Abstract
This study aims to examine the potential reasons for the current prevalence of the fusarium wilt in the oriental melon. Twenty-seven Fusarium isolates obtained from oriental melon greenhouses in 2010–2011 were identified morphologically and by analysis of elongation factor-1 alpha gene (EF-1α) and internal transcribed spacer (ITS) rDNA sequences as 6 Fusarium species (8 isolates of F. oxysporum, 8 F. commune, 5 F. proliferatum, 3 F. equiseti, 2 F. delphinoides, and 1 F. andiyazi), which were classified as same into 6 EF-1α sequence-based phylogenetic clades. Pathogenicity of the Fusarium isolates on the oriental melon was highest in F. proliferatum, next in F. oxysporum and F. andiyazi, and lowest in the other Fusarium species tested, suggesting F. proliferatum and F. oxysporum were major pathogens of the oriental melon, inducing stem rots and vascular wilts, respectively. Oriental melon and watermelon were more susceptible to F. oxysporum than shintosa and cucumber; and cucumber was most, oriental melon and watermelon, medially, and shintosa was least susceptible to F. proliferatum, whose virulence varied among and within their phylogenetic subclades. Severe root-knot galls were formed on all the crops infected with Meloidogyne incognita; however, little indication of vascular wilts or stem and/or root rots was shown by the nematode infection. These results suggest the current fungal disease in the oriental melon may be rarely due to virulence changes of the fusarium wilt pathogen and the direct cause of the severe root-knot nematode infection, but may be potentially from other Fusarium pathogen infection that produces seemingly wilting caused by severe stem rotting.
Keywords: Fusarium species, identification, pathogenicity, root-knot nematode, virulence
Plants belonging to Family Cucurbitaceae such as cucumber, oriental melon and watermelon are important vegetable crops worldwide and major greenhouse-grown fruit-bearing vegetables in Korea, of which the cultivation areas are 3,629 ha, 5,515 ha, and 12,299 ha with annual productions of 254,276 tons, 176,622 tons, and 672,914 tons, respectively (MAFRA, 2015). Particularly for the oriental melon (Cucumis melo var. makuwa Makino), the greenhouse cultivation area comprises the highest ratio (97.5%) of the total area among the other fruit-bearing vegetables with the greenhouse ratios of around 80%, indicating the growers’ preference to the continuous greenhouse-cropping of the oriental melon in limited areas, which is probably because of the high benefit return from the greenhouse farming.
The oriental melon has been suffered from a variety of diseases caused by 8 viruses, 4 bacteria, thirties of fungi and Oomycetes, and 3 root-knot nematodes (KSPP, 2009). Among all of these diseases, the most detrimental ones in the continuous greenhouse-cropping are caused by soilborne pathogens such as Fusarium species and root-knot nematodes that accumulate their propagules during the successive cropping in greenhouse conditions that are favorable for their growth and reproduction. This facility cultivation also ensures the survival of the pathogens in harshly cold weather conditions during the winter time in Korea.
Cultivation fields once infested with the fusarium wilt pathogen (Fusarium oxysporum f. sp. melonis) face significant and continuous disease problems, consequently resulting in the replant problem (Banihashemi and deZeeuw, 1975). Also the root-knot nematodes reproduce continuously without cessation in such warm-temperature conditions as in greenhouses and thereby the root-knot nematodes have been highly populated in oriental melon greenhouses because of the continuous cultivation for 20 years in Korea (Kim and Yeon, 2001; Kim et al., 2005). This is evidenced by the population dynamics of the root-knot nematodes and their serious damages due to the successive cropping of the oriental melon in Korea (Byeon et al., 2014; Cho et al., 2000; Kim, 2001a, 2001b; Kim and Yeon, 2001; Kwon et al., 1998; Park et al., 1995).
It is very difficult to control soil-borne diseases using chemical pesticides in general (Agrios, 2004). For the fusarium wilt of the oriental melon, no fungicides are commercially available to practice the disease control with a full efficiency, but the use of resistant cultivars is suggested as an efficient and environmentally friendly control strategy for the disease (Freeman et al., 2002). However, this control strategy has rarely been implemented in the oriental melon until now in Korea as no oriental melon cultivar resistant to the Korean race of the pathogen has been developed yet (Lee et al., 2015; Matsumoto et al., 2011). For the root-knot nematodes, the use of resistant cultivars is also an efficient and economical way of the nematode control, although there are several methods such as admixtures of soil, rotation and flooding reliable for their control (Byeon et al., 2014; Kim and Choi, 2001; Kinloch and Hinson, 1972; Rhoades, 1976). However, no oriental melon cultivars resistant to the root-knot nematodes have ever been commercialized in Korea yet.
In the cucurbitaceous crops, the vegetable production with grafted seedlings has been practiced for a long period of time from 1920, and currently it becomes a common practice (constituting about 96% of the total plant propagation) to grow the oriental melon during the winter time to increase cold tolerance and to prevent the fusarium wilt caused by F. oxysporum f. sp. melonis (Kim et al., 2005; Lee, 1994; Lee and Oda, 2003). Several rootstocks for grafting the oriental melon have been developed for their resistance to the fusarium wilt, among which shintosa (Cucurbit maxima × Cu. moschata) has been widely used as a rootstock plant since it was firstly used in 1920 (Lee, 1994). However, the fusarium wilt still occurs prevalently in oriental melon greenhouses with little indication of the disease subsided by the grafting rootstocks resistant to the fungal disease.
