Abstract
During human pregnancy, cytotrophoblasts (CTBs) play key roles in uterine invasion, vascular remodeling, and anchoring of the feto-placental unit. Due to the challenges associated with studying human placentation in utero, cultured primary villous CTBs are used as a model of the differentiation pathway that leads to invasion of the uterine wall. In vitro, CTBs emulate in vivo cell behaviors, such as migration, aggregation, and substrate penetration. Although some of the molecular features related to these cell behaviors have been described, the underlying mechanisms, at a global level, remain undefined at midgestation. Thus, in this study, we characterized second-trimester CTB differentiation/invasion in vitro, correlating the major morphological transitions with the transcriptional changes that occurred at these steps. After plating on Matrigel as individual cells, CTBs migrated toward each other and formed multicellular aggregates. In parallel, using a microarray approach, we observed differentially expressed (DE) genes across time, which were enriched for numerous functions, including cell migration, vascular remodeling, morphogenesis, cell communication, and inflammatory signaling. DE genes encoded several molecules that we and others previously linked to critical CTB function in vivo, suggesting that the novel DE molecules we discovered played important roles. Immunolocalization confirmed that CTBs in situ gave a signal for two of the most highly expressed genes in vitro. In summary, we characterized, at a global level, the temporal dynamics of primary human CTB gene expression in culture. These data will enable future analyses of various types of in vitro perturbations—for example, modeling disease processes and environmental exposures.
This study describes a global transcriptional analysis of time-dependent changes in gene expression occurring in primary human cytotrophoblasts as they differentiate in vitro.
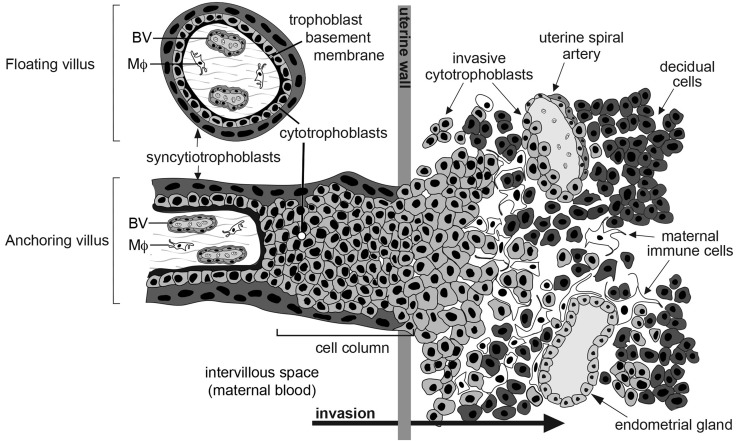
Within the intervillous space of the human placenta, networks of chorionic villi suspended in circulating maternal blood facilitate the vital exchange of nutrients, wastes, and gases between the maternal and embryonic/fetal units (Fig. 1). The villi are covered in two trophoblast layers. The outer layer is composed of multinucleated syncytiotrophoblasts, transport, and hormone-producing cells. Beneath resides a polarized layer of mononuclear cytotrophoblast (CTB) progenitors (2). Depending on location, CTBs differentiate into syncytio-trophoblasts or exit the placenta to anchor the embryo/fetus to the uterus. During the latter process, the cells detach from the trophoblast basement membrane of the villi and aggregate to form columns of unpolarized cells, which attach to the uterine wall. Invasive CTBs, also known as extravillous trophoblasts, deeply invade (interstitially) into the uterine wall, reaching the inner third of the myometrium during normal pregnancy. During remodeling of the uterine vessels, endovascular CTBs penetrate the walls of the spiral arteries, increasing their diameter, decreasing resistance, and diverting maternal blood flow to the placenta (3, 4). Due to their diverse and important roles in placental development, perturbations in CTB differentiation may be associated with several pregnancy complications, such as preeclampsia (PE) (5) ± intrauterine growth restriction, preterm labor (6), and the syndromes associated with excessive invasion (e.g., accreta, percreta, and increta) (7).
Figure 1.
Arrangement of cytotrophoblasts (CTBs) at the human maternal–fetal interface. Within the intervillous space of the human placenta, floating chorionic villi are suspended in circulating maternal blood. These structures facilitate the exchange of nutrients, wastes, and gases between the maternal and embryonic/fetal units. Villi are covered in two trophoblast layers, as follows: (1) an outer layer of multinucleated syncytiotrophoblasts and (2) a polarized layer of mononuclear CTBs. In anchoring chorionic villi during the first and second trimesters of pregnancy, CTBs, which are differentiating along the invasive pathway, exit the placenta by detaching from the trophoblast basement membrane and aggregating to form columns of unpolarized cells, which attach to the uterine wall. CTBs invade the decidua (interstitial invasion) or remodel the uterine spiral arteries (endovascular invasion), which increases their elasticity and diverts maternal blood flow to the placenta. CTB cultures contain cells isolated from floating and anchoring villi. BV, fetal blood vessel; MΦ, macrophage [modified from Damsky et al. (1)].
Influenced by numerous cues (8), CTBs modulate the expression of molecules that mediate adhesion, migration, and cell–cell communication, which underlie their broad functional capabilities. For example, as CTBs penetrate the uterine wall, they downregulate the expression of adhesion molecules that inhibit invasion, such as E-cadherin, and upregulate others that favor this process (1). To facilitate invasion, CTBs release numerous degradative molecules, including matrix metalloproteinase (MMP) family members (9), which break down basement membrane components and extracellular matrix (ECM) molecules they encounter. At the same time, the cells upregulate other factors that play a role in their unusual ability to mimic endothelial and vascular smooth muscle cells. These include other adhesion molecules [e.g., VE-cadherin (10), neural cell adhesion molecule (11)] as well as Eph/ephrin and Notch family members (12, 13), which most likely play crucial roles in the ability of endovascular CTBs to channel blood that flows through the maternal spiral arteries, which they line. Furthermore, the cells express a complex network of molecules (e.g., interleukins, chemokines, and HLA-G) that mediate their interactions with the maternal immune system (14). Determining, at a global level, the mechanisms controlling these behaviors (e.g., migration, adhesion, invasion, and immune tolerance), which are critical for CTB functions, is key to understanding normal placental development and disease.
Due to the difficulty of studying human CTBs in utero and known differences between human and rodent placentation and pregnancy, primary human cell culture models are valuable because they enable studies that address CTB functions at cellular and molecular levels (15). Over the past 30 years, protocols have been developed for isolating and culturing CTBs from placentas of various gestational ages (16, 17). Multiple steps have been introduced to improve the viability and purity of CTBs, for example, serial enzymatic digestions, Percoll gradient separations, and magnetic bead immunodepletions (18). In this context, CTBs isolated from normal pregnancies and a variety of pregnancy complications are used to study a wide range of placental cell functions in vitro.
Transcriptomic-based approaches provide a means to survey global RNA expression changes at gene and pathway levels. Additionally, the in vitro and in vivo expression of the identified molecules can be directly compared as an independent measure of their potential relevance to human pregnancy. In placental cells or tissue, global assessments of RNA expression have been used to investigate the molecular changes that occur during pregnancy and the factors that may influence expression, such as gestational age (19), tissue specification (20), species (21), and normal vs disease states (22). This approach has also been used to profile subsets of primary CTBs (23).
Our laboratory routinely isolates and cultures CTBs from first- and second-trimester placentas as well as term tissue. We use this cell culture model to study, at cellular and molecular levels, the differentiation pathway that leads to invasion of the uterine wall (16, 24). In this study, building on this work, we describe CTB morphological transitions and parallel transcriptomic changes over time in vitro as the cells acquire an invasive phenotype. The detailed characterization of this culture model during the second trimester of pregnancy will expand the utility of this system for studies of placental development, in normal pregnancy and disease, and for studies of the effects of possible perturbants such as environmental exposures.
Materials and Methods
Tissue collection
All methods in this study were approved by the University of California, San Francisco, Institutional Review Board. Informed consent was obtained from all donors. Second-trimester placentas (gestational age: 14 to 22 weeks) were collected immediately following elective terminations and placed in cytowash medium, consisting of DME/H-21 (Gibco), 12.5% fetal bovine serum (Hyclone), 1% glutamine plus (Atlanta Biologicals), 1% penicillin/streptomycin (Invitrogen), and 0.1% gentamicin (Gibco). Tissue samples were placed on ice prior to dissection.
Human primary villous CTB isolation
CTBs, consisting of both extravillous and villous CTBs, were isolated as described (16), with minor modifications, from second-trimester human placentas (gestational ages ranged from 14 to 22 weeks). In brief, the floating and anchoring chorionic villi were extensively washed in cold phosphate-buffered saline (PBS), dissected into 2- to 4-mm pieces, and filtered through a 1-mm mesh strainer to remove small pieces of tissue. CTBs were isolated according to the following steps: (1) removal of the outer syncytial layer via collagenase (Sigma-Aldrich; C-2674) digestion; (2) release of the CTBs by sequential enzymatic digestion [trypsin (Sigma-Aldrich; T8003; twice) and collagenase]; and (3) purification via Percoll density gradient centrifugation. Single cells were counted using a hemacytometer and immediately collected (0-hour samples) or transferred to a Matrigel (BD Biosciences)-coated 12-well plate. The substrate consisted of a 1:1 (volume-to-volume) mixture of Matrigel and culture medium (see later), which was incubated for 15 minutes at 37°C. CTBs were cultured at a density of 500,000 CTBs/well in 1.5 mL medium containing DME/H-21, 2% Nutridoma (Roche), 1% sodium pyruvate (Sigma-Aldrich), 1% HEPES buffer (Invitrogen), 1% glutamate plus (Atlanta Biologicals), and 1% penicillin/streptomycin (Invitrogen). Cells were incubated at 37°C in 5% CO2/95% air. At 3 hours postplating, medium was replaced to eliminate unattached cells. As previously reported, staining with anti-cytokeratin (CK) showed that cell purity was routinely ∼80% to 90% (16). Only cell preparations that met this criterion were analyzed. Because we analyzed primary CTBs, contaminants (e.g., immune and stromal cells) could contribute to the downstream analyses.
