Abstract
Objective: Stimulant medications, with methylphenidate as the main agent, are the most prescribed for the treatment of attention-deficit/hyperactivity disorder. Nevertheless, real challenges still remain for clinicians concerned with adaptation of the therapeutic regimens, in terms of doses and timing, to children's daily activities. The aim of this study was to optimize short-acting methylphenidate regimens according to specific children's needs by evaluating the performance of a particular regimen through a web-based application.
Methods: In this article, accounting for day-to-day children's activities and using up-to-date pharmacokinetic knowledge of methylphenidate, we propose a computational approach for the identification of the most suitable dosing regimens of immediate-release formulations of methylphenidate based on constraints on drug concentration and time frame of activities, defined through therapeutic boxes. To assess the performance of these regimens, time- and concentration-based therapeutic indicators, as well as a roller coaster effect, are proposed.
Results: A web-based interface that can serve as an educational tool for clinicians and patients has been developed based on the proposed approach for the evaluation of dosing regimens. Comparison of those optimal regimens identified by our method with the well-accepted regimens defined in the NIMH Collaborative Multisite Multimodal Treatment study of Children with attention-deficit/hyperactivity disorder indicates that there is still room for improvement in the current practice especially for the last dose administration to avoid side effects such as sleep disturbance.
Conclusion: The developed approach and its associated web-based interface provide an efficient way to evaluate and adapt the methylphenidate regimens to children's daily activities. In addition, this approach could be used as proof of concept to further implement combination of short- and long-acting methylphenidate.
Keywords: : population pharmacokinetics, methylphenidate, web-based application, dose adaptation, precision medicine, eHealth
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is one of the most common chronic childhood neurodevelopmental disorders, which has a worldwide prevalence of about 5%, stable across the last three decades (Polanczyk et al. 2007, 2014). ADHD is primarily characterized by developmentally inappropriate, pervasive, and impairing inattention, impulsiveness, and restlessness. It begins in childhood and affects 3%–9% of school-aged children, including 30% of pediatric outpatient referrals (Thompson and Ni Bhrolchain 2013) and 2.5% of adults (Simon et al. 2009). Stimulants are the leading medications for ADHD, with a significant growth in their prescription over the last two decades, especially in the United States, raising concerns and critics of overprescription (Garfield et al. 2012).
Methylphenidate (MPH) is the most prescribed psychostimulant for ADHD treatment (Kimko et al. 1999; Greenhill et al. 2002). By blocking the dopamine and norepinephrine transporters, MPH stimulates the increase of synaptic dopamine and norepinephrine concentrations. Oral effective doses of 0.25 and 1 mg/kg are estimated to being able to, respectively, block 50% and 75% of the dopamine transporter (Volkow et al. 1997, 1998; Spencer et al. 2006). By tracking changes in behavioral response with a high degree of temporal resolution, it was possible to demonstrate a tight correspondence between the clinical response and the plasma levels of d-MPH (Teicher et al. 2006).
In the early 2000s, different long-acting preparations were developed with the aim to replace the inconvenient frequent dosing (twice to thrice a day) of short-acting forms by a single dose to meet the children's needs for a whole day (Stein et al. 1996). While some children with ADHD still continue to use immediate-release MPH formulations, single-daily dose strategies are currently more prescribed (McCracken et al. 2003; Swanson and Hechtman 2005). However, clinical experience indicates that predesigned pharmacokinetic (PK) profiles of these drug formulations do not always fit the children's specific needs at different periods (morning routine, bus transportation, homework, and bed time).
Therefore, prescribers started to combine the use of long-acting preparations with short-acting ones to obtain optimal therapeutic outcomes (Zelnik and Terkel-Dawer 2015). However, this practice still relies heavily on prescribers' personal experience and best guesses, which is prone to errors, and associated risks of side effects, despite the large MPH safety margin.
Model-based solutions can be used as complementary tools to assist the clinician in finding the best therapeutic strategies for dose and time schedules. This practice is likely to have a preponderant influence in the coming years and might become the main alternative for dose selection in the near future (Pharmacometrics FDA 2011). The Population Pharmacokinetics (Pop-PK) approach, widely accepted as an effective approach to model relationships between drug input, exposure, and effects by taking into account the inter- and intraindividual variability, provides a convenient platform for dose adaptation (Sheiner et al. 1979).
In the context of ADHD treatment with MPH, we, in this study, propose a computational strategy that uses advanced modeling and simulation techniques to accommodate patient's specific needs and clinical constraints. As a proof of concept study, we initially focused on the optimization of multiple daily administration of immediate release (IR) MPH over a typical child's day. This approach allowed us to compare our optimization algorithm with the treatment regimen used in the NIMH Collaborative Multisite Multimodal Treatment study of Children with ADHD (MTA study) (Greenhill et al. 1996), which used IR MPH, the only preparation available when the study was designed. Using a previously reported computational strategy, we reformulated several therapeutic indicators (TIs) for the evaluation of a drug regimen's performance in terms of the daily disposition of doses and timings, by adapting them to the situation of ADHD treatment with MPH (Bonnefois et al. 2015). To maximize the outreach to the ADHD community, we materialized our computational strategy into a web-based version that supports numerical and graphical outputs to assist clinicians in their evaluation of the performance of MPH regimens.
The article is organized as follows: in Materials and Methods section, we detail the computational methodology of our daily drug regimen selection in terms of dose partitions and associated timings. Several TIs are introduced for the evaluation of drug regimen performance. In Results section, we show how the performance of MPH regimens can be optimized and measured using numerical and graphical results. We applied the same approach to the MTA regimen, for comparison. Concerned with practical utility, we have developed a web-based application, which is also presented herein. Applicability and benefits of our methodology, both in clinical practice and drug development process, are discussed in the last section.
Materials and Methods
Pop-PK model of MPH
A one-compartment Pop-PK model with first-order absorption and elimination was developed using NONMEM VII, level 3.0 (Beal et al. 2009) to describe the PK of IR formulations of MPH (Ritalin®) using data from a population of 44 male adult subjects receiving single oral doses, 26 with a dose of 10 mg and 18 of 20 mg. The descriptive statistics are reported in Table 1. Pop-PK is a modeling approach to investigate the impact of variability on the dose–concentration relationship and the extent of this variability in terms of therapeutic outcomes in a target population.
Table 1.
Characteristics of the Studied Population
| IR formulation | |||
|---|---|---|---|
| 10 mg | 20 mg | 10 or 20 mg | |
| Characteristics | |||
| Subjects | 26 | 18 | 44 |
| Age (years) | |||
| Range | 18–37 | 18–35 | 18–37 |
| Mean | 25.58 | 24.47 | 25.07 |
| SD | 4.93 | 4.47 | 4.74 |
| Height (cm) | |||
| Range | 158–188 | 165–186 | 158–188 |
| Mean | 175.92 | 174.22 | 175.23 |
| SD | 6.32 | 6.39 | 6.34 |
| Weight (kg) | |||
| Range | 59.8–92 | 59–96.8 | 59–96.8 |
| Mean | 77.4 | 74.57 | 76.25 |
| SD | 8.98 | 11.52 | 10.07 |
IR, immediate release; SD, standard deviation.
Exponential interindividual variability is mode-led for PK parameters, while the proportional error model is used to describe the unexplained residual error. A lag time is added for the delay of absorption. Moreover, since a large correlation is found between apparent clearance (CL/F) and apparent volume (V/F), a constant parameter θ is included to describe this correlation [Eq. (1a) and (1b)].
 |
 |
No information on covariates was available. The estimation of Pop-PK parameters and the associated variability are reported in Table 2. The disposition of MPH is similar in adults and children for both immediate-release and modified-release formulations (Kimko et al. 1999, 2012; Wigal et al. 2011). Indeed, using the developed model, average clearance was estimated at 254 L/h in adults. This is in accordance with the clearance estimated in two studies for school-aged children (232 or 229 L/h) (Wigal et al. 2007; Childress et al. 2016).
Table 2.
Estimated Parameter Values of Pop-PK Model of IR MPH
| Parameters | TV | CV (%) |
|---|---|---|
| CL/F (L/h) | 254 | 47.1 |
| V/F (L) | 949 | 45 |
| Ka (h−1) | 1.72 | 64.4 |
| Alag (h) | 0.442 | 7.6 |
| θ | 0.922 | — |
| Residual error (%) | — | 17.3 |
CL/F, apparent clearance; CV, coefficient of variation; IR, immediate release; Ka, absorption rate constant; MPH, methylphenidate; Pop-PK, population pharmacokinetics; V/F, apparent volume of distribution; TV, typical value; θ, correlation between CL/F and V/F.
