Abstract
Patient: Female, 51
Final Diagnosis: Breast implant associated anaplastic large cell lymphoma
Symptoms: Breast swelling
Medication: —
Clinical Procedure: Surgery
Specialty: Oncology
Objective:
Rare disease
Background:
Anaplastic large cell lymphomas (ALCL) are a rare type of primary breast lymphoma. The association between breast implants and ALCL was first described in 1997 and since then 34–173 cases have been presented. The annual incidence of breast implant-associated ALCL (BI-ALCL) is 0.1–0.3 per 100 000 women who undergo breast reconstruction, and cases are often underreported due to the rarity of these tumors. BI-ALCL arises from the inflammatory T cells surrounding the fibrous capsule, and most tumors are in situ.
Case Report:
Here, we present the case of a 51-year-old woman with ALCL following bilateral silicone breast implants. The patient presented with breast enlargement and tenderness 9 years following reconstructive surgery. Imagining studies showed fluid collection surrounding the affected breast implant. Staging studies and histocytopathology examination confirmed the presence BI-ALCL without capsular invasion or metastasis. Complete surgical excision was performed. The patient continues to be in complete remission.
Conclusions:
Due to the rarity of these tumors, establishing the diagnosis of BI-ALCL can be challenging and requires a multidisciplinary approach. Clinicians should be aware of the relationship between breast implants and BI-ALCL.
MeSH Keywords: Antigens, CD30; Breast Implants; Lymphoma, Large-Cell, Anaplastic
Background
Non-Hodgkin lymphoma (NHLs) is a heterogeneous group of hematological malignancies that can rarely involve the breast as either a primary or secondary tumor. Primary NHL of the breast account for less than 1% of all breast cancers and are predominantly B cell in origin [1]. Less than 10% of NHLs are derived from mature, CD30+ T cells [1,2]. Anaplastic large cell lymphomas (ALCL) are a rare subtype of T cell NHLs that accounts for only 3% of all adult NHLs and 6% of breast NHLs [3]. ALCLs are subcategorized as anaplastic lymphoma kinase (ALK)-positive or -negative tumors. ALK positivity is predominantly seen in children or young adults, while ALK-negative ALCL is more common in populations approximately 40–65 years of age and is managed more aggressively [4]. Approximately 1 in 500 000 women are diagnosed with ALCL in the United States annually and 3 in 100 million primarily involve the breast [5,6].
The association between breast implants and ALCL has been discussed in the literature and is an extremely rare event. The first reported case describing an association between breast implants and ACLC was presented by Keech and Creech in 1997 [7]. Since then, between 34 and 173 cases have been described [6,8]. The annual incidence of breast implant-associated ALCL (BI-ALCL) is 0.1 to 0.3 per 100 000 women who undergo breast reconstruction [9] and cases are often underreported due to the rarity of these tumors [10,11]. In 2011, the US Food and Drug Administration (FDA) cautioned that breast implants carry a very small but increased risk of developing ALCL [6]. Interestingly, a report published by Jacob (2016) showed that more women with unilateral breast cancer diagnosis are deciding to undergo double mastectomy despite lack of survival benefit, a trend that may increase the number of women opting for breast reconstructive surgery [12].
All cases of BI-ALCLs are ALK-negative and arise from the inflammatory T cells surrounding the fibrous capsule, and in advanced disease these cells can invade the native breast parenchyma [13]. The breast tissue itself does not show any malignant changes [13]. Histologically, BI-ALCLs are composed of large pleomorphic cells with kidney-shaped nuclei [10]. In addition, epithelial membrane antigen (EMA)-positive, epithelioid cells resembling breast cancer have also been described [14]. ALCL usually presents late, with a median onset of 9.2 years after surgery (range: 4 months to 25 years) [14]. Tumors are indolent and carry an excellent prognosis with a median overall survival (OS) of 12 years [14]. Of note, no difference has been observed between saline vs. silicone implants and the development of BI-ALCL [13]. We present a case report of ALK-negative ALCL following bilateral silicone breast implants.
