Abstract
The impact of incretins upon pancreatic β-cell expansion remains extremely controversial. Multiple studies indicate that incretin-based therapies can increase β-cell proliferation, and incretins have been hypothesized to expand β-cell mass. However, disagreement exists on whether incretins increase β-cell mass. Moreover, some reports indicate that incretins may cause metaplastic changes in pancreatic histology. To resolve these questions, we treated a large cohort of mice with incretin-based therapies and carried out a rigorous analysis of β-cell turnover and pancreatic histology using high-throughput imaging. Young mice received exenatide via osmotic pump, des-fluoro-sitagliptin, or glipizide compounded in diet for 2 weeks (short-term) on a low-fat diet (LFD) or 4.5 months (long-term) on a LFD or high-fat diet (HFD). Pancreata were quantified for β-cell turnover and mass. Slides were examined for gross anatomical and microscopic changes to exocrine pancreas. Short-term des-fluoro-sitagliptin increased serum insulin and induced modest β-cell proliferation but no change in β-cell mass. Long-term incretin therapy in HFD-fed mice resulted in reduced weight gain, improved glucose homeostasis, and abrogated β-cell mass expansion. No evidence for rapidly dividing progenitor cells was found in islets or pancreatic parenchyma, indicating that incretins do not induce islet neogenesis or pancreatic metaplasia. Contrasting prior reports, we found no evidence of β-cell mass expansion after acute or chronic incretin therapy. Chronic incretin administration was not associated with histological abnormalities in pancreatic parenchyma; mice did not develop tumors, pancreatitis, or ductal hyperplasia. We conclude that incretin therapies do not generate β-cells or alter pancreatic histology in young mice.
A large cohort of young male mice receiving incretin therapies of varying durations with or without metabolic stress demonstrated that incretins do not expand β-cells or alter pancreatic histology.
Type 2 diabetes (T2D) is generally characterized by the inability of the pancreatic β-cells to produce adequate amounts of insulin to meet the metabolic demand, resulting in uncontrolled blood glucose. Various potent drugs that increase insulin production in patients with T2D have been developed. Sulfonylureas such as glipizide inhibit the K+–adenosine triphosphate (ATP) channel through binding to the sulfonylurea receptor (SUR) subunit, leading to β-cell membrane depolarization and insulin release (1). Gut-derived peptides such as glucagon-like peptide-1 (GLP-1), also known as incretins, stimulate β-cell function and insulin secretion (2). Incretin-based T2D therapies increase circulating GLP-1 levels directly via GLP-1 analogs or indirectly through inhibition of dipeptidyl peptidase-4 (DPP-4)-inactivating enzyme (3). Treatment of patients with T2D with the GLP-1 analog exenatide or with DPP-4 inhibitor preserves β-cell function and dramatically improves glucose control (3). However, the complete mechanism of their beneficial actions remains poorly understood.
Several studies have proposed that incretin therapy also promotes β-cell mass expansion in control mice or partial β-cell mass restoration in diabetic mice. In rodents treated with GLP-1 receptor agonists, β-cell proliferation is increased (4–9). A few studies have demonstrated that DPP-4 inhibitors have mitogenic effects on β-cells in control rodents (10). More prominent are reports that incretins or DPP-4 inhibitors, such as des-fluoro-sitagliptin, increase β-cell mass in rodent models of obesity and diabetes (11–13). Des-fluoro-sitagliptin administration to mice rendered diabetic with a high-fat diet (HFD) and streptozotocin (STZ) resulted in an increase in β-cell mass compared with untreated mice (12, 13). Furthermore, observations of increased small islets and duct associated insulin+ cells suggesting islet neogenesis have been noted after exenatide or sitagliptin treatment in STZ-treated mice (14–17). In contrast, one study indicated that exenatide had no effect on β-cell mass after 28 days of treatment (18). Whether observations of incretin-induced β-cell expansion hold true in human patients is unknown. This fundamental question has plagued incretin research and remains unanswered because of the lack of appropriate model systems. Moreover, the acute nature of in-cretin administration in prior rodent studies requires new experiments with long-term incretin therapy to thoroughly test for β-cell mass expansion. Finally, whether incretin-based therapies such as sitagliptin might recruit specialized progenitors to contribute to β-cell mass expansion through islet neogenesis is also unclear.
A few clinical reports imply that exenatide may occasionally cause pancreatitis (19–21). More worrisome, Butler et al. (22) reported that incretin therapies induced exocrine proliferation and dysplasia in patients with T2D based on brain-dead organ donor pancreatic samples; furthermore, one pancreata contained a neuroendocrine tumor, and three others had microadenomas. Although these human studies were limited in the number of cases available for analysis, rodent studies by Butler et al. (22) similarly suggest that exenatide and sitagliptin promote pancreatic duct proliferation and pancreatitis in rats (23, 24). These observations suggest that incretin-based therapies could promote proliferation of acinar or ductal pancreas, eventually causing oncogenic transformation. However, these reports only include a small cohort of animals with rare ductal metaplastic events. In the wake of these collective findings, the conversation of incretins and adverse effects on the exocrine pancreas is ongoing. As such, we also considered any potential negative side effects on the exocrine pancreas.
Our main objective was to determine if long-term des-fluoro-sitagliptin treatment in mice induces significant β-cell mass expansion or alters pancreatic parenchyma. We continuously treated mice with des-fluoro-sitagliptin, exenatide, or glipizide for several months on low-fat diet (LFD) or HFD to induce obesity and metabolic stress similar to T2D. Pancreata were collected for rigorous quantification of β-cell proliferation and mass and for exocrine histology. By directly comparing des-fluoro-sitagliptin with exenatide and glipizide, we compared the effects of physiologic GLP-1 (via DPP-4 inhibition) vs pharmacologic GLP-1 (such as GLP-1 analogs) or glipizide (commonly used oral antidiabetic drug). Here, we describe our studies that show that long-term incretin therapies may not affect β-cell mass expansion and pancreatic histology.
Methods
Mice
Animal experiments were performed at Children’s Hospital of Philadelphia according to the Institutional Animal Care and Use Committee. Male B6129SF1/J mice (JAX 101043) were obtained from Jackson Laboratories (Bar Harbor, ME). Ten-week-old male mice received glipizide (0.02% weight-to-weight ratio) or des-fluoro-sitagliptin (Merck, Kenilworth, NJ) (25) (1.1% weight-to-weight ratio) compounded in the diet (for a target dose of 576 mg/kg) as previously described (12) or exenatide (1 nmol/kg/d) via mini osmotic pump (1004; Alzet, Cupertino, CA) for 2 weeks (short-term) or 18 weeks (long-term). Short-term cohorts received a LFD (10% kcal from fat) (D12450B; Research Diets, New Brunswick, NJ). Long-term cohorts received a LFD or HFD (60% kcal fat) (D12492; Research Diets) throughout the study. Mice were labeled via the drinking water with 5-bromo-2′-deoxyuridine (BrdU; 1 g/L) (Sigma-Aldrich, St. Louis, MO) or 5-ethynyl-2′-deoxyuridine (EdU; 0.5 g/L) (Life Technologies, Grand Island, NY) as previously described (26) for 1 week each. Intraperitoneal glucose tolerance tests (GTTs) were performed as described previously (27). Serum insulin was measured using a Mouse Ultrasensitive Insulin ELISA (Alpco Diagnostics, Salem, NH).
Immunohistochemistry and morphometry
Microscopy studies and subsequent analysis was performed at Texas Children’s Hospital. Paraffin sections were prepared as previously described (26). Primary antisera included guinea pig anti-insulin (Dako, Carpinteria, CA) and rat anti-BrdU (Accurate Chemical, Westbury, NY) followed by secondary antisera conjugated to Cy3/Cy5 (Jackson ImmunoResearch, West Grove, PA) and DAPI (Molecular Probes, Eugene, OR). EdU was detected using Click-iT EdU Alexa Fluor 647 according to the manufacturer’s protocol (Life Technologies). Slides were imaged to quantify β-cell morphometry as previously (28) using Volocity 6.1.1 (PerkinElmer, Waltham, MA). BrdU- or EdU-positive β-cell ratios to total β-cells were calculated. At least 2290 β-cells were counted per animal. Analyses were confirmed by a second independent counter. Extensive details for the number of cells counted and sections analyzed are included in Supplemental Tables (342.8KB, xlsx) .
