Abstract
The α-subunit of the heterotrimeric Gz protein, Gαz, promotes β-cell death and inhibits β-cell replication when pancreatic islets are challenged by stressors. Thus, we hypothesized that loss of Gαz protein would preserve functional β-cell mass in the nonobese diabetic (NOD) model, protecting from overt diabetes. We saw that protection from diabetes was robust and durable up to 35 weeks of age in Gαz knockout mice. By 17 weeks of age, Gαz-null NOD mice had significantly higher diabetes-free survival than wild-type littermates. Islets from these mice had reduced markers of proinflammatory immune cell infiltration on both the histological and transcript levels and secreted more insulin in response to glucose. Further analyses of pancreas sections revealed significantly fewer terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL)-positive β-cells in Gαz-null islets despite similar immune infiltration in control mice. Islets from Gαz-null mice also exhibited a higher percentage of Ki-67–positive β-cells, a measure of proliferation, even in the presence of immune infiltration. Finally, β-cell–specific Gαz-null mice phenocopy whole-body Gαz-null mice in their protection from developing hyperglycemia after streptozotocin administration, supporting a β-cell–centric role for Gαz in diabetes pathophysiology. We propose that Gαz plays a key role in β-cell signaling that becomes dysfunctional in the type 1 diabetes setting, accelerating the death of β-cells, which promotes further accumulation of immune cells in the pancreatic islets, and inhibiting a restorative proliferative response.
β-cell Gαz contributes to β-cell death and dysfunction and blocks replicative capacity in the immune-mediated nonobese diabetic (NOD) mouse model, promoting T1DM-like pathophysiology.
Type 1 diabetes mellitus (T1DM) is a result of insulin deficiency arising from immune-mediated β-cell destruction. In the US population alone, it is expected that the prevalence will increase more than 144% by 2050, which means that T1DM will be diagnosed in more than 500,000 individuals in the next 35 years (1, 2).
T1DM pathogenesis targets the β-cells in the islets of Langerhans, which are the sole producers of insulin and are necessary to maintain normoglycemia. Although the precise series of events that result in T1DM onset has not been fully elucidated, factors such as genetic susceptibility due to single nucleotide polymorphisms in the various major histocompatibility complex (MHC) loci, viral infection, endoplasmic reticulum stress, and neonatal β-cell apoptosis have all been reported to trigger T1DM development (3–7). Apoptosis of β-cells due to one or more of these initiating events can result in the release of cell debris and can facilitate immune cell exposure to β-cell antigens. Islet inflammation results in both nonspecific and targeted β-cell death, including macrophage secretion of cytokines that results in activation of nuclear factor κB (NF-κB)–mediated apoptotic pathways and granzyme- and perforin-mediated β-cell death facilitated by antigen-specific cluster of differentiation 8 (CD8)–positive T-cells (8). T1DM presents only after β-cell mass drops below the threshold necessary to maintain euglycemia (9).
We previously showed that activation of the inhibitory G protein–coupled E prostanoid receptor EP3 is highly induced in diabetic islets and plays a negative role in glucose- and hormone-stimulated insulin secretion (10, 11). We also showed that in pancreatic β-cells, EP3 is specifically coupled to the inhibitory G protein Gz, whose catalytic α-subunit, when activated, inhibits adenylate cyclase, reducing cyclic adenosine monophosphate production and blunting glucose-stimulated insulin secretion (GSIS) (10, 12–14). Nevertheless, cyclic adenosine monophosphate also has known effects on β-cell replication and survival. We showed that mice lacking Gαz are protected from developing glucose intolerance after high-fat diet feeding (15) and are resistant to multiple low-dose streptozotocin (MLD-STZ)–induced diabetes when concurrently treated with a low dose of the GLP-1 mimetic exendin-4 (Ex4) (16). Protection from high-fat diet–induced glucose intolerance appeared to be due primarily to increased β-cell replication, resulting in augmented functional β-cell mass, whereas protection from MLD-STZ resulted from both increased β-cell replication and decreased β-cell apoptosis, leading to preserved functional β-cell mass (15, 16). In addition, active Gαz was able to augment β-cell death as stimulated by the proinflammatory cytokine interleukin 1β (IL-1β) (16), suggesting that it plays a critical role in the pathophysiology of immune-mediated β-cell destruction.
The nonobese diabetic (NOD) mouse is a well-accepted T1DM model that mimics the insulin-dependent diabetes mellitus seen in humans (17, 18). Although both male and female NOD mice present with insulitis, 70% to 80% of female NOD mice typically become hyperglycemic between 12 and 16 weeks of age as an initial wave of immune infiltration stimulates β-cell death, ultimately decreasing functional β-cell mass (19). Because our previous data supported the finding that loss of Gαz can affect β-cell proliferation, function, and demise in the context of β-cell stressors, we hypothesized that genetic deficiency of Gαz could also alter T1DM progression. Thus, we investigated the consequences of the loss of Gαz in NOD mice.
Research Design and Methods
Mouse breeding and phenotyping
C57BL/6N mice containing a genomic insertion of a pGKneoR cassette at 160 base pairs downstream of the translation start site of the Gαz gene (gene symbol: Gnaz) have been described and characterized previously (15). These mice were backcrossed onto the NOD background for at least 10 generations and were single nucleotide polymorphism–genotyped by Jackson Laboratories to ensure that the background was 100% NOD, save for the genomic regions surrounding the cassette inserted into the Gnaz gene. Breeding colonies were housed in a limited-access, pathogen-free facility where all cages, enrichment, and water were sterilized before use (University of Wisconsin-Madison, Biotron) on a 12-hour light/12-hour dark cycle with ad libitum access to water and irradiated breeder chow (Teklad 2919). Gαz-null and wild-type (WT) NOD mice were generated by heterozygous matings to produce littermate pairs.
Mice with LoxP sites flanking the first protein-coding exon (exon 3) of the Gnaz gene (Gαz-flox/flox) were generated from embryonic stem cells obtained from the European Conditional Mouse Mutagenesis Program (Gnaztm1a(EUCOMM)Hmgu). These embryonic stem cells contain the Gnaz gene interrupted by a β-galactosidase/neomycin resistance gene-trap cassette inserted between exons 2 and 3. Karyotyping was performed to ensure >80% euploidy of the cells before injection into C57 Albino embryos. Male progeny displaying high percentages of coat/eye chimerism were crossed back to female C57 Albino mice; progeny carrying a founding F1 germline mutation were identified by coat/eye color and confirmed by genotyping. F1 germline mice were bred with Flp “deleter” mice (Jackson Laboratories) to excise the β-galactosidase/neomycin resistance gene-trap cassette, leaving two LoxP sites surrounding exon 3 of Gnaz that do not affect protein expression. These mice were fully backcrossed into the C57BL/6J background.
To generate the β-cell–specific Gαz knockout (KO) and control mice, we bred Gαz-flox/flox mice with RIP-CreHerr mice [“InsPr-Cre” in (20)], also fully backcrossed into the C57BL/6J background. This Cre line has low hypothalamic recombination and does not express the human growth hormone mini-gene (21, 22). Experimental animals were identified by genotyping for the WT of floxed Gnaz gene and for the presence of the Cre gene. WT Cre+ mice were used as controls.
Upon weaning, mice were housed five or fewer per cage of mixed genotypes with ad libitum access to lower-fat chow in one of two facilities: the Madison VA Animal Resource Facility (LabDiet 5001, nonirradiated; 17-week study) or investigator-accessible housing in the Biotron [(Teklad 2920X, irradiated) 35-week study, T-cell analysis, and female oral glucose tolerance test (OGTT)]. All procedures were performed according to approved protocols in accordance with the principles and guidelines established by the University of Wisconsin and Madison VA institutional animal care and use committees.
Blood glucose measurements were taken weekly from 4 weeks of age up to 35 weeks of age using a blood glucose meter (AlphaTRAK) and rat/mouse–specific test strips. At the end of the study, mice were subjected to collagenase perfusion of the pancreas to isolate pancreatic islets for in vitro analysis or pancreatic dissection for either cryofixation or paraffin embedding. A separate cohort of mice from the 17-week study was euthanized at the 4- to 5-week time point, and pancreata were collected for sectioning.
MLD-STZ induction of diabetes
MLD-STZ (Sigma; #S01230) [50 mg/kg of body weight (BW)] induction of hyperglycemia and treatment with 10 μg/kg BW Ex4 (Sigma-Aldrich; #E7144) was conducted as previously described (16).
