Abstract
Background
The feasibility of single-port video-assisted thoracic surgery (SPVATS) for primary lung cancer is not well understood. In this study, we compared SP and multi-port (MP) VATS for the surgical treatment of patients with primary lung cancer.
Methods
Surgical treatment was performed in 181 patients with primary lung cancer at Inje University Haeundae Paik Hospital between June 2012 and December 2015. A propensity-matched analysis was used to compare the postoperative outcomes and to evaluate the comparative feasibility and safety of SPVATS and MPVATS.
Results
There were 37 patients in the SPVATS group and 67 patients in the MPVATS group. Propensity matching produced 32 pairs. The operation time (210 minutes versus 200 minutes, p=0.11), volume of the estimated blood loss (170 mL versus 160 mL, p=0.19), duration of chest tube drainage (5 days versus 6 days, p=0.66), and length of hospital stay (9 days versus 10 days, p=0.89) were similar between the 2 groups.
Conclusion
In our study, SPVATS for primary lung cancer was safe and feasible in well selected patients. A prospective, randomized study with a large group and long-term follow-up is necessary to evaluate the clinical feasibility and the advantages of SPVATS for primary lung cancer.
Keywords: Video-assisted thoracic surgery, Lung neoplasms, Lobectomy
Introduction
Recently, video-assisted thoracic surgery (VATS) has been accepted as a feasible, safe, and effective approach for the treatment of primary lung cancer [1,2]. Most surgeons perform VATS for primary lung cancer with 3 or more incisions. With the evolution of the VATS technique, single-port (SP) VATS for primary lung cancer has been performed, and it has been reported to have advantages including less postoperative pain, less paresthesia, and favorable cosmetic results because only 1 intercostal space is involved [3–6]. However, other surgeons have expressed some concerns about the safety and feasibility of SPVATS because of its technical difficulties.
We have performed SPVATS and multi-port (MP) VATS for primary lung cancer. The objective of this study was to compare the safety and feasibility of SPVATS and MPVATS for primary lung cancer using a propensity-matched analysis.
Methods
Surgical treatment for primary lung cancer was performed in 181 patients at Inje University Haeundae Paik Hospital between June 2012 and December 2015. We reviewed the medical records of these patients retrospectively. Our indications for VATS to treat primary lung cancer were as follows: clinical stage I/II disease with a peripherally located tumor (less than 7 cm in size) and clinical T1/2 and clinical single-station N2 disease with a peripherally located tumor. However, patients with a history of ipsilateral pulmonary or pleural disease (e.g., pleurisy, pulmonary tuberculosis, or interstitial lung disease) and those with a history of ipsilateral thoracic surgery or neoadjuvant radiotherapy were excluded from the candidate set for SPVATS.
SPVATS was attempted in 45 patients with primary lung cancer. Among these patients, 37 patients underwent SPVATS lobectomy and systematic lymph node dissection (SLND) through a single incision measuring 4–5 cm (Fig. 1). In the others, SPVATS was converted into an open thoracotomy (n=2) or MPVATS (n=6) for various reasons such as severe pleural adhesions, technical difficulties, or major bleeding. MPVATS was performed in 67 patients with primary lung cancer. The patient characteristics are presented in Table 1.
Fig. 1.
Skin incision 1 month after the operation (arrow).
Table 1.
