receptor (Vdr) in keratinocytes delays wound re-epithelialization in mice also fed a low-calcium diet, implicating a cooperative role of Vdr and calcium signaling in this process. In this study, we examined the role of vitamin D and calcium signaling in wound healing by deleting their receptors, Vdr and the calcium-sensing receptor (Casr). Gene expression profiling of neonatal epidermis lacking both Vdr and Casr [Vdr and Casr double knockout (DKO)] specifically in keratinocytes revealed that DKO affects a number of pathways relevant to wound healing, including Vdr, β-catenin, and adherens junction (AJ) signaling. In adult skin, DKO caused a significant delay in wound closure and re-epithelialization, whereas myofibroblast numbers and matrix deposition were unaffected. The injury-induced proliferation of epidermal keratinocytes was blunted in both epidermis and hair follicles, and expression of β-catenin target genes was reduced in the DKO. Expression of E-cadherin and desmoglein 1 was reduced in the shortened leading edges of the epithelial tongues re-epithelializing the wounds, consistent with the decreased migration rate of DKO keratinocytes in vitro. These results demonstrate that Vdr and Casr are required for β-catenin–regulated cell proliferation and AJ formation essential for re-epithelialization after wounding. We conclude that vitamin D and calcium signaling in keratinocytes are required for a normal regenerative response of the skin to wounding.
Vitamin D and calcium signaling in keratinocytes are required for a normal regenerative response of the skin to wounding.
Failure to heal wounds in a timely fashion results in substantial morbidity and medical expense estimated recently to affect 6.5 million patients in the United States at a cost of >$25 billion (1). Vitamin D deficiency has been implicated as at least contributory in several studies (2, 3). We (4) and others (5, 6) have demonstrated that deletion of the vitamin D receptor (Vdr) retards wound healing. An important difference between our studies and that of others (5, 6) is our use of a model in which Vdr deletion is specifically from keratinocytes. This enabled us to focus on their role in the re-epithelialization process in contrast to global deletions of Vdr, studies that revealed the importance of Vdr in the contribution of macrophages and fibroblasts to the healing process (5, 6).
In our previous studies (4), restricting dietary calcium enhanced the deficit in wound healing caused by Vdr deletion, confirming the important role played by calcium in its interaction with vitamin D signaling in promoting keratinocyte differentiation (7). Calcium is a well-established mediator of epidermal differentiation. Its actions on the keratinocyte are mediated primarily through the calcium-sensing receptor (Casr) (8–13). Many of these effects of calcium on keratinocyte differentiation are dependent on and/or complementary to those of Vdr (14–16).
Of particular importance for the current study is the requirement for both calcium and vitamin D signaling in the formation of the adherens junction (AJ) containing the E-cadherin/catenin complex (4) involved in the migration of the keratinocytes to re-epithelialize the wound (17) and their subsequent differentiation to regenerate the epidermis (12, 13). Under normal circumstances, cells residing in the basal layer of the interfollicular epidermis (IFE), junction/isthmus, and bulge regions within the outer root sheath (ORS) of the hair follicle (HF) are responsible for the regeneration of the IFE, sebaceous glands, and HF, respectively (18–21). However, after wounding, all the cells contribute at least transiently to the regeneration of the epidermis (18, 21).
We developed a mouse model in which the entire transmembrane domain and intracellular portion of the Casr is deleted in Keratin 14 (Krt14)–expressing keratinocytes (10, 22), and we have used this model to demonstrate in vivo the role of Casr in calcium signaling within the keratinocyte. The expression of the Casr is increased by 1,25(OH)2D3, making the keratinocyte more sensitive to the actions of calcium (14). Moreover, deletion of Casr led to the reduction in Vdr expression (23). We recently showed that deletion of the Vdr in keratinocytes delays wound re-epithelialization in mice fed a low-calcium diet, implicating a cooperative role of Vdr and calcium signaling in this process. Therefore, we generated a mouse model in which receptors for both vitamin D and calcium signaling, Vdr and Casr, are removed from keratinocytes and evaluated the role of vitamin D and calcium signaling in the wound re-epithelialization process. Because Vdr deletion affects hair cycling in which resting telogen shifts to growing anagen, we examined the role of Vdr and Casr in wound re-epithelialization in telogen skin. The state of the skin is judged by skin color, because melanogenesis is strictly coupled to anagen phase (24).
Materials and Methods
Animals
Mice in which exon 7 of the Casr is floxed (Casrtm1Wch) (23) and exon 3 of the Vdr is floxed (Vdrtm1Pcn; gift from Dr. Shigeaki Kato) were bred with mice expressing Tg(Keratin14-cre)amc (Krt14 cre; Jackson Laboratory). The final breeding (female mice homozygous for the floxed genes bred with male mice homozygous for the floxed genes and transgenic for the cre) produces litters homozygous for the floxed genes, but only half have the cre [double knockout (DKO)]. The non–cre-expressing littermate mice serve as controls (CONs). The epidermis of neonatal mice was removed from the dermis as previously described (25) for microarray analysis. All other mice were raised after weaning on a low-calcium diet containing 0.01% to 0.02% calcium, 0.42% phosphorus, and 2,200 IU vitamin D/kg (TD86162; Teklad). These mice were bred into the C57Bl/6J background before use. Male mice for DKO and CON (n ≥ 3) were used to evaluate wound healing. They were studied at 12 weeks of age (telogen) with biopsy specimens obtained from skin that was clearly in telogen by visual inspection (pink). All experiments were approved by the Institutional Animal Care and Ethics Committee at the San Francisco VA Medical Center.
Wounding protocol
After shaving, two 6-mm, full-thickness skin biopsies from the upper portion of the backs of the mice were performed. Regions of the skin in telogen (pink) were selected to avoid differences in stages of HF cycling (26). The wounds were monitored during a 6- to 10-day recovery as described (4). In addition, two - mm biopsies were made in the upper back of other mice that were killed after 3 days for analyses; one of the wounds was used for histologic examination and the other to determine mRNA levels. The samples for histology were fixed by 4% paraformaldehyde and paraffin embedded. Sections across the center of the wound were obtained angled in parallel with hair follicles. The rim of tissue around the other wound in the same mouse was harvested and stored in RNAlater (Qiagen) for subsequent gene expression analyses.
