Abstract
In the last few years, the survival of patients with castration-resistant prostate cancer (CRPC) has significantly improved as a result of the development of second-generation androgen deprivation therapies such as abiraterone and second-generation antagonists such as enzalutamide. However, CRPC patients rapidly develop resistance to these drugs, in many cases because of reactivation of the therapeutic target, the androgen receptor (AR) transcription factor. Several mechanisms responsible for AR transcriptional reactivation have been demonstrated, including mutation, amplification, and rearrangement of the AR gene, transcriptional compensation by alternative steroid receptors, and mutation or copy number alteration of genes encoding AR coregulators. In addition, CRPC tumors display elevated expression of truncated AR variants (AR-Vs) that can arise from alternative splicing or underlying AR gene rearrangements. In this review, we discuss general mechanisms of resistance to androgen/AR-targeted therapies, with a focus on the role of AR-Vs in conferring resistance to abiraterone or enzalutamide in CRPC patients.
We review the role of androgen receptor variants (AR-Vs) arising from alternative splicing and genome rearrangements in resistance to AR-targeted therapies in castration-resistant prostate cancer.
Prostate cancer is the most common noncutaneous cancer in men and the second leading cause of cancer-related deaths in men. Thirteen percent of men in the United States will develop prostate cancer during their lifetime, and an estimated 26,000 US men die of prostate cancer each year (1). When confined to the prostate, local therapies such as surgery and radiotherapy are curative for many men. However, some patients display recurrence of their prostate cancer following surgery or radiotherapy. In 1941, Huggins and Hodges (2) first reported the use of androgen deprivation therapy (ADT) for men with advanced prostate cancer, suggesting a critical role for the androgen/androgen receptor (AR) axis in the progression of prostate cancer. This seminal finding formed the foundation of current ADT regimens for advanced prostate cancer, which consist of chemical castration with a luteinizing hormone–releasing hormone agonist or antagonist and direct inhibition of the AR with competitive antagonist antiandrogens. Although most patients respond to initial ADT, virtually all patients progress to the lethal stage referred to as castration-resistant prostate cancer (CRPC). In this review, we summarize the current knowledge regarding the mechanisms of CRPC resistance to second-generation androgen/AR-targeted therapies, with a focus on AR variants (AR-Vs).
AR and Mechanism of Androgen Action
The AR is a member of the class I nuclear steroid receptor family, which includes the estrogen receptor, glucocorticoid receptor (GR), progesterone receptor, and mineralocorticoid receptor. The AR gene is located on chromosome X at cytogenetic band Xq11-12 and consists of eight exons encoding for a 110-kDa protein. Structurally, the modular AR protein contains a long NH2-terminal domain (NTD) encoded by exon 1, a DNA-binding domain (DBD) encoded by exons 2 and 3, a short hinge region encoded by exons 3 and 4, and a ligand-binding domain (LBD) at the carboxyl terminus encoded by exons 4 to 8. The main physiological androgens are testosterone and dihydrotestosterone, which exert their actions by binding directly to the AR LBD. Binding of androgen to the LBD induces conformational changes in the protein that expose the nuclear localization signal in the hinge region, resulting in AR nuclear translocation. In the nucleus, androgen-bound AR binds as a dimer to androgen response elements (AREs) located predominantly in genomic enhancer regions and regulates the transcriptional output of androgen-regulated genes (Fig. 1).
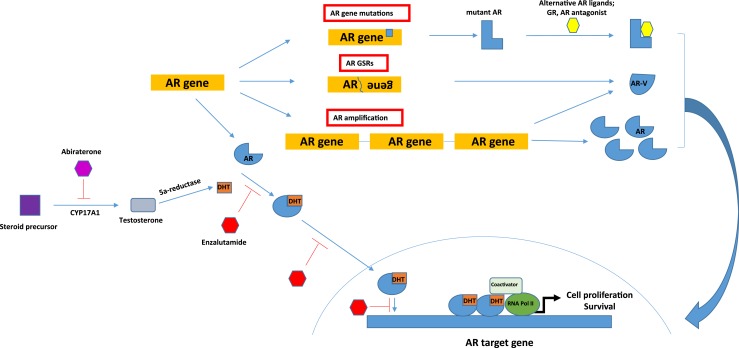
Figure 1.
A summary of molecular alterations leading to persistent AR signaling that supports castration resistance. In castration-resistant prostate cancer cells, adrenal androgens can be converted to dihydrotestosterone (DHT). DHT binds to AR in the cytoplasm and promotes translocation of AR into the nucleus, where it binds as a dimer to AREs for gene activation or repression. Abiraterone inhibits the androgen steroidogenesis enzyme CYP17A1. Enzalutamide inhibits AR signaling by competing with DHT for binding to the AR LBD. Enzalutamide also inhibits nuclear translocation and binding of AR to AREs. GSR, genomic structural rearrangement.
Mechanisms of Abiraterone and Enzalutamide Action
Abiraterone and enzalutamide are second-generation ADT and antagonist drugs, respectively, that were initially approved for treatment of CRPC following taxane chemotherapy. Because clinical trials showed increased survival of CRPC patients treated with abiraterone or enzalutamide compared with chemotherapy, these drugs are now approved for use in the prechemotherapy setting after failure of first-line androgen/AR-targeted therapy (3, 4).
The development of abiraterone was based on the finding that testosterone and dihydrotestosterone in tumor tissue from ADT-treated CRPC patients persisted at high levels sufficient to activate AR and its target genes, despite castrate levels of androgens in the circulation (5–7). These tissue androgens were subsequently found to have an intracrine origin, produced from adrenal androgens or cholesterol as precursors. These findings served as the basis for targeting intracrine androgen synthesis with abiraterone, a steroidal inhibitor of the 17α-hydroxylase and 17,20-lyase activities of the CYP17A1 androgen synthesis enzyme expressed in testes, adrenal glands, and prostate cancer cells (8, 9) (Fig. 1). Abiraterone has also been shown to be metabolized by 3β-hydroxysteroid dehydrogenase to Δ4-abiraterone, which appears to have a broader spectrum of targets than abiraterone, including direct antagonism of the AR LBD (10, 11).
