In the early 1900s, the idea emerged that hormones derived from the intestinal mucosa could be contributing glucose-lowering actions by activating pancreatic secretion (1). Since that time, many incretin peptides including glucagon-like peptide-1 (GLP-1, secreted from intestinal l cells) and gastric inhibitory polypeptide (secreted from intestinal K cells) have been discovered to have glucose-lowering properties through their actions at the pancreas and other peripheral tissue sites. Other valuable findings were that orally administered glucose induced a greater insulin secretory response than intravenously administered glucose (2) and that type 2 diabetic subjects exhibit a deficit in this incretin response (3). Although antidiabetic effects of pharmacologic gastric inhibitory polypeptide are thought to be minimal in type 2 diabetes, beneficial effects of pharmacologic GLP-1 treatment in type 2 diabetes is clear (4). Emerging from these discoveries and observations (including many not noted here), substantial interest in incretin-based therapies developed for treating type 2 diabetes. One of these incretin-based therapies relates to incretin degradation in the body. Dipeptidyl peptidase-4 (DPP4) is a protease expressed on many cell surfaces and plays a key role in the degradation and the short half-life of GLP-1. Although GLP-1 mimetics act via pharmacological activation of the GLP-1 receptor (GLP-1R), inhibition of DPP4 serves as an ancillary approach to increase endogenous GLP-1 levels. Differences exist in these therapies, including that DPP4 inhibitors lead to 1.5-fold to threefold increase in circulating GLP-1, whereas GLP-1R mimetics lead to much higher levels of pharmacologic GLP-1 molecules (4). With the global success of incretin-based therapies using GLP-1R mimetics and DPP4 inhibitors for type 2 diabetes treatments, understanding their modes of action is of great value.
GLP-1R mimetics and DPP4 inhibitors exhibit similar antidiabetic actions that extend beyond their action on pancreatic islets to affect brain, muscle, liver, adipocytes, and stomach, each of which is suggested to support their therapeutic efficacy (5). Actions include, but are not limited to, diminished appetite, decreased gastric emptying, lower hepatic glucose production, and enhanced peripheral glucose uptake (5). At the pancreas, these agents, acting via elevated GLP-1 levels, augment glucose-stimulated insulin secretion through action at the pancreatic β cell, a central hallmark of their therapeutic action. Importantly, these agents also lower glucagon secretion through action at the α cell, which occurs in a glucose-dependent manner. Along with this, both GLP-1R mimetics and DPP4 inhibitors have been suggested to enhance β-cell proliferation and limit β-cell apoptosis, thus expanding β-cell mass (5, 6). This latter action is thought to be therapeutically valuable because diminished β-cell mass is thought to be central in the genesis of type 2 diabetes. Although rodent studies suggest that these actions are central in the incretin-based mode of action, this has never been shown in humans (7). Although irrefutable evidence exists that these therapies improve glycemic control, whether incretin-based therapies increase human β-cell proliferation/mass remains an unanswered question.
With the emergence of these therapies, early concerns of pancreatitis have been closely examined and not considered to be a clinical problem (8). However, more recently incretin-based therapies have been associated with exocrine cell proliferation and dysplasia (9). Some supporting work in rodents has suggested that GLP-1–based therapies can promote exocrine and ductal cell proliferation, implicating a risk of pancreatic cancer (7, 10). Thus, an important clinical question is: what is the possibility of exocrine proliferation, dysplasia, and the risk of pancreatic cancer with incretin-based therapies?
In this issue, Cox et al. (11) have examined the effect of short- and long-term treatment with exenatide (a GLP-1R agonist) and des-fluoro-sitagliptin (a DPP4 inhibitor) on β-cell turnover, mass, and pancreatic exocrine changes through well-planned rodent studies. During short-term treatments, both incretin-based therapies improved glucose tolerance, but only sitagliptin produced a modest β-cell proliferation, without changing β-cell mass. Long-term treatment with both incretin-based therapies during high-fat challenge diminished β-cell proliferation and β-cell mass expansion. An explanation for this, as noted by the authors (11), is that the therapies reduced the metabolic demand on the rodent islet, adiposity, and insulin resistance. Through a blinded analysis by an independent pathologist, neither incretin-based treatment (short or long) produced any exocrine pancreatic proliferation or dysplasia. Taken together, these data led the Kushner group to conclude incretin-based therapy does not promote β-cell proliferation or β-cell mass expansion, and it does not alter exocrine pancreas histology.