Dosing regimens
The design of MPH dosing regimens has to account for clinical constraints and children's daily activities. More precisely, the prescriber has to consider school schedule, homework, and family routines when determining the time of the first daily administration, the total daily dose (TDD), as well as its fractioning over the day.
Time of the first daily administration
The first administration of a day should take place in the period T from 6:00 to 8:00 am, which corresponds to children's morning activities such as waking up, having breakfast, hygiene, dressing up, and leaving for school. Our method allows to test different periods of T and visualize their impact on the regimen performance.
Total daily dose
In this work, we use 0.25–1 mg/kg as the range of TDD, with dose escalation of 0.25 mg/kg. Thus, four categories of TDD adjusted by weight (WT) are obtained:
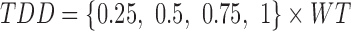 |
In clinical practice, TDD has to be rounded to the closest available dose. In the case of MPH, all available doses should be multiples of 5 mg (available dose unit). For example, for a patient of 26 kg, the minimum TDD is 0.25 × 26 = 6.5 mg, giving rise to a prescribed dose of 5 mg.
Fractioning of TDD
IR MPH is often administered using bis in die (BID) or ter in die (TID) regimens. Therefore, we can define a dosing regimen on a daily basis:
where
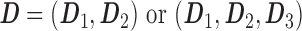 |
for BID or TID, respectively.
Selection of dosing regimens
Different dosing regimens produce different PK profiles, which can induce different therapeutic effects. For this, we adapted previous TIs to evaluate the performance of a dosing regimen, in terms of TDD, its fractioning, and schedule (Bonnefois et al. 2015). This allows identification of an optimal dosing regimen. For that purpose, we also designed an algorithm to search, in the plane of dose and time, and select the best TDD and its corresponding regimen.
PK target
The minimum effective concentration of MPH corresponds to an average plasma concentration of 6 ng/mL, obtained with a dose of 0.25 mg/kg (Volkow et al. 1998; Spencer et al. 2006). It has been reported that a dopamine transporter occupancy of 70% in healthy subjects corresponds to a range of MPH plasmatic concentrations of 15–20 ng/mL (Spencer et al. 2006, 2012; Costa et al. 2013). Therefore, the target therapeutic window (TW) of MPH is set to a range of 6 to 20 ng/mL.
Additional to these pharmacological considerations, the use of MPH can be subject to children's daily activities. Indeed, it is expected to maintain MPH therapeutic effect for a total typical period from 8:00 am to 6:00 pm, to cover school time and after school (transportation, homework). Moreover, a dose as low as 0.5 mg/kg has been shown to delay sleep start time and decrease sleep duration in a double-blind, placebo-controlled, randomized crossover study (Santisteban et al. 2014). Therefore, it is important to have MPH concentration as low as possible, below 6 ng/mL, just before bed time.
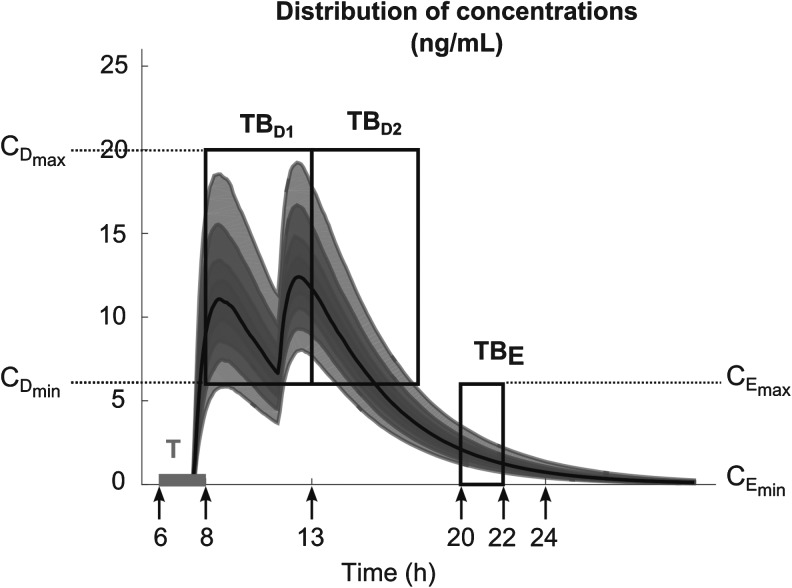
All these constraints on drug concentration and timing led us to define two rectangular zones in the time–concentration plane, referred to as therapeutic boxes (TBs), as illustrated in Figure 1. The day TB (TBD) is further divided into TBD1 and TBD2, referring to AM and PM constraints. A dosing regimen is evaluated in terms of the occurrence of its PK profiles within these boxes.
FIG. 1.
Illustration of different TBs that coordinate the PK profiles in terms of day and evening child activities. The three black outlined boxes illustrate day TB (TBD) divided into an AM box (TBD1) and a PM box (TBD2) and evening TB (TBE). Arrows from left to right refer to the following: 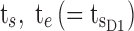 ,
, 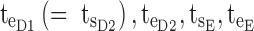 .
. 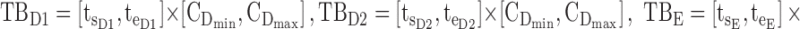 [
[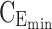 ,
, 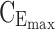 ]. For the
]. For the 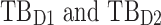 ,
, 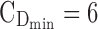 ng/mL,
ng/mL, 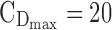 ng/mL. For the AM period,
ng/mL. For the AM period, 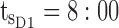 am and
am and 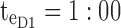 pm. For the PM period,
pm. For the PM period, 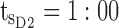 pm and
pm and 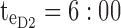 pm. For the
pm. For the 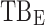
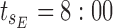 pm,
pm, 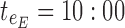 pm and
pm and 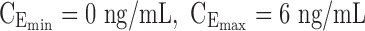 . The thick gray bar shows the range of the first administration time (noted T), from
. The thick gray bar shows the range of the first administration time (noted T), from 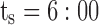 am and
am and 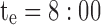 am. TB: therapeutic boxes;
am. TB: therapeutic boxes; 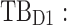 day therapeutic box during AM period;
day therapeutic box during AM period; 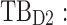 day therapeutic box during PM period;
day therapeutic box during PM period; 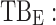 evening therapeutic box;
evening therapeutic box; 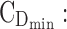 minimum effective concentration in day therapeutic box;
minimum effective concentration in day therapeutic box; 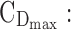 maximum concentration in day therapeutic box;
maximum concentration in day therapeutic box; 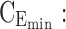 minimum concentration in evening therapeutic box;
minimum concentration in evening therapeutic box; 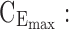 maximum concentration in evening therapeutic box; ts: starting administration time of T period; te: ending administration time of T period;
maximum concentration in evening therapeutic box; ts: starting administration time of T period; te: ending administration time of T period; 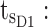 starting time of day therapeutic box in AM period;
starting time of day therapeutic box in AM period; 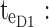 ending time of day therapeutic box in AM period;
ending time of day therapeutic box in AM period; 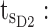 starting time of day therapeutic box in PM period;
starting time of day therapeutic box in PM period; 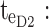 ending time of day therapeutic box in PM period;
ending time of day therapeutic box in PM period; 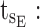 starting time of evening therapeutic box;
starting time of evening therapeutic box; 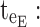 ending time of evening therapeutic box; PK: pharmacokinetic.
ending time of evening therapeutic box; PK: pharmacokinetic.
Moreover, the Roller Coaster effects (RCE), which refer to the drug effects waxing and waning in correspondence with the peaks and troughs following each administration, should be considered. Usual strategies to avoid RCE and its temporary increase of ADHD symptoms (irritability, restlessness…) include administering closer in time, giving a smaller dose shortly after the larger one, or switching to a longer acting stimulant. Reducing the RCE by minimizing the plasma concentration fluctuations over time has also been included in our selection of dosing regimens.
Criteria for dosing regimen performance
To evaluate the performance of dosing regimens, we propose two classes of TIs, in terms of time or concentration:
Time-based therapeutic indicators: In this class, the effective time TI (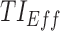 ) refers to the percentage of time during which steady-state MPH concentrations remain within the TBs. Mathematically, for the ith patient, the drug concentration–time curve
) refers to the percentage of time during which steady-state MPH concentrations remain within the TBs. Mathematically, for the ith patient, the drug concentration–time curve 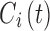 and a time period P = [a,b],
and a time period P = [a,b], 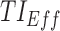 can be expressed according to Equation (2).
can be expressed according to Equation (2).
