Abstract
The cytokine leukemia inhibitory factor (LIF) is essential for rendering the uterus receptive for blastocyst implantation. In mice, LIF receptor expression (LIFR) is largely restricted to the uterine luminal epithelium (LE). LIF, secreted from the endometrial glands (GEs), binds to the LIFR, activating the Janus kinase–signal transducer and activation of transcription (STAT) 3 (Jak-Stat3) signaling pathway in the LE. JAK-STAT activation converts the LE to a receptive state so that juxtaposed blastocysts begin to implant. To specifically delete the LIFR in the LE, we derived a line of mice in which Cre recombinase was inserted into the endogenous lactoferrin gene (Ltf-Cre). Lactoferrin expression in the LE is induced by E2, and we demonstrate that Cre recombinase activity is restricted to the LE and GE. To determine the requirement of the LIFR in implantation, we derived an additional mouse line carrying a conditional (floxed) Lifrflx/flx gene. Crossing Ltf-Cre mice with Lifrflx/flx mice generated Lifrflx/Δ:LtfCre/+ females that were overtly normal but infertile. Many of these females, despite repeated matings, did not become pregnant. Unimplanted blastocysts were recovered from the Lifrflx/Δ:LtfCre/+ uteri and, when transferred to wild-type recipients, implanted normally, indicating that uterine receptivity rather than the embryo’s competency is compromised. The loss of Lifr results in both the failure for STAT3 to translocate to the LE nuclei and a reduction in the expression of the LIF regulated gene Msx1 that regulates uterine receptivity. These results reveal that uterine expression of the LIFR is essential for embryo implantation and further define the components of the LIF signaling pathway necessary for effective implantation.
The LIFR was ablated in the uterine luminal epithelium using a LIFR conditional allele. The majority of the females were infertile, as the embryos failed to implant due to uterine unresponsiveness.
In mammals, implantation, the period during which the blastocyst establishes an intimate connection with the uterus, is essential for embryonic development. In preparation for implantation, the uterus undergoes a series of changes, primarily driven by the ovarian steroid hormones that culminate in the uterus becoming transiently receptive to blastocysts. In mice, receptivity commences on the morning of the fourth day after mating (day of plug = day 1 pregnancy), with implantation, trophoblast invasion, and decidualization occurring some 12 hours later (1).
The ovarian steroid hormones estrogen (E2) and progesterone (P4) are the key factors regulating uterine preparation (2). At ovulation, high levels of E2 stimulate uterine cell proliferation (3). Subsequently, levels of P4 increase, and these suppress cell proliferation and initiate cell differentiation, culminating in the uterus, becoming potentially receptive on the fourth day after mating. On the morning of the fourth day, blastocysts are distributed along the uterine lumen, juxtaposed to the antimesometrial luminal epithelium (LE). Implantation is then initiated by a brief surge of E2 (nidatory E2) that results in the embryonic trophectoderm invading the LE. The LE adjacent to the invading trophectoderm undergoes either apoptosis or entosis (4), and the underlying stroma differentiates to form the decidua (5). Decidualization increases vascular permeability and acts to support early postimplantation embryonic growth and placenta formation, as well as restrict trophoblast invasion.
The hormonal effects on the uterus are mediated either directly or indirectly through locally produced growth factors and cytokines (6). One of these factors, essential for implantation, is leukemia inhibitory factor (LIF) (7). During ovulation, LIF is expressed in the murine glandular epithelium (GE) (8, 9). Over the next 3 days of the reproductive cycle, LIF levels decline but then transiently increase in the GE prior to implantation on D4 of pregnancy. The second increase of LIF is induced by nidatory E2, together with the tumor suppressor and transcription factor, p53 (10, 11). In a wide variety of mammalian species, including human females, a rise in uterine LIF expression is associated with the onset of implantation, implicating LIF in having an important role in regulating uterine receptivity in humans and many other mammals (12). Polymorphisms in the p53 gene, which are associated with reduced LIF levels due to reduced transcriptional activity, are frequently present among young women undergoing in vitro fertilization, potentially correlating a reduction LIF levels with infertility (13).
Uteri from Lif−/− mice show no evidence of epithelial apoptosis, stromal decidualization, or blastocyst invasion (10). However, transfer of the blastocysts from Lif−/− females to wild-type (WT) pseudopregnant recipients results in the embryos undergoing normal implantation and development (7). Furthermore, a single injection of recombinant LIF into pregnant Lif−/− mice on day 4 or 5 of pregnancy induces blastocyst implantation with development to term, revealing that during pregnancy LIF is only required to initiate implantation (10). The injection of LIF is also sufficient in replacing nidatory E2 at inducing implantation in appropriately primed ovariectomized mice. Together these findings demonstrate that E2, together with p53, induces LIF expression, with LIF mediating the effects of nidatory E2 at initiating uterine receptivity and implantation. In the absence of LIF, implantation fails and is a maternal defect (14).
LIF acts by binding to the heterodimeric receptor comprised of the LIF receptor (LIFR)β and GP130. In the uterus, both LIFRβ and GP130 are detected in the GE and LE at 3 to 4 days postcoitum (dpc) (15–17). LIFR expression is initially restricted to the uterine LE, with levels increasing from day 3 onwards after mating. On day 4 following LIF expression, LIFR levels transiently increase in the LE and to a lesser extent in the GE, thereafter expression declines. GP130 expression is restricted to the apical surface of the day 4 LE (17). Treatment of the LE with LIF induces activation of the Janus kinase and signal transducer and activator of transcription (Jak/Stat)–signaling pathway that phosphorylates the latent transcription factor Stat3 (15). Phosphorylated dimers of signal transducer and activator of transcription 3 (pSTAT3) then translocate to the nucleus (18). In the LE of Lif−/− mice, Stat3 translocation to the nucleus does not occur (15, 17). Mice that are homozygous for a deletion of the Stat3-GP130 docking site show defective implantation, and deregulation of soluble GP130 has been reported in patients with unexplained fertility (19, 20). In addition, genetic ablation of the Stat3 gene or the application of Jak/Stat inhibitors (21–23) results in defective implantation.
A deeper examination into the role of LIF signaling components in implantation is complicated due to the broad expression of these proteins, with the loss of each of the three proteins (LIFR, gp130, and Stat3) resulting in embryonic/perinatal lethality (24–26). Lifr-deficient mice die during embryogenesis or shortly after birth making it impossible to determine the LIFR’s role at regulating implantation. To define this role, we first derived a mouse line carrying conditional alleles of Lifr (Lifrflx/flx) by the introduction of LoxP sites at the 5′ and 3′ ends, flanking the extracellular domain of the Lifr gene. In addition, we established a mouse line (LtfCre/Cre) in which the lactotransferrin/lactoferrin gene (Ltf) was used to drive Cre recombinase expression in the uterine epithelia. Lactotransferrin is an iron-binding antimicrobial protein that is expressed in many secretory tissues including tear ducts, salivary glands, and mammary glands, as well as the uterine GE and LE, where it functions as part of the innate immune system (27). Mice lacking a functional Ltf gene are viable and overtly normal. In the murine GE and LE, Ltf expression is induced by E2 and inhibited by P4 and is highly expressed for 3 days after ovulation. The Cre recombinase gene was “knocked into” the Ltf locus, so placing Cre under the control of the Ltf promoter, and we show that this knock-in, in the uterus, restricts Cre expression to the LE and GE.
Here, we show that Lifrflx/Δ mice crossed with LtfCre/Cre (CB4) mice results in viable adult mice. In the females, there is a loss of the Lifr expression in the uterine LE and GE. This is associated with a failure to activate the Jak-Stat pathway upon treatment, with LIF resulting in the majority of blastocysts not implanting.
Materials and Methods
Mice
Mice were maintained at the A*STAR Biological Resource Centre facility in accordance with (28), with the protocols reviewed and approved by the A*STAR Biological Resource Centre Institutional Animal Care and Use Committee.
Derivation of the Ltf-Cre and Lifrflx/flx lines
An outline of the three transgenic and recombinant (gene-targeted) lines is presented in Supplemental Fig. 1. (7.2MB, pdf)
Transgenic Ltf-Cre mice
A 2.5-kb EcoRI-SmaI fragment from the mL14p9E plasmid containing the Ltf promoter (corresponding to bases –2637 to –18, ATG = 1) was ligated with the Cre-poly (A) cassette, to generate a Ltf-Cre transgenic mouse line by pronuclear injection of the plasmid (Supplemental Figs. 1A (7.2MB, pdf) and 2A (7.2MB, pdf) ).
Ltf-Cre (L4-Cre) transgenic animals were generated by standard pronuclear injection techniques at the National Cancer Institute at Frederick transgenic mouse facility. After pronuclear injection of the transgene, 170 mice were derived and genotyped by southern blotting to detect the presence and dosage of the transgene. Six males from the 55 genotyped as being positive for the transgene were then subsequently analyzed, by crossing with Rosa26R3 female reporter mice (29). From each female, the E13 embryo was collected and stained for β-galactosidase expression.