Enzalutamide (originally named MDV3100) was developed as a result of the finding that twofold overexpression of AR protein sensitized CRPC cells to castrate levels of androgens and converted first-generation AR antagonists such as bicalutamide into weak agonists (12). Enzalutamide is a competitive antagonist that binds to the AR LBD with higher affinity than bicalutamide and was discovered as a result of high-throughput screening against a cell model engineered to overexpress AR (13, 14). Binding of enzalutamide to the AR LBD resulted in reduced AR nuclear translocation, impaired genome-wide binding of AR to AREs, and impaired recruitment of coactivators to AR target genes (13, 14) (Fig. 1).
Mechanisms of CRPC Resistance to First- and Second-Generation AR-Targeted Therapies
CRPC metastases display distinct heterogeneity in terms of molecular landscape and clinical-pathologic features. A recent study examining the genomic features of a cohort of 150 patients with metastatic CRPC demonstrated that the majority of cases (96.4%) displayed typical adenocarcinoma histology, whereas only 2.9% of cases showed the histology of neuroendocrine prostate cancer (NEPC) (15). NEPC is an aggressive subtype of CRPC that is highly resistant to AR-targeted therapies. Histologically, NEPC is characterized by the expression of neuroendocrine markers, such as chromogranin A and synaptophysin, and reduced or absent AR expression (16). A biopsy-based study designed to compare the genomic and transcriptomic features of CRPC metastases with NEPC vs adenocarcinoma histology revealed low to absent protein expression levels of AR as well as low to absent activity of the AR transcriptional program in NEPC samples (17). Conversely, the adenocarcinoma samples displayed high expression levels of AR protein and broad activity of the AR transcriptional program (17). Because the adenocarcinoma class of CRPC is the most prevalent in patients and these tumors display evidence of active AR, the majority of CRPC studies have focused on elucidating the mechanism accounting for sustained androgen/AR signaling in CRPC. These studies have revealed diverse mechanisms including AR gene alterations, transcriptional compensation by alternative steroid receptors, mutation or copy number alteration of genes encoding AR coregulators, and expression of constitutively active AR-Vs.
AR Gene Alterations
AR amplification is the most common AR gene alteration in CRPC. Grasso et al. (18) performed a whole-exome sequencing (WES) study with CRPC metastases collected during rapid autopsies of a cohort of 50 patients who died of CRPC. Their analyses revealed that 25 of 50 patients (50%) had AR gene amplification (18). In a larger study of 150 CPRC patients performed by Robinson et al. (15), WES of bone and soft-tissue metastases collected with biopsies revealed that ∼50% of the patients had AR gene copy number gain. In contrast, The Cancer Genome Atlas WES study of 333 primary prostate cancer tissues reported <1% of cases had AR gene amplification (19). This supports the concept that AR gene amplification is an adaptive response to ADT. This concept is also consistent with work showing that overexpression of AR protein can sensitize CRPC cells to castrate levels of androgens and can convert antagonists such as bicalutamide into weak agonists (12, 13).
AR point mutations are also more frequent in CRPC than in primary prostate cancer. For example, both the Grasso et al. (18) and Robinson et al. (15) studies reported somatic AR point mutations in approximately 10% of CRPC tissues, whereas The Cancer Genome Atlas study (19) did not find evidence of somatic AR point mutations in any primary prostate cancer tissues examined. This is also consistent with results of a previous study that performed targeted AR sequencing in a cohort of 181 primary cancers and 37 CRPC metastases, revealing that somatic AR point mutations occurred only in CRPC (20). These studies have also established that AR point mutations occur more frequently in patients with prior exposure to antiandrogens, with the most common mutations occurring in residues located in the AR LBD. This aligns with mechanistic studies demonstrating that mutations in the AR LBD can confer AR agonist activity to bicalutamide and flutamide, as well as other steroids including glucocorticoids (15).
In addition to AR gene amplification and somatic point mutations, diverse AR genomic structural rearrangements (AR-GSRs) have recently been reported in CRPC. AR-GSRs are defined as rearrangement events having at least one breakpoint mapping within the AR gene body. In a targeted paired-end DNA-sequencing study of the coding and noncoding regions of the 183-kb AR gene in 30 soft-tissue metastases collected from rapid autopsy of 15 CRPC patients, AR-GSRs were frequent, with 10 of 30 metastases (six of 15 patients) displaying at least one AR-GSR event (21). AR-GSRs also occurred in two of six locally recurrent CRPC tumors but were not observed in primary prostate cancer (21). In a separate targeted paired-end DNA-sequencing study with cell-free DNA isolated from the plasma of 30 patients with CRPC, a similar diversity of AR-GSRs was observed in 15 patients (22). Collectively, these studies support the notion that AR-GSRs are frequent events in CRPC. A challenge going forward will be unraveling the functional outcomes of the diverse deletion, duplication, inversion, and translocation events that have been reported. As discussed later, there is strong evidence that many of these AR-GSR events support stable expression of diverse AR-V species that can mediate constitutive AR transcriptional activity in CRPC cells.
Given that CRPC represents a highly heterogeneous disease stage, several studies have explored AR gene alterations in the context of intratumor and intrapatient heterogeneity. For example, whole-genome sequencing of multiple metastatic sites collected from rapid autopsies of 10 CRPC patients revealed intrapatient heterogeneity wherein independent metastatic sites displayed independently evolving AR gene amplification and/or point mutation events (23). This heterogeneity was also observed with regard to AR-GSRs, wherein anatomically separate metastases from the same patient displayed distinct AR-GSR events (21). Adding additional complexity, AR-GSRs can occur in CRPC metastases that also display AR amplification, perhaps at higher frequency than in CRPC metastases displaying AR copy number neutrality (21). In contrast, a trend toward mutual exclusivity between intratumor AR-GSRs and AR point mutations has been observed (21). Despite this evidence for intrapatient heterogeneity in AR gene alterations, a recent study by Kumar et al. (24) reported substantial interpatient diversity in the genome-wide alterations occurring in CRPC but limited intrapatient genomic diversity.