F. oxysporum with more than 80 forma speciales morphologically indistinguishable shows cross-pathogenicity among cucurbitaceous vegetables (Cafri et al., 2005; Owen, 1955; Zhou and Everts, 2007). Fusarium spp. other than F. oxysporum such as F. equiseti, F. graminearum, F. moniliforme, F. proliferatum, F. sambucinum, F. solani, and F. semitectum (F. incarnatum) were reported to be related with fusarium diseases in oriental melon areas (Kim and Kim, 2004). These aspects make it difficult to find out the reasons for current fusarium wilt problems in the oriental melon. Thus, this study aims to reveal the reasons for the current prevalence of the fusarium wilts in the oriental melon greenhouses firstly by examining the distribution and pathogenicity of Fusarium species to find out the major fusarium pathogens responsible for the fusarium wilt prevalence. This includes the pathological changes of F. oxysporum and the occurrence of other Fusarium spp. inducing the disease. Secondly it was examined on any direct relationships of the root-knot nematodes with the prevalence of the wilt symptoms when they have been highly populated during the continuous greenhouse cropping so that their infections are severe (Agrios, 2004).
Materials and Methods
Isolation of Fusarium spp
Fusarium species were isolated from rhizosphere soils and stem tissues of the greenhouse-grown oriental melon in two different locations, Seongju (Gyeongbuk province) and Yeoju (Gyeonggi province), Korea, during the disease survey in 2010 and 2011. Soil samples taken from rhizospheres of the oriental melon with the presumed fusarium wilt symptoms were dried for 24 h in a laminar flow hood. The soil samples were serially diluted to make soil suspensions of the concentration of 10−3 g ml−1, and plated on Fusarium-selective medium, Komada’s agar (Komada, 1975). Stem samples from the presumably diseased oriental melon plants were rinsed with tap water to remove adhering soils and debris, and cut with a flame-sterilized razor blade into c.a. 1-cm stem tissues. These stem tissues were surface sterilized with 70% ethanol for 30 s and 1.0% sodium hypochlorite for 30 s, followed by rinsing with sterile distilled water (SDW) two times, and then placed on Komada’s agar. Putative Fusarium colonies formed on soil suspension-plated agar and mycelial pieces grown out from the stem tissue samples were transferred and cultured on potato-dextrose agar (PDA; Difco, Detroit, MI, USA) at 25°C for 10 days in an incubator. The mycelial plugs were transferred to carnation leaf agar (CLA) and cultured at 25°C for three days, followed by storing at −80°C in a deep freezer until use.
Species identification of Fusarium isolates
Species of the Fusarium isolates were identified based on microscopic and macroscopic characteristics (totally morphological characteristics) of single-spored fusarium isolates as described in other studies (Leslie and Summerell, 2006; Marasas et al., 2001; Schroers et al., 2009; Skovgaard et al., 2003). For microscopic observation, the Fusarium isolates were cultured on CLA at 25°C for 10 days in the dark for examining macroconidia, microconidia, phialides and microconidial chains (Fisher et al., 1982). For the formation of chlamydospores, the fungal isolates were cultured on Spezieller Nährstoffarmer agar (SNA) at 20°C for 14 days when they were observed under a compound light microscope or for 21 days when they were not observed at 14 days after incubation. For all microscopic observations, three agar plates were used to view the presence of the structures, if any, they were collected randomly with three replications for each plate and their morphological characteristics were examined under a compound light microscope (Axiophot; Zeiss, Oberkochen, Germany). For macroscopic observation, the cultural appearances (colony colors [pigmentations]) were observed on PDA, which were determined using Methuen handbook of color chart (Kornerup and Wanscher, 1978).
For molecular identification of the Fusarium isolates, the genomic DNA was extracted from the fungal colonies formed on PDA by single-step protocol of Thompson and Henry (1995). A portion of the elongation factor-1 alpha gene (EF-1α) was amplified using the primers, EF1: 5′-ATGGGTYAAGGAGGACAAGAC-3′ and EF2: 5′-GGAAGTACCAG-TGATGTT-3′ under the following PCR cycling conditions: 2 min at 95°C; followed by 35 cycles of 30 s at 94°C, 30 s at 54°C, and 1 min at 72°C; and a final cycle of 10 min at 72°C. In addition, PCR for internal transcribed spacer (ITS) of rDNA was performed with universal primers ITS1 and ITS4 (White et al., 1990), which was performed in a total volume of 25 μl containing 10× PCR buffer, 0.2 mM dNTP, 0.5 U of Taq DNA polymerase, 20 pmol of both primers, and 50 ng of template DNA. The PCR products were purified using a QIAquick Purification Kit (Qiagen, Santa Clarita, CA, USA). PCR products were sequenced using an automated DNA sequencer (ABI3730XL; Applied Biosystems, Foster City, CA, USA), and compared by BLAST analysis to the gene sequences of the Fusarium isolates registered in GenBank of US National Center for Biotechnology Information (NCBI; http://www.ncbi.nlm.nih.gov/Entrez/) and FUSARIUM-ID database (http://fusarium.cbio.psu.edu) (Geiser et al., 2004). Nucleotide sequences of EF-1α that is more informative for Fusarium spp. than ITS-rDNA (da Silva et al., 2014) were analyzed using molecular evolutionary genetics analysis (MEGA 5.0 version) and used to perform sequence alignment and maximum likelihood analysis to construct phylogenetic tree of the Fusarium isolates tested in this study.