Mouse islet isolation and GSIS assay
Mice were euthanized using 2,2,2-tribromoethanol (Sigma; #T48402) anesthesia followed by cervical dislocation. Intact pancreatic islets were isolated from mice using a collagenase digestion protocol (23). On the day of isolation, islets were picked into 100 µL of islet medium (RPMI 1640; Gibco; #11879020) containing 11.1 mmol/L glucose (Fisher Scientific; #D16), 10% heat-inactivated fetal bovine serum (Sigma-Aldrich; #12306C), and 1% Hepes (Sigma; #H4034) and penicillin/streptomycin (Gibco; #15070-063)) in each well of a 96-well V-bottom tissue culture-coated plate (Corning Life Sciences; #3894) according to a protocol optimized in our laboratory (24). Insulin enzyme-linked immunosorbent assays were performed essentially as described elsewhere (11).
Glucose tolerance testing
Animals were fasted for 4 to 6 hours before glucose tolerance testing. Glucose was given using an oral gavage at a dose of 2 g/kg BW. Blood glucose measurements were taken immediately before gavage and at 5, 15, 30, 60, and 120 minutes following gavage. For female mice, blood was collected using a lateral tail nick at baseline and 5 minutes after gavage. Oral glucose tolerance testing was performed at 16 and 24 weeks of age for male and female mice, respectively.
Whole pancreas staining
For all slide staining assays, 10-μm serial sections were cut on positively charged slides, with 18 sections per stop position (three per slide) and three stop positions per pancreas separated by at least 200 μm. Immune infiltration of the β-cell was determined using standard eosin (Polysciences; #17269) and hematoxylin (Sigma; #GHS280) staining. Following staining, quantification of immune infiltration was accomplished using a numerical scoring system, ranking the extent of immune infiltration from no immune cells present (0) to an islet that was completely infiltrated with immune cells (4) (Supplemental Fig. 1 (27.7MB, docx) ). A single observer who was blind to genotype identifiers analyzed all tissue sections. For islet infiltration scoring, every islet in a single, 10-μm section was scored per mouse. Staining and analysis techniques used for determining β-cell fractional area and β-cell death (as shown by TUNEL positivity) were performed as previously described (16).
The immunohistochemical staining protocol described previously was modified for optimization of Gαz analysis (16). The primary modification to the immunostaining protocol was use of the rabbit anti-Gαz primary antibody (Abcam; #AB150434), diluted 1:400 in antibody diluent with background-reducing components (Dako; #S3022). Every islet in a single 10-μm section was scored, and three distinct sections were averaged per mouse to give one biological replicate.
Quantitative real-time polymerase chain reaction
Islets from WT and Gαz-KO mice were isolated and collected for isolation of whole RNA using the Qiagen RNeasy® Kit (Qiagen; #74106) according to the manufacturer’s instructions. Complementary DNA (cDNA) was then generated with random hexamers (High-Capacity cDNA Reverse Transcription Kit, Applied Biosystems®; #4368813), and quantitative reverse transcription polymerase chain reaction (qRT-PCR) was performed using SYBR green (Roche; #04913914001) according to previously published methods (25). Quantification of messenger RNA (mRNA) expression of IL-1β (gene symbol: IL1β), interferon γ (IFNγ), tumor necrosis factor α (TNFα), nuclear factor kappa-light-chain-enhance of activated β-cell (NFκB), C-X-C motif chemokine 10 (CXCL10), chemokine (C-C motif) ligand 2 and 4 (CCL2, CCL4), and cluster of differentiation 3 and 8 (CD3, CD8) was performed by normalization of cycle times within each sample to those of β-actin. Primer sequences can be found in Table 1.
Table 1.
qRT-PCR Primer Sequences and Properties
| Gene (M. Mus.) | Orientation | Sequence (5′ → 3′) | Tm | % GC |
|---|---|---|---|---|
| NF-κB | F | TCCTGATCCAGACAAAAACTGG | 54.87 | 45.45 |
| R | ATCCCGGAGTTCATCTCATAGT | 54.97 | 45.45 | |
| IL-1β | F | CCACCTTTTGACAGTGATGAGA | 54.81 | 45.45 |
| R | GAGATTTGAAGCTGGATGCTCT | 54.98 | 45.45 | |
| IFNγ | F | TGAAAGACAATCAGGCCATCAG | 62.18 | 45.45 |
| R | GTGGGTTGTTGACCTCAAACT | 62.36 | 47.62 | |
| TNFα | F | CTCACACTCACAAACCACCAAG | 63.15 | 50 |
| R | GTAGACAAGGTACAACCCATCG | 62.03 | 50 | |
| CXCL10 | F | GGCCATAGGGAAGCTTGAAAT | 62.21 | 47.62 |
| R | CATCGTGGCAATGATCTCAACA | 62.63 | 45.45 | |
| CCL2 | F | TCATTCACCAGCAAGATGATCC | 61.99 | 45.45 |
| R | GCTTGGTGACAAAAACTACAGC | 61.82 | 45.45 | |
| CCL4 | F | GCTGTGGTATTCCTGACCAAAA | 62.38 | 45.45 |
| R | CAGTTCAACTCCAAGTCACTCA | 61.66 | 45.45 | |
| CD3 | F | CCAGTCAAGAGCTTCAGACAAG | 62.46 | 50 |
| R | TCAGTTGGTTTCCTTGGAGATG | 61.85 | 45.45 | |
| CD8 | F | CCTCAACCTGCTTTGTCTACAG | 62.50 | 50 |
| R | GCAAGAGTGGCTGAATGTAGT | 61.95 | 47.62 | |
| Gαz | F | AAGGAACATCAGGGCCAGAG | 56.40 | 55 |
| R | CAGCACTACGGTTCAGCAAC | 55.38 | 50 |
Abbreviations: F, forward; %GC, percent guanosine/cytosine content; R, reverse; Tm, melting temperature.
Intracellular cytokine staining
Splenocytes were harvested from 17- to 19-week-old female NOD or Gαz-KO mice and cultured for 3 days with 1 μg/mL plate-bound anti-CD3ε (clone 145-2C11) and 1 μg/mL soluble anti-CD28 (clone 37.51) (BD Biosciences; #553057 and #553294) in complete RPMI media with 10% fetal bovine serum. Suspensions of single cells were incubated with GolgiStop (BD Biosciences; 51-2092KZ) for 4 hours before staining. Cells were stained for surface markers, fixed and permeabilized with Cytofix/Cytoperm Plus reagents (BD Biosciences; #51-2090KZ and #51-2091KZ), stained for intracellular cytokines, and analyzed with the BD LSR II flow cytometer. Fluorescent antibodies for CD3ε (clone 145-2C11), CD8α (clone 53-6.7), TNFα (clone MP6-XT22), and IFNγ (clone XMG1.2) were purchased from BD Biosciences (#563565, #561092, #561041, and #563376, respectively). The fluorescent antibody for CD4 (clone RM4-5) was purchased from eBioscience (#11004282). Ghost Dye viability reagent was purchased from Tonbo Biosciences (#130865). Flow cytometry data were analyzed with FlowJo software.
Statistical analysis
Data are expressed as mean ± standard error of the mean unless otherwise noted. Data were compared by one- or two-way analysis of variance or Student t test as appropriate and as described in the figure legends. A P value <0.05 was considered statistically significant. Statistical analyses were performed with GraphPad Prism version 6 (GraphPad Software, San Diego, CA).
Results
Diabetes protection afforded by loss of Gαz is robust and durable
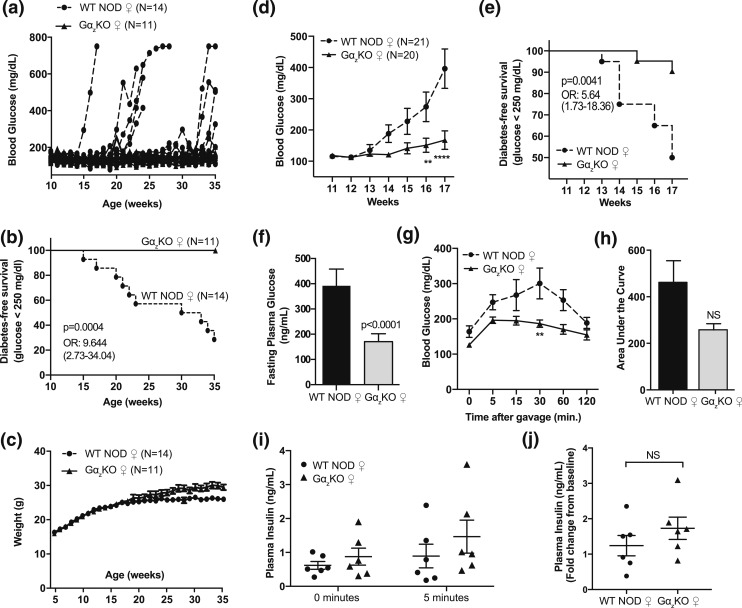
The robustness of the NOD phenotype has been reported as variable and dependent on environmental conditions (17). To determine the natural history of diabetes development in our NOD colony as well as any protection afforded by loss of Gαz, we tracked blood glucose levels of female mice weekly from 4 to 35 weeks of age. Two waves of diabetes development were apparent in the WT mice [Fig. 1(a)], with ∼45% of the mice becoming diabetic by 23 weeks of age and then a lag of 7 weeks before an additional 25% of mice became diabetic [Fig. 1(b)]. None of the 11 Gαz-KO females became hyperglycemic. Analysis of the Kaplan-Meier survival data revealed a strong protection from diabetes development, with WT females being 9.644 times more likely to develop diabetes than the Gαz-KO females [Fig. 1(b)]. Body weights did not differ between the genotypes until ∼25 weeks of age, when approximately half of the WT mice were diabetic, consistent with a wasting phenotype [Fig. 1(c)]. Therefore, we concluded that the diabetes protection phenotype in the Gαz-KO females on NOD background was robust and durable, at least through 8 to 9 months of age.