Thus for the school time, starting at 8:00 am and ending at 6:00 pm, P corresponds to D = [8:00 am, 6:00 pm] and TBD = [8:00 am, 6:00 pm] × [6, 20 ng/mL]. When separated in AM and PM periods, we have TBD1 = [8:00 am, 1:00 pm]
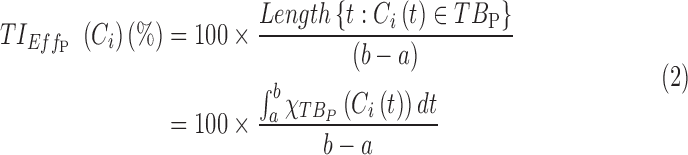 |
× [6, 20 ng/mL] and TBD2 = [1:00 pm, 6:00 pm] × [6, 20 ng/mL]. For the evening period starting at  = 8:00 pm and ending at
= 8:00 pm and ending at 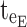 = 10:00 pm, P corresponds to E = [8:00 pm, 10:00 pm], and TBE = [8:00 pm, 10:00 pm] × [0, 6 ng/mL].
= 10:00 pm, P corresponds to E = [8:00 pm, 10:00 pm], and TBE = [8:00 pm, 10:00 pm] × [0, 6 ng/mL].
A given drug regimen can induce different PK profiles due to the PK variability. The performance of this regimen can thus be evaluated by averaging the performance over N-simulated individual PK profiles:
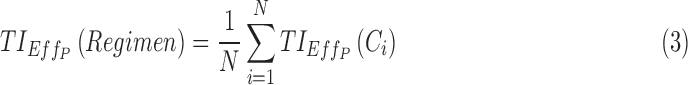 |
with N high enough (1000 in this article).
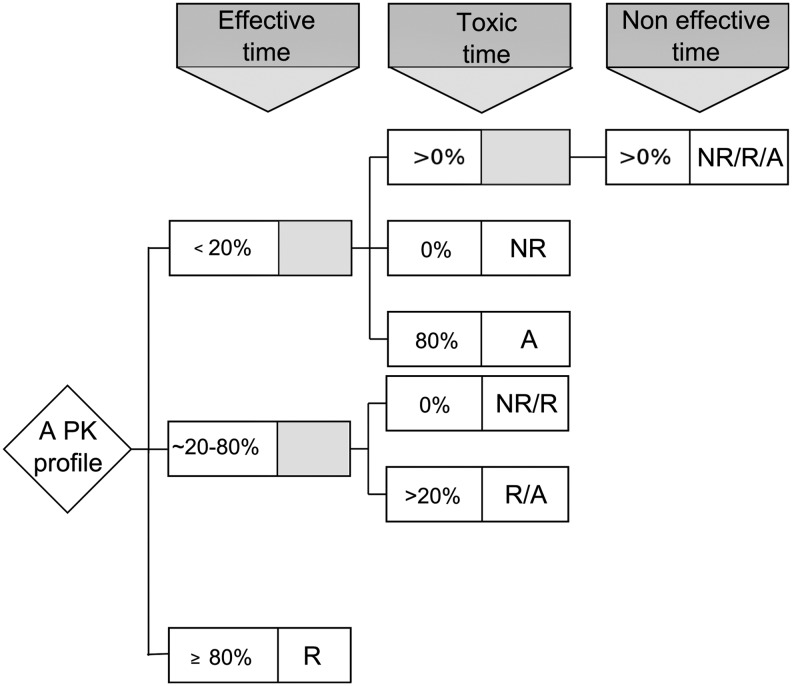
Concentration-based therapeutic indicators: As adopted in a previous work (Bonnefois et al. 2015), we make use of the three zones below, within, or beyond TW, to refer to noneffective, effective, or toxic zones, respectively. The concentration-based TIs are defined based on the categorization of individual PK profiles with respect to these zones. There are at most six exclusive categories, with three simple and three hybrid ones. An individual PK profile belongs to the simple category of nonresponders (NR), responders (R), or adverse-responders (A) if its concentration–time curve completely stays within the noneffective, effective, or toxic zone, respectively. If the concentration–time course goes through more than two zones, the hybrid category of nonresponders/responders (NR/R), responders/adverse-responders (R/A), or nonresponders/responders/adverse-responders (NR/R/A) are used. The criteria for categorization of a PK profile are described in Figure 2. For example, a PK profile will be included in R if the time it remains in noneffective and toxic zones is <20% of the considered time period, thus it stays in the effective zone >80% of time. In the following, we will choose a threshold of 80% since an effective time of 100% is unrealistic in practice. This threshold can eventually be changed to accommodate different therapeutic contexts.
FIG. 2.
Categorization of an individual PK profile regarding the time it spends in the effective, toxic, and noneffective zones. PK, pharmacokinetics; NR, nonresponders; NR/R, nonresponders/responders; R, responders; R/A, responders/adverse-responders; A, adverse-responders; NR/R/A, nonresponders/responders/adverse-responders.
We can evaluate the proportion of those N PK profiles generated from a given drug regimen and the underlying PK variability that belongs to each of the mentioned six categories (CAT). This evaluation gives rise to the probability for a given dosing regimen to be associated to a category CAT:
 |
where CAT is one of {R, A, NR, R/A, NR/R, NR/R/A}.
For the purpose of ADHD therapy, we will define two concentration-based TIs delimited to three specific time periods, AM, PM, and E, to account for the trade-off between efficacy and side effects in a day. Mathematically, they are formulated for responders R as follows:
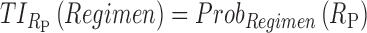 |
As mentioned above, P can be D1 or D2, and E, with effective zones of [6, 20 ng/mL] and [0, 6 ng/mL], which correspond to therapeutic boxes TBD1 or TBD2 and TBE, respectively.
Therapeutic indicator for RCE: Additional to time-based and concentration-based TIs, we also propose a new TI for the evaluation of the RCE for the PK profile Ci of the ith individual:
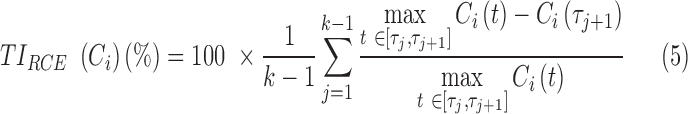 |
where 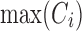 is the maximum value of Ci over the dosing interval
is the maximum value of Ci over the dosing interval 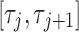 and k = 2 or 3 for BID and TID, respectively.
and k = 2 or 3 for BID and TID, respectively.
Then the performance of a given regimen can be evaluated by averaging the 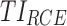 of N-simulated PK profiles:
of N-simulated PK profiles:
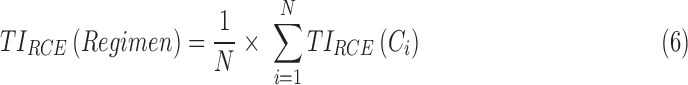 |
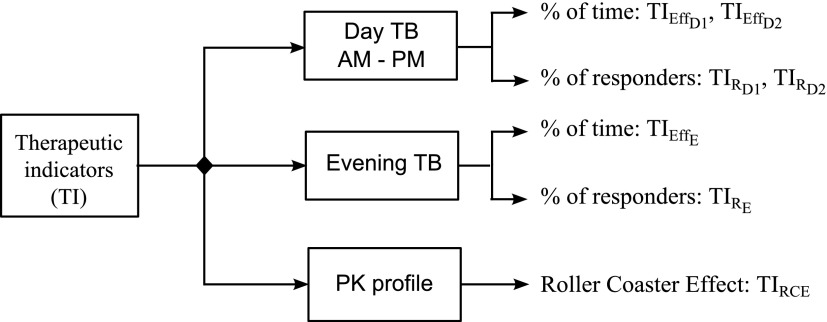
TIs used in this article are summarized in Figure 3.
FIG. 3.
TIs applied for the evaluation of a dosing regimen of MPH. TB: therapeutic box; PK: pharmacokinetics, 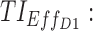 therapeutic indicator of effective time into day therapeutic box for AM period;
therapeutic indicator of effective time into day therapeutic box for AM period; 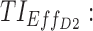 therapeutic indicator of effective time into day therapeutic box for PM period;
therapeutic indicator of effective time into day therapeutic box for PM period; 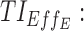 therapeutic indicator of effective time into evening therapeutic box;
therapeutic indicator of effective time into evening therapeutic box; 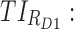 therapeutic indicator responders into day therapeutic box for AM period;
therapeutic indicator responders into day therapeutic box for AM period; 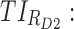 therapeutic indicator responders into day therapeutic box for PM period;
therapeutic indicator responders into day therapeutic box for PM period; 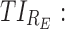 therapeutic indicator responders into evening therapeutic box;
therapeutic indicator responders into evening therapeutic box;  therapeutic indicator of roller coaster effect. All TIs are in percentage. MPH, methylphenidate; TIs, therapeutic indicators.
therapeutic indicator of roller coaster effect. All TIs are in percentage. MPH, methylphenidate; TIs, therapeutic indicators.