Ltf-Cre (CB4)
To derive the “knock-in” mouse line, CB4, the Cre recombinase, was inserted into the endogenous lactoferrin gene, just downstream of the ATG start site (Supplemental Fig. 1B (7.2MB, pdf) and Supplemental Fig. 3 (7.2MB, pdf) ). The lactoferrin (Ltf) gene consists of 17 exons and is transcribed as a single transcript. A 9-kb EcoRI fragment in pGEM3Z (mL14p9E) containing 2.6 kb of 5′ flanking sequence and the first eight exons of the Ltf gene, was subcloned from a 14-kb Ltfl phage clone (mL14 from a 129/J mouse genomic DNA library, DASH, Strategene—a gift of C. T. Teng; Supplemental Fig. 3A (7.2MB, pdf) and 3B (7.2MB, pdf) ). An XbaI fragment containing exon 1 was subcloned into pBluescript. Cre recombinase complementary DNA (cDNA) containing the SV40 poly (A) sequence was excised from the pmnCre plasmid (gift from P. Soriano) using StyI-HindIII digestion and cloned by blunt-end ligation into the StyI site at bp72 in exon 1. The resulting XbaI sequence containing Cre-pA was then recloned into mL14pE9. A neomycin (neo) cassette under the control of the PGK promoter was isolated from pPGKneobpA, using Xho1, was blunt-end ligated into the partially digested XbaI site. A thymidine kinase (TK) cassette, under the control of the MC1 promoter from pKS-TK, was cloned into the Xho1-BamH1 (in the vector) site to replace an Xho1-EcoRI fragment in the mL14p9E plasmid, thus generating the targeting vector, pLF-C-N-TK (Supplemental Fig. 3B (7.2MB, pdf) ). A 1.5-kb fragment spanning exons 5 to 8 was excised with Xho1-BamH1 (Supplemental Fig. 3B (7.2MB, pdf) ) and used as a probe for southern analysis to detect homologous recombinants (Supplemental Fig. 3D (7.2MB, pdf) ). The resulting transcript from the targeted allele (Supplemental Fig. 3B (7.2MB, pdf) ) would produce the initial 17 amino acids of Ltf followed by the Cre sequence (Supplemental Fig. 3C and 3E (7.2MB, pdf) ).
Derivation of the a conditional LifRflx/flx allele
The LifR gene contains 21 exons spanning some 50 kb. To generate the conditional LifR allele (LifRflx/flx; Supplemental Fig. 1C (7.2MB, pdf) ), two targeting vectors were constructed: pTV-LRup (pTV-LifR-fH), which generated (LifRflx/flxHyg), and pTV-LRdown (pTV-LifR-fN), which generated LifRflxlxNeo (Supplemental Fig. 4A and 4B (7.2MB, pdf) ). These vectors targeted introns 1 and 14, respectively (30). pTV-LRup was derived from pl5-5.5 Asp, a 5.7-kb KpnI fragment between exons 1 and 2 and cloned into pGEM7 (gift from C. Ware) (25). A PGK-hygro-poly (A) cassette flanked by loxP sites was blunt-end ligated into the BstEII site. The resulting construct was digested with BamHI and cloned into pBS-TK, containing the TK negative selection cassette. The 0.5-kb BamHI-XbaI fragment in p15-5.5, absent from pTV-LRup, was used as a probe for southern analysis.
The genomic DNA fragment used to make the targeting vector pTV-LRdown (pTV-LifR-fH) was derived by screening a library of 129 bacterial artificial chromosomes (BACs; RP22-129S6/SvEvTac Research Genetics) using LifR cDNA as a probe. From 27 BAC clones, three were confirmed by dot blot analysis as containing the LifR gene (RP22-175O16, RP22-417F23, and RP23-476A13). DNA from a mixture of the three BACs was digested with EcoRI; large fragments were gel purified and cloned into the EcoRI site of pGEMTEasy (Promega). Clones were screened by polymerase chain reaction (PCR) using primers targeting the fibronectin type III domains of LifR (exons 12 to 14), LR1783 forward (5′-CATTGAACGAGGAGACACAGTCAGTTTTGG-3′) and LR2197 reverse (5′-GAACGTAACAGTTGGTATCCCTGGTTAGTGC-3′), resulting in the identification of a 9kb EcoRI fragment containing exons 11 to 16. A PGKneobpA cassette flanked by loxP sites was excised from pHR68 (gift from D. Duboule) by XhoI digestion and inserted into an XhoI site within intron 14 in the same orientation as the PGK-hygro-poly (A) cassette. An MC1-TK cassette was cloned into the Sac1-Sal1 site to generate the pTV-LRdown targeting vector. The DNA fragment used for southern analysis was generated from the BAC by cloning an EcoR1-BamH1 fragment 3′ to the targeting construct.
Gene targeting in embryonic stem cells
The targeting vectors were linearized by Not1 digestion and electroporated into W9.5 129 embryonic stem (ES) cells. ES clones were cultured and selected for as described (31, 32). For the LtfCre (CB4) knock-in southern analysis, genomic DNA was digested with EcoR1. The blots were probed with a 1.0-kb Xho-Bam1 fragment from mL14p9E corresponding to the sequence immediately 3′ to the targeting vector. The probe hybridized to the 9-kb WT allele and a 7.4-kb recombinant allele due to addition of EcoR1 site within the neo cassette (Supplemental Fig. 2B (7.2MB, pdf) ). Four positive clones were identified among 144 G418-resistant clones. Clones were karyotyped to ensure diploidy, and two clones were expanded, injected into C57Bl/6 blastocysts, and transferred to B6CBAF1 recipient females. Germline chimeras were identified and transmission of the LtfCre verified by PCR (31, 32).
For derivation of the LifRflx/flx conditional mouse line, a double targeting strategy was used. Initially, the constructs pTV-LRup and and pTV-LRdown were electroporated individually to test their respective targeting efficiencies. Screening of 48 antibiotic-resistant clones using Kpn1 digestion for LRup (LifRflxH), which produced a 5.7-kb WT band, or a 4.1-kb mutant band and BamH1 digestion for LRdown (LifRflxH) that resulted a 3.9-kb WT band or a 5.1-kb recombinant band, was performed. The LRup (LifRflxH) electroporation produced 32 (67%) positive clones, whereas the LRdown (LifRflxNeo) electroporation produced 22 (46%) positive clones. The high rate of homologous recombination revealed that the LifR locus is easily targetable and that a simultaneous double-targeting strategy could be attempted. To this end, the ES cells were electroporated with a mixture containing both the pTV-LRup and pTV-LRdown targeting constructs. After 24 hours, ES cells were simultaneously selected with hygromycin (Invitrogen, Carlsbad, CA) and G418 (Invitrogen). Of 48 clones, five were confirmed to be positive by southern analysis for homologous recombination at both loci. To further ensure that both targeting vectors recombined with the LifR gene in cis and ensure that the loxP sites were functional, Cre recombinase was transiently expressed in the five ES clones by transfection of pMC1-Cre (a Cre cDNA expressed under the MC1 promoter, gift of K. Rajewsky). PCR was used to identify the deletion product of the floxed LifR. Two of five clones were injected into blastocysts to generate chimeras. Clone J8 produced germline offspring inheriting the conditional LifRflx/+ allele.
Maintenance and genotyping of mice
Germline transmission of the Ltf-Cre knock-in was confirmed by southern analysis, and mice were subsequently genotyped by PCR amplification of genomic DNA. Two pairs of PCR primers were used to genotype LtfCre (CB4) lines: Cre primers (Cre73 forward: 5′-GTGTCCAATTTACTGACCGTACACC-3′; Cre373 reverse: 5′-GACGATGAAGCATGTTTAGCTGG-3′) and Ltf primers (forward 5′-TGAGGTCACCCAGCACAGATAAAG-3′; Ltf intron1-5′-GAGAGGGTGAGATGTTCCTGTGAGTC), corresponding to sequences within the promoter through intron 1. The Ltf primers generate a 331-bp WT product or a 1.7-kb LtfCre (CB4; CreKI) product.
Mice containing the LifR+/flx alleles were identified by PCR using the following primers: LifR-CKO up-forward (U5) 5′-CCTTTTGCAGTCTCACAGGTCTTG-3′; Lifr-CKO up-reverse (U3) 5′-TGGAAAGCAGAGAAGCACAAGC-3′ to generate a 368-nucleotide (nt) WT product, a ∼2-kb hygromycin-containing fragment (Hygro-on; fH) or a 461-nt loxP-containing product (Hygro-off; fDH). Lifr-CKO down-forward (D5) 5′-gcccctttgtgacatactcgattc-3′; Lifr-CKO down-reverse (D3) 5′-TGGTAAGTCCAACTCAGCTAAGTGC-3′ to generate a 172-nt WT product, an ∼2-kb neo-containing fragment (fN) or a 272-nt loxP-containing fragment. In addition, using primers U5 together with D3, amplifies a 474-nt product in the null LifrΔ/Δ allele.
To delete the neo and hygromycin selectable markers, the LifrfH-fN/fH-fN mice were crossed with CB4 mice, and the resultant Lifrflx/+:LtfCre/+ mice were screened for loss of neo and hygromycin cassettes by PCR as described above. The deletion of the selectable markers leaves two loxP sites at introns 1 and 14 intact. To generate the complete Lifr deletion (LifrΔ/Δ), the Lifrflx/flx mice were crossed with the Ltf-Cre transgenic line (L4-Cre), used here as a general deletor to obtain LifrΔ/+:Cre/+. The Lifrflx/+:Ltf Cre/+ mice were then crossed with LifrΔ /+:Cre– mice to obtain Lifrflx/Δ::Ltf Cre/+. Females with this genotype were then used to determine the consequences of Lifr deletion in the LE. To determine the tissue specific expression of Cre activity in vivo LtfCre/Cre mice were crossed to the mouse reporter lines: Z/AP (33), Rosa26R3, and mT/mG (34).
