Abstract
Type 2 deiodinase amplifies and type 3 deiodinase depletes levels of the active form of thyroid hormone, triiodothyronine. Given the opposing activities of these enzymes, we tested the hypothesis that they counteract each other’s developmental functions by investigating whether deletion of type 2 deiodinase (encoded by Dio2) modifies sensory phenotypes in type 3 deiodinase-deficient (Dio3−/−) mice. Dio3−/− mice display degeneration of retinal cones, the photoreceptors that mediate daylight and color vision. In Dio2−/− mice, cone function was largely normal but deletion of Dio2 in Dio3−/− mice markedly recovered cone numbers and electroretinogram responses, suggesting counterbalancing roles for both enzymes in cone survival. Both Dio3−/− and Dio2−/− strains exhibit deafness with cochlear abnormalities. In Dio3−/−;Dio2−/− mice, deafness was exacerbated rather than alleviated, suggesting unevenly balanced actions by these enzymes during auditory development. Dio3−/− mice also exhibit an atrophic thyroid gland, low thyroxine, and high triiodothyronine levels, but this phenotype was ameliorated in Dio3−/−;Dio2−/− mice, indicating counterbalancing roles for the enzymes in determining the thyroid hormone status. The results suggest that the composite action of these two enzymes is a critical determinant in visual and auditory development and in setting the systemic thyroid hormone status.
Dio2 amplifies and Dio3 depletes levels of T3. The opposing activities of these enzymes suggest that their composite action provides a sensitive control over the visual and auditory systems.
Mammalian development is promoted by a progressive rise in circulating levels of triiodothyronine (T3) and thyroxine (T4), a tetra-iodinated precursor of T3. However, tissues respond to T3 in distinct ways and with different time courses. This individualized response may be determined partly in the tissue itself by the amplification or depletion of T3 levels by type 2 and type 3 deiodinases, respectively (1–4). Type 2 deiodinase (encoded by Dio2) converts T4 into T3 by outer ring 5′-deiodination whereas type 3 deiodinase (encoded by Dio3) depletes T4 and T3 by inner ring 5-deiodination, producing reverse T3 and 3,3′-diiodothyronine, respectively.
The functions of the Dio3 gene include a prominent role in sensory development. Dio3−/− mice lose retinal cones, the photoreceptors that mediate daylight vision and color vision (5) and represent a model for achromatopsia, a form of retinal degeneration with loss of cone function (6). Dio3−/− mice also exhibit deafness and premature cochlear differentiation, suggesting a protective role for type 3 deiodinase during auditory development (7). Additionally, Dio3−/− mice display retarded growth and an atrophic thyroid gland with abnormal T4 and T3 levels, reflecting defects in the pituitary–thyroid axis and in the systemic thyroid hormone status (8, 9). Dio2−/− mice exhibit a different array of phenotypes, including moderately elevated T4 levels and impaired negative feedback by T4 over the pituitary–thyroid axis (10, 11). Dio2−/− mice also display deafness with cochlear retardation similar to that caused by hypothyroidism, despite having circulating T4 and T3 levels that would normally suffice for the development of hearing (12).
The opposing activities of type 2 and type 3 deiodinases suggest that their net action may be a key determinant of development. This composite action is likely to be dynamic given the shifting, or reciprocal, patterns of expression of these deiodinases in many immature tissues (1, 13, 14), including the cochlea (7, 15). The net action may also involve a role in setting levels of T4 and T3 in the circulation, which may further modify tissue outcomes. To test whether the Dio2 and Dio3 genes counteract each other in development, we investigated whether deletion of Dio2 reverses or otherwise modifies visual and auditory phenotypes in Dio3−/− mice.
Materials and Methods
Mouse strains
Strains carrying Dio2 (10) and Dio3 (8) knockout alleles were crossed to generate wild-type (WT), Dio2−/−, Dio3−/−, and Dio2−/−;Dio3−/− groups on a similar genetic background (C57BL/6J × 129/Sv). Dio3+/−;Dio2+/− parents were crossed to generate groups for analyses. Genotyping was determined by polymerase chain reaction (PCR) as described (8, 12). Studies on mice followed American Thyroid Association guidelines (16) and approved protocols at the National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health. Most results are presented for mixed groups of males and females, unless otherwise noted. The low survival rates of Dio3−/− pups (8) and the complex breeding scheme necessary to generate requisite genotypes made it impractical in most cases to obtain sufficient numbers for groups of only one gender. There were no obvious gender-based differences in the phenotypes examined. For serum hormones in adults, separate male and female groups are presented.
Immunohistochemistry and in situ hybridization
Eyes were dissected and fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) for 3 to 5 hours at 4°C, then rinsed in PBS, cryoprotected in 30% sucrose, and embedded in OCT. For immunostaining for opsins or activated caspase 3, 10-μm-thick cryosections were incubated overnight with primary antibody in PBS with 1.5% normal goat or horse serum, 0.1% bovine serum albumin, and 0.4% Triton X-100, as described (5). Sections were rinsed in PBS then incubated with second antibody for color development (Vector Laboratories, Burlingame, CA) or with Alexa Fluor 488 goat anti-rabbit or anti-mouse secondary antibody for immunofluorescence. For in situ hybridization, eyes were fixed in 4% paraformaldehyde/PBS overnight. Cryosections were incubated with digoxigenin-labeled riboprobes made from a mouse Dio2 complementary DNA (cDNA), spanning bases 590 to 1383 downstream of the ATG start codon, as described (12). For antibodies used, see Table 1.
Table 1.
Antibodies Used
| Antibody | Peptide/Protein Target | Species | Type | Manufacturer | Catalog No. | Dilution | RRID |
|---|---|---|---|---|---|---|---|
| Anti–S opsin | S opsin | Goat | Polyclonal | Santa Cruz Biotechnology | Sc-14363 | 1:1000 | AB_2158332 |
| Anti–M opsin | M opsin | Rabbit | Polyclonal | Millipore | AB 5405 | 1:1000 | AB_177456 |
| Anti–activated caspase 3 | Caspase 3 | Rabbit | Polyclonal | Promega | G748A | 1:300 | AB_430875 |
| Anti–glutamine synthetase | Glutamine synthetase | Mouse | Monoclonal | Millipore | MAB302 | 1:300 | AB_2110656 |
Abbreviation: RRID, Research Resource Identifier.
RNA analysis
RNA sequencing datasets represent pools of six to 12 retinae from WT mice (of mixed C57BL/6J × 129/Sv background) at the stages shown in Fig. 1. An independent analysis on a separate series of samples [at embryonic day 17, postnatal day (P)6, and P14] gave similar results. Total RNA was prepared using TRIzol reagent and messenger RNA (mRNA) enriched using a Dynabeads purification kit (Thermo Fisher, Grand Island, NY). cDNA was synthesized using a SuperScript double-strand cDNA kit (Thermo Fisher) then fragmented with a Bioruptor (Diagenode, Danville, NJ) with medium output settings for 15 minutes. Libraries were constructed using a TruSeq chromatin immunoprecipitation library preparation kit (Illumina, San Diego, CA). Fifty base single-end reads were generated on an Illumina HiSeq 2500 sequencer, and prealignment of data was performed at the National Institute of Diabetes and Digestive and Kidney Diseases Genomics Facility and further analyzed using a Genomatix (Munich, Germany) platform. Data were converted using bcl2fastq software version 2.17.1.14 (Illumina). After chastity filtering, alignment was performed using BBMap version 36.02 to mouse MM9 reference genome. The accession number for the WT retina datasets on the Gene Expression Omnibus database is GSE95016.
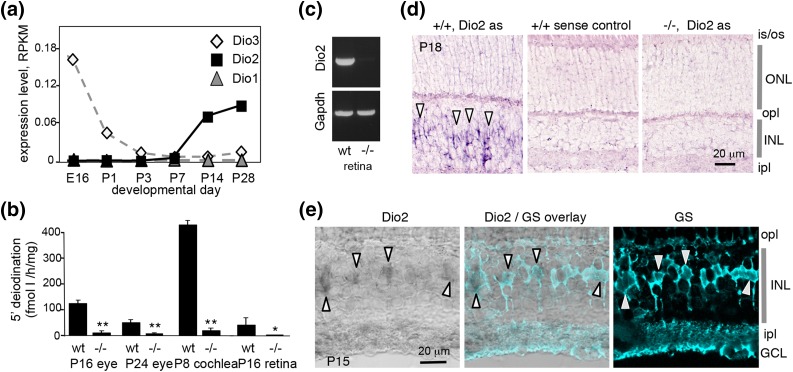
Figure 1.
