Abstract
Phthalates are used in consumer products and are known endocrine-disrupting chemicals. However, limited information is available on the effects of phthalate mixtures on female reproduction. Previously, we developed a phthalate mixture made of 35% diethyl phthalate, 21% di(2-ethylhexyl) phthalate, 15% dibutyl phthalate, 15% di-isononyl phthalate, 8% di-isobutyl phthalate, and 5% benzylbutyl phthalate that mimics human exposure. We tested the effects of prenatal exposure to this mixture on reproductive outcomes in first-filial-generation (F1) female mice and found that it impaired reproductive outcomes. However, the impact of this exposure on second-filial-generation (F2) and third-filial-generation (F3) females was unknown. Thus, we hypothesized that prenatal exposure to the phthalate mixture induces multigenerational and transgenerational effects on female reproduction. Pregnant CD-1 dams were orally dosed with vehicle (tocopherol-stripped corn oil) or a phthalate mixture (20 and 200 µg/kg/d, 200 and 500 mg/kg/d) daily from gestational day 10 to birth. Adult F1 females born to these dams were used to generate the F2 generation and adult F2 females born to F1 females were used to generate the F3 generation. F2 and F3 females were subjected to tissue collections and fertility tests. Prenatal phthalate mixture exposure increased uterine weight, anogenital distance, and body weight; induced cystic ovaries; and caused fertility complications in the F2 generation. It also increased uterine weight, decreased anogenital distance, and caused fertility complications in the F3 generation. These data suggest that prenatal exposure to the phthalate mixture induces multigenerational and transgenerational effects on female reproduction.
This study shows that prenatal exposure to the phthalate mixture induces multigenerational and transgenerational effects on female reproduction.
Phthalates are a group of synthetic chemicals widely present in consumer products, including personal care products and plastic products (1, 2). Humans are constantly exposed to a number of phthalates through their use of consumer products. This constant exposure to phthalates is supported by the frequent detection of multiple phthalate metabolites in various human fluid samples across regions and populations (3–5). Phthalate exposure is of concern because studies have shown that it is associated with increased human health risks (6). For example, urinary phthalate metabolite concentrations have been associated with high blood pressure (7, 8), diabetes (9), increased insulin resistance (10), pregnancy loss (11), and preterm birth (12). Phthalates are also considered to be endocrine-disrupting chemicals (EDCs). EDCs are defined as chemicals that disrupt normal endocrine hormone signaling at the receptor or signal transduction levels (13). In animal studies, phthalates have been shown to act as EDCs to disrupt the reproductive system in both males and females (14, 15). Phthalates are known to induce “phthalate syndrome” in male rodents by disrupting the development and function of the male reproductive system (16, 17). They are also capable of targeting the ovaries to disrupt the female reproductive system (18).
Recently, phthalates have been shown to have multigenerational or transgenerational effects (19–24). Multigenerational effects occur when the effects of an EDC are observed in more than one generation and transgenerational effects occur when the effects of an EDC are transmitted to the generations that are not directly exposed to the EDC (13). Studies have shown that long-term dietary exposure to diethyl phthalate (DEP) (19) and dibutyl phthalate (DBP) (20) induced multigenerational effects on body weight and reproductive outcomes in both male and female rats. Parental exposure to di(2-ethylhexyl) phthalate (DEHP) caused adverse transgenerational effects on stress hormones, behavior (21), and reproduction (22) in male mice in the third filial generation (F3). Prenatal exposure to DEHP in mice accelerated follicle recruitment in both the first filial generation (F1) and second filial generation (F2) (23). In addition, prenatal exposure to DEHP caused multigenerational effects on male reproduction in the F1 and F2 generations, but not in the F3 and fourth filial generation (F4) (24). However, information on the transgenerational effects of phthalates on females is limited. Moreover, little information exists on the transgenerational effects of a mixture of phthalates on female reproduction. One previous study examined the transgenerational effects of a mixture of bisphenol A (BPA), DEHP, and DBP and found that this mixture induced pubertal abnormalities, ovarian disease, and obesity in the F3 generation in female rats (25). Given that humans and animals are constantly exposed to mixtures of phthalates, it is important to further examine the effects of phthalate mixtures.
In this study, we developed a phthalate mixture consisting of DEHP, DEP, DBP, benzylbutyl phthalate (BBzP), di-isononyl phthalate (DiNP), and di-isobutyl phthalate (DiBP). This phthalate mixture was based on the urinary phthalate metabolite levels detected in pregnant women in Illinois so that we could mimic human exposure to phthalates (unpublished iKids study). Phthalate exposure is of concern because epidemiological studies have shown that it is associated with increased human health risks such as infertility and early reproductive senescence (6). The limited available experimental studies that focused on the effects of chemical mixtures either used very high doses (much higher than human daily exposure) or the mixtures were not made based on actual phthalates present in humans (25). It is important to examine environmentally relevant doses of a phthalate mixture that mimics human daily exposure to better understand the potential risks of phthalates on human and animal health. In our previous work, we showed that the selected phthalate mixture inhibited mouse antral follicle growth and steroidogenesis, reduced apoptosis, and induced oocyte fragmentation in vitro (26). In addition, we reported that prenatal exposure to this phthalate mixture from gestational day (GD) 10 to birth induced reproductive disruption in female mice in the F1 generation (27). The purpose of the current study was to expand our previous work by testing the hypothesis that exposure to an environmentally relevant phthalate mixture induces adverse transgenerational effects on female reproduction in mice. To test our hypothesis, we exposed pregnant mice to the phthalate mixture and we evaluated the female offspring born to these exposed dams in the next three generations. In this current study, we focused on the female mice from F2 and F3 generations and compared the effects of the mixture with the results obtained from F1 generation (27).
Materials and Methods
Chemicals
All the phthalates used in this study, DEP, DEHP, DBP, DiBP, DiNP, and BBzP (>98% purity) were purchased from Sigma-Aldrich (St. Louis, MO). The phthalate mixture was made of 21% DEHP, 35% DEP, 15% DBP, 8% DiBP, 5% BBzP, and 15% DiNP (27). The mixture composition was derived from levels of phthalate metabolites measured in urine samples from pregnant women in Illinois (unpublished data from the iKids study). The phthalate mixture was diluted in tocopherol-stripped corn oil (vehicle control). The doses used for this study were 20 µg/kg/d, 200 µg/kg/d, 200 mg/kg/d, and 500 mg/kg/d. This phthalate mixture and the selected doses were used in a previous study conducted by our laboratory on the effects of this phthalate mixture on female reproduction in F1 mice (27). Briefly, our two lower doses (20 and 200 µg/kg/d) were selected to mimic daily human exposure levels based on the amount of DEHP present in these two doses. Our two higher doses (200 and 500 mg/kg/d) were selected so that we can compare our study results with available single phthalate study results (27). Moreover, our doses also include some of the doses used in single phthalate studies. From the limited number of transgenerational studies on phthalates, DEHP starting at 150 mg/kg/d induced transgenerational adverse effects on reproductive outcomes in male mice (21) and DEHP at 40 µg/kg/d induced multigenerational ovarian development deficiency in female mice (23). Our 200 µg/kg/d dose contains approximately 40 µg/kg/d of DEHP and our 500 mg/kg/d dose contains approximately 100 mg/kg/d of DEHP. These doses are close to those reported to induce transgenerational or multigenerational effects in male and female reproduction in the aforementioned DEHP studies.
Animals
Adult cycling female and adult male CD-1 mice were purchased from Jackson Laboratory (Bar Harbor, ME) and allowed to acclimate to the facility for at least 2 weeks before use. The mice were housed at the University of Illinois at Urbana–Champaign, Veterinary Medicine Animal Facility in polysulfone cages. Food (Harlan Teklad 8604) and water (reverse osmosis filtered) were provided for ad libitum consumption. Temperature was maintained at 22 ± 1°C, and animals were subjected to 12-hour light–dark cycles. The Institutional Animal Care and Use Committee at the University of Illinois at Urbana–Champaign approved all procedures involving animal care, dosing, euthanasia, and tissue collection.
Study design
At 2 months of age, 60 healthy and unexposed females were mated with untreated proven breeder male mice. The presence of a vaginal sperm plug was used as a sign of successful mating and GD 1 was considered as the day of the presence of vaginal sperm plug. Female mice were separated from males and singly housed once vaginal sperm plugs were observed. These dams were considered to be the F0 generation. On GD 10, F0 dams were randomly assigned to five different treatment groups (12 F0 dams/treatment group) and then were dosed every morning at the same time until the birth of pups. F0 mice were orally dosed by gently pipetting tocopherol-stripped corn oil (vehicle control) or different doses of phthalate mixture (20 µg/kg/d, 200 µg/kg/d, 200 mg/kg/d, and 500 mg/kg/d) into their mouths. All the doses were administered based on the individual dam body weight (total volume delivered ranged from 28 to 44 µl). The pups born to the F0 dams were considered the F1 generation. The F1 generation was exposed to phthalate mixture as fetuses during dosing of the F0 dams. The F1 females were used to generate the F2 generation by breeding F1 female mice (3 months of age) with nonexposed nonlittermate adult proven male breeders. This F2 generation was exposed to phthalates as germ cells when the F0 dams were dosed with the mixture. The F2 females were then used to generate the F3 generation by breeding F2 female mice (3 months of age) with nonexposed nonlittermate adult proven male breeders. This F3 generation was the first generation that was not exposed to phthalate mixture during the dosing of F0 dams. The F3 females were then used to generate the F4 by breeding F3 female mice (3 months of age) with nonexposed nonlittermate adult proven male breeders.
Tissue collection, body weights, and organ weights
All pregnant mice were allowed to give birth naturally and the day of birth was considered as postnatal day (PND) 0 for the pups. On PNDs 1, 4, 8, and 21, at least one female F2 and F3 pup per litter (n = 3 to 11 dams/treatment group) was randomly chosen for tissue collections. On PND 60 and 13 months of age, the remaining F2 and F3 female pups were euthanized and their tissues were collected during diestrus. At each time point, sera, ovaries, uteri, and livers were collected; organ weights and body weights were recorded; and anogenital distances (AGDs) were measured. Organ weights were recorded as whole organ weights in grams, AGD was recorded in millimeters, and ovaries from the same pup were measured together and recorded as one weight. At 13 months of age, due to the enlarged cystic ovaries in some treatment groups, ovarian weights were reported for one healthy ovary per mouse. Ovaries were categorized as cystic at 13 months of age if they were dramatically enlarged in size and had visible fluid-filled cysts [see (27) for photographed example]. AGD was normalized to cubic root of body weight to account for body size effects (28). Sera were stored for measuring sex steroid hormone levels in future studies. Ovaries, uteri, and livers were snap-frozen or fixed for molecular or histological analyses in future studies. F4 pups born to the F3 mice at 3 months of age were kept until the day of weaning (PND 21) and euthanized for the measurements of body weights and AGDs. AGDs to cubic root of body weight were calculated and compared with control for F4 female mice. All tissue collections were performed without knowledge of treatment groups.