Morphometric measurement of re-epithelialization
Photographs including a ruler were obtained at each time point, digitized, and areas and perimeters of the epithelial margins measured with Bioquant (Bioquant Image Analysis Corporation). Measurements across the wound as well as between the leading edges of the epidermis were recorded and expressed as a ratio of the distance between the leading edges of the epithelial tongues to the overall diameter of the wound.
Immunohistochemistry
The paraffin sections were first treated with citrate-based antigen unmasking solutions (Vector Laboratory). They were incubated with commercially available antibodies against Vdr (Santa Cruz C-20), E-cadherin (Santa Cruz Biotechnology H-108), desmoglein 1 (Santa Cruz Biotechnology H-290) Cd44 (catalog no. IF7 Ab119863; Abcam), and α-smooth muscle actin (α-Sma; catalog no. SP171; Sigma-Aldrich). The Casr antibody is a custom-made rabbit polyclonal antibody raised against the peptide corresponding to amino acids 215 to 236 of the human keratinocyte Casr (8). The sections were then incubated with biotinylated secondary antibody, streptavidin-horseradish peroxidase using the Vectastain Elite ABC kit (Vector Laboratory), and DAB staining solution (Vector Laboratory) or Anti-Ig HRP staining kit (BD Bioscience). The sections were counterstained with Gill’s hematoxylin.
Cell proliferation
The wound sections were stained with a proliferating cell nuclear antigen (PCNA) staining kit (Invitrogen) according to the manufacturer’s instructions with minimum exposure of substrate to specifically detect cell proliferation induced by wounding. The PCNA-positive cells at the edge of the wound were quantitated by image analysis using Bioquant. The percentage of PCNA-positive cells in knockout mice compared with those in CONs was calculated. Statistical significance was evaluated by counting cells in six or more sections in each group each with three mice per group.
mRNA analyses
High-quality RNA was purified from the epidermis of neonates using the PicoPure RNA isolation kit (Thermo Fisher Scientific) according to the manufacturer’s protocol. The quantity and quality of RNA were verified using the Aligent Bioanalyzer (Agilent Technologies) for microarray analyses. RNA was also isolated from wounded skin for quantitative polymerase chain reaction (qPCR) analyses as described (4). The skin tissues were homogenized with Stat 60 RNA extraction reagent (Tel-Test) using a Polytron homogenizer (Kinematica) and the RNA extracted. The RNA was further purified by RNA easy mini kit (Qiagen) for qPCR.
Microarray
Gene expression profiles were analyzed using an Illumina beads chip-based gene array (Mouse Ref-8 v2.0; Ambion) in the University of California, Los Angeles, Neuroscience Genomics Core. The data were normalized by Genome Studio (Illumina), and analyzed using pathway software IPA (Ingenuity Systems). The array data were submitted to a public database (GEO/NCBI/NIH; http://www.ncbi.nlm.nih.gov/geo). Data for neonatal epidermis for DKO are available as an accession number GSE68725 (DKO) under superseries GSE68726.
qPCR
The RNA was reverse transcribed and gene expression was evaluated by quantitative real-time PCR, as described (4). Briefly, cDNA was synthesized and qPCR was performed using Power SYBR Green (Applied Biosystems) with the 7300 or ViiA Real-Time PCR system (Applied Biosystems ). Relative mRNA levels compared with the control gene Gapdh were calculated. We used primers that were designed and verified by Primer bank where the sequences are available (https://pga.mgh.harvard.edu/primerbank/).
Cell migration assay
Human primary keratinocytes were isolated from neonatal foreskin and maintained in Medium 154CF (Thermo Fisher Scientific) containing Human Keratinocyte Growth Supplement (Thermo Fisher Scientific) with 0.07 mM calcium. Keratinocytes were transfected with short interfering RNA for Vdr and Casr, or random short interfering RNA (siCON), as described previously (27). Confluent keratinocyte cultures were switched to 154CF media with 5 μg/mL mitomycin for 4 hours to stop their proliferation. The cultures were scratched using a 20 μL pipette tip (Rainin). Cell migration was monitored by taking phase-contrast photographs in the same field before and after scratching at over five different locations. Cell migration was assessed by measuring the open space remaining at different times after scratching, using Bioquant software. Statistical significance was evaluated by t test using at least five different fields for two independent batches of keratinocytes.
Results
Generation of mice lacking both Vdr and Casr from epidermal keratinocytes (DKO)
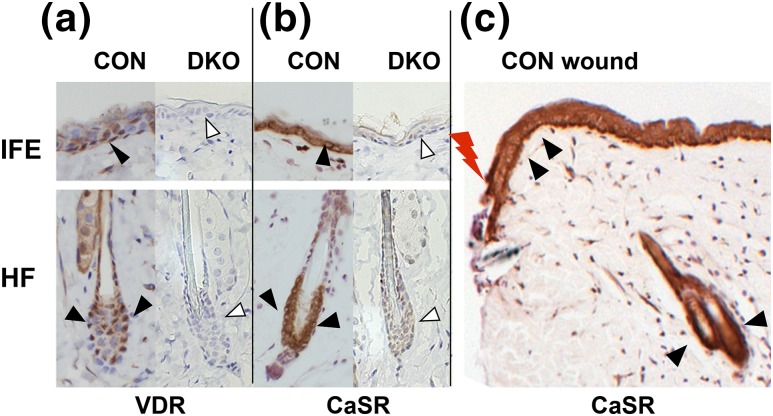
Mice expressing both floxed Vdr (28, 29) and floxed Casr (23) were bred with mice expressing Krt14 cre recombinase to generate the DKO. The triple-transgenic mice with floxed Vdr/floxed Casr with the Krt14-driven cre transgene (DKO) were compared with CONS that had floxed Vdr/floxed Casr but no cre. The efficiency of gene deletion is shown in Figure 1. Both Vdr and Casr were expressed in the keratinocytes in both the basal layer of IFE, and bulge and ORS of the HF of CONs (3 months old), but their expressions were essentially abolished in DKO skin [Fig. 1(a) and 1(b)]. Casr was abundantly expressed in the thickened epidermis at the wounding edge [Fig. 1(c)].
Figure 1.