Expression of the Dio2 gene in mouse retina. (a) Analysis of Dio1, Dio2, and Dio3 mRNA expression in WT mice by RNA sequencing. Data points represent pools of retinae. RPKM, reads per kilobase of transcript per million mapped reads. (b) Outer ring 5′-deiodination in pooled tissue homogenates from WT and Dio2−/− mice, measured using T4 substrate. The level of 5′-deiodination in eye or retina was low compared with cochlea at P8. *P < 0.05, **P < 0.001 (by Student t test), comparing Dio2−/− to WT. (c) RT-PCR detection of Dio2 mRNA in retina from WT but not Dio2−/− mice at P28; Gapdh, positive control gene. Product bands were 500 bp. The Dio2 product resided within the deleted sequences in Dio2−/− mice. (d) In situ hybridization detected Dio2 mRNA [antisense probe (as)] in the inner nuclear layer in WT but not in Dio2−/− mice. A sense probe gave minimal nonspecific signal. (e) Confocal images showing Dio2 signals detected by in situ hybridization (gray) and glutamine synthetase (GS) detected by immunofluorescence (turquoise), a marker of Müller glial cells. The overlay shows alignment of GS+ and Dio2+ cells (arrowheads). E, embryonic day; GCL, ganglion cell layer; INL, inner nuclear layer; ipl, inner plexiform layer; is/os, inner and outer segments; ONL, outer nuclear layer; opl, outer plexiform layer.
For reverse transcription polymerase chain reaction (RT-PCR), cDNA was made from retinal RNA from WT and Dio2−/− male mice at P28. Analysis of Dio2 and Gapdh genes was performed with primers as follows: Dio2 forward, 5′-CAG GTA ACA ATT ATG CCT CGG-3′, reverse, 5′-GCT GAA ATT CTT CTC CAG CCA AAC-3′; Gapdh forward, 5′-TCG GTG TGA ACG GAT TTG GCC GTA-3′, reverse, 5′-ATG GAC TGT GGT CAT GAG CCC TTC-3′. Both product bands were 500 bp.
Histology
Eyes were fixed in 2% paraformaldehyde/3% glutaraldehyde in PBS for 24 hours. The lens was removed and then the retina was rinsed in PBS and dehydrated in an ethanol gradient before embedding in glycol methacrylate plastic (5); 3-μm-thick microtome sections were stained with Gill no. 3 hematoxylin with eosin. Cochleae were fixed for 1 to 2 days, then decalcified in 0.1 M EDTA for 1 week before embedding in plastic; 4-μm-thick sections were stained with Gill no. 3 hematoxylin. Thyroid glands were fixed for 3 to 5 days, then immersed in 0.1 M EDTA for 1 week to soften the attached trachea before embedding in plastic; 6-μm-thick sections were stained with Gill no. 3 hematoxylin with eosin. Areas of the gland and follicles were measured using ImageJ and are expressed as arbitrary units (1 unit = 12.3 μm2) for five sections per gland for three to four mice. Cone and rod nuclei were counted in 208-μm-long fields of the outer nuclear layer, on three to four sections per mouse for three mice.
Auditory and visual tests
Adult mice (2 to 4 months of age) were anesthetized with avertin (0.25 mg/g body weight) for testing the auditory-evoked brainstem response (ABR) using a SmartEP system (Intelligent Hearing Systems, Miami, FL) as described (7). Amplitudes of the first peak to the following trough were measured by the SmartEP system. Adult mice (2 months of age) were anesthetized with ketamine and xylazine for measurement of the electroretinogram (ERG) for light-adapted, photopic cone responses and dark-adapted, scotopic rod responses using an Espion electrophysiology system (Diagnosys, Lowell, MA), as described (17). For dark adaptation, mice were housed overnight (∼18 hours) in darkness.
Statistical analysis
Statistical significance between several genotype groups was determined by one-way analysis of variance (ANOVA) and a Tukey post hoc test using GraphPad Prism software. A Student t test was used for other two group comparisons.
Deiodination assays and hormone measurements
Assays for type 2 deiodination activity and for T3 and T4 levels were performed as described (8, 18). Pools of dissected eyes, retinae, or cochleae were homogenized in buffer containing 20 mM Tris-HCl (pH 7.6), 5 mM dithiothreitol, and 0.25 mM sucrose. Pools of four to six eyes, six to 10 retinae, and 8 cochleae were analyzed in triplicate. Homogenates were incubated with [125I]T4 substrate, 1.2 mM EDTA, 1 mM propylthiouracil, 1 μM T3, and 20 mM dithiothreitol for 1 hour at 37°C, and then products were separated using Bio-Rad AG 50WXG (H+) resin to measure [125I]I− product. Serum total T4 and T3 values were determined by radioimmunoassay using Coat-A-Count reagents (Diagnostic Systems Laboratories, Webster, TX), as described (8). Hormones were measured for individual adult mice and for pools of two to three pups due to limited volumes of serum obtained.
Results
Dio2 expression in mouse retina
Given the role of type 3 deiodinase in cone photoreceptor function (5), we further investigated type 2 deiodinase in the retina in mice. RNA sequencing analysis revealed rising levels of Dio2 mRNA in the postnatal retina [Fig. 1(a)]. Dio3 mRNA displayed an earlier peak, as expected (5), suggesting a reciprocal developmental profile for Dio3 and Dio2 expression. Little or no Dio1 mRNA (type 1 deiodinase) was detected at any stage.
Type 2 5′-deiodination activity was detected in homogenates of WT mouse eye or dissected retina at postnatal ages [Fig. 1(b)]. This activity was attributed to type 2 deiodinase because the activity was abrogated in Dio2−/− mice and was resistant to inhibition by propylthiouracil, thereby distinguishing it from type 1 deiodinase activity. The type 2 deiodination activity in the eye or retina was low compared with that in the cochlea at P8, a known enriched source of type 2 deiodinase in mice (15). RT-PCR analysis also detected Dio2 mRNA in retina in WT mice but not in Dio2−/− mice [Fig. 1(c)].
In situ hybridization detected Dio2 mRNA in the postnatal retina in the inner nuclear layer [Fig. 1(d)], which contains several types of interneurons and Müller glia. A specific Dio2 signal was not detected on sections from Dio2−/− mice, which lack the portion of the gene used as a probe for in situ hybridization (12). The Dio2 signal was weak but localized to an irregular band of cells in a central zone of the inner nuclear layer, suggestive of expression in Müller glia. This proposal was supported by double staining for glutamine synthetase, a marker of Müller glia [Fig. 1(e)].
