Abstract
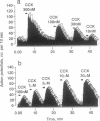
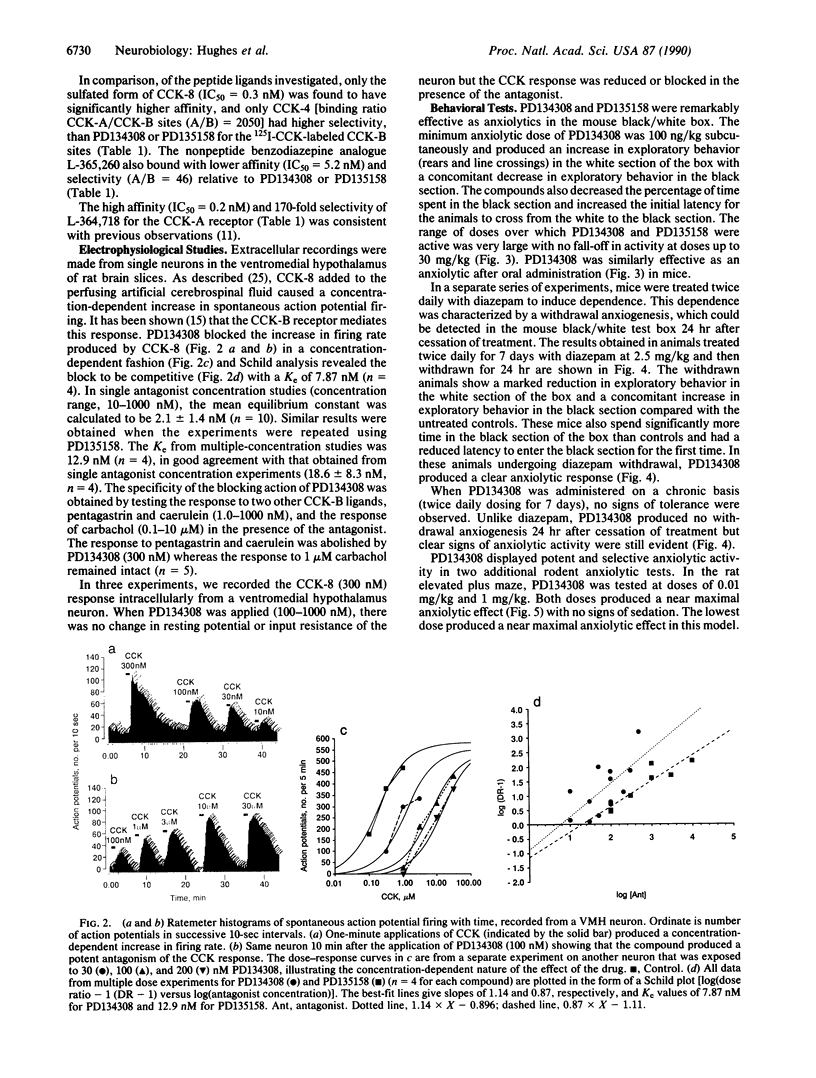
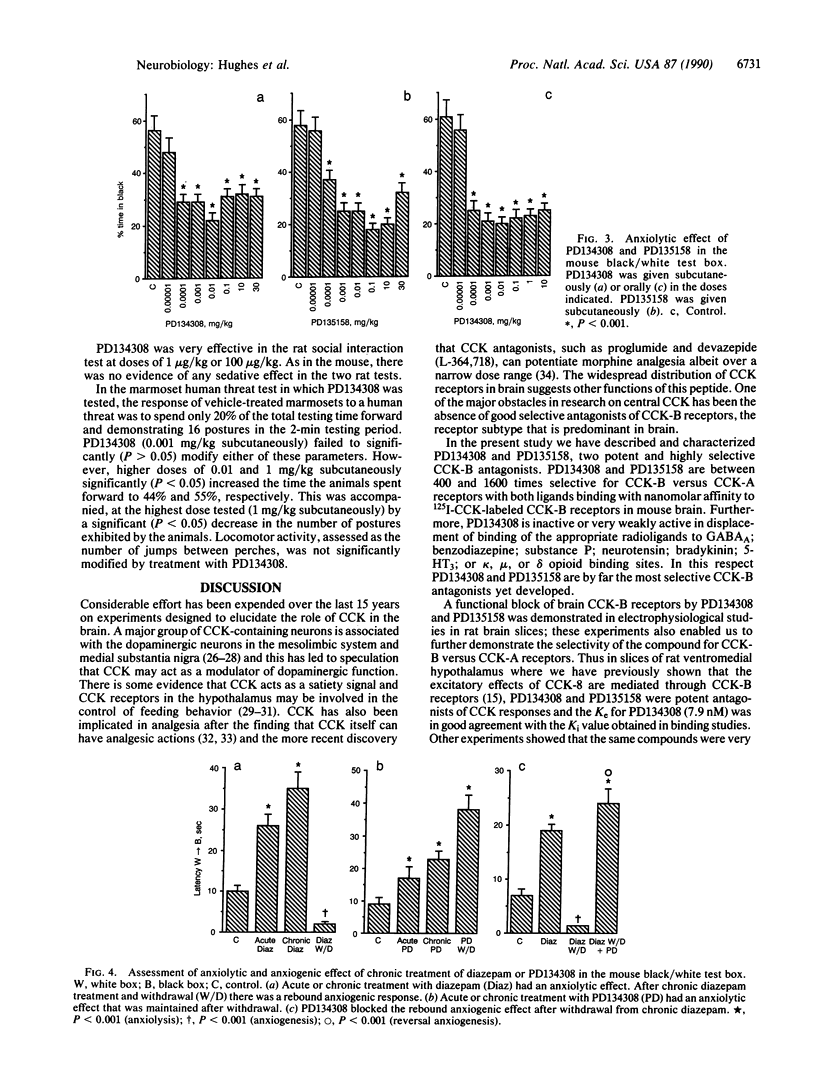
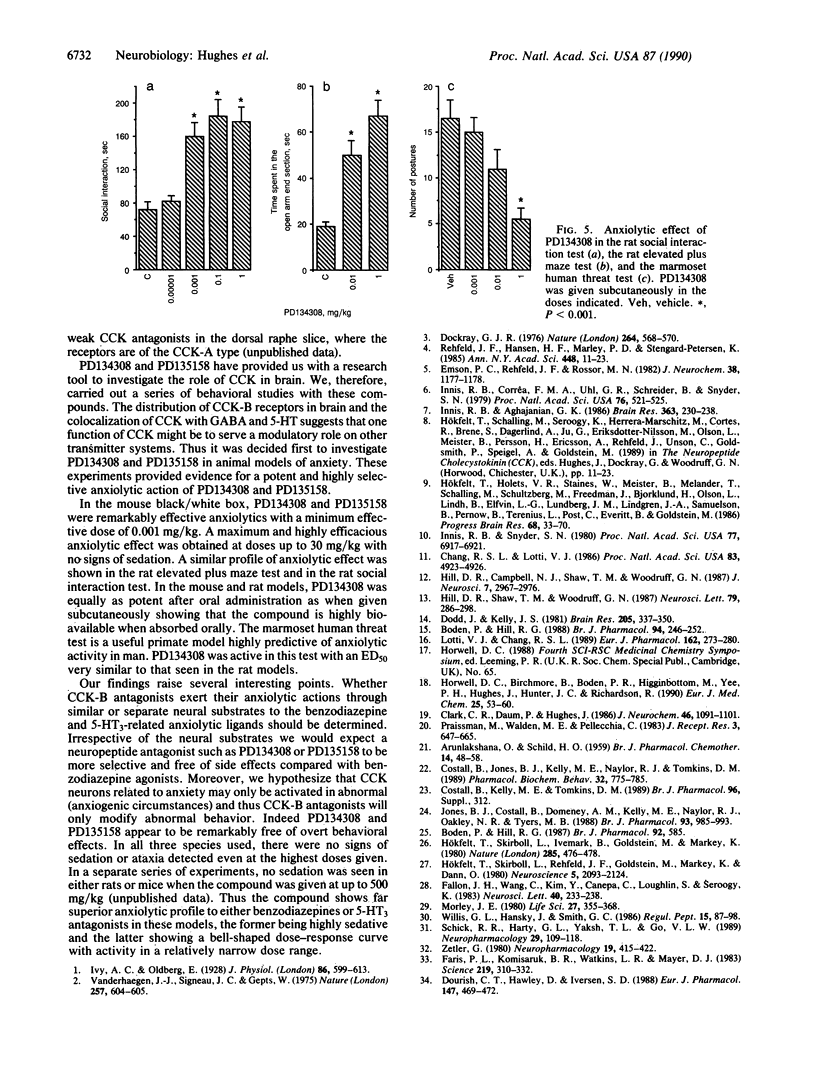
PD134308 and PD135158 are potent and selective antagonists at the cholecystokinin type B (CCK-B) receptors with IC50 values of 1.6 nM and 3.5 nM, respectively, in the radioligand binding assay and Ke values of 7.82 and 12.9 nM, respectively, in their blocking action on CCK responses in the rat lateral hypothalamic slice. PD134308 and PD135158 produced potent anxiolytic effects in the mouse black/white box test after either subcutaneous or oral administration. There was no evidence of the development of tolerance to the anxiolytic action of either PD134308 or PD135158 in mice treated twice daily for 7 days, nor was there any sign of withdrawal anxiogenesis after abrupt termination of this treatment. Both CCK-B antagonists were able to suppress the withdrawal anxiogenesis and produce an anxiolytic effect in mice previously made tolerant to diazepam. PD134308 and PD135158 produced potent anxiolytic effects in the rat elevated plus maze test and the rat social interaction test. The effects were comparable in magnitude to those seen with diazepam. However, unlike diazepam, PD134308 and PD135158 did not produce sedation. The CCK-B antagonists also showed powerful anxiolytic activity in the "marmoset human threat test." These results provide evidence of a selective role for CCK-B receptors in the control of anxiety. PD134308 and PD135158 are members of a class of anxiolytic agents that have a greatly improved profile compared with benzodiazepines or serotonin-related anxiolytics.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARUNLAKSHANA O., SCHILD H. O. Some quantitative uses of drug antagonists. Br J Pharmacol Chemother. 1959 Mar;14(1):48–58. doi: 10.1111/j.1476-5381.1959.tb00928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden P., Hill R. G. Effects of cholecystokinin and related peptides on neuronal activity in the ventromedial nucleus of the rat hypothalamus. Br J Pharmacol. 