Abstract
Two modes of gonadotropin-releasing hormone (GnRH) and luteinizing hormone (LH) secretion are necessary for female fertility: surge and episodic secretion. However, the neural systems that regulate these GnRH secretion patterns are still under investigation. The neuropeptide somatostatin (SST) inhibits episodic LH secretion in humans and sheep, and several lines of evidence suggest SST may regulate secretion during the LH surge. In this study, we examined whether SST alters the LH surge in ewes by administering a SST receptor (SSTR) 2 agonist (octreotide) or antagonist [CYN154806 (CYN)] into the third ventricle during an estrogen-induced LH surge and whether endogenous SST alters episodic LH secretion. Neither octreotide nor CYN altered the amplitude or timing of the LH surge. Administration of CYN to intact ewes during the breeding season or anestrus increased LH secretion and increased c-Fos in a subset GnRH and kisspeptin cells during anestrus. To determine if these stimulatory effects are steroid dependent or independent, we administered CYN to ovariectomized ewes. This SSTR2 antagonist increased LH pulse frequency in ovariectomized ewes during anestrus but not during the breeding season. This study provides evidence that endogenous SST contributes to the control of LH secretion. The results demonstrate that SST, acting through SSTR2, inhibits episodic LH secretion, likely acting in the mediobasal hypothalamus, but action at this receptor does not alter surge secretion. Additionally, these data provide evidence that SST contributes to the steroid-independent suppression of LH pulse frequency during anestrus.
Neither an SSTR2 agonist nor antagonist altered the LH surge. However, the latter increased mean LH in intact ewes and LH pulse frequency in ovariectomized anestrous, but not breeding-season, ewes.
Ovarian function, and therefore fertility, in females is dependent on cyclical changes in two modes of gonadotropin secretion. Surge secretion of luteinizing hormone (LH) occurs once per estrous or menstrual cycle at the end of the follicular phase in response to positive feedback effects of estradiol (E2) and induces ovulation and luteinization. Pulsatile secretion of LH is necessary for folliculogenesis and steroidogenesis and is regulated by negative feedback effects of E2 and progesterone, with progesterone inhibiting LH pulse frequency and E2 inhibiting pulse amplitude (1). These actions result in changes in LH pulse patterns during the normal ovarian cycle that are important for the control of ovulation because the increasing episodes of LH secretion during the follicular phase drive the preovulatory rise in circulating E2.
Increased LH pulse frequency also underlies the transition from infertility to reproductive competence at the onset of puberty and the transition into the breeding season. In several species, these changes are controlled by both steroid-dependent (changes in response to the negative feedback actions of E2 or testosterone) and steroid-independent (evident in gonadectomized animals) inhibition of LH pulse frequency, but the importance of each varies with species (2). In sheep, the steroid-dependent suppression, which reflects an increased ability of E2 to inhibit gonadotropin-releasing hormone (GnRH) and LH pulse frequency prior to puberty and during the nonbreeding (anestrous) season, is more dramatic than the modest decrease in GnRH and LH pulse frequency seen in ovariectomized (OVX) animals (3). In contrast, steroid-independent suppression of LH is evident and critical for prepubertal suppression of LH in the agonadal monkey (4, 5) and in children (6, 7).
The neurons that produce GnRH do not contain estrogen receptor α or progesterone receptor (8–10), implicating a role for neuronal afferents to GnRH cells that convey the negative and positive feedback signals. The neuropeptide somatostatin (SST) is produced throughout the CNS, including in the hypothalamus and preoptic area (POA), and may contribute to the regulation of both episodic and surge secretion of LH. SST exerts its effects via five different Gi/o protein-coupled receptors (SSTR1 through SSTR5). Activation of these receptors inhibits adenylate cyclase activity and opens potassium channels, resulting in membrane hyperpolarization and therefore reduced responsiveness to stimulatory input (11). Consistent with a generally inhibitory effect, SST inhibited GnRH release from rat hypothalamic explants (12) and spike firing in GnRH cells in mice (13) and rats (14). Furthermore, these actions are likely mediated primarily through SSTR2 because the SSTR2 agonist seglitide produced similar inhibition of spike firing and membrane hyperpolarization as SST in mice (13).
SST neurons in the ventromedial nucleus (VMN) of the ovine hypothalamus contain estrogen receptor α (15, 16), and several lines of evidence suggest that they may be involved in the positive feedback actions of E2 in ewes. First, the VMN is considered the site of E2-positive feedback in ewes because E2 microimplants placed into this area, but not elsewhere, induced an LH and GnRH surge (17). Second, ewes treated with E2 implants known to induce an LH surge 24 hours later had more preproSST mRNA per cell (in the VMN) than ewes that did not receive E2 implants (18). It should be noted that the increase in preproSST mRNA was observed shortly (4 hours) after E2 implants were inserted, a time when E2 inhibits LH secretion. Third, c-Fos expression was higher in SST neurons within the VMN of ewes at the time of naturally occurring LH surges (19) and in anestrous ewes treated with estradiol benzoate than in controls (16). Finally, intracerebroventricular (ICV) administration of the SSTR2 agonist octreotide blunted the LH surge and prevented the typical increase in c-Fos within GnRH cells in rats (20). More direct evidence exists for a possible role for SST in regulation of pulsatile LH secretion. Infusion of SST into the third ventricle using an early follicular phase model in ewes inhibited mean LH concentrations and the number of LH pulses (18). Similarly, intravenous SST infusion into healthy humans (women in the follicular phase of menstrual cycle and men) suppressed mean LH concentrations (21), and octreotide suppressed pulse amplitude and mean LH concentrations in women with polycystic ovarian syndrome (22).
In this study, we tested the hypothesis that SST, acting via SSTR2, acts in the hypothalamus to suppress both the LH surge and episodic LH secretion in ewes. We targeted SSTR2 based on evidence that this receptor mediates the actions of SST in rodents (13, 20) and that GnRH cells in the preoptic area of sheep contain SSTR2, but not the other SST receptor subtypes, as determined by immunohistochemistry (23). To determine the role (if any) of SST on the generation of the LH surge, we administered either an SSTR2 agonist or antagonist to ewes during an E2-induced LH surge. To determine if endogenous SST alters episodic LH secretion, we administered a SSTR2 antagonist to ovary-intact, breeding-season ewes during the luteal phase (when progesterone is the dominant suppressor of LH), to ovary-intact ewes during anestrus (when E2 is the dominant suppressor of LH), and to OVX ewes during both the breeding and anestrous seasons. Previous work in sheep had provided no evidence on the neural sites of action of SST. Therefore, we administered the SSTR2 agonist and antagonist into the third ventricle because this approach has the advantage of testing a functional role for SST signaling without a priori information of sites of action.
Materials and Methods
Animals
Multiparous mature ewes (>3 years old) of mixed breeding were maintained in a light- and temperature-controlled research building. Lighting conditions were adjusted every other week to mimic local natural lighting conditions. Ewes were fed a maintenance ration of cubed alfalfa hay and had free access to water and mineral blocks. Breeding season experiments were done in October and November, and anestrous studies were done in May and June. All procedures were approved by the West Virginia University Animal Care and Use Committee and were performed in accordance with the National Institutes of Health guidelines for the care and use of research animals.
General methods
Surgical procedures
All surgeries were performed using aseptic techniques with anesthesia maintained with 2% to 5% isofluorane in oxygen. Wool was removed from the surgical site, and skin was scrubbed with betadine solution. For OVX, the reproductive tract was externalized by midventral laparotomy, the ovarian blood supply was ligated with suture, and ovaries were removed. The remaining reproductive tract was rinsed with sterile saline to minimize adhesions and returned to the abdominal cavity. Peritoneum, subcutaneous layers, and skin were closed with suture. For neurosurgery, an 18-gauge needle was prepared and placed in the caudal, basal portion of the third ventricle using a stereotaxic procedure as previously described (24). The cannula was cemented in place with dental acrylic, and the needle hub was plugged and covered with a plastic cap. Animals were treated pre- and postoperatively with analgesics (carprofen for both surgery types and gabapentin for neurosurgeries) and antibiotics (ampicillin for both surgery types and gentamycin for neurosurgeries). All veterinary drugs were acquired from Patterson Veterinary (Bessemer, AL) except gabapentin, which was compounded locally (McCracken Pharmacy, Waynesburg, PA). Animals were allowed to recover from surgery for at least 1 week before experiments were performed.