Figure 1.
The diabetes protection phenotype of female (♀) Gαz KO NOD mice is robust and durable. (a) Timeline of diabetes development in individual Gαz-KO or WT NOD female mice. (b) Kaplan-Meier survival curve and (c) body weights of the animals shown in (a). (d) Mean blood glucose levels and (e) diabetes-free survival of female Gαz-KO NOD mice and WT NOD controls housed in a facility separate from those shown in (a). (f) The 4- to 6-hour fasting plasma glucose levels of Gαz-KO NOD and WT NOD female mice. (g) Plasma blood glucose values, (h) area under the curve, and (i) plasma insulin levels during OGTTs of 24-week-old Gαz-KO NOD and WT NOD female mice. (j) Change in plasma insulin levels at 5 minutes shown as fold-change from baseline. (d, g, i) Data were compared by two-way analysis of variance with the Tukey post hoc test. **P < 0.01; ****P < 0.0001. (d) n = 20 or 21 per group and (g, i) n = 6 per group. (f, j) Data were compared by Student t test; n = 20 or 21 per group and n = 6 per group, respectively. All error bars represent standard error of the mean. NS, not significant; OR, odds ratio.
To determine the molecular and cellular signaling events mediating the early development of diabetes, we tracked blood glucose levels from 11 to 17 weeks of age in a second cohort of female Gαz-KO and WT NOD mice. Gαz-KO NOD mice had significantly lower random-fed blood glucose levels than WT controls throughout the 17 weeks of the study, with a divergence between the genotypes beginning around week 13 [Fig. 1(d)]; this is within the expected timeline for initial diabetes development in female NOD mice (17, 18). When expressed as diabetes-free survival (blood glucose level <250 mg/dL), Gαz-KO female mice had 90% disease-free survival over the 17-week period, which was nearly double that observed in WT NOD females (50%) [Fig. 1(e)]. At week 17, the 4- to 6-hour fasting plasma glucose levels were significantly lower in Gαz-KO females than in WT animals, who overall had fasting hyperglycemia [Fig. 1(f)].
Next, WT and Gαz-KO NOD females were given an oral glucose challenge. The mean blood glucose levels of Gαz-KO NOD females was lower at each time point during the glucose challenge and was statistically lower at the 30-minute time point [Fig.1(g)]. The area under the curve was also lower in Gαz-KO NOD mice, although this was not statistically significant [Fig. 1(h)]. The mean levels of plasma insulin were also higher in the Gαz-KO NOD mice 5 minutes after the glucose challenge, whether represented as ng/mL or as the fold-change vs the level at time = 0, although these differences were also not statistically significant [Fig. 1(i) and 1(j), respectively].
Loss of Gαz protects from diabetes by affecting multiple molecular and cellular events
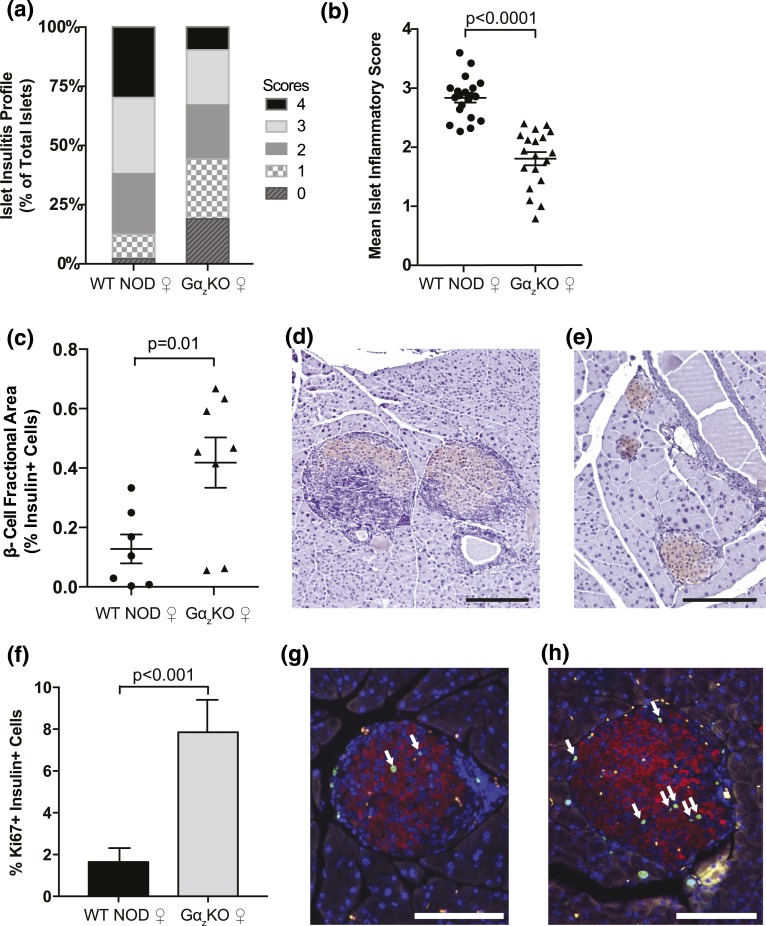
Following our conclusion that loss of Gαz led to increased protection from diabetes and improved glucose tolerance in the NOD mice, we evaluated whether this protection resulted from a decrease in immune infiltration and maintenance of functional β-cell mass. The extent of insulitis was analyzed using the scoring system described in the Methods (see Supplemental Fig. 1 (27.7MB, docx) for scoring examples). Islets from female Gαz-KO NOD mice exhibited a reduced inflammatory profile compared with those of WT controls [Fig. 2(a)]. When shown as a mean insulitis score, islets from Gαz-KO mice exhibited a 30% reduction in overall mean insulitis compared with those of WT females [Fig. 2(b)]. This reduction in islet immune infiltration correlated with a fourfold increase in β-cell fractional area as determined by immunohistochemistry [Fig. 2(c)]. Representative images of insulin and hematoxylin-stained pancreas sections from female WT NOD and Gαz-KO NOD mice are included for visualization of quantified differences [Fig. 2(d) and 2(e), respectively].
Figure 2.
Female (♀) Gαz-KO NOD mice have decreased islet insulitis and increased insulin-positive pancreas area at 17 weeks of age. Pancreata were collected from female WT and Gαz-KO mice at 17 weeks of age and subjected to hematoxylin and eosin staining to determine islet inflammation or insulin immunohistochemistry with hematoxylin counterstain to determine β-cell fractional area. (a) Percentage of islets in each of the islet inflammation scoring categories. (b) Mean islet immune infiltration score for each biological replicate. (c) Quantification of β-cell fractional area calculated from all islets counted on three sections of each mouse pancreas separated by at least 200 μm. Representative pancreas sections from WT and Gαz-KO mice are shown in panels (d) and (e), respectively. (f) Quantification of insulin+ and Ki67+ cells in 17-week-old female mice calculated by counting all islets in a pancreas section and averaging two sections separated by at least 200 μm per mouse. Representative pancreas sections from WT and Gαz-KO mice are shown in panels (g) and (h), respectively. White arrows indicate insulin+ Ki67+ cells. In all images, the scale bar represents 200 μm. (b) Data were compared by Student t test; n = 21 per group. (c) n = 7 per group. (f) n = 6 per group.