Rescue of cone photoreceptors in Dio3−/−;Dio2−/− mice
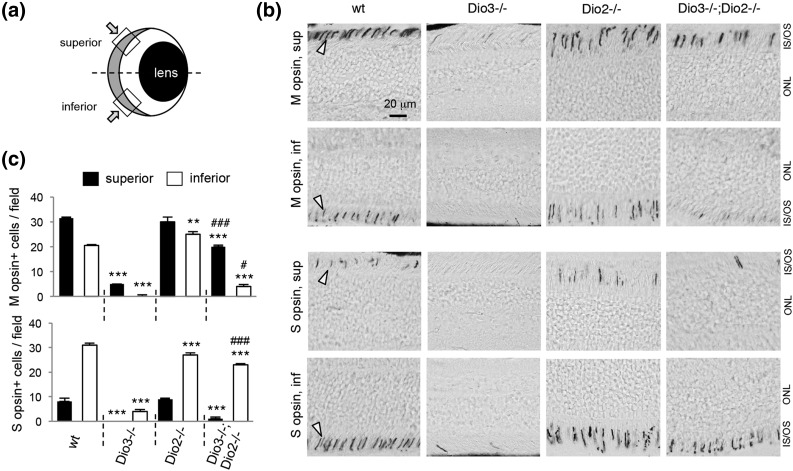
To test whether deletion of Dio2 resulted in cone defects or modified the phenotype in Dio3−/− mice, we examined opsin expression. Most mammals express two cone opsin photopigments with peak sensitivity to medium-long (M, or green) and short (S, or blue) wavelengths of light (19). In WT mice, opsins are distributed in gradients: M opsin predominates in cones in the superior retina, and S opsin predominates in the inferior retina [Fig. 2(a) and 2(b)]. Dio3−/− adult mice lacked most cones of both M and S types (5), but Dio2−/− mice displayed no obvious opsin abnormality. However, Dio3−/−;Dio2−/− mice recovered M and S opsin expression compared with Dio3−/− mice [Fig. 2(c)]. The opsin gradient was slightly exaggerated in Dio3−/−;Dio2−/− mice: M opsin was poorly restored in inferior regions, and S opsin was poorly restored in superior regions, suggesting that although cones were rescued, opsin patterning was not fully normal in the absence of both deiodinases.
Figure 2.
Immunohistochemistry for cone opsins in deiodinase-deficient mice. (a) Diagram of a vertical section through the eye depicting the superior and inferior views of the retina shown in (b). The retina is gray-shaded at the back of the eye. (b) In WT mice at 2 months of age, M opsin predominates in cones in superior (sup) and S opsin in inferior (inf) regions of the retina (arrowheads indicate stained cone segments). Dio3−/− mice lack both cone types, but Dio3−/−;Dio2−/− mice regain many M and S cones. IS/OS, inner and outer segments; ONL, outer nuclear layer. (c) Counts of M and S opsin–positive cones. Groups contained three mice. ANOVA was performed for all genotypes. **P < 0.01, ***P < 0.001, for comparisons of mutant genotypes to WT; #P < 0.05, ###P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio3−/−.
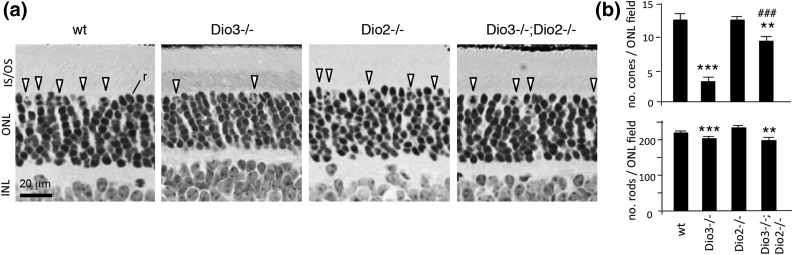
Histological analysis corroborated the recovery of cones in Dio3−/−;Dio2−/− mice. In mice, ∼3% of photoreceptors are cones and ∼97% are rods, the photoreceptor type that mediates vision in dim light (20). Cones are distinguished by their location at the edge of the outer nuclear layer and by their large nuclei compared with rods, which have small, dense nuclei [Fig. 3(a)]. In Dio3−/−;Dio2−/− mice, cone nuclei attained ∼70% of WT numbers [Fig. 3(b)]. Dio2−/− mice displayed normal numbers of cone and rod nuclei. Dio3−/− and Dio3−/−;Dio2−/− genotypes both displayed a modest loss of rods (∼12% below WT), suggesting a minor vulnerability of rods in these genotypes.
Figure 3.
Retinal histology in deiodinase-deficient adult mice. (a) Cones (arrowheads) are distinguished by their large nuclei and location in the outer zone of the outer nuclear layer on plastic sections. In contrast, the more numerous rods [representative rod (r)] have small, dense nuclei and reside throughout the outer nuclear layer. Adult mice at 2 to 3 months of age are shown. (b) Cone and rod nuclei counted in outer nuclear layer fields on 3-μm-thick plastic sections (n = 3 mice, mean ± SEM). Dio3−/− mice have a major loss of cones and minor loss of rods. Dio3−/−;Dio2−/− mice recover many cones. ANOVA was performed for all genotypes. **P < 0.01, ***P < 0.001, for comparisons of mutant genotypes to WT; ###P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio3−/−. IS/OS, inner and outer segments; INL, inner nuclear layer; ONL, outer nuclear layer.
Cell death in the postnatal retina
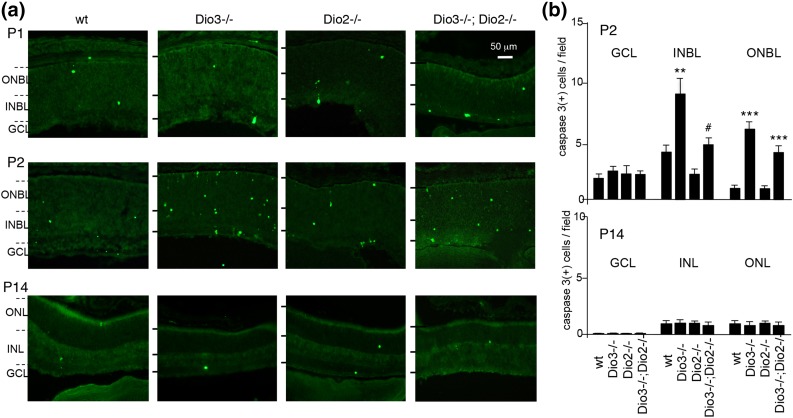
In Dio3−/− mice, cones degenerate by apoptosis in the postnatal period (5), as demonstrated by staining for activated caspase 3 [Fig. 4(a)]. In WT pups, a modest incidence of apoptosis occurs with a peak at ∼P2 and then a decline to low levels during the next 2 weeks (21). Apoptotic cells appear in shifting patterns in neonatal mice, as many immature cell types have not yet reached their final location. Cones migrate inward through the neuroblast layer and then outward to the outer zone of the outer nuclear layer as the retina matures (22). At P2, Dio3−/−;Dio2−/− mice displayed fewer caspase 3–positive cells in the neuroblast layers than do Dio3−/− mice (Fig. 4). In Dio2−/− mice, caspase 3–positive cell numbers were in the normal range.
Figure 4.
Apoptosis in the retina in deiodinase-deficient mice. (a) In the postnatal retina, cells positive for activated caspase 3 [caspase 3(+)] occur with moderate incidence in WT mice and with a higher incidence in Dio3−/− mice that is reduced in Dio3−/−;Dio2−/− mice. (b) Caspase 3(+) cell counts (mean ± SEM) determined in retinal layers at P2 and P14. ANOVA was performed for all genotypes. **P < 0.01, ***P < 0.001, for comparisons of mutant genotypes to WT; #P < 0.05, for comparisons of Dio3−/−;Dio2−/− to Dio3−/−. GCL, ganglion cell layer; ONBL/INBL, outer and inner neuroblast layers; ONL/INL, outer and inner nuclear layers.
ERG analysis
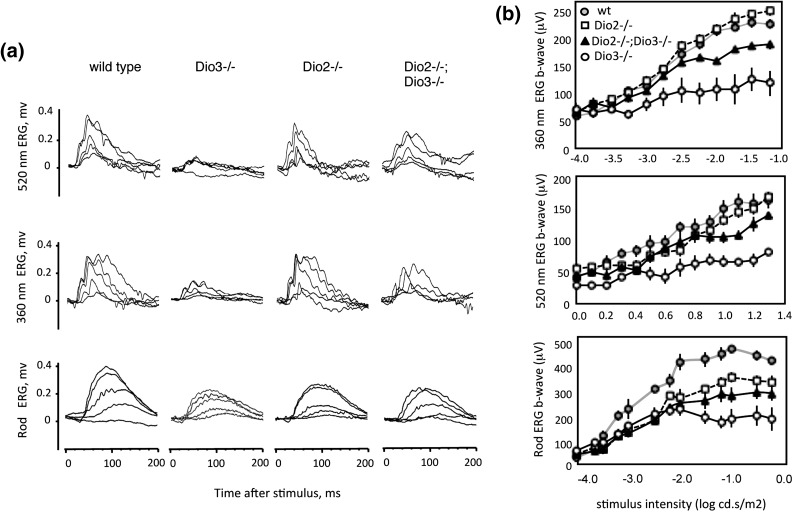
Dio3−/− mice display a defective cone ERG in response to photopic stimuli at 520 nm and 360 nm (5), wavelengths that optimally activate M and S opsins, respectively, in mice (23) [Fig. 5(a)]. Dio2−/− mice had normal responses at both wavelengths. However, compared with Dio3−/− mice, Dio3−/−;Dio2−/− mice recovered substantial responses at both wavelengths, and the ERG b-wave magnitudes approached normal over a range of stimulus intensities [Fig. 5(b)].