DKO mice were generated in which both Vdr and Casr were removed from Krt14-expressing keratinocytes. Immunohistochemistry for (a) Vdr and (b, c) Casr in 3-month-old DKO skin (arrowheads) and littermate CON skin. The Casr staining in the epidermis at the edge of the wound (lightning bolt) in CON skin is also shown. Representative images of staining in IFE and HF are shown.
Deletion of both Casr and Vdr downregulates gene expression for Vdr signaling and AJ signaling in neonatal epidermis
As a first step in examining the molecular mechanisms by which Vdr and Casr regulate epidermal regeneration, we performed gene expression profiling in the epidermis lacking Vdr and Casr, using microarray. We used neonatal epidermis to identify the causative signaling pathways and genes functioning in the epidermis before obvious phenotypic changes observed in adult skin, in particular before the disruption of HF cycling in these mice that begins around day 15. RNA was purified from the epidermis of the DKO mice and their littermate CONs (n = 3). The gene expression profiles were analyzed using a beads-based gene array including 25,600 annotated transcripts and 19,100 genes (Illumina) (data are available in GEO database GSE68726). The fold changes of DKO/CON were calculated and analyzed using IPA software (Ingenuity Systems).
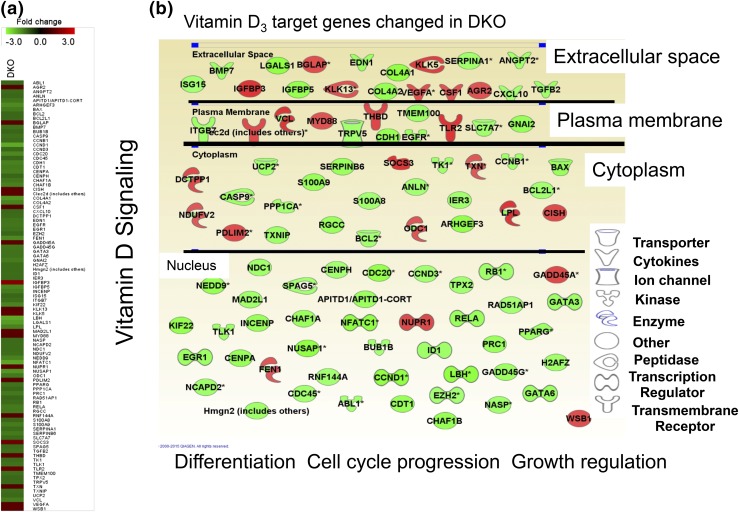
These analyses revealed that expression of vitamin D3 target genes was significantly downregulated in DKO epidermis [Fig. 2(a), heat map]. A substantial number of these downregulated vitamin D3 target genes (Fig. 2, light gray) in neonatal epidermis are up-regulated (Fig. 2, dark gray) after wounding (24 hours) in adult skin of the CON, implicating the role of these genes in wound healing (Supplemental Fig. 1 (1.3MB, pdf) ). A series of downregulated vitamin D3 target genes in DKO [Fig. 2(b), light gray] are directly or indirectly linked to growth regulation, cell cycle progression, and cell migration and differentiation (Table 1). Pathway analysis indicated that epithelial AJ signaling was the most significantly downregulated canonical pathway in DKO epidermis (Table 2). Cellular growth and proliferation were the most affected cellular functions, and 1,25(OH)2D3 and β-catenin (CTNNB1) were shown to be highly important potential upstream regulators to cause these changes in DKO (Table 3). Our study also suggested that Vdr and Casr concurrently regulate epidermal homeostasis through regulation of E-cadherin mediated AJ formation.
Figure 2.
Deletion of both Vdr and Casr downregulates Vdr signaling in neonatal epidermis. (a, b) Gene expression profiling of neonatal (days P1 to P3) epidermis lacking both Vdr and Casr (DKO). (a) The heat maps for DKO show the fold changes (KO/CON) for representative genes for vitamin D signaling. (b) The changes in vitamin D3 target genes in DKO epidermis are shown with their subcellular localization and function. The lighter elements denote downregulation, and the darker elements denote upregulation.
Table 1.
Molecular and Cellular Functions Altered in DKO Neonatal Epidermisa
| No. of Molecules | P Value | |
|---|---|---|
| Cellular growth and proliferation | 245 | 2.83E–15 to 4.69E–03 |
| Cell death and survival | 236 | 5.88E–12 to 5.26E–03 |
| Cellular development | 213 | 8.75E–09 to 4.96E–03 |
| Cell cycle | 103 | 1.06E–07 to 4.96E–03 |
| Cellular movement | 140 | 1.42E–07 to 4.04E–03 |
Genome-wide microarray profiling revealed differentially expressed genes caused by deletion of Vdr and Casr. IPA pathway analyses provided the most affected molecular and cellular functions, with statistical significance.
Table 2.
Top Canonical Pathways Altered in DKO Neonatal Epidermisa
| Ratio | P Value | |
|---|---|---|
| Epithelial AJ signaling | 19/147 | 1.8E–06 |
| Remodeling of epithelial AJ | 11/68 | 3.81E–05 |
| Cancer signaling | 8/42 | 9.32E–05 |
| Gap junction signaling | 16/176 | 2.16E–04 |
Genome-wide microarray profiling revealed differentially expressed genes caused by deletion of Vdr and Casr. IPA pathway analyses provided the top canonical pathways, with statistical significance.
Table 3.
Potential Upstream Regulators Predicted to Cause Changes in DKO Epidermisa
| Activation z Score | P Value of Overlap | |
|---|---|---|
| 1,25D3 | 1.783 | 1.81E–10 |
| CTNNB1 | –2.336 | 4.08E–08 |
| MYC | –2.645 | 2.38E–11 |
Genome-wide microarray profiling revealed differentially expressed genes caused by deletion of Vdr and Casr. IPA pathway analysis predicted potential upstream regulators that would cause these changes (with statistical significance), among which Vdr ligand (1,25(OH)2D3) is listed.
