Abstract
Vaccines reside in a complex multiscale system that includes biological, clinical, behavioral, social, operational, environmental, and economical relationships. Not accounting for these systems when making decisions about vaccines can result in changes that have little effect rather than solutions, lead to unsustainable solutions, miss indirect (e.g., secondary, tertiary, and beyond) effects, cause unintended consequences, and lead to wasted time, effort, and resources. Mathematical and computational modeling can help better understand and address complex systems by representing all or most of the components, relationships, and processes. Such models can serve as “virtual laboratories” to examine how a system operates and test the effects of different changes within the system. Here are ten lessons learned from using computational models to bring more of a systems approach to vaccine decision making: (i) traditional single measure approaches may overlook opportunities; (ii) there is complex interplay among many vaccine, population, and disease characteristics; (iii) accounting for perspective can identify synergies; (iv) the distribution system should not be overlooked; (v) target population choice can have secondary and tertiary effects; (vi) potentially overlooked characteristics can be important; (vii) characteristics of one vaccine can affect other vaccines; (viii) the broader impact of vaccines is complex; (ix) vaccine administration extends beyond the provider level; (x) and the value of vaccines is dynamic.
Keywords: Systems, vaccines, decision making, computational modeling
INTRODUCTION
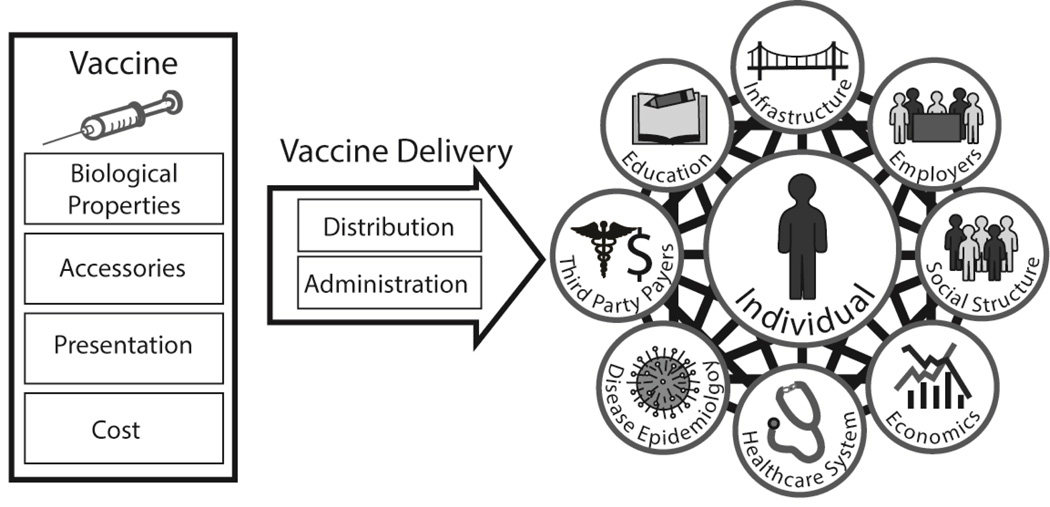
Vaccines reside in a complex multiscale system that includes biological, clinical, behavioral, social, operational, environmental, and economical relationships (Figure 1). Not accounting for these systems when making decisions about vaccines can result in “band-aids” rather than solutions, i.e., making changes that don’t have a real or sustainable effect or making less than optimal changes but not solving the underlying issues. Even if a solution is provided, it may be unsustainable. Additionally, overlooking the systems may miss indirect (e.g., secondary, tertiary, and beyond) effects of a vaccine. In fact, without understanding the system, even well-meaning efforts can lead to unintended consequences. Finally, not understanding the relevant systems can lead to wasted time, effort, and resources when developing and implementing vaccines and vaccination programs.
Figure 1.
Many lessons can be learned by using a systems approach to vaccine decision making
A system consists of various interconnected components that interact with and affect one another, though they may appear independent. Many natural and human-made systems exist throughout the universe (e.g., ecosystems, air traffic control, meteorology), making few things truly independent and nearly everything part of a system. Altering a single aspect of a system tends to affect other parts of the interconnected system, often in complex ways. Therefore, unless all aspects of the system are taken into account, unexpected results may occur with changes.
A systems approach involves understanding, considering, and addressing the entire system when making any important decision, observation, or change. Some changes, that may appear inconsequential, may have lasting significant effects. The critical first step of a systems approach is to outline an overall picture of the entire system. However, understanding a complex system with many components may be difficult. Direct and immediate one-way cause-and-effect relationships may be easy to identify, while other effects (e.g., involving intermediaries, back-and-forth interactions, delays) may not be perceived at once.
Mathematical and computational modeling can help better understand and address complex systems by representing all or most of the components, relationships, and processes. Such models can serve as “virtual laboratories” to examine how a system operates and test the effects of different changes within the system in many settings[1–41]. An example of computational modeling is air traffic control systems. An air traffic control system takes data and information from different sources (e.g., weather, environmental, runway capacity, and plane location data) and uses a computational simulation model to represent each component and process in the system, integrating everything and allowing air traffic controllers to view the system as a whole to make appropriate decisions. Without air traffic control computational systems, diagnosing system vulnerabilities, coordinating operations, developing solutions, and anticipating the impact of changes in the system or new technology would be considerably more difficult. The simulation model can anticipate consequences so that appropriate real life changes can be made (e.g., if airplanes need to alter their courses to avoid heavy traffic or storm systems). Similarly, taking a systems approach to vaccines can aid manufacturers, policymakers, and NGOs to make appropriate decisions for vaccines. Computational systems modeling for vaccines takes into account the interactions between economic modeling, biological and transmission modeling, clinical outcomes modeling, and logistics modeling. Here are ten examples of lessons learned from using computational models to take a more systems approach to vaccine decision making.