Onset of puberty
After weaning (PND 21), one F2 and F3 female per litter (n = 11 to 12 litters/treatment group) was kept for analyses of onset of puberty, estrous cyclicity, and fertility. Vaginal opening was monitored every morning as a sign of onset of puberty. Body weights were recorded on the day of weaning and on the day of vaginal opening. After vaginal opening, estrous cyclicity was monitored every morning by examining vaginal smears daily for 30 consecutive days. Days between vaginal opening and the presence of first estrus were calculated and compared with control.
Fertility tests
One F2 and F3 female per litter (n = 7 to 12 litters/treatment group) was subjected to fertility tests at 3, 6, and 9 months of age. For each fertility test, estrous cyclicity was monitored every morning for 14 consecutive days prior to mating. Estrous cyclicity was also monitored for 14 consecutive days at 13 months of age in the F2 generation. Then, female mice were housed with untreated proven breeder male mice for 2 weeks to evaluate fertility of the females. Successful mating was determined by the presence of a vaginal sperm plug. Body weights were recorded twice a week starting on the first day of estrous cyclicity examination until the birth of pups. Maintenance or loss of pregnancy was monitored by body weight gain. For every female, the presence of a vaginal sperm plug, days to pregnancy, the ability to become pregnant and maintain pregnancy, and pregnancy lengths were recorded. The ability to deliver live pups, the size of the litter, average live pup birth weight, and pup sex ratio were also recorded to evaluate the birth outcomes. To examine fertility, we used the following equations based on our previous study (27) to calculate mating index, pregnancy rate, fertility index, gestation index, and percent females who produced live pups:
Statistical analyses
Data analyses were conducted using SPSS statistical software (SPSS Inc., Chicago, IL). One-way analysis of variance was used to conduct multiple comparisons between normally distributed experimental groups and was then followed by Dunnett post hoc comparisons if equal variances were assumed or Games–Howell post hoc comparisons if equal variances were not assumed. Kruskal–Wallis H tests were used for comparison between groups if data were not normally distributed, followed by Mann-Whitney U two-independent sample tests. For enlarged cystic ovaries and the ability of females to deliver live litters, we assigned each mouse a score of 0 or 1: 0 indicates female did not have enlarged cystic ovaries or did not deliver live litter and 1 indicates female had at least one enlarged cystic ovary or delivered live litter. Then, we used Fisher’s exact tests to compare treatment groups to control. Statistical significance was assigned at P < 0.05.
Results
Effect of phthalate mixture exposure on F2 and F3 female pup tissue and body weights
In the F2 generation, prenatal exposure to the phthalate mixture significantly increased body and liver weights compared with controls on PND 1 (200 µg/kg/d), did not affect body and liver weights on PND 4, but decreased AGD (500 mg/kg/d) and increased uterine weights (200 and 500 mg/kg/d) on PND 8 compared with control (Table 1, n = 5 to 10 dams/treatment, P < 0.05). On PND 21, the mixture increased body and liver weights (200 µg/kg/d and 200 mg/kg/d), increased AGD (200 µg/kg/d and 200 mg/kg/d), and increased ovary weights (200 mg/kg/d) compared with control (Table 1, n = 5 to 10 dams/treatment, P < 0.05; 0.05 < P < 0.1, borderline significance). On PND 60, the phthalate mixture did not affect body and tissue weights or AGD compared with controls (Table 1, n = 3 to 8 dams/treatment). As the mice aged, the phthalate mixture at 200 µg/kg/d significantly increased body weights in the F2 pups at 6, 9, and 13 months of age compared with control (Table 2, n = 5 to 13 female mice/treatment, P < 0.05). However, phthalate mixture did not affect organ weights or AGD at 13 months of age compared with control (Table 1, n = 3 to 9 dams/treatment).
Table 1.
Effects of Prenatal Exposure to Phthalate Mixture on Body and Organ Weights and AGD in F2 Females
| Treatment |
Age of Pups (F2) |
|||||
|---|---|---|---|---|---|---|
| PND 1 | PND 4 | PND 8 | PND 21 | PND 60 | 13 Months | |
| Body weight, g | ||||||
| Control | 1.72 ± 0.06 | 2.87 ± 0.21 | 5.12 ± 0.24 | 10.61 ± 0.81 | 28.17 ± 0.83 | 41.23 ± 1.39 |
| 20 µg/kg/d | 1.83 ± 0.04 | 3.17 ± 0.15 | 5.80 ± 0.26 | 13.03 ± 0.21a | 26.86 ± 0.70 | 43.10 ± 1.29 |
| 200 µg/kg/d | 1.97 ± 0.07a | 3.38 ± 0.17 | 5.79 ± 0.21 | 13.22 ± 0.65a | 29.50 ± 0.84 | 47.99 ± 2.18a |
| 200 mg/kg/d | 1.77 ± 0.03 | 2.96 ± 0.15 | 5.36 ± 0.30 | 12.40 ± 0.83 | 28.01 ± 0.50 | 41.15 ± 1.67 |
| 500 mg/kg/d | 1.71 ± 0.04 | 3.04 ± 0.16 | 5.01 ± 0.26 | 11.43 ± 0.78 | 28.65 ± 1.49 | 43.30 ± 2.57 |
| Liver weight, g | ||||||
| Control | 0.0728 ± 0.0028 | 0.1035 ± 0.0078 | 0.1573 ± 0.0076 | 0.5288 ± 0.0590 | 1.5989 ± 0.0822 | 2.3058 ± 0.1218 |
| 20 µg/kg/d | 0.0762 ± 0.0020 | 0.1188 ± 0.0067 | 0.1856 ± 0.0160 | 0.6727 ± 0.0280b | 1.3901 ± 0.0570 | 2.6565 ± 0.1273 |
| 200 µg/kg/d | 0.0830 ± 0.0024a | 0.1240 ± 0.0072 | 0.1717 ± 0.0051 | 0.6820 ± 0.0370b | 1.6106 ± 0.0576 | 2.5465 ± 0.1483 |
| 200 mg/kg/d | 0.0716 ± 0.0010 | 0.0990 ± 0.0056 | 0.1613 ± 0.0103 | 0.6399 ± 0.0574 | 1.5455 ± 0.0513 | 2.4783 ± 0.1299 |
| 500 mg/kg/d | 0.0719 ± 0.0016 | 0.1052 ± 0.0067 | 0.1539 ± 0.0152 | 0.5272 ± 0.0432 | 1.5382 ± 0.0827 | 2.4591 ± 0.1202 |
| AGD, mm/∛body weight, g | ||||||
| Control | 0.8241 ± 0.0680 | 1.5136 ± 0.0900 | 1.8492 ± 0.0353 | 1.6518 ± 0.0664 | ||
| 20 µg/kg/d | 0.8823 ± 0.0263 | 1.6543 ± 0.0717 | 1.7815 ± 0.0281 | 1.6079 ± 0.0403 | ||
| 200 µg/kg/d | 1.0170 ± 0.0618 | 1.8087 ± 0.0500a | 1.8058 ± 0.0360 | 1.5544 ± 0.0498 | ||
| 200 mg/kg/d | 0.7251 ± 0.0457 | 1.7791 ± 0.0485b | 1.7307 ± 0.0597 | 1.5478 ± 0.0685 | ||
| 500 mg/kg/d | 0.5856 ± 0.0135a | 1.6329 ± 0.1231 | 1.8522 ± 0.0978 | 1.5078 ± 0.0522 | ||
| Uterine weight, g | ||||||
| Control | 0.0033 ± 0.0002 | 0.0142 ± 0.0008 | 0.1172 ± 0.0084 | 0.2243 ± 0.0333 | ||
| 20 µg/kg/d | 0.0035 ± 0.0002 | 0.0192 ± 0.0014 | 0.1068 ± 0.0041 | 0.2354 ± 0.0125 | ||
| 200 µg/kg/d | 0.0037 ± 0.0002 | 0.0232 ± 0.0016 | 0.1002 ± 0.0066 | 0.2455 ± 0.0211 | ||
| 200 mg/kg/d | 0.0058 ± 0.0005a | 0.0211 ± 0.0039 | 0.1011 ± 0.0043 | 0.1669 ± 0.0088 | ||
| 500 mg/kg/d | 0.0046 ± 0.0005a | 0.0145 ± 0.0003 | 0.1040 ± 0.0046 | 0.2512 ± 0.0589 | ||
| Ovary weight, g | ||||||
| Control | 0.0033 ± 0.0003 | 0.0141 ± 0.0010 | 0.0090 ± 0.0010 | |||
| 20 µg/kg/d | 0.0040 ± 0.0002 | 0.0141 ± 0.0010 | 0.0117 ± 0.0014 | |||
| 200 µg/kg/d | 0.0041 ± 0.0003 | 0.0130 ± 0.0009 | 0.0115 ± 0.0007 | |||
| 200 mg/kg/d | 0.0046 ± 0.0004b | 0.0142 ± 0.0009 | 0.0091 ± 0.0009 | |||
| 500 mg/kg/d | 0.0039 ± 0.0003 | 0.0130 ± 0.0015 | 0.0112 ± 0.0011 | |||
P < 0.05 (i.e., significant differences from the control).
P = 0.07 (i.e., borderline significance compared with control).
Table 2.