Patient characteristics
| Characteristic | All patients | Propensity-matched patients | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SP (n=37) | MP (n=67) | p-value | SP (n=32) | MP (n=32) | p-value | |
| Age (yr) | 66 (40–78) | 64 (40–82) | 0.56 | 66 (40–77) | 64 (46–82) | 0.75 |
|
| ||||||
| Gender | 0.13 | 0.43 | ||||
|
| ||||||
| Male | 26 (70.3) | 37 (55.2) | 22 (68.8) | 19 (59.4) | ||
|
| ||||||
| Female | 11 (29.7) | 30 (44.8) | 10 (31.3) | 13 (40.6) | ||
|
| ||||||
| Body mass index (kg/m2) | 23.4 (17.6–31.7) | 24.7 (15.5–36.6) | 0.20 | 23.5 (17.7–31.7) | 24.6 (15.5–33.7) | 0.61 |
|
| ||||||
| Smoking history | 0.17 | 0.38 | ||||
|
| ||||||
| Yes | 13 (35.1) | 16 (23.9) | 9 (28.1) | 6 (18.8) | ||
|
| ||||||
| No | 24 (64.9) | 51 (76.1) | 23 (71.9) | 26 (81.3) | ||
|
| ||||||
| Hypertension | 13 (35.1) | 24 (35.8) | 0.94 | 11 (34.4) | 12 (37.5) | 0.79 |
|
| ||||||
| Diabetes | 7 (18.9) | 11 (16.4) | 0.75 | 7 (21.9) | 5 (15.6) | 0.52 |
|
| ||||||
| Affected lobe | <0.01 | 0.47 | ||||
|
| ||||||
| Right | 14 (37.8) | 50 (74.6) | ||||
|
| ||||||
| Upper | 11 (29.7) | 15 (22.4) | 0.41 | 8 (25.0) | 9 (28.1) | 0.78 |
|
| ||||||
| Middle | 1 (2.7) | 13 (19.4) | 0.02 | 1 (3.1) | 3 (9.4) | 0.30 |
|
| ||||||
| Lower | 2 (5.4) | 22 (32.8) | 0.01 | 2 (6.3) | 5 (15.6) | 0.23 |
|
| ||||||
| Left | 23 (62.2) | 17 (25.4) | ||||
|
| ||||||
| Upper | 11 (29.7) | 11 (16.4) | 0.11 | 11 (34.4) | 9 (28.1) | 0.59 |
|
| ||||||
| Lower | 12 (32.5) | 6 (9.0) | <0.01 | 10 (31.3) | 6 (18.8) | 0.25 |
|
| ||||||
| Pulmonary function | ||||||
|
| ||||||
| Forced expiratory volume in 1 second (%) | 86.0 (61.0–131.0) | 84.5 (53.0–123.0) | 0.24 | 88.0 (66.0–131.0) | 87.5 (65.0–123.0) | 0.82 |
|
| ||||||
| Vital capacity (%) | 91.0 (73.0–124.0) | 90.0 (55.0–125.0) | 0.30 | 91.5 (73.0–124.0) | 91.5 (55.0–125.0) | 0.54 |
Values are presented as median (range) or number (%), unless otherwise stated.
SP, single-port; MP, multi-port.
All surgical procedures were performed by a single surgeon. Under general anesthesia, the patient was placed in a lateral decubitus position and one-lung ventilation was performed with a double-lumen endotracheal tube.
In SPVATS, an incision measuring 4–5 cm was made in the fifth or sixth intercostal space on the anterior axillary line. The soft tissue and intercostal muscles were retracted with an X-small wound retractor (Alexis; Applied Medical, Rancho Santa Margarita, CA, USA) to secure the intercostal space. In MPVATS, a 5-mm incision was made in the eighth intercostal space along the mid-axillary line to introduce a 5-mm thoracoscopic trocar. The second incision (4–5 cm) was made in the fifth or the sixth intercostal space along the anterior axillary line, and the third incision (1 cm) was made in the sixth intercostal space along the posterior axillary line just below the scapular tip.
A 5-mm, 30° thoracoscope (Karl Storz endoscope; Karl Storz, Tuttlingen, Germany) was used in all cases. Optimal surgical vision was achieved with pulmonary traction by using endoscopic ring forceps. The pulmonary vessels and bronchus were dissected with a 5-mm endoscopic grasper, spatula-shaped electrocautery, and a 5-mm endoscopic ultrasonic scalpel (Harmonic Ace; Ethicon Endo-Surgery, Cincinnati, OH, USA). The pulmonary vessels were divided with flexible linear staplers or Hem-o-Lok clips (Weck Surgical Instruments; Teleflex Medical, Durham, NC, USA). In patients with incomplete fissures, the fissures were divided with endoscopic linear staplers and an ultrasonic scalpel. After the lobectomy, the resected lobes were removed from the thoracic cavity using a protective bag. SLND was performed with the ultrasonic scalpel. The lymph nodes, including perinodal tissue, were removed. At the end of the procedure, a 24F chest tube was placed in the thoracic cavity through the posterior portion of the single incision.