Histology and immunohistochemistry
For whole-mount embryo analysis, embryos were fixed with 4% paraformaldehyde (PFA). β-Galactosidase activity in the Rosa26R3 crosses was detected after incubation with X-gal (5-bromo-4-chloro-3-indolyl-b-d-galactopyranoside; Invitrogen). Alkaline phosphatase activity in Z/AP mice was detected using BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium; Vector Laboratories) staining according to manufacturer’s protocol. Cre expression in the uterus was evaluated using the Gt (ROSA)26R3Sortm1Sor/J, Z/AP, and mT/mG mouse reporter lines. Frozen sections were fixed with 4% PFA and then stained with BCIP/NBT or by immunofluorescent analysis for mT or mG. Immunofluorescence analysis was performed on paraffin-embedded samples as previously described (17).
The effects of Lifr deficiency on glial cells of the central nervous system (CNS) and osteoclasts in bone were histologically characterized and by hematoxylin and eosin (H&E) staining with images being recorded on a Zeiss Axioimager. Paraffin-embedded thin sections of spinal cords (5 μm) were stained with an antibody against GFAP to identify glial cells and counterstained with Nissl stain. The osteoclasts in the long bones were identified by their unique morphology and size after H&E staining, performed using standard histological procedures.
Analysis of the CB4 mT/mEGFP reporter mice
The uteri from day 1 pregnant CB4+/+/TL +/+ or Tl +/+ mice were fixed in 4% PFA (Thermo Scientific, Rockford, IL) in phosphate-buffered saline (PBS) pH 7.4 for 24 hours, followed by fixation in 30% sucrose (Sigma, St Louis, MO) in PBS pH 7.4 for 24 hours at 4°C. Fixed uteri were cut into pieces and placed in tissue freezing medium (Electron Microscopy Sciences, Hatfield, PA) and frozen in cold heptane (Sigma) kept on dry ice. Cryosections of 10 μm were cut and stored at –70°C. For detecting membrane-targeted green fluorescent protein (mGFP) fluorescence, cryosections were dried completely at room temperature for 10 to 15 minutes. The sections were treated with chloroform: isoamyl acetate 24:1 (Sigma) for 10 seconds to eliminate background autofluorescence. The sections were dried and then rehydrated in PBS, pH 7.4, for 15 minutes. Sections were incubated in 1% sodium borohydride (Sigma) in PBS, pH 7.4, for 20 minutes followed by washing in PBS twice for 5 minutes each. The sections were treated with a mouse-on-mouse blocking reagent (Vector Laboratories, Burlingame, CA) for 1 hour and then washed in PBS twice for 5 minutes each to quench additional autofluorescence arising from antibody binding to blood vessels. The sections were incubated with DAPI (Invitrogen) to stain nuclei for 10 minutes and washed with PBS. Subsequently they were treated with 75 mM copper sulfate in ammonium acetate solution, pH 5.0 (Sigma), for 90 minutes to further decrease lipofuscin autofluorescence (35). The sections were washed in PBS and mounted in ProLong gold antifade mounting medium (Invitrogen) and viewed and photographed using a Zeiss LSM510 confocal microscope (Carl Zeiss, Jena, Germany).
Implantation analysis
For the analysis of blastocyst implantation, female mice with the desired genotype (Lifrflx/Δ: LtfCre/+) were derived and were mated with WT males of proven fertility. Plug dates were recorded. After the mice were plugged at least three times without becoming pregnant, they were killed after the fourth mating at 7 dpc. Uteri were collected and flushed to recover unimplanted blastocysts, and these were transferred to pseudopregnant recipients (7, 32).
Northern analysis
From a pool of three to five uteri, 10 µg of total RNA was prepared, according to the manufacturer’s instructions (Northern Max, Ambion), separated by electrophoresis in formaldehyde denaturing gels, and transferred onto Hybond-N+ membrane (Amersham). P-32–labeled cDNAs corresponding to Cre and lactoferrin were used as probes. After standard washing, the membranes were analyzed using a phosphorimager for 1 to 3 days.
In situ hybridization of uterine sections
Uteri were isolated and frozen in optimum cutting temperature and then sectioned at 10 µm and mounted on Superfrost slides (VWR International). In situ hybridization was performed as previously described (9). Sections were warmed to room temperature and fixed in 4% PFA. Sections were treated with Proteinase K and prehybridized in 50% (volume-to-volume ratio) formamide, 4×SSC, 5% dextran sulfate (weight-to-volume ration), 1× Denhardt’s solution, 0.25 mg/mL transfer RNA and heat-denatured salmon sperm DNA (0.5 mg/mL) for 3 hours at 42°C. A full-length Lifr cDNA clone was used to generate sense and antisense probes by in vitro transcription with (α-35S) uridine triphosphate. The hybridization was performed overnight with either S35-labeled sense or antisense probes. Slides were processed for liquid emulsion autoradiography and visualized using Kodak D-19 developer.
LIF treatment and Western analysis
LE from D3 pregnant mice was isolated from uteri by enzymatic digestion as previously described (15, 36). The purified LE was incubated at room temperature in freezing holding buffer (Specialty Media). LIF (Esgro, Millipore) was added and incubated for 2 hours. Proteins were extracted by lysis buffer containing 0.1% NP-40, 50 mM Tris-HCl, pH 7.5, 150 mM NaCl, and complete miniprotease inhibitor cocktail (Roche). Protein extracts were separated on 12% acrylamide/bisacrylamide gels and transferred to polyvinylidene difluoride membranes (Bio-Rad). Membranes were blocked in 5% nonfat dry milk in TTBS (0.1% Tween 20 in Tris-buffered saline) and incubated O/N with the following primary antibodies: pSTAT3 (Cell Signaling) and STAT3 (Cell Signaling) at 4°C. Loading was normalized using an antibody to glyceraldehyde 3-phosphate dehydrogenase. After washing in TTBS, membranes were exposed to horseradish peroxidase–conjugated secondary antibodies, incubated in enhanced chemiluminescence solution (Amersham), and processed for autoradiography on film (Kodak).
Results
Transgenic lactoferrin-directed Cre mice do not show Cre restriction to the LE
In the sexually mature uterine LE, lactoferrin (lactotransferrin, Ltf) is induced by E2, both during estrous and the first 3 days following ovulation. Its expression is then inhibited by a rise in P4 (27, 37, 38). To determine whether lactoferrin would be a tissue-specific promoter for Cre expression in the uterine LE, we initially generated a transgenic line, Ltf-Cre (Supplemental Fig. 1A (7.2MB, pdf) ) using an EcoRI-Sma1 fragment from the Ltf promoter (corresponding to sequences –2637 to –18; transcriptional start site = +1) that contains the E2-regulated element. Following pronuclear injection, we examined the offspring from six independently derived transgenic males. The offspring from all six males, when crossed to mice carrying the β-galactosidase (ROSA26R3Sortm1Sor/J) reporter gene, all showed ubiquitous expression of β-galactosidase in midgestation embryos (Supplemental Fig. 2A (7.2MB, pdf) ), indicating that the Ltf-Cre transgene was probably expressed very early in development. We retained one line, homozygous for the transgene, which subsequently served as a universal constitutive deleter line to generate the LifrΔ/Δ mice.
Insertion of Cre recombinase into the endogenous Ltf gene results in restricted Cre expression
Because the transgenic approach failed to produce mice with Cre expression restricted to the LE and GE, we then chose to “knock-in” the Cre recombinase gene into the Ltf gene so that Cre is under control of all Ltf regulatory elements. The vector for a knock-in of Cre recombinase was made by inserting a modified Cre recombinase cDNA (mnCre) within exon 1, downstream of the ATG start site of the Ltf gene (Supplemental Fig. 1 (7.2MB, pdf) and Supplemental Fig. 3A–E (7.2MB, pdf) ).
Four homologous recombinant ES clones (out of 144 clones) were identified and confirmed by southern analysis (Supplemental Fig. 3D (7.2MB, pdf) ). The resulting fusion protein was predicted to contain the initial 14 amino acids of Ltf followed by Cre sequence (Supplemental Fig. 3E (7.2MB, pdf) ). Homozygous Cre knock-in (LtfCre/Cre or CB4) mice were indistinguishable from WT siblings and did not display any overt phenotype, consistent with Ltf-null mice being viable (39). There was however some anemia and reduced levels of hemoglobin in pups derived from homozygous mothers (data not shown).