Immunolocalization of CTB antigens
At 3, 15, or 39 hours of culture, medium was removed and cells were washed once with PBS. Next, CTBs were fixed with 4% paraformaldehyde for 20 minutes, washed twice with PBS, and stored in PBS at 4°C until further processing. PBS was removed, and cold methanol was added to permeabilize the CTBs. After 5 minutes, the cells were washed with PBS three times, and 5% bovine serum albumin (BSA) (Hyclone)/PBS was added for 1 hour to block nonspecific reactivity. The blocking solution was removed, the primary antibody (in 5% BSA) was added, and the cells were incubated overnight at 4°C. Primary antibodies included the following: anti-CK [catalogue Fisher_001-clone7D3, Research Resource Identifier (RRID): AB_2631235, rat monoclonal; 1:100] (8), anti-HLA-G (catalogue Fisher_002-clone4H84, RRID:AB_2631236, mouse monoclonal; 1:100) (25), and anti-Ki-67 (Thermo Fisher Scientific catalogue RM-9106-S1, RRID: AB_149792, rabbit monoclonal; 1:100). CKs recognized by the rat antibody are highly expressed by human CTBs vs other placental cell types, possible contaminants (26). HLA-G, a major histocompatibility class Ib antigen, is specific for extravillous CTBs (25, 27). Ki-67 expression, a nuclear antigen, is used to identify proliferating cells (28). The next day, the antibody solution was removed and the CTBs were washed three times in PBS. Detection of primary antibodies was accomplished via incubation (1 hour) with species-specific secondary antibodies diluted in PBS:goat anti-rat immunoglobulin G (IgG; Alexa Fluor, 1:1000, A11081; Life Technologies); donkey anti-mouse (1:1000, A21202; Life Technologies); and goat anti-rabbit IgG (1:1000, A21206; Life Technologies). Cultures were washed with PBS three times and transferred to 1.5 mL PBS mixed with Hoechst 33342 (1:2500; Life Technologies). Phase brightfield and fluorescent photo montages were created by stitching together images (42 covering a 5-mm × 5-mm area), which were captured by using a Leica inverted microscope with a 10× objective and the tilescan function (Leica Application Suite Advanced Fluorescence).
We calculated the average fluorescence intensity associated with CK, HLA-G, or Ki-67 on a per cell basis by using Volocity software (PerkinElmer; version 6.3). First, we identified all cells or objects within each image by virtue of Hoechst staining. Initially, we excluded objects that intersected with the border of the image or were <20 µm2, which eliminated potential artifacts (e.g., cell debris). As a result of this process, ∼1% of the objects were discarded from the analysis. We applied a separation of object function, which included automated erosion and division operations to enable improved measurements of aggregated cell populations. Next, we determined the average intensity of CK, HLA-G, or Ki-67 per object. For CK or HLA-G, we used the dilate function (level 4) to measure the intensity of immunoreactivity associated with the cytoplasm and plasma membrane, respectively. Immunopositive/-negative intensity thresholds for CK or HLA-G immunostaining were determined as the mean intensity of ≥30 objects with negative signal + 1 standard deviation. Thresholds (±) for Ki-67 average intensity were calculated as the mean average intensity of ≥30 objects with immunopositive Ki-67 nuclear signals −1 standard deviation. To control for variability in overall fluorescence intensity among experiments, limits were determined on a per well basis for each experiment and time point. Percentages of cells that expressed each antigen were calculated as the number of immunopositive cells per total number of cells per stitched image. Average values were determined across ≥3 independent cultures. Images captured ∼20,000 cellsper well.
Quantification of CTB migration
To describe migration and aggregation of CTBs over time, we determined the minimum distance between nuclei using Volocity software. Objects were identified, as described in the previous section. Then we calculated the minimum distance between nuclei (centroid to centroid). This process entailed automated measurements of all possible distances between objects within each image. Three to 12 stitched images were analyzed per independent experiment (n = 3), and the average minimum distance among cells at 3, 15, and 39 hours postplating was calculated. The standard error of the mean was computed across the average of the three independent experiments. Representative images at 20× were exported in TIF format and processed via Photoshop (Adobe).
RNA isolation
Placentas for transcriptional profiling ranged in gestational age from 14 to 21 weeks (mean = 17.5 weeks). Two experiments used cells from individual placentas, and two experiments used a combination of cells that were isolated from two placentas. The contribution of female and male samples to the data was estimated by evaluating CTB expression (0 hours) of sex-specific genes: RPS4Y1, XIST, EIF1AY, and KDM5D (29) (Supplemental Fig. 1 (584.8KB, eps) ). Information about gestational age and sex is included in Supplemental Table 1 (10.3KB, xlsx) . Samples for quantitative reverse transcription polymerase chain reaction (qRT-PCR) validation ranged in gestational age from 18.3 to 22 weeks (mean = 20.3 weeks). RNA for these analyses was isolated from CTBs that were prepared from individual placentas. Briefly, immediately following CTB isolation (0 hours) or after 3, 15, 19, or 39 hours in culture, RLT lysis buffer (Qiagen) was added to either the cell pellet or culture dish well. The lysate was collected and stored at −80°C. We isolated and purified RNA using the RNeasy Micro Kit (Qiagen). The RNA concentration and quality were estimated (absorbance 260 nm/280 nm = 1.9 to 2.1) by using a Nanodrop spectrometer (Thermo Fisher Scientific). Samples destined for microarray analyses (0-, 3-, 15-, and 39-hour time points) were assessed for quality (RNA integrity number > 9) using the Agilent RNA 6000 Nano LabChip Kit and Bioanalyzer 2100 system.
Global gene expression profiling of CTBs
The analysis platform was the Affymetrix Human Genome U133 Plus 2.0 array. Sample processing and hybridization were performed by the University of California, San Francisco, Gladstone Institute, as previously described (29). Affymetrix CEL files were processed using the Affymetrix Expression Console and Transcriptome Analysis Console software packages. Raw values were normalized via the robust multiarray average algorithm. Raw and normalized data were deposited in the Gene Expression Omnibus (GSE86171). One-way analysis of variance (ANOVA) was applied to identify differentially expressed (DE) genes across time. Average fold change (FC) values were determined by calculating the ratio of average log 2 intensities between each time point and the 0-hour CTB group. Datasets were annotated using the Affymetrix Transcriptome Analysis Console database (10/1/14). In cases of multiple probes per gene, the one with the lowest P value, having the most significant change over time, was selected for comparison purposes. To define DE genes across time and among samples, we applied a cutoff of P ≤ 0.00005 (one-way ANOVA), an absolute FC ≥ 2 between any of the four time points with a false discovery rate of <1%. Hierarchical clustering of FC values was completed by using average linkage and Euclidean distance (TIGR MEV) (30). A secondary post hoc Student t test was used to determine the significance of changes between each time point and time 0 hours (cutoff applied: P ≤ 0.001, absolute FC ≥ 2).
Functional analyses of DE genes
We used DAVID (31) to identify functional enrichment of gene ontology (GO) biological processes (level 4) within our DE gene list. Genes were defined using the Affymetrix Probe ID. GO terms containing ≥15 DE genes with a P value of ≤0.005 and a fold enrichment (FE) ≥1.5 were selected as significantly overrepresented. Corresponding enrichment scores (i.e., P values and FE, were also determined for upregulated and downregulated gene clusters). As previously described (32), relative enrichment scores across GO terms were calculated as −log (p − value) × FE. We grouped GO terms based on GO classification (http://geneontology.org) to express common themes. To evaluate changes within GO biological processes on a temporal level, we calculated absolute average FC ratios of DE genes across time within enriched GOs, which were selected based on their relevancy to human placental development.
Validation of DE genes
These analyses used an independent set of CTBs cultured for 0, 3, 15, 19, and 39 hours. First, RNA was converted to complementary DNA using an ISCRIPT complementary DNA synthesis kit (Bio-Rad). Next, qRT-PCR was performed using TaqMan Universal Master Mix II, no UNG (Life Technologies), and specific TaqMan primers for CBS, CXCL6, DHCR7, DUSP2, F5, FABP7, ITGA2, MMP9, MMP12, PDXK, PEG3, and S100A7 (Supplemental Table 2 (9.1KB, xlsx) ). Selected targets were identified based on observations of robust (absolute FC > 2) and significant (P < 0.0005) changes in expression over time, and association with pathways of interest. We also included PEG3 (P = 0.005, absolute FC > 2) and MMP12 (P = 0.4) to estimate the repeatability of targets with different significance criteria. Reactions were carried out for 40 cycles. A minimum of three biological replicates was analyzed in all comparisons. Differential expression across time was calculated by using the ΔΔ cycle threshold method (normalized to glyceraldehyde-3-phosphate dehydrogenase). To determine significant changes over time, one-way ANOVA was applied (P ≤ 0.05). FC values were expressed as average log 2 ratios between each time point and the 0-hour CTB group.