Effects of Prenatal Exposure to Phthalate Mixture on Body Weight in F2 and F3 Females
| Treatments |
Age of Pups (F2) |
Age of Pups (F3) |
|||||
|---|---|---|---|---|---|---|---|
| 3 Months, g | 6 Months, g | 9 Months, g | 13 Months, g | 3 Months, g | 6 Months, g | 9 Months, g | |
| Control | 30.16 ± 1.23 | 38.93 ± 1.66 | 40.23 ± 1.04 | 41.23 ± 1.39 | 35.19 ± 1.27 | 40.87 ± 1.61 | 46.41 ± 3.27 |
| 20 µg/kg/d | 30.83 ± 1.04 | 41.74 ± 1.68 | 43.06 ± 1.37 | 43.10 ± 1.29 | 32.71 ± 1.16 | 39.11 ± 1.60 | 42.72 ± 2.21 |
| 200 µg/kg/d | 32.10 ± 0.77 | 45.83 ± 1.47a | 50.72 ± 1.89a | 47.99 ± 2.18a | 36.26 ± 1.50 | 44.00 ± 1.77 | 52.91 ± 3.37 |
| 200 mg/kg/d | 30.46 ± 0.56 | 40.07 ± 1.49 | 42.41 ± 1.85 | 41.15 ± 1.67 | 36.01 ± 0.98 | 45.62 ± 1.87 | 51.54 ± 2.54 |
| 500 mg/kg/d | 31.23 ± 1.09 | 40.75 ± 2.83 | 43.53 ± 3.09 | 43.30 ± 2.57 | 33.44 ± 2.09 | 40.74 ± 2.22 | 41.35 ± 2.80 |
P < 0.05 (i.e., significant differences from the control).
In addition, the phthalate mixture increased the number of enlarged cystic ovaries at 13 months of age in F2 generation (Table 3). Specifically, two of seven animals (28.6%) in the control group, eight of 11 animals (72.7%) in the 20 µg/kg/d group, three of seven animals (42.9%) in the 200 µg/kg/d group, three of nine animals (33.3%) in the 200 mg/kg/d group, and two of six animals (33.3%) in the 500 mg/kg/d group had at least one enlarged cystic ovary (Table 3, P > 0.05; P = 0.088 for control vs the 20 µg/kg/d group). Of these animals, three animals in 20 µg/kg/d group and one animal in the 200 µg/kg/d group had two enlarged cystic ovaries. Moreover, two animals in the 200 µg/kg/d group had blood-filled cysts, and other animals in this group had clear fluid-filled cysts (Table 3).
Table 3.
Effects of Prenatal Exposure to Phthalate Mixture on the Occurrence of Cystic Ovaries in F2 Females at 13 Months of Age
| Treatment | Total Number of Females | Number of Females With Cystic Ovaries | Number of Females With Blood-Filled Cysts | Number of Females With Clear Fluid-Filled Cysts |
|---|---|---|---|---|
| Control | 7 | 2 | 0 | 2 |
| 20 µg/kg/d | 11 | 8a | 0 | 8 |
| 200 µg/kg/d | 7 | 3 | 2 | 1 |
| 200 mg/kg/d | 9 | 3 | 0 | 3 |
| 500 mg/kg/d | 6 | 2 | 0 | 2 |
P = 0.088 (i.e., borderline significance compared with control).
In the F3 generation, the phthalate mixture significantly increased body weights on PND 1 (200 mg/kg/d), but it did not affect body and tissue weights or AGD on PND 4 and 8 compared with controls (Table 4, n = 5 to 10 dams/treatment, P < 0.05). The phthalate mixture significantly decreased AGD (200 mg/kg/d) on PND 21. Further, it increased uterine weights (200 µg/kg/d and 200 mg/kg/d) and decreased ovarian weights (200 mg/kg/d) on PND 60 compared with controls (Table 4, n = 3 to 8 dams/treatment, P < 0.05; 0.05 < P < 0.1, borderline significance). The phthalate mixture did not affect body weights of F3 females at 3, 6, and 9 months of age compared with control (Table 2, n = 5 to 10 female mice/treatment).
Table 4.
Effects of Prenatal Exposure to Phthalate Mixture on Body and Organ Weights and AGD in F3 Females
| Treatment |
Age of Pups (F3) |
||||
|---|---|---|---|---|---|
| PND 1 | PND 4 | PND 8 | PND 21 | PND 60 | |
| Body weight, g | |||||
| Control | 1.74 ± 0.04 | 3.19 ± 0.09 | 5.68 ± 0.20 | 13.25 ± 0.75 | 30.49 ± 0.92 |
| 20 µg/kg/d | 1.73 ± 0.08 | 3.16 ± 0.07 | 5.77 ± 0.29 | 14.47 ± 0.57 | 29.55 ± 1.34 |
| 200 µg/kg/d | 1.78 ± 0.03 | 3.23 ± 0.09 | 6.03 ± 0.17 | 14.23 ± 1.34 | 31.37 ± 1.17 |
| 200 mg/kg/d | 1.89 ± 0.04a | 2.94 ± 0.10 | 5.39 ± 0.35 | 14.81 ± 0.51 | 32.50 ± 2.02 |
| 500 mg/kg/d | 1.68 ± 0.04 | 3.25 ± 0.12 | 6.15 ± 0.13 | 14.82 ± 0.32 | 28.66 ± 1.06 |
| Liver weight, g | |||||
| Control | 0.0715 ± 0.0020 | 0.1134 ± 0.0054 | 0.1934 ± 0.0159 | 0.7107 ± 0.0493 | 1.7779 ± 0.1205 |
| 20 µg/kg/d | 0.0700 ± 0.0025 | 0.1087 ± 0.0019 | 0.2027 ± 0.0159 | 0.7331 ± 0.0668 | 1.6777 ± 0.1048 |
| 200 µg/kg/d | 0.0730 ± 0.0023 | 0.1145 ± 0.0043 | 0.1970 ± 0.0085 | 0.7449 ± 0.0908 | 1.7668 ± 0.0694 |
| 200 mg/kg/d | 0.0747 ± 0.0028 | 0.1055 ± 0.0060 | 0.1833 ± 0.0128 | 0.7699 ± 0.0325 | 1.7646 ± 0.1464 |
| 500 mg/kg/d | 0.0677 ± 0.0032 | 0.1082 ± 0.0041 | 0.1972 ± 0.0061 | 0.7747 ± 0.0115 | 1.6424 ± 0.0683 |
| AGD, mm/∛body weight, g | |||||
| Control | 1.0628 ± 0.0436 | 1.6609 ± 0.0934 | 1.7181 ± 0.0660 | ||
| 20 µg/kg/d | 1.0371 ± 0.0491 | 1.4396 ± 0.0799 | 1.7576 ± 0.0571 | ||
| 200 µg/kg/d | 0.9964 ± 0.0495 | 1.5840 ± 0.1310 | 1.8220 ± 0.0627 | ||
| 200 mg/kg/d | 0.9482 ± 0.0543 | 1.3258 ± 0.0555a | 1.7548 ± 0.0581 | ||
| 500 mg/kg/d | 0.9553 ± 0.0596 | 1.5925 ± 0.2694 | 1.7558 ± 0.0617 | ||
| Uterine weight, g | |||||
| Control | 0.0047 ± 0.0002 | 0.0304 ± 0.0043 | 0.1087 ± 0.0047 | ||
| 20 µg/kg/d | 0.0044 ± 0.0003 | 0.0386 ± 0.0044 | 0.1124 ± 0.0041 | ||
| 200 µg/kg/d | 0.0041 ± 0.0003 | 0.0336 ± 0.0073 | 0.1353 ± 0.0076a | ||
| 200 mg/kg/d | 0.0042 ± 0.0004 | 0.0272 ± 0.0026 | 0.1342 ± 0.0113a | ||
| 500 mg/kg/d | 0.0051 ± 0.0003 | 0.0457 ± 0.0066 | 0.1265 ± 0.0070 | ||
| Ovary weight, g | |||||
| Control | 0.0043 ± 0.0002 | 0.0158 ± 0.0014 | |||
| 20 µg/kg/d | 0.0045 ± 0.0005 | 0.0149 ± 0.0010 | |||
| 200 µg/kg/d | 0.0043 ± 0.0002 | 0.0138 ± 0.0011 | |||
| 200 mg/kg/d | 0.0047 ± 0.0004 | 0.0118 ± 0.0008b | |||
| 500 mg/kg/d | 0.0045 ± 0.0005 | 0.0135 ± 0.0012 | |||
P < 0.05 (i.e., significant differences from the control).
P = 0.07 (i.e., borderline significance compared with control).
In the F4 generation at PND 21, the phthalate mixture did not affect body weight of the females, but it significantly decreased AGD (20 and 200 µg/kg/d and 200 mg/kg/d) compared with control (Table 5, n = 2 to 7 dams/treatment, P < 0.05). Although the body weight and AGD of the F4 females in 500 mg/kg/d group appeared to be lower than controls, we could not perform statistical analyses on this group because there were only two litters in this group (Table 5).
Table 5.
Effects of Prenatal Exposure to Phthalate Mixture on Body Weight and AGD in F4 Females at PND 21
| Treatment | Body Weight, g | AGD, mm/∛body weight, g |
|---|---|---|
| Corn oil | 9.90 ± 1.03 | 2.00 ± 0.05 |
| 20 µg/kg/d | 8.32 ± 0.85 | 1.75 ± 0.02a |
| 200 µg/kg/d | 8.26 ± 0.78 | 1.75 ± 0.08a |
| 200 mg/kg/d | 8.11 ± 0.78 | 1.70 ± 0.05a |
| 500 mg/kg/d | 7.83 (n = 2) | 1.66 (n = 2) |
P < 0.05 (i.e., significant differences from the control).