As the authors note (11), multiple variables may produce this divergent outcome compared with previous studies, including mouse genetic backgrounds and treatment regimens. However, multiple studies have now reported that GLP-1 mimetics produce no substantial risk of pancreatitis and/or pancreatic cancer (12, 13), findings that are supported by Cox et al. (11). In rodents, β-cell proliferation has been extensively studied, but as highlighted in a series of review articles, has been less explored in human β cells (14–16). The study by Cox et al. (11) indicates that β-cell proliferation and mass expansion is not a substantial part of the incretin-based response. It is therefore possible that with regard to human β cells, which themselves have significantly lower proliferation rates than rodent β cells (17), incretin-based therapies do not induce any β-cell proliferation or mass expansion (18). Thus, it seems likely that it is other nonproliferative properties of incretin-based therapies that are important for their therapeutic effects. These include suppression of food intake, weight loss, and enhanced glucose-stimulated insulin secretion and suppression of glucagon.
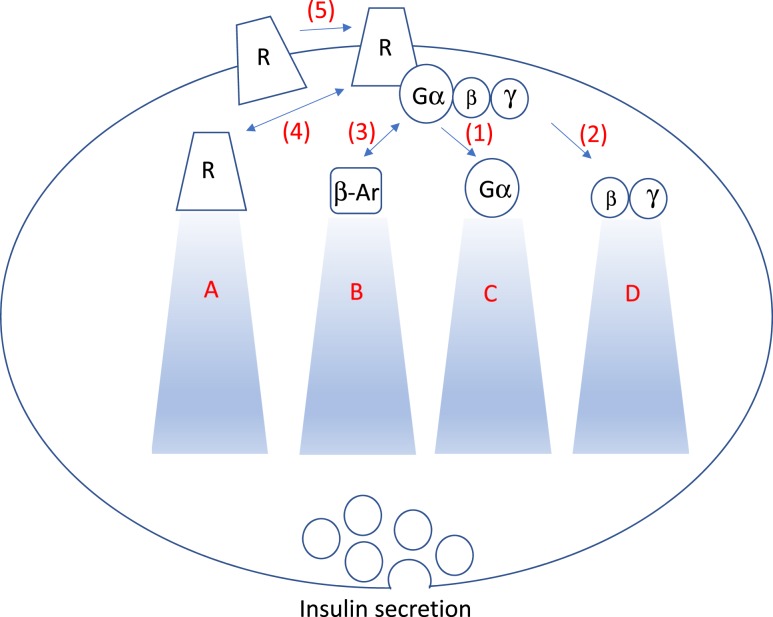
Because incretin-based therapies mediate their actions through the GLP-1R, a G-protein coupled receptor (GPCR), a higher resolution of understanding of how GPCR signaling influences β-cell function and proliferation is needed, with a focus on human β cells. GLP-1 functions through multiple signaling pathways, including the most well-characterized GPCR signaling mechanism, the Gα protein class (Fig. 1). Key observations into how G proteins influence β-cell function/proliferation have come from multiple groups, including the Weinstein group (focused on Gsα) (20), the Wess group (focused on Gqα) (21), and others (22, 23). Collectively, these data have propelled the GPCR area of research focused on β-cell function.
Figure 1.
GPCR signaling events that may be differentially contributing to aspects of β-cell function and proliferation. (1) GPCR activation leads to Gα downstream signaling effects that mediate aspects of β-cell function and proliferation. (2) Gβγ may also influence β-cell function and proliferation. (3) Evidence suggests that β-arrestin (β-Ar) mediates β-cell function/proliferation. (4) When GPCRs are activated, each receptor (R) is internalized, recycled, or degraded at differential rates. (5) GPCRs can form dimers (homodimers or heterodimers) in which each of these five steps can influence receptor signaling abilities. (A) Receptor internalization can result in rapid return to the plasma membrane or a more profound downregulation, influencing the GPCR’s signaling abilities. (B) β-arrestin, which contributes to the downregulation process, also can activate pathways such as ERK. Zhu et al. (19)demonstrated the importance of β-arrestin in β-cell function. (C) Gα proteins, in particular Gsα, Gq/11α, or Gi/oα, and downstream signals, influencing β-cell function. Multiple other G-protein modulators influence the inactivation of Gα proteins. (D) Gβγ can influence ion channel function, mitogen-activated protein kinases activation, and adenylate cyclase properties, among others.