Pot experiments for pathogenicity (virulence) tests of Fusarium isolates
Oat-meal medium (oat meal:sand:distilled water, 1:20:4, w/w/w) sterilized at 121°C for 20 min in an autoclave was used for preparing the inoculums of the Fusarium isolates. Mycelial plugs from the fungal cultures grown on PDA at 25°C for 7 days were inoculated into the oat meal medium and incubated at 25°C for 15 days. Fifteen day-old seedlings of shintosa (C. maxima × C. moschata), oriental melon (C. melo var. makuwa Makino cv. Searon-Ggul), and cucumber (Cucumis sativus L. cv. Headong-baekdadagi) and 20-day-old seedlings of watermelon (Citrullus lanatus [Thumb] Matsum & Nakai cv. Wori-Ggul) were planted in plastic pots of 10-cm-diameter filled with 130 g sterilized mixtures (1:1) of sand and bed soil (composed of 64.9% coco-peat, 15% peat-moss, 7% zeolite, 10% perlite, 2.6% dolomite, 0.03% wetting agent, and 0.47% N-P-K common fertilizer). For inoculation, the top soils in a depth of 5 cm were mixed with 10 g oat meal medium infested with the fungi, in which each seedling was planted with four replications for each treatment, arranged in a factorial design of two factors (plant × pathogen), and grown at 25 ± 2°C in a greenhouse, watering daily to field capacity. The plants were examined every seven days for symptom development until 4 weeks after inoculation, when the plants were pulled out, washed free of soil with tap water, and observed wilt or stem rot symptoms (disease severity) using the method modified from Bletsos (2005), which was based on vascular wilt severity index (disease index, DI) 0 to 5; 0 = no symptom; 1 = underground stem yellow-brownish discolored; 2 = < 30% above-ground stem brownish discolored; 3 = stem bottom region decayed; 4 = stem darkly discolored and split; 5 = whole plant dead, or those corresponding to these disease indices for others such as derived from root or stem rot symptoms.
Pathogenicity of the total Fusarium spp. isolated from the oriental melon greenhouses and reference isolates including F. oxysporum f. sp. melonis (FOM) and F. oxysporum f. sp. niveum (FON) was tested on the oriental melon with four replications, among which the isolates of F. oxysporum and F. proliferatum that were major species and highly pathogenic to the oriental melon were tested for virulence on cucurbitaceous vegetables such as oriental melon, watermelon, shintosa and cucumber.
Pot experiments for pathogenicity tests of Meloidogyne incognita
The rook-knot nematode, Meloidogyne incognita was obtained from pure cultures maintained on chili pepper (Capsicum annuum cv. Bugang) in a greenhouse (Seo et al., 2014). For inoculum preparation, the plants were uprooted and the entire root system was dipped in water to remove adhering soil. Egg masses of M. incognita were isolated by hand-picking with the help of forceps, and incubated on Baermann funnels for 3–5 days to make second-stage nematode juveniles (J2) hatched out of eggs (Son et al., 2008; Southey, 1986), and diluted to make a nematode suspension to the concentration of about 400 J2 ml−1 in SDW. Seedlings of the cucurbitaceous plants at the same growth stages as in the pathogenicity test of the Fusarium isolates were inoculated with the nematode by pouring 5 ml nematode suspension (containing about 2,000 J2 per pot) and grown at 25 ± 2°C in a greenhouse, watering daily to field capacity, in a completely randomized design of single factors (plant). At 4 weeks after inoculation, plants were uprooted from the pots and washed free of soil with tap water, and formations of the root-knot galls and eggmasses were examined with naked eyes, which were evaluated by gall index and eggmass index, respectively. The gall index was scored 0–5 assigned as 0 = 0–10%; 1 = 11–20%; 2 = 21–50%; 3 = 51–80%; 4 = 81–90%; 5 = 91–100% of galled roots (Baker, 1985); eggmass index was assigned to each eggmass number using ratings of 0 = no eggmass; 1 = 1–2, 2 = 3–10, 3 = 11–30, 4 = 31–100, and 5 ≥ 100 egg masses per root system (Roberts et al., 1990). Each treatment was replicated five times.
Statistical analysis
Pathogenicity of Fusarium isolates on the oriental melon was tested with four replications, for which comparison of the pathogenicity among clades (Fusarium spp.) was tested by the general analysis of variance for a nested experiment. For virulence of F. oxysporum isolates, inoculation experiments were conducted twice each with 4 replications, whose results were pooled to have doubled (8) replications and analyzed in a two factor factorial design for two factors of plant and pathogen (including their interactions) with both quantitative levels, conducting a nested experimental analysis for comparing virulence among subclades of F. oxysporum of which the isolates were nested in the 4 subclades. For virulence of F. proliferatum isolates on the cucurbitaceous vegetables, the plants were inoculated by the pathogen with 5 replications, conducting a nested experimental analysis for comparing virulence among its subclades as in F. oxysporum. For M. incognita, single factors (plants) of one treatment (nematode inoculation) were analyzed in a completely randomized design for their significant differences among the factors. For all of these experiments, analyses of variance were carried out using SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Fisher’s least significant difference was employed to test for significant differences among the factors at P ≤ 0.05 (two-tailed) from the critical values in the t-distribution table for all the pathogenicity and virulence tests.