Drug preparation and blood sampling procedures
Drugs for ICV injection were prepared the night before injection and sterilized with ultraviolet irradiation. Animals were treated prophylactically with gentamicin before and after the blood sampling period. For studies of surge-type LH secretion, blood samples were collected at 2- to 4-hour intervals during the expected time of the LH surge. For studies of episodic LH secretion (experiments 2, 3, and 5), blood samples were collected at 12-minute intervals from 2 hours before until 4 hours after drug or vehicle injection. For all experiments, jugular blood samples (3 to 4 mL) were collected via venipuncture and placed in heparinized tubes. Plasma was harvested and stored at −20°C until assayed for LH with radioimmunoassay.
Experiment 1: Does SST act in the hypothalamus to regulate surge-type LH secretion?
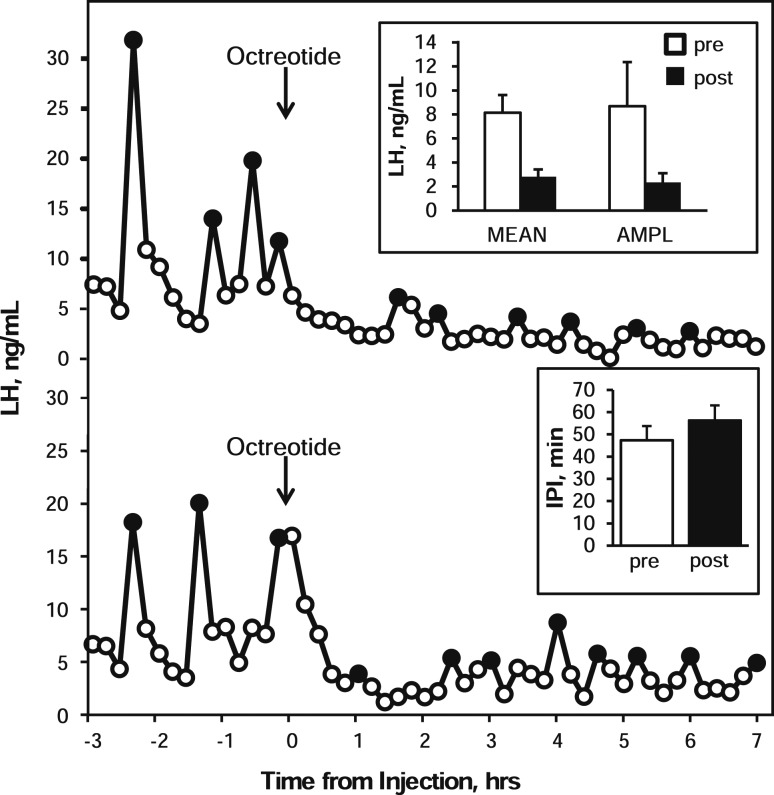
To identify an effective dose of the selective SSTR2 agonist octreotide that would alter LH secretion, in a preliminary experiment we administered 20 nmol of octreotide (Tocris Bioscience, Ellisville, MO) in 100 μL of artificial cerebrospinal fluid (aCSF) (25) to OVX ewes. LH concentrations were measured in blood samples collected at 12-minute intervals from 3 hours before to 7 hours after drug administration. Octreotide was selected for these experiments because it has been demonstrated to alter LH secretion in rats (20) and humans (22). A single 20-nmol ICV injection of octreotide suppressed mean LH (pre: 8.1 ± 1.5 ng/mL; post: 2.8 ± 0.6 ng/mL) and pulse amplitude (pre: 8.7 ± 3.7 ng/mL; post: 2.3 ± 0.8 ng/mL) for the entire postinjection sampling period (Fig. 1). However, the interpulse interval (IPI) was not obviously altered (pre: 47.3 ± 6.4 minutes; post: 56.3 ± 6.7 minutes).
Figure 1.
LH concentration patterns in two ewes receiving injections of the SSTR2 agonist octreotide (arrows) into the third ventricle. Black circles denote samples identified as pulses. Inset panels depict group mean [± standard error of the mean (SEM)] LH concentration (MEAN), LH pulse amplitude (AMPL), and interpulse interval (IPI) during the 3-hour preinjection (open bars) and 7-hour postinjection (black bars) periods. Statistical analysis was not performed because of the low number of animals (n = 3) and the lack of contemporary vehicle control injection.
We used a well-established artificial follicular phase model in OVX ewes to test the effects of a SSTR2 agonist and antagonist on the E2-induced LH surge (26). Briefly, at the time of OVX and insertion of the third ventricle cannula, a small (1 cm long) Silastic implant (inner diameter 0.34 cm, outer diameter 0.46 cm) (Dow Corning Corp., Midland, MI) containing E2 was inserted subcutaneously and remained in place for the entire experiment. Two progesterone-containing CIDRs (Zoetis, Parsippany, NJ) were also inserted vaginally at this time. Eight days later, the CIDRs were removed, and the next day four large (3 cm long) E2 implants were inserted subcutaneously and removed 3 days later. Blood samples were collected at 2-hour intervals for 36 hours starting at the time of large E2 implant insertion and then at 4-hour intervals for an additional 12 hours. This treatment protocol reliably induces an LH surge 20 to 24 hours after insertion of the large E2 implants. To test the effect of a SSTR2 agonist, six ewes were randomly assigned to receive ICV injections of either the SSTR2 agonist, octreotide (20 nmol in 100 μL), or aCSF every 8 hours for the first 24 hours of blood sampling starting at the time of E2 implant insertion. This dosing regimen was chosen based on results from the preliminary experiment. Three days after removal of large E2 implants, progesterone pretreatment, surge induction, and experimental protocol (ICV injections and blood sampling) were repeated, with each ewe receiving the alternate treatment in a cross-over design.
To test the effects of the SSTR2 antagonist, seven adult ewes were OVX, and a permanent cannula was placed into the third ventricle at least 1 week before any experimental procedure. An LH surge was induced as described previously. Ewes were randomly assigned to receive ICV injections of either the selective SSTR2 antagonist, CYN154806 (CYN) (27) (60 nmol in 100 μL; n = 4) (Tocris Bioscience, Ellisville, MO), or an equal volume of saline (n = 3) at 6-hour intervals for 24 hours starting at the insertion of the large E2 implant treatment. This dose of antagonist altered pulsatile LH secretion in experiments 2 through 5.
Experiment 2: Does endogenous SST act in the hypothalamus to regulate tonic LH secretion in intact anestrous ewes?
Eight ovary-intact anestrous ewes received a permanent cannula into the third ventricle in mid-April (anestrous season). Ewes were randomly assigned to receive a single ICV injection of CYN (60 nmol in 100 μL sterile saline) or vehicle with 6 hours of frequent blood collection. The protocol was repeated 4 days later, at which time each animal received the alternate treatment in a crossover design.
Experiment 3: Does endogenous SST act in the hypothalamus to regulate tonic LH secretion in luteal phase ewes?
Eight ovary-intact ewes received a permanent cannula into the third ventricle in mid-October (breeding season). Estrous cycles were synchronized with two intramuscular injections of prostaglandin F2α (20 mg) (Lutalyse, Zoetis, Parsippany, NJ) given 7 days apart (28). Eight days after the second injection, ewes were randomly assigned to receive a single ICV injection of CYN (60 nmol in 100 μL sterile saline) or 100 μL of vehicle, and frequent blood samples were collected for 2 hours before and 4 hours after injections as described previously. The protocol was repeated 2 days later, in which each animal received the alternate treatment in a crossover design.
Experiment 4: Effects of SSTR2 blockade on GnRH and kisspeptin cell activation
To determine whether SST may act via GnRH or kisspeptin cells, we administered CYN or saline via ICV injection and examined c-Fos expression in GnRH or kisspeptin cells using immunohistochemistry. Six days after the final sampling period in experiment 2, the same intact ewes were randomly assigned to receive either saline (n = 4) or CYN (n = 4). Animals were euthanized, and hypothalamic tissue was collected 2 hours after injection.
Ewes were treated with heparin (20,000 U) 10 minutes before and at the time of euthanasia, which was performed with an overdose of sodium pentobarbital (8 to 16 mL, intravenously; Euthasol; Patterson Veterinary, Bessemer, AL). The head was rapidly removed and perfused with 6 L of 4% paraformaldehyde in 0.1 M phosphate buffer (PB) with 0.1% NaNO3. A block of tissue including the hypothalamus and POA was removed and stored overnight in the same paraformaldehyde solution at 4°C. Tissue was then transferred to 20% sucrose in PB and maintained at 4°C. After sucrose infiltration, tissue was frozen, and 45-μm-thick coronal sections were cut with a microtome with a freezing stage and collected in 10 series (450 μm apart) and stored at −20°C in cryoprotectant solution (29).