Because important understanding of how Gα proteins mediate β-cell function and proliferation exists, focusing on other aspects of GPCR signaling and their role on β-cell function/proliferation, in particular, human β cells will help us as new incretin-based therapies emerge. GPCRs signal through pathways other than solely the Gα system, including Gβγ and β-arrestin. An understanding of the roles of these other signaling systems in human β cell function/proliferation will be important. Although the role of Gβγ subunits is not clear in β cells, studies are revealing how β-arrestin can regulate β-cell function/proliferation (19, 24). An additional level of complexity in GPCR signaling arises from: (1) each GPCR having unique constitutive levels of activity that influence the effects of agonists to regulate cellular function; (2) each GPCR being internalized and recycled to the plasma membrane at different rates; and (3) each GPCR forming complex dimers with similar and dissimilar GPCRs, which modulates their signaling capabilities (25). Moreover, many GPCRs, including the GLP-1R, which has traditionally been thought to signal by one class of G protein (i.e., Gsα), may also signal through other G proteins (26, 27). How all these signaling complexes emanating from the GLP-1R influence β-cell function and proliferation needs to be examined, with a particular focus on human β cells. With this greater understanding, GPCR agonists can be tailored to influence these signaling capabilities uniquely, allowing selectivity of certain signaling pathways over others by using biased agonists (28). This level of fine-tuning can potentially focus on certain aspects of β-cell function and/or proliferation while limiting others.
In sum, this report (11) has added important data suggesting the lack of association of incretin-based therapies with exocrine dysplasia and brought to light the question, do we need or want human β cells to proliferate in response to a diabetes therapy? If yes, are incretin-based therapies resulting in β-cell proliferation and mass expansion? Although these current therapies may not be leading to β-cell proliferation and mass expansion, can incretin-based therapies be tailored to human β-cell proliferation? The next steps are to characterize the signaling pathways that link GPCRs in human β cells with β-cell function, with or without β-cell proliferation. These next steps will also need to be supported by models that are more clinically applicable (i.e., using older mice and/or human islets).
Acknowledgments
Acknowledgments
This work was supported by the National Institutes of Health (Grant R01DK104927-01A1) and the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Career Development Grant 1IK2BX001587-01 (to B.T.L.). F.M.-J. was supported by Grant R01DK107444.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- DPP4
- dipeptidyl peptidase-4
- GLP-1
- glucagon-like peptide-1
- GLP-1R
- glucagon-like peptide-1 receptor
- GPCR
- G-protein coupled receptor.
References
- 1.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J Physiol. 1902;28(5):325–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elrick H, Stimmler L, Hlad CJ Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–1082. [DOI] [PubMed] [Google Scholar]
- 3.Nauck M, Stöckmann F, Ebert R, Creutzfeldt W. Reduced incretin effect in type 2 (non-insulin-dependent) diabetes. Diabetologia. 1986;29(1):46–52. [DOI] [PubMed] [Google Scholar]
- 4.Nauck M. Incretin therapies: highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Diabetes Obes Metab. 2016;18(3):203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahrani AA, Barnett AH, Bailey CJ. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat Rev Endocrinol. 2016;12(10):566–592. [DOI] [PubMed] [Google Scholar]
- 6.Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143(11):4397–4408. [DOI] [PubMed] [Google Scholar]
- 7.Drucker DJ. Incretin action in the pancreas: potential promise, possible perils, and pathological pitfalls. Diabetes. 2013;62(10):3316–3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drucker DJ, Sherman SI, Bergenstal RM, Buse JB. The safety of incretin-based therapies--review of the scientific evidence. J Clin Endocrinol Metab. 2011;96(7):2027–2031. [DOI] [PubMed] [Google Scholar]