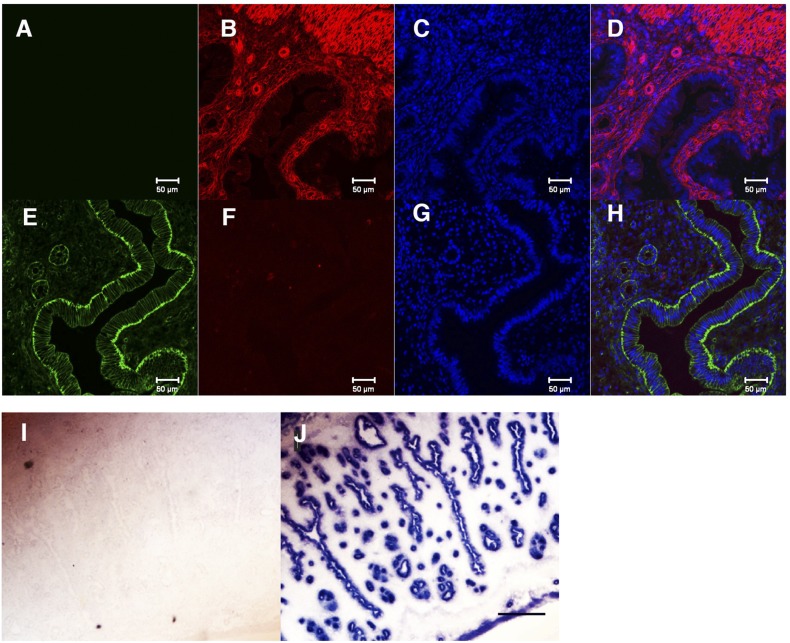
In contrast to the Ltf-Cre transgene, the Ltf-Cre insertion line (CB4) had a restricted expression profile. Northern blot analysis detected Cre recombinase transcripts and no Ltf transcripts in the uteri from homozygous CB4 mice (Supplemental Fig. 2B (7.2MB, pdf) ). To further evaluate the tissue-specific expression and activity, female CB4 mice were crossed with different reporter lines; the double-fluorescent mT/mG reporter Line (34), the β-galactosidase reporter mice Gt (ROSA26Sortm1Sor/J) (29) and the alkaline phosphatase Z/AP (33). As shown in Supplemental Fig. 2C (7.2MB, pdf) , E13 embryos generated from the Z/AP × CB4 cross, in contrast to the transgenic lines, did not show ubiquitous AP expression, but showed small discreet regions of AP expression that varied between individual embryos. CB4 female mice were then crossed with the dual reporter mT/mG male reporter mice to obtain a more precise definition of Cre activity in the different tissues, and specifically in the uterus of CB4 × mT/mG female offspring. The mT/mG mice constitutively express Tomato Red (mT) prior to Cre-mediated recombination. Following Cre introduction, expression of mT is lost and expression is switched to an mGFP, specifically in cells where Cre is functional. Strong mT expression was observed in the uterine stroma without CB4 [Fig. 1(B) and 1(D)]. After crossing with CB4 males, mG expression was only induced and localized at the LE membrane and more weakly in the GE membranes, as well as a few cells in the stroma that were probably neutrophils or macrophages expressing Ltf [Fig. 1(E) and (H)] (37). For unknown reasons, mT expression in the stroma was lost, but no mG expression was induced. In females prior to their first reproductive cycle (at ∼5 to 6 weeks) or in ovariectomized mice, mG expression was not induced in the LE or GE. However, following E2 injection (50 ng), mG was induced in the LE of ovariectomized mice (data not shown). Confirmation that Cre activity in the CB4 line was restricted to the uterine LE and GE was established by crossing the Cre mice to the β-galactosidase reporter mice Gt (ROSA26Sortm1Sor/J) where β-galactosidase activity was restricted to the LE and GE [Fig. 1(I) and 1(J)]. In other tissues from the mT/mG × CB4 mice, strong Cre activity was noted in the salivary glands, whereas in the liver (where the LIFR is highly expressed), no activity was detected. Weak activity was, however, detected in the ovarian stroma and, to a lesser extent, the heart myocardium, with no activity being detected in other adult tissues (Supplemental Fig. 5 (7.2MB, pdf) ).
Figure 1.
Cre reporter expression in CB4 × mT/mG mice is restricted to the uterine luminal and glandular epithelium. (A–D) Expression of mT of mT/mG mice is localized to the stromal and epithelial cells in uterus: (A) Control, no antibody; (B) tomato red; (C) 4′,6-diamidino-2-phenylindole; (D) merged. (E–H) Lactotransferrin-driven Cre (CB4) ablates mT expression in the LE and GE and induces mGFP specifically in the LE and GE. (E) mEGP; (F) mT; (G) 4′,6-diamidino-2-phenylindole; (H) merged. The reason that mT expression is lost in the stroma is unclear; however, mGFP is clearly restricted to the LE. Scale bar: 50 μm. (I and J) CB4 Cre induces β-galactosidase expression in the uterine GE and LE (I) β-gal+/+ expression in the absence of CB4; (J) CB4 × β-gal+/+.
Derivation Lifrflx/flx conditional allele
To ensure that as much of the Lifr gene could be deleted following recombination, loxP sites were introduced into Lifr gene flanking the exons encoding the extracellular domains the LIFR (Supplemental Fig. 4A–F (7.2MB, pdf) ). Mice homozygous for the floxed Lifr (Lifrflx/flx) allele and mice heterozygous with a deleted allele (LifrΔ/+or LifrΔ/flx) were viable and indistinguishable both in growth rate and fertility from their WT siblings.
We derived homozygous deleted LifrΔ/Δ mice by crossing the Lifrflx/flx mice with the Ltf-Cre constitutive deleter line and compared their phenotype to the phenotype of the previously described Lifr−/− mice (25). The Lifr−/− mice die perinataly, and viable LifrΔ/Δ offspring were identified at birth which then subsequently died within a few hours after birth (n = 38 newborns analyzed; P > 0.001). LifrΔ/Δ E16 and E19 embryos were identified (n = 7) at normal Mendelian ratios and showed no overt abnormalities apart from being smaller. Compared with WT littermates, LifrΔ/Δ mice were 8% (E14) and 27% (E19) lighter and 5% (E14) and 12% (E19) shorter in body length (data not shown). Such growth retardation may have been due defective placental development, as reported for the Lifr−/− mice (25), because the placenta expresses high levels of the LIFR. Lifr−/− mice have increased osteoclast numbers in the long bones and defects in glial cell differentiation in the CNS. We observed a similar reduction in glial cell numbers, by GFAP staining in both E16 and E19 LifrΔ/Δ embryos (Supplemental Fig. 6A (7.2MB, pdf) ). We also observed increased osteoclast numbers in the long bones, visualized by H&E staining (Supplemental Fig. 6B (7.2MB, pdf) ). Because of the perinatal lethality of the LifrΔ/Δ mice, the mice were maintained with a Lifrflx/flx genotype.
Loss of Lifr in the LE of Lifrflx/flx: LtfCre/Cre mice results in infertility
We derived Lifrflx/Δ:LtfCre/+ females to determine if LE/GE expression of the LIFR is required for implantation. Mice with either of these genotypes were, in size and overall health, indistinguishable from WT siblings, indicating that any non LE-GE expression of LtfCre/Cre did not significantly delete Lifrflx/flx in other adult tissues, such as the liver, where the LIFR is highly expressed (40) or during development, which would result in potential lethality.
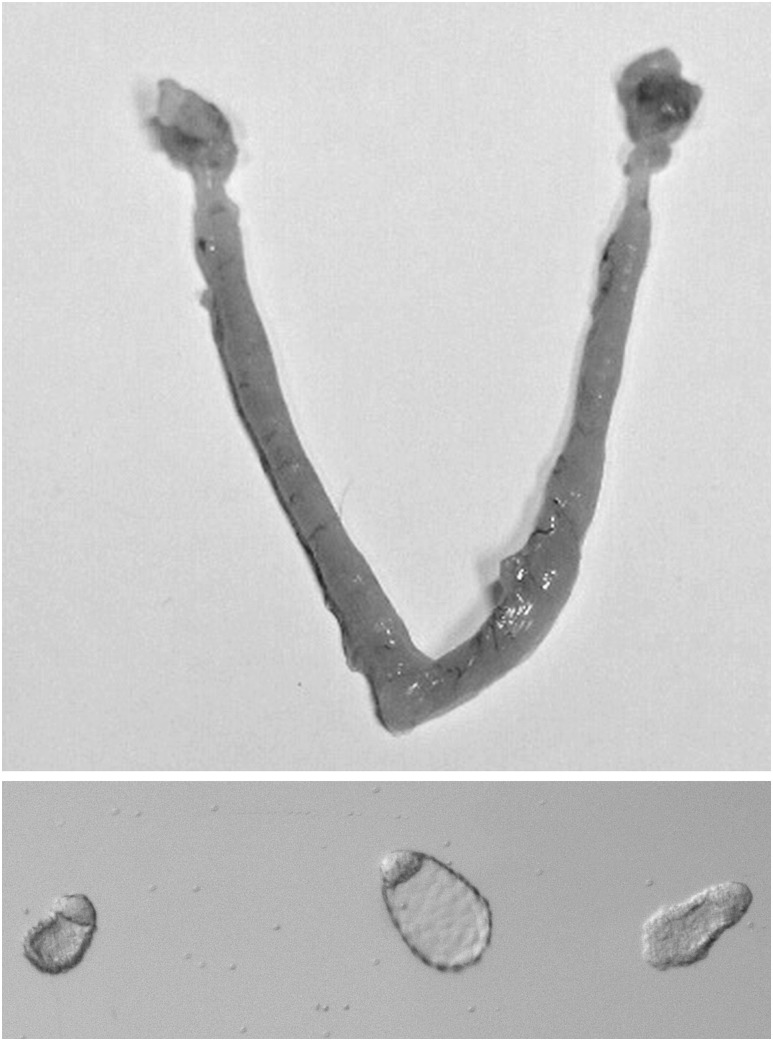
To analyze the consequences of Lifr loss in the LE on blastocyst implantation, females with the desired genotype (Lifrflx/Δ:LtfCre/+) were mated with WT males of proven fertility. Plug dates were recorded from a total of 11 females. After each of the females had mated for at least three cycles without becoming pregnant, a fourth mating was initiated. The females were then killed at 7 days after the last mating (7 dpc). Uteri were collected, examined for the presence of implantation sites and then flushed to isolate unimplanted blastocysts. From the 11 females, 27 hatched, unimplanted blastocysts were recovered from six of seven mice that showed no indication of implantation sites [Fig. 2(A)], indicating their uteri did not support implantation. The remaining four females showed evidence of implantation with a total of seven decidual swellings being identified in the four mice. The recovered blastocysts [Fig. 2(B)] were morphologically similar to normal blastocysts undergoing implantational delay, in that they were elongated and were not enclosed in a zona. To verify that the embryos were unaffected by loss of Lifr expression, the blastocysts were transferred to WT pseudopregnant recipients. Twelve blastocysts were transferred to pseudopregnant WT recipients, and seven pups were subsequently born. These data indicate that implantation failure in Lifr flx/Δ:LtfCre+ LE mice is a consequence of a defective uterus rather than defective blastocysts. The uteri from which the blastocysts were recovered showed no evidence of a decidual response or any indication that implantation had been initiated (data not shown).