Deletion of both Vdr and Casr delays wound re-epithelialization
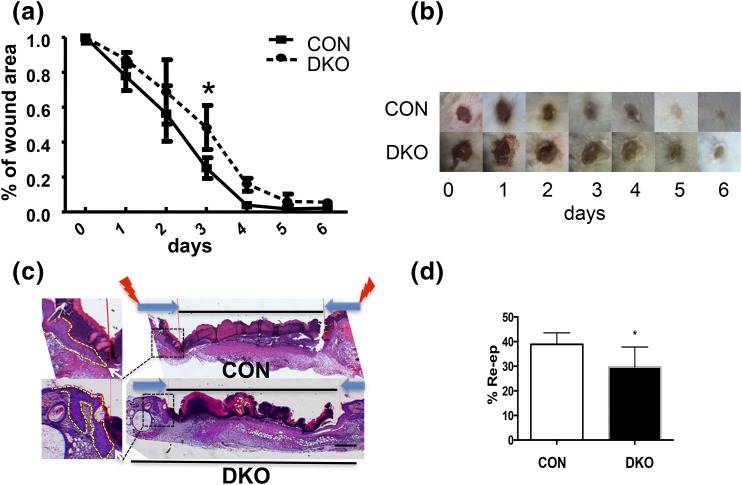
We then examined the impact of DKO on wound healing. In the first set of experiments, the healing process of 6-mm, full-thickness skin biopsy wounds from the upper back of 3-month-old DKO mice and littermate CONs was monitored with daily photographs, and the area of the open wound was determined. Wound closure was delayed in DKO mice compared with CONs, as shown by the sizes and images of the wounds [Fig. 3(a) and 3(b)]. Wound re-epithelialization was determined in histologic sections of 3-mm wounds obtained at day 3 that demonstrated the delay in re-epithelialization in DKO skin as shown in images [Fig. 3(c)] and with calculation of the distance for the leading edge of the epithelial tongues to travel to close the wounds [Fig. 3(d)].
Figure 3.
Deletion of both Vdr and Casr delays re-epithelialization after wounding. (a) Six-mm, full-thickness skin biopsy wounds were made on the backs of 3-month-old DKO mice and their CON littermates. The areas of the wounds were measured 0 to 6 days later and normalized to the original wound area (time 0) in DKO and CON mice. The bars enclose the mean ± standard deviation (SD). *P < 0.05 (n = 7 to 8). (b) Representative 0- to 6-day wound photographs of DKO and CON mice are shown. (c) Three-mm, full-thickness skin biopsy wounds were made on the back skin of 3-month-old DKO and CON mice, and the re-epithelialization was evaluated histologically at day 3 by hematoxylin and eosin staining. Images at higher magnification (boxed, right) show the edge (white arrows) of epithelial tongues (dotted lines, left) from which the distance was measured to evaluate the re-epithelialization. (d) Percent re-epithelialization was quantitatively evaluated by analysis of different cross-sections (n = 6; three mice each). Percent re-epithelialization was defined as the distance traveled by both epithelial margins [large dark arrows in (c), right] divided by the distance needed to travel to fully re-epithelialize the wound [lightning bolts in (c), right]. The bars enclose mean ± SD. Statistical significance was evaluated by t test. *, P < 0.05.
In mouse skin, contraction of the wound involves myofibroblasts within the dermis (30). To examine whether deletion of Vdr and Casr from keratinocytes influenced the dermal matrix and, in particular, the number of myofibroblasts in the dermis, we evaluated collagen deposition within granulation tissue and the distribution of the α−Sma–positive myofibroblasts (Supplemental Fig. 2A and 2B (1.3MB, pdf) ). We did not observe clear differences in either comparing the DKO and CON mice. Moreover, the mRNA levels of myofibroblast and matrix markers in the wounds of DKO mice were not different from those markers within wounds of the littermate CONs (Supplemental Fig. 2C (1.3MB, pdf) ). These results demonstrate that DKO specifically in the epidermis delays wound re-epithelialization but not matrix deposition and myofibroblast numbers. Therefore, our attention turned toward events occurring in the epidermis that contribute to the delay in re-epithelialization of wounds in the DKO mice.
Deletion of both Vdr and Casr blunts injury-induced proliferation and activation of β-catenin signaling
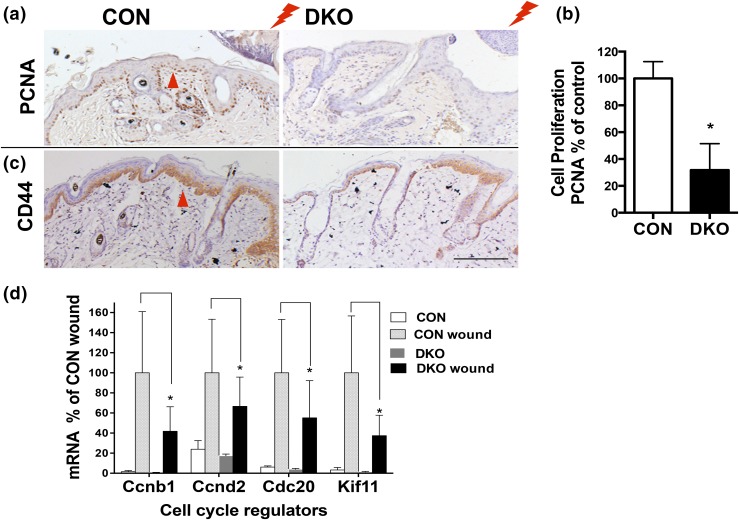
Wound-induced cell proliferation was evaluated by PCNA staining. Three days after creating 3-mm, full thickness wounds by biopsy on the back skin of DKO mice and CON mice (3 months old), proliferation of cells at the wound edge was substantially increased in the stratum basale and ORS of the CONs but less in the DKO mice [Fig. 4(a) and 4(b)]. Microarray analysis indicated that in the DKO β-catenin signaling was reduced compared with CONs (Table 1; Supplemental Fig. 3 (1.3MB, pdf) ). β-catenin signaling mediates injury-induced cell proliferation (19). Therefore, we evaluated β-catenin signaling in wounded skin of CON and DKO mice by measuring the β-catenin target gene Cd44. The expression of Cd44 was induced at the leading edge of wounds in CONs, but it was less induced in DKO mice [Fig. 4(c)]. Similarly, the expression of β-catenin target genes Ccnd1 and other cell cycle regulators was highly induced by wounding in CONs but less so in DKO mice [Fig. 4(d)]. These results suggest that the combined deletion of Vdr and Casr blunts injury-induced keratinocyte proliferation by limiting the stimulation of β-catenin signaling in the keratinocytes.