Results
Isolation and species identification of Fusarium isolates
A total of 27 Fusarium isolates were obtained from 216 soil and plant samples collected from the two oriental melon-growing areas of Seongju (20 isolates; including 5 F. oxysporum isolates, 7 F. commune isolates, 2 F. proliferatum isolates, and 6 other Fusarium species isolates) and Yeoju (7 isolates; including 3 F. oxysporum isolates, one F. commune isolate, and 3 F. proliferatum isolates) in 2010 and 2011 (Table 1). The species identifications of these Fusarium isolates were determined when their morphological and molecular genetic characteristics show their species identities in common. For morphological (macro- and microscopic) characteristics, vegetative and asexual reproductive structures were examined, including colony color (pigmentation), micro- and macroconidia, and chlamydospores (Fig. 1). Based on their microscopic and macroscopic characteristics, showing little variation within the same species and variable degrees of variations depending on Fusarium species and morphological characteristics, these Fusarium isolates were identified as 6 Fusarium spp. including F. oxysporum (8 isolates), F. commune (8 isolates), F. proliferatum (5 isolates), F. equiseti (3 isolates), F. delphinoides (2 isolates), and F. andiyazi (1 isolate) (Table 1, Fig. 1). Among these species, F. oxysporum, F. commune and F. proliferatum were the major populations in the oriental melon-growing greenhouses in Korea.
Table 1.
Identification of Fusarium isolates from greenhouse-grown oriental melons by morphological characteristics and gene sequencing analysis of elongation factor-1 alpha gene (EF-1α) and rDNA internal transcribed spacer (ITS)
| Isolation area* | Isolate | Morphological characteristic | Identification ¶ | Most identical GenBank accession no. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Pigment | Microconidia† | Chlamy dospore‡ | Macroconidia | EF-1α | ITS | |||||
|
| ||||||||||
| No. of septa | Apical cell§ | Basal cell|| | ||||||||
| SB | F4 | Orange | E, O | R, S | 2–3 | T | F | F. oxysporum | KT005896.1 | KU984712.1 |
| YI | F8 | Violet | E, O | S | 3 | C | F | KU128954.1 | KU872840.1 | |
| YG | F9 | Violet | E, O | S | 2–3 | T | F | KP761170.1 | KU195688.1 | |
| YI | F10 | Orange | E, K, O | S | 3 | T | F | KT794174.1 | KU195687.1 | |
| SJ | F19 | Violet | E, O | R | 2–3 | C | F | KT006896.1 | KU931554.1 | |
| SJ | F20 | Violet | E, O | S | 3 | TC | F | JF740824.1 | KU097256.1 | |
| SY | F21 | Orange | E, O | S | 3 | TC | F | KJ920413.1 | KU195686.1 | |
| SB | F23 | Violet | E | R | 3 | TC | SC | KT884662.1 | KU984712.1 | |
| SY | F2 | Violet | E, O | S | 2 | SC | F | F. commune | KU341327.1 | KT982281.1 |
| SB | F3 | Violet | E | S | 1–3 | SC | F | KU341327.1 | KT982281.1 | |
| YD | F11 | Orange | E | S | 1–3 | SC | F | KU341325.1 | KU341324.1 | |
| SB | F15 | Violet | E | S | 1–3 | SC | F | JX289893.1 | KT982280.1 | |
| SW | F17 | Orange | E | R, S | 1–3 | SC | F | KP868559.1 | KU341323.1 | |
| SJ | F24 | Pale orange | E | S | 2–3 | SC | F | KC430630.1 | KU341324.1 | |
| SY | F25 | Violet | E, O | S | 3 | SC | F | KP868659.1 | KT982281.1 | |
| SW | F28 | Orange | E, O | S | 2–3 | SC | F | KP868659.1 | KU341424.1 | |
| YI | F5 | Violet | E | nd | 3–5 | C | Pd | F. proliferatum | KU847810.1 | KP267107.1 |
| YD | F6 | Greyish orange | E, O | nd | 3 | C | Pd | KM462980.1 | KT803067.1 | |
| YI | F7 | Orange | E | nd | 3 | C | Pd | KP9649061 | KP267107.1 | |
| SW | F14 | Violet | E | nd | 3 | C | Pd | KP9649061 | KP267107.1 | |
| SY | F18 | Violet | E, O | nd | 2–3 | C | Pd | KP964907.1 | KT462721.1 | |
| SY | F1 | Pale orange | nd | Cl | 3 | TE | PF | F. equiseti | KP267226.1 | KU041860.1 |
| SJ | F22 | Orange | nd | Ch, Cl | 3 | TE | PF | DQ842087.1 | KU041860.1 | |
| SY | F29 | Orange | nd | Ch | 5 | TE | PF | FJ895283.1 | KU041860.1 | |
| SJ | F26 | Orange | S | Ch | 3 | C | S | F. delphinoides | AB817172.1 | KU296244.1 |
| SW | F27 | Orange | S | Ch | 2 | C | S | EU926292.1 | KU296244.1 | |
| SY | F16 | Dark pink | E | nd | 3 | SC | P | F. andiyazi | KT257545.1 | KP245748.1 |
| FOM** | Violet | E, K, O | S | 3 | T | F | F. oxysporum f. sp. melonis | |||
| FON | Violet | E, O | S | 3 | T | F | F. oxysporum f. sp. niveum | |||
Isolation areas: Seongju-gun, Byeokjin-myeon (SB), Jocheon-myeon (SJ), Yongam-myeon (SY), Wolhyang-myeon (SW); Yeoju-gun, Ipo-ri (YI), Gyesan-ri (YG), Daesin-ri (YD).
E, elliptical; O, oval; K, kidney; nd, not detected; S, straight shaped.
R, rough; S, smooth; Cl, clumps; Ch, chains.
T, tapered; C, curved; TC, tapered and curved; SC, slightly curved; TE, tapered and elongate.
F, foot shaped; SC, slightly curved; Pd, poorly developed; PF, prominently foot shape; P, pedicillate.