Case Report
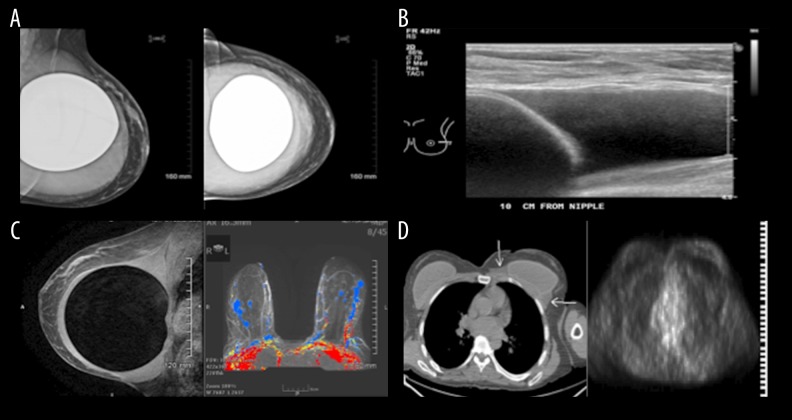
Our patient was a 51-year-old white woman who underwent bilateral breast augmentation in 2007 with McGhan Style 120 silicone implants from lot # 1315735. Six weeks prior to presentation, she noted an increase in the size of one of her breasts. Reviewing recent photographs, it was apparent the discrepancy in breast size began approximately 3 months prior. She denied any other symptoms such as pain, redness, fever, chills, night sweats, nipple discharge, or focal masses in either breast. Upon initial consultation, a mammogram and breast ultrasound revealed a large effusion surrounding the left implant, but no masses within the capsule or in either breast (Figure 1). A 200-ml collection of mildly turbid, straw-colored fluid was aspirated and sent for histological examination. Clinical and radiographic evaluations of the right breast were normal (Figure 1). A breast MRI noted a large loculated fluid collection surrounding the fibrous capsule of the left breast implant, without enhancement of the capsule or within either breast (Figure 1).
Figure 1.
Imaging studies. Evaluation studies included breast mammography, ultrasound, magnetic resonance imaging (MRI), computed tomography (CT), and positron emission tomography (PET). (A) Mammogram. MLO and CC views demonstrated a smooth soft tissue density area completely surrounding the silicone breast implant. (B) Ultrasonography of the left breast showed an anechoic fluid collection. (C) MRI of the breasts. Sagittal view T2W sequence demonstrated the bright signal intensity fluid-like collection surrounding the dark signal intensity central silicone breast implant. This was not enhancing, as seen on the axial post contrast (colored image) subtraction vibrant sequences. (D) PET/CT study. No FDG uptake was observed in the left breast fluid density (white arrows CT) surrounding the implant. The right breast implant is unremarkable.
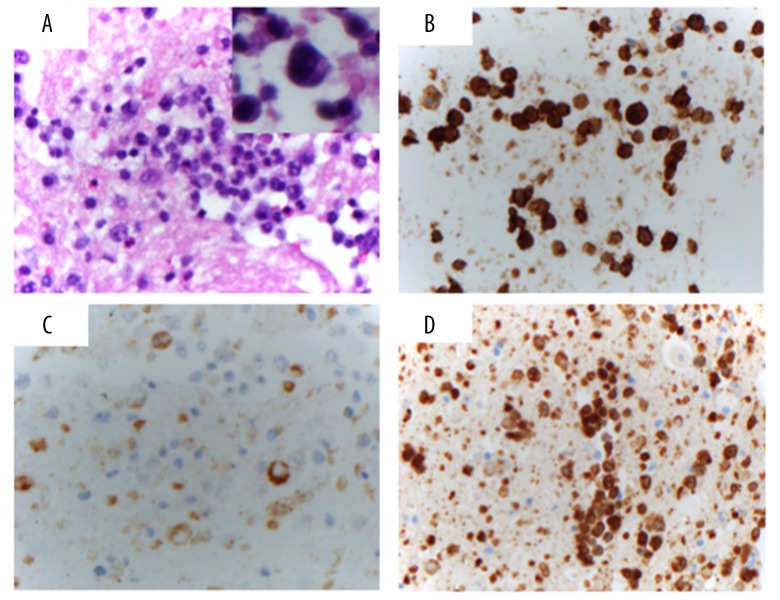
Histological examination of the fluid revealed scattered large atypical cells admixed within a population rich in small lymphocytes, suggestive of a lymphoid malignancy. Immunohistochemistry (IHC) demonstrated that the atypical cells were positive for CD45, CD3, CD30, TIA1, and granzyme B, but were negative for ALK1. A subset of large cells was positive for EMA. CD20, a marker for B cells, was negative (Figure 2).
Figure 2.
Histology and Immunohistochemical (IHC) studies. (A) Cell block stained with hematoxylin and eosin (40×) showing atypical lymphocytes with enlarged nuclei and prominent nucleoli (inset, 100× magnification of an atypical large cell). (B) CD30 stains the majority of the cells (40×). (C) EMA stains a subset of cells, mainly the most atypical enlarged cells (40×). (D) CD3 stains most of the cells (40×).