TEN LESSONS LEARNED
Lesson 1: Traditional single (or limited) measure approaches may overlook opportunities
Using a single or only a few measures to assess the burden of a disease or the impact of an intervention can lead to substantial over- or under-estimates. One example is Chagas’ disease, which has traditionally been considered a neglected tropical disease primarily affecting Latin America, which in turn may have kept development of a vaccine for Chagas Disease lower on the global priority list. The challenge is that the clinical effects of Chagas Disease do not even beginning manifesting until over a decade after infection has occurred, meaning that traditional measures such as short term mortality and immediate direct medical costs will capture little of its impact[42–44]. Instead, accounting for the full spectrum of effects requires looking further into the future and incorporating future morbidity measures and productivity losses. Moreover, focusing only on Latin America overlooks other parts of the world that are affected such as North American and Europe. When using a computational model developed by the Public Health Computational and Operations Research (PHICOR) team that accounted for the downstream clinical sequalae such as congestive heart failure, future productivity losses, and other parts of the world, the annual global cost ($7.2 billion) of Chagas Disease exceeds that of both cervical cancer and rotavirus [17, 45].
With Chagas resulting in considerable costs throughout a patient’s lifetime, it is not surprising that a vaccine to prevent Trypanosoma cruzi (which causes Chagas Disease) infection would be highly cost-effective if the cost was less than or equal to $200 and even cost savings if the vaccine cost was $20, both with efficacy ≥ 75%, even if the infection risk was as low as 5%, based on computational modeling studies [18]. Therefore, not accounting for future morbidity and mortality and productivity losses may have severely underestimated the value of a Trypanosoma cruzi vaccine.
Lesson 2: Complex interplay among many vaccine, population, and disease characteristics
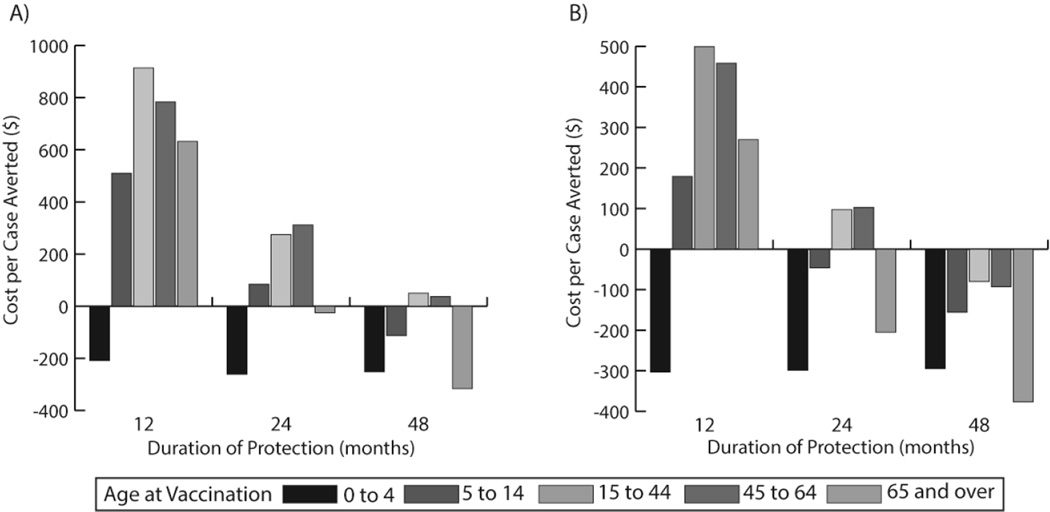
The characteristics of a vaccine (e.g., biological and physical properties), target population, pathogen, and disease are all interwoven so that changing one can affect the others. For instance, a computational model developed by PHICOR shows how varying the cost, efficacy, and duration of protection for a potential norovirus vaccine interact with each other and affect the potential impact on different age groups [7]. A 75% efficacious vaccine would annually prevent 6,125 cases per 10,000 vaccines among children 0–4 years old. Decreasing efficacy to 25% would then reduce the number of cases prevented to only 2,036 cases (0–4 years old, 48 months protection) per 10,000 vaccines given. In an older age group (15–44 years old), the vaccine with 25% efficacy only prevented 100 cases per 10,000 vaccines administered with 12 months protection. Cost savings across all efficacies can also occur when administered to children under 5 years old provided that the vaccine cost is ≤$25 (Figure 2). More expensive vaccines can also have cost savings, but need higher efficacy and longer periods of protection [7]. By taking into account these vaccine, population, and disease characteristics, human norovirus vaccine developers can be advised as to the necessary parameters for maximum population benefit.
Figure 2.
Cost per Norovirus case averted by vaccination with $25 vaccine cost and (a) 50% vaccine efficacy or (b) 75% vaccine efficacy
Lesson 3: Accounting for perspective can identify synergies
Different decision makers have different interests and therefore will respond to different measures and perspectives. For example, employers may be most interested in how a vaccine may affect their profits (revenues minus costs). Therefore, demonstrating that influenza vaccine is cost-effective to society or third party payers may not convince employers to pay for or offer vaccination. However, showing how an influenza vaccine can save productivity losses with employees missing fewer days of work can be compelling. A computational model developed by PHICOR showed that employer sponsored influenza vaccination is inexpensive (<$35/vaccinated employee) or can even be cost savings for employers [21]. The cost of vaccinating versus the cost of employees missing work due to influenza or influenza like virus were modeled, varying profession type and wage, transmission coefficient (R), serological attack rate, and percent of symptomatic employees. For normal seasonal influenza, median cost per employee unvaccinated ranged from $20 to −$38, with negative values indicating cost savings for employers. The cost of vaccinating versus the cost of employees missing work due to influenza or influenza like illness was cost savings if employees are contagious (R=1.2, 1.6) and cost-effective otherwise (R=0).