Selection of the best regimen
Based on the quantitative evaluation of a dosing regimen described above, we can exhaustively go through a set of potential drug regimens to find those that maximize or minimize the above TIs. In this context of ADHD therapy, 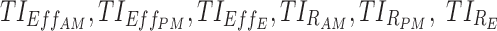 have to be maximized, while
have to be maximized, while 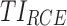 has to be minimized.
has to be minimized.
To select the best regimen, a mono-objective approach would target a particular TI by determining the regimen that maximizes (or minimizes) this TI, through testing all possible fractionated doses and dosing times. However, it is preferable to use a multiobjective approach that combines these mono-objectives of TIs by assigning different weights “left to the discretion of practitioners.” The performance of each dosing regimen is then given by Equation (7).
The mono-objective approach provides a best regimen for each TI. However, we cannot expect to have such a unique regimen fulfilling all mono-objectives at the same time.
The mono-objective approach provides a best regimen for each TI. However we cannot expect to have such a unique regimen fulfilling all mono-objectives at the same time. Thus the multiobjective approach to evaluate regimen performance is a more rational choice.
A web-based application
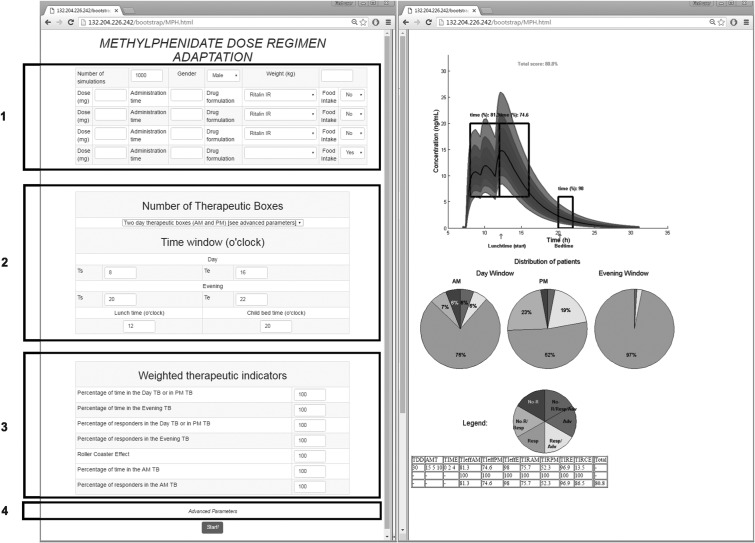
An exhaustive testing of possible combinations of doses and timings is time-consuming. Hence, we have developed a web-based application that requires less computational time and provides an educational tool for clinicians and parents to select and visualize the performance of dosing regimens. Screen captures of this web-interface are shown in Figure 4.
FIG. 4.
Screen capture of the web-based application. Left image includes blocks to be filled by the user: in block 1, patient covariates and the tested regimen (dose and time); in block 2, the time constraints; in block 3, weight values for different TIs; and in block 4, advanced parameters as the concentration ranges of therapeutic boxes and the desired threshold. Right image reports numerical and graphical representations of the performance of a given dose regimen. TB: therapeutic box; TDD: total daily dose; AMT: amount of doses (in mg); 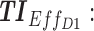 therapeutic indicator of effective time into day therapeutic box for AM period;
therapeutic indicator of effective time into day therapeutic box for AM period; 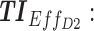 therapeutic indicator of effective time into day therapeutic box for PM period;
therapeutic indicator of effective time into day therapeutic box for PM period; 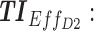 therapeutic indicator of effective time into evening therapeutic box;
therapeutic indicator of effective time into evening therapeutic box; 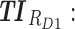 therapeutic indicator responders into day therapeutic box for AM period;
therapeutic indicator responders into day therapeutic box for AM period; 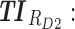 therapeutic indicator responders into day therapeutic box for PM period;
therapeutic indicator responders into day therapeutic box for PM period; 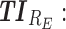 therapeutic indicator responders into evening therapeutic box;
therapeutic indicator responders into evening therapeutic box; 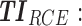 therapeutic indicator of roller coaster effect; All TIs are in percentage; Total is the performance of the associated regimen.
therapeutic indicator of roller coaster effect; All TIs are in percentage; Total is the performance of the associated regimen.
The left side of Figure 4 is the input part, which consists of four blocks: 1. Dosing regimen constraints; 2. Constraints modulated by child activities; 3. Weighting assignment; 4. Advanced parameters such as concentration ranges and effective time threshold. The right side of Figure 4 is the output part, which displays numerical and graphical performance results, as will be explained in Results section.
All algorithms are programmed in Matlab (MathWorks, version 2015b) and compiled into an executable file that can be implemented on any web server.
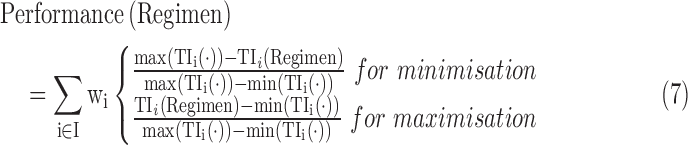 |
where 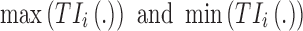 are the maximum and minimum of
are the maximum and minimum of 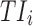 for all considered regimens,
for all considered regimens,  , R
, R and wi are weights with
and wi are weights with 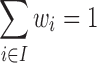 .
.
The prescriber can enter any combination of doses and times of administration, and fill the beginning and ending times of the TB, which gives the system the flexibility to adapt to any prescription and schedule. Moreover, values of TW can be adapted in advanced parameters since no well-established TW is defined. In addition, the clinician can individualize the performance indicators by assigning a specific weight for each TI n. For example, if a patient does not present sleep problems, the weight associated to the evening TIs can be decreased, so the importance of these indicators will be reduced in the evaluation of the performance of the regimen. At the opposite for a patient whose sleep is easily disturbed by stimulant, the clinician can increase the weight on the evening indicators. Changing the weight of the TI could also balance the relative importance of the morning or the afternoon, minimize and maximize a possible roller coaster effect.
Results
Numerical results and graphical representations are used to show the performance of the obtained optimal regimens for different types of prescriptions such as BID and TID. In the following section, we will illustrate the developed approach through two avenues: finding the best regimen by fractioning different TDDs, given daily patient constraints, and improving the performance of a regimen by changing daily dose and time schedule.
Fractioning of a given TDD
To find the best regimens by fractioning a given TDD and illustrate their performance, we consider a typical example of a 7½-year-old boy weighing 26 kg, for whom the TDD could range from 5 to 40 mg. Here is his daily routine: he wakes up at 7:00 am and starts school at 9:00 am. At 12:00 pm, he goes back home for lunch and is back to class at 1:00 pm. School is over at 3:00 pm. He goes to the homework club until 3:30 pm. Then, he gets home by 4:00 pm and then plays for an hour. Supper is at 6:00 pm and his bedtime routine starts at 7:30 pm to get into bed at 8:00 pm. During this school/home work/transportation time (8:00 am–6:00 pm), the expected concentration should be between the minimum effective concentration 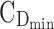 = 6 ng/mL and the maximum effective concentration
= 6 ng/mL and the maximum effective concentration 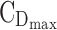 = 20 ng/mL. During the evening period (8:00–10:00 pm), the concentrations are expected to remain below
= 20 ng/mL. During the evening period (8:00–10:00 pm), the concentrations are expected to remain below 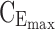 = 6 ng/mL, as depicted in Figure 1.
= 6 ng/mL, as depicted in Figure 1.
To determine the best regimen of BID and TID, we adopted an exhaustive search for all possible administration times and fragmented doses for each TDD (from 10 to 40 mg/day by 5 mg). Since the minimum available unit dose of IR MPH is 5 mg, all fragmented doses should be multiples of 5 mg. Moreover, since absorption of IR MPH is rapid and the time to the peak of blood concentration (tmax) is between 1 and 3 h (Quinn et al. 2007; Markowitz and Patrick 2008), hourly based administration times were chosen in the search process.
In this example, equal weights have been attributed to all seven TIs for the sake of simplicity. Equation (7) is used to evaluate and compare the performance between dosing regimens.
Numerical evaluation of regimen performance
The best BID and TID regimens found through the search process are reported in Table 3, along with their respective TI values and performance. As expected, the TID regimen shows higher TI values and a better overall performance compared to BID (81.5% vs. 73.3%). This finding complies with the usual practice of frequent, three to five daily administrations (Mendelkin 2013). However, if the tolerance threshold for the regimen's performance is relaxed to 70%, for example, then, BID could be a viable alternative, depending on patient and caregivers' preferences.