We retrospectively analyzed our experience of patients who underwent successful SPVATS and MPVATS for primary lung cancer (37 and 67 patients). We performed a statistical analysis with PASW SPSS statistics ver. 18.0 (SPSS Inc., Chicago, IL, USA). The SP and MP pairs were matched according to the propensity score generated using a logistic regression model that included the following parameters: age, sex, body mass index, percentage of forced expiratory volume in 1 second and vital capacity, clinical T and N stages, the affected lobe, completeness of the fissure, and the extent of pleural adhesion. The 1-to-1 match was achieved using the nearest-neighbor matching algorithm. Continuous variables were obtained as median with range and were compared according to various clinicopathological factors using the Mann-Whitney test. The categorical variables were compared using the chi-square test or the Fisher exact test. All p-values<0.05 were considered to indicate statistical significance.
Results
Table 1 shows the characteristics of patients who underwent SPVATS and MPVATS, before and after matching. There were no significant differences between the 2 groups after matching. The distribution of the affected lobe, which would contribute to potential bias, did not show statistically significant variance after propensity matching (p<0.001 versus p=0.471).
Table 2 shows the postoperative outcomes. The median operation time in the SPVATS and the MPVATS group was similar (210 minutes [range, 100 to 360 minutes] versus 200 minutes [range, 90 to 320 minutes], p=0.11). The median amount of blood loss was 170 mL (range, 20 to 400 mL) versus 160 mL (range, 70 to 500 mL) (p=0.19). The median number of dissected lymph nodes was 26 (range, 10 to 53) versus 23 (range, 14 to 48) (p=0.59). The median number of explored nodal stations was 6 (range, 4 to 9) versus 7 (range, 2 to 9) (p=0.25). The median size of the tumors was 2.5 cm (range, 1.3 to 6.0 cm) versus 2.5 cm (range, 1.0 to 5.5 cm) (p =0.53). In the microscopic examination, all patients underwent R0 resection. The median duration of chest tube drainage was 5 days (range, 2 to 18 days) versus 6 days (range, 2 to 11 days) (p=0.66). The median duration of the intensive care unit stay and hospital stay was 1 day (range, 1 to 2 days) versus 1 day (range, 1 to 2 days) (p=0.17), and 9 days (range, 6 to 22 days) versus 10 days (range, 5 to 28 days) (p=0.89).
Table 2.
Postoperative outcomes
| Variable | All patients | Propensity-matched patients | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SP (n=37) | MP (n=67) | p-value | SP (n=32) | MP (n=32) | p-value | |
| Pleural adhesion | 12 (32.4) | 20 (29.9) | 0.79 | 9 (28.1) | 8 (25.0) | 0.78 |
|
| ||||||
| Incomplete fissure | 26 (70.3) | 47 (70.1) | 0.99 | 22 (68.8) | 22 (68.8) | 1.00 |
|
| ||||||
| Operation time (min) | 210 (100–495) | 200 (90–380) | 0.41 | 210 (100–360) | 200 (90–320) | 0.11 |
|
| ||||||
| Blood loss (mL) | 170 (20–450) | 180 (70–500) | 0.44 | 170 (20–400) | 160 (70–500) | 0.19 |
|
| ||||||
| Tumor diameter (cm) | 2.3 (1.3–6.0) | 2.5 (0.7–6.5) | 0.49 | 2.5 (1.3–6.0) | 2.5 (1.0–5.5) | 0.53 |
|
| ||||||
| No. of dissected lymph nodes | 25 (10–63) | 28 (10–75) | 1.00 | 26 (10–53) | 23 (14–48) | 0.59 |
|
| ||||||
| Explored nodal stations | 6 (4–9) | 6 (2–9) | 0.09 | 6 (4–9) | 7 (2–9) | 0.24 |
|
| ||||||
| Pathologic stages | 0.60 | 0.79 | ||||
|
| ||||||
| O | 1 (1.5) | |||||
|
| ||||||
| IA | 23 (62.2) | 32 (47.8) | 19 (59.4) | 20 (62.5) | ||
|
| ||||||
| IB | 4 (10.8) | 13 (19.4) | 6 (18.8) | 6 (18.8) | ||
|
| ||||||
| IIA | 5 (13.5) | 12 (17.9) | 3 (9.4) | 3 (9.4) | ||
|
| ||||||
| IIB | 1 (2.7) | 1 (1.5) | 0 (0) | 1 (3.1) | ||
|
| ||||||
| IIIA | 4 (10.8) | 8 (11.9) | 4 (12.5) | 2 (6.3) | ||
|
| ||||||
| Chest tube (day) | 5 (2–18) | 6 (2–29) | 0.56 | 5 (2–18) | 6 (2–11) | 0.66 |
|
| ||||||
| Intensive care unit stay (day) | 1 (1–2) | 1 (1–26) | 0.45 | 1 (1–2) | 1 (1–2) | 0.17 |
|
| ||||||
| Hospital stay (day) | 9 (6–22) | 11 (5–29) | 0.63 | 9 (6–22) | 10 (5–28) | 0.89 |
|
| ||||||
| Wound size (cm) | 4.5 (3.5–5.8) | - | 4.5 (3.5–5.5) | - | ||
|
| ||||||
| Histologic type | 0.92 | 1.00 | ||||
|
| ||||||
| Adenocarcinoma | 26 (70.3) | 48 (71.6) | 23 (71.9) | 23 (71.9) | ||
|
| ||||||
| Squamous cell carcinoma | 8 (21.6) | 15 (22.4) | 7 (21.9) | 7 (21.9) | ||
|
| ||||||
| Other | 3 (8.1) | 4 (6.0) | 2 (6.3) | 2 (6.3) | ||
|
| ||||||
| Conversion | 8 (17.8) | 0 | 0.01 | 5 (15.6) | 0 | 0.07 |
Values are presented as median (range) or number (%), unless otherwise stated.