Effect of phthalate mixture exposure on F2 and F3 pubertal outcomes
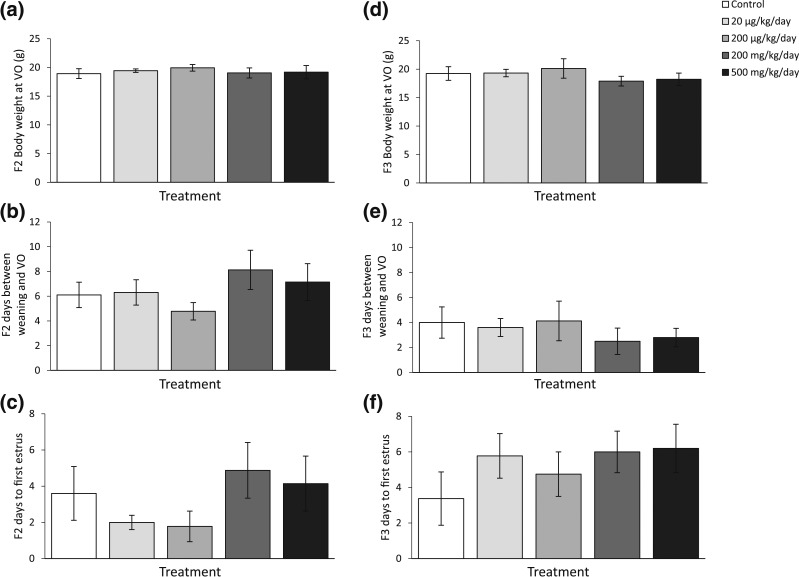
To monitor puberty, we recorded the age at vaginal opening, body weight at vaginal opening, and days between vaginal opening to first estrus in both F2 and F3 generations. Our results indicate that the phthalate mixture did not affect body weight at vaginal opening, days between weaning and vaginal opening, or days between vaginal opening and first estrus in F2 and F3 females compared with control [Fig. 1(a–c), n = 7 to 10 females/treatment of F2; Fig. 1(d–f), n = 5 to 9 females/treatment of F3].
Figure 1.
The effects of prenatal exposure to phthalate mixture on pubertal outcomes in F2 and F3 females. The effects of prenatal exposure to phthalate mixture on body weights at vaginal opening (VO) in F2 and F3 females are shown in panels (a) and (d), respectively. The effects of prenatal exposure to phthalate mixture on days between weaning and VO in F2 and F3 females are shown in panels (b) and (e), respectively. The effects of prenatal exposure to phthalate mixture on days between VO and the presence of first estrus in F2 and F3 females are shown in panels (c) and (f), respectively. Graphs represent means ± standard error of the mean from 7 to 10 females per treatment group for F2 and 5 to 9 females per treatment group for F3 dams.
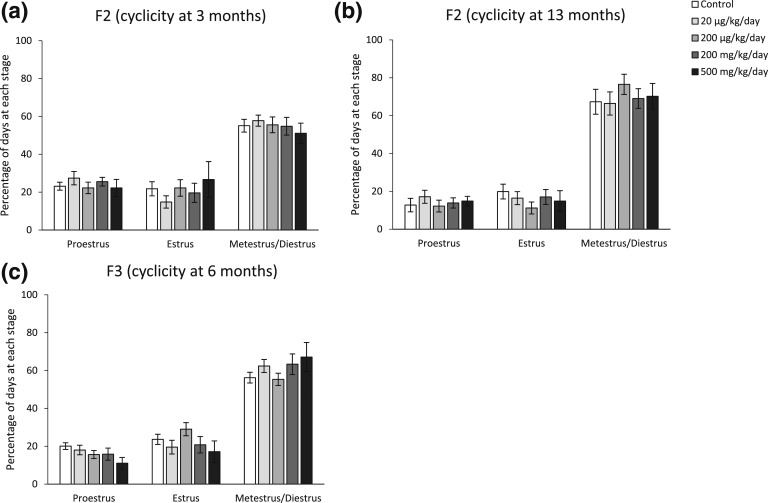
Effect of phthalate mixture exposure on F2 and F3 cyclicity over time
Estrous cyclicity was monitored for 14 consecutive days during adulthood at selected ages in both the F2 and F3 generations. The phthalate mixture did not affect estrous cyclicity in either the F2 or F3 generation at the selected ages [Fig. 2(a) and 2(b), n = 6 to 10 females/treatment of F2. Fig. 2(c), n = 5 to 10 females/treatment of F3]. However, in the F2 females at 13 months of age, one animal in control and two animals in the 20 µg/kg/d group were not cycling and were in persistent diestrus, and one animal in the 20 µg/kg/d group was not cycling and in persistent estrus.
Figure 2.
The effects of prenatal exposure to phthalate mixture on estrous cyclicity at selected ages in F2 and F3 females. (a) Estrous cyclicity at 3 months of age in F2 females. (b) Estrous cyclicity at 13 months in F2 females. (c) Estrous cyclicity at 6 months in F3 females. Graphs represent means ± standard error of the mean from 6 to 10 females per treatment group for F2 females and 5 to 10 females per treatment group for F3 females.
Effect of phthalate mixture exposure on F2 and F3 fertility over time
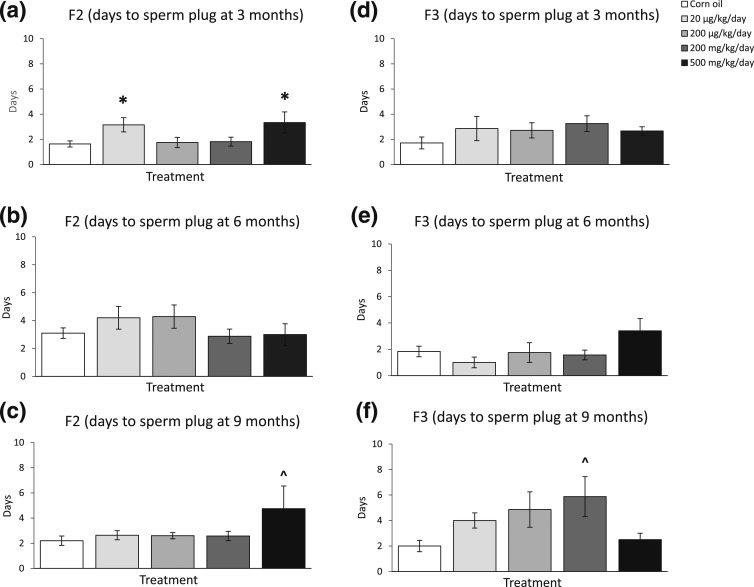
Fertility tests were performed at 3, 6, and 9 months of age for the F2 and F3 female mice. In the F2 generation, the phthalate mixture at 20 µg/kg/d and 500 mg/kg/d significantly increased the time female mice needed to mate with males at 3 months [Fig. 3(a), n = 7 to 13 females/treatment, P < 0.05]. It did not affect the days to mate with males at 6 months of age [Fig. 3(b), n = 6 to 13 females/treatment], and it (500 mg/kg/d) borderline significantly increased the days to mate at 9 months of age in the F2 generation compared with control [Fig. 3(c), n = 6 to 12 females/treatment, P = 0.06]. In the F3 generation, the phthalate mixture did not affect the days female mice needed to mate with males at 3 and 6 months of age [Fig. 3(d) and 3(e), n = 5 to 10 females/treatment], but it (200 mg/kg/d) borderline significantly increased the days to mate at 9 months of age compared with control [Fig. 3(f), n = 4 to 8 females/treatment, P = 0.07].
Figure 3.
The effects of prenatal exposure to phthalate mixture on time to pregnancy at 3, 6, and 9 months of age in F2 and F3 females. The effects of prenatal exposure to phthalate mixture on time to pregnancy at 3 months of age in F2 and F3 females are shown in panels (a) and (d), respectively. The effects of prenatal exposure to phthalate mixture on time to pregnancy at 6 months of age in F2 and F3 females are shown in panels (b) and (e), respectively. The effects of prenatal exposure to phthalate mixture on time to pregnancy at 9 months of age in F2 and F3 females are shown in panels (c) and (f), respectively. Graphs represent means ± standard error of the mean from 6 to 13 females per treatment group for F2 females and 4 to 10 females per treatment group for F3 females. *P < 0.05 (i.e., significant differences from the control); ^P = 0.05 < P < 0.1 (i.e., borderline significance compared with control).
Different fertility indices were calculated based on breeding and pregnancy complications. In the F2 generation during the fertility tests at the 3-month time point, one female in the control, 20 and 200 µg/kg/d, and 200 mg/kg/d groups successfully mated with a male but did not become pregnant; one female in the 200 µg/kg/d group did not mate with the male; and one female in the 500 mg/kg/d group had dystocia. These breeding, pregnancy, and delivering complications resulted in reduced fertility-related indices; however, all the fertility-related indices were statistically similar in phthalate mixture treated groups compared with control (Table 6, n = 7 to 13 females/treatment). In the F2 generation during the fertility test at the 6-month time point, all the control females were fertile and maintained their pregnancies; two females in the 20 µg/kg/d group and one female in each of the 200 and 500 mg/kg/d groups successfully mated but did not become pregnant; and one female in the 200 µg/kg/d group did not mate with the male, resulting in the reduction of some fertility-related indices in the phthalate mixture treated group (Table 6, n = 6 to 13 females/treatment). In the F2 generation during the fertility test at the 9-month time point, one female in the control group and 500 mg/kg/d group experienced pregnancy loss, one female in 200 µg/kg/d had dystocia, and two females in 200 mg/kg/d group never mated with males. At this age, fertility-related indices were not significantly affected by phthalate mixture treatment compared with control (Table 6, n = 6 to 12 females/treatment).
Table 6.