Pathology
Pancreatic sections from long-term treatment cohorts were stained with hematoxylin and eosin. Slides were scanned digitally via Aperio (Leica Biosystems, Buffalo Grove, IL). In total, there were 521 stained slides, with four or five sections per slide, from the head and tail pancreas. Slides were scored by an independent pathologist (R.A.W.), and subsequently the code was broken to tabulate the findings. Evaluations were performed for the following: (1) to assess the “normalcy” of the ductular system based on experience and compared with control; (2) to report on histopathological findings, including hyperplasia, atrophy, differences in ductular cell size and shape, dilations, cysts, inflammation, and inspissated secretion; (3) to report histopathological observations in individual, group and overall incidence and severity scores (0 = none, 1 = minimal, 2 = slight, 3 = mild, 4 = moderate, 5 = severe); (4) to report distribution scores (O = none, F = focal, M = multifocal, D = diffuse); and (5) to provide narrative or conclusion as to the histologic presence or lack of hyperplasia or other changes in the ductular system of the exocrine pancreas in this study. Subsequently, head pancreas sections for all eight groups (262 slides) were reanalyzed to determine the incidence and appearance of pancreatic duct glands (PDGs) in response to the report by Butler et al. (22).
Statistics
Results are reported as means ± standard error of the mean and compared with a one-way or two-way analysis of variance where appropriate.
Results
Short-term incretin therapy improves glucose tolerance
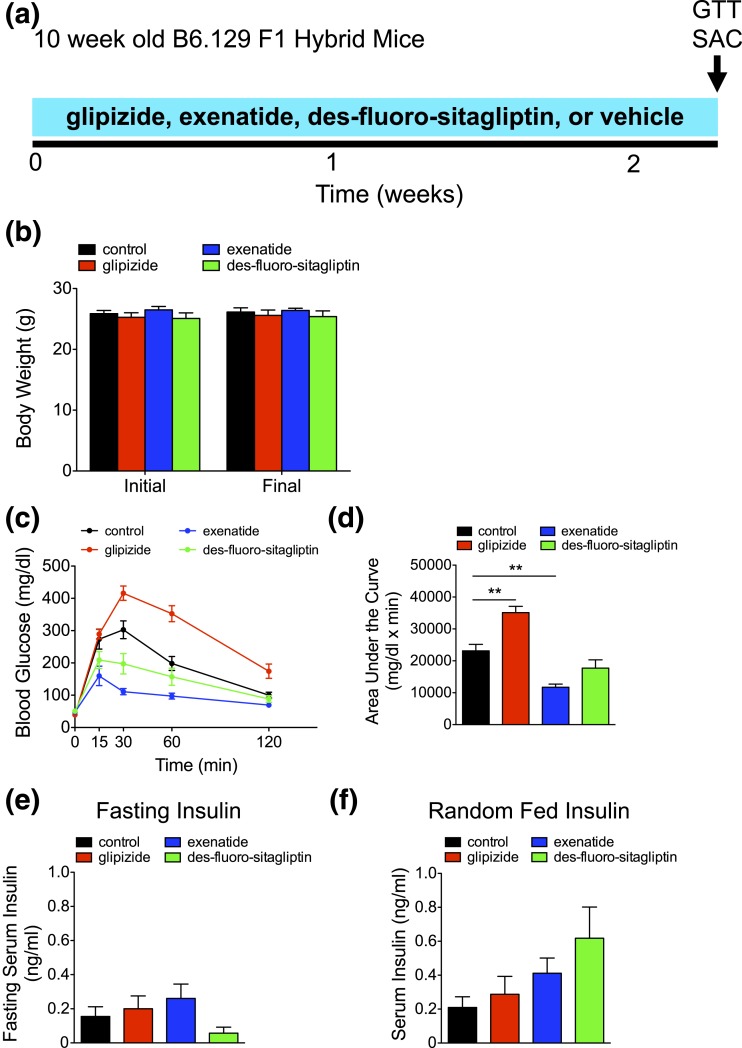
Ten-week-old B6.129 F1 hybrid mice were administered incretin therapies via des-fluoro-sitaglipitin or exenatide or the sulfonylurea glipizide for ∼2 weeks [Fig. 1(a)]. The therapies were well tolerated with minimal impact on body weight [Fig. 1(b)]. Short-term exenatide significantly improved glucose tolerance, although des-fluoro-sitaglipitin had little impact [Fig. 1(c) and 1(d)]. In contrast, glipizide impaired glucose tolerance, a seemingly paradoxical response for an antidiabetic therapy. Continuous inhibition of K+-ATP SUR might prevent β-cells from sensing glucose flux (29). Indeed, genetic lesions of K+-ATP SUR are associated with glucose intolerance (30, 31). Fasting insulin was not significantly different between groups [Fig. 1(e)]. Random-fed insulin was insignificantly altered but demonstrated a two- and threefold increase with exenatide and des-fluoro-sitaglipitin, respectively, compared with control [Fig. 1(f); Supplemental Table 1 (342.8KB, xlsx) ], consistent with the effects of incretins to potentiate insulin secretion. Short-term glipizide had little to no impact on serum insulin [Fig. 1(e) and 1(f); Supplemental Table 1 (342.8KB, xlsx) ].
Figure 1.
Short-term incretin therapy improves glucose tolerance. (a) Timing of short-term diabetes therapy (glipizide, exenatide, des-fluoro-sitagliptin, or vehicle [control]), GTT, and kill (SAC) in 10-week-old B6.129 F1 hybrid mice. (b) Initial and final body weight (in grams). (c) Intraperitoneal GTT was performed, with the corresponding area under the curve analysis in (d). (e and f) Fasting and random-fed serum insulin (ng/mL). Means ± standard error of the mean (n = 5 animals/group). **P < 0.01.
Short-term des-fluoro-sitagliptin therapy increases β-cell proliferation
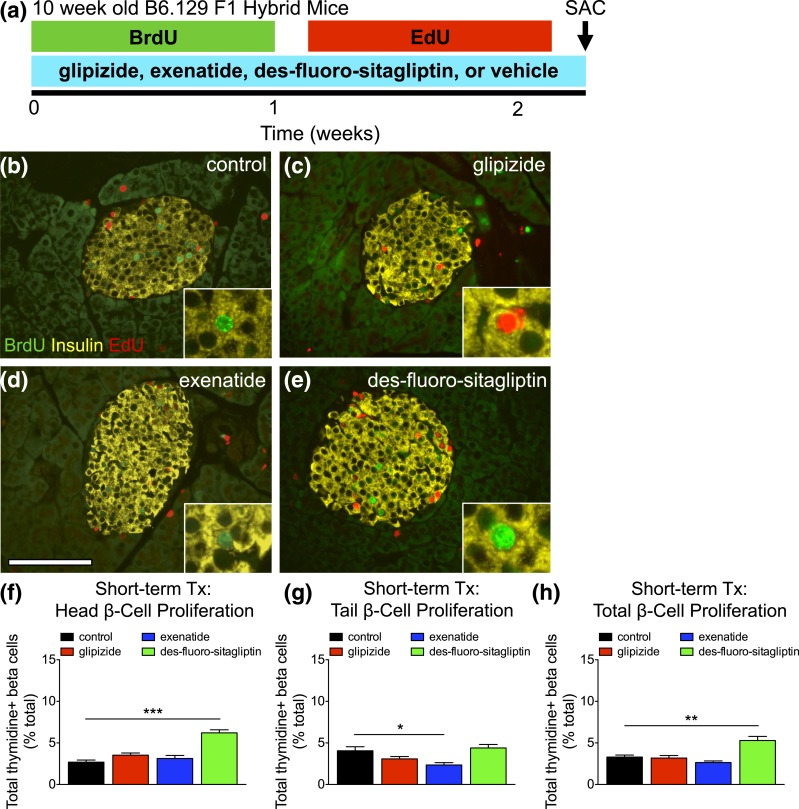
To test the impact of short-term incretins upon β-cell proliferation, we administered thymidine analogs for 2 weeks prior to kill [Fig. 2(a)]. Two-week thymidine incorporation in β-cells of control mice was ∼3% [Fig. 2(b–h); Supplemental Table 2 (342.8KB, xlsx) ]. Glipizide and exenatide had no effect on β-cell proliferation in the total pancreas. In contrast, des-fluoro-sitaglipitin significantly increased β-cell proliferation, with 5.3% of β-cells in the total pancreas incorporating thymidine during 2 weeks of labeling. Thus, des-fluoro-sitaglipitin increases β-cell proliferation over the short term.