SP, single-port; MP, multi-port.
In the SPVATS group, we analyzed the impact of several clinicopathological factors on the operation time, amount of blood loss, duration of drainage, and duration of the hospital stay (Table 3). In patients with an N(+) stage, the amount of blood loss was significantly higher (p=0.01). Patients with an incomplete fissure required a longer operation time (p =0.03) and had more blood loss (p =0.03). The duration of the hospital stay was longer in patients younger than 60 years of age and in females. However, in the multivariate analysis, these differences were statistically insignificant.
Table 3.
Univariate analysis of single-port video-assisted thoracic surgery in terms of the impact of several clinicopathological factors on the operation time, amount of blood loss, duration of drainage, and duration of hospital stay
| Variable | Operation time (min) | Blood loss (mL) | Duration of drainage (day) | Hospital stay (day) | ||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||
| Median (range) | p-value | Median (range) | p-value | Median (range) | p-value | Median (range) | p-value | |
| Age (yr) | 0.83 | 0.37 | 0.05 | 0.02 | ||||
|
| ||||||||
| ≤60 | 213.3 (105–306) | 170.0 (20–300) | 4.4 (2–11) | 7.8 (6–10) | ||||
|
| ||||||||
| >60 | 195.0 (100–495) | 176.7 (20–450) | 6.3 (3–18) | 10.0 (6–22) | ||||
|
| ||||||||
| Sex | 0.08 | 0.01 | 0.21 | 0.03 | ||||
|
| ||||||||
| Male | 217.5 (100–495) | 176.0 (20–450) | 6.0 (2–18) | 10.3 (6–11) | ||||
|
| ||||||||
| Female | 176.7 (140–300) | 170.0 (20–340) | 5.2 (3–7) | 8.4 (6–22) | ||||
|
| ||||||||
| N stage | 0.77 | 0.01 | 0.20 | 0.27 | ||||
|
| ||||||||
| N (+) | 210.0 (100–360) | 192.5 (20–450) | 5.3 (2–16) | 9.0 (6–18) | ||||
|
| ||||||||
| N (−) | 180.0 (130–495) | 70.0 (60–200) | 7.3 (6–18) | 11.5 (10–22) | ||||
|
| ||||||||
| Tumor size (cm) | 0.49 | 0.35 | 0.79 | 0.56 | ||||
|
| ||||||||
| ≤3 | 186.7 (100–495) | 166.7 (20–430) | 5.9 (2–16) | 9.5 (6–18) | ||||
|
| ||||||||
| >3 | 230.0 (105–495) | 220.0 (60–450) | 5.0 (2–18) | 11.5 (10–22) | ||||
|
| ||||||||
| Adhesion | 0.16 | 0.49 | 0.83 | 0.49 | ||||
|
| ||||||||
| − | 200.0 (100–360) | 190.0 (20–450) | 5.6 (2–18) | 9.0 (6–22) | ||||
|
| ||||||||
| + | 227.5 (130–495) | 165.0 (20–330) | 6.0 (4–16) | 9.8 (6–18) | ||||
|
| ||||||||
| Fissure | 0.03 | 0.03 | 0.93 | 0.59 | ||||
|
| ||||||||
| Complete | 175.0 (100–270) | 90.0 (40–280) | 5.7 (3–11) | 9.7 (7–15) | ||||
|
| ||||||||
| Incomplete | 221.7 (105–495) | 200.0 (20–450) | 5.7 (2–18) | 9.3 (6–22) | ||||
|
| ||||||||
| Upper lobe | 0.35 | 0.63 | 0.27 | 0.57 | ||||
|
| ||||||||
| Yes | 216.0 (105–495) | 183.3 (20–450) | 5.8 (2–18) | 9.3 (7–22) | ||||
|
| ||||||||
| No | 178.3 (100–350) | 160.0 (40–300) | 5.6 (2–10) | 9.5 (6–17) | ||||
The postoperative complications are presented in Table 4. Prolonged chest tube drainage for more than 7 days was required for 6 patients in each group. The causes of prolonged chest tube drainage were persistent air leakage and a persistent large amount of drainage of more than 3 mL/kg/day. Among patients who had persistent air leakage, 3 patients in each group received chemical pleurodesis with talc powder or minocycline. In the SPVATS group, chylothorax occurred in a patient who underwent left upper lobectomy on postoperative day 4. Initially, we attempted conservative treatment. The patient was made to fast for 7 days after the diagnosis of chylothorax, and chemical pleurodesis was attempted. However, conservative treatment was unsuccessful. We performed an SPVATS ligation of the thoracic duct through the right eighth intercostal space on postoperative day 12. The chest tube drain was successfully removed on postoperative day 5 after the second operation. There was no surgical mortality.