For detection of GnRH and c-Fos, dual-label immunohistochemistry was performed on one complete series of hemisections throughout the hypothalamus. All steps were performed at room temperature with mild agitation unless noted differently. Tissue was rinsed in 0.1 M PB eight times (15 minutes each) and stored overnight in PB at 4°C. Tissue was rinsed 12 additional times in 0.1 M phosphate-buffered saline (PBS) (15 minutes each). Endogenous peroxidase activity was quenched by incubation in 1% H2O2 in PBS, and the tissue was rinsed four times for 5 minutes each in PBS (typical rinsing step). Tissue was incubated in 4% normal goat serum (NGS; Jackson Immunoresearch, West Groove, PA) in PBS containing 0.04% triton X-100 (PBST) for 1 hour and then incubated in rabbit anti–c-Fos serum (dilution: 1:2000 with 4% NGS in PBST; SC-52, RRID:AB_2106783; Santa Cruz Biotechnology, Dallas, TX) for 17 hours. Tissue was rinsed and incubated sequentially in biotinylated goat anti-rabbit immunoglobulin (dilution: 1:500 in PBST with 4% NGS; BA-1000; Jackson Immunoresearch) for 1 hour, ABC-elite (dilution 1:500 in PBS; Vector Laboratory Burlingame, CA) for 1 hour, and nickel-enhanced 3,3′diaminobenzidene solution (10 mg per 50 mL PB with 2 mL of 2% NiSO4 and 20 μL of 30% H2O2 to produce a black reaction product; Sigma-Aldrich, Saint Louis, MO) for 10 minutes with intervening rinse steps. Tissue was then rinsed in PB, placed into 1% H2O2 in PBS, rinsed, incubated in blocking solution containing 4% NGS in PBST for 1 hour, and incubated in rabbit anti-GnRH serum (dilution: 1:2000 in PBST with 4% NGS, 20075, RRID:AB_572248; Immunostar, Hudson, WI) for 1 hour. Tissue was rinsed in PBS and incubated sequentially in biotinylated goat anti-rabbit immunoglobulin (dilution: 1:500 in PBST with 4% NGS), ABC-elite (dilution: 1:500 in PBS), and 3,3′-diaminobenzidine (10 mg per 50 mL PB with 20 μL of 30% H2O2 to produce a brown reaction product) for 10 minutes. Tissue was rinsed in PB and mounted onto superfrost slides (Fisher Scientific, Pittsburgh, PA). Once tissue was dried, cover glass was applied with Eukitt (Fisher Scientific) mounting media.
The total number of GnRH cells and the percentage of GnRH cells that contained c-Fos was determined in the diagonal Band of Broca, POA, anterior hypothalamic area, and the mediobasal hypothalamus (MBH) (30) using a bright field microscope (Axiovert 400CFL, Zeiss) with a 20× objective. Values were averaged per animal. All cell counts were made by a single observer blinded to treatment groups.
Detection of c-Fos within kisspeptin cells was performed in one series of hemisections throughout the arcuate nucleus (ARC), which contains the population of kisspeptin-neurokinin B-dynrophin (KNDy) neurons (31, 32), and in two to four hemi sections from the POA as described previously except that 10% H2O2 and 20% NGS were used in blocking solutions and a kisspeptin antibody (dilution: 1:10,000 in PBST with 4% NGS; AB9754, RRID:AB_2296529; Millipore, Billerica, MA) was used after detection of c-Fos. The total numbers of kisspeptin cells and kisspeptin cells that contained c-Fos were determined in two to four sections in the rostral, middle, and caudal portions of the ARC (32) and POA. The mean number of kisspeptin cells per hemisection and the percentage of kisspeptin cells that contained c-Fos were averaged per animal. All cell counts were made by a single observer blinded to treatment groups using a bright-field microscope (VS120; Olympus, Tokyo, Japan) with a 20× objective. Antibodies used to detect c-Fos (32, 33), GnRH (34), and kisspeptin (35) have been validated and used extensively in sheep. Additional details about these antibodies are presented in Table 1.
Table 1.
Primary Antibodies
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog #, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used | RRID |
|---|---|---|---|---|---|---|
| c-Fos | FOS human, mouse, rat | Rabbit anti-c-Fos polyclonal antibody, unconjugated | Santa Cruz Biotechnology, SC-52 | Rabbit; polyclonal | 1:2000 | AB_2106783 |
| GnRH | Luteinizing hormone–releasing hormone | LHRH | ImmunoStar, 20075 | Rabbit; polyclonal | 1:2000 | AB_572248 |
| Kisspeptin | Mouse kisspeptin 10 | Anti-kisspeptin antibody | Millipore, AB9754 | Rabbit; polyclonal | 1:10,000 | AB_2296529 |
Abbreviation: RRID, research resource identifier.
Experiment 5: Does endogenous SST suppress LH in a steroid-independent manner?
To determine whether the stimulatory effects of SSTR2 blockade observed in experiment 3 were dependent or independent of gonadal steroid inhibition of LH secretion, we administered CYN to six OVX ewes during anestrus (June). Seven to 10 days after OVX, animals were randomly assigned to receive a single ICV injection of CYN (60 nmol in 100 μL sterile saline) or vehicle, with frequent blood samples collected for 6 hours. The animals received the alternate treatment 5 days later in a crossover design.
In light of the results of the first part of this study, we next determined if endogenous SST mediates the steroid-independent suppression of LH caused by inhibitory photoperiod by giving CYN to eight OVX ewes during the breeding season (mid-November). Animals were randomly assigned to receive treatments (CYN or saline as described previously) 6 to 9 days after OVX and received the alternate treatment 2 days later.
Data analysis
Radioimmunoassay
LH concentrations were measured in duplicate as described previously (36) using a double–liquid-phase radioimmunoassay with reagents provided by the National Hormone and Peptide Program. The sensitivity of the assay averaged 0.077 ng/tube (NIH S24), and the intra- and interassay coefficients of variation were 5.8% and 8.3%, respectively. Progesterone concentrations were determined using a radioimmunoassay kit (MP Bioscience, Santa Anna, CA) according to the manufacturer’s directions. Progesterone was measured in duplicate in a single assay with a sensitivity of 0.012 ng/tube and an intra-assay coefficient of variation of 10.1%.
Statistical analysis
For experiment 1, the time of maximal LH secretion and the amplitude of the LH surge (maximal concentration minus LH concentration of the first sample collected at time of insertion of large E2 implants) were compared between treatment groups with t tests (paired t tests for experiments conducted as cross-over designs). In studies of episodic LH secretion, pulses were identified with established criteria (1); briefly, a pulse had to be 2 assay standard deviations greater than the preceding nadir, the pulse had to occur within two samples of the preceding nadir, and the amplitude had to be greater than the assay sensitivity. The total number of GnRH or kisspeptin cells and the percentage of each that contained c-Fos within each area were compared between treatment groups with independent t tests.
For experiments examining episodic LH secretion, mean LH concentration and LH pulse amplitude (maximal pulse value minus preceding nadir) were determined during the 2-hour preinjection and 4 hour postinjection sampling periods and analyzed by two-way analysis of variance (ANOVA) with repeated measures (time and treatment). Tukey’s multiple comparisons test was used to determine differences between individual points where appropriate. LH IPI was determined during the 4-hour postinjection period and was compared between treatment groups with a paired t test. Postinjection pulse latency (time from injection to the first pulse) and the amplitude of the first pulse after injection were compared between treatment groups with a paired t test. P < 0.05 was considered statistically significant.
Results
Effects of SSTR2 agonist and antagonist on surge-type LH secretion
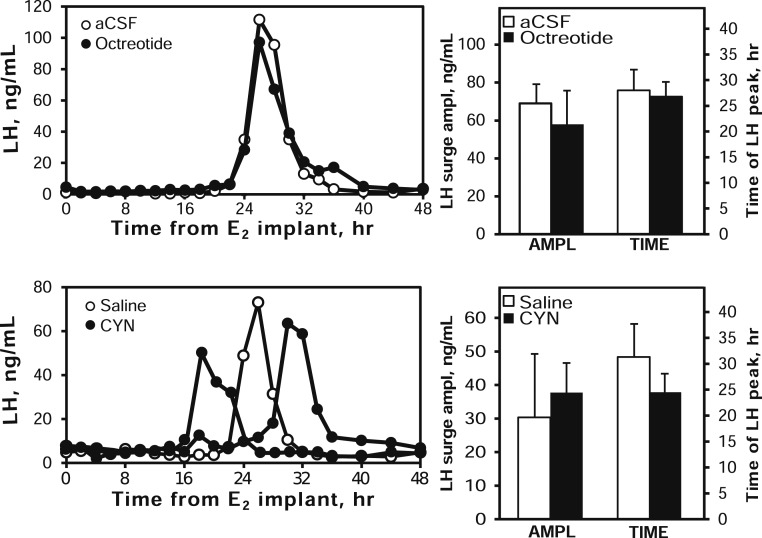
Estradiol treatment induced an LH surge in all animals. Administration of the SSTR2 agonist octreotide did not alter the amplitude of the LH surge or the timing of the LH peak compared with aCSF-treated animals (Fig. 2, upper panels). Similarly, administration of the SSTR2 antagonist CYN did not alter the amplitude or the peak time of the LH surge compared with saline-treated animals (Fig. 2, lower panels).