References: Leslie and Summerell (2006) for F. oxysporum, F. proliferatum, and F. equiseti; Skovgaard et al. (2003) for F. commune; Schroers et al. (2009) for F. delphinoides; and Marasas et al. (2001) for F. andiyazi.
FOM, F. oxysporum f. sp. melonis; FON, F. oxysporum f. sp. niveum provided from Rural Development Administration, Korea.
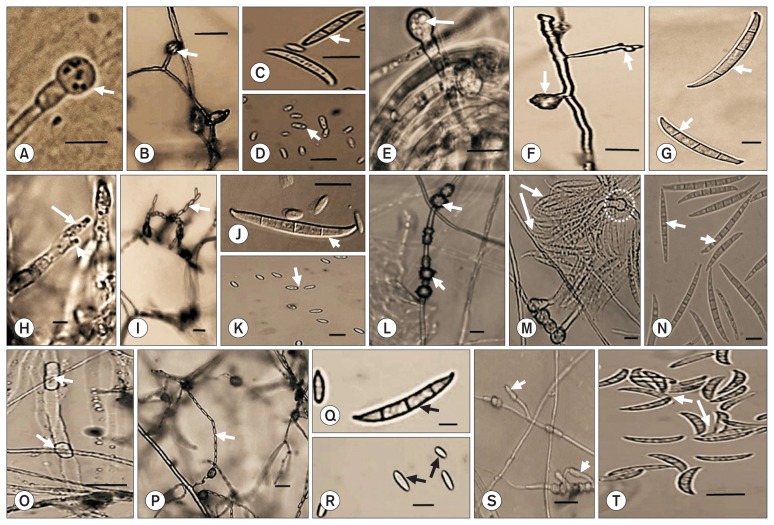
Fig. 1.
Morphological characteristics of Fusarium spp. isolated from greenhouse-grown oriental melons identified as F. oxysporum (A–D), F. commune (E–G), F. proliferatum (H–K), F. equiseti (L–N), F. andiyazi (O–R), and F. delphinoides (S, T), showing arrows pointing to chlamydospores (A, E, L), pseudochlamydospores (O), monophialides (B, S) bearing false head (B), polyphialides (F, H), microconidial chains (I, P), microconidia (D, K, R) and macroconidia (C, G, J, M, N, Q, T). Circle (M) indicates monophialide. Scale bars = 10 μm.
For F. oxysporum, colony color on PDA was orange or violet; microconidia were abundant, single-celled, oval, elliptical to kidney-shaped; macroconidia were fusiform with 26.6–39.1 × 3.5–5.04 μm in size, which was differentiated from those of the other species examined; i.e., F. commune, 25.7–56.3 × 3.6–5.8 μm; F. proliferatum, 24.9–50.1 × 3.0–6.4 μm; F. equiseti, 43.9–53.4 × 3.9–6.7 μm; F. delphinoides, 14.5–23.0 × 3.7–4.7 μm; and F. andiyazi, 24.1–27.1 × 2.5–2.9 μm (data not shown), 3 to 4 celled (2–3-septate), apical cells either tapered, curved or both, basal cells typically foot-shaped or occasionally slightly curved; and chlamydospores with either smooth, rough or both (Table 1, Fig. 1). For the other Fusarium spp., their morphological characteristics differed from those of F. oxysporum and from each other in variable degrees depending on the species; however, they were all coincided with the species specifications previously described (Table 1). F. oxysporum was the most variable in the morphological characteristics compared to the other major Fusarium species (F. commune and F. proliferatum), especially in macroconidial shapes forming multi-shaped apical and basal cells relative to the mono-shaped cells in the other two (Table 1). Colony color was one of the most variable characteristics compared to other microscopic characteristics as the major species had 2–3 different colony colors on PDA. For all major species, the least variation occurred in the formation and shape of chlamydospores for which no chlamydospore was formed in F. proliferatum. Monophialides were formed in F. oxysporum, but polyphialides, in the other major Fusarium species (Fig. 1).
In molecular analysis using the DNA sequences of EF-1α amplified with primers EF1 and EF2 and ITS-rDNA amplified with primers ITS1 and ITS4, all 27 isolates were also identified as the same species as identified by the morphological characteristics, showing Fusarium species most highly identical to those of accession numbers listed in NCBI with the same Fusarium species for both EF-1α and ITS-rDNA sequences (Table 1), which were compared with those listed in FUSARIUM-ID database (http://fusarium.cbio.psu.edu) to confirm their identities.