Figure 5.
ERG analysis in deiodinase-deficient mice. (a) Representative traces for cone (upper two rows) and rod ERG (lower row) in 2- to 3-month-old mice. Photopic stimuli at 520 nm (green) and 360 nm (blue) wavelengths indicate M and S opsin responses, respectively. Cone traces are shown for five intensities: 1.26, 2.51, 5.011, 10, and 38 (P) cd⋅s/m2 at 520 nm, and 0.00032, 0.001, 0.0032, 0.01, and 0.0316 (P) cd⋅s/m2 at 360 nm. Rod traces are for intensities of 0.0001, 0.000316, 0.001, 0.0063, and 0.01 (P) cd⋅s/m2. (b) Magnitude of the ERG b-wave for a range of stimulus intensities (mean ± SEM) for cones (upper two plots) and rods (lower plot). Most groups included 10 to 12 mice; the Dio3−/− group included four mice. For Dio3−/−;Dio2−/− mice, the magnitude differed from Dio3−/− mice (P < 0.01) for intensities >4 cd⋅s/m2 at 520 nm and >0.0018 cd⋅s/m2 at 360 nm. For rods, magnitudes are different from WT for each mutant genotype for intensities >0.00063 cd⋅s/m2 (P < 0.01).
We observed a reduced rod ERG in Dio3−/− mice as expected (5) and in Dio2−/− mice, suggesting that rod function was modestly impaired in the absence of either type 3 or type 2 deiodinase. In Dio3−/−;Dio2−/− mice, the rod b-wave magnitude was somewhat increased compared with Dio3−/− mice and approached the level in Dio2−/− mice.
ABRs
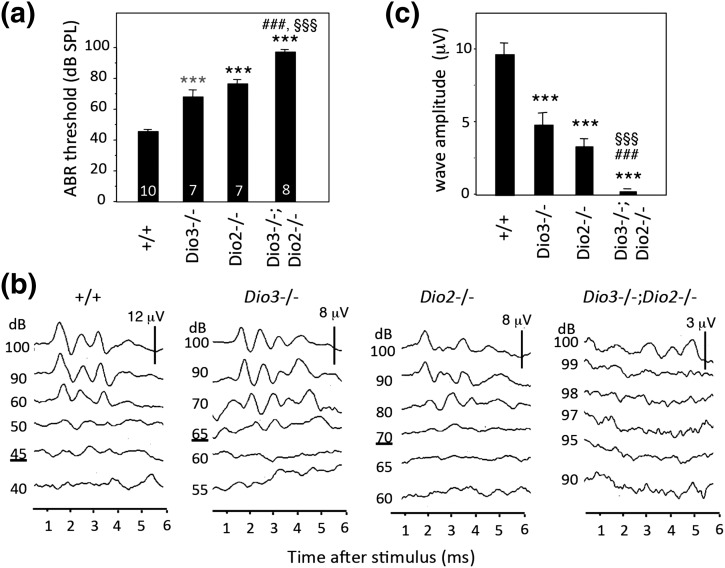
Both Dio3−/− and Dio2−/− mouse strains exhibit deafness with a defective ABR (7, 12). Given that both deiodinases are expressed in the immature cochlea, we tested whether deletion of Dio2 would ameliorate auditory defects in Dio3−/− mice. In Dio3−/− and Dio2−/− adult mice, auditory thresholds were elevated for a click (broadband frequency) stimulus [Fig. 6(a)]. Thresholds were 68 ± 5 and 76 ± 3 dB sound pressure level (SPL) for Dio3−/− and Dio2−/− genotypes, respectively, and 46 ± 2 dB SPL for WT [mean ± standard error of the mean (SEM)] [Fig. 6(a)]. However, in Dio3−/−;Dio2−/− mice, the threshold was 97± 2 dB SPL, worse than for either single mutant (P < 0.001). Dio3−/−;Dio2−/− mice displayed similarly exacerbated defects in response to pure tone stimuli at 8, 16, and 32 kHz frequencies that span the sensitive range of hearing in mice (not shown).
Figure 6.
ABR in deiodinase-deficient mice. (a) Mean thresholds (±SEM) for a click stimulus for adult mice (2 to 3 months old); numbers are noted within columns. The threshold was further elevated in Dio3−/−;Dio2−/− mice compared with Dio3−/− or Dio2−/− mice. ANOVA was performed for all genotypes. ***P < 0.001, for comparisons of mutants to WT; ###P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio3−/−; §§§P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio2−/−. (b) Representative ABR traces in response to a click stimulus at intensities noted on the left. A typical WT waveform shows three to four major peaks within 5 ms of the stimulus. Thresholds are underlined. No specific waveform was detected for the Dio3−/−;Dio2−/− mice. Vertical bar indicates response scale (μV). Note the higher thresholds and diminished responses for Dio3−/− and Dio2−/− mice. (c) Amplitude of waveforms (peak 1 to following trough) evoked with a click applied at equal intensity (90 dB SPL) for each genotype. ANOVA was performed for all genotypes. ***P < 0.001, for comparisons of mutant genotypes to WT; ###P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio3−/−; §§§P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio2−/−.
Figure 6(b) shows representative waveform traces with three to four peaks detected within 5 ms of the stimulus in WT mice. In contrast, in many Dio3−/−;Dio2−/− mice, no specific peaks could be evoked even with a maximal stimulus at 100 dB SPL. The waveforms that were evoked in the single mutant strains displayed significantly lower magnitudes than in WT mice when subjected to equivalent stimuli [Fig. 6(c)].
Cochlear morphology
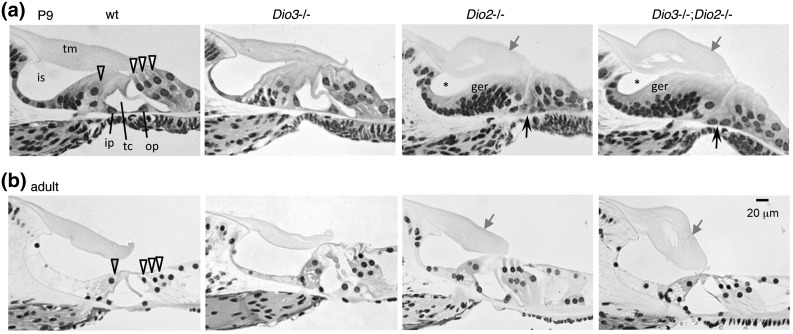
The cochlear abnormalities in Dio3−/− (7) and Dio2−/− (12) mice suggest that type 3 deiodinase constrains and type 2 deiodinase stimulates the rate of postnatal cochlear remodeling prior to the onset of hearing (at ∼P13). In WT mice at P9, the organ of Corti, which contains the mechanosensory hair cells, approaches a nearly mature morphology with three rows of outer hair cells and one row of inner hair cells on either side of an open tunnel of Corti [Fig. 7(a)]. The hair cells are elongated above the Deiters support cells, and the greater epithelial ridge, a transient cell-dense structure, has largely regressed to form the inner sulcus cavity below the tectorial membrane. The tectorial membrane extends to the hair cells and is essential for auditory transduction (24). The morphological status of the organ of Corti in Dio3−/− pups is accelerated in the first few neonatal days, but by P9, it appears relatively normal whereas in Dio2−/− pups, the morphology is retarded. In Dio2−/− pups, the greater epithelial ridge displayed only initial signs of regression, the hair cells lacked an upright structure, the tunnel of Corti was unopened, and the tectorial membrane was enlarged. Dio3−/−;Dio2−/− mice displayed a similar retardation as in Dio2−/− mice. Thus, deletion of Dio2 resulted in retardation or over-reversal rather than correction of the Dio3−/− phenotype.