Figure 4.
Combined deletion of Vdr and Casr blunts injury-induced cell proliferation and activation of β-catenin signaling. (a) PCNA staining at the wounding edge (lightning bolt) of DKO and littermate CON mice (3 days after wounding at 3 months old; arrowhead). The representative images for three analyses are shown. (b) Cell proliferation in IFE was quantified by Bioquant, shown in the bar graph with statistical significance. Mean ± SD shown (n = 6). *P < 0.05. (c) Immunohistochemistry for the β-catenin target gene Cd44 (arrowhead) at wound sections similar to those in (a) for DKO and CON. (d) The mRNA levels for β-catenin–regulated Ccnd1 and other cell cycle regulators in control and wounded skin in DKO and CON mice were evaluated by qPCR. Statistical significance compared with wounded CON skin vs wounded DKO skin is shown by an asterisk (*P < 0.05). Mean ± SD shown (n = 3).
Deletion of Vdr and Casr reduced migration of keratinocytes and decreased AJ formation
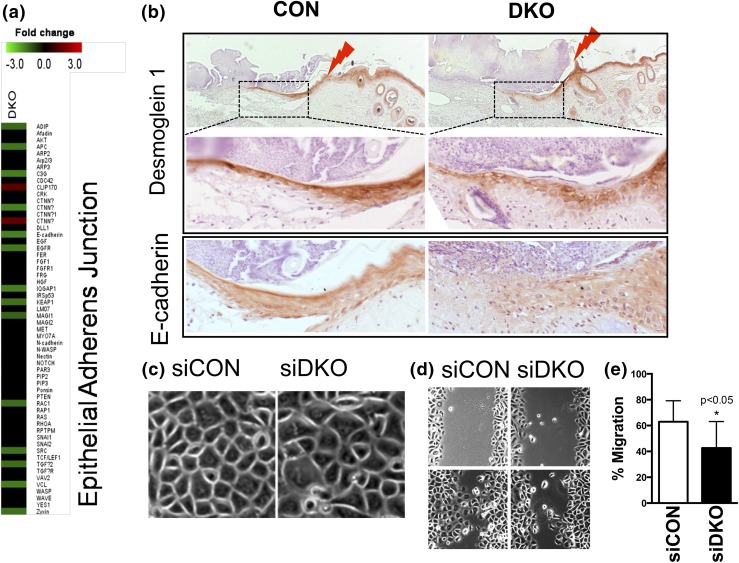
Pathway analysis of the microarray data demonstrated a reduction in epithelial AJ formation of which the E-cadherin/catenin complex is a part [Table 1; Fig. 5(a); Supplemental Fig. 3 (1.3MB, pdf) ]. Previous in vitro studies showed that Vdr and Casr are each required for formation of the E-cadherin/catenin complex (10, 27). Therefore, we examined the expression of E-cadherin as well as the desmosome component, desmoglein 1, also controlled by calcium signaling, at the leading edges of the epithelium re-epithelializing the wound in both CON and DKO mice [Fig. 5(b)]. Both were highly expressed in the CON mice epithelia. Desmoglein 1 was expressed in the upper layers of the epithelium, whereas E-cadherin was expressed throughout the epithelium. However, in the DKO mice, their expression was reduced. Moreover, the expression pattern of desmoglein 1 was disorganized [Fig. 5(b), middle panels], and that of E-cadherin was limited to a shorter migration zone [Fig. 5(b), bottom panels].
Figure 5.
Deletion of both Vdr and Casr (DKO) impairs adhesion and migration of keratinocytes to re-epithelize the wound. (a) DKO downregulation of AJ signaling in epidermis as shown in microarray analyses. (b) Immunohistochemistry for desmoglein 1 (upper two panels) and E-cadherin (bottom panels) on the wound sections of CON (left) and DKO (right) mice (3 days after wounding at 3 months of age). The upper panels for desmoglein 1 are of low magnification, with the boxed regions shown in the middle panels at higher magnification. The lightning bolts show wound margins. Representative images from two analyses are shown. (c) The phase-contrast morphology of human keratinocytes transfected by siDKO compared with siCON (phase microscopy). (d, e) Migration capability was assessed by the scratch assay. Confluent cultures of transfected cells were treated to block proliferation, and they were scratched by pipette tips. After 16 hours, the migration rate was quantitated by measuring the amount of open area remaining in the scratch region through Bioquant software, and the results expressed as the ratio of closure after 16 hours to the original area of the scratch. Mean ± SD shown (n = 12). *P < 0.05.
To determine whether the decreased rate of re-epithelialization in the DKO mice was due to a reduction in adhesion, migration, or both, we tested whether decreasing the expression of Vdr and Casr with short interfering RNA (siDKO) in cultures of human keratinocytes would alter these processes. The typical honeycombed appearance of confluent keratinocytes was disrupted in siDKO cells, indicating reduced adhesion capability [Fig. 5(c)]. Cell migration rate was also decreased in siDKO cells compared with siCON cells, as shown in the results from the scratch test [Fig. 5(d) and 5(e)]. These data demonstrate that Vdr and Casr regulate adhesion and migration of keratinocytes, essential processes for wound re-epithelialization.
Discussion
Calcium and vitamin D signaling are well established as major regulators of keratinocyte differentiation, and their actions can be both compensatory and mutually interdependent (7). These points are well illustrated by the comparable impact both Casr and Vdr deletions have on epidermal differentiation (23, 31, 32) in vivo and their interdependency on keratinocyte differentiation in vitro (4, 14–16). These compensatory but interacting aspects of calcium and vitamin D signaling on wound healing, however, have received little attention, although our previous study demonstrating that a low-calcium diet potentiated the delay in wound healing caused by deletion of the Vdr from keratinocytes (4) indicated this interaction during wound healing as well. With these earlier observations in mind, in this study, we examined the impact of the combined deletion of Vdr and Casr in keratinocytes on the wound healing process.