The occurrence of pandemic influenza, with 20% serologic attack rates and R=0, employer sponsored vaccination led to cost savings per employee vaccinated (−$598 to −$25). Cost savings increased as R=1.2 (−$843 to −$66), and further increased (−$882 to −$82) when R=1.6 A 30% serologic attack rate led to even greater cost savings per employee vaccinated. When R=0, cost savings were −$1,116 to −$44, and when R=1.2 led to cost savings of −$1,200 to −$110. R=1.6 resulted in the highest cost savings (−$1,273 to −$132) per employee vaccinated. Therefore, administering the influenza vaccine can be cost-effective or even cost savings from an employer’s perspective.
Lesson 4: Do not overlook the distribution system
In order to work, vaccines must first reach the population. Vaccine supply chains are the complex system of locations, equipment, vehicles, people, and processes that must be coordinated to take vaccines from their manufacturers to where they are ultimately administered. As many vaccines degrade when exposed to higher temperatures, vaccine supply chains must keep these vaccines at lower temperatures. However, when temperatures are too low, some vaccines may freeze and thus destabilize.
Before a new vaccine is introduced to the market, decision makers should determine whether existing supply chains can handle the new vaccine. For example, using the HERMES (Highly Extensible Resource for Modeling Supply Chains) software platform, the HERMES Logistics Team developed a discrete simulation model of the Niger World Health Organization (WHO) expanded program on immunization (EPI) supply chain [46]. Using this HERMES-generated model, we then evaluated the impact of introducing the rotavirus and/or pneumococcal vaccines to the EPI regimen in in Niger [13]. The Niger vaccine supply chain includes for levels, starting from the Central Store in Niamey and including 7 Regional Stores, Districts stores, and over 600+ integrated health clinics (IHC). The simulation showed that as of 2008, significant bottlenecks existed at District and IHC levels, which impaired many vaccines from reaching their destinations and many people who arrived for vaccination at different clinics not getting vaccinated (vaccine availability=69%). Therefore, introducing either the rotavirus or pneumococcal vaccine would only exacerbate their existing storage and transport bottlenecks and thus further inhibit the flow of all vaccines to their intended locations. When only PCV-7, RV (259.8cm3) or RV (17.1cm3) was introduced, overall vaccine availability fell to 29%, 13%, or 51%, respectively due to significant storage and transport bottlenecks. Vaccine availabilities were 26% when both PCV-7 and the smallest presentation of RV (17.1cm3) were introduced. The capacity of the distribution system must be assessed and addressed prior to introduction of new vaccines to prevent bottlenecks from impeding delivery of all vaccines.
Lesson 5: Target population choice can have secondary, tertiary, etc. effect
Often, the primary driving force behind target population selection is clinical benefit and potentially cost-effectiveness. However, as HERMES work in Thailand demonstrated, the choice of target population and timing of vaccination for influenza has a large impact on vaccine supply chains, affecting availability of both the influenza vaccine and other routine vaccines. Previously, less than 1% of the population received the influenza vaccine despite Thailand’s well developed public health infrastructure The HERMES Logistics Team utilized the HERMES software to develop a computational discrete-event simulation model of Thailand’s National Immunization Program (NIP) supply chain in Trang Province to model the impact of different coverage rates and time-frames for influenza vaccination among the target population of high risk individuals (e.g. elderly >65, healthcare workers, etc.) [3].
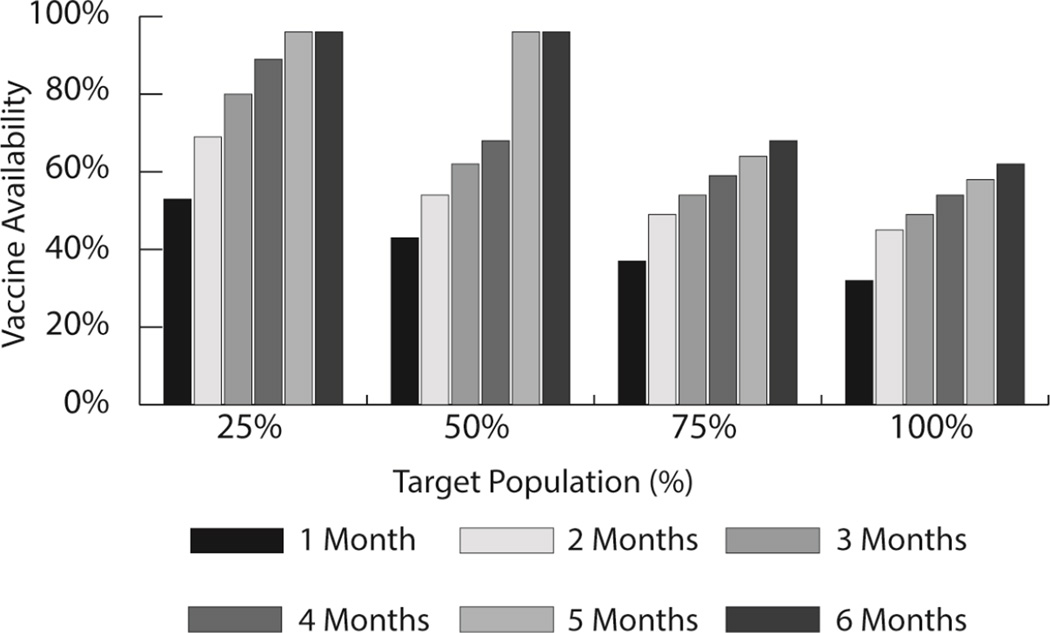
Simulations demonstrated that introduction of the influenza vaccine created bottlenecks in the supply chain and thus reduced the availability of both influenza and other NIP vaccines. Transport capacity utilization from the province to the hospitals increased from 31% at baseline to 69% and 109% for target population sizes of 50% and 100%, respectively. The impact of influenza vaccine introduction on storage capacity at the provincial level followed similar patterns. The high transport needs were somewhat mitigated with an increased vaccination time frame from one to six months. Vaccine availabilities for NIP vaccines decreased from 95% to 94% with the introduction of the influenza vaccine. Influenza vaccine availability increased as vaccination time frame increased to 6 months (Figure 3). However, vaccines were still unavailable for many when the percent of the target population was increased from 25% to 100%. Adjustments must be made to the supply chain before increasing coverage of the influenza vaccine or else availability of all vaccines will suffer. This model demonstrates the importance of considering vaccine supply chain capacity when determining an appropriate target population and coverage goals for the influenza vaccine.
