A hallmark of chronic hepatitis B virus (HBV) infection is the functional impairment and depletion of antiviral T cells. In this issue of JEM, Pallett et al. identify a reservoir of functional HBV-specific T cells among liver-resident T cells.
Abstract
A hallmark of chronic hepatitis B virus (HBV) infection is the functional impairment and depletion of antiviral T cells. In this issue of JEM, Pallett et al. (https://doi.org/10.1084/jem.20162115) identify a reservoir of functional HBV-specific T cells among liver-resident T cells.

Insight from Fabian J. Bolte and Barbara Rehermann
More than 240 million people worldwide are chronically infected with the hepatitis B virus (HBV) and are at risk of developing liver cirrhosis and hepatocellular carcinoma. HBV is a small, circular DNA virus that forms a mini-chromosome as its transcriptional template within the nucleus of hepatocytes. Therefore, HBV replication can be suppressed but the virus cannot be eliminated by nucleoside analogue therapy.
Host immune responses play a significant role in the outcome of HBV infection (Park and Rehermann, 2014). The majority of patients who acquire HBV infection during adulthood recover spontaneously with vigorous T cell and antibody responses that provide long-term control of small traces of persisting virus. In contrast, almost all patients who are infected at birth develop chronic infection. In chronic HBV infection, HBV-specific immune responses are profoundly impaired via multiple mechanisms, including the antigen-dependent induction of inhibitory molecules such as PD-1, Tim-3, and CTLA-4 (Boni et al., 2007; Rehermann, 2013). This results in an “exhausted” phenotype of antigen-specific T cells, similar to that observed in HIV infection and cancer.
Even though a small percentage of patients recover spontaneously from chronic HBV infection, all efforts to restore immune responses in patients with chronic HBV infection have failed. A partial recovery of HBV-specific T cell function has been achieved in vitro by stimulation with viral peptides in the presence of antibodies against inhibitory receptors (Fisicaro et al., 2010). However, it is not known whether the same can be achieved in vivo and whether these cells would maintain their functionality in the immunotolerant environment of the liver.
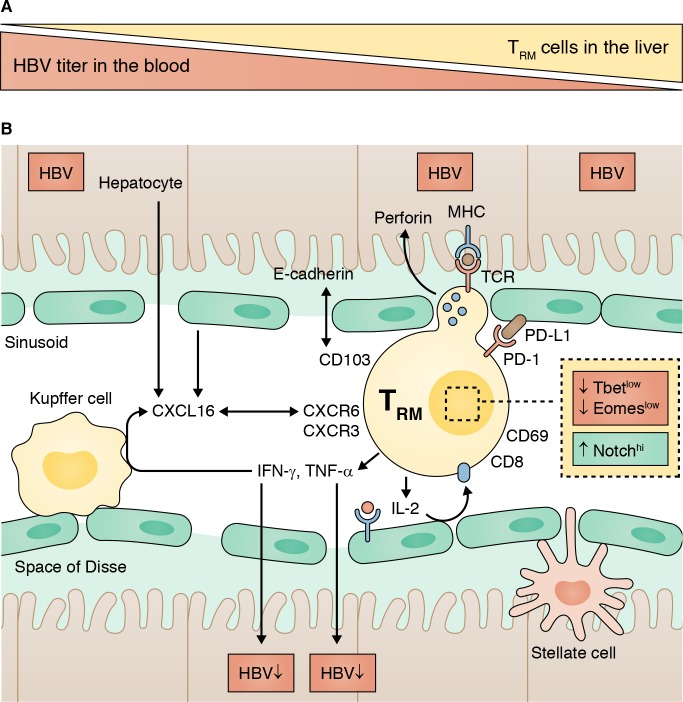
In this issue, Pallett et al. describe a distinct population of tissue-resident memory T (TRM) cells that are enriched in the liver of patients with chronic HBV infection as compared with uninfected controls. TRM cells were identified by their surface expression of CD69, which negatively regulates sphingosine-1-phosphate receptor 1 (S1P1)–mediated egress of T cells from tissues (Skon et al., 2013; Mackay et al., 2015). TRM cells lack expression of lymph node–homing molecules CD62L and CCR7. In their study, Pallett et al. (2017) distinguish between CD69+CD103− and CD69+CD103+ TRM cells based on the expression of the αEβ7 integrin (CD103), which binds to e-cadherin on epithelial cells. CD69 and CD103 have also been described as key markers for TRM cells in several other tissues, including skin, lung, and intestine (Mackay et al., 2013; Mueller and Mackay, 2016).