Table 3.
Best BID and TID Regimens with Their TIs Value and Performance
| BID | TID | |
|---|---|---|
| Dose (mg) | 20, 15 | 15, 5, 10 |
| Time (h) | 7:30, 11:30 | 7:30, 9:30, 12:30 |
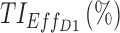 |
75.8 | 82.6 |
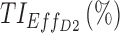 |
78.5 | 83 |
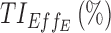 |
94.8 | 95.5 |
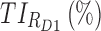 |
59.1 | 74.8 |
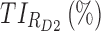 |
53.2 | 63.9 |
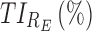 |
91.6 | 92.3 |
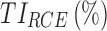 |
60.3 | 78.2 |
| Performance (%) | 73.3 | 81.5 |
TIs: therapeutic indicators; 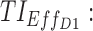 therapeutic indicator of effective time into day therapeutic box for AM period;
therapeutic indicator of effective time into day therapeutic box for AM period; 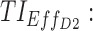 therapeutic indicator of effective time into day therapeutic box for PM period;
therapeutic indicator of effective time into day therapeutic box for PM period; 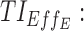 therapeutic indicator of effective time into evening therapeutic box;
therapeutic indicator of effective time into evening therapeutic box; 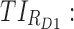 therapeutic indicator responders into day therapeutic box for AM period;
therapeutic indicator responders into day therapeutic box for AM period; 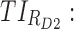 therapeutic indicator responders into day therapeutic box for PM period;
therapeutic indicator responders into day therapeutic box for PM period; 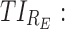 therapeutic indicator responders into evening therapeutic box;
therapeutic indicator responders into evening therapeutic box; 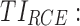 therapeutic indicator of roller coaster effect. All TIs are in percentage.
therapeutic indicator of roller coaster effect. All TIs are in percentage.
Graphical representation of regimen performance
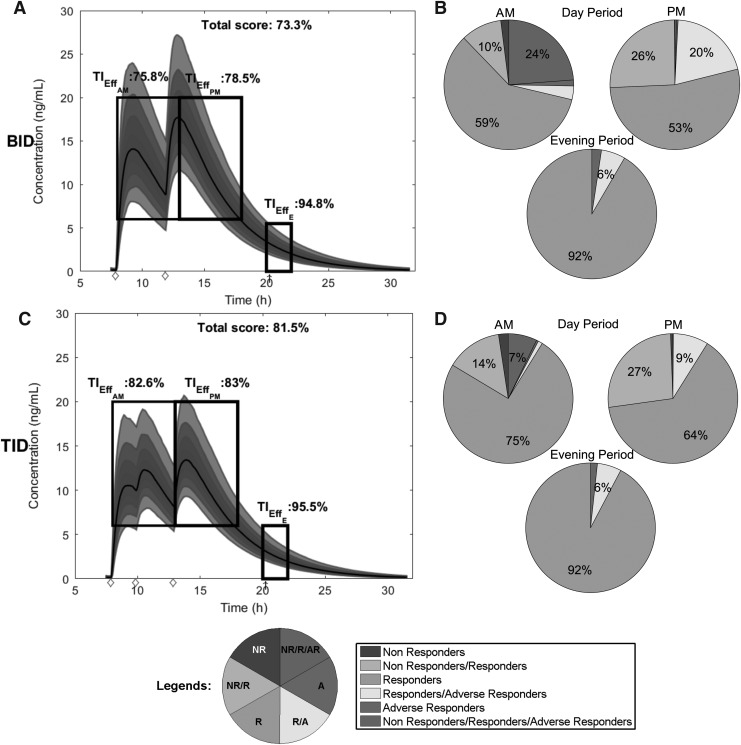
We can also graphically visualize the performance of the dosing regimens using the web-based application. In Figure 5, we reproduce graphical representations of the simulated PK profiles for both regimens and their corresponding TIs. The simulated PK profiles are reported in the left column of Figure 5, where their distribution ranges from the lower 10% to the upper 90%, along with their median PK profiles in solid black lines. TBs (TBD1, TBD2, and TBE) represented by black outlined rectangles, are also inscribed within time–concentration spaces to indicate their content in terms of therapeutic concentrations. The values of three time-based TIs, 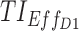 ,
, 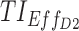 , and
, and 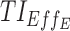 are reported over their corresponding TBs. We can observe that, for these best regimens, their predicted median concentrations are entirely located in TBD1, TBD2, and TBE. The total score of each regimen's performance is given.
are reported over their corresponding TBs. We can observe that, for these best regimens, their predicted median concentrations are entirely located in TBD1, TBD2, and TBE. The total score of each regimen's performance is given.
FIG. 5.
Graphical representation of time- and concentration-based TIs for the optimized BID and TID regimens. Distribution of MPH concentrations at steady state during a 24-hour time interval for a 26 kg child for the best BID and TID, respectively in (A, C). The middle line represents the median of concentrations. The TB for AM and PM is indicated with the first and the second black box with both 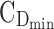 = 6 ng/mL and
= 6 ng/mL and 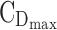 = 20 ng/mL and [8:00 am, 1:00 pm] or [1:00 pm, 6:00 pm], respectively. The evening TB is defined by the third black box with
= 20 ng/mL and [8:00 am, 1:00 pm] or [1:00 pm, 6:00 pm], respectively. The evening TB is defined by the third black box with 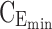 = 0 ng/mL and below
= 0 ng/mL and below 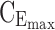 = 6 ng/mL between 8:00 pm and 10:00 pm. Black arrow and diamonds represent the bedtime and administration times, respectively. Probabilities of PK profiles during school and evening periods belonging to six therapeutic categories were represented for BID and TID, respectively in (B, D).
= 6 ng/mL between 8:00 pm and 10:00 pm. Black arrow and diamonds represent the bedtime and administration times, respectively. Probabilities of PK profiles during school and evening periods belonging to six therapeutic categories were represented for BID and TID, respectively in (B, D). 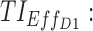 therapeutic indicator of effective time into day therapeutic box for AM period;
therapeutic indicator of effective time into day therapeutic box for AM period; 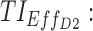 therapeutic indicator of effective time into day therapeutic box for PM period;
therapeutic indicator of effective time into day therapeutic box for PM period; 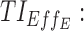 therapeutic indicator of effective time into evening therapeutic box. All TIs are in percentage; BID, bis in die; MPH, methylphenidate; PK, pharmacokinetic; TB, therapeutic box; TIs, therapeutic indicators; TID, ter in die.
therapeutic indicator of effective time into evening therapeutic box. All TIs are in percentage; BID, bis in die; MPH, methylphenidate; PK, pharmacokinetic; TB, therapeutic box; TIs, therapeutic indicators; TID, ter in die.
The percentage of the six concentration-based TIs of a drug regimen is represented by pie charts in the right column of Figure 5, for day and evening periods. This graphical representation is used for its easy interpretation by clinicians. The global proportion of the profiles, which belong to categories A or R/A, provides a clinically useful indicator (here, for the evening period, 8.4% for BID and 7.7% for TID, corresponding to yellow and red portions of the pie charts added together).
Comparison with MTA study
To evaluate our approach, we compared the optimal regimen with the regimen used in the NIMH Collaborative Multisite Multimodal Treatment study of Children with ADHD (MTA study) (Greenhill et al. 1996), a landmark study in the field. In the MTA study, the first dose was given between 7:00 and 8:00 am, the “noon” dose was given between 11:00 am and 12:00 pm, and the last dose was given between 3:00 and 4:00 pm. The first two doses were the same, but the last dose was sculpted (low dose: 5 mg TID for a total dose of 15 mg, intermediate dose: 10, 10, and 5 mg for a total dose of 25 mg, and high dose: 20, 20, and 10 mg for children weighing >25 kg; or 15, 15, and 5 mg for children with smaller weight). As in the example above, a 26 kg child is selected, which allowed considering a TDD of up to 50 mg, with 20, 20, and 10 mg partition.
Since the administration times were not optimized, all eight possible administration time schedules were tested for 1000 virtual children weighing 26 kg, as mentioned in Table 4.
Table 4.