Figure 3.
Average influenza vaccine availability across varying vaccination campaign time-frames and target population sizes
Lesson 6: Potentially overlooked characteristics can be important
Decision makers may focus on certain vaccine characteristics such as efficacy and duration of action because these seem to directly affect disease prevention and control but may overlook other characteristics such as the vial size. In actuality, practically all vaccine characteristics can in some way affect disease prevention and control. For example, switching from multi-dose vials to single dose vials prevents dose wastage and contamination. However, single dose vials are more costly and create more medical waste and packaging, which can be problematic to the delivery of the vaccines. The HERMES Logistics Team modeled the impact of changing from multi-dose vials to single dose vials in the Niger vaccine supply chain.
To minimize vaccine wastage costs, single or low dose presentations were more cost-effective for low demand clinics, while large multi-dose vials were more cost-effective for high demand areas[32]. However, when the measles vaccine was switched to a lower dose presentation, the increased number of vials created bottlenecks throughout the supply chain and lowered vaccine availability from 90% to 80%, since lower dose vials take up more room than the multi-dose vials [1]. Additionally, added medical waste disposal and logistics costs (e.g., storage and transport) more than tripled the annual costs of vaccine administration to $586,214 with single dose vials ($179,779 with the 10-dose vial). Switching to a measles vaccine presentation other than the 10 dose per vial presentation overwhelmed the system, leading to higher costs and lower vaccine availability.
Ultimately, the choice of appropriate vial size can greatly impact the success of vaccine delivery, but the correct choice may depend on the vaccine supply chain, vaccine characteristics, immunization size session and goals of decision makers [12].
Lesson 7: Characteristics of one vaccine can affect other vaccines
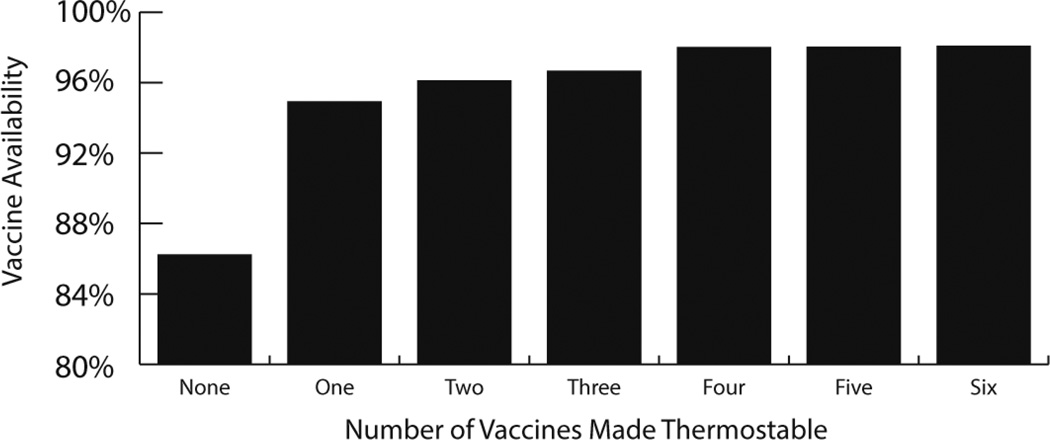
Since all vaccines sit within systems with other vaccines, changing a single characteristic of one vaccine could potentially affect other vaccines. For example, making a single vaccine more thermostable could benefit other vaccines that are not altered. Using a HERMES-generated simulation model of the Niger immunization supply chain, our HERMES Logistics Team explored the impact on vaccine availability of making one or more vaccines thermostable (Figure 4). Making the pentavalent vaccine thermostable increased the availabilities of all vaccines to at least 93% (93–97%). A thermostable pentavalent vaccine improved all vaccine availabilities more than having any other single vaccine be thermostable. When more vaccines were made thermostable in addition to the pentavalent vaccine, vaccine availability further increased 1–2%. Additional gains to vaccine availability were limited after three vaccines (pentavalent, tetanus toxoid, and yellow fever) were made thermostable [28]. These results show that the focus of developing thermostable vaccines need not necessarily be to remove the cold chain completely, but rather to alleviate bottlenecks in the supply chain by focusing on introducing this characteristic to a single or few vaccines.
Figure 4.