Comparisons between second-trimester and term samples
We assessed the expression profiles of genes identified to be DE in CTB culture in an RNA sequencing (RNA-seq) dataset generated by the Epigenome Roadmap Project (20), which included CTBs purified, in our laboratory, from second-trimester and term placentas (0 hours). We compared the average reads per kilobase per million mapped reads (RPKM) expression values of second-trimester and term CTBs with differences in expression between these time periods. The official gene symbol was used to align the two datasets (merge function) (33).
Immunostaining of tissue sections
We used an immunolocalization approach to investigate the expression, at the protein level, of transcripts that were abundant as well as DE over time in cultured CTBs. For this purpose, we analyzed tissue sections of the maternal–fetal interface during the second trimester of pregnancy. The general method we used was published (34). Briefly, biopsies were fixed in 3% paraformaldehyde, dehydrated in increasing sucrose concentrations, and embedded in OCT (Thermo Fisher Scientific). Immunolocalization of proteins was performed using species-specific primary antibodies diluted in blocking buffer (PBS with 3% BSA and 0.05% Tween 20) for anti-CK (rat polyclonal; 1:100) (8), anti-NOTUM (Sigma-Aldrich; catalogue SAB3500082, RRID:AB_10604118, rabbit polyclonal, 1:100), and anti-EFEMP1 (Abcam; catalogue ab14926, RRID:AB_301517, rabbit polyclonal, 1:100) for 1 hour at 37°C. Detection of primary antibodies was done via incubation (1 hour at 37°C) with species-specific secondary antibodies diluted in blocking buffer: goat anti-rat IgG (Alexa Fluor, 1:1000, A11081; Life Technologies) and goat anti-rabbit IgG (1:1000, A21206; Life Technologies). Sections were washed with PBS three times and coverslipped with Vectashield containing 4′6-diamidino-2-phenylindole (Vector Bio-Laboratories). For these analyses, tissue sections from at least 12 placentas were evaluated. Images were acquired using a Leica inverted microscope with a 10× or 20× objective. Representative photomicrographs were exported in TIF format and processed via Photoshop (Adobe). No specific immunoreactivity was detected staining with the primary or secondary antibody alone or with an irrelevant isotype-matched antibody.
Results
Characterization of cultured CTBs
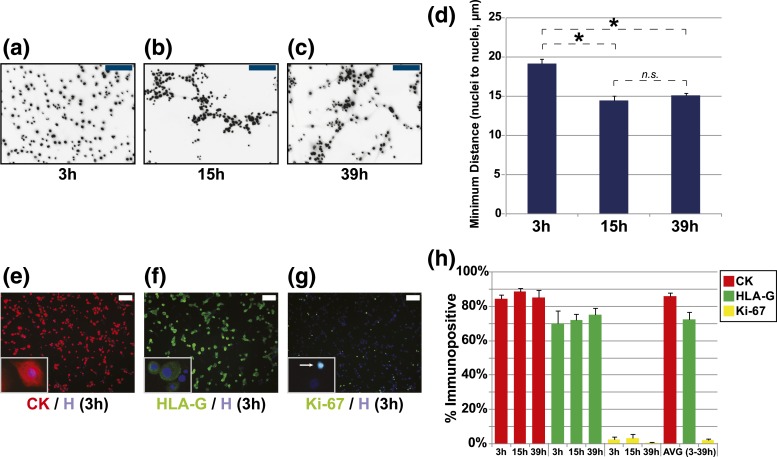
CTBs, isolated from floating and anchoring chorionic villi of second-trimester placentas, were plated on Matrigel and cultured for up to 39 hours. Within 3 hours, CTBs started to spread on the matrix and extend cellular projections [Fig. 2(a–c)]. By 15 hours, CTBs formed multicellular aggregates. Time-lapse imaging indicated a high level of cell movement within the aggregates at higher resolution (data not shown). Furthermore, as previously described (16), CTBs did not fuse to form multinucleated syncytiotrophoblasts. To complement our visual observations, we quantified the distance of each CTB to its closest neighbor at 3, 15, and 39 hours. We observed a significant difference in the average minimum distance among cells at 15 hours (Δ↓4.5 µm) as compared with 3 hours [P ≤ 0.05; Fig. 2(d)]. Cell aggregation was similar at 15 and 39 hours (P ≥ 0.05). Using a semiquantitative approach that combined immunofluorescence localization and high-throughput content image analysis, we calculated (on average) that the majority of cells in our model expressed CK (86.1 ± 1.6%) [Fig. 2(e)] and HLA-G (72.3 ± 4.4%) [Fig. 2(f)]. In contrast, only 2.1 ± 0.6% of cells expressed Ki-67 [Fig. 2(g)]. The expression of CK, HLA-G, and Ki-67 did not change as a function of time in culture [quantified in Fig. 2(h)]. As a whole, these observations suggested that our tissue culture system models CTB exit from the cell cycle aggregation, a proxy for differentiation.
Figure 2.
Morphological transitions of cultured primary human CTBs over 39 hours. (a–c) CTBs were incubated for 3, 15, and 39 hours before the nuclei were visualized by Hoechst staining. (d) The average minimum distance among CTB nuclei (n = 3). Asterisks signify P ≤ 0.05 (t test). Representative images of immunostaining for (e) CK, (f) HLA-G, and (g) Ki-67 in CTBs at 3 hours; nuclei were stained with Hoechst. (h) Average percentage of cells that stained for these antigens as a function of time in culture. Images are representative of ≥3 independent experiments. Scale bars = 100 µm. n.s., not significant.
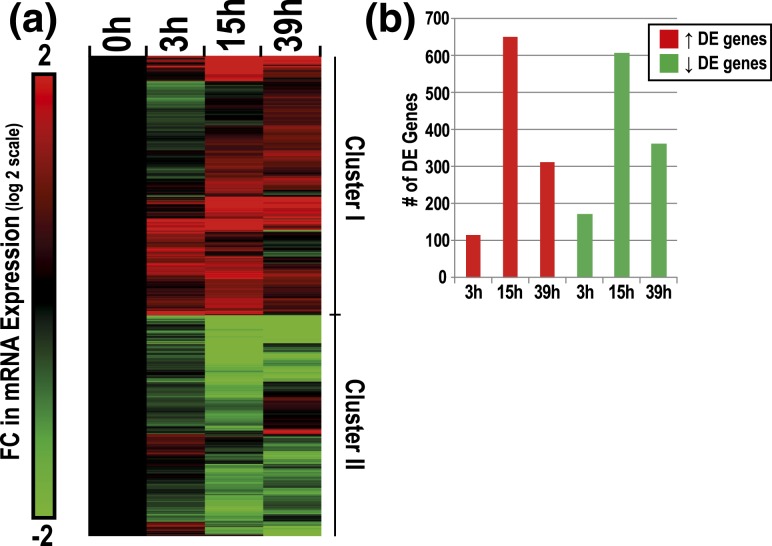
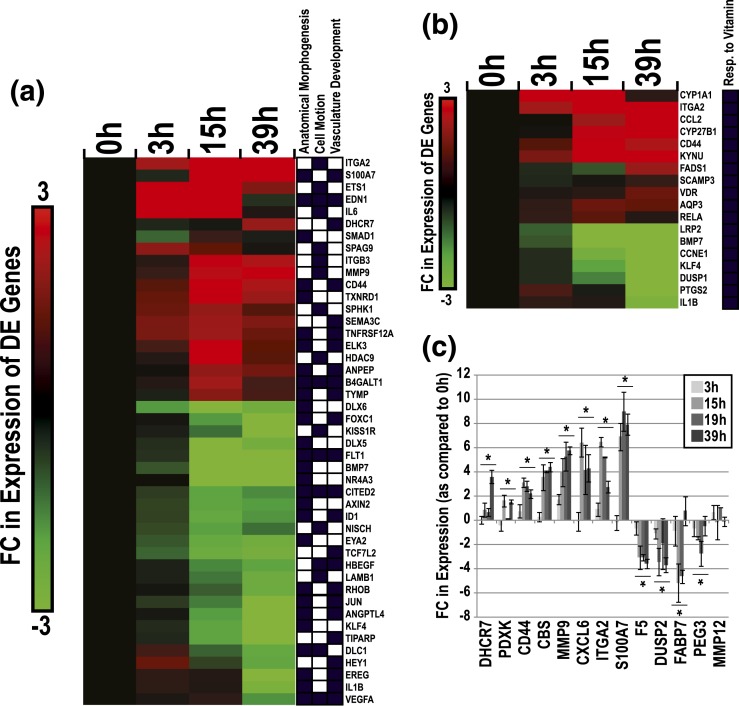
We identified 2232 genes as significantly DE across time (0–39 hours) as CTBs differentiated in culture (ANOVA, P ≤ 0.00005, absolute FC ≥ 2) [Fig. 3(a)]. Within this gene set, time-dependent changes were highly dynamic. Approximately 54% of DE genes followed a positive trend across time (Cluster I), and conversely, 46% of DE genes displayed a negative trend (Cluster II). As compared with the 0-hour data, the largest differences, in terms of absolute magnitude and significance, were observed at 15 hours [Fig. 3(b)]. These analyses suggested robust time-dependent changes in the transcriptome of CTBs during the initial 39 hours of culture.
Figure 3.