Phylogenetic relations of the Fusarium species
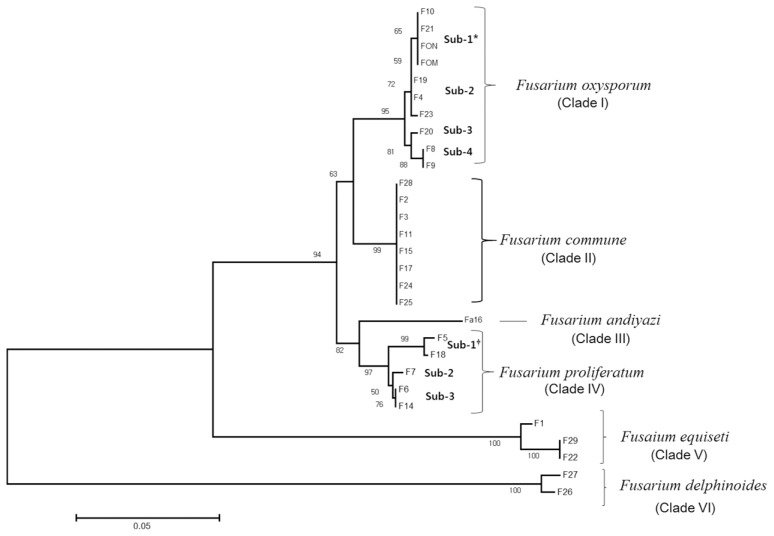
The 27 Fusarium isolates obtained from the oriental melon fields and FOM and FON provided from the Rural Development Administration, Korea, were analyzed in their phylogenetic relations based on their EF-1α gene sequences (Fig. 2). The total 29 Fusarium isolates were clustered into 6 distinct clades (I–VI) that were matched to Fusarium species identified by morphological and molecular characteristics, as clade I: 10 isolates of F. oxysporum (F4, F8, F9, F10, F19, F20, F21, F23, FOM, FON), clade II: 8 of F. commune (F2, F3, F11, F15, F17, F24, F25, F28), clade III: one F. andiyazi isolate (F1), clade IV: 5 F. proliferatum isolates (F5, F6, F7, F14, F18), clade V: 3 of F. equiseti (F1, F22, F29), and clade VI: 2 of F. delphinoides (F26, F27), respectively (Fig. 2). Within the clade of the major Fusarium species, the phylogenetic relationships varied most in the clade I (F. oxysporum) by subgrouping into 4 subclades (subclade 1 [F10, F21, FOM, FON], subclade 2 [F4, F19, F23], subclade 3 [F20], and subclade 4 [F8, F9]), and lowest in the clade III (F. commune) with no subgroup differentiated as in their microscopic characteristics, respectively; however, clade IV (F. proliferatum) with low variations in the microscopic characteristics showed phylogenetic variations similar to clade I (F. oxysporum) to have three subclades; F5 and F18 in subclade 1, F7 in subclade 2, and F6 and F14 in subclade 3 (Fig. 2).
Fig. 2.
Phylogenetic analysis using the maximum likelihood method on the basis of elongation factor-1 alpha gene sequencing. The numbers beside branches represent the percentage of congruent cluster in 1,000 bootstrap trials. The bar indicates 5% sequence dissimilarity. An asterisk (*) indicates subclades of Fusarium oxysporum. A dagger (†) indicates subclades of Fusarium proliferatum.
Pathogenicity of Fusarium isolates on oriental melon
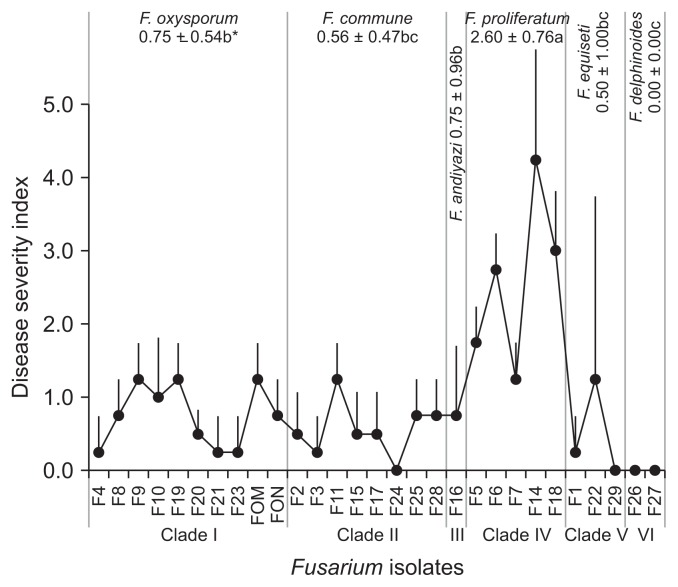
All of 27 Fusarium isolates obtained from the oriental melon greenhouses and reference isolates FOM and FON were subjected to pathogenicity test on the oriental melon, showing their DIs from 0.0 (F. delphinoides F26, F27) to 4.3 (F. proliferatum F14) (Fig. 3). Among the major Fusarium spp., F. proliferatum with the highest pathogenicity (DI of 2.6) to the oriental melon and F. andiyazi and F. oxysporum with the next DI of 0.75 showed higher pathogenicity than F. commune and F. equiseti with the lower DIs of 0.56 and 0.50, respectively; F. delphinoides was totally non-pathogenic to the oriental melon with DI of 0.0. For F. oxysporum, the disease severities were generally low on the oriental melon, showing mostly DI of 0–3 and rarely DI of 4 with typical vascular wilt symptoms of average DI of 0.75, but no oriental melon plants with DI of 5 was found in this study (Fig. 3, 4). For F. proliferatum, the disease severities were much higher than F. oxysporum; however, the disease was not authentic vascular wilts but stem rots that eventually caused plant death seemingly caused by severe wilting (Fig. 3, 5).
Fig. 3.
Pathogenicity of Fusarium isolates in different clades (Fusarium spp.) on the oriental melon as expressed by disease severity index at 4 weeks after inoculation. *Averages with the same letters denote no significant difference among the Fusarium spp. at P ≤ 0.05 by least significant difference test.
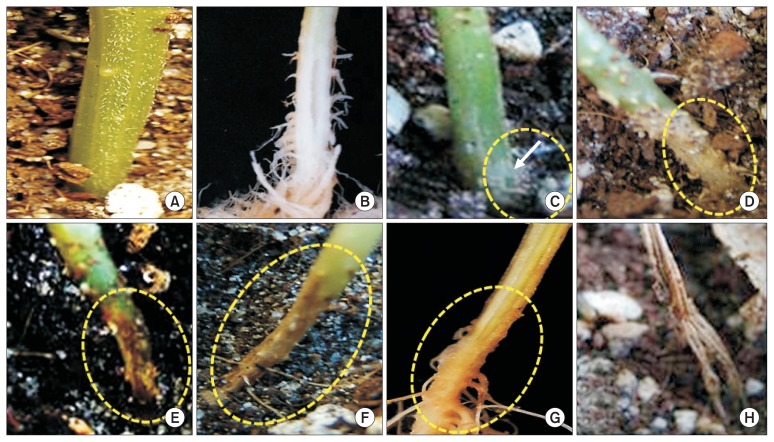
Fig. 4.