Figure 2.
Top panels: Effect of SSTR2 agonist (octreotide, black circles and bars) and vehicle (aCSF, open circles and bars) on the estrogen-induced LH surge. LH concentrations during E2-induced LH surges in one animal are shown in the left panel; the right panel presents mean (± SEM) LH surge amplitude and time (hours after E2 implants) of maximum LH value. Bottom panels: Effect of SSTR2 antagonist (CYN, black circles and bars) and vehicle (saline, open circles and bars) on the estrogen-induced LH surge. The left panel depicts LH concentrations during an E2-induced LH surge in one control and two CYN-treated animals to illustrate variability in timing of the surge; the right panel presents mean (± SEM) LH surge amplitude and time of maximum LH values.
Effects of SSTR2 antagonist in anestrous ewes
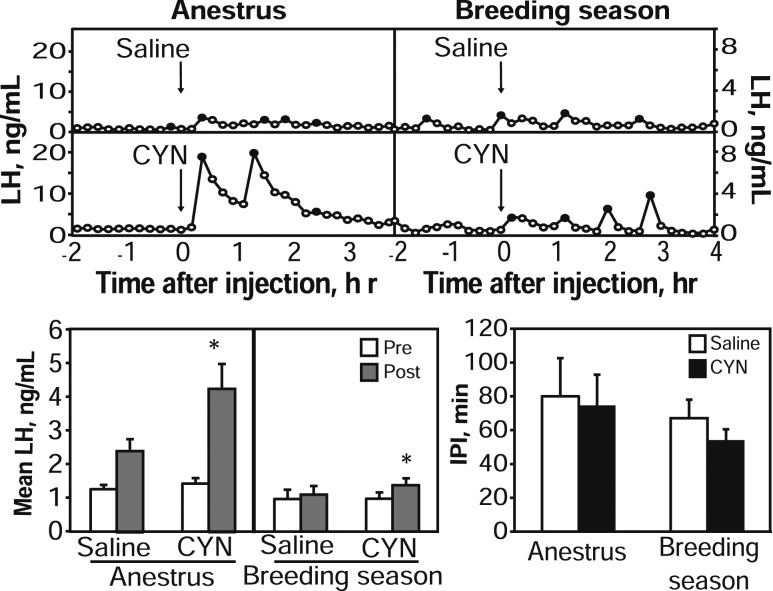
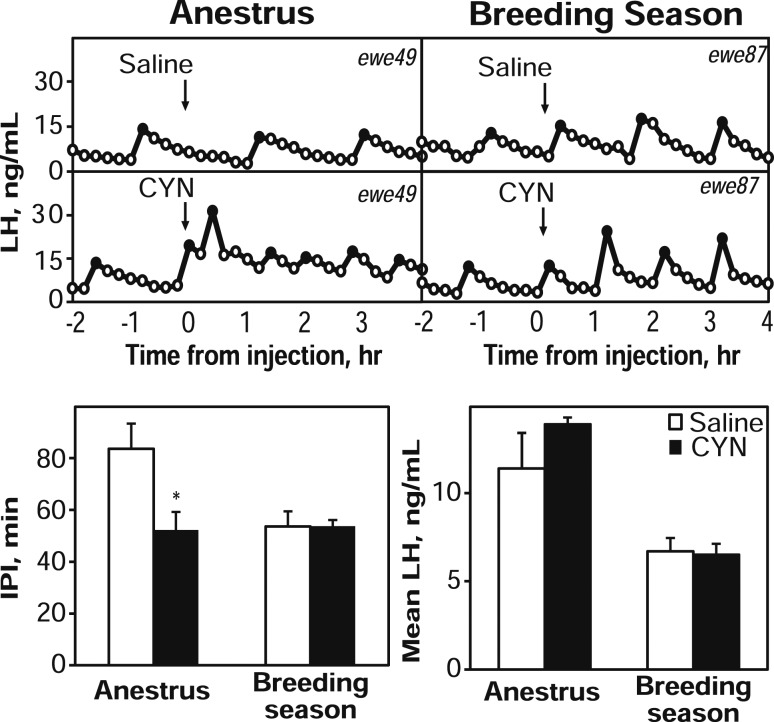
One ewe apparently had an LH surge during one sampling period (evident by very high and sustained LH concentration patterns) and was thus removed from analysis. A single injection of CYN into anestrous ewes induced pulse-like LH secretion in all seven ewes within 36 minutes. Four of these ewes also had an LH pulse within 36 minutes after injection of saline (Fig. 3). For mean LH, two-way ANOVA revealed a significant time × treatment interaction such that CYN significantly increased mean LH concentrations (pre: 1.4 ± 0.2 ng/mL; post: 4.3 ± 0.7 ng/mL), whereas saline injections did not alter mean LH (pre: 1.3 ± 0.1 ng/mL; post: 2.4 ± 0.4 ng/mL). Pulse amplitude during the postinjection period tended (P = 0.089) to be greater in animals that received CYN (4.7 ± 0.9 ng/mL) than in animals that received saline (2.7 ± 0.5). Neither IPI (Fig. 3) nor timing of the first pulse (data not shown) was different between the two treatments.
Figure 3.
Top: Representative LH concentration patterns in one anestrous intact ewe (left) and another luteal phase ewe (right) receiving saline (top panels) or CYN (bottom panels) as ICV injection (arrows). Black points represent samples identified as pulses. Note differences in y-axes between seasons. Bottom panels: Mean LH (± SEM) concentrations during the 2 hour preinjection (open bars) and 4 hour postinjection (gray bars) sampling periods for saline or CYN treatments are shown on the left, and mean IPI (± SEM) values during the 4-hour sampling period after saline (open bars) or CYN (black bars) are shown on the right. *P < 0.05 versus preinjection period.
Effects of SSTR2 antagonist in luteal phase ewes
The synchronization protocol using prostaglandin resulted in luteal phase concentrations of progesterone (>1 ng/mL) in seven of eight ewes on both sampling days; one ewe with low progesterone concentrations was excluded from analysis. Two-way ANOVA for mean LH revealed a significant time × treatment interaction such that a single ICV injection of CYN into luteal phase ewes produced a modest but significant increase in mean LH concentrations (pre: 1.0 ± 0.2 ng/mL; post: 1.4 ± 0.2 ng/mL), whereas saline injections did not alter mean LH concentrations (pre: 1.0 ± 0.3 ng/mL; post: 1.1 ± 0.3 ng/mL) (Fig. 3). The first pulse after CYN injection occurred sooner (24 ± 4.5 minutes) than the first pulse after saline injection (58 ± 10.3 minutes). Neither IPI (Fig. 3) nor pulse amplitude (data not shown) was significantly altered by treatment with this antagonist.