The effect of making multiple vaccines thermostable on the vaccine availabilities of all WHO EPI vaccines
Lesson 8: The broader impact of vaccines is complex
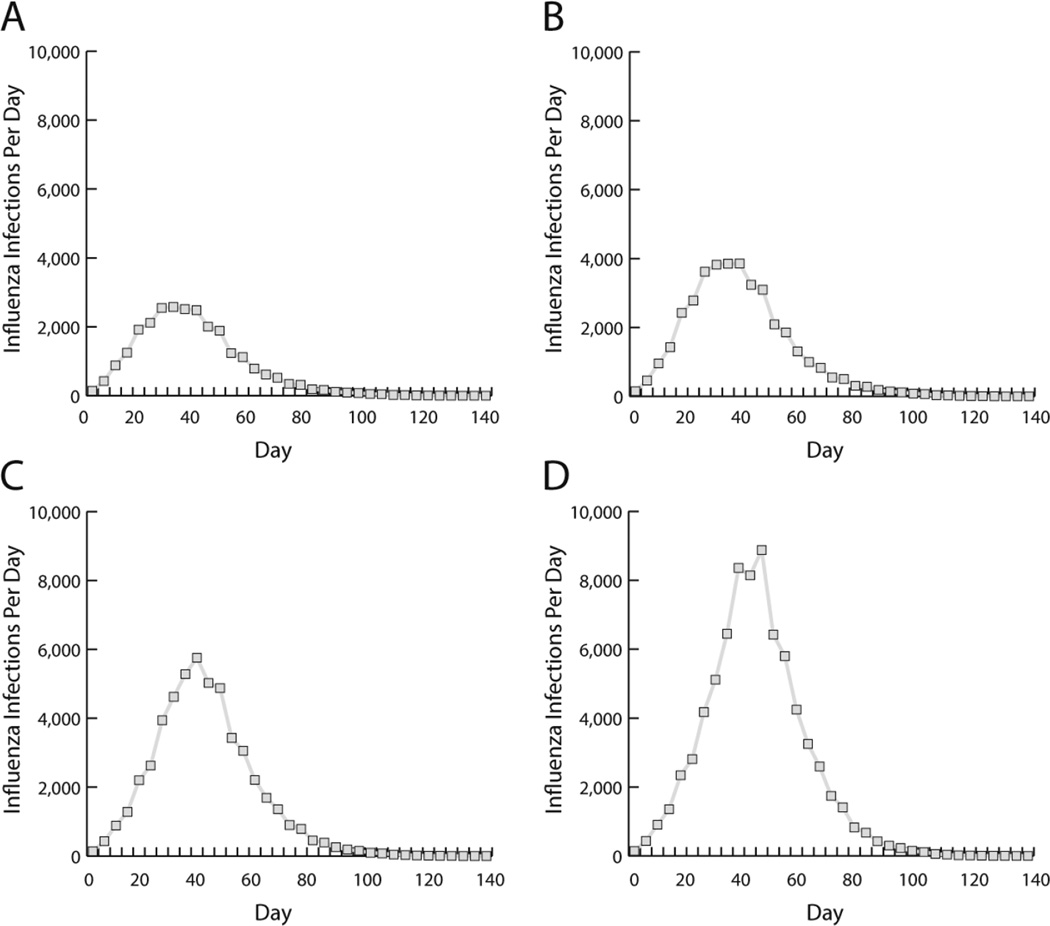
Our world is highly interconnected so that one population, sector, or location can easily affect another. Therefore, studies and measures focusing only on a specific population, location, or sector may overlook potential broader benefits of a vaccine. For instance, arguments for immunizing lower income populations often revolve around humanitarian and ethical principles, i.e., it is the right thing to do. Such arguments may not resonate with higher income populations who may believe that they are distant enough from low income populations. Potentially more compelling would be demonstrating how immunization rates in lower income communities can drive infection risk among high income populations. Using our agent-based model of the Washington D.C. metropolitan area, we demonstrated the effects of delaying influenza immunization to high or low income populations when limited vaccines are available (e.g., pandemic influenza) [47].
Delays of 10 days in immunizing the two lowest income counties causing low coverage in these communities increased the total number of influenza infections throughout the entire region by 0.68% and number of new infections per day during an influenza epidemic by 1,477 throughout the entire region (Figure 5). Increasing the delay to 30 days, increased the total number of influenza infections by 2.75% and number of new infections per day by 7,405 throughout the entire region. Influenza rates in low income communities affected all other communities due to high density and heavy mixing and high levels of travel to other locations for work. Thus, the selfless, altruistic behavior of ensuring equal access to vaccines can lead to selfish, utilitarian benefits by lowering the total number of influenza infections.
Figure 5.
Number of new influenza infections each day when two lowest-income counties experience vaccination delays of (a) 0 days, (b) 10 days, (c) 20 days, or (d) 30 days compared to other counties
Lesson 9: Vaccine administration extends beyond the provider level
Changing how vaccine administration occurs can seem relevant mainly to the person administering the vaccine. However, the impact of such changes can extend far beyond the provider level. For instance, introducing a technology that changes the mode of vaccine administration such as a microneedle patch (MNP) can expand the settings where vaccination can occur (e.g., self-administration) and increase vaccination compliance, which in turn can alter how the influenza vaccine can alter influenza transmission and the seasonal influenza epidemic curve. To explore these complex relationships, the PHICOR Team working with collaborators from PATH and the Georgia Institute of Technology developed an integrated economic and epidemiological model that represented the spread of seasonal influenza in the United States and explored different administration scenarios and the resulting economic value of each [24]. With healthcare provider administration, MNP would be cost effective at all MNP price points ($9.50–$30) and market shares of 20% or higher assuming the same efficacy and compliance as current vaccines. A MNP would be economically dominant compared to baseline when the MNP price was at $9.50 and market shares of 30% or higher. To assess the impact of self-administration, two specific scenarios were also explored. Assuming the same efficacy and compliance as current technologies, option one with 72.7% of MNP self-administered resulted in lower administration costs but an increase in influenza cases due to lower administration success. Option two with 36.4% of MNP self-administered was not cost effective, but the increase in influenza cases was not significant. However, in both scenarios, efficacy or compliance increases of at least 3% allowed MNPs to be cost-effective and even dominant in some scenarios. By exploring these scenarios using a systems approach, investigators are able to comprehensively map vaccine administration systems in order to effectively design policies and inform decision makers.