Figure 7.
Cochlear histology in deiodinase-deficient mice. (a) At P9, a few days before hearing begins in WT mice, the organ of Corti is not fully mature but displays an open tunnel of Corti (tc) between the inner pillar (ip) and outer pillar (op) cells. The inner and outer hair cells (gray-filled and open arrowheads, respectively) are elongated, and the inner sulcus (is) is open beneath the tectorial membrane (tm). Morphology is similar in WT and Dio3−/− mice, but in Dio2−/− and Dio3−/−;Dio2−/− genotypes it is retarded: the tunnel of Corti has not opened (black arrow), the hair cells have not elongated, the inner sulcus is only partly open (*), many cells remain in the greater epithelial ridge (ger), and the tectorial membrane is enlarged (gray arrow). (b) In adults (2 to 3 months old), the morphology in Dio2−/− and Dio3−/−;Dio2−/− genotypes has largely caught up with that in WT and Dio3−/− mice, but the tectorial membrane remains enlarged.
In adults, the morphology in Dio3−/−;Dio2−/− mice still resembled that in Dio2−/− mice: in both genotypes, overall morphology had caught up with that in WT mice but the tectorial membrane was persistently enlarged.
Thyroid hormones and thyroid gland morphology
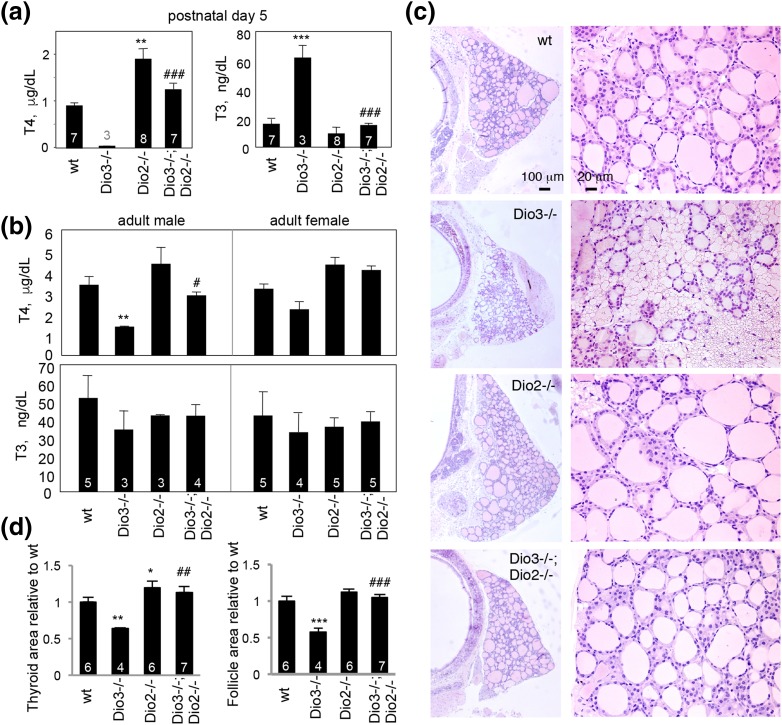
In addition to defects in the retina and cochlea, Dio2−/− (10) and Dio3−/− (8) mice have abnormalities in systemic T4 and T3 levels that could contribute to phenotypes. Therefore, we investigated whether deletion of both genes in Dio3−/−;Dio2−/− mice modified serum levels of T4 and T3. Dio3−/− pups are known to exhibit an extreme phenotype of undetectable T4 and high T3 (8), but in Dio3−/−;Dio2−/− pups at P5, T4 was increased and T3 decreased to approximately normal ranges [Fig. 8(a)]. Dio2−/− pups had a high T4 level, as expected from previous evidence at juvenile ages (10, 12).
Figure 8.
Thyroid hormone levels and thyroid gland histology. (a) In pups at P5, serum T4 was undetectable in Dio3−/− pups, but in Dio3−/−;Dio2−/− pups it was similar to WT. T3 was high in Dio3−/− pups, but in Dio3−/−;Dio2−/− pups it was similar to WT. Numbers of pools of serum (males and females) tested are noted within columns (or above column for T4, Dio3−/−). Pools contained males and females because of scarcity of the Dio3−/− genotype in the litters, as explained in Materials and Methods. ANOVA was performed for all genotypes. **P < 0.01, ***P < 0.001, for comparisons of mutant genotypes to WT; ###P < 0.001, for comparisons of Dio3−/−;Dio2−/− to Dio3−/−. (b) In 2- to 3-month-old adults, T4 is lower in Dio3−/− mice than in WT (males, **P < 0.01); in females, T4 is lower but not significantly. In Dio3−/−;Dio2−/− mice, T4 is elevated compared with Dio3−/− mice (#P < 0.05 in males). Number of mice is noted within columns. (c) In Dio3−/− mice (2 to 3 months old), the thyroid gland is small and has many atrophic follicles. In Dio3−/−;Dio2−/− mice, the gland has a near normal size. (d) Areas of a thyroid gland lobe or follicle are smaller in Dio3−/− than in WT mice (mean ± SEM) but they have near normal dimensions in Dio3−/−;Dio2−/− mice. Number of mice is noted within columns. Groups included males and females, except the Dio2−/− group, which included only males; no differences were obvious between males and females. *P < 0.05, **P < 0.01, ***P < 0.001, compared with WT; ##P < 0.01, ###P < 0.001, Dio3−/−;Dio2−/− compared with Dio3−/−.
By weaning and adult ages, Dio3−/− mice eventually acquire detectable levels of T4, although still below normal, whereas T3 drops to subnormal levels (8). Dio2−/− mice retain slightly high T4 beyond weaning age (10, 12). In Dio3−/−;Dio2−/− mice at 2 to 3 months of age, both T4 and T3 were in the normal range [Fig. 8(b)], suggesting a persistent stabilization of T4 and T3 levels in the circulation.
In Dio3−/−;Dio2−/− mice, the thyroid gland recovered a more normal size and follicular content than did the atrophic gland in Dio3−/− mice (8) [Fig. 8(c) and 8(d)]. In Dio2−/− mice, the gland was slightly enlarged. In WT or Dio3−/−;Dio2−/− mice, most follicles (69% and 62%, respectively) were medium-sized (100 to 200 area units) or larger. In contrast, in Dio3−/− mice, most follicles (70%) were small (<100 area units), and only 30% were medium-sized or larger. In Dio2−/− mice, the distribution of follicle sizes resembled that in WT mice. The results indicate counteracting roles for Dio3 and Dio2 genes in determining thyroid gland morphology and systemic levels of T4 and T3.
Developmental weight gain
Dio3−/−;Dio2−/− mice thrived reasonably well and gained more weight than Dio3−/− mice at juvenile ages (8). At 5 weeks of age, weights of WT and Dio3−/− males were 18.5 ± 1.7 and 11.5 ± 3.7 g, respectively (mean ± SEM; n = 7; P < 0.001). Dio3−/−;Dio2−/− males weighed 15.3 ± 2.6 g, representing a 30% improvement compared with Dio3−/− males (n = 10, P = 0.01). Recovery of weight continued into adulthood. Young females displayed similar trends, but after 5 weeks of age, both Dio3−/−;Dio2−/− and Dio3−/− genotypes were of similar weight, and neither was significantly different than WT female mice (n = 6, P = 0.9).
Discussion
This study shows that deletion of Dio2 strongly modifies phenotypes in Dio3−/− mice and supports the proposal that the composite action of both type 2 and type 3 deiodinases is critical for mammalian development. We suggest that such combined action offers enhanced control over T3 functions in sensitive tissues during development. The outcomes vary between tissues and may involve both direct and indirect influences to different extents at distinct stages, as discussed later.