Transcriptional Compensation by Alternative Steroid Receptors
Increased expression or activity of other steroid receptors, such as the GR, has been identified as another mechanism of CRPC resistance to AR-targeted therapies. GR expression levels were upregulated in two independent antiandrogen-resistant xenograft prostate cancer models (25), CPRC metastases (24), and CRPC cells isolated from bone marrow aspirates of enzalutamide-resistant CRPC patients (25). In addition to GR, the levels of estrogen receptor and progesterone receptor may be increased in a subset of CRPC metastases (24). Mechanistically, GR and AR share overlapping genome-wide−binding specificity and ensuing transcriptomes because of a common DNA recognition element (26). Indeed, in model systems, inhibition of AR led to the upregulation of GR expression in a subset of prostate cancer cells, and activation of GR was sufficient to confer enzalutamide resistance (25). This study concluded that GR upregulation functions as a bypass mechanism of AR blockade. Another study confirmed these results by demonstrating the efficacy of GR antagonists, including mifepristone, in mitigating the survival of prostate cancer cells conferred by GR activation (27). The study further suggested that the GR-regulated prosurvival gene SGK1 may mediate the effects of GR in promoting CRPC progression.
Alterations in AR Coregulators
Genomic and transcriptomic alterations in coregulators of the AR pathway are also associated with resistance to AR-targeted therapies in CRPC. For example, mutations in FOXA1, the gene encoding a pioneer factor that facilitates AR chromatin binding and transcriptional activation, occurred at a higher frequency in CRPC [12% (15)] than in primary prostate cancer [4% (28)]. Nuclear receptor coactivators 1 and 2, coactivators that enhance transcriptional activity of AR and other steroid receptors, were shown to be overexpressed in 20% of primary prostate cancer and 63% of CRPC (20). A recent study that examined genomic copy number alterations in circulating tumor cells from 16 patients with CRPC resistant to abiraterone or enzalutamide revealed copy number alterations affecting multiple genes encoding AR coregulators, including copy number gains in FOXA1 (31.25%), NCOA2 (43.75%), and BRD4 (43.75%) (29). Collectively, these genomic findings underscore the critical roles of AR coregulators in mediating resistance to androgen/AR-targeted therapies, including second-generation drugs. There is also a wealth of nongenomic data supporting critical roles for diverse AR coregulators in various stages of prostate cancer development and progression. However, details of these AR coregulators are beyond the scope of this review. Readers are referred to recent reviews on this topic published elsewhere (30, 31).
The Role of AR-Vs in Resistance to Enzalutamide and Abiraterone in CRPC
Overview of AR-Vs
The first clues to the existence of truncated forms of the AR in prostate cancer originated from studies demonstrating expression of LBD-deleted AR protein species in CRPC 22Rv1 cells arising from calpain-mediated cleavage of the AR hinge region (32). However, a subsequent study using RNA interference to knock down AR expression in 22Rv1 cells found that two discrete small interfering RNA (siRNA) species blocked expression of full-length and truncated AR species differentially (33). Because differential siRNA sensitivity was incompatible with a proteolysis mechanism, this led to the hypothesis that alternative splicing was the underlying mechanism. This prompted the use of 3′ rapid amplification of complementary DNA ends to characterize the 3′ end of this putative transcript, resulting in discovery of the first AR-Vs. These species were found to harbor the AR NTD and some or all of the AR DBD but lacked the AR LBD by virtue of splicing of an intronic exon harboring an in-frame translation stop codon (33).
Subsequent studies have identified additional AR-Vs from prostate cancer cell lines, xenografts, and tissues from prostate cancer patients, including the frequently expressed AR-V7 (34, 35). The majority of these AR-Vs share structural similarities with the first reported AR-Vs, harboring the same NTD/DBD domains as the full-length AR but lacking the LBD because of alternatively spliced 3′ terminal exons. These structural properties are expected to yield constitutively active, ligand-independent forms of the AR. In line with this, most AR-Vs that have been interrogated functionally display constitutive transcriptional activity (33, 36, 37), although some AR-Vs have been reported as conditionally active (38). On the basis of these findings, a priority has been to understand the mechanisms underlying AR-V expression in prostate cancer. As discussed next, important AR gene rearrangement−dependent and alternative splicing-dependent mechanisms have emerged in recent studies.
Gene rearrangement−dependent AR-Vs
Perhaps the best-characterized mechanism underlying AR-V expression in CRPC is structural rearrangement of the AR gene, also termed AR-GSR. For example, the first cell line model (22Rv1) in which AR-Vs were discovered and functionally validated was found to harbor a 35-kb intragenic tandem duplication containing AR exon 3 as well as several of the 3′ terminal cryptic exons (CEs) expressed in AR-Vs (39). This tandem duplication included AR exon CE3, the 3′ terminal exon of AR-V7 that is highly expressed in these cells (39). A subsequent study in the LuCaP 86.2 xenograft model revealed an 8579-bp genomic deletion of AR exons 5–7, which provided a mechanism for synthesis of the truncated ARv567es AR-V in this model (40). This putative causal link between AR-GSRs and AR-Vs was confirmed in a subsequent study that used genome engineering to delete AR exons 5, 6, and 7 in a prostate cancer cell line. This deletion led to a switch in AR expression from full-length AR to ARv567es, which was constitutively active and resistant to enzalutamide (41). The associations between AR-V expression in CRPC and underlying AR-GSRs were further strengthened by the findings that expression of AR-V7 in the CWR-R1 cell line was linked to a 48-kb deletion in AR intron 1 (40) and that ARv567es expression in the LuCaP 136 xenograft was linked to genomic inversion of a segment of the AR gene harboring AR exons 5, 6, and 7 (41).
The association between AR-GSRs and AR-Vs has recently been extended to clinical tumors. In addition to remarkable diversity in the breakpoint locations and rearrangement classes that have been reported for AR-GSRs in CRPC (21, 22), AR-GSRs also display varying degrees of clonal enrichment in individual tumors. Importantly, tumors displaying AR-GSRs with high clonal enrichment were all found to express unique AR-GSR−dependent AR-Vs (21). These AR-Vs harbored the AR NTD/DBD core, were constitutively active, and could support ligand-independent growth when expressed in prostate cancer cells (21). This raises a scenario where individual patients with CRPC are likely to express unique AR-V species due to unique AR-GSRs and where these key events would go undetected with existing targeted measurement strategies. This argues that unbiased methods are needed for studies seeking to capture the full contributions of AR-GSRs and AR-Vs to therapeutic responses in CRPC.