To further investigate the increased β-cell area seen in Gαz-KO NOD females, pancreas sections were immunofluorescently labeled to identify nuclear Ki-67, a marker of actively replicating cells. Gαz-KO NOD female islets exhibited a dramatic increase in insulin+, Ki-67+ cells compared with those of WT NOD females, suggesting that increased β-cell replication was in part responsible for enlarged β-cell area [Fig. 2(f–h)].
Finally, we repeated the 17-week observational study with male NOD mice, for which the prevalence of outright hyperglycemia was lower, to determine whether they exhibited any protective phenotype with regard to insulitis and/or glucose tolerance. We found male Gαz-KO mice had slightly lower mean random-fed blood glucose levels than WT controls at weeks 16 and 17, but this was not statistically significant (Supplemental Fig. 2A) (27.7MB, docx) . None of the male Gαz-KO mice became hyperglycemic by 17 weeks of age, whereas two of 23 male WT mice (8.6%) became diabetic (Supplemental Fig. 2B) (27.7MB, docx) . Interestingly, though, insulitis scores mimicked those observed in female mice, with the Gαz-KO animals being relatively more protected from severe insulitis, as plotted by percentage of islets in each scoring category (Supplemental Fig. 2C) (27.7MB, docx) or mean insulitis score for each animal (Supplemental Fig. 2D) (27.7MB, docx) . Male Gαz-KO mice trended toward lower peak blood glucose levels during OGTTs, a lower glucose area under the curve, and a higher fasting blood glucose level, although none of these measurements were statistically different from those of WT mice (Supplemental Fig. 2E–G) (27.7MB, docx) . Next, to directly measure β-cell function, we harvested islets from three nondiabetic mice of each genotype, matched as closely as possible for fasting blood glucose levels, and performed in vitro GSIS assays. In response to 16.7 mM of stimulatory glucose, islets from Gαz-KO mice secreted >45% more insulin than WT islets, shown as a percentage of total islet insulin (Supplemental Fig. 2H) (27.7MB, docx) . Interestingly, however, the insulin content of Gαz-KO islets was also significantly increased (by about 45%) (Supplemental Fig. 2I) (27.7MB, docx) .
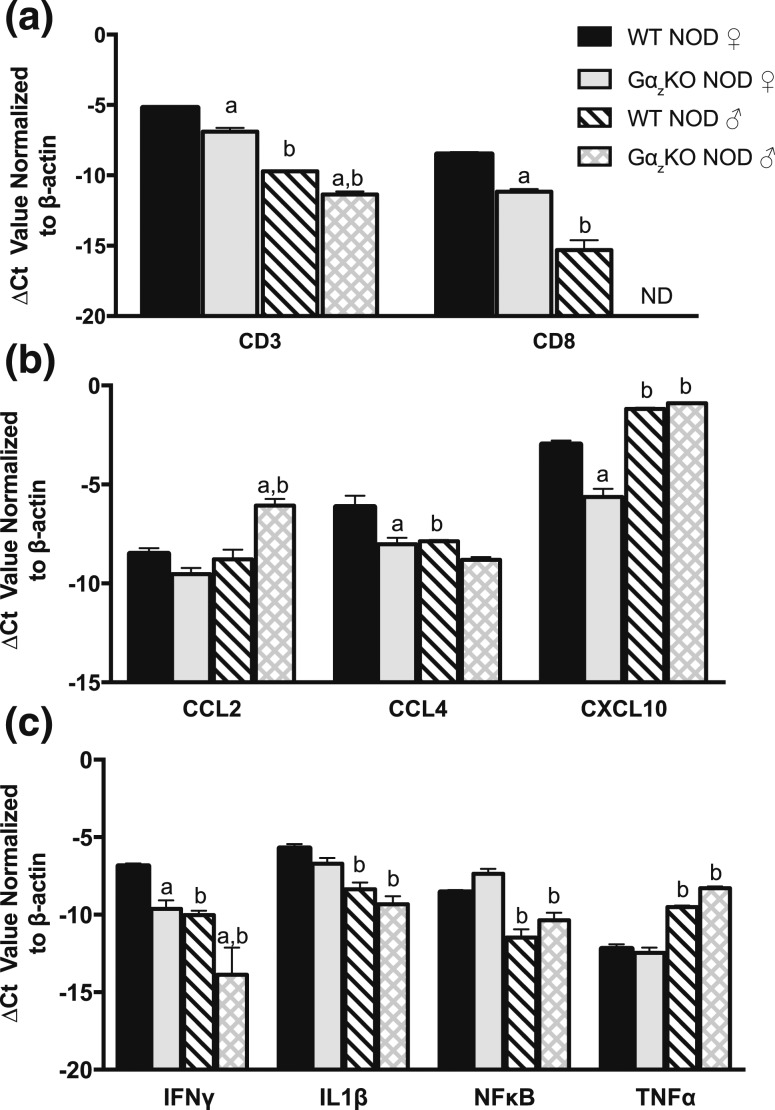
Loss of Gαz improves NOD islet inflammatory profile
Islets were isolated from both male and female WT and Gαz-KO mice at 17 weeks of age. Following isolation, cDNA samples were prepared, and we investigated the mRNA expression of various immune cell-surface markers and a panel of chemokines and cytokines. We found that the transcripts for CD3 (a marker of T-cell lineage) and CD8 (exclusive to cytotoxic T-cells) were significantly reduced in both Gαz-KO males and females compared with respective WT controls [Fig. 3(a)]. Interestingly, a significant decrease was also observed in both CD3 and CD8 transcripts when Gαz-KO NOD male islets were compared with Gαz-KO NOD female islets. We investigated the levels of various chemoattractant molecules, including CCL2, also known as monocyte chemoattractant protein 1; CCL4, also known as macrophage inflammatory protein 1 β; and CXCL10, also known as interferon γ–induced protein 10. We found that CCL2 transcript was increased only in islets from Gαz-KO NOD males [Fig. 3(b)]. CCL4 transcript levels were lower in all groups compared with those in WT NOD females [Fig. 3(b)]. Interestingly, although CXCL10 was lower in Gαz-KO NOD female islets, it was increased in both WT and Gαz-KO NOD male islets [Fig. 3(b)]. Similar to the expression pattern seen in CD3 and CD8, we found that IFNγ was significantly lower in Gαz-KO islets than in WT NOD islets, relative to sex [Fig. 3(c)]. IL-1β, a key inflammatory player, and NF-κB, a proapoptotic factor, were both decreased only at the transcript level in male NOD islets compared with female islets, with respect to genotype [Fig. 3(c)]. Islet transcript levels of TNFα were higher in male NOD islets than in those of NOD females, relative to genotype [Fig. 3(c)].
Figure 3.
Transcripts of important inflammatory players are different between islets of WT and Gαz-KO animals, regardless of sex. T-cell markers Islets were isolated from both male and female Gαz-KO and WT NOD animals and subjected to mRNA expression analysis for (a) T-cell markers, (b) chemoattractant molecules, and (c) cytokines known to be important in T1DM pathology. Data were compared by two-way analysis of variance with Tukey post hoc test, with the exception of CD8, which was compared using Student t test; N = 3 to 5 per group. (a) P < 0.05 vs WT NOD, respective to sex; (b) P < 0.05 vs female, respective to genotype. Ct, cycle threshold; ND, not detectable.
Loss of Gαz protects NOD β-cells from apoptosis upon initial immune infiltration
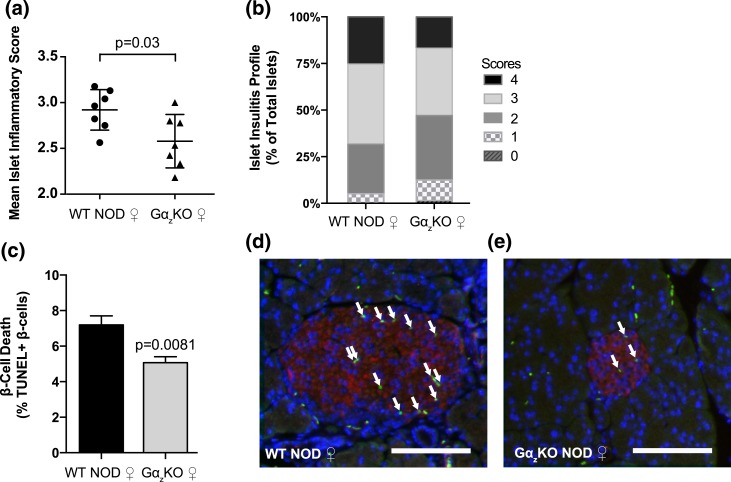
To gain more insight into the mechanisms by which loss of Gαz protects female NOD mice from becoming hyperglycemic, pancreata from 4-week-old female mice were collected and analyzed using our established insulitis scoring system. Although there was a small but statistically significant reduction in the mean immune infiltration score [Fig. 4(a)], both WT and Gαz-KO female mice had a majority of islets in grades 2 to 4, indicating partial to complete islet immune infiltration [Fig. 4(b)]. These results suggest Gαz does not play a role in initial immune cell infiltration in the NOD model. Next, the percentage of TUNEL+ β-cells, a marker for β-cell apoptosis, was quantified on pancreas sections from 4-week-old female WT and Gαz-KO mice costained with insulin and 4′,6-diamidino-2-phenylindole (DAPI). Gαz-KO pancreas sections showed a 30% reduction in the percentage of TUNEL+, insulin+ cells, indicative of a reduction in β-cell apoptosis [Fig. 4(c)]. As expected, TUNEL+ β-cells tended to surround the invading immune infiltrate, which can be observed as clusters of small DAPI-positive cells in representative sections [Fig. 4(d) and 4(e)].