Retinal photoreceptors
T3 controls the differentiation and survival of cone photoreceptors. The expression of M opsin and the patterning of M and S opsins over the retina depend on a cone-specific thyroid hormone receptor (25) and adequate T3 and T4 in the circulation (26–29). However, an excess of T3 or deletion of Dio3 leads to cone cell death, indicating that the T3 signal must also be appropriately constrained (5). Our findings on the Dio2 gene suggest a possible additional need for amplification of T3 in the postnatal retina.
A role for type 2 deiodinase in the mammalian retina was implied by an earlier report of outer ring deiodination activity in the rat retina (30). In the postnatal mouse retina, we detected type 2 deiodination, although at low levels. In situ hybridization localized Dio2 mRNA in Müller glial cells, in agreement with a report that mentioned detection of Dio2 mRNA by microarray analysis in flow-sorted Müller cells (31). Moreover, we detected little or no Dio2 mRNA in isolated cones and rods (unpublished data), cell types that constitute ∼70% of the retinal cell population. These findings suggest similarities between the retina and the brain, in which Dio2 is expressed mostly in glia rather than neurons (32). Müller glial projections span the layers of the retina from the outer limiting membrane to the inner surface of the ganglion cell layer and form an interface with the vasculature that provides homeostatic support for photoreceptors and other retinal neurons (33). Thus, in addition to their role in intercellular transfer of ions and metabolites, Müller glia may form part of a cellular pathway for transfer of T3 in the retina.
Dio2−/− mice had no overt cone defects but did have a moderately impaired rod ERG, suggesting a subtle role for type 2 deiodinase on rod function. We previously noted a reduced rod ERG in Dio3−/− mice (5). We cannot exclude a subtle role for type 2 deiodinase on nonphotoreceptor cell types or on nondevelopmental processes such as aging. However, the major impact of deletion of Dio2 was revealed in Dio3−/− mice, which resulted in rescue of cones. This rescue potentially reflects adjustments both of locally generated T3 in the retina and of the high systemic T3 levels that occur in young Dio3−/− mice. Future investigation of these possibilities may be aided by cell-specific deletions of Dio2 and Dio3 genes.
A Dio2 knockout in zebrafish (34) reduced the size of the eye and ear, suggesting a conserved role for the Dio2 gene in these sensory organs. Knockdown of Dio2 or Dio3 in zebrafish also delayed growth of the eye, delayed opsin expression, and partially disorganized the retinal layers (35). Both Dio2 and Dio3 genes are also expressed in the chick embryonic retina (36), suggesting that a composite action by both enzymes in the retina may be widely conserved.
The recovery of cones in Dio3−/− mice following the deletion of Dio2 may have wider implications for retinal degeneration and loss of vision (37). Most known forms of retinal degeneration do not result from defective T3 signaling, although a high free T4 level in adult human populations has been associated with an increased risk of age-related macular degeneration (38), and cone defects arise in rare cases of resistance to thyroid hormone (39). However, in mouse models of achromatopsia (Pde6c mutant) and Leber congenital amaurosis (Rpe65 mutant) that do not originate from disrupted T3 signaling, the loss of cones at juvenile ages was reported to be partly reduced by chemically induced hypothyroidism (40) or virus-mediated overexpression of Dio3 (41). Thus, T3 may be a secondary influence over photoreceptor degeneration caused by other factors. Deiodinases may offer a potential target for the design of interventions to ameliorate the loss of photoreceptors.
Auditory system
The development of hearing requires T3 (42), and deafness is a risk in endemic iodine deficiency, congenital hypothyroidism (43), and resistance to thyroid hormone (44). The expression patterns of Dio3 and Dio2 genes in the mouse cochlea indicate that substantial control over auditory development is governed by deiodination within the auditory system (7). Our study demonstrates that deletion of Dio2 in Dio3−/− mice worsens rather than neutralizes the deafness in Dio3−/− mice. Thus, in the auditory system, deiodinases do not evenly balance each other’s function and may serve partly independent roles. In Dio3−/−;Dio2−/− mice, cochlear morphology is retarded as in Dio2−/− mice, which may be interpreted as an over-reversal of the premature differentiation in Dio3−/− mice.
It is noteworthy that the deafness in Dio3−/−;Dio2−/− mice is exacerbated despite circulating levels of T4 and T3 that should suffice for auditory development, implying that altered deiodination within the auditory system is a major cause of the phenotype. We speculate that the severe phenotype in Dio3−/−;Dio2−/− mice may involve a double hit at different times or in different cell types in the auditory system. Not all defects need reside in the cochlea. T3 controls cochlear remodeling and function (42, 45) but also promotes the maturation of auditory relay centers in the brainstem (46), in which Dio2 mRNA is detected in rat pups (47). T3 also promotes myelination of the eighth nerve (48) and maturation of the ossicles and middle ear (49). In certain brain regions, type 2 deiodination is thought to generate T3 (50), whereas type 3 deiodination suppresses T3 responses (51). Conceivably, both processes promote the maturation of the central auditory pathway.
The cellular pathway that transfers T3 within the compartmentalized cochlea is poorly understood but presumably involves the coordinated action of deiodinases and membrane transporters for the uptake and release of T4 or T3 by specific cell types (52). However, the definition of these cell types and their anatomical proximity to cells that respond to T3 awaits improved methods for detection of deiodinases with precise cellular resolution.
Thyroid hormone status and other implications
In addition to tissue-intrinsic consequences of deletion of type 2 deiodinase in modifying phenotypes in Dio3−/− mice, there may be an indirect influence of the relatively normalized systemic levels of T4 and T3. This could result from known functions of the Dio2 gene in the hypothalamic–pituitary–thyroid axis, perhaps consistent with the rescued thyroid gland morphology in Dio3−/−;Dio2−/− mice. Deletions in mice indicate a role for type 2 deiodinase in the anterior pituitary in the control of thyrotropin production and in the mediobasal hypothalamus in the control of thyrotropin-releasing hormone (10, 53). In the neonatal rat hypothalamus, a rise of activating deiodination follows an earlier peak of inactivating deiodination, suggesting that functional maturation of the hypothalamic–pituitary–thyroid axis may require the balanced activities of both type 3 and type 2 deiodinases (1). Immunohistochemical studies also suggest that both deiodinases are expressed in regions of the human hypothalamus (54, 55). However, other peripheral means may modify the systemic thyroid hormone status. Combined deletions of Dio2 and Dio1 (56) or of all three Dio3, Dio2, and Dio1 deiodinase genes (57) suggest that peripheral clearance by type 1 or type 3 deiodinase modifies the levels of T4 and T3 in the circulation.
In summary, our study suggests that the composite action of the Dio3 and Dio2 genes is critical for visual and auditory development and for setting the thyroid hormone status. Dio3−/− male mice also display hypogonadism that can be partly rescued by deletion of the Dio2 gene (58). Dual patterns of Dio3 and Dio2 expression characterize the immature retina, cochlea (7), brain regions (1), skeletal muscle (4), and brown adipose tissue (59) in mammals and also occur in nonmammalian species (60, 61), suggesting that together these genes provide a widely adaptable developmental mechanism.
Acknowledgments
We thank Val Galton (Dartmouth Medical School) for advice and Harold Smith and Sijung Yun at the Genomics Facility at the National Institute of Diabetes and Digestive and Kidney Diseases for next-generation sequencing.
Acknowledgments
This work was supported by the intramural research program at National Institute of Diabetes and Digestive and Kidney Diseases at the National Institutes of Health (to L.N., H.L., and D.F.) and National Institutes of Health Grant DK095908 (to A.H.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ABR
- auditory-evoked brainstem response
- ANOVA
- analysis of variance
- cDNA
- complementary DNA
- ERG
- electroretinogram
- M
- medium-long wavelength
- mRNA
- messenger RNA
- P
- postnatal day
- PBS
- phosphate-buffered saline
- PCR
- polymerase chain reaction
- RT-PCR
- reverse transcription–polymerase chain reaction
- S
- short wavelength
- SEM
- standard error of the mean
- SPL
- sound pressure level
- T3
- triiodothyronine
- T4
- thyroxine
- WT
- wild-type.