The Effects of Prenatal Exposure to Phthalate Mixture on Fertility at the 3-, 6-, and 9-Month Time Points in the F2 Generation
| Treatment | Total Female | Plugged Female | Pregnant Females | Delivered Female | Mating Index | Pregnancy Rate | Fertility Index | Gestational Index |
|---|---|---|---|---|---|---|---|---|
| 3-Month time point | ||||||||
| Control | 9 | 9 | 8 | 8 | 100 | 89 | 89 | 100 |
| 20 µg/kg/d | 13 | 13 | 12 | 12 | 100 | 92 | 92 | 100 |
| 200 µg/kg/d | 10 | 9 | 8 | 8 | 90 | 90 | 89 | 100 |
| 200 mg/kg/d | 12 | 12 | 11 | 11 | 100 | 92 | 92 | 100 |
| 500 mg/kg/d | 6 | 6 | 6 | 5 | 100 | 100 | 100 | 83 |
| 6-Month time point | ||||||||
| Control | 10 | 10 | 10 | 10 | 100 | 100 | 100 | 100 |
| 20 µg/kg/d | 13 | 13 | 11 | 11 | 100 | 85 | 85 | 100 |
| 200 µg/kg/d | 10 | 9 | 9 | 9 | 90 | 90 | 100 | 100 |
| 200 mg/kg/d | 11 | 11 | 10 | 10 | 100 | 91 | 91 | 100 |
| 500 mg/kg/d | 6 | 6 | 5 | 5 | 100 | 83 | 83 | 100 |
| 9-Month time point | ||||||||
| Control | 9 | 9 | 9 | 8 | 100 | 100 | 100 | 89 |
| 20 µg/kg/d | 12 | 12 | 12 | 12 | 100 | 100 | 100 | 100 |
| 200 µg/kg/d | 10 | 10 | 10 | 10 | 100 | 100 | 100 | 100 |
| 200 mg/kg/d | 10 | 8 | 8 | 8 | 80 | 80 | 100 | 100 |
| 500 mg/kg/d | 6 | 6 | 6 | 5 | 100 | 100 | 100 | 83 |
In the F3 generation during fertility tests at the 3-month time point, one female in the 200 µg/kg/d and 500 mg/kg/d groups successfully mated but did not become pregnant; one female in the 200 mg/kg/d group did not mate with the male and another female in this group lost her pregnancy, whereas in controls and the 20 µg/kg/d group, all animals successfully delivered live pups. These complications led to several reduced fertility-related indices in the three highest treatment groups (Table 7, n = 5 to 10 females/treatment). In the F3 generation during the 6-month time point fertility test, none of the animals in the control, 200 µg/kg/d, and 500 mg/kg/d groups had complications; however, one female in the 20 µg/kg/d group lost her pregnancy and another female never mated with male, and two females in 200 mg/kg/d group successfully mated with males but never became pregnant. As a result, the 20 µg/kg/d and 200 mg/kg/d groups showed relatively lower fertility-related indices compared with other groups (Table 7, n = 5 to 10 females/treatment). At the 9-month time point in the F3 generation, two control females lost their pregnancy; one female lost her pregnancy and the other one never mated with a male in the 20 µg/kg/d group; one female successfully mated with a male but did not become pregnant in the 200 µg/kg/d group; one female never mated with a male, one female successfully mated but did not become pregnant, and another female lost her pregnancy in the 200 mg/kg/d group; and one animal successfully mated with a male but did not become pregnant in the 500 mg/kg/d group. All these complications resulted in reduced fertility-related indices in all groups (Table 7, n = 4 to 10 females/treatment).
Table 7.
Effects of Prenatal Exposure to Phthalate Mixture on Fertility at the 3-, 6-, and 9-Month Time Points in the F3 Generation
| Treatment | Total Female | Plugged Female | Pregnant Females | Delivered Female | Mating Index | Pregnancy Rate | Fertility Index | Gestational Index |
|---|---|---|---|---|---|---|---|---|
| 3-Month time point | ||||||||
| Control | 8 | 8 | 8 | 8 | 100 | 100 | 100 | 100 |
| 20 µg/kg/d | 10 | 10 | 10 | 10 | 100 | 100 | 100 | 100 |
| 200 µg/kg/d | 8 | 8 | 7 | 7 | 100 | 88 | 88 | 100 |
| 200 mg/kg/d | 10 | 9 | 9 | 8 | 90 | 90 | 100 | 89 |
| 500 mg/kg/d | 5 | 5 | 4 | 4 | 100 | 80 | 80 | 100 |
| 6-Month time point | ||||||||
| Control | 7 | 7 | 7 | 7 | 100 | 100 | 100 | 100 |
| 20 µg/kg/d | 10 | 9 | 9 | 8 | 90 | 90 | 100 | 89 |
| 200 µg/kg/d | 8 | 8 | 8 | 8 | 100 | 100 | 100 | 100 |
| 200 mg/kg/d | 10 | 10 | 8 | 8 | 100 | 80 | 80 | 100 |
| 500 mg/kg/d | 5 | 5 | 5 | 5 | 100 | 100 | 100 | 100 |
| 9-Month time point | ||||||||
| Control | 7 | 7 | 7 | 5 | 100 | 100 | 100 | 71 |
| 20 µg/kg/d | 10 | 9 | 9 | 8 | 90 | 90 | 100 | 89 |
| 200 µg/kg/d | 7 | 7 | 6 | 6 | 100 | 86 | 86 | 100 |
| 200 mg/kg/d | 9 | 8 | 7 | 6 | 89 | 78 | 88 | 86 |
| 500 mg/kg/d | 4 | 4 | 3 | 3 | 100 | 75 | 75 | 100 |
Effect of phthalate mixture exposure on the ability of producing live pups across generations
In the F1 generation, at the 3-month time point, 91% of control females, 83% of the females in the 200 mg/kg/d group, and 91% of the females in the 500 mg/kg/d group produced live pups. Further, all females in the two lowest dose groups produced live pups (Table 8, n = 11 to 12 females/treatment, P > 0.05). At the 6-month time point, all groups showed reduced percentages of females who produced live pups. Although females exposed to the three highest doses of mixture produced relatively fewer live litters, none of the groups showed statistically significant reduction in percentages compared with control (Table 8, n = 10 to 12 females/treatment, P > 0.05). Similarly, at the 9-month time point, the percentage of females delivering live pups was reduced across all groups including controls. As a result, no treatment group produced fewer live pups compared with the control group (Table 8, n = 7 to 11 females/treatment, P > 0.05).
Table 8.
Effects of Prenatal Exposure to Phthalate Mixture on the Ability to Produce Live Pups at the 3-, 6-, and 9-Month Time Points in the F1, F2, and F3 Generations
| Treatment |
F1 |
F2 |
F3 |
|||
|---|---|---|---|---|---|---|
| Total Female | % Produced Live Pups | Total Female | % Produced Live Pups | Total Female | % Produced Live Pups | |
| 3-Month time point | ||||||
| Control | 11 | 91 | 9 | 89 | 8 | 100 |
| 20 µg/kg/d | 12 | 100 | 13 | 85 | 10 | 100 |
| 200 µg/kg/d | 11 | 100 | 10 | 80 | 8 | 88 |
| 200 mg/kg/d | 12 | 83 | 12 | 92 | 10 | 80 |
| 500 mg/kg/d | 11 | 91 | 6 | 83 | 5 | 40a |
| 6-Month time point | ||||||
| Control | 10 | 90 | 10 | 100 | 7 | 100 |
| 20 µg/kg/d | 11 | 91 | 13 | 77 | 10 | 70 |
| 200 µg/kg/d | 11 | 64 | 10 | 90 | 8 | 88 |
| 200 mg/kg/d | 12 | 58 | 11 | 91 | 10 | 80 |
| 500 mg/kg/d | 11 | 82 | 6 | 83 | 5 | 60 |
| 9-Month time point | ||||||
| Control | 7 | 57 | 9 | 89 | 7 | 71 |
| 20 µg/kg/d | 11 | 73 | 12 | 100 | 10 | 70 |
| 200 µg/kg/d | 10 | 60 | 10 | 90 | 7 | 86 |
| 200 mg/kg/d | 10 | 80 | 10 | 80 | 9 | 67 |
| 500 mg/kg/d | 9 | 56 | 6 | 83 | 4 | 75 |
P < 0.05 (i.e., significant differences from the control).
In the F2 generation, at the 3-month time point, some animals in all groups including controls did not deliver live pups, resulting in reduced percentages of females producing live pups in all groups (Table 8, n = 6 to 13 females/treatment, P > 0.05). At the 6-month time point, all animals in the control group gave birth to live pups, but fewer females in the phthalate mixture-treated groups gave birth to live pups. However, the lower percentages in phthalate mixture treated groups were not statistically different from controls (Table 8, n = 6 to 13 females/treatment, P > 0.05). At the 9-month time point, several more animals in the control, 200 µg/kg/d, 200 and 500 mg/kg/d groups did not produce live pups, resulting in lower percentages of females producing live pups in all groups and no significant differences between groups (Table 8, n = 6 to 12 females/treatment, P > 0.05).
In the F3 generation, at the 3-month time point, all females in the control and 20 µg/kg/d groups produced live pups; however, females in the three highest dose groups produced fewer live pups compared with control, especially at the 500 mg/kg/d dose (Table 8, n = 5 to 10 females/treatment; P < 0.05 for the 500 mg/kg/d group vs control). At the 6-month time point, although females in all phthalate mixture–treated groups produced fewer live pups compared with controls, the differences between each treatment group and controls were not statistically significant (Table 8, n = 5 to 10 females/treatment; P > 0.05). At the 9-month time point, not all the animals in control group produced live pups. As a result, the percentage of females producing live pups in the phthalate mixture treated groups were comparable to the control group (Table 8, n = 4 to 10 females/treatment, P > 0.05).