Figure 4.
Loss of Gαz did not dramatically alter insulitis by 4 weeks of age, but it did reduce β-cell death. Pancreata were collected from female (♀) WT and Gαz-KO mice at 4 weeks of age and subjected to hematoxylin and eosin staining. Insulitis was quantified using the previously described scoring system. (a) Percentage of islets in each of the scoring categories and (b) mean islet immune infiltration score for each biological replicate. (c–e) Additional pancreata were collected at 4 weeks of age and subjected to immunofluorescence analysis for insulin staining (red) and TUNEL assay (green), with a DAPI nuclear counterstain (blue). (c) Quantification of TUNEL+ β-cell nuclei calculated from all islets counted on three sections of each mouse pancreas separated by at least 200 μm. Representative TUNEL-stained pancreas sections from (d) WT mice and (e) Gαz-KO mice are shown, with white arrows indicating TUNEL+ β-cell nuclei. In both images, the scale bar represents 100 μm. Data were compared by Student t test. (a, b) n = 7 per group; (c) n = 5 per group. All error bars represent standard error of the mean.
The protective effect of Gαz loss on hyperglycemia is β-cell specific
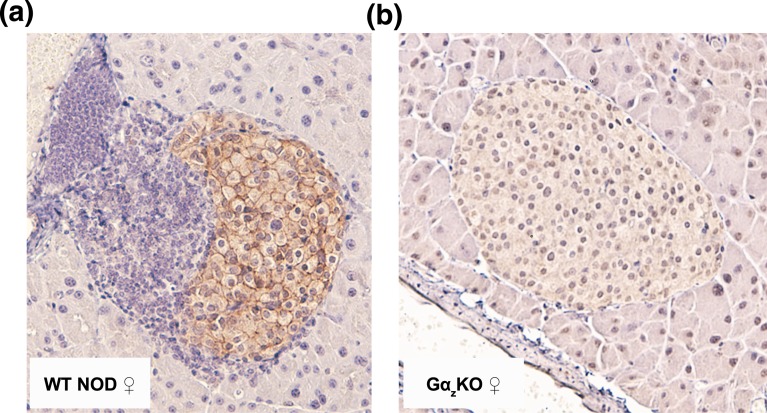
Gαz has a relatively limited expression pattern, with protein expression being confirmed only in the brain, pancreatic islet, and platelets (12, 26–28). Nevertheless, we wanted to confirm that the effects on islet immune infiltration and β-cell replication, function, and death were due to Gαz expression being lost from the islet cells and not the invading immune cells. Pancreata from WT and Gαz-KO female mice were immunohistochemically stained for Gαz, confirming that Gαz was specifically expressed in the islet cells (localized around the plasma membrane as expected for a membrane-associated protein) and not in the exocrine pancreas or invading immune infiltrate [Fig. 5(a)]. A Gαz-KO islet was provided as a negative control for antibody specificity [Fig. 5(b)].
Figure 5.
Gαz is expressed in islets but not in the islet-infiltrating immune cells. Representative islet images from both (a) WT and (b) Gαz-KO NOD pancreata that were subjected to immunohistochemical analysis for Gαz protein expression (brown) and counterstained with hematoxylin (purple).
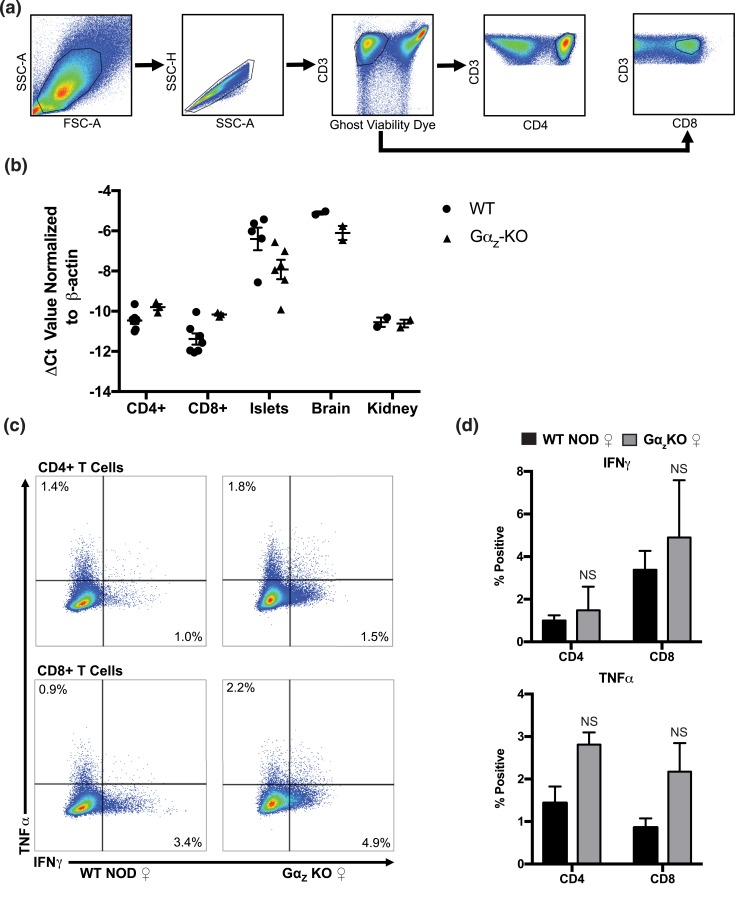
To confirm a lack of expression/effect of Gαz in T-cells, we isolated RNA from CD4+ and CD8+ splenic T-cells and performed qRT-PCR analyses. Gαz levels were not significantly different between the genotypes and were significantly lower than known Gαz-expressing tissues, including islet and brain [Fig. 6(a)], and were essentially identical to a known negative control for Gαz expression, the kidney. Islet and brain Gαz-null samples did express an mRNA construct amplifiable by the Gnaz qRT-PCR primers used, but because the DNA cassette used to generate the KO interfered with the translation start site, production of Gαz protein was completely absent in these tissues in the KO mouse (10, 29). We then investigated whether T-cell cytokine production was altered between WT and KO mice. Splenic CD4+ and CD8+ cells did not show any significant difference in the production of type 1 T helper (Th1) cell cytokines TNFα and IFNγ, suggesting that protection from hyperglycemia observed in Gαz mice may be due to loss of Gαz in the β-cells and not the immune cells [Fig. 6(b–d)].
Figure 6.
Gαz mRNA is not expressed in splenic T-cells and does not significantly alter their type 1 T helper cell response. Splenic T-cells were harvested from female (♀) WT or Gαz-KO mice at 17 to 19 weeks of age and separated into CD4+ and CD8+ populations by flow cytometry. (a) Gating strategy to obtain CD4+ and CD8+ T-cell populations. (b) Expression of Gnaz transcript in CD4+ and CD8+ T-cells, islets, brain, and kidney samples from WT NOD and Gαz-KO animals. (c) Representative scatter plots for intracellular cytokine staining of TNFα and IFNγ in CD4+ and CD8+ T-cell populations stimulated with CD3ε and CD28 antibodies for 3 days. (d) Quantification of percent positive cells from data displayed in (c). Data were compared by Student t test; n = 3 to 5 per group. All error bars represent standard error of the mean. NS, not significant.