Characterization of changes in CTB gene expression as a function of time in culture. (a) Hierarchical clustering of time-dependent DE genes in CTBs (2232 genes total, P ≤ 0.00005, absolute FC ≥ 2). Upregulated or downregulated genes over time in culture were designated as either cluster I or cluster II, respectively. Average FC values are displayed as the ratio of average intensities between each time point and the average 0-hour CTB values (log 2 scale). (b) Post hoc t test analysis of DE genes per time point (t test, P ≤ 0.001, absolute FC ≥ 2).
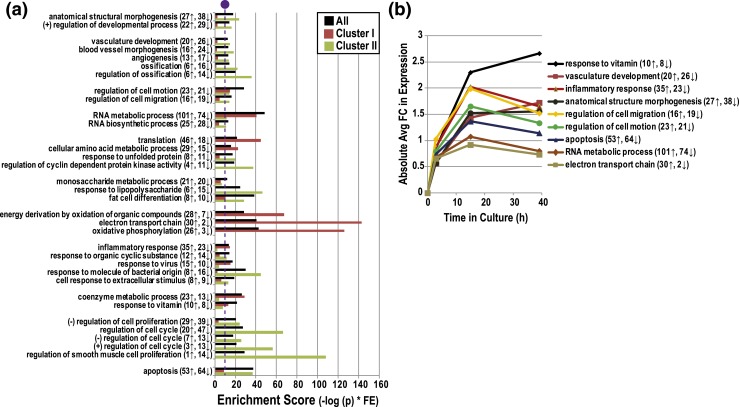
As to the DE subset, we observed an enrichment of genes involved in a diverse range of GO biological processes, including anatomical structural morphogenesis, vasculature development, cell motion, RNA metabolism, translation, monosaccharide metabolism, energy derivation by oxidation of organic compounds, inflammatory response, coenzyme metabolism, regulation of cell proliferation, and apoptosis (P ≤ 0.005, FE ≥ 1.5) [Fig. 4(a)]. We also conducted separate GO analyses for Clusters I or II. On average, genes that were involved in RNA metabolism and energy derivation were upregulated over time. This was particularly true for RNAs encoding proteins that function in the electron transport chain (30↑, 2↓). Enriched categories of genes that were predominately downregulated over time in culture included response to lipopolysaccharide (6↑, 15↓) and regulation of proliferation (20↑, 47↓).
Figure 4.
Functional GO enrichment analysis of CTB genes that were upregulated or downregulated as a function of time in culture. (a) GO terms that were overrepresented (biological processes) among DE genes whose expression changed during CTB culture (P ≤ 0.005; FE ≥1.5, total number changed ≥15; bar denotes significance). (b) Absolute average FC ratios of DE genes within selected enriched GO terms. The number of upregulated or downregulated DE genes in each category. (+), positive; (−), negative.
With regard to selected GO categories, expression of genes related to inflammatory responses, anatomical structure morphogenesis, regulation of cell migration/motion, apoptosis, RNA metabolism, and electron transport chain changed in a time-dependent manner, peaking at 15 hours [absolute FC; Fig. 4(b)]. For example, at this time point there was a fourfold change in expression of genes that are involved in inflammatory responses (log 2 = 2.0). In contrast, the expression of genes related to vitamin responses and vascular development peaked at 39 hours with the highest observed FCs, 6.4 (log 2 = 2.7) and 3.3 (log 2 = 1.7), respectively.
Due to our interest in the mechanisms underlying CTB differentiation/invasion and vascular mimicry, we mapped DE genes (additional filter; absolute FC > 4) associated with three GO biological processes: anatomical structure morphogenesis (28 genes), cell motion (the family term of cell migration; 18 genes), and vasculature development (23 genes) [Fig. 5(a)]. This gene subset included ITGA2, S100A7, ETS1, EDN1, IL6, DHCR7, MMP9, and CD44, which were upregulated over time, and DLX6, FOXC1, KISS1R, DLX5, FLT1, BMP7, NR4A3, and CITED2, which were downregulated. Molecules linked to response to vitamin were also clustered due to their strong regulation and the influence of vitamins on pregnancy outcome. This subset included p450 enzymes (CYP1A1, -27B1), the vitamin D receptor, CD44, and aquaporin (AQP)3, which were all upregulated in culture [Fig. 5(b)]. We also listed DE genes with the largest FCs in our culture system. This subset included a variety of molecules that are linked to the aforementioned categories and additional pathways, including chemokines and inflammatory molecules (e.g., CFB, SAA1, TNFAIP6, CXCL5, and CXCL6), collagen endopeptidases (MMP3, -10), solute carriers (SCL22A1, -6A11, -1A6), and structural components (e.g., KRT6A, KRT6B, ITGA2, and LAMB3) (Supplemental Table 3 (12.4KB, xlsx) ).
Figure 5.
DE genes within enriched categories relevant to CTB invasion. (a) Hierarchical clustering plot of DE genes related to enriched GO terms (anatomical structure morphogenesis, cell motion, and vascular development) in CTBs over time in culture. DE genes that passed a secondary filter (absolute FC > 4; log 2 = 2) are shown. Black boxes signify GO associations. (b) DE genes related to the enriched GO term: response to vitamin. (c) qRT-PCR verification of gene expression changes over time in culture. Asterisks indicate significant changes (ANOVA, P ≤ 0.05; bars denote standard error of the mean).
For a subset of DE genes, we validated expression changes observed by microarray via a qRT-PCR approach and RNA samples isolated from a second set of CTB samples [Fig. 5(c)]. DHCR7, PDXK, CD44, CBS, MMP9, CXCL6, ITGA2, and S100A7 were upregulated with time in culture (ANOVA, P < 0.05). F5, DUSP2, FABP7, and PEG3 were downregulated (ANOVA, P < 0.05). MMP12 mRNA levels, which did not change according to the microarray data, were also unchanged when assayed by qRT-PCR. Our qRT-PCR and microarray results were highly correlated in terms of FC in expression (as compared with the 0-hour time point) (r = 0.88; not shown). FC values for genes (PDXK, PEG3), which were not significantly regulated in the microarray data (0.05 > x > 0.0005), correlated between the two analytical methods.
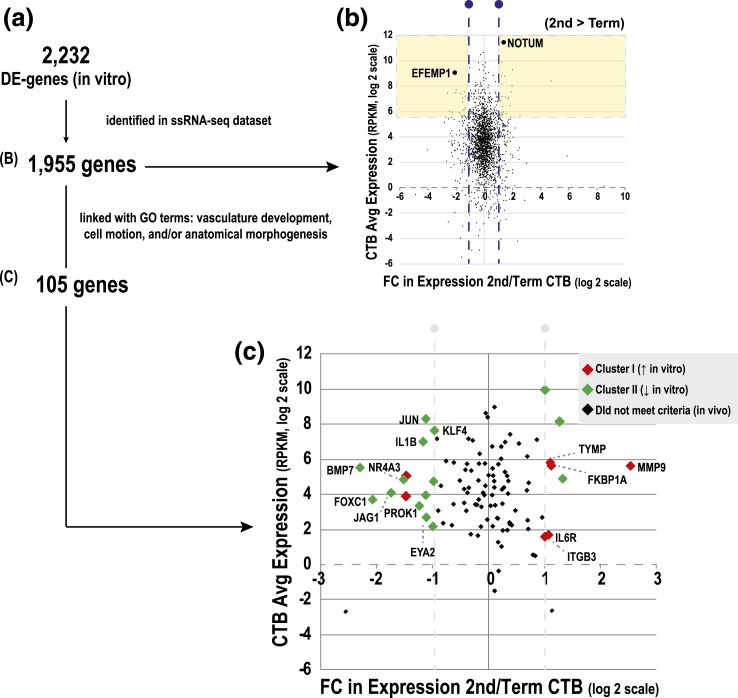
We tested the hypothesis that gene expression changes in vitro parallel, in some cases, genes and pathways that are upregulated in second trimester, when the placenta is still remodeling the uterine wall, as compared with term, when this process is completed. Thus, we interrogated ssRNA-seq profiles of freshly isolated CTBs from second-trimester and term placentas (20) to determine transcripts (identified as DE in vitro) whose expression was dependent on gestational age. Of the 2232 DE genes in vitro, 1955 had RPKM values [Fig. 6(a)]. We plotted the average abundance of this subset vs the FC difference in expression between second-trimester and term CTBs [Fig. 6(b)]. Approximately 95% of the genes we compared were expressed >1 RPKM (log 2 scale = 0). Approximately 16% of genes were DE >twofold between the two gestational ages (blue lines, log 2 scale = 1). Forty-seven genes that were in the 10th percentile by abundance (>58.5 RPKM) were also gestationally regulated (>twofold). This subset included molecules upregulated in term vs second-trimester CTBs (e.g., EFEMP1) or upregulated in second-trimester vs term CTBs (e.g., NOTUM). The majority of genes that were modulated during gestation (77%) were downregulated (green vs red) in culture. Furthermore, we conducted additional analyses using the subset of genes (105 total) that was associated with pathways underlying CTB differentiation/invasion: anatomical structure morphogenesis, cell motion, and vasculature development [Fig. 6(c)]. This subset included molecules upregulated in term vs second-trimester CTBs (e.g., BMP7, KLF4, and JAG1) or upregulated in second-trimester vs term CTBs (e.g., MMP9, ITGB3, and IL6R). Thus, the latter cross comparison identified molecules that are known regulators of CTB invasion, suggesting that potentially novel candidates that emerged from this analysis could be interesting to study in this context.
Figure 6.