Effect of phthalate mixture exposure on F3 and F4 pup birth outcomes
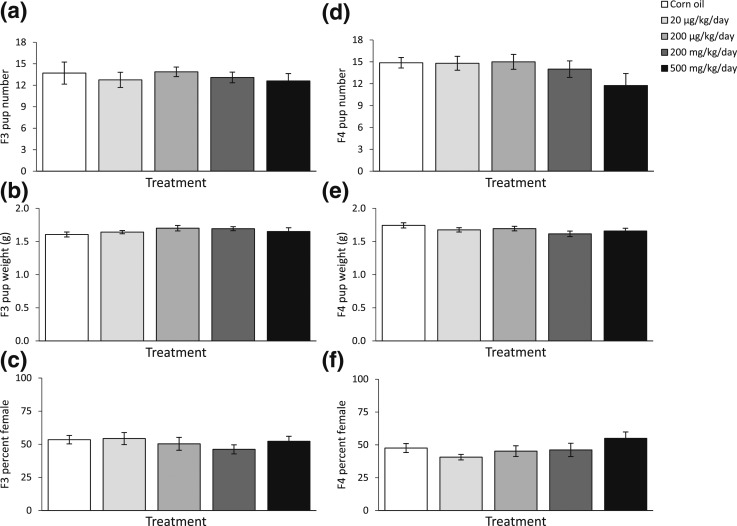
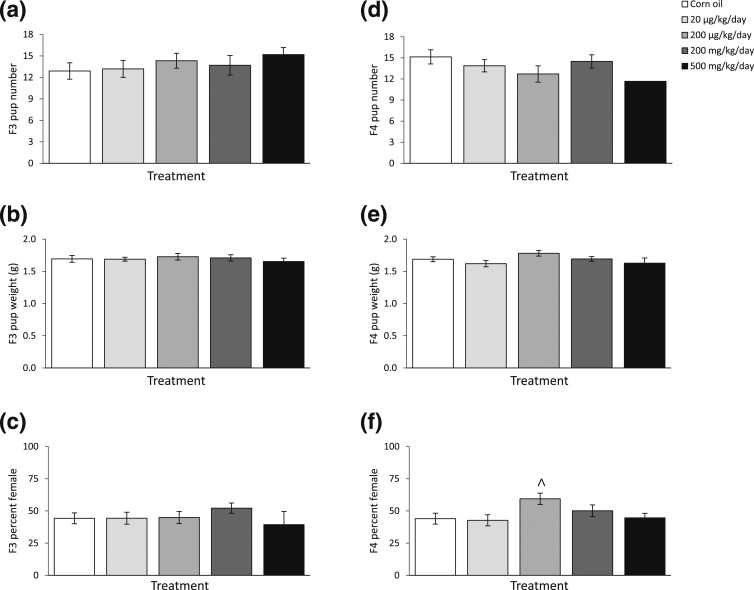
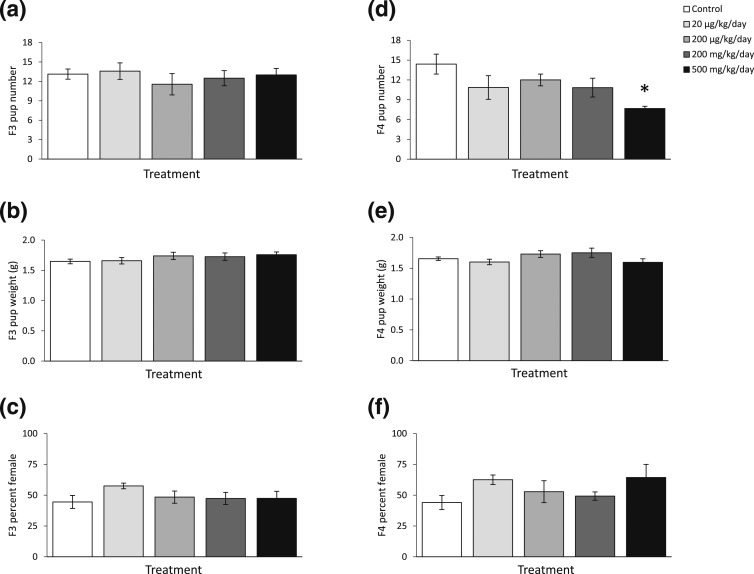
Litter size, average live pup body weight, and percentages of female pups in F3 and F4 generations were recorded and calculated at each fertility test in the F2 and F3 generations. After the fertility tests conducted at the 3-month time point on the F2 and F3 generations, the phthalate mixture did not affect F3 and F4 litter size, average live pup body weight, or percentages of female pups compared with control [Fig. 4(a–c), n = 7 to 13 dams/treatment of F2. Fig. 4(d–f), n = 5 to 10 dams/treatment of F3]. After fertility tests conducted at the 6-month time point on the F2 and F3 generations, the phthalate mixture did not affect F3 birth outcomes [Fig. 5(a–c), n = 6 to 13 females/treatment], it only borderline significantly increased the percentage of female pups at 200 µg/kg/d dose in the F4 generation compared with control [Fig. 5(f), n = 5 to 10 females/treatment, P = 0.07]. After fertility tests conducted at the 9-month time point on the F2 and F3 generations, the phthalate mixture did not affect F3 birth outcomes compared with control [Fig. 6(a–c), n = 6 to 12 females/treatment], but it reduced the litter size in the F4 generation compared with control [Fig. 6(d), n = 5 to 7 females/treatment].
Figure 4.
The effects of prenatal exposure to phthalate mixture on F3 and F4 birth outcomes at the 3-month time point in the F2 and F3 female fertility tests. Litter sizes of F3 and F4 are shown in panels (a) and (d), respectively. Average pup birth weights for F3 and F4 pups are shown in panels (b) and (e), respectively. Percentages of F3 and F4 pups that were female are shown in panels (c) and (f), respectively. Graphs represent means ± standard error of the mean from 5 to 10 dams per treatment group for F3 pups and for F4 pups.
Figure 5.
The effects of prenatal exposure to phthalate mixture on F3 and F4 birth outcomes at the 6-month time point in the F2 and F3 female fertility tests. Litter sizes of F3 and F4 are shown in panels (a) and (d), respectively. Average pup birth weights for F3 and F4 pups are shown in panels (b) and (e), respectively. Percentages of F3 and F4 pups that were female are shown in panels (c) and (f), respectively. Graphs represent means ± standard error of the mean from 6 to 13 dams per treatment group for F3 pups and 5 to 10 dams per treatment group for F4 pups. ^P = 0.07 (i.e., borderline significance compared with control).
Figure 6.
The effects of prenatal exposure to phthalate mixture on F3 and F4 birth outcomes at the 9-month time point in the F2 and F3 female fertility tests. Litter sizes of F3 and F4 are shown in panels (a) and (d), respectively. Average pup birth weights for F3 and F4 pups are shown in panels (b) and (e), respectively. Percentages of F3 and F4 pups that were female are shown in panels (c) and (f), respectively. Graphs represent means ± standard error of the mean from five to 12 dams per treatment group for F3 pups and five to seven dams per treatment group for F4 pups. *P < 0.05 (i.e., significant differences from the control).
Discussion
In our previous study, we reported that prenatal exposure to an environmentally relevant phthalate mixture significantly decreased AGD, increased uterine weight, disrupted estrous cyclicity, reduced fertility-related indices, caused breeding complications, and induced enlarged cystic ovaries in the F1 generation of mice (27). In this current study, we provided additional information on the effects of this phthalate mixture on female reproduction in the F2 and F3 generations and compared the effects caused by phthalate mixture exposure across generations. Our results showed that phthalate mixture exposure adversely affected tissue weights and AGD in both the F2 and F3 generations. It also increased body weight and induced enlarged cystic ovaries in F2 generation, and it caused breeding and pregnancy complications at different ages in both the F2 and F3 generations. Phthalate mixture exposure also reduced the ability of dams to produce live pups and affected the pup AGD in all three generations.
Our data showed the phthalate mixture consistently increased body weight in the F2 generation at all ages, but not in the F3 generation. In the F2 generation, we observed that the phthalate mixture at 200 µg/kg/d increased body weight on PNDs 1 and 21, and 3, 6, 9, and 13 months of age. This phthalate mixture-induced increase in body weight is similar to that observed in response to some other chemicals. Specifically, a mixture of BPA, DEHP, and DBP induced obesity in the F3 generation of female rats (25). Interestingly, the overweight phenotype was observed at a low dose of the mixture (BPA 25 mg/kg/d, DEHP 375 mg/kg/d, and DBP 33 mg/kg/d) (25). This is similar to the findings in our study because we showed that one of the lower doses of our mixture (200 µg/kg/d) increased body weight. The mechanism by which phthalate mixture exposure increased body weight is unknown. However, phthalates have been shown to induce obesity by acting on peroxisome proliferator-activated receptors (PPARs) (29–31). It is also interesting that the phthalate mixture increased body weight only at the second lowest dose, indicating that different doses of the phthalate mixture may act differently to induce different effects. Further, we only observed a phthalate mixture-induced increase in body weight in the F2 generation and not the F1 or F3 generations (except for at PND 1 in the F3 generation), but the reasons for this are unknown. Future studies should examine the mechanisms underlying the different effects of different doses and different outcomes in the F1, F2, and F3 generations.
Our data also indicate exposure to the phthalate mixture increased uterine weight in both the F2 and F3 generations. In the F2 generation, the phthalate mixture increased uterine weight at PND 8, an age well before weaning. In the F3 generation, the phthalate mixture increased uterine weight at PND 60, an age at which mice are adults. The reasons for phthalate mixture induced increase in uterine weight are unknown. Because uterine weight is affected by estrogen levels, we speculate that perhaps the phthalate mixture increased estrogen levels in the pups. It is also possible that in adult mice, the phthalate mixture-induced increase in uterine weight may be the result of disrupted hormone levels or disrupted uterine development. Disrupted estrogen levels in both neonates and adults could be due to the phthalate mixture interfering with the steroidogenic pathway. A previous study has shown that acute early life exposure to DEHP resulted in long term disruptions in steroidogenesis (32). Moreover, in vitro exposure to this phthalate mixture dramatically disrupts the steroidogenic pathway in cultured mouse antral follicles by disrupting hormone levels, enzyme expression, and receptor expression (26). Further, studies have shown that phthalates have the ability to cause epigenetic changes (33). Thus, it is possible that the phthalate mixture disrupted the steroidogenic pathway through epigenetic changes, which persist across generations to affect uterine weights.
Phthalate mixture exposure trended to increase the incidence of enlarged cystic ovaries in the F2 generation. Although we saw an increase in enlarged cystic ovaries in response to the phthalate mixture, this increase was not statistically different from controls. Our experiments were not designed to study the incidence of cystic ovaries in female mice, so our sample size was small and likely did not have enough power for us to detect statistical differences. Our observation of enlarged cystic ovaries is similar to what we observed in response to the phthalate mixture in the F1 females (27). Our results are also consistent with the results from studies on the transgenerational effects of a mixture of plastic derivatives (BPA, DEHP, and DBP) on reproduction (25, 34). In those studies, female rats from both the F1 and F3 generations had several ovarian diseases at 1 year of age, including polycystic ovaries, with animals in the F3 generation experiencing more severe ovarian diseases than the F1 generation (25). As suggested by Nilsson et al. (34), it is possible that environmental toxicant-induced transgenerational ovarian disease is due to exposure during a critical exposure window, which is around sex determination, and due to an alteration in the transcriptome and epigenome of granulosa cells. In our study, animals were exposed to phthalate mixture around sex determination; thus, our exposure window overlapped with the critical exposure window that is suggested by Nilsson et al. (34) to cause ovarian diseases. Further examination of the transcriptome and epigenome in phthalate mixture treated ovaries would provide more information to confirm our speculation. Future studies should also examine the ovaries in F3 generation at 13 months of age to determine if this effect is transgenerational.
Exposure to the phthalate mixture differentially affected AGD in the F2, F3, and F4 generations. Specifically, it increased AGD in the F2 generation, but decreased AGD in the F3 and F4 generations. AGD is determined by prenatal androgen levels. Therefore, it is possible that the phthalate mixture increased androgen levels in the F1 generation, leading to an increase in AGD in F2 pups. In the F2 and F3 generations, it is likely that the phthalate mixture reduced androgen levels, leading to reduced AGD in the F3 and F4 pups. Future studies should examine the androgen levels in all four generations of females to provide more information on the mechanisms underlying phthalate mixture induced effects on AGD.