Outer (upper) and internal (lower) symptoms caused by Fusarium oxysporum, showing the non-inoculated healthy stem tissues (A) and stem tissue decays (vascular tissue discolorations) (yellow circles) at 4 weeks after inoculation corresponding to wilt severity index (disease index, DI) of 0 = no symptoms (A); 1 = underground stem yellow-brownish discolored, showing the decay of outermost stem tissues (arrow) (B); 2 = < 30% above-ground stem brownish discolored (C); 3 = stem bottom region decayed (D); 4 = stem darkly discolored and split (E). DI of 5 (plant death) was not observed in our study.
Fig. 5.
Outer (A, C–F, H) and internal (B, G) symptoms caused by Fusarium proliferatum, showing the non-inoculated healthy stem tissues (A, B) and stem tissue decays (stem tissue rot) (yellow circles) at 4 weeks after inoculation corresponding to disease severity index of 0 = no symptoms (A, B); 1 = underground outermost stem decays (arrow) (C); 2 = < 30% above-ground stem decays (D); 3 = stem bottom region decayed (E); 4 = stem darkly decayed (F) and corresponding stem tissue rots (G); and 5 = plant death (H).
Virulence of F. oxysporum and F. proliferatum isolates on cucurbitaceous vegetables
F. oxysporum includes the forma speciales that cause the fusarium wilts in the cucurbitaceous vegetables. F. proliferatum was one of the major Fusarium species showing the highest pathogenicity to the oriental melon in the pathogenicity test on oriental melon mentioned above. Thus all isolates of these two Fusarium species were tested for virulence in detail on the cucurbitaceous crops such as oriental melon, watermelon, shintosa and cucumber. For F. oxysporum, the disease severities were generally low for all crop species tested, showing mostly DI of 0–3 and rarely DI of 4 with typical vascular wilt symptoms of average DI of 0.75 on all crops examined, but no DI of 5 was found in this study (Table 2). The virulence varied significantly depending on the plants and Fusarium isolates for all plant species with the significantly higher DIs in the oriental melon (DI = 1.08) and watermelon (DI = 0.90) than in cucumber (DI = 0.53) and shintosa (DI = 0.49), and highest in F4 (DI = 0.97), F8 (DI = 0.97), and F9 (DI = 0.88) as in FOM (DI = 0.97) and FON (DI = 1.03) for average DI on all plant species examined (Table 2). FOM and 4 isolates such as F4, F9, F19, and F23 showing generally high virulence on all crops tested were more virulent to oriental melon and watermelon than shintosa and cucumber (hereafter termed as FOM type), while FON and 3 isolates such as F8, F10, and F20 showed similar virulence on all crops tested (termed as FON type), and only F21 showed low virulence on all crops tested (minor type) (Table 2). Virulence was not significantly different among subclades of F. oxysporum on oriental melon and watermelon that were mostly more susceptible to the pathogens than shintosa and cucumber. However, their virulences were significantly different from one another on shintosa and cucumber, on which the highest virulence was noted in subclade 4 containing F. oxysporum F8 and F9 (Table 2).
Table 2.
Virulence of Fusarium oxysporum isolates in different subclades in the phylogenetic tree of elongation factor-1 alpha gene sequences on cucurbitaceous vegetables at 4 weeks after inoculation
| Sub clade* | Isolate† | Disease index‡ | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Watermelon | Oriental melon | Shintosa | Cucumber | Total | |||||||
| 1 | F10 | 0.75 ± 0.89ab§X|| | 0.97 ± 0.85aX | 0.75 ± 0.71cdX | 0.91 ± 0.62aXY | 0.88 ± 0.71aX | 0.63 ± 0.54abYZ | 0.63 ± 0.52bcdX | 0.50 ± 0.51bZ | 0.75 ± 0.69ab | 0.96 ± 0.75a |
| F21 | 0.38 ± 0.52bX | 0.25 ± 0.46dX | 0.25 ± 0.46cdX | 0.13 ± 0.35eX | 0.25 ± 0.45c | ||||||
| FOM | 1.38 ± 1.06aX | 1.75 ± 0.46aX | 0.38 ± 0.46bcdY | 0.38 ± 0.52deY | 0.97 ± 0.64a | ||||||
| FON | 1.38 ± 0.92aX | 0.88 ± 0.83cX | 1.00 ± 0.83aX | 0.88 ± 0.64abcX | 1.03 ± 0.73a | ||||||
| 2 | F4 | 1.00 ± 0.763abY | 0.92 ± 0.78aX | 1.75 ± 1.49aX | 1.17 ± 0.96aX | 0.63 ± 1.49aYZ | 0.25 ± 0.29bY | 0.50 ± 0.53cdeZ | 0.25 ± 0.41bY | 0.97 ± 0.82a | 0.56 ± 0.69b |
| F19 | 0.75 ± 1.04abXY | 1.13 ± 0.64bcX | 0.00 ± 0.00dZ | 0.13 ± 0.35eYZ | 0.50 ± 0.51bc | ||||||
| F23 | 1.00 ± 0.53abX | 0.63 ± 0.74cdXY | 0.13 ± 0.74dY | 0.13 ± 0.35eY | 0.47 ± 0.50bc | ||||||
| 3 | F20 | 1.00 ± 0.76abX | 1.00 ± 0.76aX | 0.88 ± 0.64cX | 0.88 ± 0.64aXY | 0.63 ± 0.64abcX | 0.63 ± 0.74abYZ | 0.38 ± 0.52deX | 0.38 ± 0.52bZ | 0.72 ± 0.68a | 0.72 ± 0.68ab |
| 4 | F8 | 0.88 ± 0.35abX | 0.63 ± 0.55aZ | 1.00 ± 0.76bcX | 1.25 ± 0.76aX | 0.75 ± 0.76abX | 0.69 ± 0.49aYZ | 1.25 ± 0.71aX | 1.13 ± 0.62aXY | 0.97 ± 0.57a | 0.63 ± 0.72b |
| F9 | 0.38 ± 0.74bY | 1.50 ± 0.76abX | 0.63 ± 0.76abcY | 1.00 ± 0.53abXY | 0.88 ± 0.64a | ||||||
| Total | 0.90 ± 0.93X | 1.08 ± 1.40X | 0.49 ± 0.33Y | 0.53 ± 0.43Y | 0.75 ± 0.62 | ||||||
Subgroup of F. oxysporum clade as shown in Fig. 2.