Figure 2.
Unimplanted blastocysts isolated from the uteri of Lifrflx/Δ:LtfCre/+ mice. Upper panel: Lifrflx/Δ:LtfCre/+ uterus at 7 dpc showing no evidence of embryo implantation. Lower panel: Unimplanted blastocysts flushed from the uteri of a Lifrflx/Δ:LtfCre/+ female at day 7 after mating with a fertile male. The blastocysts are not enclosed by a zona pellucida and are morphologically typical for blastocysts undergoing implantational delay.
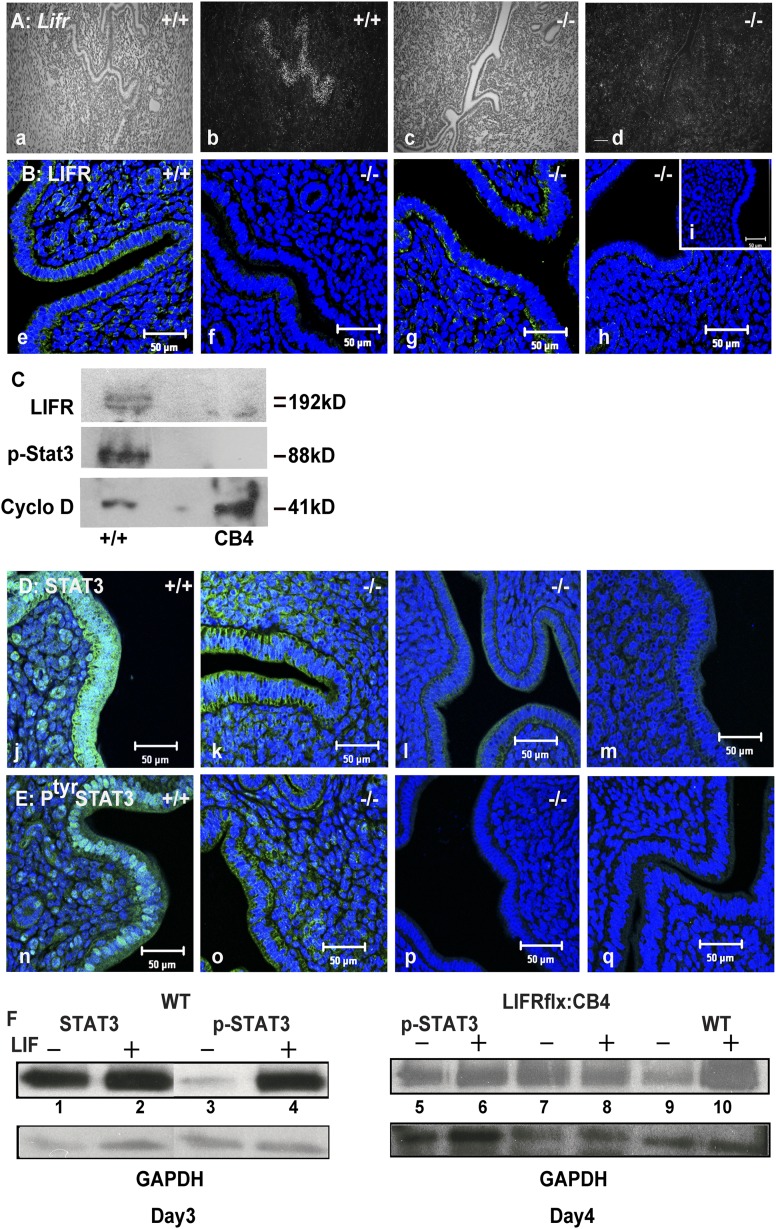
The fact that seven implantation sites were detected in four of the Lifr flx/Δ:LtfCre+ females, even after four rounds of mating, indicated that Ltf-Cre mediated deletion, may, occasionally not be fully penetrant. To analyze this, we preformed in situ hybridization and Immunostaining on uterine sections from the females. In situ hybridization, using a full-length Lifr cDNA as a probe, hybridized to Lifr transcripts in the LE of the WT uterus. The Lifr hybridization signal in the LE was weak on day 3 dpc, and increased in intensity between days 4 and 5 dpc, in agreement with previous reports (16, 17) [Fig. 3(A), panel b]. No signal was detected using the sense probe. Screening for the Lifr in Lifrflx/Δ:LtfCre/+mice revealed the complete absence of Lifr transcripts from three Lifr flx/Δ:LtfCre+ individuals [Fig. 3(A), panels c and d]. However, a substantial hybridization signal, indicative of Lifr transcripts, was found in the LE of the fourth mouse (not shown). These results were supported by western analysis and immunostaining of the LIFR protein [Fig. 3(B), panels e–h, and 3(C)] where LIFR was detectable in the basal and apical sides of the LE of WT mice 2 hours after an injection of nidatory E2 [Fig. 3(B), panel e], whereas in the LE from three Lifr−/−, it was either undetectable by immunostaining or western analysis in two individuals [Fig. 3(B), panels f and h, and 3(C)] and weakly expressed in the LE of another mouse [Fig. 3(B), panel g].
Figure 3.
Loss of LIFR expression in the uteri of Lifrflx/Δ:LtfCre/+ mice is associated with a failure to phosphorylate STAT3 in the luminal epithelium. (A) Lifr transcripts (by in situ hybridization) are absent in the LE in the Lifrflx/Δ:LtfCre/+ uteri on day 4 of gestation (panel a: WT bright field; panel b: WT dark field showing hybridization to the LE; panel c: Lifrflx/Δ:LtfCre/+ bright field; panel d: Lifrflx/Δ:LtfCre/+ dark field showing loss of the hybridization signal). (B) Immunostaining of LIFR expression in the LE shows loss of the LIFR in two ovariectomized mated Lifrflx/Δ:LtfCre/+ females 2 hours after E2 injection on day 4 after mating compared with WT controls (panel e: WT; panel f: D4 cLIFR−/− 1; panel g: D4 cLIFR−/− 2; panel h: day 7 cLIFR−/−; panel i: negative control). (C) Western analysis showing of LIFR and STAT3 in the LE. Isolated LE from Lifrflx/Δ:LtfCre/+ and WT mice day 4, 2 hours after E2 injection at 4 dpc; also shows loss of the two LIFR proteins and absence of pSTAT3 in the LE from Lifrflx/Δ:LtfCre/+ mice. CyclinD was used as a loading control. (D) Stat3 expression is reduced in the LE of Lifrflx/Δ:LtfCre/+ mice. There is no nuclear localization of STAT3 in the LE of ovariectomized Lifrflx/Δ:LtfCre/+ 4-dpc females 2 hours after E2 injection compared with WT (panel j: WT with strong STAT3 expression in the LE and GE; panel k: D4 cLIFR−/−; panel l: D7 cLIFR−/− 2; panel m: negative control). (E) Nuclear localization of pSTAT3 is absent in the LE of ovariectomized pregnant Lifrflx/Δ:LtfCre/+ females. Two hours after E2 injection at 4 dpc, WT LE shows pSTAT3 nuclear localization (panel n: WT showing strong nuclear localization; panel o: D4 cLIFR−/− 1; panel p: D7 cLIFR−/− 2; panel q: negative control). (F) Western analysis of STAT3 and pSTAT3 levels on extracts from the LE of WT and Lifrflx/Δ:LtfCre/+ mice on days 3 and 4 following LIF treatment of isolated LE. In the left panel (D3), there is robust expression of STAT3 in both treated and untreated WT LE (lanes 1 and 2). Following LIF treatment, pSTAT3 levels are increased (lanes 3 and 4). On the right, pSTAT3 levels in the Lifrflx/Δ:LtfCre/+ LE from day-4 uteri are reduced and do not increase following LIF treatment (lanes 5–8), as seen in WT LE (lanes 9 and 10). Glyceraldehyde 3-phosphate dehydrogenase was used as a loading control. Scale bars: 50 μm.