Lesson 10: The value of vaccines is dynamic (i.e., time-dependent)
Another key thing to keep in mind is that the value and impact of a vaccine is time-dependent. In July 2016, the United Kingdom eliminated the infant dose of meningococcal C (MenC) conjugate vaccine from its National Vaccine Programme schedule since infants are now well protected by herd immunity [48]. Prior to introduction of the MenC vaccination in the UK in 1998/1999, there were a 955 of MenC per year [49]. The success of the vaccination program led to only 1 infant case in 2014/2015, essentially eradicating the disease in infants [50]. Doses administered later give similar immunity to the disease making the initial booster no longer necessary or cost effective [51].
The value of vaccines also includes the match (or mismatch) between when the timing of vaccine administration and the timing of pathogen transmission and disease onset. For instance, each year, the influenza begins sometime in autumn or winter and ends sometime in late winter or spring. The peak of the seasonal influenza epidemic falls somewhere in the middle of these times and can vary from year to year. Vaccine administered during an influenza season cannot prevent influenza cases and transmission that has already occurred. The impact of earlier vaccination depends on transmission dynamics, the R0, the amount of preexisting immunity, and seasonal peak. Earlier vaccination shifts the epidemic curve later in influenza season and reduces the peak [23]. Using our agent-based model of Allegheny County, Pennsylvania, with a peak in February or later, vaccinating individuals by the end of September could avert 9,634–17,794 influenza cases, $0.6–$1.4 million in direct costs, $2.1–$4.0 million in productivity losses, and 35–64 quality adjusted life years (QALYs). With an earlier vaccination peak (e.g. December), vaccination by September would avert an additional 33,139 cases, $2.2 million in direct costs, $7.2 million in productivity losses, and 120 QALYs (R0=1.2, no preexisting immunity). Vaccinating just children by the end of September could avert 11,366 cases, saving $0.6 million in direct costs, $2.3 million in productivity losses, and 42 QALYs. These estimates would be comparable to a 5% increase in coverage of vaccination at any time in all age groups. The complexity in understanding the time-dependent value of a vaccine demonstrates the importance of a systems based approach to design, plan, and implement vaccine delivery interventions.
CONCLUSIONS
These are just ten examples of how vaccines reside in complex systems and decision making without considering these systems could led to the wrong decisions. Unaided, decision makers may not be able to fully understand these systems and the implications of different choices. Systems approaches have transformed decision making in other industries and do so in the vaccine world.
Acknowledgments
This work was supported by the Agency for Healthcare Research and Quality (AHRQ) via grant R01HS023317, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) Office of Behavioral and Social Sciences Research (OBSSR) and the Global Obesity Prevention Center (GOPC) via grant U54HD070725, NICHD via grant U01 HD086861, the Bill and Melinda Gates Foundation, the National Institute Medical Sciences (NIGMS) via the MIDAS agreement 5 U24 GM110707 and USAID via grant AID-OAA-A-15-00064. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Assi TM, Brown ST, Djibo A, Norman BA, Rajgopal J, Welling JS, et al. Impact of changing the measles vaccine vial size on Niger's vaccine supply chain: a computational model. BMC public health. 2011;11:425. doi: 10.1186/1471-2458-11-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Assi TM, Brown ST, Kone S, Norman BA, Djibo A, Connor DL, et al. Removing the regional level from the Niger vaccine supply chain. Vaccine. 2013;31:2828–2834. doi: 10.1016/j.vaccine.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Assi TM, Rookkapan K, Rajgopal J, Sornsrivichai V, Brown ST, Welling JS, et al. How influenza vaccination policy may affect vaccine logistics. Vaccine. 2012;30:4517–4523. doi: 10.1016/j.vaccine.2012.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bacon KM, Hotez PJ, Kruchten SD, Kamhawi S, Bottazzi ME, Valenzuela JG, et al. The potential economic value of a cutaneous leishmaniasis vaccine in seven endemic countries in the Americas. Vaccine. 2013;31:480–486. doi: 10.1016/j.vaccine.2012.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey RR, Stuckey DR, Norman BA, Duggan AP, Bacon KM, Connor DL, et al. Economic value of dispensing home-based preoperative chlorhexidine bathing cloths to prevent surgical site infection. Infection control and hospital epidemiology. 2011;32:465–471. doi: 10.1086/659763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartsch SM, Hotez PJ, Hertenstein DL, Diemert DJ, Zapf KM, Bottazzi ME, et al. Modeling the economic and epidemiologic impact of hookworm vaccine and mass drug administration (MDA) in Brazil, a high transmission setting. Vaccine. 2016;34:2197–2206. doi: 10.1016/j.vaccine.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bartsch SM, Lopman BA, Hall AJ, Parashar UD, Lee BY. The potential economic value of a human norovirus vaccine for the United States. Vaccine. 2012;30:7097–7104. doi: 10.1016/j.vaccine.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49:1784–1792. doi: 10.1086/649013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haidari LA, Brown ST, Constenla D, Zenkov E, Ferguson M, de Broucker G, et al. The economic value of increasing geospatial access to tetanus toxoid immunization in Mozambique. Vaccine. 