Table 4.
Postoperative complications
| Variable | All patients | Propensity-matched patients | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| SP (n=37) | MP (n=67) | p-value | SP (n=32) | MP (n=32) | p-value | |
| Prolonged chest tube drainage (>7 day) | 7 | 16 | 0.56 | 6 | 6 | 1.00 |
|
| ||||||
| Persistent air leakage (>5 day) | 4 | 7 | 4 | 4 | ||
|
| ||||||
| Chemical pleurodesis | 3 | 7 | 3 | 3 | ||
|
| ||||||
| Large amount of drainage (>3 mL/kg/day) | 2 | 9 | 2 | 2 | ||
|
| ||||||
| Chylothorax | 1 | - | - | - | ||
|
| ||||||
| Pneumonia | 2 | 6 | 0.52 | 2 | 0 | 0.15 |
|
| ||||||
| Wound dehiscence | 1 | 3 | 0.65 | 1 | 1 | 1.00 |
|
| ||||||
| Delirium | 1 | 1 | 0.67 | 1 | 0 | 0.31 |
SP, single-port; MP, multi-port.
In the SPVATS group, there were 8 (17.8%) conversions. In 6 patients, SPVATS was converted into MPVATS due to strong adhesions, particularly around the interlobar lymph node, and because of difficulty in achieving the optimal angle. In 2 patients, the operation was converted into open thoracotomy due to massive bleeding resulting from a pulmonary artery injury.
Discussion
VATS is now a standard option for performing intrathoracic procedures. In 2004, Rocco et al. [7] reported the first SPVATS wedge resection. Initially, SPVATS was performed for diagnostic purposes and minor pulmonary resection [8–10]. However, over time, SPVATS for major pulmonary resection has been increasingly performed.
In 2011, Gonzalez et al. [11] reported a case of SPVATS lobectomy; this was the world’s first report of a major SPVATS resection. They reported that SPVATS lobectomy is less invasive and has many advantages in terms of postoperative pain, paresthesia, and cosmetic effects because only 1 intercostal space is involved. Recently, some authors have reported their experiences with SPVATS lobectomy and compared SPVATS and MPVATS lobectomy for primary lung cancer. They have concluded that no significant differences exist between SPVATS and MPVATS with respect to operation time, blood loss, number of dissected lymph nodes, hospital stay, morbidity, and mortality [12,13].
Gonzalez-Rivas et al. [3] reported that SPVATS upper lobectomy has some aspects that are more difficult than any other surgical procedure performed on the lobe. In our experience, the most complicated aspect of SPVATS lobectomy is the stapling of the upper lobar arteries because of the difficulty of achieving the optimal angle for stapling while approaching the apical area with the stapler. When the use of a stapler was not feasible, we used Hem-o-Lok clips. We used these clips in 13 patients for dividing the pulmonary artery branches (Table 5). These clips were used most frequently in left upper lobectomy procedures. We usually used these clips in lower lobectomy procedures for the division of superior segmental arteries. In MPVATS, Hem-o-Lok clips were not used.