Mapping CTB genes that were DE in culture (microarray) to CTB gene expression changes from second trimester to term (RNA-seq). (a) The RNA-seq dataset was generated from CTBs that were isolated in our laboratory (20). In total, 1955/2232 DE genes could be compared. (b) The average abundance of transcripts of DE genes (y-axis, RPKM, log 2 scale) vs the FC difference between second-trimester and term CTBs (x-axis, log 2 scale). (c) DE genes related to GO terms: anatomical morphogenesis, cell motion, and/or vascular development. Genes are labeled based on patterns of regulation over time in culture.
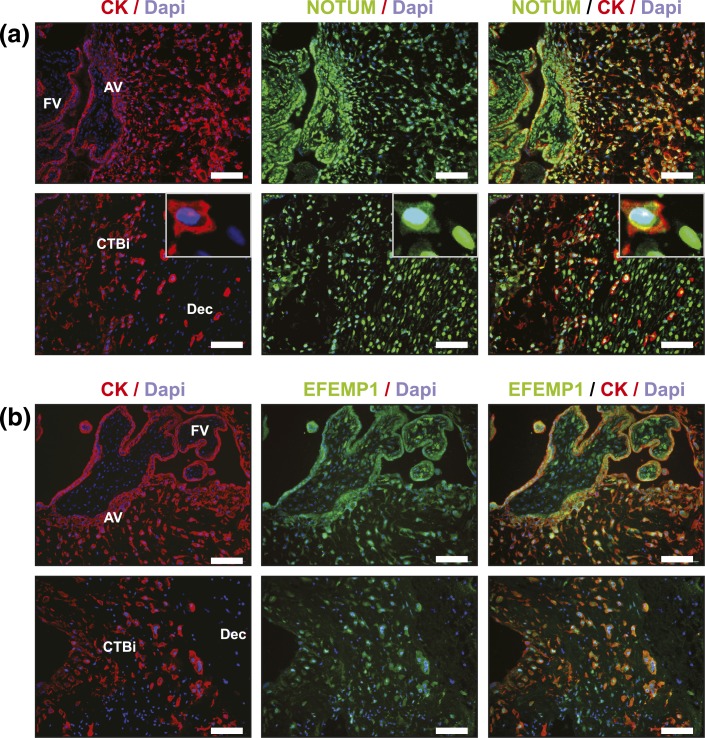
Next, in tissue sections of second-trimester biopsies of the maternal–fetal interface, we evaluated protein expression of NOTUM and EFEMP1 using an immunofluorescence approach. These two molecules stood out in our transcriptomic analyses as follows: (1) highly abundant; (2) significantly DE over time in cultured CTBs; and (3) DE between second trimester and term. In second-trimester tissue sections (n = 12), NOTUM [green; Fig. 7(a)] was expressed in all trophoblast subpopulations that we examined, including CTBs within the floating villi and cell columns (upper panels) as well as the placental cells that invaded more deeply into the decidua (lower panels). Expression of NOTUM was also apparent in non-CK–positive cells within the villous core and decidua. As to modulation as a function of invasion, the major finding was that NOTUM expression tended to move from the cytoplasm to the nucleus. EFEMP1 was also widely expressed among the CTB subpopulations [Fig. 7(b)]. In general, antibody reactivity, which was highest in villous CTBs (upper panels), decreased as the cells invaded the uterus (lower panels). Thus, these analyses suggested that NOTUM and EFEMP1 have interesting patterns of modulation as a function of CTB differentiation/invasion.
Figure 7.
Immunolocalization of NOTUM and EFEMP1, which were DE in cultured CTBs. Representative images of floating and anchoring villi (FV, AV, respectively; upper panels) and invasive (i) CTBs within the decidua (Dec; lower panels). Tissue sections of second-trimester samples were immunostained for (a) NOTUM or (b) EFEMP1. The samples were costained with anti-CK (trophoblast marker) and 4′6-diamidino-2-phenylindole (Dapi; nuclear dye). Tissue sections from ≥12 placentas were evaluated. Scale bars = 100 µm.
Discussion
Model systems in which TBs isolated from human placentas are placed in culture provide important opportunities for studying the mechanisms and cellular functions underlying normal development and disease. We published protocols for isolating CTBs and culturing them on three-dimensional substrates (16, 24). Targeted analyses showed that under these conditions the cells differentiate along the pathway that leads to migration away from the placenta proper and invasion of the uterine wall (1, 8). In this work, we expanded these reports by completing a global transcriptomic analysis that described in detail the changes in gene expression that paral-leled the CTB morphological transitions as the cells acquired an invasive phenotype in vitro. Later we discuss these results in the context of CTB differentiation in vivo.
Quantification of CTB migration in vitro
We developed a semiautomated pipeline for doing high throughput image content analysis and applied this approach to our CTB differentiation model. As described previously, the majority of cells expressed CK, which identifies all TB subpopulations, and HLA-G, which is upregulated at the protein level as the cells invade the uterine wall (25, 26). Isolation and culture of second-trimester CTBs on Matrigel enabled their attachment (by 3 hours), after which they spread on the substrate, becoming highly migratory. In the process, they migrated toward each other, forming multicellular aggregates while extending elongated filapodia (by 15 hours). In agreement with previous studies, we showed that cultured CTBs that differentiated along the invasive pathway had a very low rate of proliferation (∼2% immunopositive for Ki-67 expression). We quantified these transitions by measuring the average minimum distance between the cell nuclei at three time points. This approach enabled us to quantify migratory activity and aggregation across hundreds of thousands of cells. In this study, we established baseline conditions that, in future experiments, can be used to evaluate the effects of potential perturbants, including pharmaceuticals, environmental chemicals, and disease states (35), on CTB behavior.
Transcriptomic changes in cultured CTBs
In parallel with the described morphological transitions, we observed robust, global RNA expression changes over time in cultured CTBs (Fig. 3). Using a conservative approach (false discovery rate <1%; P ≤ 0.00005; absolute FC ≥2), ∼10% of genes were significantly DE over time. Our results suggested changes in specific pathways related to CTB functions in vivo, including morphogenesis, vasculature development, cell migration, cell communication, vitamin/nutrition, and inflammatory responses. These pathways suggested an interplay between molecules that drive cell behaviors in CTBs (remodeling, migration), placental development, and cell–cell communication. The data highlighted many interesting processes with known or potential relevance to CTB interactions with maternal cells in vivo and novel regulators.
Migration and morphogenesis pathways
At a transcriptomic level, several of the DE molecules played critical roles in CTB migration and/or morphogenesis [Figs. 4 and 5(a)]. This subset included MMP9, which was upregulated (>ninefold) in culture. In parallel, we observed the increased expression of four other MMP family members (MMP3, -8, -10, and -14). As a class, MMPs are critical for CTB invasion and differentiation with MMP9 playing an especially important role (in vitro and in vivo) (36–38). Lower expression levels of MMP9 are associated with PE (39), and the MMP9 (–1562C/T) variant is linked with PE susceptibility (40). Integrin expression was also modulated, including ITGA2 (↑), ITGB3 (↑) (Fig. 5), ITGB6 (↑), and ITGB5 (↓) (data not shown). Regulated expression of integrins and their ECM ligands is an integral part of the CTB differentiation pathway that leads to formation of cell columns and uterine invasion (41, 42). For example, in first-trimester placentas, column CTBs express high levels of ITGA6/B4 and low levels of ITGA5/B1, whereas invasive extravillous CTBs have the opposite expression pattern (43). To our knowledge, expression of ITGA2 has yet to be described in human CTBs. We identified many other genes that were DE in culture with proposed links to CTB invasion/differentiation and placental development. They included the following: (1) ETS1 (↑), a transcription factor that regulates expression of MMPs and other molecules important for uterine invasion (44, 45); (2) the secreted preproprotein/signaling peptide, EDN1 (↑), which is involved in invasion and vasoconstriction (46); and (3) KISS1R1 (↓), a G protein–coupled receptor that selectively binds to kisspeptins and represses TB migration/invasion (47). Our results suggested that CTB differentiation in vitro recapitulated many of the molecular switches that occur as CTBs acquire invasive/migratory properties in vivo.
Vascular and immune pathways
CTB endovascular invasion garners a supply of maternal blood that enables exchange of nutrients, wastes, and gases at the maternal–fetal interface. In our culture model, DE genes included molecules that play important roles in vascular development [Fig. 5(a)], such as CD44 (↑), a cell adhesion surface receptor for hyaluronic acid and a major component of the ECM. These receptor–ligand complexes regulate intracellular signaling pathways critical for CTB invasion and remodeling of the decidua (48). Other DE molecules in this class included VEGFA and FLT1, which were downregulated. Increased expression of these molecules underlies maternal vascular dysfunction in PE (49). Additionally, several inflammatory mediators were DE in our model. For example, CXCL6 [Fig. 5(c)] and interleukin-6 [Fig. 5(a)] were upregulated. In HTR-8/SVneo cells, interleukin-6 promotes migration, invasion, and modulation of integrin profiles (50). In the same cell line (and in primary CTBs), CXCL6 reduces TB migration/invasion by reducing MMP2 activity (51). Other DE inflammatory molecules included numerous chemokines and interleukins. These results provided a global transcriptional context for previous (13) and future investigations using this model system to study inflammatory mediators and their mechanistic roles in CTB differentiation/invasion, vascular remodeling, and immune interactions with the mother.