1988 May;94(1):246–252. doi: 10.1111/j.1476-5381.1988.tb11521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang R. S., Lotti V. J. Biochemical and pharmacological characterization of an extremely potent and selective nonpeptide cholecystokinin antagonist. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4923–4926. doi: 10.1073/pnas.83.13.4923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark C. R., Daum P., Hughes J. A study of the cerebral cortex cholecystokinin receptor using two radiolabelled probes: evidence for a common CCK 8 and CCK 4 cholecystokinin receptor binding site. J Neurochem. 1986 Apr;46(4):1094–1101. doi: 10.1111/j.1471-4159.1986.tb00623.x. [DOI] [PubMed] [Google Scholar]
- Costall B., Jones B. J., Kelly M. E., Naylor R. J., Tomkins D. M. Exploration of mice in a black and white test box: validation as a model of anxiety. Pharmacol Biochem Behav. 1989 Mar;32(3):777–785. doi: 10.1016/0091-3057(89)90033-6. [DOI] [PubMed] [Google Scholar]
- Dockray G. J. Immunochemical evidence of cholecystokinin-like peptides in brain. Nature. 1976 Dec 9;264(5586):568–570. doi: 10.1038/264568a0. [DOI] [PubMed] [Google Scholar]
- Dodd J., Kelly J. S. The actions of cholecystokinin and related peptides on pyramidal neurones of the mammalian hippocampus. Brain Res. 1981 Feb 2;205(2):337–350. doi: 10.1016/0006-8993(81)90344-9. [DOI] [PubMed] [Google Scholar]
- Dourish C. T., Hawley D., Iversen S. D. Enhancement of morphine analgesia and prevention of morphine tolerance in the rat by the cholecystokinin antagonist L-364,718. Eur J Pharmacol. 1988 Mar 15;147(3):469–472. doi: 10.1016/0014-2999(88)90183-5. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Rehfeld J. F., Rossor M. N. Distribution of cholecystokinin-like peptides in the human-brain. J Neurochem. 1982 Apr;38(4):1177–1179. doi: 10.1111/j.1471-4159.1982.tb05369.x. [DOI] [PubMed] [Google Scholar]
- Fallon J. H., Wang C., Kim Y., Canepa N., Loughlin S., Seroogy K. Dopamine- and cholecystokinin-containing neurons of the crossed mesostriatal projection. Neurosci Lett. 1983 Oct 10;40(3):233–238. doi: 10.1016/0304-3940(83)90044-7. [DOI] [PubMed] [Google Scholar]