Figure 2.
Short-term des-fluoro-sitagliptin treatment (Tx) increases β-cell proliferation. (a) Timing of short-term diabetes therapy (glipizide, exenatide, des-fluoro-sitagliptin, or vehicle [control]), thymidine labeling (BrdU and EdU 1 week each), GTT, and kill (SAC) in 10-week-old B6.129 F1 hybrid mice. (b–e) Islet staining for BrdU (green), insulin (yellow), and EdU (red) in short-term treated mice with (b) vehicle (control), (c) glipizide, (d) exenatide, or (e) des-fluoro-sitagliptin. Scale bar, 100 μm. (f–h) Cumulative β-cell proliferation, measured by thymidine+ (BrdU and EdU) insulin+ cells as percent of total β-cells for the (f) head, (g) tail, and (h) total pancreas. Mean ± standard error of the mean (≥2427 β-cells/pancreas; n = 5 animals/group). *P < 0.05; **P < 0.01; ***P < 0.001.
Incretin therapy does not induce rapidly dividing progenitors in islets or pancreatic parenchyma
Several studies have observed an increase in the num-ber of small islets or hormone+ cells in ducts, possibly suggesting increased neogenesis (14–17). To objectively detect β-cell neogenesis, we used sequential administration of two thymidine analogs and quantified cells undergoing multiple rounds of cell division, as previously described (9). This approach will test if β-cells expand via highly proliferative transit-amplifying progenitors, as observed in skin or intestine. However, BrdU+/EdU+ copositive β-cells were very rare [Supplemental Fig. 1(a) (3.9MB, pdf) ], as evident in representative images shown in Fig. 2(b–e) and quantified in Supplemental Table 3 (342.8KB, xlsx) . Moreover, in the current study short-term incretin therapies had minimal or no impact on β-cell proliferation, further indicating a lack of highly replicative progenitor cells. These studies confirm our previous observations that β-cells expand in response to incretins via self-renewal, rather than a highly proliferative progenitor cell population (9).
Rapidly dividing cells within the exocrine pancreas would indicate hyperplasia after incretin therapy, such as was suggested by Butler et al. (22) to occur in T2D pancreata within PDGs. Sporadic BrdU+/EdU+ copositive exocrine cells were observed in various pancreata [Supplemental Fig. 1(b) (3.9MB, pdf) ]. However, the cells were generally rare, and no association with incretin treatment was observed [Supplemental Fig. 1(b) (3.9MB, pdf) ; Supplemental Table 3 (342.8KB, xlsx) )]. Thus, incretin therapies do not induce highly proliferative progenitor cells within the exocrine pancreas in young mice.
Short-term incretin therapy does not expand β-cells
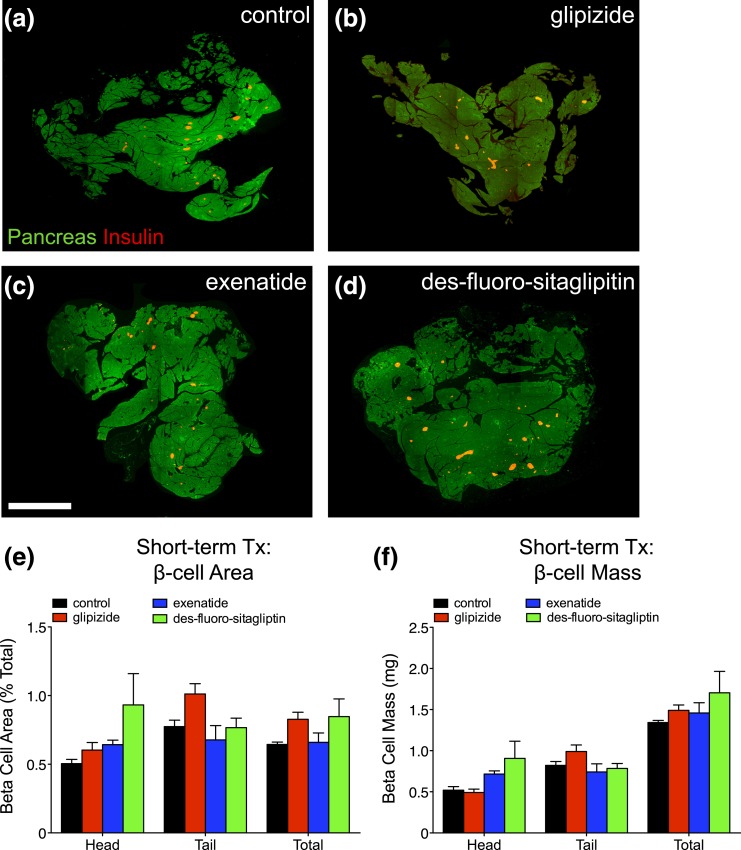
To determine whether incretins expand β-cells, we performed extensive histomorphometry to quantify β-cell mass [Fig. 3(a–d)]. β-cell area in mice treated with glipizide, exenatide, and des-fluoro-sitaglipitin was comparable to controls in the head, tail, and total pancreas [Fig. 3(e); Supplemental Table 4 (342.8KB, xlsx) )]. Similarly, β-cell mass after glipizide, exenatide, and des-fluoro-sitaglipitin therapy was unchanged from controls. Thus, short-term incretin therapy does not expand β-cell mass in young mice.
Figure 3.
Short-term incretin therapy (Tx) does not expand β-cells. (a–d) Total pancreas (green) and total β-cell area (red) generated by composites of low-power image scans from pancreatic sections of short-term treated mice with (a) vehicle (control), (b) glipizide, (c) exenatide, or (d) des-fluoro-sitagliptin. Scale bar, 2 mm. (e) β-cell area (% total) and (f) β-cell mass (in milligrams) for the head, tail, and total pancreas. Mean ± standard error of the mean (21–28 sections/pancreas; n = 5 animals/group).