Information on the transgenerational effects of phthalates on AGD is limited and inconsistent. One study focusing on the male lineage showed that DEHP exposure increased AGD in the F3 males compared with controls in mice (21). Another similarly designed study showed that DEHP exposure only decreased AGD in the F1 generation but showed no effect in the F2 and F3 generations of male mice (22). Differences among studies are probably due to the different lineages followed in each study. Our study focused on the female line and the other two studies focused on the male line. Differences could also be due to the differences between chemicals and the dosages used in the studies. Our study, as well as other studies, has shown that phthalates do not have linear dose response curves (18, 32). Thus, it is possible that different doses can trigger different effects.
Phthalate mixture exposure did not affect puberty outcomes (age at vaginal opening and age at first estrus) in F1 (27), F2, or F3 female mice. Most of the previous studies on the effects of phthalate exposure on puberty focused on direct exposure or the F1 generation and the results are inconsistent [reviewed in (14, 15)]. However, in one DEHP transgenerational study on male puberty, exposure to DEHP delayed pubertal onset in the F1 generation, but not in the F2 and F3 generations (22). Further, exposure to a mixture of BPA, DEHP, and DBP delayed pubertal onset in F1 female rats, but this exposure increased the incidence of early pubertal onset in F3 females compared with controls (25, 35). Our findings are somewhat similar to the DEHP transgenerational study (22) because it also showed that phthalate exposure did not cause multigenerational or transgenerational effects on puberty onset. The differences between our findings and the other mixture studies are probably due to the several factors. First, the animal species are different in that we used mice and the other study group used rats. Second, the dosing windows are different because we dosed the animals shortly after sex determination, but the other studies exposed rats during sex determination. Third, the mixture used in our study consisted only of phthalates, but the other studies used BPA and phthalates. BPA has been shown to cause transgenerational effects on puberty onset in mice (36). Thus, it is possible that the transgenerational effects on puberty onset caused by the mixture of BPA, DEHP, and DBP were mainly due to the effects of BPA.
Our results showed that phthalate mixture exposure did not affect estrous cyclicity in the F2 and F3 generations. In contrast, we reported a dramatic disruption of estrous cyclicity in the F1 generation shortly after puberty onset (27). Interestingly, we observed that three out of 11 animals in the lowest phthalate mixture treatment group in the F2 generation were not cycling at all by 13 months of age. This observation is likely the result of earlier onset of reproductive senescence. This is also in accordance with our findings of enlarged cystic ovaries in the F2 generation, in which we observed the highest incidence of enlarged cystic ovaries in the lowest treatment group. Future hormone and ovarian follicle histological analyses are needed to examine whether these animals experienced early reproductive senescence. To our knowledge, other information on the transgenerational effects of phthalates on estrous cyclicity is unavailable.
Phthalate mixture exposure increased time to pregnancy in the F2 and F3 generations. In our previous study, we observed that the phthalate mixture significantly increased the number of days that the F1 females needed to become pregnant at the 3-month time point (27). This increased time to pregnancy was also observed in the F2 generation at 3 and 9 months of age, as well as at the 9-month time point in the F3 generation. It is likely that the reason for the increased time to pregnancy observed in the F1 generation at the 3-month time point was due to disrupted estrous cyclicity at this same time-point (27). In contrast to results obtained in the F1 generation, we did not observe disrupted estrous cyclicity in the F2 or F3 generations. However, DEHP has been shown to have transgenerational effects on stress hormones and behaviors in female mice (21). Thus, it is possible that phthalate mixture exposure may induce disruptions in behavior that affect the willingness of F2 and F3 females to mate with the males.
Our data on F2 and F3 females also showed that phthalate mixture induced pregnancy complications, but to a lesser extent than observed in the F1 generation (27). The reasons for the phthalate-induced pregnancy abnormalities are unknown. Exposure to DEHP disrupts ovarian functions in multiple generations (18, 23), and normal ovarian function is very important for pregnancy. Thus, disruption of ovarian function could lead to complications in maintaining pregnancy. Interestingly, the effects of the phthalate mixture on fertility gradually declined in the F2 and F3 generations compared with the F1 generation. This similar trend has been reported in a male transgenerational study (24). Specifically, a study on male rats showed that DEHP exposure dramatically reduced conception rate in the F1 generation to 50% and it reduced conception rate in the F2 generation to 75%, but it did not affect male fertility in F3 and F4 generations (24). Differences among generations are likely due to the differences among the time of exposure in different generations. The F1 generation was directly exposed to the chemicals as developing fetuses, the F2 generation was directly exposed as germ cells in the F1 generation, and F3 and F4 generations were not exposed to the chemicals.
Our fertility tests in the F3 generation at the 9-month time point showed that phthalate mixture significantly reduced litter size at highest dose. These results are consistent with previous studies on single phthalates. DBP exposure through gestation reduced the number of live pups per litter in rats (37). DEHP exposure from PND 5 through weaning, mating, and pregnancy at 20 and 40 μg/kg/d significantly reduced litter size compared with control in mice (38). DBP exposure at 500 mg/kg/d in pregnant rats reduced litter size in the F1, F2, and F3 generations compared with control (39). Interestingly, gestational exposure to DEHP at 20 mg/kg/d increased litter size in the F3 generation in rats (40). The reduced litter size observed in our study could be due to disrupted ovarian and uterine function. It has been shown that DBP exposure decreases the number of corpora lutea and the weight of placenta, and it increases resorptions and pre- and postimplantation loss in the F1, F2, and F3 generations of rats (39). Moreover, DEHP exposure induces multigenerational acceleration of follicle recruitment in F1 and F2 mice (23). It is possible that these effects could be passed down to the F3 generation, resulting in fewer follicles available at 9 months of age and a reduction in litter size. However, future examination is needed to investigate the causes of this reduction in litter size, especially with regards to the effects of phthalate mixture on ovarian and uterine functions.
Several female mice in both the F2 and F3 generations gave birth only to dead pups, but the percentage of females that produced live litters was lowest in the F3 generation. Reduced ability to produce live litters has been reported in single phthalate direct exposure studies. For example, dietary exposure to DEHP at 500 mg/kg/d induced 100% abortion rate in mice (41). Further, two major types of dead litters were observed in our study: intact dead pups (either in nests or spread out in the cage) and damaged dead pups. Several scenarios could explain our observations. First, it is possible that more female mice experienced dystocia than we observed, and dystocia led to the death of pups. We only considered a female to have dystocia when we could detect she was in pain and was giving birth for more than 4 hours during the light cycle. It is likely that some of the females experienced dystocia during the dark hours and we missed the opportunity to detect their condition. Second, it is possible that the phthalate mixture disrupted maternal behavior so that the dams neglected newborn litters, leading to pups being found dead on the day of birth. Unlike live litters, pups from some of the dead litters were spread far apart from each other in the cage, or they were cannibalized by the dams. In both situations, no milk spots were observed, indicating the dams did not feed the pups. This maternal negligence hypothesis is supported by a study on DEHP, which showed that perinatal exposure to DEHP aggravated anxiety- and depression-like behaviors in mice (42). Further, another study showed that DEHP induced transgenerational effects on stress hormones and behavior in mice (21). Alternatively, it is possible that phthalate mixture disrupted fetal development to induce abnormalities in the fetus, resulting in deaths of the entire litter.
In conclusion, our data show that exposure to an environmentally relevant phthalate mixture induced some multigenerational and transgenerational effects on female reproduction. We observed multigenerational effects on body weight and enlarged cystic ovaries and transgenerational effects on fertility and birth outcomes. Our study provided some of the first information on the multigenerational and transgenerational effects of a phthalate mixture on female reproductive health. Future studies should investigate the mechanisms underlying the phthalate mixture-induced effects on female reproduction. Because we observed some effects of the phthalate mixture in the F3 generation, further epigenetic studies are particularly important to understand the transgenerational effects of this phthalate mixture. A limited number of studies on DEHP exposure suggest that the mechanisms that underlie the transgenerational effects in females involve epigenetic and/or imprinted genes. For example, prenatal DEHP exposure can affect DNA methylation of imprinted genes in the oocytes of F1 and F2 mice (43). DEHP exposure through the diet causes transgenerational effects through estrogen receptor 1, which are mediated by PPARα-dependent pathways (44). Further analyses on the animals from our study with a focus on DNA methylation, imprinted genes, and the PPARs will help us understand the mechanisms involved in the toxicity of our phthalate mixture. Moreover, given that both women and men are constantly exposed to many phthalates, future studies should focus on the impact of phthalate mixture exposure in both females and males on reproductive outcomes.
Acknowledgments
We thank all the members of the J.A.F. laboratory for their assistance and constructive input. We also thank Dr. Rebecca Smith for her help with statistical analyses.
Acknowledgments
This work was supported by National Institutes of Health Grant P01 ES022848 (J.A.F.), Environmental Protection Agency Grant RD-83459301 (J.A.F.), National Institutes of Health Grant R56 ES025147 (J.A.F.), and an Environmental Toxicology Fellowship (C.Z.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AGD
- anogenital distance
- BBzP
- benzylbutyl phthalate
- BPA
- bisphenol A
- DBP
- dibutyl phthalate
- DEHP
- di(2-ethylhexyl) phthalate
- DEP
- diethyl phthalate
- DiBP
- di-isobutyl phthalate
- DiNP
- di-isononyl phthalate
- EDC
- endocrine-disrupting chemical
- F0
- parental generation
- F1
- first filial generation
- F2
- second filial generation
- F3
- third filial generation
- F4
- fourth filial generation
- GD
- gestational day
- PND
- postnatal day
- PPAR
- proliferator-activated receptor.