Staging work-up was done and noted minimal abnormal FDG uptake (less than the mediastinal blood pool uptake) within the left breast effusion (Figure 1). Bilateral sub-centimeter, minimally FDG avid axillary adenopathy was thought to be inflammatory as opposed to neoplastic (Figure 1). After aspiration, the left breast with implant remained at baseline size, comparable to the right breast.
Relevant history in this pre-menopausal woman included menarche at 11, G6P3, first live birth at 25, and excision of a left breast lipoma in 2007 prior to implants. Medications included vortioxetine, lisdexamfetamine, armodafinil, and multivitamins. Family history noted breast and ovarian carcinomas in her mother (at the age of 68 and 71, respectively), glioblastoma in her father, and colon cancer in a paternal grandfather. Social history was non-contributory, except for 2–4 alcohol portions per week and a remote history of smoking (20 pack years, quit 30 years ago).
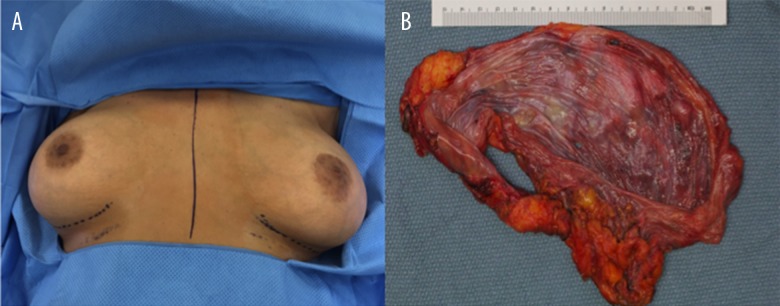
Physical exam results were normal except for intact subpectoral breast implants bilaterally and well-healed scars from the implant (Figure 3A). The left breast continues to appear modestly larger than the right. The patient underwent bilateral capsulectomy and extraction of silicone implants (Figure 3B).
Figure 3.
Gross anatomical representation of the case. (A) Photograph of the breast status after bilateral augmentation depicts asymmetry in the patient’s left breast prior to surgery. (B) En bloc removal of the breast implant, the inner surface of smooth glistening implant capsule with no distinctive lesions.
Discussion
Since its first description in 1997, the association between breast implants and ACLC has generated much attention, including recent reports from several regulatory agencies, including the US FDA [6,7]. All patients diagnosed with BI-ALCL have been women, with a median age of occurrence of 54 years (range, 28–87 years) [9]. In the largest meta-analysis of BI-ALCL, 37 reports documenting 79 cases globally have been published and an additional 94 unpublished cases were presented [8].
Patients with BI-ACLC present most commonly with breast discomfort or enlargement due to swelling, without breast pain requiring further follow-up. The late onset of these symptoms following breast implants placement is a common characteristic of BI-ALCL [15]. Development of breast mass, contractures, and, in rare cases, lymphadenopathy, ulcerations, rash, pruritus, lymphomatoid papulosis, and B symptoms have also been reported, although less frequently [8,10,15]. Follow-up imagining studies with ultrasound and mammogram often reveals a fluid collection or a seroma confined to the capsular space [11]. Interestingly, 28 out of 71 (39.4%) patients with BI-ALCL have had a prior history of breast cancer that required reconstructive surgery (with 14 cases unreported) and 5 out of 71 (7%) had a previous history of lymphomas (with 17 cases unreported) [15].
BI-ALCL arises from mature, CD30+ T cells surrounding the implant capsule, and 65.5% of cases are in situ tumors [11,13]. Although treatment guidelines are lacking, retrospective studies have shown that complete surgical excision, which includes removal of the implant, total capsulectomy, and complete removal of any disease or mass with negative margins, is recommended for all patients with BI-ALCL [11]. Patients with complete surgical excision showed improved event-free survival (EFS) and median OS when compared to patients who underwent only limited surgery, chemotherapy, or radiation therapy [11,15]. The most commonly used chemotherapy regimen is cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), which had been used in 44 out of 51 cases of BI-ALCL reported (86.3%) [11,14]. BI-ALCL patients treated with chemotherapy have a relapse rate of 29%, perhaps related to the more aggressive clinical behavior leading to clinicians to use it post-surgically [11,15]. Several case reports have also described unusually aggressive and treatment-refractory forms of BI-ALCL, suggesting that subtypes of BI-ACLC may also exist [16,17].