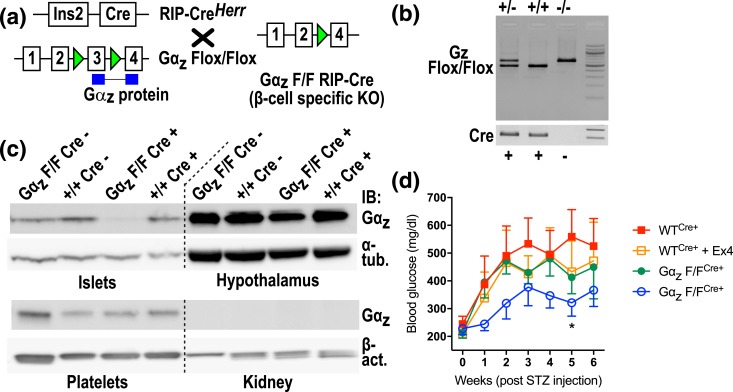
Finally, we used the MLD-STZ procedure, which includes an immune component but is initiated by β-cell destruction, to specifically test the role of β-cell Gαz in β-cell survival to insult. First, we bred C57BL/6J mice containing exon 3 of Gαz flanked by LoxP sites with mice expressing Cre under the control of the rat insulin promoter to generate experimental animals [Fig. 7(a)]. Animals were distinguished by the presence of one or two bands upon polymerase chain reaction amplification of genomic DNA [Fig. 7(b)]. Genotyping polymerase chain reaction primers was designed to detect the deleted allele; any animals showing this pattern were not used for experiments (data not shown). Isolation of tissues known to express Gαz (islets, hypothalamus, and platelets) confirmed the β-cell–specificity of the Gαz deletion in Gαz-flox/flox;Cre-positive animals [Fig. 7(c)].
Figure 7.
Validation of β-cell–specific deletion of Gαz-null and protection from streptozotocin-induced diabetes. (a) Breeding strategy to generate the β-cell–specific Gαz-null mouse. Green triangles indicate LoxP sites. Blue boxes represent the Gαz protein gene product that is disrupted by Cre-mediated recombination. F/F indicates the “floxed” (i.e., recombined) allele. (b) Representative genotyping results to identify experimental animals. (c) Gαz protein expression in multiple tissues demonstrates islet-specific loss of Gαz protein. Hypothalamus and platelets are positive controls for Gαz expression, whereas the kidney serves as a negative control. Alpha-tubulin (α-tub.) or beta-actin (β-act.) was used as a loading control. (d) Blood glucose levels after MLD-STZ induction show protection from severe hyperglycemia in the Gαz-F/F, Cre+ animals concurrently treated with 10 μg/kg exendin-4 (Ex4). Data were compared by two-way analysis of variance. All error bars represent the standard error of the mean. *P < 0.05 vs WTCre+; n = 4 or 5 per group.
Experimental animals were subjected to MLD-STZ induction with and without the addition of 10 μg/kg/d Ex4, which we previously demonstrated was sufficient to protect full-body Gαz-null C57BL/6N mice from severe hyperglycemia induced by streptozotocin (16). Indeed, although WT mice treated with Ex4 or mice lacking β-cell Gαz trended toward lower blood glucose values during the 6-week experimental time course, only the combination of β-cell–specific Gαz loss and Ex4 treatment was able to protect mice from developing severe hyperglycemia, phenocopying our results from the full-body KO [Fig. 7(c)] (16) and implicating β-cell Gαz as an initiating factor in β-cell loss in T1DM.
Discussion
The NOD mouse is a well-accepted model of T1DM because it replicates the key pathogenic characteristics of the human disease (17, 18). Female NOD mice develop early insulitis that typically progresses to overt diabetes. Furthermore, although the development of overt hyperglycemia in male NOD animals is of lower penetrance, they exhibit other important pathogenic aspects of T1DM, including β-cell dysfunction and insulitis. We previously showed that loss of Gαz protects mice from both diet-induced glucose intolerance, a model of type 2 diabetes mellitus, and streptozotocin-mediated β-cell insult, a chemically induced model of T1DM (10, 15, 16). In both models, there were key improvements in β-cell replication, survival, or both, lending credence to testing the impact of the Gαz-KO mutation in a physiologically relevant T1DM model.
We previously showed Gαz-KO C57BL/6N mice are protected from developing severe hyperglycemia after MLD-STZ induction of diabetes only when coadministered a subtherapeutic dose of the GLP-1 mimetic Ex4 (16). In the NOD model, Gαz loss alone was sufficient to protect from the development of severe hyperglycemia. These results are not necessarily discordant because MLD-STZ induces a rapid, immune-mediated loss of functional β-cell mass with recovery dependent not only on β-cell survival but also on the mounting of a β-cell replication response. In the NOD model, we propose that Gαz is primarily playing a role in the initial apoptotic event, which then induces further migration of immune cells to the islet. When uncontrolled, this immune infiltration propagates continued cell death, as apoptotic and necrotic cells can be potent activators of dendritic cells and other antigen-presenting cells (30). This cycle causes the rapid immune-mediated destruction of pancreatic β-cells, resulting in T1DM pathogenesis. Gαz-KO mice still have immune infiltration into the islets (Fig. 2; Supplemental Fig. 2 (27.7MB, docx) ); therefore, we do not suspect that loss of Gαz is playing a role in the initial immune onslaught. Rather, we propose that in the absence of Gαz, the progression of insulitis is reduced or halted and β-cell survival and function are maintained. This hypothesis is supported by the decrease in islet immune infiltration scores from 4 to 5 weeks to 17 weeks of age in Gαz-null NOD mice [compare Fig. 4(a) and 4(b) to Fig. 2(a) and 2(b)].
T1DM is foremost an autoimmune, inflammatory condition; thus, the primary and often most successful therapeutic targets focus on these mediators and pathways. It is well accepted that T1DM pathogenesis is T-cell–mediated; therefore, we were interested specifically in CD3+, a marker of T-cell lineage, and CD8+, a cell surface marker specific to cytotoxic T-cells (31, 32), both of which were reduced in Gαz-KO NOD islets [Fig. 3(a)]. Furthermore, IFNγ is secreted specifically by Th1 cells and is known to bind to receptors directly on β-cells that can induce apoptotic pathways via STAT1 and NF-κB (33). Because T1DM is a Th1 cell–mediated disease, high IFNγ and low interleukin-4 values are correlated with pathogenic β-cell insulitis (34). Although we do not see a difference in islet mRNA expression of NF-κB between WT and Gαz-KO mice [in fact, it is slightly increased in KO mice; Fig. 3(c)], IFNγ transcript expression is reduced eightfold in Gαz-KO NOD islets, regardless of sex [Fig. 3(c)]. In mice at 17 weeks of age, when islets were collected for cytokine/chemokine expression profiling, the initiation of apoptotic events had long since passed, which could explain why we did not see a change in NF-κB expression [Fig. 3(c)]. Reduction in expression of and likely local levels of IFNγ would lead to decreased expression of MHC II on antigen-presenting cells, such as macrophages and dendritic cells present in and around the islet. A reduction in MHC II expression would indicate a reduced capacity for MHC II to present antigen epitopes to other adaptive immune cells (35), thereby reducing the further activation of β-cell–specific inflammatory events. Reduced expression of IFNγ is likely necessary to prevent insulitis and maintain β-cell survival and function because of its ability to influence the cyclic apoptotic nature through multiple pathways.
Interestingly—and to our surprise—we found that TNFα was dramatically upregulated in islets of male NOD mice, regardless of genotype [Fig. 3(c)]. There is some evidence that TNFα offers a protective role in β-cells at later stages of insulitis (36–38). We believe these findings could offer some insight into the divergence in hyperglycemic development and β-cell pathogenesis seen between male and female NOD mice and should be a topic of further investigation.
The effects of inflammatory mediators in the pathogenesis of T1DM continue to be thoroughly investigated because of the direct and profound ties to the disease (39–42). However, cytokines and their local and systemic effects are highly influenced by the presence or absence of other cytokines. In vivo, cytokines are present in a milieu and are not likely to be found in isolation (43). It is the mixture of these proteins that leads to inflammatory phenotypes of chemoattraction and cytotoxicity. In addition, these molecules can have broad actions owing to a wide distribution of receptors making their therapeutic potential rather limited (44–46). Our finding that loss of Gαz mitigates some of these key inflammatory events is exciting. Gαz has an attractive therapeutic potential, in that targeting a single protein results in alterations in other key pathways that themselves are difficult to target. A role for Gαz in natural killer cells has been previously suggested (46–49), although these results have not been replicated.