Vitamin and nutrient regulation pathways in CTBs
In general, the importance of vitamins and other nutrients for pregnancy health and placental development is well-recognized (52–54). However, the underlying mechanistic links remain unresolved at molecular and cellular levels. In cultured CTBs, genes associated with the GO term, response to vitamin, were among some of the most dramatically differentially expressed in our dataset [Fig. 5(b)]. This subset included members of the vitamin D signaling pathway—the primary p450-activating enzyme (CYP27B1) and the vitamin D receptor. The expression of both increased with time in culture, suggesting enhanced CTB responsiveness to compounds of this class as they differentiate along the invasive pathway. This observation is in line with published results regarding the relationships between vitamin D and the following: (1) extravillous TB invasiveness (55); (2) calciotropic hormone regulation (56); and (3) pregnancy complications [e.g., PE (57)]. In addition, we observed increased expression of CBS, a member of the folate metabolism/cysteine-synthesis pathway [Fig. 5(c)]. CBS is the critical regulator of homocysteine production during pregnancy, and altered levels may underlie impaired decidualization/changes in uterine–gene expression (58), PE, pregnancy loss, and congenital birth defects (54). The cytochrome p450 enzymes—CYP1A1, -1A2, -1B1—which play roles in xenobiotic/drug metabolism as well as endogenous regulation of fatty acid metabolism, angiogenesis, and epithelial differentiation (59), were also upregulated in culture. The functions of these enzymes, with dual roles in metabolizing exogenous and endogenous compounds, are poorly understood in the context of placental development and function. Additionally, other molecules in this subset, such as AQP3 and AQP9, were upregulated in culture. These membrane proteins, which act as access points for water molecules, may play key roles during mammalian pregnancy (60, 61). Whether these molecules, which are highly regulated in our system, also play roles in CTB differentiation is an interesting possibility.
DE genes in second-trimester vs term CTBs
Using an ssRNA-seq dataset generated as part of the Epigenome Roadmap Project (20), we defined a subset of genes (initially observed to be DE in vitro in CTBs) that were DE between second trimester vs term [Fig. 6(a)]. This analysis revealed several abundant molecules (top 10% expression, yellow shading) that were associated with faulty CTB invasion and/or placental disease, e.g., FSTL3 (62), FLT1 (63), ADAM12 (64), TFPI2 (65, 66), F5 (67), and HSD17B1 (68) (Supplemental Table 4 (14KB, xlsx) ), as well as new molecules yet to be studied in this context (e.g., EFEMP1 and NOTUM) [Fig. 6(b)]. In a follow-up analysis, we identified genes that were up- or downregulated as a function of CTB differentiation/invasion in vitro, focusing on a subset of the component processes—morphogenesis, cell motion, and vascular development. Then we asked whether they were also regulated as a function of gestational age [Fig. 6(c)]. Molecules with known functions during CTB differentiation, such as MMP9 (36–38) and ITGB3 (69), were upregulated in cultured CTBs, and the transcripts were expressed at higher levels during second trimester relative to term. In general, these findings further supported the concept that genes and pathways critical to CTB differentiation and placental development were actively modulated in our cell culture model. Furthermore, these analyses highlighted new molecules yet to be studied in this context. For example, we identified two genes, NOTUM and EFEMP1, as potentially involved in CTB differentiation. In vitro, aggregating CTBs downregulated their expression at the RNA level. Immunolocalization of NOTUM and EFEMP1, in tissue sections of second-trimester placentas, confirmed expression of these molecules at the protein level in villous CTB progenitors, as well as in CTBs transiting through the columns and within the uterine wall. NOTUM, a carboxylesterase, was recently identified as a key inhibitor of WNT signaling (70) and critical for neural/head induction in Xenopus (71). Despite the recognized importance of WNT signaling in TB invasion (72), nothing is known about the potential role(s) of NOTUM in placental development. In our analyses, CTB invasion was associated with movement of NOTUM from the cytoplasmic to the nuclear compartment. To our knowledge, this phenomenon has not been previously reported.
The ECM glycoprotein, EFEMP1, acts as a regulator of MMP expression and cancer metastasis (73). In an estrogen-dependent manner, EFEMP1 inhibits WNT/B-catenin signaling pathways and thereby the epithelial-to-mesenchymal transition of endometrial carcinoma cells (74). Previously, EFEMP1 was shown to be expressed in human CTBs that reside within the smooth chorion layer of fetal membranes (75). In this study, we add new data, suggesting expression of EFEMP1 in the placenta during midgestation and differential patterning of EFEMP1 in villous CTBs (higher expression) as compared with CTBs invading the uterus (lower expression). In general, our study provides evidence of modulation of NOTUM and EFEMP1 expression in CTBs within the maternal–fetal interface, suggesting possible roles of these two molecules in TB differentiation.
Conclusion
Primary cultures of human villous CTBs are an important model system for studying their adhesion, migration, and invasion—behaviors that are critical determinants of pregnancy outcomes. In this study, using a transcriptomic-based approach, we profiled CTB gene expression as the cells differentiated along the invasive pathway in vitro. The DE genes were involved in key biological pathways that are critical to placentation in vivo: cell migration, vascular remodeling, morphogenesis, and inflammation. The rich datasets that were generated provide a foundation of gene expression profiles against which the effects of numerous variables, including environmental and pharmacological compounds, can be evaluated.
Acknowledgments
We thank Cheryl Godwin de Medina for patient recruitment, Gabriel Goldfien and Yan Zhou for technical assistance, and Jason Farrell and Nicomedes Abello for tissue collection.
Acknowledgments
This work was supported by the National Institute of Environmental Health Sciences (Grants P01ES022841 and K99ES023846) and the Environmental Protection Agency (Grant RD83543301).
Disclosure Summary: The authors have nothing to disclose.
Appendix:
Primary Antibodies
| Peptide/Protein Target | RRID | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|---|
| Cytokeratin | AB_2631235 | Clone 7D3 | Fisher_001-clone7D3 | Rat; monoclonal | 1:100 | |
| HLA-G | AB_2631236 | Clone 4H84 | Fisher_002-clone4H84 | Mouse; monoclonal | 1:100 | |
| Ki-67 | AB_149792 | Ki-67 | Thermo Fisher Scientific, RM-9106-S1 | Rabbit; monoclonal | 1:100 | |
| NOTUM | AB_10604118 | NOTUM | Sigma-Aldrich, SAB3500082 | Rabbit; polyclonal | 1:100 | |
| EFEMP1 | AB_301517 | TYTQCTDGYEWDPVRQQC | EFEMP1 | Abcam, ab14926 | Rabbit; polyclonal | 1:100 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- ANOVA
- analysis of variance
- AQP
- aquaporin
- BSA
- bovine serum albumin
- CK
- cytokeratin
- CTB
- cytotrophoblast
- DE
- differentially expressed
- ECM
- extracellular matrix
- FC
- fold change
- FE
- fold enrichment
- GO
- gene ontology
- IgG
- immunoglobulin G
- MMP
- matrix metalloproteinase
- PBS
- phosphate-buffered saline
- PE
- preeclampsia
- qRT-PCR
- quantitative reverse transcription polymerase chain reaction
- RNA-seq
- RNA sequencing
- RPKM
- reads per kilobase per million mapped reads.