- Faris P. L., Komisaruk B. R., Watkins L. R., Mayer D. J. Evidence for the neuropeptide cholecystokinin as an antagonist of opiate analgesia. Science. 1983 Jan 21;219(4582):310–312. doi: 10.1126/science.6294831. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Campbell N. J., Shaw T. M., Woodruff G. N. Autoradiographic localization and biochemical characterization of peripheral type CCK receptors in rat CNS using highly selective nonpeptide CCK antagonists. J Neurosci. 1987 Sep;7(9):2967–2976. doi: 10.1523/JNEUROSCI.07-09-02967.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill D. R., Shaw T. M., Woodruff G. N. Species differences in the localization of 'peripheral' type cholecystokinin receptors in rodent brain. Neurosci Lett. 1987 Aug 31;79(3):286–289. doi: 10.1016/0304-3940(87)90445-9. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Holets V. R., Staines W., Meister B., Melander T., Schalling M., Schultzberg M., Freedman J., Björklund H., Olson L. Coexistence of neuronal messengers--an overview. Prog Brain Res. 1986;68:33–70. doi: 10.1016/s0079-6123(08)60230-7. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Rehfeld J. F., Skirboll L., Ivemark B., Goldstein M., Markey K. Evidence for coexistence of dopamine and CCK in meso-limbic neurones. Nature. 1980 Jun 12;285(5765):476–478. doi: 10.1038/285476a0. [DOI] [PubMed] [Google Scholar]
- Hökfelt T., Skirboll L., Rehfeld J. F., Goldstein M., Markey K., Dann O. A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience. 1980;5(12):2093–2124. doi: 10.1016/0306-4522(80)90127-x. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Aghajanian G. K. Cholecystokinin-containing and nociceptive neurons in rat Edinger-Westphal nucleus. Brain Res. 1986 Jan 22;363(2):230–238. doi: 10.1016/0006-8993(86)91008-5. [DOI] [PubMed] [Google Scholar]
- Innis R. B., Corrêa F. M., Uhl G. R., Schneider B., Snyder S. H. Cholecystokinin octapeptide-like immunoreactivity: histochemical localization in rat brain. Proc Natl Acad Sci U S A. 1979 Jan;76(1):521–525. doi: 10.1073/pnas.76.1.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis R. B., Snyder S. H. Distinct cholecystokinin receptors in brain and pancreas. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones B. J., Costall B., Domeney A. M., Kelly M. E., Naylor R. J., Oakley N. R., Tyers M. B. The potential anxiolytic activity of GR38032F, a 5-HT3-receptor antagonist. Br J Pharmacol. 