Comparison of the TIs (±SD) and the Performance (±SD) Between Optimal TID Regimen (Last Regimen) and Regimens Defined in MTA Study for 1000 Patients with 1000 Iterations
| Doses (mg) | Times (h) |
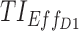 (%) (%)
|
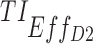 (%) (%)
|
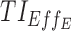 (%) (%)
|
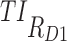 (%) (%)
|
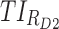 (%) (%)
|
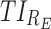 (%) (%)
|
Performance (%) |
|---|---|---|---|---|---|---|---|---|
| 5, 5, 5 | 7, 11, 14 | 13.9 (±0.7) | 35.2 (±1.2) | 98.3 (±0.3) | 3.2 (±0.6) | 21.1 (±1.3) | 97.3 (±0.5) | 44.8 (±0.5) |
| [7,8], [11,12], [15,16] | [6.6 ± 0.6: 16.7 ± 0.7] | [18.8 ± 0.9, 37.3 ± 1.3] | [91.8 ± 0.7, 96.8 ± 0.5] | [1 ± 0.3, 3.4 ± 0.6] | [5.5 ± 0.7, 25.2 ± 1.4] | [87.6 ± 1, 95 ± 0.7] | [37.2 ± 0.2, 43.3 ± 0.4] | |
| 10, 10, 5 | 7, 11, 14 | 58.1 (±1.1) | 76.6 (±0.9) | 96.3 (±0.5) | 35.1 (±1.5) | 61.1 (±1.6) | 93.8 (±0.7) | 70.2 (±0.7) |
| [7,8], [11,12], [15,16] | [44.1 ± 1.1, 58.5 ± 1] | [60.8 ± 1.1, 81.7 ± 0.9] | [82.3 ± 0.9, 93.8 ± 0.6] | [21 ± 1.3, 35.5 ± 1.5] | [39.9 ± 1.6, 70.9 ± 1.5] | [72.5 ± 1.4, 89.7 ± 0.9] | [58.3 ± 0.6, 67.3 ± 0.8] | |
| 15, 15, 5 | 7, 11, 14 | 75.5 (±0.9) | 83.8 (±0.8) | 87.9 (±0.9) | 53.6 (±1.6) | 72.2 (±1.5) | 81.3 (±1.2) | 75.7 (±0.9) |
| [7,8], [11,12], [15,16] | [66.1 ± 0.7, 75.7 ± 0.9) | [78 ± 0.9, 84.1 ± 0.9] | [64.3 ± 1.2, 83.4 ± 0.9] | [33.4 ± 1.5, 53.9 ± 1.6] | [60.4 ± 1.6, 73.2 ± 1.4] | [49.8 ± 1.6, 74.6 ± 1.3] | [62.1 ± 0.8, 73.7 ± 0.9] | |
| 20, 20, 10 | 7, 11, 14 | 71.9 (±0.9) | 68.1 (±1.1) | 55 (±1.3) | 47.1 (±1.5) | 52.2 (±1.6) | 39.5 (±1.6) | 55 (±1.1) |
| [7,8], [11,12], [15,16] | [59.4 ± 0.8, 76.4 ± 0.8] | [60.2 ± 1.2, 74.9 ± 0.9] | [20 ± 1, 42.8 ± 1.3] | [24.8 ± 1.4, 56.5 ± 1.6] | [44.1 ± 1.5, 58.1 ± 1.6] | [8.7 ± 0.9, 26.8 ± 1.4] | [41.3 ± 0.8, 53.3 ± 1] | |
| 20, 10, 5 | 7,11,14 | 81.3 (±0.8) | 90.5 (±0.5) | 94.9 (±0.5) | 66.5 (±1.5) | 83.1 (±1.2) | 91 (±0.9) | 84.6 (±0.6) |
In italic, performance below 60%; In bold, performance between 60 and 80%; Underlined, performance ≥80%.
TIs: therapeutic indicators; SD, standard deviation; 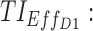 therapeutic indicator of effective time into day therapeutic box for AM period;
therapeutic indicator of effective time into day therapeutic box for AM period; 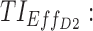 therapeutic indicator of effective time into day therapeutic box for PM period;
therapeutic indicator of effective time into day therapeutic box for PM period; 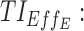 therapeutic indicator of effective time into evening therapeutic box;
therapeutic indicator of effective time into evening therapeutic box; 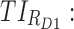 therapeutic indicator responders into day therapeutic box for AM period;
therapeutic indicator responders into day therapeutic box for AM period; 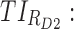 therapeutic indicator responders into day therapeutic box for PM period;
therapeutic indicator responders into day therapeutic box for PM period; 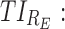 therapeutic indicator responders into evening therapeutic box;
therapeutic indicator responders into evening therapeutic box; 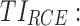 therapeutic indicator of roller coaster effect; all TIs are in percentage; [xmin ± SDxmin, xmax ± SDxmax] correspond to the minimum value with its SD and maximum value with its SD of TI of MTA regimens, respectively.
therapeutic indicator of roller coaster effect; all TIs are in percentage; [xmin ± SDxmin, xmax ± SDxmax] correspond to the minimum value with its SD and maximum value with its SD of TI of MTA regimens, respectively.
Using these administration times coupled with the above dose partitions, the average regimens' performances and their respective standard deviations are calculated with 1000 repetitions of 1000 children and summarized in Table 4.
The best regimen is for a TDD of 35 mg, divided into three doses of 20, 10, and 5 mg taken at 7:00 am, 11:00 am, and 2:00 pm. With regard to the total dose, the TIs show an inverse U curve, as the indicators deteriorate for a low dose of 15 mg and a high dose of 50 mg. However, it is known that among the responders to MPH, about 22% need a low dose (15 mg/day), 25% a moderate dose (16–34 mg/day), and 30% a high dose (35 mg/day or greater) for an optimal therapeutic response (Greenhill et al. 2001). A correction factor should be introduced as a fixed TW does not take in account this difference in dose–response.
So the most valid comparison is between the same dose of 35 mg, but with different times of administration in our optimization algorithm and in the expert-based MTA schedule. To compare them thoroughly, we computed both time-based and concentration-based indicators for three time periods by dividing the day into morning and afternoon periods, in addition to the evening period. Moreover, the performance of the obtained optimal regimen is statistically higher compared with the MTA regimen (unilateral paired t-test for the difference between performance of means, mdiff, using the t-test function in R, 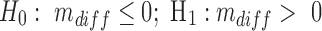 , with α = 1%). Based on a simulated dataset of 1000 patients as mentioned above, this gives
, with α = 1%). Based on a simulated dataset of 1000 patients as mentioned above, this gives 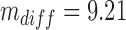 ,
, 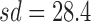 , test-value = 12.6, df = 999, and p-value = 3 × 10−34. This test was repeated 1000 times, with 1000 simulated patients each time, and the same conclusions for the hypothesis test were obtained. Clinically, the optimal and MTA regimens can be considered having the same value for
, test-value = 12.6, df = 999, and p-value = 3 × 10−34. This test was repeated 1000 times, with 1000 simulated patients each time, and the same conclusions for the hypothesis test were obtained. Clinically, the optimal and MTA regimens can be considered having the same value for 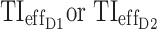 (5.6% or 6.4%, respectively, Table 4). However, a higher difference of >15% is obtained for the evening, with
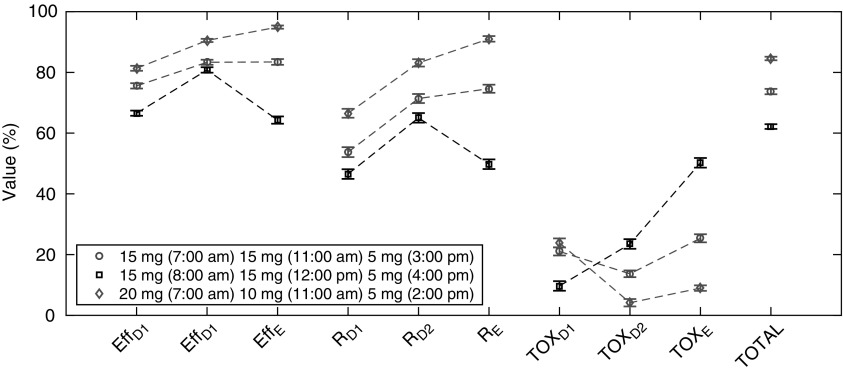
(5.6% or 6.4%, respectively, Table 4). However, a higher difference of >15% is obtained for the evening, with 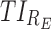 = 91% vs. 74.6% for the optimal and MTA regimens, respectively. All this gives rise to a difference of 10.9% in the global performance (84.6% vs. 73.7%). Thus, the optimal regimen should be preferred, especially if the evening period is given a higher priority. Until now, we retained only the proportion of responders. However, with the additional consideration of adverse-responders, the optimal regimen is consistently superior to the MTA regimen in terms of all TIs, Figure 6, with the only exception of a slightly higher proportion of adverse-responders in the morning, compared to the 15 mg MTA dose at 7:00 am.