AR splicing variants
Another mechanism proposed for AR-V expression in prostate cancer is alternative splicing, although this mechanism has been investigated only for AR-V7. For example, pharmacologic suppression of AR gene transcription in VCaP and LNCaP95, two prostate cancer cell lines positive for AR-V7, led to decreased expression of AR-V7 messenger RNA (mRNA) as well as full-length AR mRNA (42). Additionally, treatment of these cells with enzalutamide, which is known to increase transcription of the AR gene (43), led to increased expression of AR-V7 and full-length AR mRNA. These findings led to the conclusion that AR-V7 expression was linked to the transcription rate of the AR gene. Indeed, enzalutamide treatment in VCaP cells resulted in increased recruitment of the splicing factors ASF/SF2 and U2AF65 to the 3′ splice recognition site of AR exon CE3, the 3′ terminal exon of AR-V7. Moreover, siRNA knockdown of these splicing factors significantly reduced expression of AR-V7 in both VCaP and LNCaP95 cells, although these manipulations also reduced expression of full-length AR. Taken together, these data suggest that ADT-induced AR gene transcription and the subsequent recruitment of splicing factors to AR pre-mRNA contribute to AR-V7 splicing in prostate cancer cells. However, as elaborated in the following section, AR gene amplification may be an important contributing factor in this mechanism. In line with this, VCaP cells displayed high-level amplification of the AR gene (40), and increased AR gene copy number was associated with increased AR-V7 mRNA expression in CRPC metastases (21).
AR-Vs in CRPC progression
One intriguing result from genomic and transcriptomic studies of clinical specimens is that the relative ratios of AR-Vs such as AR-V7 as a function of overall AR expression are not significantly changed in CRPC metastases compared with primary prostate cancer or even nonmalignant prostate tissue (15). Nevertheless, there appears to be a strong clinical correlation between AR-V7 expression and CRPC progression. How can this be the case? One explanation may be AR gene amplification, which appears to promote increased expression of full-length AR and AR-V7 but may not affect the relative expression ratios of these species (21). For example, RT-PCR analysis of AR-V7 mRNA levels in a cohort of prostate cancer patients revealed higher expression of AR-V7 in CRPC than in hormone-naive prostate cancer (36). Subsequent studies demonstrated that high levels of AR-V7 mRNA expression or nuclear AR-V7 protein expression in metastatic CRPC tissues was associated with CRPC progression during therapy with enzalutamide and abiraterone as well as with shortened overall survival (44–46). Although these differences in AR-V7 expression levels may be explained by AR gene amplification, there is great interest in evaluating the utility of AR-V7 and perhaps other AR-Vs as predictive biomarkers of treatment response. These efforts are discussed in more detail in following sections.
Functional Role of AR-Vs in Resistance to Abiraterone and Enzalutamide in CRPC
Functional evidence supporting roles for AR-V7 and other AR-Vs as drivers of resistance to abiraterone and enzalutamide has been derived mainly from CRPC cell line models expressing high levels of endogenous AR-V7. In this regard, it is important to keep in mind that many of these models also harbor underlying AR-GSRs. Therefore, data from these models may be relevant only to clinical CRPC, where AR-V expression is driven by AR-GSR events, and not cases where AR-Vs are expressed at much lower levels because of alternative splicing. On the other hand, data from models where expression of AR-V7 is not linked to underlying AR-GSRs, such as the AR-amplified VCaP cell line, may be more relevant to clinical specimens that are AR amplified but AR-GSR negative.
In 22Rv1 cells, which harbor an underlying AR-GSR (39), siRNA-mediated knockdown of AR-V7 inhibited ligand-independent cell growth and restored sensitivity to enzalutamide, suggesting that AR-Vs are sufficient for resistance to enzalutamide in 22Rv1 cells (47). This was confirmed in a study by Cao et al. (48), which showed that 22Rv1 cells were more responsive to enzalutamide treatment both in vitro and in vivo after knockdown of AR-V7. In CWR-R1 cells, which represent another AR-GSR−positive cell model expressing high levels of AR-V7 (40), cell growth was found to be androgen independent and enzalutamide resistant. However, cell growth was inhibited by knockdown of AR-V7 (47).
In contrast to these AR-GSR− and AR-V7−positive models, VCaP cells are AR-GSR negative but AR-V7 positive. These cells may better reflect AR-V7 expression in clinical CRPC, where AR-V7 levels are associated with AR copy number (21). In this regard, it is important to note that growth of VCaP cells remains sensitive to treatment with enzalutamide (13). This may be explained by the observation that even under castrate conditions, AR-V7 mRNA expression levels are approximately 1% of full-length AR expression levels in VCaP cells (49). Nevertheless, siRNA-mediated knockdown of AR-V7 in VCaP cells reduced androgen-independent expression of AR transcriptional targets by approximately 30%, indicating some degree of AR-V7 function. On the basis of these findings, it was hypothesized that acute increases in AR-V7 expression in response to androgen/AR-targeted therapies may maintain a basal level of AR transcriptional activity to support cell survival. However, these levels of transcriptional activity are not sufficient to drive growth of CRPC tumors that remain AR dependent (49). This finding contrasts with data from a model of enzalutamide-resistant LNCaP cells, which display higher levels of AR-V7 and full-length AR expression than parental LNCaP cells do. In this model, Yamamoto et al. (50) demonstrated that an antisense oligonucleotide inhibiting expression of both full-length AR and AR-V7 had indistinguishable effects on cell growth compared with an antisense oligonucleotide that inhibited full-length AR only. These data suggest that in enzalutamide-resistant LNCaP cells, full-length AR remained the main driver of persistent AR signaling (47, 50). Collectively, this work points to highly context-dependent roles for AR-Vs as functional drivers of CRPC resistance to AR-targeted therapies. Focused studies are needed to address whether underlying AR-GSR and/or AR gene amplification events could be the basis for this context dependence.
AR-Vs as Predictive Biomarkers of Resistance to Abiraterone and Enzalutamide
Although biopsies of CRPC metastases have been important for understanding the molecular landscape of genomic and transcriptomic alterations occurring at this disease stage, this method of tissue sampling presents logistic challenges for biomarker development. Therefore, there has been substantial focus on developing methods and assays for examining circulating tumor cells (CTCs) as well as cell-free DNA and RNA in the blood of patients with CRPC. Compared with conventional biopsies, these methods are noninvasive and can be performed longitudinally to reflect dynamic changes in tumor evolution. Moreover, considering the genomic heterogeneity between discrete metastatic sites in individual patients, CTCs and/or cell-free DNA and RNA may provide a more comprehensive profile of the burden of genomic and transcriptomic alterations of the metastases.