We first showed that Casr and Vdr were expressed in IFE and HF, those cells that respond to wounding with proliferation and migration to regenerate the epidermis (18, 21). When we examined the transcriptome of the epidermis of mice in which both Vdr and Casr were deleted, we were struck by how profound an effect the combined deletion had when Vdr signaling elements were specifically assessed even in neonatal epidermis, which phenotypically looked normal (Fig. 2; Supplemental Fig. 3 (1.3MB, pdf) ). These studies pointed us to two pathways that play important roles in the response of skin to wounding, namely, β-catenin signaling, given its role in proliferation producing the cells that subsequently regenerate the epidermis (18, 21); and the formation of the E-cadherin/catenin complex that not only is essential for epidermal differentiation (13) but enables migration of keratinocytes as a unit across the wound to re-epithelialize it (17). Indeed, the combined deletion of Vdr and Casr blunted the proliferative response to wounding (Fig. 4) and the migration of the epithelium across the wound in adult skin (Figs. 3 and 5).
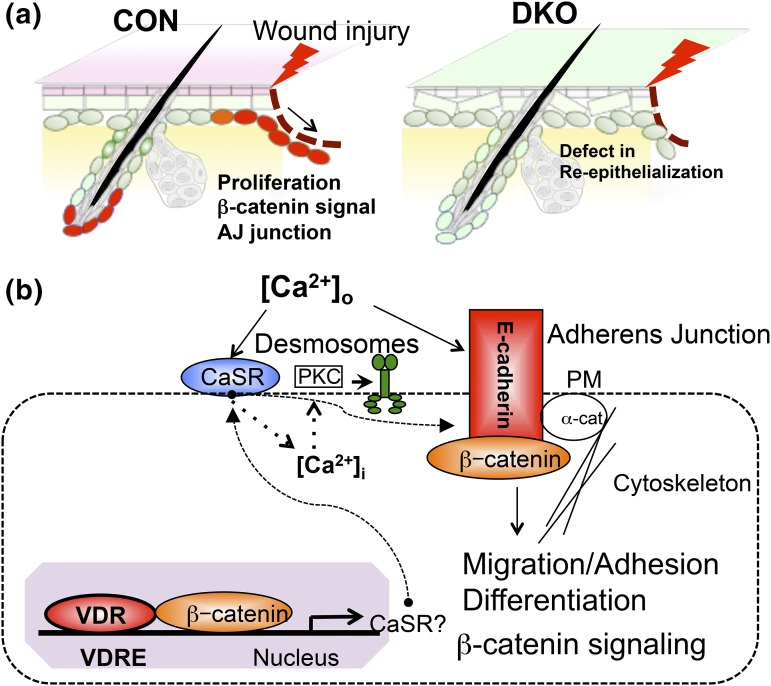
Our working model is shown in Figure 6. Figure 6(a) summarizes our findings illustrating the reduction in proliferation and re-epithelialization probably caused by the loss of β-catenin signaling and E-cadherin/catenin (AJ junction) formation. Our concept of potential molecular mechanisms by which vitamin D and calcium signaling interact to regulate proliferation and migration of keratinocytes during wound re-epithelialization is shown in Figure 6(b).
Figure 6.
A proposed model in which Vdr and calcium signaling concurrently control wound re-epithelialization through regulation of AJ formation and β-catenin signaling. (a) Summary showing deficiency in both Vdr and Casr prevents proliferation and migration of keratinocytes to delay wound (lightning bolt) re-epithelialization of epidermis. (b) A proposed action of vitamin D and calcium signaling, in which they cross-talk to regulate AJ formation, β-catenin signaling through AJ signaling, and desmosome function through calcium-regulated PKCα activity. Both Vdr and Casr concurrently control β-catenin signaling and AJ signaling, which govern adhesion, migration, and differentiation of regenerating keratinocytes at the wound edge.
First, Vdr induces genes including Casr regulating differentiation of the HF and epidermis (7) but also acts in partnership with β-catenin to bind to VDRE/LEF response elements to regulate β-catenin target genes such as Cnnd1 to promote cell cycle progression (33). Calcium, mediated by the Casr, stimulates E-cadherin/catenin complex formation (10). This complex provides a reservoir of β-catenin in the membrane but also includes α-catenin, the link of this complex to the cytoskeleton enabling cell migration as well as cell adhesion, and the enzymes phosphatidyl inositol 3 kinase and phosphatidyl inositol 4-phosphate 5-kinase 1α involved in generating signaling molecules that promote differentiation (12, 13). In particular, phosphatidyl inositol 3 kinase and phosphatidyl inositol 4-phosphate 5-kinase 1α sequentially phosphorylate PIP and PIP2 to PIP3, which activates PLC-γ1. PLC-γ cleaves PIP2 to form IP3 and diacylglycerol; IP3 releases calcium from intracellular stores important for the acute response to wounding, and diacylglycerol along with calcium activates PKCα. The role of PKCα is of particular interest. We have shown its role in calcium-induced keratinocyte differentiation (34), but mice lacking PKCα also have delayed re-epithelialization in response to wounding, whereas if the PKCα is constitutively activated, re-epithelialization is accelerated (35). This regulation of re-epithelialization is thought to be due to a change in the adhesiveness of desmosomes. Lack of PKCα results in hyperadhesive desmosomes blocking migration, but when PKCα is present and active, the desmosomes become calcium sensitive and less adhesive, permitting migration (35). Thus, in the DKO, a reduction in desmosome formation as reflected by the decreased expression of desmoglein (Fig. 5) as well as decreased calcium signaling required to activate PKCα may also contribute to the blunting of re-epithelialization.
In this study, we demonstrated a role for Vdr and Casr in epidermal keratinocytes in wound healing by using a conditional knockout model in which Vdr and Casr are deleted from Krt14-expressing epithelia. However, it is still possible that deletion of these genes in epithelia may indirectly influence other cells such as immune cells. In fact, we observed that injury-induced macrophage recruitment into the dermis is reduced in DKO wounds (data not shown).
Our data indicate that vitamin D signaling is beneficial for wound re-epithelialization in skin. The Vdr may work with other nuclear hormone receptors that are also involved in cutaneous wound repair (36). The retinoid X receptor may function with Vdr by forming a complex to activate transcription of vitamin D3 target genes. Other steroid hormone receptors such as the glucocorticoid receptor are not known to interact with Vdr and are likely to exert their effects on wound healing independent of Vdr.