- 9.Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC. Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors. Diabetes. 2013;62(7):2595–2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gier B, Matveyenko AV, Kirakossian D, Dawson D, Dry SM, Butler PC. Chronic GLP-1 receptor activation by exendin-4 induces expansion of pancreatic duct glands in rats and accelerates formation of dysplastic lesions and chronic pancreatitis in the Kras(G12D) mouse model [published correction appears in Diabetes. 2012;61(18):2195.]. Diabetes. 2012;61(5):1250–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox AR, Lam CJ, Rankin MM, Rios JS, Chavez J, Bonnyman CW, King KB, Wells RA, Anthony D, Tu JX, Kim JJ, Li C, Kushner JA. Incretin therapies do not expand β-cell mass or alter pancreatic histology in young male mice. Endocrinology. 2017;158(6):1701–1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Monami M, Nreu B, Scatena A, Cresci B, Andreozzi F, Sesti G, Mannucci E. Safety issues with Glucagon-Like peptide-1 receptor agonists: pancreatitis, pancreatic cancer, and cholelithiasis. data from randomised controlled trials [published online ahead of print February 28, 2017]. Diabetes Obes Metab. doi: 10.1111/dom.12926. [DOI] [PubMed] [Google Scholar]
- 13.Azoulay L, Filion KB, Platt RW, Dahl M, Dormuth CR, Clemens KK, Durand M, Juurlink DN, Targownik LE, Turin TC, Paterson JM, Ernst P; Canadian Network for Observational Drug Effect Studies Investigators . Incretin based drugs and the risk of pancreatic cancer: international multicentre cohort study. BMJ. 2016;352:i581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart AF, Hussain MA, García-Ocaña A, Vasavada RC, Bhushan A, Bernal-Mizrachi E, Kulkarni RN. Human β-cell proliferation and intracellular signaling: part 3. Diabetes. 2015;64(6):1872–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernal-Mizrachi E, Kulkarni RN, Scott DK, Mauvais-Jarvis F, Stewart AF, Garcia-Ocaña A. Human β-cell proliferation and intracellular signaling part 2: still driving in the dark without a road map. Diabetes. 2014;63(3):819–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulkarni RN, Mizrachi EB, Ocana AG, Stewart AF. Human β-cell proliferation and intracellular signaling: driving in the dark without a road map. Diabetes. 2012;61(9):2205–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rankin MM, Kushner JA. Adaptive beta-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58(6):1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linnemann AK, Baan M, Davis DB. Pancreatic β-cell proliferation in obesity. Adv Nutr. 2014;5(3):278–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhu L, Almaça J, Dadi PK, Hong H, Sakamoto W, Rossi M, Lee RJ, Vierra NC, Lu H, Cui Y, McMillin SM, Perry NA, Gurevich VV, Lee A, Kuo B, Leapman RD, Matschinsky FM, Doliba NM, Urs NM, Caron MG, Jacobson DA, Caicedo A, Wess J. β-arrestin-2 is an essential regulator of pancreatic β-cell function under physiological and pathophysiological conditions. Nat Commun. 2017;8:14295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie T, Chen M, Zhang QH, Ma Z, Weinstein LS. Beta cell-specific deficiency of the stimulatory G protein alpha-subunit Gsalpha leads to reduced beta cell mass and insulin-deficient diabetes. Proc Natl Acad Sci USA. 2007;104(49):19601–19606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakajima K, Jain S, Ruiz de Azua I, McMillin SM, Rossi M, Wess J. Minireview: Novel aspects of M3 muscarinic receptor signaling in pancreatic β-cells. Mol Endocrinol. 2013;27(8):1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimple ME, Joseph JW, Bailey CL, Fueger PT, Hendry IA, Newgard CB, Casey PJ. Galphaz negatively regulates insulin secretion and glucose clearance. J Biol Chem. 2008;283(8):4560–4567. [DOI] [PubMed] [Google Scholar]
- 23.Regard JB, Kataoka H, Cano DA, Camerer E, Yin L, Zheng YW, Scanlan TS, Hebrok M, Coughlin SR. Probing cell type-specific functions of Gi in vivo identifies GPCR regulators of insulin secretion. J Clin Invest. 2007;117(12):4034–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mancini AD, Bertrand G, Vivot K, Carpentier É, Tremblay C, Ghislain J, Bouvier M, Poitout V. β-arrestin recruitment and biased agonism at free fatty acid receptor 1. J Biol Chem. 2015;290(34):21131–21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajagopal S, Shenoy SK. GPCR desensitization: acute and prolonged phases [published online ahead of print January 28, 2017]. Cell Signal. S0898-6568(17)30030-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montrose-Rafizadeh C, Avdonin P, Garant MJ, Rodgers BD, Kole S, Yang H, Levine MA, Schwindinger W, Bernier M. Pancreatic glucagon-like peptide-1 receptor couples to multiple G proteins and activates mitogen-activated protein kinase pathways in Chinese hamster ovary cells. Endocrinology. 1999;140(3):1132–1140. [DOI] [PubMed] [Google Scholar]
- 27.Ferré S, Casadó V, Devi LA, Filizola M, Jockers R, Lohse MJ, Milligan G, Pin JP, Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol Rev. 2014;66(2):413–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Costa-Neto CM, Parreiras-E-Silva LT, Bouvier M. A pluridimensional view of biased agonism. Mol Pharmacol. 2016;90(5):587–595. [DOI] [PubMed] [Google Scholar]