There was an apparent correlation between fertility (ability to support implantation) and detectable Lifr mRNA signal in the LE; the pregnant female was still LIFR positive, whereas the infertile ones showed no LIFR signal. These results indicate that Cre recombinase, under the control of the Ltf promoter in Lifrflx/Δ:LtfCre/+ females, was effective in the tissue-specific ablation of the Lifr in the majority of mice analyzed.
STAT3 activation by LIF is inhibited in the Lifrflx/Δ:LtfCre+ LE
The binding of LIF to the LIFR/gp130 receptor complex activates the JAK/STAT pathway (41). Previously, we demonstrated that STAT3 is constitutively expressed in the LE during the preimplantation period but is only phosphorylated and translocates to the nuclei in the LE, but not the GE, in response to LIF treatment on days 3 and 4 after mating (15, 17). To determine whether STAT3 is phosphorylated in the LE of Lifrflx/Δ:LtfCre/+ mice, we performed western and immunofluorescence analysis on whole uteri and LE extracts from mice on days 3 and 4 after mating [Fig. 3(D–F)]. We isolated and purified LE from WT females at 3 dpc and incubated the LE with LIF as previously described (15). Treatment of the WT LE, with LIF, resulted in a robust increase of phosphorylated Stat3 [Fig. 3(F), lanes 1–4]. As with previous observations, the overall levels of STAT3 did not change in response to LIF treatment, but pSTAT3 levels were strongly increased [Fig. 3(F), lane 4] (15). Extracts of LE from WT and Lifrflx/Δ:LtfCre/+mice from 4 dpc were analyzed following LIF treatment using an antibody specific for pSTAT3. Although treatment of WT LE with LIF resulted in STAT3 phosphorylation (Fig. 3, lanes 9 and 10), in the Lifrflx/Δ:LtfCre/+, LE extracts STAT3 phosphorylation did not change [Fig. 3(F), lanes 5–8].
These results were confirmed by Immunostaining uteri from ovariectomized WT and Lifrflx/Δ:LtfCre/+ mice 2 hours after the injection of nidatory E2 (n = 3 each genotype). STAT3 protein was detected in the cytoplasm of WT and Lifrflx/Δ:LtfCre/+ LE at varying levels [Fig. 3(D), panels j–m]; however, only in the WT LE was pSTAT3 localization to the nuclei detected [Fig. 3(E), panel n]—not in the LE nuclei from the Lifrflx/Δ:LtfCre/+ mice [Fig. 3(E), panels o–q].
MSX1 uterine expression persists in the Lifrflx/Δ:LtfCre/+ LE
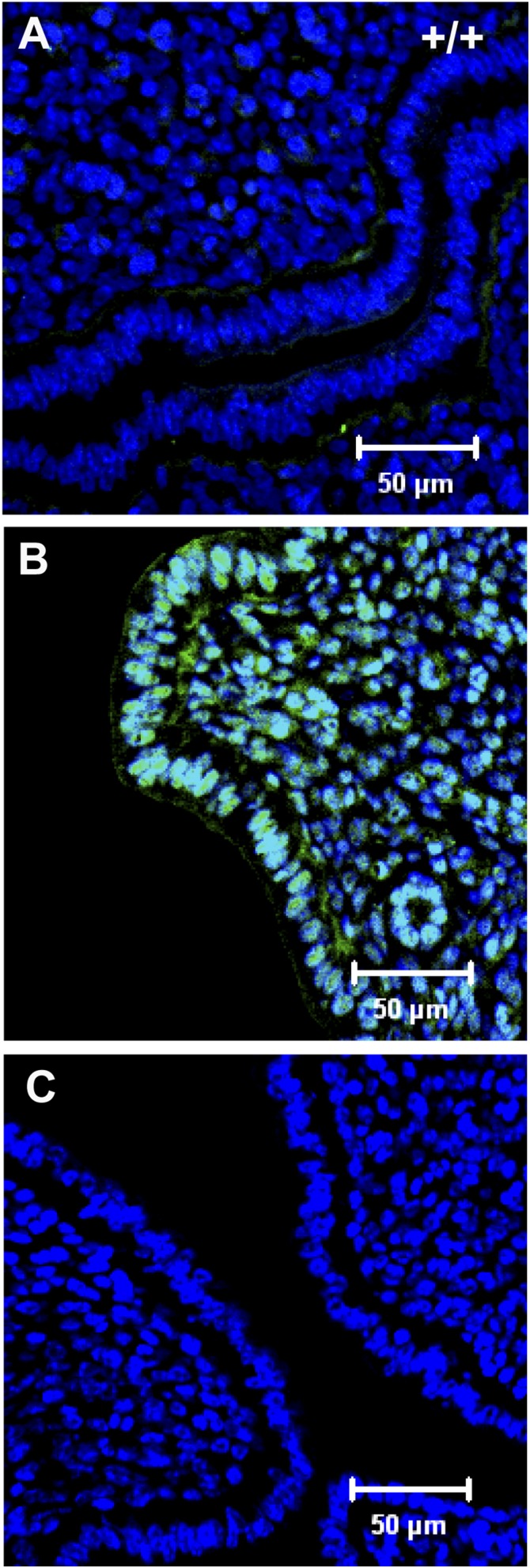
One of the genes whose expression is inhibited in the LE by LIF is the homeobox gene Msx1 (17, 42, 43, 44). In WT mice on day 4 dpc, MSX1 expression is detectable in the uterine LE (42, 44). However, within 2 to 3 hours after either E2 or LIF injection, MSX1 expression is completely suppressed [Fig. 4(A)]. In the Lifrflx/Δ:LtfCre/+ uteri, MSX1 expression was sustained and even appeared to have spread into some stromal cell nuclei [Fig. 4(B)] by 3 hours after E2 injection. This supports previous findings that LIF induction on day 4 suppresses MSX1 expression and that this suppression is necessary to render the uterus receptive to implanting blastocysts.
Figure 4.
MSX1 expression is disrupted in the uteri of Lifrflx/Δ:LtfCre/+ mice after E2 induction. (A) Two hours after E2 injection at 4 dpc, MSX1 expression has disappeared from the WT uterus. (B) In the uteri of Lifrflx/Δ:LtfCre/+ mice, MSX1 expression persists in the LE and has extended in stromal cells. (C) Negative control.
Discussion
Embryo implantation is a highly complex yet coordinated process that is driven by the ovarian steroid hormones E2 and P4. These hormones operate by activation of many autocrine and paracrine signaling molecules in different tissue compartments of the uterus. Among these signaling molecules, the cytokine LIF is essential. LIF’s action on the LE results in the change in the expression of at least 50 transcription factors within 1 hour of LIF treatment. These transcription factors, in turn, activate a multitude of physiological pathways (17, 45). Analysis of how these different factors act in concert, resulting in successful blastocyst implantation, has been hampered by the lack of an appropriate in vitro system to dissect the molecular and cellular events. Consequently, it has been necessary to resort to studying the process in vivo, primarily through genetic routes in which mutations are introduced into genes suspected of being important to implantation. However, deletion of many of these genes results in embryonic or developmental abnormalities that prevent female mice from reaching a reproductive maturity. This issue can be sidestepped using the Cre-Lox system to conditionally regulate gene expression.
At least five Cre lines of mice have been derived to specifically delete genes in the uterus, these being PR-Cre (46), Amhr2-Cre (47), Wnt7a-Cre (48), Sprr2f-Cre (49), and recently, another Ltf line, Ltf-iCre (50). Each of these lines has advantages and disadvantages at directing gene ablation in different cellular compartments. A major issue with many of these lines is that the expression of the genes driving Cre may not be restricted to a specific cell type in the uterus. For instance, PR is expressed in all cellular compartments in the uterus, as well the ovary, mammary gland, and pituitary, so making it difficult to define gene function in uterine biology, if the floxed gene is expressed in additional cell types. Furthermore, some of these genes are expressed, either during embryogenesis or in the maturing postnatal uterus, and so disrupting uterine development rather than physiological function during adult reproduction.
Here, we have described the derivation of an Ltf-Cre line (CB4) and showed that it can be used to specifically ablate gene expression in the uterine LE and GE. The mouse line, CB4 (LtfCre/Cre), has Cre recombinase that was inserted into the lactoferrin (Ltf) gene, so placing Cre expression under control of the E2/P4 regulated Ltf promoter. We used the CB4 line to specifically delete a floxed conditional allele of the LIF receptor in the uterine LE. LIF initiates implantation by binding to the heterodimeric receptor comprised of the LIFR and gp130 proteins that are expressed in the LE. Binding of LIF to the heterodimeric receptor activates the JAK/STAT signaling pathway in the LE (15, 17, 18, 41), resulting in the LE becoming receptive to the juxtaposed blastocysts. Determining the requirement for the LIFR in implantation has been hindered by the fact that Lifr-nulls die at birth, so preventing the analysis of LIFR function in different adult tissues (25).
In the LE and GE of Lifrflx/Δ:LtfCre/+ mice, Lifr transcripts and protein were eliminated, indicating that the Ltf–Cre resulted in deletion the Lifrflx/Δ alleles. The majority of the Lifrflx/Δ:LtfCre/+ females, despite repeated mating with males of proven fertility, did not become pregnant or produce offspring. These females were carrying unimplanted blastocysts, and following transfer to pseudopregnant WT recipients, the majority of these blastocysts implanted and developed to term, a result identical to that seen in the Lif-null mice (7). These results demonstrate that LIFR and GP130 expression in the LE, are the signaling receptor components required by LIF to initiate the onset of LE receptivity followed by embryo implantation.