2016;34:4161–4165. doi: 10.1016/j.vaccine.2016.06.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haidari LA, Brown ST, Ferguson M, Bancroft E, Spiker M, Wilcox A, et al. The economic and operational value of using drones to transport vaccines. Vaccine. 2016;34:4062–4067. doi: 10.1016/j.vaccine.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haidari LA, Connor DL, Wateska AR, Brown ST, Mueller LE, Norman BA, et al. Only adding stationary storage to vaccine supply chains may create and worsen transport bottlenecks. Journal of public health management and practice : JPHMP. 2013;19(Suppl 2):S65–S67. doi: 10.1097/PHH.0b013e31828a83fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haidari LA, Wahl B, Brown ST, Privor-Dumm L, Wallman-Stokes C, Gorham K, et al. One size does not fit all: The impact of primary vaccine container size on vaccine distribution and delivery. Vaccine. 2015;33:3242–3247. doi: 10.1016/j.vaccine.2015.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee BY, Assi TM, Rajgopal J, Norman BA, Chen SI, Brown ST, et al. Impact of introducing the pneumococcal and rotavirus vaccines into the routine immunization program in Niger. American journal of public health. 2012;102:269–276. doi: 10.2105/AJPH.2011.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee BY, Assi TM, Rookkapan K, Connor DL, Rajgopal J, Sornsrivichai V, et al. Replacing the measles ten-dose vaccine presentation with the single-dose presentation in Thailand. Vaccine. 2011;29:3811–3817. doi: 10.1016/j.vaccine.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee BY, Assi TM, Rookkapan K, Wateska AR, Rajgopal J, Sornsrivichai V, et al. Maintaining vaccine delivery following the introduction of the rotavirus and pneumococcal vaccines in Thailand. PloS one. 2011;6:e24673. doi: 10.1371/journal.pone.0024673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee BY, Bacon KM, Bailey R, Wiringa AE, Smith KJ. The potential economic value of a hookworm vaccine. Vaccine. 2011;29:1201–1210. doi: 10.1016/j.vaccine.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee BY, Bacon KM, Bottazzi ME, Hotez PJ. Global economic burden of Chagas disease: a computational simulation model. The Lancet Infectious diseases. 2013;13:342–348. doi: 10.1016/S1473-3099(13)70002-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BY, Bacon KM, Connor DL, Willig AM, Bailey RR. The potential economic value of a Trypanosoma cruzi (Chagas disease) vaccine in Latin America. PLoS neglected tropical diseases. 2010;4:e916. doi: 10.1371/journal.pntd.0000916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee BY, Bacon KM, Shah M, Kitchen SB, Connor DL, Slayton RB. The economic value of a visceral leishmaniasis vaccine in Bihar state, India. The American journal of tropical medicine and hygiene. 2012;86:417–425. doi: 10.4269/ajtmh.2012.10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee BY, Bacon KM, Wateska AR, Bottazzi ME, Dumonteil E, Hotez PJ. Modeling the economic value of a Chagas' disease therapeutic vaccine. Human vaccines & immunotherapeutics. 2012;8:1293–1301. doi: 10.4161/hv.20966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee BY, Bailey RR, Wiringa AE, Afriyie A, Wateska AR, Smith KJ, et al. Economics of employer-sponsored workplace vaccination to prevent pandemic and seasonal influenza. Vaccine. 2010;28:5952–5959. doi: 10.1016/j.vaccine.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee BY, Bailey RR, Wiringa AE, Assi TM, Beigi RH. Antiviral medications for pregnant women for pandemic and seasonal influenza: an economic computer model. Obstetrics and gynecology. 2009;114:971–980. doi: 10.1097/AOG.0b013e3181bdbfed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee BY, Bartsch SM, Brown ST, Cooley P, Wheaton WD, Zimmerman RK. Quantifying the economic value and quality of life impact of earlier influenza vaccination. Medical care. 2015;53:218–229. doi: 10.1097/MLR.0000000000000302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee BY, Bartsch SM, Mvundura M, Jarrahian C, Zapf KM, Marinan K, et al. An economic model assessing the value of microneedle patch delivery of the seasonal influenza vaccine. Vaccine. 2015;33:4727–4736. doi: 10.1016/j.vaccine.2015.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee BY, Bartsch SM, Willig AM. The economic value of a quadrivalent versus trivalent influenza vaccine. Vaccine. 2012;30:7443–7446. doi: 10.1016/j.vaccine.2012.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BY, Brown ST, Cooley P, Potter MA, Wheaton WD, Voorhees RE, et al. Simulating school closure strategies to mitigate an influenza epidemic. Journal of public health management and practice : JPHMP. 2010;16:252–261. doi: 10.1097/PHH.0b013e3181ce594e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee BY, Brown ST, Cooley PC, Zimmerman RK, Wheaton WD, Zimmer SM, et al. A computer simulation of employee vaccination to mitigate an influenza epidemic. American journal of preventive medicine. 2010;38:247–257. doi: 10.1016/j.amepre.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee BY, Cakouros BE, Assi TM, Connor DL, Welling J, Kone S, et al. The impact of making vaccines thermostable in Niger's vaccine supply chain. Vaccine. 2012;30:5637–5643. doi: 10.1016/j.vaccine.2012.06.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee BY, Connor DL, Kitchen SB, Bacon KM, Shah M, Brown ST, et al. Economic value of dengue vaccine in Thailand. The American journal of tropical medicine and hygiene. 2011;84:764–772. doi: 10.4269/ajtmh.2011.10-0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee BY, Connor DL, Wateska AR, Norman BA, Rajgopal J, Cakouros BE, et al. Landscaping the structures of GAVI country vaccine supply chains and testing the effects of radical redesign. Vaccine. 2015;33:4451–4458. doi: 10.1016/j.vaccine.2015.07.033. [DOI] [PubMed] [Google Scholar]