In our study, we confirmed that Gαz is not expressed in the infiltrating immune cells, whereby its expression is clearly apparent around the islet cell membranes, and therefore propose that the changes seen in the islet cytokine and chemokine mRNA profiles are a result of the absence of Gαz acting in the β-cell to mediate apoptosis and paracrine signaling. We previously showed that overexpression of both WT and constitutively active Gαz in the INS-1 (832/3) rat insulinoma cell line increased cytotoxicity, decreased viability, and increased caspase cleavage, particularly in response to IL-1β, indicating a role for Gαz in promoting stress-induced β-cell death pathways (16). In support of this, Gαz-KO C57BL/6N islets are resistant to IL-1β–induced upregulation of mRNA encoding C/EBP homologous protein, Tribbles homologue 3, and Caspase 9, functional regulators of β-cell death (16). Our MLD-STZ study with the β-cell–specific Gαz-null C57BL/6J mouse is fully consistent with a β-cell–centric effect of Gαz loss in diabetes protection [Fig. 7(d)]. We expect that Gαz is acting in a similar manner in the NOD mouse islets but were unable to test the intrinsic susceptibility to β-cell death with isolated islets in this model because of the rapid insulitis that occurred, which is statistically different between genotypes even at 4 weeks of age (Fig. 4).
Altered β-cell function and survival can potentially affect the function, activation, and/or migration of immune cells. Consistent with this, we observed a lesser degree of insulitis in Gαz-KO mice most likely because of reduced antigen presentation and/or activation of immune cells. However, our initial assessment of T-cell function/cytokine production did not show a significant difference between KO and WT mice. More detailed immunophenotyping of T-cells and/or other immune cells that are critically implicated in T1DM is necessary to identify the contribution of these cells to the phenotype. A β-cell–specific KO of Gαz in the NOD background will confirm whether the protection afforded the Gαz-KO NOD mice is β-cell autonomous.
In summary, Gαz is a crucial mediator of T1DM pathogenesis in a well-accepted mouse model of the disease and as a single modulator is able to exert action on multiple facets of the disease. Combined, these characteristics provide a relevant rationale for specifically targeting the action of Gαz to promote β-cell function and survival in early T1DM.
Acknowledgments
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grant K01 DK102488 (to F.E.) and Juvenile Diabetes Research Foundation (JDRF) Grant 5-CDA-2014-184-A-N (to F.E.); American Diabetes Association Grant 1-14-BS-115 (to M.E.K.), NIH Grant R01 DK102598 (to M.E.K.), and JDRF Grant 17-2011-608 (to M.E.K.). A.L.B. was supported in part by a Summer Undergraduate Research Fellowship from the American Society for Pharmacology and Experimental Therapeutics. M.T.C. was supported in part by a Minority Undergraduate Research Supplement from the American Diabetes Association. J.W. was supported in part by the Molecular and Applied Nutrition Training Program T32 DK007665 to the University of Wisconsin-Madison Department of Nutritional Sciences. The flow cytometry data were collected with resources and facilities provided by the University of Wisconsin Carbone Cancer Center–Flow Cytometry Laboratory with funding from Support Grant P30 CA014520. This material is the result of work supported with resources and the use of facilities at the William S. Middleton Memorial VA Hospital located in Madison, Wisconsin, and the George E. Wahlen VA Hospital located in Salt Lake City, Utah. M.E.K. is the guarantor of this work.
Author contributions: M.E.K., T.E.G., and F.E. conceptualized the experiments. M.T.C., R.J.F., A.L.B., N.A.T., Q.E.H., J.C., K.C., M.D.S., E.L., H.B., J.W., and H.N.W. performed the experiments and analyzed the data. M.T.C., R.J.F., F.E., and M.E.K. prepared and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| Gαz | Rabbit anti-Gαz | Abcam, AB150434 | Rabbit; monoclonal | 1:400 | AB_150434 |
| Insulin | GP anti-insulin | Dako, A056401-2 | Guinea pig; polyclonal | 1:500 | AB_2617169 |
| CD28 | Anti-mouse CD28 | BD Biosciences, 553295 | Hamster anti-mouse; monoclonal | 1 μg/mL | AB_394764 |
| CD3ε | Anti-mouse CD3ε | BD Biosciences, 553058 | Hamster anti-mouse; monoclonal | 1 μg/mL | AB_394591 |
| CD8α | Anti-mouse CD8α | BD Biosciences, 561093 | Rat anti-mouse; monoclonal | AB_561093 | |
| TNFα | Anti-mouse TNF | BD Biosciences, 554418 | Rat anti-mouse; monoclonal | AB_395379 | |
| INFγ | Anti-mouse IFNγ | BD Biosciences, 557735 | Rat anti-mouse; monoclonal | AB_396843 | |
| CD4 | Anti-mouse CD4 | eBioscience, 56-0042-80 | Rat anti-mouse; monoclonal | AB_494002 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- CCL2
- chemokine (C-C motif) ligand 2
- CCL4
- chemokine (C-C motif) ligand 4
- CD3
- cluster of differentiation 3
- CD8
- cluster of differentiation 8
- cDNA
- complementary DNA
- CXCL10
- C-X-C motif chemokine 10
- DAPI
- 4′,6-diamidino-2-phenylindole
- Ex4
- exendin-4
- Gαz
- G protein α-subunit
- GSIS
- glucose-stimulated insulin secretion
- IL-1β
- interleukin 1β
- IFNγ
- interferon γ
- KO
- knockout
- MHC
- major histocompatibility complex
- MLD-STZ
- multiple low-dose streptozotocin
- mRNA
- messenger RNA
- NF-κB
- nuclear factor κB
- NOD
- nonobese diabetic
- OGTT
- oral glucose tolerance test
- qRT-PCR
- quantitative reverse transcription polymerase chain reaction
- T1DM
- type 1 diabetes mellitus
- Th1
- type 1 T helper
- TNFα
- tumor necrosis factor α
- TUNEL
- terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling
- WT
- wild-type.
References
- 1.Imperatore G, Boyle JP, Thompson TJ, Case D, Dabelea D, Hamman RF, Lawrence JM, Liese AD, Liu LL, Mayer-Davis EJ, Rodriguez BL, Standiford D; SEARCH for Diabetes in Youth Study Group . Projections of type 1 and type 2 diabetes burden in the U.S. population aged <20 years through 2050: dynamic modeling of incidence, mortality, and population growth. Diabetes Care. 2012;35(12):2515–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dabelea D, Mayer-Davis EJ, Saydah S, Imperatore G, Linder B, Divers J, Bell R, Badaru A, Talton JW, Crume T, Liese AD, Merchant AT, Lawrence JM, Reynolds K, Dolan L, Liu LL, Hamman RF; SEARCH for Diabetes in Youth Study . Prevalence of type 1 and type 2 diabetes among children and adolescents from 2001 to 2009. JAMA. 2014;311(17):1778–1786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coppieters KT, Boettler T, von Herrath M. Virus infections in type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1):a007682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burren OS, Adlem EC, Achuthan P, Christensen M, Coulson RM, Todd JA. T1DBase: update 2011, organization and presentation of large-scale data sets for type 1 diabetes research. Nucleic Acids Res. 2011;39(Suppl 1):D997–D1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49(1):1–7. [DOI] [PubMed] [Google Scholar]
- 6.Engin F, Yermalovich A, Nguyen T, Hummasti S, Fu W, Eizirik DL, Mathis D, Hotamisligil GS. Restoration of the unfolded protein response in pancreatic β cells protects mice against type 1 diabetes [published correction appears in Sci Transl Med. 2013;5(214):214er11]. Sci Transl Med. 2013;5(211):211ra156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Engin F. ER stress and development of type 1 diabetes. J Investig Med. 2016;64(1):2–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pirot P, Cardozo AK, Eizirik DL. Mediators and mechanisms of pancreatic beta-cell death in type 1 diabetes. Arq Bras Endocrinol Metabol. 2008;52(2):156–165. [DOI] [PubMed] [Google Scholar]
- 9.Mathis D, Vence L, Benoist C. beta-Cell death during progression to diabetes. Nature. 2001;414(6865):792–798. [DOI] [PubMed] [Google Scholar]
- 10.Kimple ME, Joseph JW, Bailey CL, Fueger PT, Hendry IA, Newgard CB, Casey PJ. Galphaz negatively regulates insulin secretion and glucose clearance. J Biol Chem. 2008;283(8):4560–4567. [DOI] [PubMed] [Google Scholar]