Histologically, BI-ALCL is composed of large pleomorphic cells that express markers of T cell lineage, including CD3, CD30, TIA1, and granzyme B [10]. In addition, cells lack B cells markers (CD20, CCD79a, and PAX5). All cases of BI-ALCL are ALK-negative. In contrast to systemic ALK-negative ALCL, BI-ALCL have an excellent prognosis despite the lack of ALK expression. Once the diagnosis of ALCL is established, differentiating systemic ALK-negative ALCL from primary BI-ALCL is very important due to striking differences in the clinical behavior and management of both conditions. Furthermore, 40% to 70% of patients with BI-ALCL are positive for epithelial membrane antigen (EMA), which may lead clinicians to incorrectly diagnose cases as primary or recurrent triple-negative breast carcinomas [13]. The indolent presentation of BI-ALCL may further delay diagnosis and lead clinicians to diagnose other diseases. A detailed history and physical exam and lack of systemic signs may aid clinicians in the proper diagnosis of BI-ALCL, and differentiate it from other more aggressive forms of ALK-negative ALCL.
The pathophysiology of BI-ALCL is poorly defined. Chronic inflammation secondary to a foreign body (i.e., the implants) has been hypothesized to be responsible for the development of BIALCL. The development of hematological malignancies due to chronic inflammation, as seen with mucosa-associated lymphoid tissue lymphomas (MALTomas) in the background of chronic Helicobacter pylori infection, is well documented [18]. The immunogenic potential and pro-inflammatory role of silicone in breast implants has also been described previously [19–24]. Increased antibody titers and granulomatous reactions have been observed following silicone breast implantation [19–24]. In addition, breast implants have also been associated with the development of several autoimmune disorders, including scleroderma, morphea, systemic lupus erythematous (SLE), rheumatoid arthritis, CREST syndrome, and “human adjuvant disease” [19]. Although silicone has largely been recognized as a biologically inert material, a subset of individuals may have an increased sensitivity to implants, leading to a more robust foreign body immune response or chronic inflammation and the development of BI-ALCL. In fact, a stronger immune response has also been observed in implants with textured surfaces vs. smooth surfaces, irrespective of the implant filling material [8]. The excellent response of BI-ALCL to complete surgical excision therapy also suggests that foreign body removal is sufficient to withdraw the antigenic stimulation that promotes chronic inflammation. The tumorigenic potential of silicone and the role of infection, or biofilm, in the development of BI-ALCL have also been described, but have not gained much acceptance [15,25,26].
Conclusions
Due to the rarity of these tumors and broad differential diagnosis as described above, a coordinated response among plastic surgeons, oncologists, radiologist, and pathologists is required for the management of patients diagnosed with BIALCL. Clinicians should be aware of the relationship that exists between breast implants and ALCL. Future studies are required to develop optimal guidelines for prevention, treatment, and management of BI-ALCL.
References:
- 1.Alexander DD, Mink PJ, Adami HO, et al. The non-Hodgkin lymphomas: A review of the epidemiologic literature. Int J Cancer. 2007;120(Suppl. 12):1–39. doi: 10.1002/ijc.22719. [DOI] [PubMed] [Google Scholar]
- 2.Talwalkar SS, Miranda RN, Valbuena JR, et al. Lymphomas involving the breast: A study of 106 cases comparing localized and disseminated neoplasms. Am J Surg Pathol. 2008;32:1299–309. doi: 10.1097/PAS.0b013e318165eb50. [DOI] [PubMed] [Google Scholar]
- 3.Hapgood G, Savage KJ. The biology and management of systemic anaplastic large cell lymphoma. Blood. 2015;126:17–25. doi: 10.1182/blood-2014-10-567461. [DOI] [PubMed] [Google Scholar]
- 4.Drexler HG, Gignac SM, von Wasielewski R, et al. Pathobiology of NPM-ALK and variant fusion genes in anaplastic large cell lymphoma and other lymphomas. Leukemia. 2000;14:1533–59. doi: 10.1038/sj.leu.2401878. [DOI] [PubMed] [Google Scholar]
- 5.Altekruse SF, Kosary CL, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2007. National Cancer institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2007/, table 19.28, based on November 2009 SEER data submission, poster to the SEER web site, 2010. [Google Scholar]
- 6.The Center for Devices and Radiological Health USFaDA Anaplastic large cell lymphoma (ALCL) in women with breast implants: Preliminary FDA findings and analysis. http://www.fda.ogv/downloads/medicaldevices/productsandmedicalprocedures/implantsnadprosthetics/breastimplants/UCM240003.pdf.