Lifr transcript loss in the LE was, in a few mice, incomplete, with some residual Lifr signal persisting in about 15% of the LE cells. Such mosaic expression may well account for some Lifrflx/Δ:LtfCre/+ LE females being subfertile, with a reduced number of implantation sites. Several reasons may account for the apparent partial loss, including insufficient levels of expression of Cre from the Ltf promoter, Ltf expression varying between individual LE cells and the distance between the loxP sites in the floxed Lifr allele, reducing the efficiency of recombination. However, the patchy expression of Cre activity, both in embryos and in the uterus, as revealed in the reporter mice, suggests that variable expression of Cre is a possibility. These observations suggest that LIFR expression in a few LE cells may be sufficient to allow for the implantation of a few blastocysts, probably in the regions of the LE where LIFR was still expressed, suggesting that changes in the LE in response to LIF that allow embryo implantation are highly localized and do not “spread” to neighboring cells.
Many studies have shown that deletion, mutation, or pharmacological inhibition of either the Stat3 or the Gp130 genes results in female infertility and implantation failure. Gp130-null mice die in utero due to defective cardiac and vascular development. However, a mouse line homozygous for a truncation of a C-terminal region of gp130, which eliminates the STAT3 binding and activation site, has defective implantation, similar to the LIF-deficient mice (19). Pharmacological inhibition of the JAK/STAT pathway has also supported the notion that STAT3 and JAK are required for implantation (21, 51). Recently, the conditional deletion of either Stat3 or Gp130 in the uterus using the PgrCre/+ was reported (23, 52). Loss of either factor resulted in female infertility and implantation failure confirming their requirement for implantation, although because Pgr is expressed in both the uterine epithelium and stroma, this makes it difficult to interpret which tissue is affected by loss of either factor.
Stimulation of the WT-LE with LIF resulted in efficient phosphorylation of STAT3 and nuclear translocation in the WT LE, but not in LE from the Lifrflx/Δ:LtfCre/+ mice, where pSTAT3 levels were reduced and did not show increased phosphorylation following LIF treatment. The reduced levels of STAT3 and phosphorylation dynamics may have been partly due to a breakdown in the autoregulation of STAT3 levels due to a failure to increase expression of the negative regulator SOCS3 that inhibits STAT3 phosphorylation and that is transcriptionally induced by pSTAT3. The failure to activate the JAK-STAT pathway in the LE probably was responsible for the persistent expression of the homeobox gene MSX1, in which LIF mediated suppression of MSX1 expression is required for the uterus to become receptive (42). Together with previous reports, these findings demonstrate the centrality of the LIF-Lifr/gp130-JAK/STAT3 signaling pathway in regulating uterine receptivity and implantation.
Based on our reporter analysis, the CB4 Ltf-Cre line was largely effective in expressing Cre recombinase in the LE and in deleting LifR function in this cellular compartment. However, Ltf-Cre is strongly expressed in other tissues, especially the salivary gland and more weakly in the heart. Ablation of the Lifr in these tissues would probably not affect reproductive functions in the uterus. We also compared the effectiveness of the Wnt7a-Cre line at deleting the LifR in the uterus and we were only able to derive one female (out of 75 offspring tested) that was of the Lifrflx/Δ: Wnt7a Cre/+ genotype, whereas we derived 21 Lifrflx/Δ:LtfCre/+ females from 81 offspring. This may have been due (based on our observation) to Wnt7a –Cre expression in the developing CNS, so ablating LifR that is essential to the developing CNS (data not shown). Cell-specific ablation of any gene of interest in the uterus may therefore require the judicious choice of a specific line of Cre mice.
Although all these results have been obtained primarily in mice, they are relevant to human reproductive health. In women with unexplained fertility defects, altered expression of soluble gp130 has been reported (20), and reduced fertility is associated with specific polymorphisms in the transcription factor p53 and its regulator Mdm2 affecting the levels of LIF expression in the uterus (23, 53). In summary, our results show that defective loss and/or function of the LIFR in the LE adversely results in reduced numbers of implanting blastocysts. The levels of LIFR expression and its functional activity in the LE change during the preimplantation phase and suggest that incorrect expression/function of the LIFR during reproductive cycles maybe an additional factor contributing to infertility in human reproduction.
Acknowledgments
We thank Ms. Debbie Swing National Cancer Institute at Frederick for help generating transgenic mouse lines. This work was funded in part by the Intramural Research Program of the National Cancer Institute and by the Singapore Agency for Science, Technology and Research (A*STAR).
Current affiliation: C.L. Stewart’s current affiliation is the Institute of Medical Biology, 138648 Singapore.
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog No., and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| LIFR (C19) | Anti-LIFR | Santacruz Biotechnology, sc-659 | Rabbit; polyclonal | 1:200 to 500 | AB_2136105 | |
| Phospho Y705 STAT3 | Anti-phospho Y705 STAT3 | Bioworld Technology, BS4181 | Rabbit; polyclonal | 1:75 to 750 | AB_1664102 | |
| STAT3 (79D7) | Anti-STAT3 | Cell Signaling, catalog no. 4904S | Rabbit; monoclonal | 1:50 | AB_331269 | |
| MSX1 | Anti-MSX1 | Abcam, ab73883 | Mouse; monoclonal | 1:500 | AB_1269479 | |
| Goat anti-rabbit IgG secondary | Anti-rabbit HRP | Dako, P0448 | Goat; polyclonal | 1:50 | AB_2617138 | |
| Alexa fluor secondary Ab | Anti-rabbit | A-11034 | Goat; polyclonal | 1:10 | AB_2576217 |
Abbreviations: Ab, antibody; HRP, horseradish peroxidase; IgG, immunoglobulin G; RRID, Research Resource Identifier.
Footnotes
- BAC
- bacterial artificial chromosome
- cDNA
- complementary DNA
- CNS
- central nervous system
- dpc
- days postcoitum
- E2
- estrogen
- ES
- embryonic stem
- GE
- endometrial gland
- H&E
- hematoxylin and eosin
- Jak
- Janus kinase
- LE
- luminal epithelium
- LIF
- leukemia inhibitory factor
- LIFR
- leukemia inhibitory factor receptor
- Ltf
- lactoferrin gene
- mGFP
- membrane-targeted green fluorescent protein
- neo
- neomycin
- nt
- nucleotide
- P4
- progesterone
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- PFA
- paraformaldehyde
- pSTAT3
- phosphorylated dimers of signal transducer and activator of transcription 3
- STAT
- signal transducer and activator of transcription
- TK
- thymidine kinase
- WT
- wild-type.