References
- 1.Damsky CH, Fitzgerald ML, Fisher SJ. Distribution patterns of extracellular matrix components and adhesion receptors are intricately modulated during first trimester cytotrophoblast differentiation along the invasive pathway, in vivo. J Clin Invest. 1992;89(1):210–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Red-Horse K, Zhou Y, Genbacev O, Prakobphol A, Foulk R, McMaster M, Fisher SJ. Trophoblast differentiation during embryo implantation and formation of the maternal-fetal interface. J Clin Invest. 2004;114(6):744–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y, Fisher SJ, Janatpour M, Genbacev O, Dejana E, Wheelock M, Damsky CH. Human cytotrophoblasts adopt a vascular phenotype as they differentiate: a strategy for successful endovascular invasion? J Clin Invest. 1997;99(9):2139–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Damsky CH, Fisher SJ. Preeclampsia is associated with failure of human cytotrophoblasts to mimic a vascular adhesion phenotype: one cause of defective endovascular invasion in this syndrome? J Clin Invest. 1997;99(9):2152–2164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ball E, Bulmer JN, Ayis S, Lyall F, Robson SC. Late sporadic miscarriage is associated with abnormalities in spiral artery transformation and trophoblast invasion. J Pathol. 2006;208(4):535–542. [DOI] [PubMed] [Google Scholar]
- 6.Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345(6198):760–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jauniaux E, Jurkovic D. Placenta accreta: pathogenesis of a 20th century iatrogenic uterine disease. Placenta. 2012;33(4):244–251. [DOI] [PubMed] [Google Scholar]
- 8.Chen JZ, Sheehan PM, Brennecke SP, Keogh RJ. Vessel remodelling, pregnancy hormones and extravillous trophoblast function. Mol Cell Endocrinol. 2012;349(2):138–144. [DOI] [PubMed] [Google Scholar]
- 9.Cohen M, Bischof P. Factors regulating trophoblast invasion. Gynecol Obstet Invest. 2007;64(3):126–130. [DOI] [PubMed] [Google Scholar]
- 10.Kokkinos MI, Murthi P, Wafai R, Thompson EW, Newgreen DF. Cadherins in the human placenta--epithelial-mesenchymal transition (EMT) and placental development. Placenta. 2010;31(9):747–755. [DOI] [PubMed] [Google Scholar]
- 11.Blankenship TN, King BF. Macaque intra-arterial trophoblast and extravillous trophoblast of the cell columns and cytotrophoblastic shell express neural cell adhesion molecule (NCAM). Anat Rec. 1996;245(3):525–531. [DOI] [PubMed] [Google Scholar]
- 12.Hunkapiller NM, Gasperowicz M, Kapidzic M, Plaks V, Maltepe E, Kitajewski J, Cross JC, Fisher SJ. A role for Notch signaling in trophoblast endovascular invasion and in the pathogenesis of pre-eclampsia. Development. 2011;138(14):2987–2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Red-Horse K, Kapidzic M, Zhou Y, Feng KT, Singh H, Fisher SJ. EPHB4 regulates chemokine-evoked trophoblast responses: a mechanism for incorporating the human placenta into the maternal circulation. Development. 2005;132(18):4097–4106. [DOI] [PubMed] [Google Scholar]
- 14.Hemberger M. Immune balance at the foeto-maternal interface as the fulcrum of reproductive success. J Reprod Immunol. 2013;97(1):36–42. [DOI] [PubMed] [Google Scholar]
- 15.Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, Lubzens E, Huppertz B. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta. 2011;32(Suppl):S49–S54. [DOI] [PubMed] [Google Scholar]
- 16.Hunkapiller NM, Fisher SJ. Chapter 12. Placental remodeling of the uterine vasculature. Methods Enzymol. 2008;445:281–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF III. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118(4):1567–1582. [DOI] [PubMed] [Google Scholar]
- 18.Douglas GC, King BF. Isolation of pure villous cytotrophoblast from term human placenta using immunomagnetic microspheres. J Immunol Methods. 1989;119(2):259–268. [DOI] [PubMed] [Google Scholar]
- 19.Mikheev AM, Nabekura T, Kaddoumi A, Bammler TK, Govindarajan R, Hebert MF, Unadkat JD. Profiling gene expression in human placentae of different gestational ages: an OPRU Network and UW SCOR Study. Reprod Sci. 2008;15(9):866–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundaje A, Meuleman W, Ernst J, Bilenky M, Yen A, Heravi-Moussavi A, Kheradpour P, Zhang Z, Wang J, Ziller MJ, Amin V, Whitaker JW, Schultz MD, Ward LD, Sarkar A, Quon G, Sandstrom RS, Eaton ML, Wu YC, Pfenning AR, Wang X, Claussnitzer M, Liu Y, Coarfa C, Harris RA, Shoresh N, Epstein CB, Gjoneska E, Leung D, Xie W, Hawkins RD, Lister R, Hong C, Gascard P, Mungall AJ, Moore R, Chuah E, Tam A, Canfield TK, Hansen RS, Kaul R, Sabo PJ, Bansal MS, Carles A, Dixon JR, Farh KH, Feizi S, Karlic R, Kim AR, Kulkarni A, Li D, Lowdon R, Elliott G, Mercer TR, Neph SJ, Onuchic V, Polak P, Rajagopal N, Ray P, Sallari RC, Siebenthall KT, Sinnott-Armstrong NA, Stevens M, Thurman RE, Wu J, Zhang B, Zhou X, Beaudet AE, Boyer LA, De Jager PL, Farnham PJ, Fisher SJ, Haussler D, Jones SJ, Li W, Marra MA, McManus MT, Sunyaev S, Thomson JA, Tlsty TD, Tsai LH, Wang W, Waterland RA, Zhang MQ, Chadwick LH, Bernstein BE, Costello JF, Ecker JR, Hirst M, Meissner A, Milosavljevic A, Ren B, Stamatoyannopoulos JA, Wang T, Kellis M; Roadmap Epigenomics Consortium . Integrative analysis of 111 reference human epigenomes. Nature. 2015;518(7539):317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barreto RS, Bressan FF, Oliveira LJ, Pereira FT, Perecin F, Ambrósio CE, Meirelles FV, Miglino MA. Gene expression in placentation of farm animals: an overview of gene function during development. Theriogenology. 2011;76(4):589–597. [DOI] [PubMed] [Google Scholar]
- 22.Zhou Y, Gormley MJ, Hunkapiller NM, Kapidzic M, Stolyarov Y, Feng V, Nishida M, Drake PM, Bianco K, Wang F, McMaster MT, Fisher SJ. Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J Clin Invest. 2013;123(7):2862–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tilburgs T, Crespo AC, van der Zwan A, Rybalov B, Raj T, Stranger B, Gardner L, Moffett A, Strominger JL. Human HLA-G+ extravillous trophoblasts: immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci USA. 2015;112(23):7219–7224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher SJ, Cui TY, Zhang L, Hartman L, Grahl K, Zhang GY, Tarpey J, Damsky CH. Adhesive and degradative properties of human placental cytotrophoblast cells in vitro. J Cell Biol. 1989;109(2):891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMaster MT, Librach CL, Zhou Y, Lim KH, Janatpour MJ, DeMars R, Kovats S, Damsky C, Fisher SJ. Human placental HLA-G expression is restricted to differentiated cytotrophoblasts. J Immunol. 1995;154(8):3771–3778. [PubMed] [Google Scholar]
- 26.Maldonado-Estrada J, Menu E, Roques P, Barré-Sinoussi F, Chaouat G. Evaluation of cytokeratin 7 as an accurate intracellular marker with which to assess the purity of human placental villous trophoblast cells by flow cytometry. J Immunol Methods. 2004;286(1-2):21–34. [DOI] [PubMed] [Google Scholar]
- 27.Kovats S, Main EK, Librach C, Stubblebine M, Fisher SJ, DeMars R. A class I antigen, HLA-G, expressed in human trophoblasts. Science. 1990;248(4952):220–223. [DOI] [PubMed] [Google Scholar]
- 28.Kaya B, Nayki U, Nayki C, Ulug P, Oner G, Gultekin E, Yildirim Y. Proliferation of trophoblasts and Ki67 expression in preeclampsia. Arch Gynecol Obstet. 2015;291(5):1041–1046. [DOI] [PubMed] [Google Scholar]
- 29.Winn VD, Haimov-Kochman R, Paquet AC, Yang YJ, Madhusudhan MS, Gormley M, Feng KT, Bernlohr DA, McDonagh S, Pereira L, Sali A, Fisher SJ. Gene expression profiling of the human maternal-fetal interface reveals dramatic changes between midgestation and term. Endocrinology. 2007;148(3):1059–1079. [DOI] [PubMed] [Google Scholar]
- 30.Saeed AI, Bhagabati NK, Braisted JC, Liang W, Sharov V, Howe EA, Li J, Thiagarajan M, White JA, Quackenbush J. TM4 microarray software suite. Methods Enzymol. 2006;411:134–193. [DOI] [PubMed] [Google Scholar]
- 31.Huang DW, Sherman BT, Tan Q, Kir J, Liu D, Bryant D, Guo Y, Stephens R, Baseler MW, Lane HC, Lempicki RA DAVID Bioinformatics Resources: expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007;35(Web Server issue):W169–W175. [DOI] [PMC free article] [PubMed]
- 32.Robinson JF, Verhoef A, van Beelen VA, Pennings JL, Piersma AH. Dose-response analysis of phthalate effects on gene expression in rat whole embryo culture. Toxicol Appl Pharmacol. 2012;264(1):32–41. [DOI] [PubMed] [Google Scholar]
- 33.Team RC. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2014. [Google Scholar]
- 34.Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, Alitalo K, Damsky C, Fisher SJ. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160(4):1405–1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hromatka BS, Drake PM, Kapidzic M, Stolp H, Goldfien GA, Shih IeM, Fisher SJ. Polysialic acid enhances the migration and invasion of human cytotrophoblasts. Glycobiology. 2013;23(5):593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo J, , Qiao F,, Yin X doi: 10.1007/s11596-011-0260-3. Impact of silencing MMP9 gene on the biological behaviors of trophoblasts. J Huazhong Univ Sci Technolog Med Sci. 2011;31(2):241–245. [DOI] [PubMed]