= 91% vs. 74.6% for the optimal and MTA regimens, respectively. All this gives rise to a difference of 10.9% in the global performance (84.6% vs. 73.7%). Thus, the optimal regimen should be preferred, especially if the evening period is given a higher priority. Until now, we retained only the proportion of responders. However, with the additional consideration of adverse-responders, the optimal regimen is consistently superior to the MTA regimen in terms of all TIs, Figure 6, with the only exception of a slightly higher proportion of adverse-responders in the morning, compared to the 15 mg MTA dose at 7:00 am.
FIG. 6.
Values of each TI (the first six) with their standard deviation, with the addition of associated toxicity values and the performance (TOTAL) of three regimens: 15 mg (7:00 am), 15 mg (11:00 am), and 5 mg (3:00 pm); 15 mg (8:00 am), 15 mg (12:00 pm), and 5 mg (4:00 pm); 20 mg (7:00 am), 10 mg (11:00 am), and 5 mg (2:00 pm). Weight for each TI is the same. 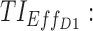 therapeutic indicator of effective time into day therapeutic box for AM period;
therapeutic indicator of effective time into day therapeutic box for AM period; 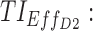 therapeutic indicator of effective time into day therapeutic box for PM period;
therapeutic indicator of effective time into day therapeutic box for PM period; 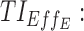 therapeutic indicator of effective time into evening therapeutic box;
therapeutic indicator of effective time into evening therapeutic box; 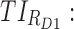 therapeutic indicator responders into day therapeutic box for AM period;
therapeutic indicator responders into day therapeutic box for AM period; 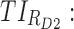 therapeutic indicator responders into day therapeutic box for PM period;
therapeutic indicator responders into day therapeutic box for PM period; 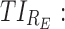 therapeutic indicator responders into evening therapeutic box; All TIs are in percentage; TOXD1: probability for patient to have experienced toxicity into day therapeutic box for AM period; TOXD2: probability for patient to have experienced toxicity into day therapeutic box for PM period; TOXE: probability for patient to have experienced toxicity into evening therapeutic box; TOTAL: performance of tested regimens using the six TIs.
therapeutic indicator responders into evening therapeutic box; All TIs are in percentage; TOXD1: probability for patient to have experienced toxicity into day therapeutic box for AM period; TOXD2: probability for patient to have experienced toxicity into day therapeutic box for PM period; TOXE: probability for patient to have experienced toxicity into evening therapeutic box; TOTAL: performance of tested regimens using the six TIs.
Application to the daily practice: changing dose and time schedule
It is possible to improve the MPH therapeutic outcomes through a rational choice of drug regimens using our approach. A clinician could test some deviation from an established regimen, such as the MTA, to assess the benefit of changing the dose, and/or timing. The clinician could test step by step a change in the dosage or time of administration and assess the impact of the change.
The advantage of this approach is to be highly flexible and adapt to any schedule of the daily routine of a specific child. Moreover, it is also possible to put some constraint on the PK profile, in addition to defining individual TB. For example, the clinician could test the impact of minimizing the peaks and troughs on the TIs for a child with a clear RCE. Clearly, the complexity of this regimen adjustment using multiple TIs is not reliably feasible based either exclusively on experience or the PK parameters provided in the information sheet.
Discussion
With the objective to select a dosing regimen that takes the best benefit from MPH and accommodates for the daily activities of children with ADHD, we proposed a computational strategy to evaluate and visualize the steady-state MPH PK profiles when different doses and time schedules are selected. Taking into account the PK variability, we developed a simulation-based algorithm by adapting several TIs that reflect various aspects of drug disposition in relationship to the TW and key day and evening periods. The relative weight of these TIs is selected by the clinician based on an individual knowledge of the child's daily life. Being concerned with the translational value of our approach, we have materialized it into a web-based application, which is also patient-friendly, and can easily be used by clinicians in their routine practice as well as patients and their families for a better understanding and control of their situations.
The rational for the choice of a range 6–20 ng/mL for TW has been explained above. The recommended target MPH drug concentration range is between 13 and 22 ng/mL (with a laboratory alert for concentration above 44 ng/mL) in the Arbeitsgemeinschaft für Neuro-psychopharmakologie und Pharmakopsychiatrie (AGNP) consensus guidelines for therapeutic drug monitoring in psychiatry (Hiemke et al. 2011). However, this recommended target TW is likely too high because the plasma level of MPH was found under the limit of 13 ng/mL in 65% of the subjects treated with the recommended doses of oral release osmotic system (OROS) MPH (Yorbik et al. 2015). In addition, this TW has to be adapted according to the interindividual differences found in pharmacodynamic studies. MPH responders can be divided in roughly equal proportions between those needing a low, an intermediate, or a high dose of MPH for an optimal therapeutic response (Greenhill et al. 2001). It is not known whether these differences in optimal doses are related to differences in the plasma concentration required to block 75% of the dopamine or norepinephrine transporter, which would necessitate moving the TW along the concentration axis, or to differences in presystemic first-pass metabolism, and/or a rapid rate of elimination, which would necessitate the addition of a clearance component in our model. The maximal value of 6 ng/mL for the evening TB is somewhat arbitrary. It might be increased in some individuals who could tolerate higher doses without sleep problems. However, the optimal MPH dosage is relatively stable within individuals. The titration-determined dose and end-of-maintenance dose were significantly correlated (0.52–0.68) in the MTA study (Vitiello et al. 2001). Once the optimal total dose is known through the initial titration process, and the TW adapted, our approach could be safely used to adapt the medication for the specific needs of this individual. This approach provides not only a clinical but also an educational tool, as the clinician can test in silico changes in prescription and have a first glimpse of what he can expect before trying any changes with the patient. It may also be an educational tool for the parents/patients as the concept of a target TW can be grasped intuitively.
This proof of concept study focused on IR MPH. However, IR MPH is rarely used alone in contemporary treatment in North America, but rather in combination with long-acting MPH. Moreover, in developing countries, foreign brand or generic forms of long-acting medications may not be distributed, not be covered by insurance, or their prices may be beyond the purchasing power of most families. Some brand forms of IR MPH manufactured locally are then the only available formulation (Khodadust et al. 2012). Incorporating long-acting formulations into the model will be the very important next step. Various modified-release MPH products include different percentages of IR MPH, for example, Concerta® with 22% of the total dose, compared to 50% for Biphentin®. With regard to the long-acting components, specific modified-release mechanisms such as Spheroidal Oral Drug Absorption System (SODAS®), Osmotic [Controlled] Release Oral [Delivery] System (OROS), or other formulations result in different plasma concentration profiles that can be modeled using the Pop-PK approach. By using the algorithm developed for IR MPH, we will be able to estimate the performance of any combination of long- and short-acting products, whatever the strength and the time of administration, while taking in account the specifics that each child needs.
Moreover, long-acting medications have been explicitly designed to mimic regimens of administration of short-acting medication defined by experts. The objective was to obtain the same PK profile, but without having the child taking medication in school setting, which is inconvenient and potentially stigmatizing. However, we have showed that these expert-based regimens may not always be the optimal ones, and our approach could provide new targets for the design of future sustained released preparations.
Conclusion
In the current work, we proposed a web-based computational platform to help in choosing dosing regimens of immediate release formulations of methylphenidate, while being subjected to daily activities. The adopted approach could be used as proof of concept to further implement combination of short and long-acting methylphenidate.
Clinical Significance
We acknowledge that the benefit of using our approach in a real-life clinical setting has yet to be demonstrated. However, given the long-running series of controversies erupted over children's treatment with stimulants, it seems imperative to use this medication as safely as possible, which means using an effective dose, while avoiding exposing children to unnecessary high dose of medication in a long-term treatment. Mathematical modeling can greatly facilitate an otherwise largely empirical approach to MPH dosing individualization.
Acknowledgments
Data were kindly provided by Pharmascience, Inc. The authors acknowledge the financial support of The Natural Sciences and Engineering Research Council of Canada (NSERC)-Industrial Chair in Pharmacometrics, jointly supported by Novartis, Pfizer, and InVentiv Health, as well as Fonds de recherche du Québec—Nature et technologies (FRQNT) and NSERC. National Natural Science Foundation of China (NSFC: No. 11501358) is also acknowledged.
Disclosures
No competing financial interests exist.