Predictive Utility of AR-V Detection in CTCs
Most of the studies on AR-Vs in CTCs have been based on measuring mRNA levels of AR-Vs by reverse transcription-polymerase chain reaction or RNA-sequencing approaches. For example, a single-cell RNA-sequencing study of CTCs demonstrated that 33 of 73 CTCs isolated from 13 CRPC patients expressed at least one type of AR-V, with AR-V7 being the most abundant AR-V detected (26 of 73 CTCs) (51). In addition, ARv567es was detected in 18 of 73 CTCs, which is intriguing because ARv567es has been consistently linked to AR-GSRs involving exons encoding the AR LBD (21, 40, 41). Expression of other AR-Vs, such as AR-V1, AR-V3, and AR-V4, was observed in seven of 73 CTCs (51). Remarkably, 13 of 73 CTCs displayed expression of more than one type of AR-V in this study.
Because of this high frequency of detection, AR-V7 has been investigated by numerous groups as a potential predictive or treatment-selection biomarker for CRPC patients who have failed first-line androgen/AR-targeted therapies and are candidates for therapy with second-generation androgen/AR-targeted therapies. In a study of 62 CRPC patients by Antonarakis et al. (52), AR-V7 mRNA was detected in the CTCs of 19% of patients who were selected to receive therapy with abiraterone and 39% of patients selected to receive therapy with enzalutamide. The presence of AR-V7–positive CTCs correlated with lower prostate-specific antigen (PSA) response rates, shorter progression-free survival, and reduced overall survival in both treatment groups (52). In a study by Steinestel et al. (53), the presence of AR-V7 mRNA in CTCs correlated with the metastatic status of the disease. Moreover, a significant correlation was found between the presence of AR-V7 in CTCs and the number of prior therapies that the patients had undergone. This agrees with results from another study showing that conversions from AR-V7–negative to AR-V7–positive CTCs occurred in some CRPC patients during treatment with abiraterone or enzalutamide, although it should be noted that conversions occurring in the opposite direction were also observed (54).
Growing evidence has supported a role for AR-Vs in determining response/resistance to first- and second-line taxane chemotherapy in prostate cancer. For instance, in contrast to the finding that patients with AR-V7–positive CTCs were more likely to display progression during therapy with abiraterone or enzalutamide, Antonarakis et al. (55) reported that the presence of AR-V7 in CTCs was not associated with resistance to taxane chemotherapy. This finding was consistent with results from a cohort of 29 CRPC patients initiating cabazitaxel therapy, for whom the detection of AR-V7 mRNA in CTCs was not associated with clinical outcomes in terms of CTC and PSA response rate, progression-free survival, and overall survival (56). Furthermore, the Antonarakis et al. study (55) found that patients with AR-V7–positive CTCs prior to therapy had better clinical outcomes when treated with taxane chemotherapy than when treated with abiraterone or enzalutamide. This concept was confirmed in a study by Scher et al. (57), who examined nuclear expression of AR-V7 protein in CTCs from 161 CRPC patients initiating antiandrogen therapy or taxane chemotherapy. In this study, patients who displayed AR-V7–positive CTCs prior to therapy exhibited superior clinical outcomes with taxane chemotherapy compared with AR-targeted therapies. Collectively, these studies suggest that the presence of AR-V7 in CTCs may serve as a treatment-selection biomarker favoring docetaxel and cabazitaxel chemotherapy over AR-targeted therapies for CRPC patients. This concept needs to be tested in large prospective biomarker-based trials.
Predictive Utility of AR-V Detection in Blood
In addition to AR-V detection in CTCs, the presence of AR-7 mRNA in whole blood has been evaluated as a biomarker of resistance to ADT in CRPC patients. For example, Del Re et al. (58) reported the development of a sensitive digital droplet polymerase chain reaction method to interrogate plasma-derived exosomal RNA from a cohort of 36 CRPC patients receiving therapy with abiraterone or enzalutamide. They found that patients with a positive digital droplet polymerase chain reaction signal for AR-V7 mRNA in exosomal RNA had shorter survival than patients who were negative for AR-V7 mRNA. In a similar study involving a larger cohort of 132 CRPC patients initiating therapy with abiraterone or enzalutamide, increased levels of PSA and AR-V7 mRNA transcripts in blood were associated with shorter time to treatment failure and shorter overall survival (59). Finally, Todenhöfer et al. (60) examined the levels of AR-V7 mRNA transcripts as well as several AR target genes in the whole blood of CRPC patients treated with abiraterone. They found that among the mRNA transcripts tested, only the detection of AR-V7 was associated with inferior clinical outcomes.
Conclusions
Widespread clinical use of second-generation androgen/AR-targeted therapies has improved the survival of CRPC patients while also underscoring the critical role of sustained AR signaling under conditions of AR-targeted therapy. Resistance to abiraterone and enzalutamide occurs through various mechanisms and represents a contemporary clinical challenge. Understanding the nuances of these resistance mechanisms and developing specific assays to monitor or predict their occurrence are expected to reveal new insights into CRPC biology and lead to the development of new therapies for CRPC. This opportunity is being exploited to interrogate AR-Vs in CRPC, which have been linked in multiple studies to clinical resistance to abiraterone and enzalutamide. Although recent efforts have focused on monitoring AR-V7 in CTCs or blood of CRPC patients, there are myriad AR-Vs with clinical relevance that have not been captured in these studies. This is true especially for patient-specific AR-Vs that arise from underlying AR-GSR events. Regardless of successes with these biomarker approaches, it remains unclear whether detection of AR-Vs such as AR-V7 reflects an AR-V–driven resistance mechanism or reflects high overexpression of AR. In cases where AR-Vs are driving resistance, the molecular structure of AR-Vs represents a therapeutic challenge that cannot be overcome with current drugs. Therefore, the development of inhibitors that selectively target AR-Vs will be important in complementing current treatment regimens for patients with CRPC. In addition, understanding the specific contexts in which AR-Vs are functioning as the main transcriptional drivers of AR signaling will be important for appropriate design of future clinical trials with these therapeutics.