1988 Apr;93(4):985–993. doi: 10.1111/j.1476-5381.1988.tb11489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti V. J., Chang R. S. A new potent and selective non-peptide gastrin antagonist and brain cholecystokinin receptor (CCK-B) ligand: L-365,260. Eur J Pharmacol. 1989 Mar 21;162(2):273–280. doi: 10.1016/0014-2999(89)90290-2. [DOI] [PubMed] [Google Scholar]
- Morley J. E. The neuroendocrine control of appetite: the role of the endogenous opiates, cholecystokinin, TRH, gamma-amino-butyric-acid and the diazepam receptor. Life Sci. 1980 Aug 4;27(5):355–368. doi: 10.1016/0024-3205(80)90183-6. [DOI] [PubMed] [Google Scholar]
- Praissman M., Walden M. E., Pellecchia C. Identification and characterization of a specific receptor for cholecystokinin on isolated fundic glands from guinea pig gastric mucosa using a biologically active 125I-CCK-8 probe. J Recept Res. 1983;3(5):647–665. doi: 10.3109/10799898309041952. [DOI] [PubMed] [Google Scholar]
- Rehfeld J. F., Hansen H. F., Marley P. D., Stengaard-Pedersen K. Molecular forms of cholecystokinin in the brain and the relationship to neuronal gastrins. Ann N Y Acad Sci. 1985;448:11–23. doi: 10.1111/j.1749-6632.1985.tb29902.x. [DOI] [PubMed] [Google Scholar]
- Schick R. R., Harty G. J., Yaksh T. L., Go V. L. Sites in the brain at which cholecystokinin octapeptide (CCK-8) acts to suppress feeding in rats: a mapping study. Neuropharmacology. 1990 Feb;29(2):109–118. doi: 10.1016/0028-3908(90)90050-2. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen J. J., Signeau J. C., Gepts W. New peptide in the vertebrate CNS reacting with antigastrin antibodies. Nature. 1975 Oct 16;257(5527):604–605. doi: 10.1038/257604a0. [DOI] [PubMed] [Google Scholar]
- Willis G. L., Hansky J., Smith G. C. Central and peripheral proglumide administration and cholecystokinin-induced satiety. Regul Pept. 1986 Aug;15(1):87–98. doi: 10.1016/0167-0115(86)90079-0. [DOI] [PubMed] [Google Scholar]
- Zetler G. Analgesia and ptosis caused by caerulein and cholecystokinin octapeptide (CCK-8). Neuropharmacology. 1980 May;19(5):415–422. doi: 10.1016/0028-3908(80)90047-7. [DOI] [PubMed] [Google Scholar]