Long-term incretin therapy improves glucose homeostasis, even on a HFD
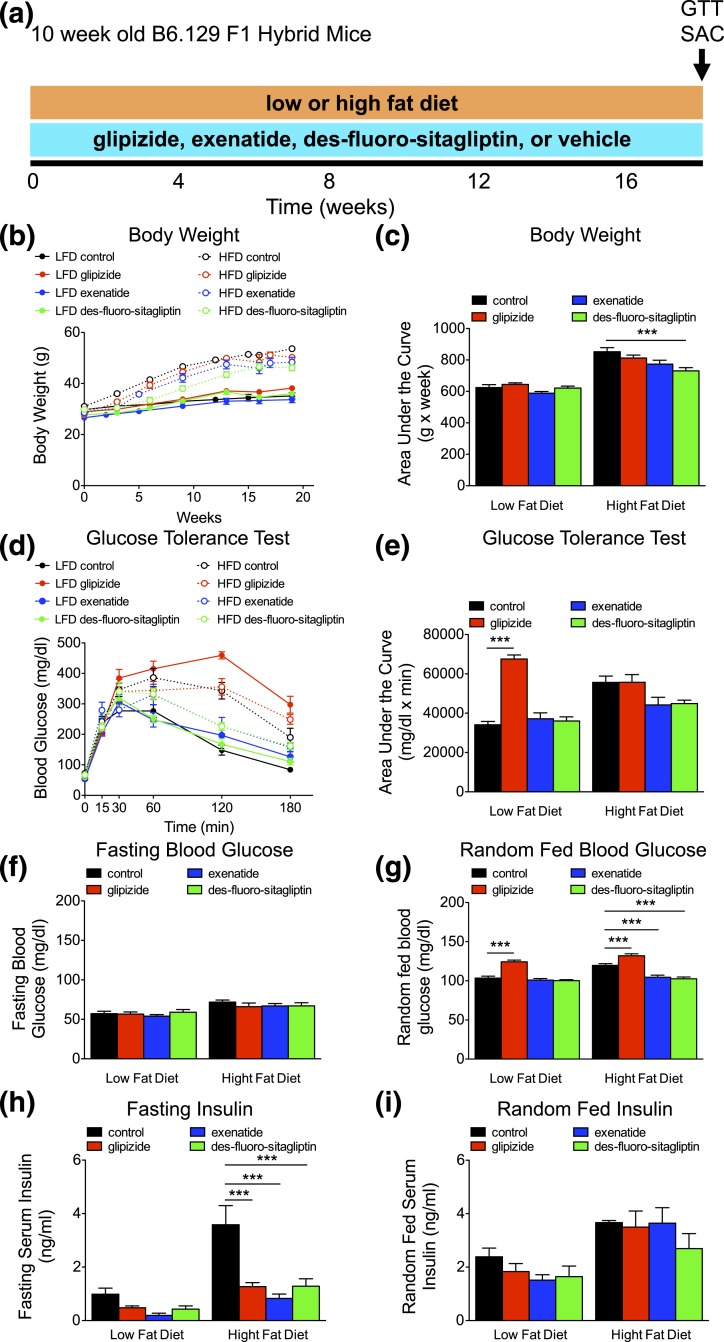
To test the impact of long-term incretin therapy on β-cell function and mass, we administered glipizide or incretin drugs for 18 weeks [Fig. 4(a)]. Additionally, mice were placed on a LFD or a HFD to determine the effect of incretin therapy on β-cells during metabolic stress. As expected, HFD exposure for 18 weeks induced substantial weight gain in all groups (P < 0.001) [Fig. 4(b) and 4(c); Supplemental Table 5 (342.8KB, xlsx) ]. Notably, mice on long-term des-fluoro-sitaglipitin therapy gained significantly less weight on a HFD compared with control mice [Fig. 4(b) and 4(c)]. Long-term incretin therapy had no effect on glucose tolerance in mice fed a LFD. As expected from our short-term studies and prior reports (29–31), long-term glipizide-treated LFD-fed mice exhibited impaired glucose tolerance [Fig. 4(d) and 4(e); Supplemental Table 5 (342.8KB, xlsx) ]. Glucose tolerance was impaired in both control and glipizide HFD-treated mice (P < 0.05) [Fig. 4(d) and 4(e); Supplemental Table 5 (342.8KB, xlsx) ]. In contrast, long-term therapy with exenatide or des-fluoro-sitaglipitin during increased metabolic stress abrogated HFD-induced metabolic changes. Fasting glucose was elevated with HFD feeding (P < 0.001) [Fig. 4(f); Supplemental Table 5 (342.8KB, xlsx) ]. Random-fed glucose was higher with long-term glipizide-treated LFD and HFD groups, especially in HFD mice, considering that control HFD-fed mice exhibited elevated (P < 0.001) glucose compared with LFD controls [Fig. 4(g); Supplemental Table 5 (342.8KB, xlsx) ]. This further reveals that continuous long-term glipizide treatment disturbs glucose control in mice. Random-fed glucose in HFD-fed mice was maintained with incretin therapy, equivalent to LFD-fed mice receiving vehicle or incretin therapy. Fasting insulin was increased (P < 0.001) in control HFD-fed mice [Fig. 4(h); Supplemental Table 5 (342.8KB, xlsx) ]. However, fasting insulin was lower with glipizide and incretin therapy compared with control mice with HFD [Fig. 4(h) and 4(i); Supplemental Table 5 (342.8KB, xlsx) ], consistent with lower insulin requirements due to lower body weight and improved glucose tolerance. Overall, incretin therapy significantly enhanced glucose control during metabolic stress with HFD feeding.
Figure 4.
Long-term incretin therapy improves glucose homeostasis even on a HFD. (a) Timing of long-term diabetes therapy (glipizide, exenatide, des-fluoro-sitagliptin, or vehicle [control]), diet administration (low or high fat), GTT, and kill (SAC) in 10-week-old B6.129 F1 hybrid mice. (b) Body weight (in grams) recorded over time with the corresponding area under the curve analysis in (c). (d) Intraperitoneal GTT was performed, with the corresponding area under the curve analysis in (e). (f) Fasting and (g) average random-fed blood glucose (mg/dL). Means ± standard error of the mean (n = 8–12 animals/group). (h and i) Fasting and random-fed serum insulin (ng/mL). Means ± standard error of the mean (n = 4–12 animals/group). ***P < 0.001 vs control within diet (one-way analysis of variance).
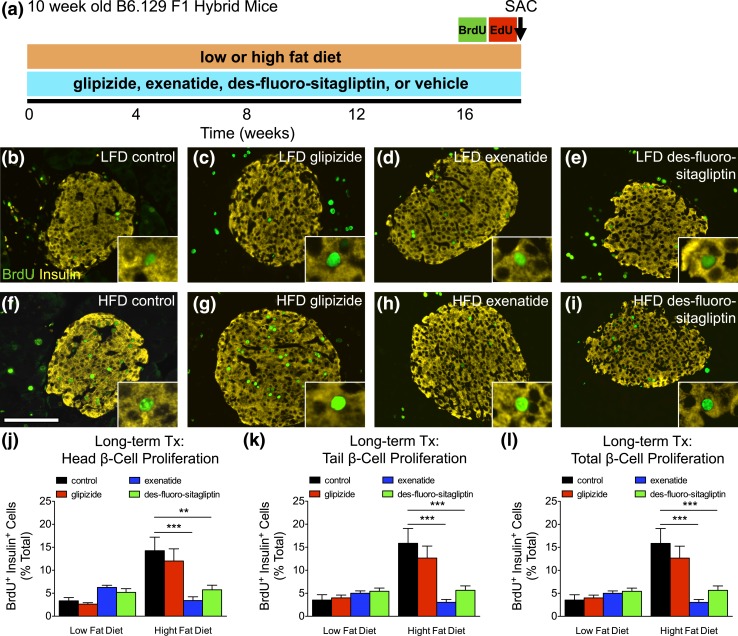
HFD-induced β-cell proliferation is abrogated with long-term incretin therapy
We measured β-cell proliferation with thymidine analogs to test if des-fluoro-sitaglipitin stimulates β-cell expansion in response to metabolic stress [Fig. 5(a–i)]. β-Cell proliferation was increased (P < 0.001) in control HFD-fed mice compared with LFD [Fig. 5(j–l); Supplemental Table 6 (342.8KB, xlsx) ]. β-Cell proliferation was similarly elevated in HFD glipizide-treated mice. However, β-cell proliferation was significantly lower in both exenatide and des-fluoro-sitaglipitin groups on a HFD and was comparable to that in LFD-fed mice. Thus, incretins seem to reduce metabolic stress–induced compensatory β-cell proliferation (Fig. 5) while improving glucose homeostasis (Fig. 4).
Figure 5.
HFD-induced β-cell proliferation is abrogated with long-term incretin therapy. (a) Timing of long-term diabetes therapy (glipizide, exenatide, des-fluoro-sitagliptin, or vehicle [control]), thymidine labeling (BrdU and EdU, 1 week each), GTT, and kill (SAC) in 10-week-old B6.129 F1 hybrid mice. (b–i) Islet staining for BrdU (green) or insulin (yellow) in mice placed on a LFD (top row) or a HFD (bottom row). Scale bar, 100 μm. (j–l) Cumulative β-cell proliferation measured by BrdU+ insulin+ cells as percent of total β-cells (unable to quantify EdU staining) for the (j) head, (k) tail, and (l) total pancreas. Means ± standard error of the mean (≥2290 β-cells/pancreas; n = 8–12 animals/group). **P < 0.01; ***P < 0.001 vs control HFD by one-way analysis of variance.