- 37.Librach CL, Werb Z, Fitzgerald ML, Chiu K, Corwin NM, Esteves RA, Grobelny D, Galardy R, Damsky CH, Fisher SJ. 92-kD type IV collagenase mediates invasion of human cytotrophoblasts. J Cell Biol. 1991;113(2):437–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Plaks V, Rinkenberger J, Dai J, Flannery M, Sund M, Kanasaki K, Ni W, Kalluri R, Werb Z. Matrix metalloproteinase-9 deficiency phenocopies features of preeclampsia and intrauterine growth restriction. Proc Natl Acad Sci USA. 2013;110(27):11109–11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kolben M, Lopens A, Bläser J, Ulm K, Schmitt M, Schneider KT, Tschesche H. Proteases and their inhibitors are indicative in gestational disease. Eur J Obstet Gynecol Reprod Biol. 1996;68(1-2):59–65. [DOI] [PubMed] [Google Scholar]
- 40.Rahimi Z, Rahimi Z, Shahsavandi MO, Bidoki K, Rezaei M. MMP-9 (-1562 C:T) polymorphism as a biomarker of susceptibility to severe pre-eclampsia. Biomarkers Med. 2013;7(1):93–98. [DOI] [PubMed] [Google Scholar]
- 41.Aplin JD, Jones CJ, Harris LK. Adhesion molecules in human trophoblast: a review. I. Villous trophoblast. Placenta. 2009;30(4):293–298. [DOI] [PubMed] [Google Scholar]
- 42.Harris LK, Jones CJ, Aplin JD. Adhesion molecules in human trophoblast: a review. II. Extravillous trophoblast. Placenta. 2009;30(4):299–304. [DOI] [PubMed] [Google Scholar]
- 43.Damsky CH, Librach C, Lim KH, Fitzgerald ML, McMaster MT, Janatpour M, Zhou Y, Logan SK, Fisher SJ. Integrin switching regulates normal trophoblast invasion. Development. 1994;120(12):3657–3666. [DOI] [PubMed] [Google Scholar]
- 44.Takai N, Ueda T, Narahara H, Miyakawa I. Expression of c-Ets1 protein in normal human placenta. Gynecol Obstet Invest. 2006;61(1):15–20. [DOI] [PubMed] [Google Scholar]
- 45.Kessler CA, Stanek JW, Stringer KF, Handwerger S. ETS1 induces human trophoblast differentiation. Endocrinology. 2015;156(5):1851–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cervar M, Puerstner P, Kainer F, Desoye G. Endothelin-1 stimulates the proliferation and invasion of first trimester trophoblastic cells in vitro: a possible role in the etiology of pre-eclampsia? J Investig Med. 1996;44(8):447–453. [PubMed]
- 47.Hiden U, Bilban M, Knöfler M, Desoye G. Kisspeptins and the placenta: regulation of trophoblast invasion. Rev Endocr Metab Disord. 2007;8(1):31–39. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi H, Takizawa T, Matsubara S, Ohkuchi A, Kuwata T, Usui R, Matsumoto H, Sato Y, Fujiwara H, Okamoto A, Suzuki M, Takizawa T. Extravillous trophoblast cell invasion is promoted by the CD44-hyaluronic acid interaction. Placenta. 2014;35(3):163–170. [DOI] [PubMed] [Google Scholar]
- 49.Fan X, Rai A, Kambham N, Sung JF, Singh N, Petitt M, Dhal S, Agrawal R, Sutton RE, Druzin ML, Gambhir SS, Ambati BK, Cross JC, Nayak NR. Endometrial VEGF induces placental sFLT1 and leads to pregnancy complications. J Clin Invest. 2014;124(11):4941–4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jovanović M, Vićovac L. Interleukin-6 stimulates cell migration, invasion and integrin expression in HTR-8/SVneo cell line. Placenta. 2009;30(4):320–328. [DOI] [PubMed] [Google Scholar]
- 51.Zhang H, Hou L, Li CM, Zhang WY. The chemokine CXCL6 restricts human trophoblast cell migration and invasion by suppressing MMP-2 activity in the first trimester. Hum Reprod. 2013;28(9):2350–2362. [DOI] [PubMed] [Google Scholar]
- 52.De-Regil LM, Palacios C, Lombardo LK, Peña-Rosas JP. Vitamin D supplementation for women during pregnancy. Cochrane Database Syst Rev. 2016;1(1):CD008873. [DOI] [PubMed] [Google Scholar]
- 53.Wei SQ. Vitamin D and pregnancy outcomes. Curr Opin Obstet Gynecol. 2014;26(6):438–447. [DOI] [PubMed] [Google Scholar]
- 54.Mislanova C, Martsenyuk O, Huppertz B, Obolenskaya M. Placental markers of folate-related metabolism in preeclampsia. Reproduction. 2011;142(3):467–476. [DOI] [PubMed] [Google Scholar]
- 55.Chan SY, Susarla R, Canovas D, Vasilopoulou E, Ohizua O, McCabe CJ, Hewison M, Kilby MD. Vitamin D promotes human extravillous trophoblast invasion in vitro. Placenta. 2015;36(4):403–409. [DOI] [PubMed] [Google Scholar]
- 56.O’Brien KO, Li S, Cao C, Kent T, Young BV, Queenan RA, Pressman EK, Cooper EM. Placental CYP27B1 and CYP24A1 expression in human placental tissue and their association with maternal and neonatal calcitropic hormones. J Clin Endocrinol Metab. 2014;99(4):1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma R, Gu Y, Zhao S, Sun J, Groome LJ, Wang Y. Expressions of vitamin D metabolic components VDBP, CYP2R1, CYP27B1, CYP24A1, and VDR in placentas from normal and preeclamptic pregnancies. Am J Physiol Endocrinol Metab. 2012;303(7):E928–E935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nuño-Ayala M, Guillén N, Arnal C, Lou-Bonafonte JM, de Martino A, García-de-Jalón JA, Gascón S, Osaba L, Osada J, Navarro MA. Cystathionine β-synthase deficiency causes infertility by impairing decidualization and gene expression networks in uterus implantation sites. Physiol Genomics. 2012;44(14):702–716. [DOI] [PubMed] [Google Scholar]
- 59.Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6(12):947–960. [DOI] [PubMed] [Google Scholar]
- 60.Liu H, Koukoulas I, Ross MC, Wang S, Wintour EM. Quantitative comparison of placental expression of three aquaporin genes. Placenta. 2004;25(6):475–478. [DOI] [PubMed] [Google Scholar]
- 61.Prat C, Bouvier D, Comptour A, Marceau G, Belville C, Clairefond G, Blanc P, Gallot D, Blanchon L, Sapin V. All-trans-retinoic acid regulates aquaporin-3 expression and related cellular membrane permeability in the human amniotic environment. Placenta. 2015;36(8):881–887. [DOI] [PubMed] [Google Scholar]
- 62.Guo J, Tian T, Lu D, Xia G, Wang H, Dong M. Alterations of maternal serum and placental follistatin-like 3 and myostatin in pre-eclampsia. J Obstet Gynaecol Res. 2012;38(7):988–996. [DOI] [PubMed] [Google Scholar]
- 63.March MI, Geahchan C, Wenger J, Raghuraman N, Berg A, Haddow H, Mckeon BA, Narcisse R, David JL, Scott J, Thadhani R, Karumanchi SA, Rana S. Circulating angiogenic factors and the risk of adverse outcomes among Haitian women with preeclampsia. PLoS One. 2015;10(5):e0126815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Aghababaei M, Beristain AG. The Elsevier Trophoblast Research Award Lecture: Importance of metzincin proteases in trophoblast biology and placental development: a focus on ADAM12. Placenta. 2015;36(Suppl 1):S11–S19. [DOI] [PubMed] [Google Scholar]
- 65.Zhou Q, Xiong Y, Chen Y, Du Y, Zhang J, Mu J, Guo Q, Wang H, Ma D, Li X doi: 10.1177/1076029611429785. Effects of tissue factor pathway inhibitor-2 expression on biological behavior of BeWo and JEG-3 cell lines. Clin Appl Thromb Hemost. 2012;18(5):526–533. [DOI] [PubMed]
- 66.Xiong Y, Zhou Q, Jiang F, Zhou S, Lou Y, Guo Q, Liang W, Kong D, Ma D, Li X. Changes of plasma and placental tissue factor pathway inhibitor-2 in women with preeclampsia and normal pregnancy. Thromb Res. 2010;125(6):e317–e322. [DOI] [PubMed] [Google Scholar]
- 67.Fong FM, Sahemey MK, Hamedi G, Eyitayo R, Yates D, Kuan V, Thangaratinam S, Walton RT. Maternal genotype and severe preeclampsia: a HuGE review. Am J Epidemiol. 2014;180(4):335–345. [DOI] [PubMed] [Google Scholar]
- 68.Ishibashi O, Ohkuchi A, Ali MM, Kurashina R, Luo SS, Ishikawa T, Takizawa T, Hirashima C, Takahashi K, Migita M, Ishikawa G, Yoneyama K, Asakura H, Izumi A, Matsubara S, Takeshita T, Takizawa T. Hydroxysteroid (17-β) dehydrogenase 1 is dysregulated by miR-210 and miR-518c that are aberrantly expressed in preeclamptic placentas: a novel marker for predicting preeclampsia. Hypertension. 2012;59(2):265–273. [DOI] [PubMed] [Google Scholar]
- 69.Chung TW, Park MJ, Kim HS, Choi HJ, Ha KT. Integrin αVβ3 and αVβ5 are required for leukemia inhibitory factor-mediated the adhesion of trophoblast cells to the endometrial cells. Biochem Biophys Res Commun. 2016;469(4):936–940. [DOI] [PubMed] [Google Scholar]
- 70.Kakugawa S, Langton PF, Zebisch M, Howell SA, Chang TH, Liu Y, Feizi T, Bineva G, O’Reilly N, Snijders AP, Jones EY, Vincent JP. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519(7542):187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang X, Cheong SM, Amado NG, Reis AH, MacDonald BT, Zebisch M, Jones EY, Abreu JG, He X. Notum is required for neural and head induction via Wnt deacylation, oxidation, and inactivation. Dev Cell. 2015;32(6):719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sonderegger S, Husslein H, Leisser C, Knofler M. doi: 10.1016/j.placenta.2006.11.003. Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 2007;28(Suppl A):S97–S102. [DOI] [PMC free article] [PubMed]
- 73.Wang Z, Cao CJ, Huang LL, Ke ZF, Luo CJ, Lin ZW, Wang F, Zhang YQ, Wang LT. EFEMP1 promotes the migration and invasion of osteosarcoma via MMP-2 with induction by AEG-1 via NF-κB signaling pathway. Oncotarget. 2015;6(16):14191–14208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yang T, Zhang H, Qiu H, Li B, Wang J, Du G, Ren C, Wan X. EFEMP1 is repressed by estrogen and inhibits the epithelial-mesenchymal transition via Wnt/β-catenin signaling in endometrial carcinoma. Oncotarget. 2016;7(18):25712–25725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moore RM, Redline RW, Kumar D, Mercer BM, Mansour JM, Yohannes E, Novak JB, Chance MR, Moore JJ. Differential expression of fibulin family proteins in the para-cervical weak zone and other areas of human fetal membranes. Placenta. 2009;30(4):335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]