Table 5.
Resected lobe and number of cases using Hem-o-Lok clips
| Variable | Single-port (n=37) | Multi-port (n=67) | p-value |
|---|---|---|---|
| Resected lobe | 0.03 | ||
| Right upper lobe | 1/11 (9.1) | 0/15 | |
| Right middle lobe | 1/1 (100) | 0/13 | |
| Right lower lobe | 0/2 (0) | 0/22 | |
| Left upperlobe | 7/11 (63.6) | 0/11 | |
| Left lower lobe | 4/12 (33.3) | 0/6 |
Values are presented as number/total number (%).
After the lobectomy, we performed SLND in every case. The most difficult part of SLND in SPVATS was the subcarinal and the paratracheal lymph node dissection after the left lobectomy; these nodes were located deep and were difficult to expose. In case of left lower lobectomy, subcarinal lymph node dissection before the division of the left lower bronchus was helpful in exposing the subcarinal area to ensure good anterior retraction of the left lower bronchus. In our study, SPVATS was converted into MPVATS or open thoracotomy in 8 patients (17.8%). This rate is relatively high in comparison with other reports. According to the previously published literature, the conversion rate was 2.0%–10.0% [2–6].
In our opinion, this difference can be explained by proficiency with the surgical procedure, the surgical indications, and the frequency of pleural adhesions. A number of conversion cases were in the beginning phase of SPVATS. Over time and with experience, the number of conversion cases has decreased. The surgical indications for SPVATS in most studies that reported a very low conversion rate were clinical stage I disease [4,6]. However, our indications for SPVATS were broader, including clinical stage II disease and clinical stage T1/2 with single-station N2 disease. In other reports, the rate of pleural adhesion was low (2.0%–30.0%) [3,4]. In our study, pleural adhesion was found in 18 (40.0%) of the candidates (n=45) considered for SPVATS. In 6 of these patients, SPVATS was converted into 3-port VATS.
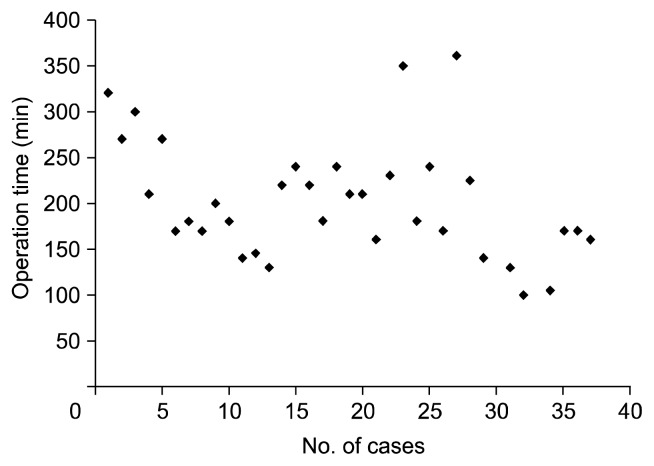
The operation time of SPVATS for primary lung cancer was 132–227 minutes [4–6]. Our median operation time was 210 minutes (range, 100 to 495 minutes). Over time and with experience, the operation time decreased (correlation coefficient=−0.47, p< 0.01) (Fig. 2).
Fig. 2.
Relationship between the operation time and the number of cases (correlation coefficient=−0.47, p<0.01).
The safety of the patient is the most important consideration, so particularly in cases of major vessel injury, using additional ports or converting to open thoracotomy should be considered without hesitation. In our study, there was major bleeding due to a pulmonary artery injury in 2 patients. In these cases, SPVATS was converted into open thoracotomy, and this major bleeding was successfully controlled.
It has been argued that SPVATS for primary lung cancer has disadvantages in terms of safety and oncological efficacy as compared to MPVATS for primary lung cancer. This is similar to the debate between VATS and open thoracotomy that occurred in the initial steps of VATS [14]. In the past 20 years, VATS has become the standard procedure for treating primary lung cancer. If its effectiveness and safety are proven, SPVATS will emerge as a useful option among the various surgical approaches for primary lung cancer.