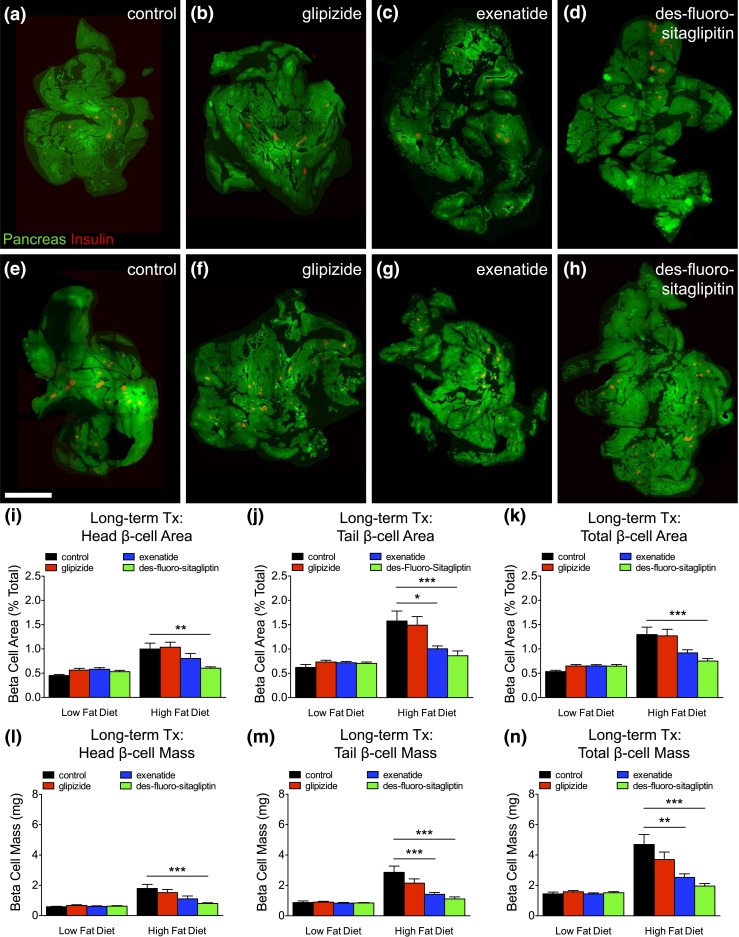
HFD-induced β-cell mass expansion is reduced with long-term incretins
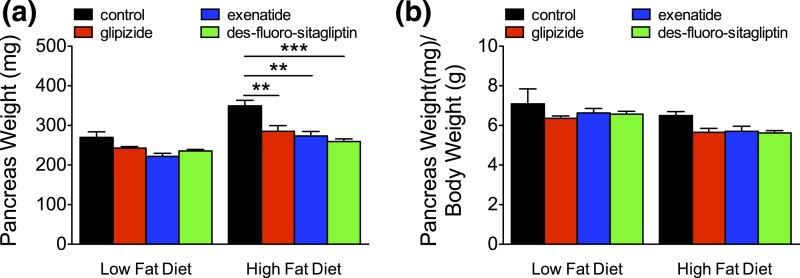
We performed β-cell morphometry [Fig. 6(a–h)] to determine the impact of reduced compensatory β-cell proliferation during metabolic stress. On a LFD, glipizide or incretin therapy had no effect on β-cell area and mass compared with controls [Fig. 6(i–n); Supplemental Table 7 (342.8KB, xlsx) ]. HFD feeding in control or glipizide-treated mice significantly increased (P < 0.001) β-cell area and mass. HFD-induced β-cell expansion was reduced with long-term exenatide therapy and completely abrogated with des-fluoro-sitaglipitin. Changes in β-cell mass were not due to reduced pancreas mass, accounting for differences in body weight (Fig. 7; Supplemental Table 7 (342.8KB, xlsx) ). These findings further indicate that long-term incretin therapy reduces the metabolic demand associated with HFD feeding, eliminating compensatory β-cell mass expansion.
Figure 6.
HFD-induced β-cell expansion is reduced with long-term incretin therapy. (a–h) Total pancreas (green) and total β-cell area (red) generated by composites of low-power image scans from pancreatic sections of long-term treated mice with (a and e) vehicle (control), (b and f) glipizide, (c and g) exenatide, or (d and h) des-fluoro-sitagliptin. Scale bar, 2 mm. (i–k) β-Cell area (% total) and (l–n) β-cell mass (in milligrams) for the (i and l) head, (j and m) tail, and (k and n) total pancreas. Means ± standard error of the mean (19–37 sections/pancreas; n = 8–12 animals/group). *P < 0.05; **P < 0.01; ***P < 0.001 vs control HFD by one-way analysis of variance.
Figure 7.
Long-term incretin therapy does not change pancreas weight in HFD-fed mice. (a) Pancreas weight (in milligrams) and (b) pancreas weight (in milligrams) normalized to body weight (in grams) from mice with long-term therapy on a LFD or and HFD. Means ± standard error of the mean (n = 8–12 animals/group). **P < 0.01; ***P < 0.001 vs control by one-way analysis of variance.
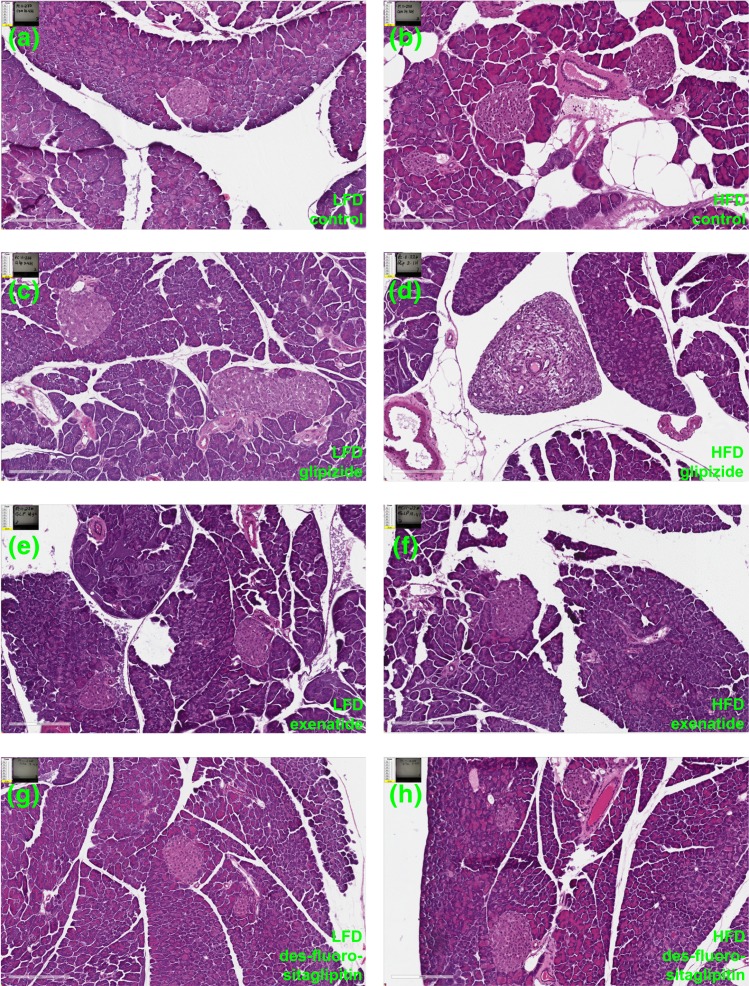
Long-term incretin therapy does not alter pancreatic histology in young mice
Previous studies suggested that incretin therapy might induce exocrine duct metaplasia (22, 23). As a result, we carried out an extensive study of pancreas histopathology on hematoxylin and eosin–stained slides (Fig. 8; Supplemental Figs. 2–9 (3.9MB, pdf) ). A trained rodent pathologist examined 521 pancreas head and tail slides distributed across all four treatment groups on both a LFD and HFD (n = 8–11/group). Slides were evaluated for changes in exocrine duct hyperplasia, atrophy, size, inflammation, and PDG changes (see Methods). Small amounts of tissue necrosis and inflammation were observed sporadically in mice of all groups (Supplemental Tables 8–10 (342.8KB, xlsx) ). Histopathological changes were focal, mild in severity, and considered incidental, with no relation to any particular treatment. Ductal changes were observed in only one mouse among all groups. Within a glipizide-treated mouse on a HFD, mild ductular hyperplasia was observed in the pancreas head and was considered secondary to inflammation at the site and most likely unrelated to treatment [Fig. 8(d); Supplemental Table 9 (342.8KB, xlsx) ]. Subsequently, all pancreas head slides (262 slides) were evaluated to determine the incidence and appearance of PDGs to address concerns reported by Butler et al. (22). PDGs were observed in the larger interlobular ducts and the larger excretory ducts, with no evidence of hyperplasia or other proliferation of PDGs in any group. Thus, long-term incretin therapies had no effect on ductal hyperplasia, inflammation, or oncogenic transformation in young adult mice.
Figure 8.
Long-term incretin therapy does not alter pancreas histology. Hematoxylin and eosin images from Aperio scans of pancreatic sections from mice on a LFD (a–d) or a HFD (e–h) with long-term treatment of (a and e) vehicle (control), (b and f) glipizide, (c and g) exenatide, or (d and h) des-fluoro-sitagliptin. Scale bar, 200 μm.