References
- 1.Abrahamsohn PA, Zorn TM. Implantation and decidualization in rodents. J Exp Zool. 1993;266(6):603–628. [DOI] [PubMed] [Google Scholar]
- 2.Finn CA, Martin L. The control of implantation. J Reprod Fertil. 1974;39(1):195–206. [DOI] [PubMed] [Google Scholar]
- 3.Zhu L, Pollard JW. Estradiol-17beta regulates mouse uterine epithelial cell proliferation through insulin-like growth factor 1 signaling. Proc Natl Acad Sci USA. 2007;104(40):15847–15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Sun X, Dey SK. Entosis allows timely elimination of the luminal epithelial barrier for embryo implantation. Cell Reports. 2015;11(3):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Denker HW. Implantation: a cell biological paradox. J Exp Zool. 1993;266(6):541–558. [DOI] [PubMed] [Google Scholar]
- 6.Large MJ, DeMayo FJ. The regulation of embryo implantation and endometrial decidualization by progesterone receptor signaling. Mol Cell Endocrinol. 2012;358(2):155–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Köntgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359(6390):76–79. [DOI] [PubMed] [Google Scholar]
- 8.Shen MM, Leder P. Leukemia inhibitory factor is expressed by the preimplantation uterus and selectively blocks primitive ectoderm formation in vitro. Proc Natl Acad Sci USA. 1992;89(17):8240–8244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt H, Brunet LJ, Stewart CL. Uterine expression of leukemia inhibitory factor coincides with the onset of blastocyst implantation. Proc Natl Acad Sci USA. 1991;88(24):11408–11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen JR, Cheng JG, Shatzer T, Sewell L, Hernandez L, Stewart CL. Leukemia inhibitory factor can substitute for nidatory estrogen and is essential to inducing a receptive uterus for implantation but is not essential for subsequent embryogenesis. Endocrinology. 2000;141(12):4365–4372. [DOI] [PubMed] [Google Scholar]
- 11.Hu W, Feng Z, Teresky AK, Levine AJ. p53 Regulates maternal reproduction through LIF. Nature. 2007;450:721–724. [DOI] [PubMed] [Google Scholar]
- 12.Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci USA. 1996;93(7):3115–3120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kang HJ, Feng Z, Sun Y, Atwal G, Murphy ME, Rebbeck TR, Rosenwaks Z, Levine AJ, Hu W. Single-nucleotide polymorphisms in the p53 pathway regulate fertility in humans. Proc Natl Acad Sci USA. 2009;106(24):9761–9766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng JG, Rodriguez CI, Stewart CL. Control of uterine receptivity and embryo implantation by steroid hormone regulation of LIF production and LIF receptor activity: towards a molecular understanding of “the window of implantation.” Rev Endocr Metab Disord. 2002;3(2):119–126. [DOI] [PubMed] [Google Scholar]
- 15.Cheng JG, Chen JR, Hernandez L, Alvord WG, Stewart CL. Dual control of LIF expression and LIF receptor function regulate Stat3 activation at the onset of uterine receptivity and embryo implantation. Proc Natl Acad Sci USA. 2001;98(15):8680–8685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131(2):341–349. [DOI] [PubMed] [Google Scholar]
- 17.Rosario GX, Hondo E, Jeong JW, Mutalif R, Ye X, Yee LX, Stewart CL. The LIF-mediated molecular signature regulating murine embryo implantation. Biol Reprod. 2014;91(3):66. [DOI] [PubMed] [Google Scholar]
- 18.Auernhammer CJ, Melmed S. Leukemia-inhibitory factor-neuroimmune modulator of endocrine function. Endocr Rev. 2000;21(3):313–345. [DOI] [PubMed] [Google Scholar]
- 19.Ernst M, Inglese M, Waring P, Campbell IK, Bao S, Clay FJ, Alexander WS, Wicks IP, Tarlinton DM, Novak U, Heath JK, Dunn AR. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194(2):189–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherwin JR, Smith SK, Wilson A, Sharkey AM. Soluble gp130 is up-regulated in the implantation window and shows altered secretion in patients with primary unexplained infertility. J Clin Endocrinol Metab. 2002;87(8):3953–3960. [DOI] [PubMed] [Google Scholar]
- 21.Catalano RD, Johnson MH, Campbell EA, Charnock-Jones DS, Smith SK, Sharkey AM. Inhibition of Stat3 activation in the endometrium prevents implantation: a nonsteroidal approach to contraception. Proc Natl Acad Sci USA. 2005;102(24):8585–8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohamet L, Heath JK, Kimber SJ. Determining the LIF-sensitive period for implantation using a LIF-receptor antagonist. Reproduction. 2009;138(5):827–836. [DOI] [PubMed] [Google Scholar]
- 23.Sun X, Bartos A, Whitsett JA, Dey SK. Uterine deletion of Gp130 or Stat3 shows implantation failure with increased estrogenic responses. Mol Endocrinol. 2013;27(9):1492–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeda K, Noguchi K, Shi W, Tanaka T, Matsumoto M, Yoshida N, Kishimoto T, Akira S. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94(8):3801–3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ware CB, Horowitz MC, Renshaw BR, Hunt JS, Liggitt D, Koblar SA, Gliniak BC, McKenna HJ, Papayannopoulou T, Thoma B, Cheng L, Donovan PJ, Peschon JJ, Bartlett PF, Willis CR, Wright BD, Carpenter, MK, Davison BL, Gearing DP. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121(5):1283–1299. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida K, Taga T, Saito M, Suematsu S, Kumanogoh A, Tanaka T, Fujiwara H, Hirata M, Yamagami T, Nakahata T, Hirabayashi T, Yoneda Y, Tanaka K, Wang WZ, Mori C, Shiota K, Yoshida N, Kishimoto T. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93(1):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng CT. Factors regulating lactoferrin gene expression. Biochem Cell Biol. 2006;84(3):263–267. [DOI] [PubMed] [Google Scholar]
- 28.National Research Council, Division on Earth and Life Studies, Institute for Laboratory Animal Research, Commission on Life Sciences. Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press; 1996.
- 29.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21(1):70–71. [DOI] [PubMed] [Google Scholar]
- 30.Chambers I, Cozens A, Broadbent J, Robertson M, Lee M, Li M, Smith A. Structure of the mouse leukaemia inhibitory factor receptor gene: regulated expression of mRNA encoding a soluble receptor isoform from an alternative 5′ untranslated region. Biochem J. 1997;328(Pt 3):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Köntgen F, Stewart CL. Simple screening procedure to detect gene targeting events in embryonic stem cells. Methods Enzymol. 1993;225:878–890. [DOI] [PubMed] [Google Scholar]
- 32.Stewart CL. Production of chimeras between embryonic stem cells and embryos. Methods Enzymol. 1993;225:823–855. [DOI] [PubMed] [Google Scholar]
- 33.Lobe CG, Koop KE, Kreppner W, Lomeli H, Gertsenstein M, Nagy A. Z/AP, a double reporter for cre-mediated recombination. Dev Biol. 1999;208(2):281–292. [DOI] [PubMed] [Google Scholar]
- 34.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45(9):593–605. [DOI] [PubMed] [Google Scholar]
- 35.Schnell SA, Staines WA, Wessendorf MW. Reduction of lipofuscin-like autofluorescence in fluorescently labeled tissue. J Histochem Cytochem. 1999;47(6):719–730 [DOI] [PubMed] [Google Scholar]
- 36.Bigsby RM, Cooke PS, Cunha GR. A simple efficient method for separating murine uterine epithelial and mesenchymal cells. Am J Physiol. 1986;251(5 Pt 1):E630–E636. [DOI] [PubMed] [Google Scholar]
- 37.McMaster MT, Teng CT, Dey SK, Andrews GK. Lactoferrin in the mouse uterus: analyses of the preimplantation period and regulation by ovarian steroids. Mol Endocrinol. 1992;6(1):101–111. [DOI] [PubMed] [Google Scholar]
- 38.Teng CT, Liu Y, Yang N, Walmer D, Panella T. Differential molecular mechanism of the estrogen action that regulates lactoferrin gene in human and mouse. Mol Endocrinol. 1992;6(11):1969–1981. [DOI] [PubMed] [Google Scholar]
- 39.Ward PP, Mendoza-Meneses M, Cunningham GA, Conneely OM. Iron status in mice carrying a targeted disruption of lactoferrin. Mol Cell Biol. 2003;23(1):178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hilton DJ, Nicola NA, Waring PM, Metcalf D. Clearance and fate of leukemia-inhibitory factor (LIF) after injection into mice. J Cell Physiol. 1991;148(3):430–439. [DOI] [PubMed] [Google Scholar]
- 41.Nicola NA, Babon JJ. Leukemia inhibitory factor (LIF). Cytokine Growth Factor Rev. 2015;26(5):533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daikoku T, Cha J, Sun X, Tranguch S, Xie H, Fujita T, Hirota Y, Lydon J, DeMayo F, Maxson R, Dey SK. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev Cell. 2011;21(6):1014–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nallasamy S, Li Q, Bagchi MK, Bagchi IC. Msx homeobox genes critically regulate embryo implantation by controlling paracrine signaling between uterine stroma and epithelium. PLoS Genet. 2012;8(2):e1002500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daikoku T, Song H, Guo Y, Riesewijk A, Mosselman S, Das SK, Dey SK. Uterine Msx-1 and Wnt4 signaling becomes aberrant in mice with the loss of leukemia inhibitory factor or Hoxa-10: evidence for a novel cytokine-homeobox-Wnt signaling in implantation. Mol Endocrinol. 2004;18(5):1238–1250. [DOI] [PubMed] [Google Scholar]
- 45.Rosario GX, Stewart CL. The multifaceted actions of leukaemia inhibitory factor in mediating uterine receptivity and embryo implantation. Am J Reprod Immunol. 2016;75(3):246–255. [DOI] [PubMed] [Google Scholar]
- 46.Soyal SM, Mukherjee A, Lee KY, Li J, Li H, DeMayo FJ, Lydon JP. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41(2):58–66. [DOI] [PubMed] [Google Scholar]
- 47.Arango NA, Kobayashi A, Wang Y, Jamin SP, Lee HH, Orvis GD, Behringer RR. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75(7):1154–1162. [DOI] [PubMed] [Google Scholar]
- 48.Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107(45):19272–19277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Contreras CM, Akbay EA, Gallardo TD, Haynie JM, Sharma S, Tagao O, Bardeesy N, Takahashi M, Settleman J, Wong KK, Castrillon DH. Lkb1 inactivation is sufficient to drive endometrial cancers that are aggressive yet highly responsive to mTOR inhibitor monotherapy. Dis Model Mech. 2010;3(3-4):181–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Daikoku T, Ogawa Y, Terakawa J, Ogawa A, DeFalco T, Dey SK. Lactoferrin-iCre: a new mouse line to study uterine epithelial gene function. Endocrinology. 2014;155(7):2718–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakamura H, Kimura T, Koyama S, Ogita K, Tsutsui T, Shimoya K, Taniguchi T, Koyama M, Kaneda Y, Murata Y. Mouse model of human infertility: transient and local inhibition of endometrial STAT-3 activation results in implantation failure. FEBS Lett. 2006;580(11):2717–2722. [DOI] [PubMed] [Google Scholar]
- 52.Lee JH, Kim TH, Oh SJ, Yoo JY, Akira S, Ku BJ, Lydon JP, Jeong JW. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 2013;27(7):2553–2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Subramani E, Madogwe E, Ray CD, Dutta SK, Chakravarty B, Bordignon V, Duggavathi R, Chaudhury K. Dysregulated leukemia inhibitory factor and its receptor regulated signal transducers and activators of transcription 3 pathway: a possible cause for repeated implantation failure in women with dormant genital tuberculosis? Fertil Steril. 2016;105(4):1076–1084.e5. [DOI] [PubMed] [Google Scholar]