Effects of SSTR2 antagonist on GnRH and kisspeptin cell activation
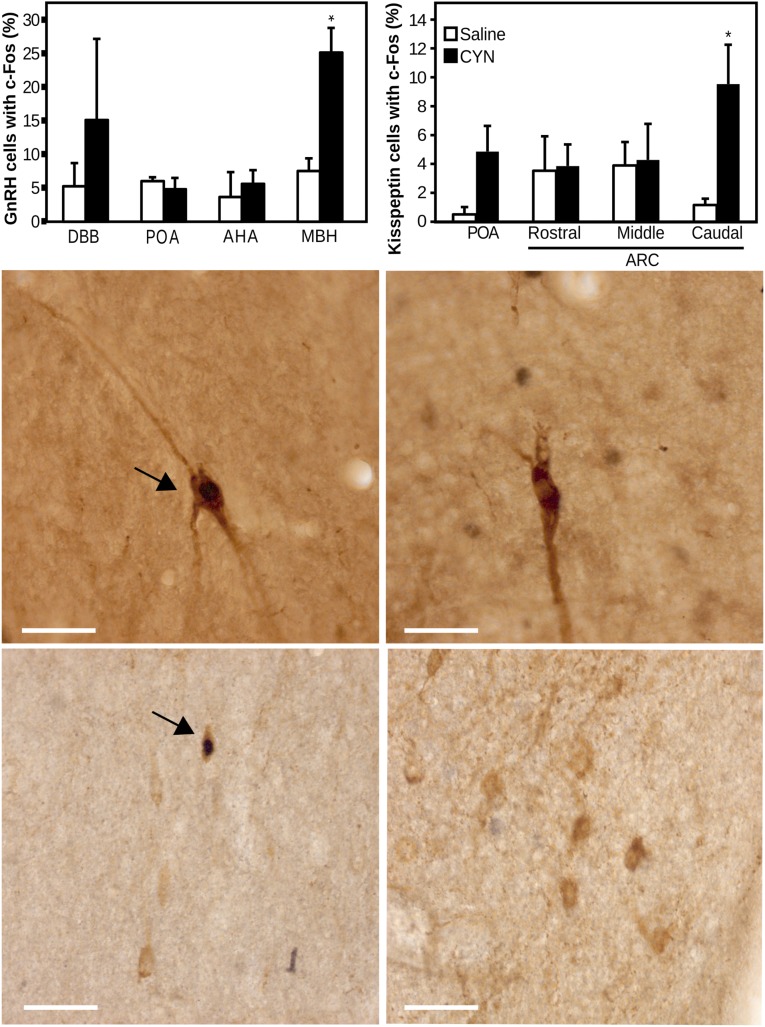
Ewes that received CYN had a significantly greater percentage of GnRH cells that contained c-Fos within the MBH compared with ewes that received saline (Fig. 4). There were no significant differences in the percentage of GnRH cells that contained c-Fos in the diagonal band of Broca, POA, or anterior hypothalamic area between treatment groups. CYN had no significant effect on the total number of GnRH cells in any of these areas (Table 2).
Figure 4.
Top panels: Percentage (± standard error of the mean) of GnRH and kisspeptin immunoreactive cells that contain c-Fos in ewes that received either saline (open bars) or CYN (black bars). *P < 0.05 versus saline-treated ewes. AHA, anterior hypothalamic area; DBB, diagonal band of Broca. Lower panels: Representative photomicrographs of GnRH (mediobasal hypothalmus, middle) and kisspeptin (caudal ARC, bottom) immunoreactive soma (gray) in ewes treated with CYN (left) or saline (right). Arrowheads indicate GnRH or kisspeptin soma that contain nuclear c-Fos (black). Scale bar, 50 µm.
Table 2.
CYN Did Not Affect the Number of GnRH Neurons
| DBB | POA | AHA | MBH | |
|---|---|---|---|---|
| Saline | 7.0 ± 2.9 | 65.5 ± 18.2 | 25.5 ± 5.2 | 51 ± 8.9 |
| CYN | 6.8 ± 2.9 | 74.5 ± 13.8 | 22.3 ± 6.4 | 27.8 ± 4.3 |
Abbreviations: AHA, anterior hypothalamic area; DBB, diagonal band of Broca.
Ewes that received CYN had a significantly greater, though modest, percentage of kisspeptin cells that contained c-Fos within the caudal aspect of the ARC compared with ewes that received saline (Fig. 4). CYN treatment did not alter the percentage of kisspeptin cells that contained c-Fos in other portions of the ARC or POA. There was also no difference in the total number of kisspeptin cells between treatment groups in any area. Although there appeared to be a large numerical difference between treatment groups in the percentage of GnRH and kisspeptin cells that contained c-Fos in the diagonal band of Broca and the POA, respectively, these differences were not statistically significant. This might be due to the relatively low number of animals (n = 4/group) but more likely reflects the variability inherent in estimating percentages because there is a small number of total cells in these areas (Table 3).
Table 3.
CYN Did Not Affect the Number of Kisspeptin Neurons
| POA | Rostral ARC | Middle ARC | Caudal ARC | |
|---|---|---|---|---|
| Saline | 10.9 ± 5.89 | 16.7 ± 7.1 | 65.5 ± 11.2 | 46.1 ± 14.2 |
| CYN | 8.4 ± 3.8 | 17.2 ± 9.2 | 30.5 ± 9.6 | 39.5 ± 7.1 |
Effects of SSTR2 antagonist in OVX ewes
After CYN injection, OVX ewes during anestrus had a significantly shorter IPI (52.3 ± 6.9 minutes) than after saline injection (83.7 ± 9.6 minutes) (Fig. 5). Similarly, the first pulse after CYN injection tended to occur sooner (P = 0.06; 32.0 ± 6.7 minutes) than after saline injection (64.0 ± 14 minutes). In contrast, during the breeding season, the IPI after CYN injection did not differ from that after saline injection, nor did the timing of the first pulse. Of note, CYN administration in anestrus reduced the IPI to a duration essentially the same as that seen in OVX during the breeding season. CYN treatment did not alter pulse amplitude (data not shown) or mean LH concentrations within either season, but mean LH levels were higher during the nonbreeding season than in the breeding season (Fig. 5) because there was an increase in LH pulse amplitude during anestrus (anestrus, 11.0 ± 2.1 ng/mL versus breeding season, 6.7 ± 0.8 ng/mL), which has been seen in previous studies (37, 38).
Figure 5.
Top panels: Representative LH patterns in one OVX ewe during anestrus (left) and another ewe in the breeding season (right) receiving ICV injection (arrows) of saline (top panels) or CYN (bottom panels). Black points represent samples identified as pulses. Bottom panels: Mean (± SEM) IPI (left panel) and mean LH concentration (right panel) during the 4-hour postinjection period in OVX ewes receiving saline (open bars) and CYN (black bars) during anestrus (left) or during the breeding season (right). *P < 0.05 versus saline injection.
Analysis of guide cannula placement
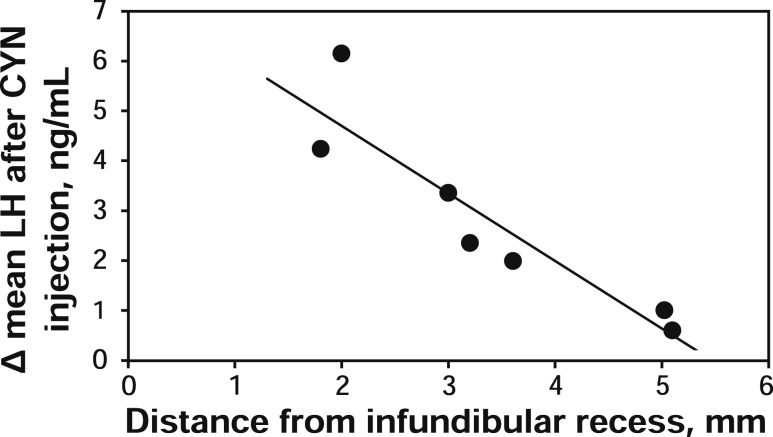
Cannulae placed into the third ventricle were aimed so that the tip was 2 to 3 mm directly above the infundibular recess (24), but there was some variation in this position. To determine if this variation affected the response to the SSTR2 antagonist, the distance from infundibular recess to the tip of the cannula was measured on radiographs captured during neurosurgery and compared with LH responses for each experiment. As illustrated in Fig. 6, for experiment 2 (intact, anestrous ewes), there was a negative relationship (R2 = 0.84) between the distance of the cannula tip to the infundibular recess and the change in mean LH concentration (mean LH concentration after CYN injection minus mean LH concentration preinjection). Animals with the cannula tip placed further rostral and dorsal had the smallest LH response. No significant relationship between cannula placement and LH response after CYN or octreotide injection were observed in any other experiment, although there was less variability in guide cannula placement and response to CYN in the other experiments.
Figure 6.
Change in mean LH concentration after CYN injection in intact anestrous ewes (experiment 2) versus chronic cannula position (distance from tip of cannula to infundibular recess on the lateral radiograph taken during neurosurgery).
Discussion
This report demonstrates a role for endogenous SST in the regulation of GnRH secretion in any species. Previous work in several species has shown that SST or SST receptor agonists can alter GnRH cell activity or LH secretion, but the current study is the first to use a SST receptor antagonist to determine whether endogenous SST suppresses LH secretion. Using this approach, we demonstrate that the SSTR2 antagonist, CYN, stimulates pulsatile LH secretion and c-Fos expression in MBH GnRH neurons, but not surge secretion.