(A) TRM cells are enriched in the HBV-infected liver, and their frequency correlates inversely with HBV titer. (B) CD8+ TRM cells reside in the liver sinusoids. They express the tissue-homing marker CD69, and a subset additionally expresses the integrin CD103. TRM cells display a unique transcriptional profile (T-betlo, Eomeslo, Notchhi). Despite high expression levels of the inhibitory receptor PD-1, they readily produce antiviral cytokines upon in vitro stimulation.
In the liver, CD69+CD103− cells comprise a heterogenous population of memory T cells that includes unconventional T cells such as mucosal associated invariant T (MAIT) cells and γδ-T cells, some of which are also present in the peripheral blood. In contrast, CD69+CD103+ TRM cells are absent in the blood and account for ∼20% of memory CD8 T cells in the liver of patients with chronic HBV infection (∼10% in uninfected controls). Using t-distributed stochastic neighbor embedding (tSNE) multidimensional analysis, Pallett et al. (2017) demonstrate that CD69+CD103+ TRM cells express a uniqe transcriptional signature (T-betloEomesloBlimp-1loHobitlo). They also express specific chemokine receptors such as CXCR6 and CXCR3. Thus, CXCL16, released by activated myeloid cells, may mediate the migration of CXCR6+ TRM to the site of inflammation.
Similar to other liver-resident cells such as Kupffer cells, TRM cells are localized in the vascular space of the liver sinusoids, from where they interact with hepatocytes through the fenestrated liver sinusoidal epithelial cell (LSEC) layer. In this location, TRM cells are exposed to the inhibitor molecule PD-L1, which is up-regulated on hepatocytes and LSECs in viral hepatitis (Mühlbauer et al., 2006). This is important because TRM cells express high levels of the exhaustion markers PD-1 and CD39. PD-1–PD-L1 interaction has been reported as the main mechanism that “turns off” the antiviral cytokine production of highly functional HBV-specific memory T cells injected into HBV transgenic mice (Isogawa et al., 2005). Using both MHC multimer staining and stimulation with HBV-specific peptides, Pallett et al. (2017) show that ∼90% of HBV-specific T cells in the liver of patients with chronic HBV infection have a TRM phenotype (either CD69+CD103− or CD69+CD103+ cells). Despite high expression of PD-1 and CD39, they readily produce IFNγ, TNFα, and IL-2 upon in vitro stimulation. IL-2 production was most strikingly enhanced within the subset of CD69+CD103+ TRM cells, suggesting that they are independent from CD4+ T cell–derived IL-2 and able to overcome PD-L1–mediated inhibition by LSECs (Maini and Schurich, 2010).
Pallett et al. (2017) propose that HBV-specific TRM cells are positioned at the right place to exert antiviral effector functions. Of particular interest is the production of antiviral cytokines because IFNγ- and TNFα-mediated control of HBV replication has previously been demonstrated (Guidotti et al., 1999). In addition, TRM cells express high levels of perforin, which may contribute to direct killing of infected hepatocytes. Interestingly, Pallett et al. (2017) observed an inverse correlation between TRM frequency and HBV viral load with the highest TRM frequency in patients with HBV-DNA titers of <2,000 IU/ml (cut-off for an inactive HBV carrier). This raises hope that TRM cells can contribute to the functional cure of HBV, i.e., to the immune-mediated control of small amounts of virus that are continuously transcribed from the cccDNA. Indeed, TRM cells were also present in the liver of two donors who had spontaneously recovered from chronic HBV infection (negative for HBsAg and HBV DNA and positive for anti-HBs and anti-HBc), suggesting the long-term maintenance of virus-specific memory CD8 T cells in the liver. However, the frequency of TRM cells did not differ in patients stratified by the phase of chronic HBV infection (HBeAg status, alanine aminotransferase activity, and liver inflammation). Thus, it will be important to further define the role of intrahepatic TRM cells during the course of chronic HBV infection.