- 7.Keech JA, Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997;100:554–55. doi: 10.1097/00006534-199708000-00065. [DOI] [PubMed] [Google Scholar]
- 8.Brody GS, Deapen D, Taylor CR, et al. Anaplastic large cell lymphoma occurring in women with breast implants: Analysis of 173 cases. Plast Reconstr Surg. 2015;135:695–705. doi: 10.1097/PRS.0000000000001033. [DOI] [PubMed] [Google Scholar]
- 9.de Jong D, Vasmel WL, de Boer JP, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008;300:2030–35. doi: 10.1001/jama.2008.585. [DOI] [PubMed] [Google Scholar]
- 10.Peters W. Update on anaplastic large cell lymphoma in women with breast implants. Plast Surg (Oakv) 2014;22:267–69. doi: 10.4172/plastic-surgery.1000894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clemens MW, Medeiros LJ, Butler CE, et al. complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016;34:160–68. doi: 10.1200/JCO.2015.63.3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacob JA. More women with breast cancer opt for bilateral mastectomy despite lack of survival benefit. JAMA. 2016;315:2154–56. doi: 10.1001/jama.2016.3584. [DOI] [PubMed] [Google Scholar]
- 13.Xu J, Wei S. Breast implant-associated anaplastic large cell lymphoma: Review of a distinct clinicopathologic entity. Arch Pathol Lab Med. 2014;138:842–46. doi: 10.5858/arpa.2013-0068-RS. [DOI] [PubMed] [Google Scholar]
- 14.Clemens MW, Miranda RN. Coming of age: Breast implant-associated anaplastic large cell lymphoma after 18 years of investigation. Clin Plast Surg. 2015;42:605–13. doi: 10.1016/j.cps.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Ye X, Shokrollahi K, Rozen WM, et al. Anaplastic large cell lymphoma (ALCL) and breast implants: breaking down the evidence. Mutat Res Rev Mutat Res. 2014;762:123–32. doi: 10.1016/j.mrrev.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Alobeid B, Sevilla DW, El-Tamer MB, et al. Aggressive presentation of breast implant-associated ALK-1 negative anaplastic large cell lymphoma with bilateral axillary lymph node involvement. Leuk Lymphoma. 2009;50:831–33. doi: 10.1080/10428190902795527. [DOI] [PubMed] [Google Scholar]
- 17.Carty MJ, Pribaz JJ, Antin JH, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg. 2011;128:112e–18e. doi: 10.1097/PRS.0b013e318221db96. [DOI] [PubMed] [Google Scholar]
- 18.Witkowska M, Smolewski P. Helicobacter pylori infection, chronic inflammation, and genomic transformations in gastric MALT lymphoma. Mediators Inflamm. 2013;2013:523170. doi: 10.1155/2013/523170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoshida SH, Chang CC, Teuber SS, et al. Silicon and silicone: Theoretical and clinical implications of breast implants. Regul Toxicol Pharmacol. 1993;17:3–18. doi: 10.1006/rtph.1993.1002. [DOI] [PubMed] [Google Scholar]
- 20.Wolfram D, Rabensteiner E, Grundtman C, et al. T regulatory cells and TH17 cells in peri-silicone implant capsular fibrosis. Plast Reconstr Surg. 2012;129:327e–37e. doi: 10.1097/PRS.0b013e31823aeacf. [DOI] [PubMed] [Google Scholar]
- 21.Thompson PA, Lade S, Webster H, et al. Effusion-associated anaplastic large cell lymphoma of the breast: time for it to be defined as a distinct clinicopathological entity. Haematologica. 2010;95:1977–79. doi: 10.3324/haematol.2010.026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vojdani A, Brautbar N, Campbell AW. Antibody to silicone and native macromolecules in women with silicone breast implants. Immunopharmacol Immunotoxicol. 1994;16:497–523. doi: 10.3109/08923979409019737. [DOI] [PubMed] [Google Scholar]
- 23.Kossovsky N, Stassi J. A pathophysiological examination of the biophysics and bioreactivity of silicone breast implants. Semin Arthritis Rheum. 1994;24:18–21. doi: 10.1016/0049-0172(94)90105-8. [DOI] [PubMed] [Google Scholar]
- 24.Brautbar N, Campbell A, Vojdani A. Silicone breast implants and autoimmunity: Causation, association, or myth? J Biomater Sci Polym Ed. 1995;7:133–45. doi: 10.1163/156856295x00652. [DOI] [PubMed] [Google Scholar]
- 25.de Martel C, Ferlay J, Franceschi S, et al. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012;13:607–15. doi: 10.1016/S1470-2045(12)70137-7. [DOI] [PubMed] [Google Scholar]
- 26.Brinton LA. The relationship of silicone breast implants and cancer at other sites. Plast Reconstr Surg. 2007;120:94S–102S. doi: 10.1097/01.prs.0000286573.72187.6e. [DOI] [PubMed] [Google Scholar]