Acknowledgments
Acknowledgments
Financial support was provided by National Institutes of Health Grant R01CA174777 (to S.M.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ADT
- androgen deprivation therapy
- AR
- androgen receptor
- ARE
- androgen response element
- AR-GSR
- AR genomic structural rearrangement
- AR-V
- AR variant
- CE
- cryptic exon
- CRPC
- castration-resistant prostate cancer
- CTC
- circulating tumor cell
- DBD
- DNA-binding domain
- GR
- glucocorticoid receptor
- LBD
- ligand-binding domain
- mRNA
- messenger RNA
- NEPC
- neuroendocrine prostate cancer
- NTD
- NH2-terminal domain
- PSA
- prostate-specific antigen
- siRNA
- small interfering RNA
- WES
- whole-exome sequencing.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 2.Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate [reprint]. J Urol. 2002;168(1):9–12. [DOI] [PubMed]
- 3.Ryan CJ, Smith MR, de Bono JS, Molina A, Logothetis CJ, de Souza P, Fizazi K, Mainwaring P, Piulats JM, Ng S, Carles J, Mulders PF, Basch E, Small EJ, Saad F, Schrijvers D, Van Poppel H, Mukherjee SD, Suttmann H, Gerritsen WR, Flaig TW, George DJ, Yu EY, Efstathiou E, Pantuck A, Winquist E, Higano CS, Taplin ME, Park Y, Kheoh T, Griffin T, Scher HI, Rathkopf DE; COU-AA-302 Investigators . Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beer TM, Tombal B. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(18):1755–1756. [DOI] [PubMed] [Google Scholar]
- 5.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10(21):7121–7126. [DOI] [PubMed] [Google Scholar]
- 6.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–4657. [DOI] [PubMed] [Google Scholar]
- 7.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–4571. [DOI] [PubMed] [Google Scholar]
- 9.de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr, Saad F, Staffurth JN, Mainwaring P, Harland S, Flaig TW, Hutson TE, Cheng T, Patterson H, Hainsworth JD, Ryan CJ, Sternberg CN, Ellard SL, Fléchon A, Saleh M, Scholz M, Efstathiou E, Zivi A, Bianchini D, Loriot Y, Chieffo N, Kheoh T, Haqq CM, Scher HI; COU-AA-301 Investigators . Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Bishop AC, Alyamani M, Garcia JA, Dreicer R, Bunch D, Liu J, Upadhyay SK, Auchus RJ, Sharifi N. Conversion of abiraterone to D4A drives anti-tumour activity in prostate cancer. Nature. 2015;523(7560):347–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li Z, Alyamani M, Li J, Rogacki K, Abazeed M, Upadhyay SK, Balk SP, Taplin ME, Auchus RJ, Sharifi N. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533(7604):547–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, Rosenfeld MG, Sawyers CL. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10(1):33–39. [DOI] [PubMed] [Google Scholar]
- 13.Tran C, Ouk S, Clegg NJ, Chen Y, Watson PA, Arora V, Wongvipat J, Smith-Jones PM, Yoo D, Kwon A, Wasielewska T, Welsbie D, Chen CD, Higano CS, Beer TM, Hung DT, Scher HI, Jung ME, Sawyers CL. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324(5928):787–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scher HI, Fizazi K, Saad F, Taplin ME, Sternberg CN, Miller K, de Wit R, Mulders P, Chi KN, Shore ND, Armstrong AJ, Flaig TW, Fléchon A, Mainwaring P, Fleming M, Hainsworth JD, Hirmand M, Selby B, Seely L, de Bono JS; AFFIRM Investigators . Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367(13):1187–1197. [DOI] [PubMed] [Google Scholar]
- 15.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC, Attard G, Beltran H, Abida W, Bradley RK, Vinson J, Cao X, Vats P, Kunju LP, Hussain M, Feng FY, Tomlins SA, Cooney KA, Smith DC, Brennan C, Siddiqui J, Mehra R, Chen Y, Rathkopf DE, Morris MJ, Solomon SB, Durack JC, Reuter VE, Gopalan A, Gao J, Loda M, Lis RT, Bowden M, Balk SP, Gaviola G, Sougnez C, Gupta M, Yu EY, Mostaghel EA, Cheng HH, Mulcahy H, True LD, Plymate SR, Dvinge H, Ferraldeschi R, Flohr P, Miranda S, Zafeiriou Z, Tunariu N, Mateo J, Perez-Lopez R, Demichelis F, Robinson BD, Schiffman M, Nanus DM, Tagawa ST, Sigaras A, Eng KW, Elemento O, Sboner A, Heath EI, Scher HI, Pienta KJ, Kantoff P, de Bono JS, Rubin MA, Nelson PS, Garraway LA, Sawyers CL, Chinnaiyan AM. Integrative clinical genomics of advanced prostate cancer [published correction appears in Cell. 2015;162(2):454]. Cell. 2015;161(5):1215–1228.