The results by Pallett et al. (2017) are promising because a therapeutic expansion of HBV-specific TRM cells (e.g., by sequential antigen-specific and cytokine-dependent stimulation as proposed in this study) may result in improved viral control. This may require new routes of antigen delivery because it has been recently shown that intravenous administration of a malaria vaccine expands pathogen-specific CD8 T cells in the liver, suggesting that durable protection against malaria can be mediated by tissue-resident T cells (Ishizuka et al., 2016). Alternatively, it would be of interest to investigate whether the function of TRM cells can be enhanced in vivo by altering the tolerogenic environment of the liver (e.g., by PD-1/PD-L1 blockade).
These are exciting new research directions, but they also raise formidable challenges in terms of monitoring these liver-directed and immune-modulatory therapies. Whereas patients who spontaneously resolve HBV infection exhibit readily detectable HBV-specific memory T cells in the peripheral blood (Rehermann et al., 1996), any therapeutic strategy that targets TRM cells and their environment can only be assessed by performing liver biopsies.
In conclusion, Pallett et al. (2017) have demonstrated the presence of an abundant population of HBV-specific memory T cells that are resident in the liver. These cells display a distinct phenotype and are strategically positioned for site-specific immune surveillance and immune responses. It is critical to further decipher their role to develop effective immunotherapeutic approaches for chronic HBV infection.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute for Diabetes, Digestive, and Kidney Diseases, National Institutes of Health.
References
- Boni C., et al. J. Virol. 2007 doi: 10.1128/JVI.02844-06. [DOI] [Google Scholar]
- Fisicaro P., et al. Gastroenterology. 2010 doi: 10.1053/j.gastro.2009.09.052. [DOI] [Google Scholar]
- Guidotti L.G., et al. Science. 1999 doi: 10.1126/science.284.5415.825. [DOI] [Google Scholar]
- Ishizuka A.S., et al. Nat. Med. 2016 doi: 10.1038/nm.4110. [DOI] [Google Scholar]
- Isogawa M., et al. Immunity. 2005 doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Mackay L.K., et al. Nat. Immunol. 2013 doi: 10.1038/ni.2744. [DOI] [Google Scholar]
- Mackay L.K., et al. J. Immunol. 2015 doi: 10.4049/jimmunol.1402256. [DOI] [Google Scholar]
- Maini M.K., and Schurich A. J. Hepatol. 2010 doi: 10.1016/j.jhep.2009.12.017. [DOI] [Google Scholar]
- Mueller S.N., and Mackay L.K. Nat. Rev. Immunol. 2016 doi: 10.1038/nri.2015.3. [DOI] [PubMed] [Google Scholar]
- Mühlbauer M., et al. J. Hepatol. 2006 doi: 10.1016/j.jhep.2006.05.007. [DOI] [Google Scholar]
- Pallett L.J., et al. J. Exp. Med. 2017 doi: 10.1084/jem.20162115. [DOI] [Google Scholar]
- Park S.H., and Rehermann B. Immunity. 2014 doi: 10.1016/j.immuni.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehermann B., et al. Nat. Med. 1996 doi: 10.1038/nm1096-1104. [DOI] [PubMed] [Google Scholar]
- Rehermann B. Nat. Med. 2013 doi: 10.1038/nm.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skon C.N., et al. Nat. Immunol. 2013 doi: 10.1038/ni.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]