Discussion
Through short- and long-term administration of two different incretin therapies, we find that under the current conditions exenatide and des-fluoro-sitaglipitin improve glucose control but do not expand β-cell mass or induce pancreatic histological alterations. Short-term incretins potentiated serum insulin and improved glucose tolerance with a small increase in β-cell proliferation but caused no change in β-cell mass or neogenesis. Long-term incretins reduced HFD-induced weight gain and improved glucose homeostasis without HFD-associated increase in β-cell proliferation and mass. Importantly, long-term administration of exenatide or des-fluoro-sitaglipitin did not alter pancreatic acinar histology or induce metaplasia of pancreatic duct glands.
Although researchers and clinicians continue to consider the potential β-cell mitogenic benefits of incretin therapy in humans (3, 32, 33), the impact of incretins in human pancreata remains unresolved due to the lack of rigorous quantitative methodologies or appropriate model systems. One potential model of T2D combined STZ with increased metabolic demand (HFD+STZ) in rodents, but this model is limited for the study of β-cell expansion due to possible toxic effects of STZ on surviving β-cells (34, 35), and the extreme β-cell deficiency of STZ might not directly apply to human T2D, where much more β-cell mass is typically present (22). To address these potential limitations, we used HFD alone as a robust stimulus for metabolic stress and obesity to model prominent features of T2D. Because prior reports on incretin-induced β-cell expansion were mostly performed with short-term incretin administration (1–2 months), we carried out short-term (∼2 weeks) and long-term (4.5 months) therapies with multiple T2D drugs followed by rigorous β-cell quantification. Thus, our combined approach and methodologies allowed us to thoroughly interrogate the effects of incretin therapy on β-cell expansion.
We provide data that indicate incretins may have little (if any) impact on β-cell mass expansion. Several previous reports have indicated that incretin therapy via administration of a GLP-1 receptor agonist or DPP-4 inhibitor stimulates β-cell proliferation and increases β-cell mass in control nondiabetic rodents (4–10). Incretin therapy is used clinically in patients with diabetes to improve glucose control through increased β-cell function and insulin secretion, but the potential added benefit of increasing β-cell mass is appealing. In agreement with these prior studies, we found that short-term treatment (∼2 weeks) with des-fluoro-sitaglipitin stimulated β-cell proliferation in young mice. However, the magnitude of β-cell proliferation increase was quite small (1.8%), suggesting that incretin therapy may not contribute substantially to β-cell expansion. Indeed, we found that, when administered for 4.5 months, des-fluoro-sitaglipitin and exenatide did not significantly affect β-cell mass in young mice on a LFD.
Our studies further inform the potential of incretins to augment β-cells in T2D. Previous studies in HFD+STZ mice suggested that β-cell proliferation and mass might favorably respond to incretins (15, 36). However, the limitations of the HFD+STZ model noted earlier likely preclude its use to study incretin-induced β-cell proliferation for T2D. Thus, we tested if incretin therapies expand β-cells in a HFD model of T2D-associated metabolic stress. In contrast to previous reports, we found long-term (4.5 months) treatment with exenatide or des-fluoro-sitaglipitin did not stimulate β-cell proliferation or mass expansion on a HFD. Indeed, HFD-induced β-cell proliferation and mass expansion was abrogated by incretin therapy. Incretin therapy alleviated the increased metabolic demand associated with HFD through increased insulin secretion and reduced weight gain, resulting in improved glucose tolerance and likely also peripheral insulin sensitivity. Notably, our results of enhanced insulin sensitivity and unaltered β-cell expansion are consistent with a study of short-term exenatide in HFD mice (18). In summary, our long-term incretin studies suggest that incretins do not augment β-cell mass expansion in response to increased metabolic demand from HFD in mice.
Furthermore, our study addresses outstanding controversies regarding possible roles of incretins to stimulate β-cell neogenesis. Pospisilik et al. (15) suggested that increased neogenesis occurred after sitaglipitin treatment based on observations of more small islets. Similarly, Xu et al. (4) reported an observation of extra islet neogenic single hormone+ cells or small clusters (fewer than eight cells) with exenatide treatment. Others have also reported islet neogenesis with incretin therapy (14, 16, 17). However, we previously reported that exenatide does not recruit highly proliferative progenitors to contribute to β-cell expansion (9). As before, we examined incretin-treated pancreatic sections for evidence of a rapidly dividing progenitor cell population, quantifying β-cells that had undergone multiple rounds of replication (BrdU+ and EdU+). However, such highly proliferative cells (which were the products of rapid cell division) were very rarely observed (five total β-cells in all groups). Moreover, the lack of substantial β-cell mass expansion after incretin therapies strongly suggests that any contribution from ductal islet neogenesis, if present, might be minimal. Thus, incretin therapies appear to have little or no effect on β-cell neogenesis.
We considered if differences in our study might account for conflicting observations with previous reports. Various strains (C57Bl6, ICR, db/db, wild type, B6.129 hybrid) have been used to study incretins in β-cell expansion (6–9, 11, 12, 17, 18, 36). Here we used low-dose continuous (1 nmol/kg/d), in contrast to our previous study (10 nmol/kg, subcutaneously) (6, 9) that reported exenatide increased β-cell proliferation. Differences in β-cell proliferation might reflect different doses or route (osmotic pump vs subcutaneously). The literature is similarly rife with conflicting reports, with various incretin doses, strains, and species sometimes (but not always) expanding β-cells (37–39). Whether observed β-cell mitogenic effects of incretins in rats will translate to humans is unclear, but, because β-cell turnover in humans is extremely low (40), the effects of incretin therapies might be minimal.
We arbitrarily limited our study to young male F1 hybrid B6129SF1/J mice because our study still involved 100 mice with the many treatment groups and large replicates. Additional cohorts would have expanded our study beyond the practical limit of analysis. Studies performed in our laboratory routinely use F1 hybrid B6129SF1/J mice (6, 9, 27, 41–43). We chose this strain because it closely approximates the mixed genetic background of laboratory knockout mice (commonly derived from SV129-derived embryonic stem cells and crossed into c57B6). Consequently, we are very familiar with these mice, including prior studies with HFD and exenatide, which can be used as a direct comparison for measurements of glucose homeostasis and β-cell mass. Nevertheless, the inherent limitations of our study (a single sex, genetic background, and age) must be considered. It remains possible that differences in these conditions could lead other investigators to could come to different conclusions about the impact of incretin therapies on β-cell expansion.
Our findings refine observations regarding the role of incretin therapies to maintain glucose homeostasis in response to metabolic demand. Our studies revealed that des-fluoro-sitaglipitin improves glucose control in the short term via insulin potentiation (Fig. 1) and in the long term through improved insulin sensitivity via reduced weight gain on a HFD (Fig. 4) without β-cell expansion (Fig. 6). One prior study administering exenatide for 4 weeks hints at a similar effect on HFD-induced weight gain (18). Indeed, we observed lower serum insulin levels in HFD-fed mice after incretin therapy, with lower body weight and improved glucose tolerance, indicating enhanced peripheral insulin sensitivity. Thus, long-term incretin therapy regulates body weight during increased metabolic demand to maintain glucose homeostasis, but the exact mechanism is unclear.
Our study also provides further evidence regarding the potential impact of incretins on pancreatic parenchyma. Scattered reports that incretin therapy might possibly induce ductal metaplasia and formation of adenocarcinomas are concerning and could negate their use as insulin potentiators for T2D if the risk outweighed the benefits. Butler et al. (22) have published two studies in rats treated with incretin therapy suggesting an association with pancreatitis and ductal metaplasia (23, 24). The first study was performed in human islet amyloid polypeptide transgenic rats fed a HFD (23). However, their findings differed from other reports (44), and also human islet amyloid polypeptide rats can develop spontaneous lesions and pancreatitis in the absence of incretin therapy (45). The second study (24) also used a rodent strain with exocrine tumors (KrasG12D mice develop pancreas cancer). Butler et al. (22) have also published an analysis of human T2D pancreas samples exposed to incretin therapies. The authors described a marked increase in exocrine cell proliferation and dysplasia in patients with T2D receiving incretin therapy. Additionally, an influence of incretin therapy on the endocrine pancreas was suggested by α-cell hyperplasia and glucagon-expressing microadenomas. Notably, several comments and a follow-up report suggest that the conclusions from the Butler et al. (22) study could not be supported (46–49). The main critiques included: (1) T2D groups with our without incretin therapy were not appropriately matched for age, duration, or advanced stage of diabetes; (2) it was unclear whether some patients actually had type 1 diabetes; (3) there were variable staining and methodological issues for quantification; (4) the observed neuroendocrine tumors and PanIN lesions appear to be due to increasing age rather than incretin therapy; and (5) there were too few samples to adequately interrogate the larger population (46–49). Thus, more extensive analyses across a much larger data set are required to resolve this outstanding issue in human pancreas.