In conclusion, in our experience, SPVATS for primary lung cancer is safe and feasible in well-selected patients. However, this study has some limitations in that it was a retrospective analysis, and the number of patients considered was very small. A randomized, prospective, and comparative study with a large group of patients and long-term follow-up is necessary to evaluate the clinical feasibility and the advantages of SPVATS for primary lung cancer.
Acknowledgments
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund no. KTCS04–070).
Footnotes
Conflict of interest
No potential conflicts of interest relevant to this article are reported.
References
- 1.Howington JA, Blum MG, Chang AC, Balekian AA, Murthy SC. Treatment of stage I and II non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e278S–313S. doi: 10.1378/chest.12-2359. [DOI] [PubMed] [Google Scholar]
- 2.Scott WJ, Allen MS, Darling G, et al. Video-assisted thoracic surgery versus open lobectomy for lung cancer: a secondary analysis of data from the American College of Surgeons Oncology Group Z0030 randomized clinical trial. J Thorac Cardiovasc Surg. 2010;139:976–81. doi: 10.1016/j.jtcvs.2009.11.059. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg. 2013;95:426–32. doi: 10.1016/j.athoracsur.2012.10.070. [DOI] [PubMed] [Google Scholar]
- 4.Hirai K, Takeuchi S, Usuda J. Single-port video-assisted thoracic surgery for early lung cancer: initial experience in Japan. J Thorac Dis. 2016;8(Suppl 3):S344–50. doi: 10.3978/j.issn.2072-1439.2016.02.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sihoe AD. Uniportal video-assisted thoracic (VATS) lobectomy. Ann Cardiothorac Surg. 2016;5:133–44. doi: 10.21037/acs.2016.03.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hsu PK, Lin WC, Chang YC, et al. Multiinstitutional analysis of single-port video-assisted thoracoscopic anatomical resection for primary lung cancer. Ann Thorac Surg. 2015;99:1739–44. doi: 10.1016/j.athoracsur.2015.01.041. [DOI] [PubMed] [Google Scholar]
- 7.Rocco G, Martin-Ucar A, Passera E. Uniportal VATS wedge pulmonary resections. Ann Thorac Surg. 2004;77:726–8. doi: 10.1016/S0003-4975(03)01219-0. [DOI] [PubMed] [Google Scholar]
- 8.Rocco G, La Rocca A, La Manna C, et al. Uniportal video-assisted thoracoscopic surgery pericardial window. J Thorac Cardiovasc Surg. 2006;131:921–2. doi: 10.1016/j.jtcvs.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 9.Alar T, Ozcelik C. Single-incision thoracoscopic surgery of pleural effusions for diagnosis and treatment. Surg Endosc. 2013;27:4333–6. doi: 10.1007/s00464-013-3060-y. [DOI] [PubMed] [Google Scholar]
- 10.Kang DK, Min HK, Jun HJ, Hwang YH, Kang MK. Early outcomes of single-port video-assisted thoracic surgery for primary spontaneous pneumothorax. Korean J Thorac Cardiovasc Surg. 2014;47:384–8. doi: 10.5090/kjtcs.2014.47.4.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez D, Paradela M, Garcia J, Dela Torre M. Singleport video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg. 2011;12:514–5. doi: 10.1510/icvts.2010.256222. [DOI] [PubMed] [Google Scholar]
- 12.Hirai K, Takeuchi S, Usuda J. Single-incision thoracoscopic surgery and conventional video-assisted thoracoscopic surgery: a retrospective comparative study of perioperative clinical outcomes. Eur J Cardiothorac Surg. 2016;49(Suppl 1):i37–41. doi: 10.1093/ejcts/ezv320. [DOI] [PubMed] [Google Scholar]
- 13.Shen Y, Wang H, Feng M, Xi Y, Tan L, Wang Q. Single-versus multiple-port thoracoscopic lobectomy for lung cancer: a propensity-matched study. Eur J Cardiothorac Surg. 2016;49(Suppl 1):i48–53. doi: 10.1093/ejcts/ezv358. [DOI] [PubMed] [Google Scholar]
- 14.Sihoe AD. Reasons not to perform uniportal VATS lobectomy. J Thorac Dis. 2016;8(Suppl 3):S333–43. doi: 10.3978/j.issn.2072-1439.2016.02.41. [DOI] [PMC free article] [PubMed] [Google Scholar]