Although several lines of correlative evidence in sheep, and pharmacological data in rodents, support a role for SST in surge type LH secretion, neither a SSTR2 agonist nor antagonist altered surge secretion in our experiments. Several methodological or theoretical considerations could account for the lack of effect of these drugs. First, as with all pharmacological studies, it is possible that an effective concentration of the drug was not achieved in the necessary locations to elicit an effect on LH surge secretion, but these drugs did affect episodic LH secretion. Second, SST could be acting through one of the other somatostatin receptors, although SSTR2 is the most likely receptor to be involved in control of GnRH secretion. Third, it is well established that several redundant systems contribute to the generation of the LH surge (39) so that compensatory pathways could mask any effects caused by altered somatostatin signaling. Fourth, based largely on data from humans (21, 40, 41), it is possible that SST acts at the pituitary to suppress the response of the gonadotrope to GnRH. Despite these caveats, the simplest explanation for the lack of effect of SSTR2 agonist and antagonist is that SST does not play an essential role in the generation of the LH surge in sheep.
Two groups have reported an increase in c-Fos, a marker for neuronal activation, in SST cells within the VMN around the time of the LH surge in sheep (16, 19). One possible explanation for the discrepancy between these observations and our pharmacological data is that the cells that contain SST in the VMN are activated during the time of the surge but contain another signaling molecule or molecules important for surge secretion in sheep. Alternatively, it is possible that SST cells in the VMN are involved with inducing estrous behavior because E2 implants placed into the VMN, but not other areas, induce sexual receptivity in ewes (42). However, one report that c-Fos increased within SST cells in the VMN in animals during the LH surge but not during the portion of estrous behavior that preceded the surge (19) argues against this possibility.
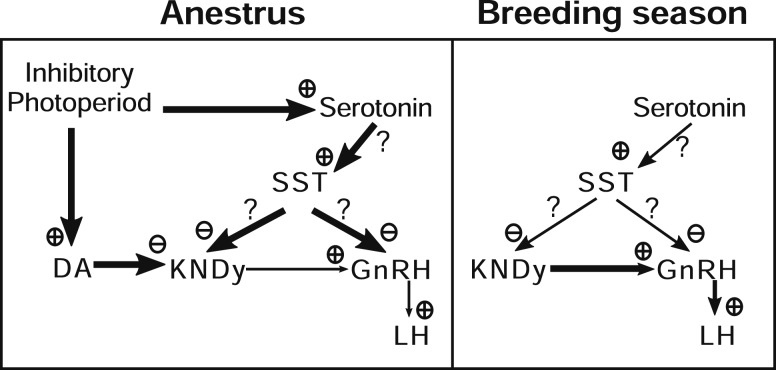
Perhaps the most dramatic effects of the SSTR2 antagonist on episodic LH secretion occurred in OVX animals, in which CYN administered to OVX ewes decreased IPI during anestrus but not during the breeding season. Interestingly, SSTR2 blockade during anestrus reduced LH IPI to levels similar to those observed for OVX ewes during the breeding season. Thus, we propose that SST acts during anestrus to mediate the steroid-independent actions of photoperiod. The only other signaling molecule that has been implicated in steroid-independent suppression of LH during anestrus is serotonin. Similar to our current findings, serotonin receptor blockade increased LH pulse frequency in OVX ewes in anestrus but not during the breeding season (43–45). Serotonin depletion during anestrus also increased LH pulse frequency in OVX ewes but had no effect in OVX ewes treated with E2 (44).
Because both SST and serotonin have been implicated in the steroid-independent actions of photoperiod, these systems may interact. The ovine hypothalamus has abundant serotonergic fiber innervation (46), and mRNA for the relevant receptor 5-HT2 (47) is concentrated in the ventral portion of the MBH, including the VMN (48). It is therefore possible that serotonin inhibits LH secretion via SST neurons that are located in the VMN. This possibility would also be consistent with the theory that serotonin suppresses LH via an inhibitory interneuron because 5-HT2 receptor activation releases diacylglycerol and inositol triphosphate, resulting in increased protein kinase C activity and Ca2+ influx, which are generally considered excitatory signaling events (49).
In contrast to the seasonal difference in response to CYN seen in OVX ewes, administration of this antagonist to ovary-intact animals increased mean LH concentrations in both seasons, although the effect size was larger in anestrous ewes. Although LH pulse patterns are similar in ovary-intact luteal phase and anestrous ewes, these patterns reflect different negative feedback actions of ovarian steroids. During anestrus, the heightened sensitivity to E2 negative feedback (50) produced by the inhibitory photoperiod is due, at least in part, to activation of inhibitory A15 dopaminergic neurons (51–53), which suppresses kisspeptin release from KNDy neurons in the ARC (31, 32, 54). During the breeding season, E2 and progesterone act together to suppress GnRH and LH pulse frequency and appear to act directly on KNDy neurons (36, 39, 55), which contain receptors for both E2 and progesterone (56, 57). Thus, the greater response in ovary-intact anestrous ewes could reflect the different inhibitory systems active at this time of year. Alternatively, this greater response might be due to the combination of steroid-independent and a more modest steroid-dependent suppression of LH by SST during anestrus because serotonergic inhibition of pulse frequency is sometimes evident in the presence of E2 (45).
Because CYN was administered into the third ventricle, the pharmacological data provide no indication of possible sites of action. However, the robust increase in the percentage of GnRH-ir cells that contained c-Fos only within the MBH supports the possibility of an action in this region. Alternatively, this could reflect an indirect action of CYN because an increase in episodic LH secretion by an endogenous opioid receptor antagonist or a pheromonal stimulus also selectively increased c-Fos in MBH GnRH neurons (58). Two other observations point to an action of CYN in the MBH. First, the negative correlation between the LH response and distance of the cannula site from the infundibular recess within the third ventricle is more consistent with an action in the MBH than in the POA. Second, there was a modest increase in the percentage of kisspeptin-ir cells in the caudal aspect of the ARC that contain c-Fos after SSTR2 blockade. The possibility that SST acts on neural afferents to GnRH cells such as the KNDy neurons of the ARC (35, 59), which have been implicated in the generation of GnRH pulses (60–64), had not been previously evaluated in any species. Thus, the stimulatory effects of SST2R blockade could be mediated by actions directly on a subset of GnRH neurons in the MBH that have been implicated in pulsatile LH secretion (58), on KNDy neurons, or a combination of these substrates (Fig. 7).
Figure 7.
Proposed model for SST action within the hypothalamus that mediates the steroid-independent control of LH secretion in sheep between the anestrous (left) and the breeding seasons (right). The inhibitory photoperiod in anestrus stimulates serotonergic neurons that increase the activity of SST neurons in the VMN. These SST neurons in turn inhibit GnRH pulse frequency, which could be mediated directly on GnRH neurons, indirectly through KNDy neurons, or indirectly through some other neuron type. This system is distinct from the steroid-dependent actions of photoperiod, which act via A15 dopaminergic (DA) neurons. See text for a more detailed discussion.
In summary, these experiments demonstrate that endogenous SST, acting at least partly through SSTR2, inhibits episodic LH secretion but does not alter LH surge secretion. Furthermore, blockade of SSTR2 in ovary-intact ewes increased mean LH in both the breeding season and anestrus, whereas in OVX ewes, SSTR2 blockade increased LH IPI only in anestrus. The use of an SSTR2 antagonist allowed us to test the role of endogenous SST in the regulation of LH secretion. Together, these studies provide evidence that SST contributes to steroid-independent suppression of LH during anestrus and may also have a minor role in the inhibitory actions of ovarian steroids on LH secretion.
Acknowledgments
We thank Miroslav Valent, Gail Sager, and John Connors for technical assistance with radioimmunoassay and animal surgeries; Drs. Margaret Minch and Jennifer Fridley for veterinary care; and Dr. Al Parlow and the National Hormone and Peptide program for reagents used to measure LH.
Acknowledgments
This work was supported by National Institutes of Health (NIH) Grants R01-HD039916, R01-HD082135, and P20GM103434 to the West Virginia IDeA Network for Biomedical Research Excellence. Imaging experiments and image analysis were performed in the West Virginia University (WVU) Microscope Imaging Facility, which has been supported by the WVU Cancer Institute and NIH Grants P20 RR016440, P30 GM103488, and P20 GM103434.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- aCSF
- artificial cerebrospinal fluid
- ANOVA
- analysis of variance
- ARC
- arcuate nucleus
- CYN
- CYN154806
- E2
- estradiol
- GnRH
- gonadotropin-releasing hormone
- ICV
- intracerebroventricular
- IPI
- interpulse interval
- KNDy
- kisspeptin-neurokinin B-dynrophin
- LH
- luteinizing hormone
- MBH
- mediobasal hypothalamus
- OVX
- ovariectomized
- PB
- phosphate buffer
- PBS
- phosphate-buffered saline
- PBST
- PBS containing 0.04% Triton X-100
- POA
- preoptic area
- SEM
- standard error of the mean
- SST
- somatostatin
- SSTR
- somatostatin receptor
- VMN
- ventromedial nucleus.