- 11.Kimple ME, Keller MP, Rabaglia MR, Pasker RL, Neuman JC, Truchan NA, Brar HK, Attie AD. Prostaglandin E2 receptor, EP3, is induced in diabetic islets and negatively regulates glucose- and hormone-stimulated insulin secretion. Diabetes. 2013;62(6):1904–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimple ME, Nixon AB, Kelly P, Bailey CL, Young KH, Fields TA, Casey PJ. A role for G(z) in pancreatic islet beta-cell biology. J Biol Chem. 2005;280(36):31708–31713. [DOI] [PubMed] [Google Scholar]
- 13.Kimple ME, Hultman RC, Casey PJ. Signaling through Gz In: Bradshaw RA, Dennis EA, eds. Handbook of Cell Signaling. 2nd ed Burlington, MA: Elsevier; 2009:1649–1653. [Google Scholar]
- 14.Kimple ME, Moss JB, Brar HK, Rosa TC, Newgard CB, Casey PJ. The effects of Gαz signaling on pancreatic β-cell function and mass. FASEB J. 2012;26(1 Suppl):615.7. [Google Scholar]
- 15.Kimple ME, Moss JB, Brar HK, Rosa TC, Truchan NA, Pasker RL, Newgard CB, Casey PJ. Deletion of GαZ protein protects against diet-induced glucose intolerance via expansion of β-cell mass. J Biol Chem. 2012;287(24):20344–20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brill AL, Wisinski JA, Cadena MT, Thompson MF, Fenske RJ, Brar HK, Schaid MD, Pasker RL, Kimple ME. Synergy between Gαz deficiency and GLP-1 analog treatment in preserving functional beta-cell mass in experimental diabetes. Mol Endocrinol. 2016;30(5):543–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson MA, Leiter EH. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5(6):601–604. [DOI] [PubMed] [Google Scholar]
- 18.Delovitch TL, Singh B. The nonobese diabetic mouse as a model of autoimmune diabetes: immune dysregulation gets the NOD. Immunity. 1997;7(6):727–738. [DOI] [PubMed] [Google Scholar]
- 19.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29(1):1–13. [DOI] [PubMed] [Google Scholar]
- 20.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127(11):2317–2322. [DOI] [PubMed] [Google Scholar]
- 21.Oropeza D, Jouvet N, Budry L, Campbell JE, Bouyakdan K, Lacombe J, Perron G, Bergeron V, Neuman JC, Brar HK, Fenske RJ, Meunier C, Sczelecki S, Kimple ME, Drucker DJ, Screaton RA, Poitout V, Ferron M, Alquier T, Estall JL. Phenotypic characterization of MIP-CreERT1Lphi mice with transgene-driven islet expression of human growth hormone. Diabetes. 2015;64(11):3798–3807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wicksteed B, Brissova M, Yan W, Opland DM, Plank JL, Reinert RB, Dickson LM, Tamarina NA, Philipson LH, Shostak A, Bernal-Mizrachi E, Elghazi L, Roe MW, Labosky PA, Myers MG Jr, Gannon M, Powers AC, Dempsey PJ. Conditional gene targeting in mouse pancreatic ß-cells: analysis of ectopic Cre transgene expression in the brain. Diabetes. 2010;59(12):3090–3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neuman JC, Truchan NA, Joseph JW, Kimple ME. A method for mouse pancreatic islet isolation and intracellular cAMP determination. J Vis Exp. 2014;Jun 25(88):e50374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Truchan NA, Brar HK, Gallagher SJ, Neuman JC, Kimple ME. A single-islet microplate assay to measure mouse and human islet insulin secretion. Islets. 2015;7(3):e1076607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davis DB, Lavine JA, Suhonen JI, Krautkramer KA, Rabaglia ME, Sperger JM, Fernandez LA, Yandell BS, Keller MP, Wang IM, Schadt EE, Attie AD. FoxM1 is up-regulated by obesity and stimulates beta-cell proliferation. Mol Endocrinol. 2010;24(9):1822–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagnon AW, Manning DR, Catani L, Gewirtz A, Poncz M, Brass LF. Identification of Gz alpha as a pertussis toxin-insensitive G protein in human platelets and megakaryocytes. Blood. 1991;78(5):1247–1253. [PubMed] [Google Scholar]
- 27.Zigman JM, Westermark GT, LaMendola J, Steiner DF. Expression of cone transducin, Gz alpha, and other G-protein alpha-subunit messenger ribonucleic acids in pancreatic islets. Endocrinology. 1994;135(1):31–37. [DOI] [PubMed] [Google Scholar]
- 28.Hinton DR, Blanks JC, Fong HK, Casey PJ, Hildebrandt E, Simons MI. Novel localization of a G protein, Gz-alpha, in neurons of brain and retina. J Neurosci. 1990;10(8):2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hultman R, Kumari U, Michel N, Casey PJ. Gαz regulates BDNF-induction of axon growth in cortical neurons. Mol Cell Neurosci. 2014;58:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman RM, Turley S, Mellman I, Inaba K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J Exp Med. 2000;191(3):411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bach JF, Mathis D. The NOD mouse. Res Immunol. 1997;148(5):285–286. [DOI] [PubMed] [Google Scholar]
- 32.Tisch R, McDevitt H. Insulin-dependent diabetes mellitus. Cell. 1996;85(3):291–297. [DOI] [PubMed] [Google Scholar]
- 33.Novak J, Beaudoin L, Griseri T, Lehuen A. Inhibition of T cell differentiation into effectors by NKT cells requires cell contacts. J Immunol. 2005;174(4):1954–1961. [DOI] [PubMed] [Google Scholar]
- 34.Rabinovitch A, Suarez-Pinzon WL, Sorensen O, Bleackley RC, Power RF. IFN-gamma gene expression in pancreatic islet-infiltrating mononuclear cells correlates with autoimmune diabetes in nonobese diabetic mice. J Immunol. 1995;154(9):4874–4882. [PubMed] [Google Scholar]
- 35.Diana J, Simoni Y, Furio L, Beaudoin L, Agerberth B, Barrat F, Lehuen A. Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat Med. 2013;19(1):65–73. [DOI] [PubMed] [Google Scholar]
- 36.Christen U, Von Herrath MG. Apoptosis of autoreactive CD8 lymphocytes as a potential mechanism for the abrogation of type 1 diabetes by islet-specific TNF-alpha expression at a time when the autoimmune process is already ongoing. Ann N Y Acad Sci. 2002;958:166–169. [DOI] [PubMed] [Google Scholar]
- 37.Green EA, Flavell RA. The temporal importance of TNFalpha expression in the development of diabetes. Immunity. 2000;12(5):459–469. [DOI] [PubMed] [Google Scholar]
- 38.Grewal IS, Grewal KD, Wong FS, Picarella DE, Janeway CA Jr, Flavell RA. Local expression of transgene encoded TNF alpha in islets prevents autoimmune diabetes in nonobese diabetic (NOD) mice by preventing the development of auto-reactive islet-specific T cells. J Exp Med. 1996;184(5):1963–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nepom GT, Ehlers M, Mandrup-Poulsen T. Anti-cytokine therapies in T1D: Concepts and strategies. Clin Immunol. 2013;149(3):279–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinovitch A, Suarez-Pinzon W, El-Sheikh A, Sorensen O, Power RF. Cytokine gene expression in pancreatic islet-infiltrating leukocytes of BB rats: expression of Th1 cytokines correlates with beta-cell destructive insulitis and IDDM. Diabetes. 1996;45(6):749–754. [DOI] [PubMed] [Google Scholar]
- 41.Rabinovitch A. An update on cytokines in the pathogenesis of insulin-dependent diabetes mellitus. Diabetes Metab Rev. 1998;14(2):129–151. [DOI] [PubMed] [Google Scholar]
- 42.Rabinovitch A, Suarez-Pinzon WL. Roles of cytokines in the pathogenesis and therapy of type 1 diabetes. Cell Biochem Biophys. 2007;48(2-3):159–163. [DOI] [PubMed] [Google Scholar]
- 43.Boldison J, Wong FS. Immune and pancreatic β cell interactions in type 1 diabetes. Trends Endocrinol Metab. 2016;27(12):856–867. [DOI] [PubMed] [Google Scholar]
- 44.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. [DOI] [PubMed] [Google Scholar]
- 45.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. [DOI] [PubMed] [Google Scholar]
- 46.Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. [DOI] [PubMed] [Google Scholar]
- 47.Al-Aoukaty A, Rolstad B, Maghazachi AA. Functional coupling of NKR-P1 receptors to various heterotrimeric G proteins in rat interleukin-2-activated natural killer cells. J Biol Chem. 1997;272(50):31604–31608. [DOI] [PubMed] [Google Scholar]
- 48.Maghazachi AA, Al-Aoukaty A, Naper C, Torgersen KM, Rolstad B. Preferential involvement of Go and Gz proteins in mediating rat natural killer cell lysis of allogeneic and tumor target cells. J Immunol. 1996;157(12):5308–5314. [PubMed] [Google Scholar]
- 49.Maghazachi AA, Al-Aoukaty A. Chemokines activate natural killer cells through heterotrimeric G-proteins: implications for the treatment of AIDS and cancer. FASEB J. 1998;12(11):913–924. [DOI] [PubMed] [Google Scholar]