References
- 1.Kaplan MM, Yaskoski KA. Maturational patterns of iodothyronine phenolic and tyrosyl ring deiodinase activities in rat cerebrum, cerebellum, and hypothalamus. J Clin Invest. 1981;67(4):1208–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. [DOI] [PubMed] [Google Scholar]
- 3.St Germain DL, Galton VA, Hernandez A. Minireview: defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150(3):1097–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dentice M, Marsili A, Zavacki A, Larsen PR, Salvatore D. The deiodinases and the control of intracellular thyroid hormone signaling during cellular differentiation. Biochim Biophys Acta. 2013;1830(7):3937–3945. [DOI] [PMC free article] [PubMed]
- 5.Ng L, Lyubarsky A, Nikonov SS, Ma M, Srinivas M, Kefas B, St Germain DL, Hernandez A, Pugh EN Jr, Forrest D. Type 3 deiodinase, a thyroid-hormone-inactivating enzyme, controls survival and maturation of cone photoreceptors. J Neurosci. 2010;30(9):3347–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Michaelides M, Hunt DM, Moore AT. The cone dysfunction syndromes. Br J Ophthalmol. 2004;88(2):291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng L, Hernandez A, He W, Ren T, Srinivas M, Ma M, Galton VA, St Germain DL, Forrest D. A protective role for type 3 deiodinase, a thyroid hormone-inactivating enzyme, in cochlear development and auditory function. Endocrinology. 2009;150(4):1952–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez A, Martinez ME, Fiering S, Galton VA, St Germain D. Type 3 deiodinase is critical for the maturation and function of the thyroid axis. J Clin Invest. 2006;116(2):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hernandez A, Martinez ME, Liao XH, Van Sande J, Refetoff S, Galton VA, St Germain DL. Type 3 deiodinase deficiency results in functional abnormalities at multiple levels of the thyroid axis. Endocrinology. 2007;148(12):5680–5687. [DOI] [PubMed] [Google Scholar]
- 10.Schneider MJ, Fiering SN, Pallud SE, Parlow AF, St Germain DL, Galton VA. Targeted disruption of the type 2 selenodeiodinase gene (DIO2) results in a phenotype of pituitary resistance to T4. Mol Endocrinol. 2001;15(12):2137–2148. [DOI] [PubMed] [Google Scholar]
- 11. Arrojo E Drigo R, Fonseca TL, Werneck-de-Castro JP, Bianco AC. Role of the type 2 iodothyronine deiodinase (D2) in the control of thyroid hormone signaling. Biochim Biophys Acta. 2013;1830(7):3956–3964. [DOI] [PMC free article] [PubMed]
- 12.Ng L, Goodyear RJ, Woods CA, Schneider MJ, Diamond E, Richardson GP, Kelley MW, Germain DL, Galton VA, Forrest D. Hearing loss and retarded cochlear development in mice lacking type 2 iodothyronine deiodinase. Proc Natl Acad Sci USA. 2004;101(10):3474–3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates JM, St Germain DL, Galton VA. Expression profiles of the three iodothyronine deiodinases, D1, D2, and D3, in the developing rat. Endocrinology. 1999;140(2):844–851. [DOI] [PubMed] [Google Scholar]
- 14.Kester MH, Martinez de Mena R, Obregon MJ, Marinkovic D, Howatson A, Visser TJ, Hume R, Morreale de Escobar G. Iodothyronine levels in the human developing brain: major regulatory roles of iodothyronine deiodinases in different areas. J Clin Endocrinol Metab. 2004;89(7):3117–3128. [DOI] [PubMed] [Google Scholar]
- 15.Campos-Barros A, Amma LL, Faris JS, Shailam R, Kelley MW, Forrest D. Type 2 iodothyronine deiodinase expression in the cochlea before the onset of hearing. Proc Natl Acad Sci USA. 2000;97(3):1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianco AC, Anderson G, Forrest D, Galton VA, Gereben B, Kim BW, Kopp PA, Liao XH, Obregon MJ, Peeters RP, Refetoff S, Sharlin DS, Simonides WS, Weiss RE, Williams GR; American Thyroid Association Task Force on Approaches and Strategies to Investigate Thyroid Hormone Economy and Action . American Thyroid Association guide to investigating thyroid hormone economy and action in rodent and cell models. Thyroid. 2014;24(1):88–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ng L, Lu A, Swaroop A, Sharlin DS, Swaroop A, Forrest D. Two transcription factors can direct three photoreceptor outcomes from rod precursor cells in mouse retinal development. J Neurosci. 2011;31(31):11118–11125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernández A, Obregón MJ. T3 potentiates the adrenergic stimulation of type II 5′-deiodinase activity in cultured rat brown adipocytes. Am J Physiol. 1996;271(1 Pt 1):E15–E23. [DOI] [PubMed] [Google Scholar]
- 19.Nathans J. The evolution and physiology of human color vision: insights from molecular genetic studies of visual pigments. Neuron. 1999;24(2):299–312. [DOI] [PubMed] [Google Scholar]
- 20.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. I. Structural analysis using light and electron microscopy. J Comp Neurol. 1979;188(2):245–262. [DOI] [PubMed] [Google Scholar]
- 21.Young RW. Cell death during differentiation of the retina in the mouse. J Comp Neurol. 1984;229(3):362–373. [DOI] [PubMed] [Google Scholar]
- 22.Rich KA, Zhan Y, Blanks JC. Migration and synaptogenesis of cone photoreceptors in the developing mouse retina. J Comp Neurol. 1997;388(1):47–63. [PubMed] [Google Scholar]
- 23.Lyubarsky AL, Falsini B, Pennesi ME, Valentini P, Pugh EN Jr. UV- and midwave-sensitive cone-driven retinal responses of the mouse: a possible phenotype for coexpression of cone photopigments. J Neurosci. 1999;19(1):442–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lukashkin AN, Richardson GP, Russell IJ. Multiple roles for the tectorial membrane in the active cochlea. Hear Res. 2010;266(1-2):26–35. [DOI] [PubMed] [Google Scholar]
- 25.Ng L, Hurley JB, Dierks B, Srinivas M, Saltó C, Vennström B, Reh TA, Forrest D. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat Genet. 2001;27(1):94–98. [DOI] [PubMed] [Google Scholar]
- 26.Roberts MR, Srinivas M, Forrest D, Morreale de Escobar G, Reh TA. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc Natl Acad Sci USA. 2006;103(16):6218–6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pessôa CN, Santiago LA, Santiago DA, Machado DS, Rocha FA, Ventura DF, Hokoç JN, Pazos-Moura CC, Wondisford FE, Gardino PF, Ortiga-Carvalho TM. Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Invest Ophthalmol Vis Sci. 2008;49(5):2039–2045. [DOI] [PubMed] [Google Scholar]
- 28.Lu A, Ng L, Ma M, Kefas B, Davies TF, Hernandez A, Chan CC, Forrest D. Retarded developmental expression and patterning of retinal cone opsins in hypothyroid mice. Endocrinology. 2009;150(3):1536–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Glaschke A, Weiland J, Del Turco D, Steiner M, Peichl L, Glösmann M. Thyroid hormone controls cone opsin expression in the retina of adult rodents. J Neurosci. 2011;31(13):4844–4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ientuile R, Macaione S, Russo P, Pugliese G, Di Giorgio RM. Phenolic and tyrosyl ring deiodination in thyroxine from rat retina during postnatal development. Eur J Biochem. 1984;142(1):15–19. [DOI] [PubMed] [Google Scholar]
- 31.Nelson BR, Ueki Y, Reardon S, Karl MO, Georgi S, Hartman BH, Lamba DA, Reh TA. Genome-wide analysis of Müller glial differentiation reveals a requirement for Notch signaling in postmitotic cells to maintain the glial fate. PLoS One. 2011;6(8):e22817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guadaño-Ferraz A, Obregón MJ, St Germain DL, Bernal J. The type 2 iodothyronine deiodinase is expressed primarily in glial cells in the neonatal rat brain. Proc Natl Acad Sci USA. 1997;94(19):10391–10396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Reichenbach A, Bringmann A. New functions of Müller cells. Glia. 2013;61(5):651–678. [DOI] [PubMed] [Google Scholar]