- 16.Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, Robinson BD, Troncoso P, Rubin MA. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol. 2014;38(6):756–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, Marotz C, Giannopoulou E, Chakravarthi BV, Varambally S, Tomlins SA, Nanus DM, Tagawa ST, Van Allen EM, Elemento O, Sboner A, Garraway LA, Rubin MA, Demichelis F. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22(3):298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grasso CS, Wu YM, Robinson DR, Cao X, Dhanasekaran SM, Khan AP, Quist MJ, Jing X, Lonigro RJ, Brenner JC, Asangani IA, Ateeq B, Chun SY, Siddiqui J, Sam L, Anstett M, Mehra R, Prensner JR, Palanisamy N, Ryslik GA, Vandin F, Raphael BJ, Kunju LP, Rhodes DR, Pienta KJ, Chinnaiyan AM, Tomlins SA. The mutational landscape of lethal castration-resistant prostate cancer. Nature. 2012;487(7406):239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell. 2015;163(4):1011–1025. [DOI] [PMC free article] [PubMed]
- 20.Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL. Integrative genomic profiling of human prostate cancer. Cancer Cell. 2010;18(1):11–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henzler C, Li Y, Yang R, McBride T, Ho Y, Sprenger C, Liu G, Coleman I, Lakely B, Li R, Ma S, Landman SR, Kumar V, Hwang TH, Raj GV, Higano CS, Morrissey C, Nelson PS, Plymate SR, Dehm SM. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7:13668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De Laere B, van Dam PJ, Whitington T, Mayrhofer M, Diaz EH, Van den Eynden G, Vandebroek J, Del-Favero J, Van Laere S, Dirix L, Grönberg H, Lindberg J. Comprehensive profiling of the androgen receptor in liquid biopsies from castration-resistant prostate cancer reveals novel intra-AR structural variation and splice variant expression patterns [published online ahead of print January 16, 2017]. Eur Urol. doi: 10.1016/j.eururo.2017.01.011 S0302-2838(17)30018-0. [DOI] [PubMed]
- 23.Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, Brewer DS, Kallio HM, Högnäs G, Annala M, Kivinummi K, Goody V, Latimer C, O’Meara S, Dawson KJ, Isaacs W, Emmert-Buck MR, Nykter M, Foster C, Kote-Jarai Z, Easton D, Whitaker HC, Neal DE, Cooper CS, Eeles RA, Visakorpi T, Campbell PJ, McDermott U, Wedge DC, Bova GS; ICGC Prostate UK Group. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353–357. [DOI] [PMC free article] [PubMed]
- 24.Kumar A, Coleman I, Morrissey C, Zhang X, True LD, Gulati R, Etzioni R, Bolouri H, Montgomery B, White T, Lucas JM, Brown LG, Dumpit RF, DeSarkar N, Higano C, Yu EY, Coleman R, Schultz N, Fang M, Lange PH, Shendure J, Vessella RL, Nelson PS. Substantial interindividual and limited intraindividual genomic diversity among tumors from men with metastatic prostate cancer. Nat Med. 2016;22(4):369–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arora VK, Schenkein E, Murali R, Subudhi SK, Wongvipat J, Balbas MD, Shah N, Cai L, Efstathiou E, Logothetis C, Zheng D, Sawyers CL. Glucocorticoid receptor confers resistance to antiandrogens by bypassing androgen receptor blockade. Cell. 2013;155(6):1309–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sahu B, Laakso M, Pihlajamaa P, Ovaska K, Sinielnikov I, Hautaniemi S, Jänne OA. FoxA1 specifies unique androgen and glucocorticoid receptor binding events in prostate cancer cells. Cancer Res. 2013;73(5):1570–1580. [DOI] [PubMed] [Google Scholar]
- 27.Isikbay M, Otto K, Kregel S, Kach J, Cai Y, Vander Griend DJ, Conzen SD, Szmulewitz RZ. Glucocorticoid receptor activity contributes to resistance to androgen-targeted therapy in prostate cancer. Horm Cancer. 2014;5(2):72–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbieri CE, Baca SC, Lawrence MS, Demichelis F, Blattner M, Theurillat JP, White TA, Stojanov P, Van Allen E, Stransky N, Nickerson E, Chae SS, Boysen G, Auclair D, Onofrio RC, Park K, Kitabayashi N, MacDonald TY, Sheikh K, Vuong T, Guiducci C, Cibulskis K, Sivachenko A, Carter SL, Saksena G, Voet D, Hussain WM, Ramos AH, Winckler W, Redman MC, Ardlie K, Tewari AK, Mosquera JM, Rupp N, Wild PJ, Moch H, Morrissey C, Nelson PS, Kantoff PW, Gabriel SB, Golub TR, Meyerson M, Lander ES, Getz G, Rubin MA, Garraway LA. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat Genet. 2012;44(6):685–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Li J, Kemeny G, Bitting RL, Beaver J, Somarelli JA, Ware KE, Gregory S, Armstrong AJ. Whole genomic copy number alterations in circulating tumor cells from men with abiraterone or enzalutamide-resistant metastatic castration-resistant prostate cancer. Clin Cancer Res. 2016;23(5):1346–1357. [DOI] [PubMed]
- 30.Culig Z. Androgen receptor coactivators in regulation of growth and differentiation in prostate cancer. J Cell Physiol. 2016;231(2):270–274. [DOI] [PubMed]
- 31.Foley C, Mitsiades N. Moving beyond the androgen receptor (AR): targeting AR-interacting proteins to treat prostate cancer. Horm Cancer. 2016;7(2):84–103. [DOI] [PMC free article] [PubMed]
- 32.Libertini SJ, Tepper CG, Rodriguez V, Asmuth DM, Kung HJ, Mudryj M. Evidence for calpain-mediated androgen receptor cleavage as a mechanism for androgen independence. Cancer Res. 2007;67(19):9001–9005. [DOI] [PubMed] [Google Scholar]
- 33.Dehm SM, Schmidt LJ, Heemers HV, Vessella RL, Tindall DJ. Splicing of a novel androgen receptor exon generates a constitutively active androgen receptor that mediates prostate cancer therapy resistance. Cancer Res. 2008;68(13):5469–5477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dehm SM, Tindall DJ. Alternatively spliced androgen receptor variants. Endocr Relat Cancer. 2011;18(5):R183–R196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu C, Luo J. Decoding the androgen receptor splice variants. Transl Androl Urol. 2013;2(3):178–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu R, Dunn TA, Wei S, Isharwal S, Veltri RW, Humphreys E, Han M, Partin AW, Vessella RL, Isaacs WB, Bova GS, Luo J. Ligand-independent androgen receptor variants derived from splicing of cryptic exons signify hormone-refractory prostate cancer. Cancer Res. 2009;69(1):16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo Z, Yang X, Sun F, Jiang R, Linn DE, Chen H, Chen H, Kong X, Melamed J, Tepper CG, Kung HJ, Brodie AM, Edwards J, Qiu Y. A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 2009;69(6):2305–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hu R, Isaacs WB, Luo J. A snapshot of the expression signature of androgen receptor splicing variants and their distinctive transcriptional activities. Prostate. 