- 31.Lee BY, Haidari LA, Lee MS. Modelling during an emergency: the 2009 H1N1 influenza pandemic. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2013;19:1014–1022. doi: 10.1111/1469-0691.12284. [DOI] [PubMed] [Google Scholar]
- 32.Lee BY, Norman BA, Assi TM, Chen SI, Bailey RR, Rajgopal J, et al. Single versus multi-dose vaccine vials: an economic computational model. Vaccine. 2010;28:5292–5300. doi: 10.1016/j.vaccine.2010.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee BY, Stalter RM, Bacon KM, Tai JH, Bailey RR, Zimmer SM, et al. Cost-effectiveness of adjuvanted versus nonadjuvanted influenza vaccine in adult hemodialysis patients. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2011;57:724–732. doi: 10.1053/j.ajkd.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee BY, Tai JH, McGlone SM, Bailey RR, Wateska AR, Zimmer SM, et al. The potential economic value of a 'universal' (multi-year) influenza vaccine. Influenza and other respiratory viruses. 2012;6:167–175. doi: 10.1111/j.1750-2659.2011.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee BY, Ufberg PJ, Bailey RR, Wiringa AE, Smith KJ, Nowalk AJ, et al. The potential economic value of a Staphylococcus aureus vaccine for neonates. Vaccine. 2010;28:4653–4660. doi: 10.1016/j.vaccine.2010.04.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee BY, Wateska AR, Bailey RR, Tai JH, Bacon KM, Smith KJ. Forecasting the economic value of an Enterovirus 71 (EV71) vaccine. Vaccine. 2010;28:7731–7736. doi: 10.1016/j.vaccine.2010.09.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee BY, Wiringa AE. The 2009 H1N1 influenza pandemic: a case study of how modeling can assist all stages of vaccine decision-making. Human vaccines. 2011;7:115–119. doi: 10.4161/hv.7.1.13740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mueller LE, Haidari LA, Wateska AR, Phillips RJ, Schmitz MM, Connor DL, et al. The impact of implementing a demand forecasting system into a low-income country's supply chain. Vaccine. 2016;34:3663–3669. doi: 10.1016/j.vaccine.2016.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norman BA, Nourollahi S, Chen SI, Brown ST, Claypool EG, Connor DL, et al. A passive cold storage device economic model to evaluate selected immunization location scenarios. Vaccine. 2013;31:5232–5238. doi: 10.1016/j.vaccine.2013.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajgopal J, Connor DL, Assi TM, Norman BA, Chen SI, Bailey RR, et al. The optimal number of routine vaccines to order at health clinics in low or middle income countries. Vaccine. 2011;29:5512–5518. doi: 10.1016/j.vaccine.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Song Y, Tai JH, Bartsch SM, Zimmerman RK, Muder RR, Lee BY. The potential economic value of a Staphylococcus aureus vaccine among hemodialysis patients. Vaccine. 2012;30:3675–3682. doi: 10.1016/j.vaccine.2012.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sosa-Estani S, Viotti R, Segura EL. Therapy, diagnosis and prognosis of chronic Chagas disease: insight gained in Argentina. Memorias do Instituto Oswaldo Cruz. 2009;104(Suppl 1):167–180. doi: 10.1590/s0074-02762009000900023. [DOI] [PubMed] [Google Scholar]
- 43.Rassi A, Jr, Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–1402. doi: 10.1016/S0140-6736(10)60061-X. [DOI] [PubMed] [Google Scholar]
- 44.Wilson LS, Strosberg AM, Barrio K. Cost-effectiveness of Chagas disease interventions in Latin America and the Caribbean: Markov models. The American journal of tropical medicine and hygiene. 2005;73:901–910. [PubMed] [Google Scholar]
- 45.Bloom DC, ET, Jane-Llopis E, et al. World Economic Forum. Geneva: Harvard School of Public Health; 2011. The global economic burden of noncommunicable diseases. [Google Scholar]
- 46.World Health Organization. WHO prequalified vaccine
- 47.Lee BY, Brown ST, Bailey RR, Zimmerman RK, Potter MA, McGlone SM, et al. The benefits to all of ensuring equal and timely access to influenza vaccines in poor communities. Health affairs. 2011;30:1141–1150. doi: 10.1377/hlthaff.2010.0778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.PH England and NHS. Removal of the infant dose of meningococcal serogroup C (MenC) conjugate vaccine given at three months from 1 July 2016. 2016 Mar 24; [Google Scholar]
- 49.Campbell H, Borrow R, Salisbury D, Miller E. Meningococcal C conjugate vaccine: the experience in England and Wales. Vaccine. 2009;27(Suppl 2):B20–B29. doi: 10.1016/j.vaccine.2009.04.067. [DOI] [PubMed] [Google Scholar]
- 50.PH England. Invasive meningococcal disease (laboratory reports in England): 2014/2015 annual data by epidemiological year. Health Protection Report [Google Scholar]
- 51.Pace D, Khatami A, McKenna J, Campbell D, Attard-Montalto S, Birks J, et al. Immunogenicity of reduced dose priming schedules of serogroup C meningococcal conjugate vaccine followed by booster at 12 months in infants: open label randomised controlled trial. Bmj. 2015;350:h1554. doi: 10.1136/bmj.h1554. [DOI] [PMC free article] [PubMed] [Google Scholar]