- 34.Houbrechts AM, Delarue J, Gabriëls IJ, Sourbron J, Darras VM. Permanent deiodinase type 2 deficiency strongly perturbs zebrafish development, growth, and fertility. Endocrinology. 2016;157(9):3668–3681. [DOI] [PubMed] [Google Scholar]
- 35.Houbrechts AM, Vergauwen L, Bagci E, Van Houcke J, Heijlen M, Kulemeka B, Hyde DR, Knapen D, Darras VM. Deiodinase knockdown affects zebrafish eye development at the level of gene expression, morphology and function. Mol Cell Endocrinol. 2016;424:81–93. [DOI] [PubMed] [Google Scholar]
- 36.Trimarchi JM, Harpavat S, Billings NA, Cepko CL. Thyroid hormone components are expressed in three sequential waves during development of the chick retina. BMC Dev Biol. 2008;8:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wright AF, Chakarova CF, Abd El-Aziz MM, Bhattacharya SS. Photoreceptor degeneration: genetic and mechanistic dissection of a complex trait. Nat Rev Genet. 2010;11(4):273–284. [DOI] [PubMed] [Google Scholar]
- 38.Chaker L, Buitendijk GH, Dehghan A, Medici M, Hofman A, Vingerling JR, Franco OH, Klaver CC, Peeters RP. Thyroid function and age-related macular degeneration: a prospective population-based cohort study—the Rotterdam Study. BMC Med. 2015;13:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weiss AH, Kelly JP, Bisset D, Deeb SS. Reduced L- and M- and increased S-cone functions in an infant with thyroid hormone resistance due to mutations in the THRβ2 gene. Ophthalmic Genet. 2012;33(4):187–195. [DOI] [PubMed] [Google Scholar]
- 40.Ma H, Thapa A, Morris L, Redmond TM, Baehr W, Ding XQ. Suppressing thyroid hormone signaling preserves cone photoreceptors in mouse models of retinal degeneration. Proc Natl Acad Sci USA. 2014;111(9):3602–3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang F, Ma H, Belcher J, Butler MR, Redmond TM, Boye SL, Hauswirth WW, Ding XQ. Targeting iodothyronine deiodinases locally in the retina is a therapeutic strategy for retinal degeneration. FASEB J. 2016;30(12):4313–4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Forrest D, Ng L. Thyroid hormone and the mammalian auditory system. In: Bass AH, Sisneros JA, Popper AN, Fay RR, eds. Hearing and Hormones. Springer Handbook of Auditory Research. Vol. 57. New York, NY: Springer; 2016:163–189.
- 43.Lichtenberger-Geslin L, Dos Santos S, Hassani Y, Ecosse E, Van Den Abbeele T, Léger J. Factors associated with hearing impairment in patients with congenital hypothyroidism treated since the neonatal period: a national population-based study. J Clin Endocrinol Metab. 2013;98(9):3644–3652. [DOI] [PubMed] [Google Scholar]
- 44.Brucker-Davis F, Skarulis MC, Pikus A, Ishizawar D, Mastroianni MA, Koby M, Weintraub BD. Prevalence and mechanisms of hearing loss in patients with resistance to thyroid hormone. J Clin Endocrinol Metab. 1996;81(8):2768–2772. [DOI] [PubMed] [Google Scholar]
- 45.Mustapha M, Fang Q, Gong TW, Dolan DF, Raphael Y, Camper SA, Duncan RK. Deafness and permanently reduced potassium channel gene expression and function in hypothyroid Pit1dw mutants. J Neurosci. 2009;29(4):1212–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friauf E, Wenz M, Oberhofer M, Nothwang HG, Balakrishnan V, Knipper M, Löhrke S. Hypothyroidism impairs chloride homeostasis and onset of inhibitory neurotransmission in developing auditory brainstem and hippocampal neurons. Eur J Neurosci. 2008;28(12):2371–2380. [DOI] [PubMed] [Google Scholar]
- 47.Guadaño-Ferraz A, Escámez MJ, Rausell E, Bernal J. Expression of type 2 iodothyronine deiodinase in hypothyroid rat brain indicates an important role of thyroid hormone in the development of specific primary sensory systems. J Neurosci. 1999;19(9):3430–3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knipper M, Bandtlow C, Gestwa L, Köpschall I, Rohbock K, Wiechers B, Zenner HP, Zimmermann U. Thyroid hormone affects Schwann cell and oligodendrocyte gene expression at the glial transition zone of the VIIIth nerve prior to cochlea function. Development. 1998;125(18):3709–3718. [DOI] [PubMed] [Google Scholar]
- 49.Cordas EA, Ng L, Hernandez A, Kaneshige M, Cheng SY, Forrest D. Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology. 2012;153(3):1548–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crantz FR, Silva JE, Larsen PR. An analysis of the sources and quantity of 3,5,3′-triiodothyronine specifically bound to nuclear receptors in rat cerebral cortex and cerebellum. Endocrinology. 1982;110(2):367–375. [DOI] [PubMed] [Google Scholar]
- 51.Hernandez A, Quignodon L, Martinez ME, Flamant F, St Germain DL. Type 3 deiodinase deficiency causes spatial and temporal alterations in brain T3 signaling that are dissociated from serum thyroid hormone levels. Endocrinology. 2010;151(11):5550–5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sharlin DS, Visser TJ, Forrest D. Developmental and cell-specific expression of thyroid hormone transporters in the mouse cochlea. Endocrinology. 2011;152(12):5053–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fonseca TL, Correa-Medina M, Campos MP, Wittmann G, Werneck-de-Castro JP, Arrojo e Drigo R, Mora-Garzon M, Ueta CB, Caicedo A, Fekete C, Gereben B, Lechan RM, Bianco AC. Coordination of hypothalamic and pituitary T3 production regulates TSH expression. J Clin Invest. 2013;123(4):1492–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alkemade A, Friesema EC, Unmehopa UA, Fabriek BO, Kuiper GG, Leonard JL, Wiersinga WM, Swaab DF, Visser TJ, Fliers E. Neuroanatomical pathways for thyroid hormone feedback in the human hypothalamus. J Clin Endocrinol Metab. 2005;90(7):4322–4334. [DOI] [PubMed] [Google Scholar]
- 55.Kalló I, Mohácsik P, Vida B, Zeöld A, Bardóczi Z, Zavacki AM, Farkas E, Kádár A, Hrabovszky E, Arrojo E Drigo R, Dong L, Barna L, Palkovits M, Borsay BA, Herczeg L, Lechan RM, Bianco AC, Liposits Z, Fekete C, Gereben B. A novel pathway regulates thyroid hormone availability in rat and human hypothalamic neurosecretory neurons. PLoS One. 2012;7(6):e37860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Galton VA, Schneider MJ, Clark AS, St Germain DL. Life without thyroxine to 3,5,3′-triiodothyronine conversion: studies in mice devoid of the 5′-deiodinases. Endocrinology. 2009;150(6):2957–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galton VA, de Waard E, Parlow AF, St Germain DL, Hernandez A. Life without the iodothyronine deiodinases. Endocrinology. 2014;155(10):4081–4087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martinez ME, Karaczyn A, Stohn JP, Donnelly WT, Croteau W, Peeters RP, Galton VA, Forrest D, St Germain D, Hernandez A. The type 3 deiodinase Is a critical determinant of appropriate thyroid hormone action in the developing testis. Endocrinology. 2016;157(3):1276–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hall JA, Ribich S, Christoffolete MA, Simovic G, Correa-Medina M, Patti ME, Bianco AC. Absence of thyroid hormone activation during development underlies a permanent defect in adaptive thermogenesis. Endocrinology. 2010;151(9):4573–4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown DD. The role of deiodinases in amphibian metamorphosis. Thyroid. 2005;15(8):815–821. [DOI] [PubMed] [Google Scholar]
- 61.Darras VM, Van Herck SL. Iodothyronine deiodinase structure and function: from ascidians to humans. J Endocrinol. 2012;215(2):189–206. [DOI] [PubMed] [Google Scholar]