2011;71(15):1656–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y, Alsagabi M, Fan D, Bova GS, Tewfik AH, Dehm SM. Intragenic rearrangement and altered RNA splicing of the androgen receptor in a cell-based model of prostate cancer progression. Cancer Res. 2011;71(6):2108–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li Y, Hwang TH, Oseth LA, Hauge A, Vessella RL, Schmechel SC, Hirsch B, Beckman KB, Silverstein KA, Dehm SM. AR intragenic deletions linked to androgen receptor splice variant expression and activity in models of prostate cancer progression. Oncogene. 2012;31(45):4759–4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nyquist MD, Li Y, Hwang TH, Manlove LS, Vessella RL, Silverstein KA, Voytas DF, Dehm SM. TALEN-engineered AR gene rearrangements reveal endocrine uncoupling of androgen receptor in prostate cancer. Proc Natl Acad Sci USA. 2013;110(43):17492–17497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu LL, Xie N, Sun S, Plymate S, Mostaghel E, Dong X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene. 2014;33(24):3140–3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cai C, He HH, Chen S, Coleman I, Wang H, Fang Z, Chen S, Nelson PS, Liu XS, Brown M, Balk SP. Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1. Cancer Cell. 2011;20(4):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hörnberg E, Ylitalo EB, Crnalic S, Antti H, Stattin P, Widmark A, Bergh A, Wikström P. Expression of androgen receptor splice variants in prostate cancer bone metastases is associated with castration-resistance and short survival. PLoS One. 2011;6(4):e19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qu Y, Dai B, Ye D, Kong Y, Chang K, Jia Z, Yang X, Zhang H, Zhu Y, Shi G. Constitutively active AR-V7 plays an essential role in the development and progression of castration-resistant prostate cancer. Sci Rep. 2015;5:7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Welti J, Rodrigues DN, Sharp A, Sun S, Lorente D, Riisnaes R, Figueiredo I, Zafeiriou Z, Rescigno P, de Bono JS, Plymate SR. Analytical validation and clinical qualification of a new immunohistochemical assay for androgen receptor splice variant-7 protein expression in metastatic castration-resistant prostate cancer. Eur Urol. 2016;70(4):599–608. [DOI] [PMC free article] [PubMed]
- 47.Li Y, Chan SC, Brand LJ, Hwang TH, Silverstein KA, Dehm SM. Androgen receptor splice variants mediate enzalutamide resistance in castration-resistant prostate cancer cell lines. Cancer Res. 2013;73(2):483–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao B, Qi Y, Zhang G, Xu D, Zhan Y, Alvarez X, Guo Z, Fu X, Plymate SR, Sartor O, Zhang H, Dong Y. Androgen receptor splice variants activating the full-length receptor in mediating resistance to androgen-directed therapy. Oncotarget. 2014;5(6):1646–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu Z, Chen S, Sowalsky AG, Voznesensky OS, Mostaghel EA, Nelson PS, Cai C, Balk SP. Rapid induction of androgen receptor splice variants by androgen deprivation in prostate cancer. Clin Cancer Res. 2014;20(6):1590–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamamoto Y, Loriot Y, Beraldi E, Zhang F, Wyatt AW, Al Nakouzi N, Mo F, Zhou T, Kim Y, Monia BP, MacLeod AR, Fazli L, Wang Y, Collins CC, Zoubeidi A, Gleave M. Generation 2.5 antisense oligonucleotides targeting the androgen receptor and its splice variants suppress enzalutamide-resistant prostate cancer cell growth. Clin Cancer Res. 2015;21(7):1675–1687. [DOI] [PubMed] [Google Scholar]
- 51.Miyamoto DT, Zheng Y, Wittner BS, Lee RJ, Zhu H, Broderick KT, Desai R, Fox DB, Brannigan BW, Trautwein J, Arora KS, Desai N, Dahl DM, Sequist LV, Smith MR, Kapur R, Wu CL, Shioda T, Ramaswamy S, Ting DT, Toner M, Maheswaran S, Haber DA. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349(6254):1351–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antonarakis ES, Lu C, Wang H, Luber B, Nakazawa M, Roeser JC, Chen Y, Mohammad TA, Chen Y, Fedor HL, Lotan TL, Zheng Q, De Marzo AM, Isaacs JT, Isaacs WB, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer. N Engl J Med. 2014;371(11):1028–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steinestel J, Luedeke M, Arndt A, Schnoeller TJ, Lennerz JK, Wurm C, Maier C, Cronauer MV, Steinestel K, Schrader AJ. Detecting predictive androgen receptor modifications in circulating prostate cancer cells [published online ahead of print April 23, 2015]. Oncotarget. doi: 10.18632/oncotarget.3925. [DOI] [PMC free article] [PubMed]
- 54.Nakazawa M, Lu C, Chen Y, Paller CJ, Carducci MA, Eisenberger MA, Luo J, Antonarakis ES. Serial blood-based analysis of AR-V7 in men with advanced prostate cancer. Ann Oncol. 2015;26(9):1859–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Antonarakis ES, Lu C, Luber B, Wang H, Chen Y, Nakazawa M, Nadal R, Paller CJ, Denmeade SR, Carducci MA, Eisenberger MA, Luo J. Androgen receptor splice variant 7 and efficacy of taxane chemotherapy in patients with metastatic castration-resistant prostate cancer. JAMA Oncol. 2015;1(5):582–591. [DOI] [PMC free article] [PubMed]
- 56.Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, Hamberg P, Meulenbeld HJ, De Laere B, Dirix LY, van Soest RJ, Lolkema MP, Martens JW, van Weerden WM, Jenster GW, Foekens JA, de Wit R, Sleijfer S. Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol. 2015;68(6):939–945. [DOI] [PubMed]
- 57.Scher HI, Lu D, Schreiber NA, Louw J, Graf RP, Vargas HA, Johnson A, Jendrisak A, Bambury R, Danila D, McLaughlin B, Wahl J, Greene SB, Heller G, Marrinucci D, Fleisher M, Dittamore R. Association of AR-V7 on circulating tumor cells as a treatment-specific biomarker with outcomes and survival in castration-resistant prostate cancer. JAMA Oncol. 2016;2(11):1441–1449. [DOI] [PMC free article] [PubMed]
- 58.Del Re M, Biasco E, Crucitta S, Derosa L, Rofi E, Orlandini C, Miccoli M, Galli L, Falcone A, Jenster GW, van Schaik RH, Danesi R. The detection of androgen receptor splice variant 7 in plasma-derived exosomal RNA strongly predicts resistance to hormonal therapy in metastatic prostate cancer patients. Eur Urol. 2017;71(4):680–687. [DOI] [PubMed]
- 59.Qu F, Xie W, Nakabayashi M, Zhang H, Jeong SH, Wang X, Komura K, Sweeney CJ, Sartor O, Lee GM, Kantoff PW. Association of AR-V7 and prostate-specific antigen RNA levels in blood with efficacy of abiraterone acetate and enzalutamide treatment in men with prostate cancer. Clin Cancer Res. 2017;23(3):726–734. [DOI] [PMC free article] [PubMed]
- 60.Todenhöfer T, Azad A, Stewart C, Gao J, Eigl BJ, Gleave ME, Joshua AM, Black PC, Chi KN. AR-V7 Transcripts in whole blood RNA of patients with metastatic castration resistant prostate cancer correlate with response to abiraterone acetate. J Urol. 2017;197(1):135–142. [DOI] [PubMed]