Nevertheless, abundant evidence suggests that incretins may alter pancreatic parenchyma. Rouse et al. (50) used HFD feeding alone followed by short-term (6 weeks) treatment with exenatide or sitagliptin. Incretin therapy was associated with changes in pancreas histology, which is indicative of pancreatitis and duct hyperplasia, supporting the findings of Butler et al. (22). Moreover, increased pancreas weight has been observed after incretin therapy in association with exocrine cell proliferation and pancreas pathology (24, 51, 52). In sharp contrast, we show here that long-term administration of exenatide or des-fluoro-sitagliptin in a large cohort of mice (n =38) on a LFD or HFD demonstrates no evidence of duct hyperplasia, pancreatitis, or tumors and did not affect pancreas weight.
In summary, our studies show that long-term incretin therapies in mice may not induce β-cell mass expansion or alter pancreatic acinar cell histology. Notably, incretin therapy abrogates HFD-induced β-cell mass expansion through reduced weight gain during increased metabolic stress. No evidence for a rapidly dividing progenitor cell was found. β-Cell function and peripheral insulin sensitivity decline with age, potentially limiting the effectiveness of incretin therapy. Future studies will consider the effects of incretin therapy on β-cells in advanced age. Importantly, after 4.5 months of incretin therapy, none of the mice developed tumors, pancreatitis, or ductal hyperplasia. In light of our findings, the beneficial impact of incretin therapies for individuals with T2D probably relate more to augmented insulin secretion and improved metabolic parameters than expansion of β-cell mass.
Acknowledgments
The authors thank S. Vale, C. Blalock, D. Kettlewell, T. LaSpina, K. DePrado, and C. Yee of the Texas Children's Diabetes and Endocrinology Center for their administrative expertise and support and Nancy A. Thornberry, Bei B. Zhang, and Harvey L. Katzeff for thoughtful discussion.
Acknowledgments
This work was supported by Merck (Diabetes IISP 36669), The Robert and Janice McNair Foundation, National Institutes of Health Grant 1R01AG040110, Diabetes Research Center at Baylor College of Medicine Grant DRC - P30DK079638, and the Pathology and Histology Core at Baylor College of Medicine with funding from National Institutes of Health Grant NCI P30-CA125123.
Acknowledgments
Author contributions: A.R.C., C.J.L., M.M.R., K.B.K., and R.A.W. conceived and designed the experiments, performed the experiments, analyzed the data, wrote the manuscript, and approved the final version. J.S.R., J.C., C.W.B., D.A., J.X.T., and J.J.K. performed the experiments, analyzed the data, wrote the manuscript, and approved the final version. C.L. conceived and designed the experiments, wrote the manuscript, and approved the final version. J.A.K. conceived and designed the experiments, analyzed the data, and wrote the manuscript. J.A.K. is also the guarantor of this work.
Disclosure Summary: J.A.K. has served on the advisory board of Johnson & Johnson and currently serves on the advisory board for Lexicon and is supported in part by a research grant from the Investigator-Initiated Studies Program of Merck Sharp & Dohme Corp. The opinions expressed in this article are those of the authors and do not necessarily represent those of Merck Sharp & Dohme Corp. The remaining authors have nothing to disclose.
Appendix.
Antibody Table
| Peptide/Protein Target | Name of Antibody | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|
| Insulin | Polyclonal guinea pig anti-insulin | Dako, A0564 | Guinea pig | 1:1000 | AB_10013624 |
| 5-bromo-2'-deoxyuridine | Rat mab Anti-BrdU | Accurate Chemicals & Scientific Corporation, OBT0030G | Rat | 1:250 | AB_609567 |
Abbreviation: RRID, Research Resource Identifier.
Footnotes
- ATP
- adenosine triphosphate
- BrdU
- 5-bromo-2′-deoxyuridine
- DPP-4
- dipeptidyl peptidase-4
- EdU
- 5-ethynyl-2′-deoxyuridine
- GLP-1
- glucagon-like peptide-1
- GTT
- glucose tolerance test
- HFD
- high-fat diet
- LFD
- low-fat diet
- PDG
- pancreatic duct gland
- STZ
- streptozotocin
- SUR
- sulfonylurea receptor
- T2D
- type 2 diabetes.
References
- 1.Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest. 2005;115(8):2047–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. [DOI] [PubMed] [Google Scholar]
- 4.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. [DOI] [PubMed] [Google Scholar]
- 5.Buteau J, Spatz ML, Accili D. Transcription factor FoxO1 mediates glucagon-like peptide-1 effects on pancreatic beta-cell mass. Diabetes. 2006;55(5):1190–1196. [DOI] [PubMed] [Google Scholar]
- 6.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58(6):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in beta-cell proliferation restricts the capacity of beta-cell regeneration in mice. Diabetes. 2009;58(6):1312–1320. [DOI] [PMC free article] [PubMed]
- 8. doi: 10.1210/me.2011-1119. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21980072&dopt=Abstract Tschen SI, Georgia S, Dhawan S, Bhushan A. Skp2 is required for incretin hormone-mediated β-cell proliferation. Mol Endocrinol. 2011;25(12):2134–2143. [DOI] [PMC free article] [PubMed]
- 9.Teta M, Rankin MM, Long SY, Stein GM, Kushner JA. Growth and regeneration of adult beta cells does not involve specialized progenitors. Dev Cell. 2007;12(5):817–826. [DOI] [PubMed] [Google Scholar]
- 10.Duttaroy A, Voelker F, Merriam K, Zhang X, Ren X, Subramanian K, Hughes TE, Burkey BF. The DPP-4 inhibitor vildagliptin increases pancreatic beta cell mass in neonatal rats. Eur J Pharmacol. 2011;650(2-3):703–707. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Brubaker PL. Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia. 2002;45(9):1263–1273. [DOI] [PubMed] [Google Scholar]
- 12.Mu J, Woods J, Zhou YP, Roy RS, Li Z, Zycband E, Feng Y, Zhu L, Li C, Howard AD, Moller DE, Thornberry NA, Zhang BB. Chronic inhibition of dipeptidyl peptidase-4 with a sitagliptin analog preserves pancreatic beta-cell mass and function in a rodent model of type 2 diabetes. Diabetes. 2006;55(6):1695–1704. [DOI] [PubMed] [Google Scholar]
- 13.Han SJ, Choi SE, Kang Y, Jung JG, Yi SA, Kim HJ, Lee KW, Kim DJ. Effect of sitagliptin plus metformin on β-cell function, islet integrity and islet gene expression in Zucker diabetic fatty rats. Diabetes Res Clin Pract. 2011;92(2):213–222. [DOI] [PubMed] [Google Scholar]
- 14.Tourrel C, Bailbé D, Meile MJ, Kergoat M, Portha B. Glucagon-like peptide-1 and exendin-4 stimulate beta-cell neogenesis in streptozotocin-treated newborn rats resulting in persistently improved glucose homeostasis at adult age. Diabetes. 2001;50(7):1562–1570. [DOI] [PubMed] [Google Scholar]
- 15.Pospisilik JA, Martin J, Doty T, Ehses JA, Pamir N, Lynn FC, Piteau S, Demuth HU, McIntosh CH, Pederson RA. Dipeptidyl peptidase IV inhibitor treatment stimulates beta-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes. 2003;52(3):741–750. [DOI] [PubMed] [Google Scholar]
- 16.Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S. GLP-1/exendin-4 facilitates beta-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract. 2006;73(1):107–110. [DOI] [PubMed] [Google Scholar]
- 17.Ansarullah LY, Lu Y, Holstein M, DeRuyter B, Rabinovitch A, Guo Z. Stimulating β-cell regeneration by combining a GPR119 agonist with a DPP-IV inhibitor. PLoS One. 2013;8(1):e53345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arakawa M, Ebato C, Mita T, Hirose T, Kawamori R, Fujitani Y, Watada H. Effects of exendin-4 on glucose tolerance, insulin secretion, and beta-cell proliferation depend on treatment dose, treatment duration and meal contents. Biochem Biophys Res Commun. 2009;390(3):809–814. [DOI] [PubMed] [Google Scholar]