References
- 1.Goodman RL, Karsch FJ. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology. 1980;107(5):1286–1290. [DOI] [PubMed] [Google Scholar]
- 2.Goodman R. Neuroendocrine control of gonadotropin secretion: comparative aspects. In: Plant TM, Zeleznik A, eds. Physiology of Reproduction. Cambridge, MA: Academic Press; 2015:1537–1574. [Google Scholar]
- 3.Goodman RL, Bittman EL, Foster DL, Karsch FJ. Alterations in the control of luteinizing hormone pulse frequency underlie the seasonal variation in estradiol negative feedback in the ewe. Biol Reprod. 1982;27(3):580–589. [DOI] [PubMed] [Google Scholar]
- 4.Pohl CR, deRidder CM, Plant TM. Gonadal and nongonadal mechanisms contribute to the prepubertal hiatus in gonadotropin secretion in the female rhesus monkey (Macaca mulatta). J Clin Endocrinol Metab. 1995;80(7):2094–2101. [DOI] [PubMed] [Google Scholar]
- 5.Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985;116(4):1341–1350. [DOI] [PubMed] [Google Scholar]
- 6.Winter JSD, Faiman C. Serum gonadotropin in concentrations in agonadal children and adults. J Clin Endocrinol Metab. 1972;35(4):561–564. [DOI] [PubMed] [Google Scholar]
- 7.Ropelato MG, Escobar ME, Gottlieb S, Bergadá C. Gonadotropin secretion in prepubertal normal and agonadal children evaluated by ultrasensitive time-resolved immunofluorometric assays. Horm Res. 1997;48(4):164–172. [DOI] [PubMed] [Google Scholar]
- 8.Skinner DC, Caraty A, Allingham R. Unmasking the progesterone receptor in the preoptic area and hypothalamus of the ewe: no colocalization with gonadotropin-releasing neurons. Endocrinology. 2001;142(2):573–579. [DOI] [PubMed] [Google Scholar]
- 9.Herbison AE, Theodosis DT. Immunocytochemical identification of oestrogen receptors in preoptic neurones containing calcitonin gene-related peptide in the male and female rat. Neuroendocrinology. 1992;56(5):761–764. [DOI] [PubMed] [Google Scholar]
- 10.Lehman MN, Karsch FJ. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-endorphin-immunoreactive neurons contain estrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology. 1993;133(2):887–895. [DOI] [PubMed] [Google Scholar]
- 11.Reisine T, Bell GI. Molecular biology of somatostatin receptors. Endocr Rev. 1995;16(4):427–442. [DOI] [PubMed] [Google Scholar]
- 12.Rotsztejn WH, Drouva SV, Epelbaum J, Kordon C. Somatostatin inhibits in vitro release of luteinizing hormone releasing hormone from rat mediobasal hypothalamic slices. Experientia. 1982;38(8):974–975. [DOI] [PubMed] [Google Scholar]
- 13.Bhattarai JP, Kaszás A, Park SA, Yin H, Park SJ, Herbison AE, Han SK, Abrahám IM. Somatostatin inhibition of gonadotropin-releasing hormone neurons in female and male mice. Endocrinology. 2010;151(7):3258–3266. [DOI] [PubMed] [Google Scholar]
- 14.Koyama M, Yin C, Ishii H, Sakuma Y.. Somatostatin inhibition of GnRH neuronal activity and the morphological relationship between GnRH and somatostatin neurons in rats . 2012;153:806–814. [DOI] [PubMed] [Google Scholar]
- 15.Herbison AE. Neurochemical identity of neurones expressing oestrogen and androgen receptors in sheep hypothalamus. J Reprod Fertil Suppl. 1995;49:271–283. [PubMed] [Google Scholar]
- 16.Scanlan N, Dufourny L, Skinner DC. Somatostatin-14 neurons in the ovine hypothalamus: colocalization with estrogen receptor alpha and somatostatin-28(1-12) immunoreactivity, and activation in response to estradiol. Biol Reprod. 2003;69(4):1318–1324. [DOI] [PubMed] [Google Scholar]
- 17.Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A. Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology. 1998;139(4):1752–1760. [DOI] [PubMed] [Google Scholar]
- 18.Pillon D, Caraty A, Fabre-Nys C, Lomet D, Cateau M, Bruneau G. Regulation by estradiol of hypothalamic somatostatin gene expression: possible involvement of somatostatin in the control of luteinizing hormone secretion in the ewe. Biol Reprod. 2004;71(1):38–44. [DOI] [PubMed] [Google Scholar]
- 19.Fergani C, Routly JE, Jones DN, Pickavance LC, Smith RF, Dobson H. Activation of cells containing estrogen receptor alpha or somatostatin in the medial preoptic area, arcuate nucleus, and ventromedial nucleus of intact ewes during the follicular phase, and alteration after lipopolysaccharide. Biol Reprod. 2014;91(6):141. [DOI] [PubMed] [Google Scholar]
- 20.Van Vugt HH, Swarts HJM, Van de Heijning BJM, Van der Beek EM. Centrally applied somatostatin inhibits the estrogen-induced luteinizing hormone surge via hypothalamic gonadotropin-releasing hormone cell activation in female rats. Biol Reprod. 2004;71(3):813–819. [DOI] [PubMed] [Google Scholar]
- 21.Samuels MH, Henry P, Ridgway EC. Effects of dopamine and somatostatin on pulsatile pituitary glycoprotein secretion. J Clin Endocrinol Metab. 1992;74(1):217–222. [DOI] [PubMed] [Google Scholar]
- 22.Prelević GM, Wurzburger MI, Balint-Perić L, Nesić JS. Inhibitory effect of sandostatin on secretion of luteinising hormone and ovarian steroids in polycystic ovary syndrome. Lancet. 1990;336(8720):900–903. [DOI] [PubMed] [Google Scholar]
- 23.Hastie P, Evans N, Robinson J. Distribution of somatostatin receptors in the hypothalamus of control and prenatally androgenised ewes. Endocrine Abstracts. 2009;19:P214. [Google Scholar]
- 24.Foradori CD, Goodman RL, Adams VL, Valent M, Lehman MN. Progesterone increases dynorphin a concentrations in cerebrospinal fluid and preprodynorphin messenger ribonucleic Acid levels in a subset of dynorphin neurons in the sheep. Endocrinology. 2005;146(4):1835–1842. [DOI] [PubMed] [Google Scholar]
- 25.Grachev P, Li XF, Hu MH, Li SY, Millar RP, Lightman SL, O’Byrne KT. Neurokinin B signaling in the female rat: a novel link between stress and reproduction. Endocrinology. 2014;155(7):2589–2601. [DOI] [PubMed] [Google Scholar]
- 26.Skinner DC, Harris TG, Evans NP. Duration and amplitude of the luteal phase progesterone increment times the estradiol-induced luteinizing hormone surge in ewes. Biol Reprod. 2000;63(4):1135–1142. [DOI] [PubMed] [Google Scholar]
- 27.Nunn C, Schoeffter P, Langenegger D, Hoyer D. Functional characterisation of the putative somatostatin sst2 receptor antagonist CYN 154806. Naunyn Schmiedebergs Arch Pharmacol. 2003;367(1):1–9. [DOI] [PubMed] [Google Scholar]
- 28.Deaver DR, Stilley NJ, Dailey RA, Inskeep EK, Lewis PE. Concentrations of ovarian and pituitary hormones following prostaglandin F2 alpha-induced luteal regression in ewes varies with day of the estrous cycle at treatment. J Anim Sci. 1986;62(2):422–427. [DOI] [PubMed] [Google Scholar]
- 29.Watson RE Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7(1):155–159. [DOI] [PubMed] [Google Scholar]
- 30.Lehman MN, Robinson JE, Karsch FJ, Silverman AJ. Immunocytochemical localization of luteinizing hormone-releasing hormone (LHRH) pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J Comp Neurol. 1986;244(1):19–35. [DOI] [PubMed] [Google Scholar]
- 31.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, Pereira A, Iqbal J, Caraty A, Ciofi P, Clarke IJ. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148:5752–5760. [DOI] [PubMed] [Google Scholar]