References
- Beal S, Sheiner LB, Boeckmann A, Bauer RJ: NONMEM User's Guides (1989–2009). Icon Development Solutions, Ellicott City, MD, 2009 [Google Scholar]
- Bonnefois G, Barrière O, Nekka F, Li J: A computational strategy for dose adaptation at the population and group levels. IOSR J Pharm Biol Sci 10:52–63, 2015 [Google Scholar]
- Costa A, Riedel M, Pogarell O, Menzel-Zelnitschek F, Schwarz M, Reiser M, Moller HJ, Rubia K, Meindl T, Ettinger U: Methylphenidate effects on neural activity during response inhibition in healthy humans. Cereb Cortex 23:1179–1189, 2013 [DOI] [PubMed] [Google Scholar]
- Childress A, Newcorn J, Stark JG, McMahen R, Tengler M, Sikes C: A single-dose, single-period pharmacokinetic assessment of an extended-release orally disintegrating tablet of methylphenidate in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 26:505–512, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield CF, Dorsey ER, Zhu S, Huskamp HA, Conti R, Dusetzina SB, Higashi A, Perrin JM, Kornfield R, Alexander GC: Trends in attention deficit hyperactivity disorder ambulatory diagnosis and medical treatment in the United States, 2000–2010. Acad Pediatr 12:110–116, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenhill LL, Abikoff HB, Arnold LE, Cantwell DP, Conners CK, Elliott G, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, March JS, Newcorn J, Pelham WE, Severe JB, Swanson JM, Vitiello B, Wells K: Medication treatment strategies in the MTA Study: Relevance to clinicians and researchers. J Am Acad Child Adolesc Psychiatry 35:1304–1313, 1996 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Pliszka S, Dulcan MK, Bernet W, Arnold V, Beitchman J, Benson RS, Bukstein O, Kinlan J, McClellan J, Rue D, Shaw JA, Stock S; American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 41:26S–49S, 2002 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, Arnold LE, Abikoff HB, Bukstein OG, Conners CK, Elliott GR, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Newcorn JH, Severe JB, Wells K, Wigal T: Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry 40:180–187, 2001 [DOI] [PubMed] [Google Scholar]
- Hiemke C, Baumann P, Bergemann N, Conca A, Dietmaier O, Egberts K, Fric M, Gerlach M, Greiner C, Grunder G, Haen E, Havemann-Reinecke U, Jaquenoud Sirot E, Kirchherr H, Laux G, Lutz UC, Messer T, Muller MJ, Pfuhlmann B, Rambeck B, Riederer P, Schoppek B, Stingl J, Uhr M, Ulrich S, Waschgler R, Zernig G: AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: Update 2011. Pharmacopsychiatry 44:195–235, 2011 [DOI] [PubMed] [Google Scholar]
- Khodadust N, Jalali A, Ahmadzad-Asl M, Khademol N, Shirazi E: Comparison of two brands of methylphenidate (Stimdate® vs. Ritalin®) in children and adolescents with attention deficit hyperactivity disorder: A double-blind, randomized clinical trial. Iran J Psychiatry Behav Sci 6:26–32, 2012 [PMC free article] [PubMed] [Google Scholar]
- Kimko HC, Cross JT, Abernethy DR: Pharmacokinetics and clinical effectiveness of methylphenidate. Clin Pharmacokinet 37:457–470, 1999 [DOI] [PubMed] [Google Scholar]
- Kimko HC, Gibianski E, Gibianski L, Starr HL, Berwaerts J, Massarella J, Wiegand F: Population pharmacodynamic modeling of various extendedrelease formulations of methylphenidate in children with attention deficit hyperactivity disorder via meta-analysis. J Pharmacokinet Pharmacodyn 39:161–176, 2012 [DOI] [PubMed] [Google Scholar]
- Markowitz JS, Patrick KS: Differential pharmacokinetics and pharmacodynamics of methylphenidate enantiomers. Does chirality matter? J Clin Psychopharmacol 28:S54–S61, 2008 [DOI] [PubMed] [Google Scholar]
- McCracken JT, Biederman J, Greenhill LL, Swanson JM, McGough JJ, Spencer TJ, Posner K, Wigal S, Pataki C, Zhang Y, Tulloch S: Analog classroom assessment of a once-daily mixed amphetamine formulation, SLI381 (Adderall XR), in children with ADHD. J Am Acad Child Adolesc Psychiatry 42:673–683, 2003 [DOI] [PubMed] [Google Scholar]
- Mendelkin T: A Physician's Perspective. Int J Interdiscip Educ 1, 2013 [Google Scholar]
- Pharmacometrics FDA: Annual Report 2010. 2011. Available at www.fda.gov/downloads/aboutfda/centersoffices/cder/ucm248099.pdf [Accessed December10, 2014]
- Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA: The worldwide prevalence of ADHD: A systematic review and metaregression analysis. Am J Psychiatry 164:942–948, 2007 [DOI] [PubMed] [Google Scholar]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA: ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol 43:434–442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn D, Bode T, Reiz JL, Donnelly GAE, Darke AC: Single-dose pharmacokinetics of multilayer-release methylphenidate and immediate-release methylphenidate in children with attention-deficit/hyperactivity disorder. J Clin Pharmacol 47:760–766, 2007 [DOI] [PubMed] [Google Scholar]
- Santisteban JA, Stein MA, Bergmame L, Gruber R: Effect of extended-release dexmethylphenidate and mixed amphetamine salts on sleep: A double-blind, randomized, crossover study in youth with attention-deficit hyperactivity disorder. CNS Drugs 28:825–833, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheiner LB, Beal S, Rosenberg B, Marathe VV: Forecasting individual pharmacokinetics. Clin Pharmacol Ther 26:294–305, 1979 [DOI] [PubMed] [Google Scholar]
- Simon V, Czobor P, Balint S, Meszaros A, Bitter I: Prevalence and correlates of adult attention-deficit hyperactivity disorder: Meta-analysis. Br J Psychiatry 194:204–211, 2009 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Ciccone PE, Madras BK, Dougherty DD, Bonab AA, Livni E, Parasrampuria DA, Fischman AJ: PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry 163:387–395, 2006 [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Bonab AA, Dougherty DD, Mirto T, Martin J, Clarke A, Fischman AJ: Understanding the central pharmacokinetics of spheroidal oral drug absorption system (SODAS) dexmethylphenidate: A positron emission tomography study of dopamine transporter receptor occupancy measured with C-11 altropane. J Clin Psychiatry 73:346–352, 2012 [DOI] [PubMed] [Google Scholar]
- Stein MA, Blondis TA, Schnitzler ER, O'Brien T, Fishkin J, Blackwell B, Szumowski E, Roizen NJ: Methylphenidate dosing: Twice daily versus three times daily. Pediatrics 98:748–756, 1996 [PubMed] [Google Scholar]
- Swanson JM, Hechtman L: Using long-acting stimulants: Does it change ADHD treatment outcome? Can Child Adolesc Psychiatr Rev 14:2–3, 2005 [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Polcari A, Foley M, Valente E, McGreenery CE, Chang WW, McKay G, Midha KK: Methylphenidate blood levels and therapeutic response in children with attention-deficit hyperactivity disorder: I. Effects of different dosing regimens. J Child Adolesc Psychopharmacol 16:416–431, 2006 [DOI] [PubMed] [Google Scholar]
- Thompson E, Ni Bhrolchain C: The epidemiology of community paediatric outpatient referrals 2006. Child Care Health Dev 39:50–54, 2013 [DOI] [PubMed] [Google Scholar]
- Vitiello B, Severe JB, Greenhill LL, Arnold LE, Abikoff HB, Bukstein OG, Elliott GR, Hechtman L, Jensen PS, Hinshaw SP, March JS, Newcorn JH, Swanson JM, Cantwell DP: Methylphenidate dosage for children with ADHD over time under controlled conditions: Lessons from the MTA. J Am Acad Child Adolesc Psychiatry 40:188–196, 2001 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE: Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature 386:827–830, 1997 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Gatley SJ, Logan J, Ding YS, Hitzemann R, Pappas N: Dopamine transporter occupancies in the human brain induced by therapeutic doses of oral methylphenidate. Am J Psychiatry 155:1325–1331, 1998 [DOI] [PubMed] [Google Scholar]
- Wigal SB, Gupta S, Heverin E, Starr HL: Pharmacokinetics and therapeutic effect of OROS methylphenidate under different breakfast conditions in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 21:255–263, 2011 [DOI] [PubMed] [Google Scholar]
- Yorbik O, Mutlu C, Ozilhan S, Eryilmaz G, Isiten N, Alparslan S, Saglam E: Plasma methylphenidate levels in youths with attention deficit hyperactivity disorder treated with OROS formulation. Ther Drug Monit 37:347–352, 2015 [DOI] [PubMed] [Google Scholar]
- Zelnik N, Terkel-Dawer R: The clinical profile of children with ADHD that require OROS-methylphenidate combined with shorter-acting formulations. Atten Defic Hyperact Disord 7:313–318, 2015 [DOI] [PubMed] [Google Scholar]