- 19.Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141(1):150–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denker PS, Dimarco PE. Exenatide (exendin-4)-induced pancreatitis: a case report. Diabetes Care. 2006;29(2):471. [DOI] [PubMed] [Google Scholar]
- 21.Soranna D, Bosetti C, Casula M, Tragni E, Catapano AL, Vecchia CL, Merlino L, Corrao G. Incretin-based drugs and risk of acute pancreatitis: a nested-case control study within a healthcare database [published correction appears in Diabetes Res Clin Pract. 2017;125:68]. Diabetes Res Clin Pract. 2015;108(2):243–249. [DOI] [PubMed] [Google Scholar]
- 22.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62(7):2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matveyenko AV, Dry S, Cox HI, Moshtaghian A, Gurlo T, Galasso R, Butler AE, Butler PC. Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin. Diabetes 2009;58(7):1604–1615. [DOI] [PMC free article] [PubMed]
- 24.Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model [published correction appears in Diabetes. 2012;61(8):2195] Diabetes. 2012;61(5):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim D, Wang L, Beconi M, Eiermann GJ, Fisher MH, He H, Hickey GJ, Kowalchick JE, Leiting B, Lyons K, Marsilio F, McCann ME, Patel RA, Petrov A, Scapin G, Patel SB, Roy RS, Wu JK, Wyvratt MJ, Zhang BB, Zhu L, Thornberry NA, Weber AE. (2R)-4-oxo-4-[3-(trifluoromethyl)-5,6-dihydro[1,2,4]triazolo[4,3-a]pyrazin-7(8H)-yl]-1-(2,4,5-trifluorophenyl)butan-2-amine: a potent, orally active dipeptidyl peptidase IV inhibitor for the treatment of type 2 diabetes. J Med Chem. 2005;48(1):141–151. [DOI] [PubMed] [Google Scholar]
- 26.Tuttle AH, Rankin MM, Teta M, Sartori DJ, Stein GM, Kim GJ, Virgilio C, Granger A, Zhou D, Long SH, Schiffman AB, Kushner JA. Immunofluorescent detection of two thymidine analogues (CldU and IdU) in primary tissue. J Vis Exp. 2010;7(46):2166. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rankin MM, Wilbur CJ, Rak K, Shields EJ, Granger A, Kushner JA. β-Cells are not generated in pancreatic duct ligation-induced injury in adult mice. Diabetes. 2013;62(5):1634–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He LM, Sartori DJ, Teta M, Opare-Addo LM, Rankin MM, Long SY, Diehl JA, Kushner JA. Cyclin D2 protein stability is regulated in pancreatic beta-cells. Mol Endocrinol. 2009;23(11):1865–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Remedi MS, Nichols CG. Chronic antidiabetic sulfonylureas in vivo: reversible effects on mouse pancreatic beta-cells. PLoS Med. 2008;5(10):e206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shiota C, Larsson O, Shelton KD, Shiota M, Efanov AM, Hoy M, Lindner J, Kooptiwut S, Juntti-Berggren L, Gromada J, Berggren PO, Magnuson MA. Sulfonylurea receptor type 1 knock-out mice have intact feeding-stimulated insulin secretion despite marked impairment in their response to glucose. J Biol Chem. 2002;277(40):37176–37183. [DOI] [PubMed] [Google Scholar]
- 31.Seghers V, Nakazaki M, DeMayo F, Aguilar-Bryan L, Bryan J. Sur1 knockout mice. A model for K(ATP) channel-independent regulation of insulin secretion. J Biol Chem. 2000;275(13):9270–9277. [DOI] [PubMed] [Google Scholar]
- 32.Portha B, Tourrel-Cuzin C, Movassat J.. Activation of the GLP-1 receptor signalling pathway: a relevant strategy to repair a deficient beta-cell mass. Exp Diabetes Res. 2011;2011:376509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pettus J, Hirsch I, Edelman S. GLP-1 agonists in type 1 diabetes. Clin Immunol. 2013;149(3):317–323. [DOI] [PubMed] [Google Scholar]
- 34.Lenzen S. The mechanisms of alloxan- and streptozotocin-induced diabetes. Diabetologia. 2008;51(2):216–226. [DOI] [PubMed] [Google Scholar]
- 35.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 36.Poucher SM, Cheetham S, Francis J, Zinker B, Kirby M, Vickers SP. Effects of saxagliptin and sitagliptin on glycaemic control and pancreatic β-cell mass in a streptozotocin-induced mouse model of type 2 diabetes. Diabetes Obes Metab. 2012;14(10):918–926. [DOI] [PubMed] [Google Scholar]
- 37.Brubaker PL, Drucker DJ. Minireview: glucagon-like peptides regulate cell proliferation and apoptosis in the pancreas, gut, and central nervous system. Endocrinology. 2004;145(6):2653–2659. [DOI] [PubMed] [Google Scholar]
- 38.Tudurí E, López M, Diéguez C, Nadal A, Nogueiras R. Glucagon-like peptide 1 analogs and their effects on pancreatic islets. Trends Endocrinol Metab. 2016;27(5):304–318. [DOI] [PubMed] [Google Scholar]
- 39.Lamont BJ, Andrikopoulos S. Hope and fear for new classes of type 2 diabetes drugs: is there preclinical evidence that incretin-based therapies alter pancreatic morphology? J Endocrinol. 2014;221(1):T43–T61. [DOI] [PubMed] [Google Scholar]
- 40.Meier JJ, Butler AE, Saisho Y, Monchamp T, Galasso R, Bhushan A, Rizza RA, Butler PC. Beta-cell replication is the primary mechanism subserving the postnatal expansion of beta-cell mass in humans. Diabetes. 2008;57(6):1584–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rankin MM, Kushner JA. Aging induces a distinct gene expression program in mouse islets. Islets. 2010;2(6):345–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox AR, Lam CJ, Rankin MM, King KA, Chen P, Martinez R, Li C, Kushner JA. Extreme obesity induces massive beta cell expansion in mice through self-renewal and does not alter the beta cell lineage. Diabetologia. 2016;59(6):1231–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cox AR, Lam CJ, Bonnyman CW, Chavez J, Rios JS, Kushner JA. Angiopoietin-like protein 8 (ANGPTL8)/betatrophin overexpression does not increase beta cell proliferation in mice. Diabetologia. 2015;58(7):1523–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aston-Mourney K, Subramanian SL, Zraika S, Samarasekera T, Meier DT, Goldstein LC, Hull RL. One year of sitagliptin treatment protects against islet amyloid-associated β-cell loss and does not induce pancreatitis or pancreatic neoplasia in mice. Am J Physiol Endocrinol Metab. 2013;305(4):E475–E484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chadwick KD, Fletcher AM, Parrula MC, Bonner-Weir S, Mangipudy RS, Janovitz E, Graziano MJ, Roy D, Reilly TP. Occurrence of spontaneous pancreatic lesions in normal and diabetic rats: a potential confounding factor in the nonclinical assessment of GLP-1-based therapies. Diabetes. 2014;63(4):1303–1314. [DOI] [PubMed] [Google Scholar]
- 46.Heine RJ, Fu H, Kendall DM, Moller DE. Comment on: Butler et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62:2595–2604. Diabetes. 2013;62(10):e16–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Engel SS, Golm GT, Lauring B. Comment on: Butler et al. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes 2013;62:2595–2604. Diabetes. 2013;62(10):e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bonner-Weir S, In’t Veld PA, Weir GC. Reanalysis of study of pancreatic effects of incretin therapy: methodological deficiencies. Diabetes Obes Metab. 2014;16(7):661–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Harja E, Lord J, Skyler JS. An analysis of characteristics of subjects examined for incretin effects on pancreatic pathology. Diabetes Technol Ther. 2013;15(8):609–618. [DOI] [PubMed] [Google Scholar]
- 50.Rouse R, Xu L, Stewart S, Zhang J. High fat diet and GLP-1 drugs induce pancreatic injury in mice. Toxicol Appl Pharmacol. 2014;276(2):104–114. [DOI] [PubMed] [Google Scholar]
- 51.Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-associated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 2009;58(9):2148–2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ellenbroek JH, Töns HA, Westerouen van Meeteren MJ, de Graaf N, Hanegraaf MA, Rabelink TJ, Carlotti F, de Koning EJ. Glucagon-like peptide-1 receptor agonist treatment reduces beta cell mass in normoglycaemic mice. Diabetologia. 2013;56(9):1980–1986. [DOI] [PubMed] [Google Scholar]