- 32.Merkley CM, Porter KL, Coolen LM, Hileman SM, Billings HJ, Drews S, Goodman RL, Lehman MN. KNDy (kisspeptin/neurokinin B/dynorphin) neurons are activated during both pulsatile and surge secretion of LH in the ewe. Endocrinology. 2012;153(11):5406–5414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grachev P, Porter KL, Coolen LM, McCosh RB, Connors JM, Hileman SM, Lehman MN, Goodman RL. Surge-like luteinising hormone secretion induced by retrochiasmatic area NK3R activation is mediated primarily by arcuate kisspeptin neurones in the ewe. J Neuroendocrinol. 2016;28(6):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weems PW, Witty CF, Amstalden M, Coolen LM, Goodman RL, Lehman MN. κ-opioid receptor is colocalized in GnRH and KNDy cells in the female ovine and rat brain. Endocrinology. 2016;157(6):2367–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merkley CM, Coolen LM, Goodman RL, Lehman MN. Evidence for changes in numbers of synaptic inputs onto KNDy and GnRH neurones during the preovulatory LH surge in the ewe. J Neuroendocrinol. 2015;27(7):624–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goodman RL, Coolen LM, Anderson GM, Hardy SL, Valent M, Connors JM, Fitzgerald ME, Lehman MN. Evidence that dynorphin plays a major role in mediating progesterone negative feedback on gonadotropin-releasing hormone neurons in sheep. Endocrinology. 2004;145(6):2959–2967. [DOI] [PubMed] [Google Scholar]
- 37.Goodman RL, Legan SJ, Ryan KD, Foster DL, Karsch FJ. Importance of variations in behavioural and feedback actions of oestradiol to the control of seasonal breeding in the ewe. J Endocrinol. 1981;89(2):229–240. [DOI] [PubMed] [Google Scholar]
- 38.Thomas GB, Pearce DT, Oldham CM, Martin GB, Lindsay DR. Effects of breed, ovarian steroids and season on the pulsatile secretion of LH in ovariectomized ewes. J Reprod Fertil. 1988;84(1):313–324. [DOI] [PubMed] [Google Scholar]
- 39.Goodman RL, Inskeep EK. Control of the ovarian cycle of the sheep. In: Plant TM, Zeleznik AJ. eds. . Knobil and Neill’s Physiology of Reproduction. Cambridge, MA: Academic Press; 2015:1259–1305. [Google Scholar]
- 40.Millar RP, Klaff LJ, Barron J, Levitt NS, Ling N. Somatostatin-28 inhibits LHRH-stimulated gonadotrophin secretion in man. Clin Endocrinol (Oxf). 1982;17(2):103–107. [DOI] [PubMed] [Google Scholar]
- 41.Lightman SL, Fox P, Dunne MJ. The effect of SMS 201-995, a long-acting somatostatin analogue, on anterior pituitary function in healthy male volunteers. Scand J Gastroenterol Suppl. 1986;119:84–95. [DOI] [PubMed] [Google Scholar]
- 42.Blache D, Fabre-Nys CJ, Venier G. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res. 1991;546(2):241–249. [DOI] [PubMed] [Google Scholar]
- 43.Meyer SL, Goodman RL. Separate neural systems mediate the steroid-dependent and steroid-independent suppression of tonic luteinizing hormone secretion in the anestrous ewe. Biol Reprod. 1986;35(3):562–571. [DOI] [PubMed] [Google Scholar]
- 44.Whisnant CS, Goodman RL. Further evidence that serotonin mediates the steroid-independent inhibition of luteinizing hormone secretion in anestrous ewes. Biol Reprod. 1990;42(4):656–661. [DOI] [PubMed] [Google Scholar]
- 45.Le Corre S, Chemineau P. Control of photoperiodic inhibition of luteinizing hormone secretion by dopaminergic and serotonergic systems in ovariectomized Ile-de-France ewes supplemented with oestradiol. J Reprod Fertil. 1993;97(2):367–373. [DOI] [PubMed] [Google Scholar]
- 46.Tillet Y. Immunocytochemical localization of serotonin-containing neurons in the myelencephalon, brainstem and diencephalon of the sheep. Neuroscience. 1987;23(2):501–527. [DOI] [PubMed] [Google Scholar]
- 47.Le Corre S, Chemineau P. Serotonergic 5HT2 receptors mediate the inhibitory action of serotonin on luteinizing hormone secretion in ovariectomized, estradiol-treated ewes that are refractory to short days. Biol Reprod. 1993;49(1):140–147. [DOI] [PubMed] [Google Scholar]
- 48.Pelletier J, Auzan C, Daveau A, Clauser E, Chemineau P. Sheep 5HT2A receptors: partial cloning of the coding sequence and mRNA localization by in situ hybridization in the ewe hypothalamus. Cell Tissue Res. 1999;295(2):231–239. [DOI] [PubMed] [Google Scholar]
- 49.Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol Rev. 1994;46(2):157–203. [PubMed] [Google Scholar]
- 50.Legan SJ, Karsch FJ, Foster DL. The endocrin control of seasonal reproductive function in the ewe: a marked change in response to the negative feedback action of estradiol on luteinizing hormone secretion. Endocrinology. 1977;101(3):818–824. [DOI] [PubMed] [Google Scholar]
- 51.Havern RL, Whisnant CS, Goodman RL. Dopaminergic structures in the ovine hypothalamus mediating estradiol negative feedback in anestrous ewes. Endocrinology. 1994;134(4):1905–1914. [DOI] [PubMed] [Google Scholar]
- 52.Billings HJ, Connors JM, Altman SN, Hileman SM, Holaskova I, Lehman MN, McManus CJ, Nestor CC, Jacobs BH, Goodman RL. Neurokinin B acts via the neurokinin-3 receptor in the retrochiasmatic area to stimulate luteinizing hormone secretion in sheep. Endocrinology. 2010;151(8):3836–3846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Thiéry JC, Martin GB, Tillet Y, Caldani M, Quentin M, Jamain C, Ravault JP. Role of hypothalamic catecholamines in the regulation of luteinizing hormone and prolactin secretion in the ewe during seasonal anestrus. Neuroendocrinology. 1989;49(1):80–87. [DOI] [PubMed] [Google Scholar]
- 54.Goodman RL, Maltby MJ, Millar RP, Hileman SM, Nestor CC, Whited B, Tseng AS, Coolen LM, Lehman MN. Evidence that dopamine acts via kisspeptin to hold GnRH pulse frequency in check in anestrous ewes. Endocrinology. 2012;153(12):5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148(3):1150–1157. [DOI] [PubMed] [Google Scholar]
- 56.Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A. Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor alpha. Neurosci Lett. 2006;401(3):225–230. [DOI] [PubMed] [Google Scholar]
- 57.Foradori CD, Coolen LM, Fitzgerald ME, Skinner DC, Goodman RL, Lehman MN. Colocalization of progesterone receptors in parvicellular dynorphin neurons of the ovine preoptic area and hypothalamus. Endocrinology. 2002;143(11):4366–4374. [DOI] [PubMed] [Google Scholar]
- 58.Boukhliq R, Goodman RL, Berriman SJ, Adrian B, Lehman MN. A subset of gonadotropin-releasing hormone neurons in the ovine medial basal hypothalamus is activated during increased pulsatile luteinizing hormone secretion. Endocrinology. 1999;140(12):5929–5936. [DOI] [PubMed] [Google Scholar]
- 59.Smith JT, Li Q, Yap KS, Shahab M, Roseweir AK, Millar RP, Clarke IJ. Kisspeptin is essential for the full preovulatory LH surge and stimulates GnRH release from the isolated ovine median eminence. Endocrinology. 2011;152(3):1001–1012. [DOI] [PubMed] [Google Scholar]
- 60.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ohkura S, Takase K, Matsuyama S, Mogi K, Ichimaru T, Wakabayashi Y, Uenoyama Y, Mori Y, Steiner RA, Tsukamura H, Maeda KI, Okamura H. Gonadotrophin-releasing hormone pulse generator activity in the hypothalamus of the goat. J Neuroendocrinol. 2009;21(10):813–821. [DOI] [PubMed] [Google Scholar]
- 62.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, Clifton DK, Mori Y, Tsukamura H, Maeda K, Steiner RA, Okamura H. Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. J Neurosci. 2010;30(8):3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rance NE, Krajewski SJ, Smith MA, Cholanian M, Dacks P. Neurokinin B and the hypothalamic regulation of reproduction. Brain Res. 2011;1027:116–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29(38):11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]