References
- 1.Koniecki D, Wang R, Moody RP, Zhu J. Phthalates in cosmetic and personal care products: concentrations and possible dermal exposure. Environ Res. 2011;111(3):329–336. [DOI] [PubMed] [Google Scholar]
- 2.Chiellini F, Ferri M, Morelli A, Dipaola L, Latini G. Perspectives on alternatives to phthalate plasticized poly(vinyl chloride) in medical devices applications. Prog Polym Sci. 2013;38(7):1067–1088. [Google Scholar]
- 3.Jensen MS, Anand-Ivell R, Nørgaard-Pedersen B, Jönsson BA, Bonde JP, Hougaard DM, Cohen A, Lindh CH, Ivell R, Toft G. Amniotic fluid phthalate levels and male fetal gonad function. Epidemiology. 2015;26(1):91–99. [DOI] [PubMed] [Google Scholar]
- 4.Pan Y, Jing J, Dong F, Yao Q, Zhang W, Zhang H, Yao B, Dai J. Association between phthalate metabolites and biomarkers of reproductive function in 1066 Chinese men of reproductive age. J Hazard Mater. 2015;300:729–736. [DOI] [PubMed] [Google Scholar]
- 5.Hines EP, Calafat AM, Silva MJ, Mendola P, Fenton SE. Concentrations of phthalate metabolites in milk, urine, saliva, and Serum of lactating North Carolina women. Environ Health Perspect. 2009;117(1):86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hauser R, Calafat AM. Phthalates and human health. Occup Environ Med. 2005;62(11):806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trasande L, Sathyanarayana S, Spanier AJ, Trachtman H, Attina TM, Urbina EM. Urinary phthalates are associated with higher blood pressure in childhood. J Pediatr. 2013;163(3):747–53.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trasande L, Attina TM. Association of exposure to di-2-ethylhexylphthalate replacements with increased blood pressure in children and adolescents. Hypertension. 2015;66(2):301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.James-Todd T, Stahlhut R, Meeker JD, Powell SG, Hauser R, Huang T, Rich-Edwards J. Urinary phthalate metabolite concentrations and diabetes among women in the National Health and Nutrition Examination Survey (NHANES) 2001-2008. Environ Health Perspect. 2012;120(9):1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Trasande L, Spanier AJ, Sathyanarayana S, Attina TM, Blustein J. Urinary phthalates and increased insulin resistance in adolescents. Pediatrics. 2013;132(3):e646–e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Toft G, Jönsson BA, Lindh CH, Jensen TK, Hjollund NH, Vested A, Bonde JP. Association between pregnancy loss and urinary phthalate levels around the time of conception. Environ Health Perspect. 2012;120(3):458–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferguson KK, McElrath TF, Ko YA, Mukherjee B, Meeker JD. Variability in urinary phthalate metabolite levels across pregnancy and sensitive windows of exposure for the risk of preterm birth. Environ Int. 2014;70:118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skinner MK. Endocrine disruptors in 2015: epigenetic transgenerational inheritance. Nat Rev Endocrinol. 2016;12(2):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit Rev Toxicol. 2013;43(3):200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kay VR, Bloom MS, Foster WG. Reproductive and developmental effects of phthalate diesters in males. Crit Rev Toxicol. 2014;44(6):467–498. [DOI] [PubMed] [Google Scholar]
- 16.Foster PM. Disruption of reproductive development in male rat offspring following in utero exposure to phthalate esters. Int J Androl. 2006;29(1):140–147. [DOI] [PubMed] [Google Scholar]
- 17.Gray LE Jr., Ostby J, Furr J, Price M, Veeramachaneni DN, Parks L. Perinatal exposure to the phthalates DEHP, BBP, and DINP, but not DEP, DMP, or DOTP, alters sexual differentiation of the male rat. Toxicol Sci. 2000;58(2):350–365. [DOI] [PubMed] [Google Scholar]
- 18.Hannon PR, Flaws JA. The effects of phthalates on the ovary. Front Endocrinol (Lausanne). 2015;6:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fujii S, Yabe K, Furukawa M, Hirata M, Kiguchi M, Ikka T. A two-generation reproductive toxicity study of diethyl phthalate (DEP) in rats. J Toxicol Sci. 2005;30(Spec No):97–116. [DOI] [PubMed] [Google Scholar]
- 20.Wine RN, Li LH, Barnes LH, Gulati DK, Chapin RE. Reproductive toxicity of di-n-butylphthalate in a continuous breeding protocol in Sprague-Dawley rats. Environ Health Perspect. 1997;105(1):102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinnies KM, Doyle TJ, Kim KH, Rissman EF. Transgenerational effects of di-(2-Ethylhexyl) phthalate (DEHP) on stress hormones and behavior. Endocrinology. 2015;156(9):3077–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doyle TJ, Bowman JL, Windell VL, McLean DJ, Kim KH. Transgenerational effects of di-(2-ethylhexyl) phthalate on testicular germ cell associations and spermatogonial stem cells in mice. Biol Reprod. 2013;88(5):112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang XF, Zhang T, Han Z, Liu JC, Liu YP, Ma JY, Li L, Shen W. Transgenerational inheritance of ovarian development deficiency induced by maternal diethylhexyl phthalate exposure. Reprod Fertil Dev. 2015;27(8):1213–1221. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Wu S, Wen S, Shen L, Peng J, Yan C, Cao X, Zhou Y, Long C, Lin T, He D, Hua Y, Wei G. The mechanism of environmental endocrine disruptors (DEHP) induces epigenetic transgenerational inheritance of cryptorchidism. PLoS One. 2015;10(6):e0126403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manikkam M, Tracey R, Guerrero-Bosagna C, Skinner MK. Plastics derived endocrine disruptors (BPA, DEHP and DBP) induce epigenetic transgenerational inheritance of obesity, reproductive disease and sperm epimutations. PLoS One. 2013;8(1):e55387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou C, Flaws JA. Effects of an environmentally relevant phthalate mixture on cultured mouse antral follicles. Toxicol Sci. 2017;156(1):217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou C, Gao L, Flaws JA. Prenatal exposure to an environmentally relevant phthalate mixture disrupts reproduction in F1 female mice. Toxicol Appl Pharmacol. 2017;318:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gallavan RH Jr, Holson JF, Stump DG, Knapp JF, Reynolds VL. Interpreting the toxicologic significance of alterations in anogenital distance: potential for confounding effects of progeny body weights. Reprod Toxicol. 1999;13(5):383–390. [DOI] [PubMed] [Google Scholar]
- 29.Newbold RR, Padilla-Banks E, Jefferson WN, Heindel JJ. Effects of endocrine disruptors on obesity. Int J Androl. 2008;31(2):201–208. [DOI] [PubMed] [Google Scholar]
- 30.Baillie-Hamilton PF. Chemical toxins: a hypothesis to explain the global obesity epidemic. J Altern Complement Med. 2002;8(2):185–192. [DOI] [PubMed] [Google Scholar]
- 31.Grün F, Blumberg B. Perturbed nuclear receptor signaling by environmental obesogens as emerging factors in the obesity crisis. Rev Endocr Metab Disord. 2007;8(2):161–171. [DOI] [PubMed] [Google Scholar]
- 32.Hannon PR, Niermann S, Flaws JA. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Taxicol Sci. 2016;150(1):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh S, Li SS. Epigenetic effects of environmental chemicals bisphenol A and phthalates. Int J Mol Sci. 2012;13(8):10143–10153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nilsson E, Larsen G, Manikkam M, Guerrero-Bosagna C, Savenkova MI, Skinner MK. Environmentally induced epigenetic transgenerational inheritance of ovarian disease. PLoS One. 2012;7(5):e36129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manikkam M, Guerrero-Bosagna C, Tracey R, Haque MM, Skinner MK. Transgenerational actions of environmental compounds on reproductive disease and identification of epigenetic biomarkers of ancestral exposures. PLoS One. 2012;7(2):e31901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ziv-Gal A, Wang W, Zhou C, Flaws JA. The effects of in utero bisphenol A exposure on reproductive capacity in several generations of mice. Toxicol Appl Pharmacol. 2015;284(3):354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y, Jiang X, Chen B. Reproductive and developmental toxicity in F1 Sprague-Dawley male rats exposed to di-n-butyl phthalate in utero and during lactation and determination of its NOAEL. Reprod Toxicol. 2004;18(5):669–676. [DOI] [PubMed] [Google Scholar]
- 38.Zhang XF, Zhang LJ, Li L, Feng YN, Chen B, Ma JM, Huynh E, Shi QH, De Felici M, Shen W. Diethylhexyl phthalate exposure impairs follicular development and affects oocyte maturation in the mouse. Environ Mol Mutagen. 2013;54(5):354–361. [DOI] [PubMed] [Google Scholar]
- 39.Mahaboob Basha P, Radha MJ. Gestational di-n-butyl phthalate exposure induced developmental and teratogenic anomalies in rats: a multigenerational assessment. Environ Sci Pollut Res Int. 2017;24(5):4537–4551. [DOI] [PubMed] [Google Scholar]
- 40.Meltzer D, Martinez-Arguelles DB, Campioli E, Lee S, Papadopoulos V. In utero exposure to the endocrine disruptor di(2-ethylhexyl) phthalate targets ovarian theca cells and steroidogenesis in the adult female rat. Reprod Toxicol. 2015;51:47–56. [DOI] [PubMed] [Google Scholar]
- 41.Schmidt JS, Schaedlich K, Fiandanese N, Pocar P, Fischer B. Effects of di(2-ethylhexyl) phthalate (DEHP) on female fertility and adipogenesis in C3H/N mice. Environ Health Perspect. 2012;120(8):1123–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu X, Yang Y, Wang R, Wang Y, Ruan Q, Lu Y. Perinatal exposure to di-(2-ethylhexyl) phthalate affects anxiety- and depression-like behaviors in mice. Chemosphere. 2015;124:22–31. [DOI] [PubMed] [Google Scholar]
- 43.Li L, Zhang T, Qin XS, Ge W, Ma HG, Sun LL, Hou ZM, Chen H, Chen P, Qin GQ, Shen W, Zhang XF. Exposure to diethylhexyl phthalate (DEHP) results in a heritable modification of imprint genes DNA methylation in mouse oocytes. Mol Biol Rep. 2014;41(3):1227–1235. [DOI] [PubMed] [Google Scholar]
- 44.Kawano M, Qin XY, Yoshida M, Fukuda T, Nansai H, Hayashi Y, Nakajima T, Sone H. Peroxisome proliferator-activated receptor α mediates di-(2-ethylhexyl) phthalate transgenerational repression of ovarian Esr1 expression in female mice. Toxicol Lett. 2014;228(3):235–240. [DOI] [PubMed] [Google Scholar]