These observations are of potential clinical significance. As noted in the at the beginning of this article, vitamin D deficiency is associated with poor wound healing (2, 3), and a randomized clinical trial of oral vitamin D supplementation demonstrated improved wound healing of patients with diabetic foot ulcers compared with placebo (37). Moreover, the superiority of calcium-alginate dressings compared with other wound care products (38) suggests a role for calcium in wound repair. Thus, maintaining good vitamin D and calcium nutrition and/or direct application of calcitriol or its analogs to the wound may accelerate wound healing in conditions where wound healing is delayed.
In summary, we have demonstrated that vitamin D and calcium signaling in keratinocytes is required for a normal wound re-epithelialization process. The mechanisms implicated are those regulated by both β-catenin signaling and E-cadherin/catenin complex formation.
Acknowledgments
We thank A. Menendez and W. Mayer for their assistance in breeding and maintaining the mice, and Rochelle Elp for technical assistance.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AR050023 (D.D.B.) and R01 AR056256 (C.T.), and US Department of Defense Grant CA110338 (D.D.B.). This work was also supported by the National Natural Science Foundation of China Grants 81301360 and 81573075 (L.H.), and the Science Foundation of Tianjin Medical University Grant 2013KY06 (L.H.).
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibodies Used
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | RRID | Dilution Used |
|---|---|---|---|---|---|---|
| Vdr | Unknown | Vdr | Santa Cruz, C-20 | Rabbit | AB_632070 | 200 times |
| Casr | Human Casr 215-236 | Casr | Custom made | Rabbit | AB_2636871 | 200 times |
| E-cadherin | Unknown | E-cadherin | Santa Cruz, H-108 | Rabbit | AB_2076666 | 200 times |
| Dsg1 (demoglein1) | Unknown | Desmoglein 1 | Santa Cruz, H-290 | Rabbit | AB_2293011 | 200 times |
| α-Sma | Unknown | α-Sma | Sigma-Aldrich, SP171 | Rabbit | AB_262054 | 200 times |
| Cd44 | Unknown | Cd44 | Abcam, IM7 AB119863 | Rabbit | AB_10898986 | 1000 times |
Footnotes
- AJ
- adherens junction
- Casr
- calcium-sensing receptor
- CON
- control
- DKO
- double knockout
- HF
- hair follicle
- IFE
- interfollicular epidermis
- Krt14
- Keratin 14
- ORS
- outer root sheath
- PCNA
- proliferating cell nuclear antigen
- qPCR
- quantitative polymerase chain reaction
- siCON
- control cells transfected with random short interfering RNA
- siDKO
- DKO cells transfected with short interfering RNA for Vdr and Casr
- Vdr
- vitamin D receptor.
References
- 1.Sen CK. Wound healing essentials: let there be oxygen. Wound Repair Regen. 2009;17(1):1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burkievcz CJ, Skare TL, Malafaia O, Nassif PA, Ribas CS, Santos LR. Vitamin D deficiency in patients with chronic venous ulcers. Rev Col Bras Cir. 2012;39(1):60–63. [PubMed] [Google Scholar]
- 3.Zubair M, Malik A, Meerza D, Ahmad J. 25-Hydroxyvitamin D [25(OH)D] levels and diabetic foot ulcer: is there any relationship? Diabetes Metab Syndr. 2013;7(3):148–153. [DOI] [PubMed] [Google Scholar]
- 4.Oda Y, Tu CL, Menendez A, Nguyen T, Bikle DD. Vitamin D and calcium regulation of epidermal wound healing. J Steroid Biochem Mol Biol. 2016;164:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luderer HF, Nazarian RM, Zhu ED, Demay MB. Ligand-dependent actions of the vitamin D receptor are required for activation of TGF-β signaling during the inflammatory response to cutaneous injury. Endocrinology. 2013;154(1):16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Song L, Papaioannou G, Zhao H, Luderer HF, Miller C, Dall’Osso C, Nazarian RM, Wagers AJ, Demay MB. The vitamin D receptor regulates tissue resident macrophage response to injury. Endocrinology. 2016;157(10):4066–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bikle DD. Vitamin D metabolism and function in the skin. Mol Cell Endocrinol. 2011;347(1-2):80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu CL, Chang W, Bikle DD. The extracellular calcium-sensing receptor is required for calcium-induced differentiation in human keratinocytes. J Biol Chem. 2001;276(44):41079–41085. [DOI] [PubMed] [Google Scholar]
- 9.Tu CL, Chang W, Bikle DD. Phospholipase cgamma1 is required for activation of store-operated channels in human keratinocytes. J Invest Dermatol. 2005;124(1):187–197. [DOI] [PubMed] [Google Scholar]
- 10.Tu CL, Chang W, Xie Z, Bikle DD. Inactivation of the calcium sensing receptor inhibits E-cadherin-mediated cell-cell adhesion and calcium-induced differentiation in human epidermal keratinocytes. J Biol Chem. 2008;283(6):3519–3528. [DOI] [PubMed] [Google Scholar]
- 11.Xie Z, Singleton PA, Bourguignon LY, Bikle DD. Calcium-induced human keratinocyte differentiation requires src- and fyn-mediated phosphatidylinositol 3-kinase-dependent activation of phospholipase C-gamma1. Mol Biol Cell. 2005;16(7):3236–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie Z, Bikle DD. The recruitment of phosphatidylinositol 3-kinase to the E-cadherin-catenin complex at the plasma membrane is required for calcium-induced phospholipase C-gamma1 activation and human keratinocyte differentiation. J Biol Chem. 2007;282(12):8695–8703. [DOI] [PubMed] [Google Scholar]
- 13.Xie Z, Chang SM, Pennypacker SD, Liao EY, Bikle DD. Phosphatidylinositol-4-phosphate 5-kinase 1alpha mediates extracellular calcium-induced keratinocyte differentiation. Mol Biol Cell. 2009;20(6):1695–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratnam AV, Bikle DD, Cho JK. 1,25 dihydroxyvitamin D3 enhances the calcium response of keratinocytes. J Cell Physiol. 1999;178(2):188–196. [DOI] [PubMed] [Google Scholar]
- 15.Su MJ, Bikle DD, Mancianti ML, Pillai S. 1,25-Dihydroxyvitamin D3 potentiates the keratinocyte response to calcium. J Biol Chem. 1994;269(20):14723–14729. [PubMed] [Google Scholar]
- 16.Bikle DD, Ng D, Tu CL, Oda Y, Xie Z. Calcium- and vitamin D-regulated keratinocyte differentiation. Mol Cell Endocrinol. 2001;177(1-2):161–171. [DOI] [PubMed] [Google Scholar]
- 17.Li L, Hartley R, Reiss B, Sun Y, Pu J, Wu D, Lin F, Hoang T, Yamada S, Jiang J, Zhao M. E-cadherin plays an essential role in collective directional migration of large epithelial sheets. Cell Mol Life Sci. 2012;69(16):2779–2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mascré G, Dekoninck S, Drogat B, Youssef KK, Broheé S, Sotiropoulou PA, Simons BD, Blanpain C. Distinct contribution of stem and progenitor cells to epidermal maintenance. Nature. 2012;489(7415):257–262. [DOI] [PubMed] [Google Scholar]
- 19.Lim X, Tan SH, Koh WL, Chau RM, Yan KS, Kuo CJ, van Amerongen R, Klein AM, Nusse R. Interfollicular epidermal stem cells self-renew via autocrine Wnt signaling. Science. 2013;342(6163):1226–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jensen KB, Collins CA, Nascimento E, Tan DW, Frye M, Itami S, Watt FM. Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell. 2009;4(5):427–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Plikus MV, Gay DL, Treffeisen E, Wang A, Supapannachart RJ, Cotsarelis G. Epithelial stem cells and implications for wound repair. Semin Cell Dev Biol. 2012;23(9):946–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. doi: 10.1126/scisignal.1159945. Chang W, Tu C, Chen TH, Bikle D, Shoback D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci Signal. 2008;1(35):ra1. [DOI] [PMC free article] [PubMed]
- 23.Tu CL, Crumrine DA, Man MQ, Chang W, Elalieh H, You M, Elias PM, Bikle DD. Ablation of the calcium-sensing receptor in keratinocytes impairs epidermal differentiation and barrier function. J Invest Dermatol. 2012;132(10):2350–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Slominski A, Paus R. Melanogenesis is coupled to murine anagen: toward new concepts for the role of melanocytes and the regulation of melanogenesis in hair growth. J Invest Dermatol. 1993;101(1 suppl)90S–97S. [DOI] [PubMed] [Google Scholar]
- 25.Lichti U, Anders J, Yuspa SH. Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat Protoc. 2008;3(5):799–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ansell DM, Kloepper JE, Thomason HA, Paus R, Hardman MJ. Exploring the “hair growth-wound healing connection”: anagen phase promotes wound re-epithelialization. J Invest Dermatol. 2011;131(2):518–528. [DOI] [PubMed] [Google Scholar]
- 27.Oda Y, Chalkley RJ, Burlingame AL, Bikle DD. The transcriptional coactivator DRIP/mediator complex is involved in vitamin D receptor function and regulates keratinocyte proliferation and differentiation. J Invest Dermatol. 2010;130(10):2377–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshizawa T, Handa Y, Uematsu Y, Takeda S, Sekine K, Yoshihara Y, Kawakami T, Arioka K, Sato H, Uchiyama Y, Masushige S, Fukamizu A, Matsumoto T, Kato S. Mice lacking the vitamin D receptor exhibit impaired bone formation, uterine hypoplasia and growth retardation after weaning. Nat Genet. 1997;16(4):391–396. [DOI] [PubMed] [Google Scholar]
- 29.Reyes-Fernandez PC, Fleet JC. Compensatory changes in calcium metabolism accompany the loss of vitamin d receptor (VDR) from the distal intestine and kidney of mice. J Bone Miner Res. 2016;31(1):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rittié L. Cellular mechanisms of skin repair in humans and other mammals. J Cell Commun Signal. 2016;10(2):103–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bikle DD, Elalieh H, Chang S, Xie Z, Sundberg JP. Development and progression of alopecia in the vitamin D receptor null mouse. J Cell Physiol. 2006;207(2):340–353. [DOI] [PubMed] [Google Scholar]
- 32.Xie Z, Komuves L, Yu QC, Elalieh H, Ng DC, Leary C, Chang S, Crumrine D, Yoshizawa T, Kato S, Bikle DD. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J Invest Dermatol. 2002;118(1):11–16. [DOI] [PubMed] [Google Scholar]
- 33.Pálmer HG, Anjos-Afonso F, Carmeliet G, Takeda H, Watt FM. The vitamin D receptor is a Wnt effector that controls hair follicle differentiation and specifies tumor type in adult epidermis. PLoS One. 2008;3(1):e1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang LC, Ng DC, Bikle DD. Role of protein kinase C alpha in calcium induced keratinocyte differentiation: defective regulation in squamous cell carcinoma. J Cell Physiol. 2003;195(2):249–259. [DOI] [PubMed] [Google Scholar]
- 35.Thomason HA, Cooper NH, Ansell DM, Chiu M, Merrit AJ, Hardman MJ, Garrod DR. Direct evidence that PKCα positively regulates wound re-epithelialization: correlation with changes in desmosomal adhesiveness. J Pathol. 2012;227(3):346–356. [DOI] [PubMed] [Google Scholar]
- 36.Rieger S, Zhao H, Martin P, Abe K, Lisse TS. The role of nuclear hormone receptors in cutaneous wound repair. Cell Biochem Funct. 2015;33(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Razzaghi R, Pourbagheri H, Momen-Heravi M, Bahmani F, Shadi J, Soleimani Z, Asemi Z. The effects of vitamin D supplementation on wound healing and metabolic status in patients with diabetic foot ulcer: A randomized, double-blind, placebo-controlled trial. J Diabetes Complications. 2017;31(4):766–772. [DOI] [PubMed] [Google Scholar]
- 38.Brenner M, Hilliard C, Peel G, Crispino G, Geraghty R, OʼCallaghan G. Management of pediatric skin-graft donor sites: a randomized controlled trial of three wound care products. J Burn Care Res. 2015;36(1):159–166. [DOI] [PubMed] [Google Scholar]