F. oxysporum isolates from oriental melon greenhouses and F. oxysporum f. sp. melonis (FOM) and F. oxysporum f. sp. niveum (FON) provided from Rural Development Administration, Korea.
Disease index of 0 to 5; 0 = no symptom; 1 = underground stem yellow-brownish discolored; 2 = < 30% above-ground stem brownish discolored; 3 = stem bottom region decayed; 4 = stem darkly discolored and split; 5 = whole plant dead, which is modified from Bletsos (2005).
Means with the same letters (a, b, c) are not significantly different within the same column at P ≤ 0.05 by least significant difference (LSD) test.
Means with the same letters (X, Y, Z) are not significantly different within the same row at P ≤ 0.05 by LSD test.
Virulence of F. proliferatum isolates were mostly higher on all cucurbitaceous vegetables than the other isolates belonging to other Fusarium species examined, showing the average DI of 2.50 compared to the average DI of 0.75 for F. oxysporum isolates; however, the symptoms were caused by stem tissue rots but not by typical internal symptoms related to the fusarium wilt (vascular tissue decays) (Table 3, Fig. 5). Contrary to F. oxysporum isolates, the F. proliferatum isolates showed the highest virulence on cucumber, next on oriental melon and watermelon, and the lowest on shintosa, on all of which the virulence was significantly differentiated among the subclades with subclade 1 with medial DI of 2.50, subclade 2 with lowest DI of 0.95 and subclade 3 with highest DI of 3.28.
Table 3.
Virulence of Fusarium proliferatum isolates in different subclades in the phylogenetic tree of elongation factor-1 alpha gene sequences on cucurbitaceous vegetables at 4 weeks after inoculation
| Sub-clade* | Isolate | Disease severity index† | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Watermelon | Oriental melon | Shintosa | Cucumber | Total | |||||||
| 1 | F5 | 1.40 ± 0.55c‡Y§ | 2.90 ± 0.72bX | 1.80 ± 0.45cdX | 2.40 ± 0.22bY | 0.40 ± 0.55cZ | 1.70 ± 0.63aZ | 1.80 ± 0.45cX | 3.00 ± 0.64bX | 1.35 ± 0.75b | 2.50 ± 0.84b |
| F18 | 4.40 ± 0.89aX | 3.00 ± 0.00bY | 3.00 ± 0.71aY | 4.20 ± 0.84abX | 3.65 ± 0.93a | ||||||
| 2 | F7 | 0.60 ± 0.55dY | 0.60 ± 0.55cY | 1.20 ± 0.84dX | 1.20 ± 0.84cX | 0.60 ± 0.55cY | 0.60 ± 0.55bY | 1.40 ± 0.89cX | 1.40 ± 0.89cX | 0.95 ± 0.76b | 0.95 ± 0.76b |
| 3 | F6 | 5.00 ± 0.00aX | 3.70 ± 0.27aX | 2.60 ± 0.55bcY | 3.40 ± 0.69aY | 1.20 ± 0.45bcZ | 1.50 ± 0.64aZ | 5.00 ± 0.00aX | 4.50 ± 0.35aW | 3.45 ± 1.70a | 3.28 ± 1.48a |
| F14 | 2.40 ± 0.55bY | 4.20 ± 0.84aX | 1.80 ± 0.84bZ | 4.00 ± 0.71bX | 3.10 ± 1.25a | ||||||
| Total | 2.76 ± 0.51Y | 2.56 ± 0.53Y | 1.40 ± 0.62Z | 3.28 ± 0.58X | 2.50 ± 0.56 | ||||||
Subclades of clade IV (F. proliferatum) as shown in Fig. 2.
Disease index of 0 to 5; 0 = no symptom; 1 = underground stem yellow-brownish discolored; 2 = < 30% above-ground stem brownish discolored; 3 = stem bottom region decayed; 4 = stem darkly discolored and split; 5 = whole plant dead.
Means with the same letters (a, b, c, d) are not significantly different within the same column at P ≤ 0.05 by least significant difference (LSD) test.
Means with the same letters (W, X, Y, Z) are not significantly different within the same row at P ≤ 0.05 by LSD test.
Virulence of rook-knot nematode on cucurbitaceous crops
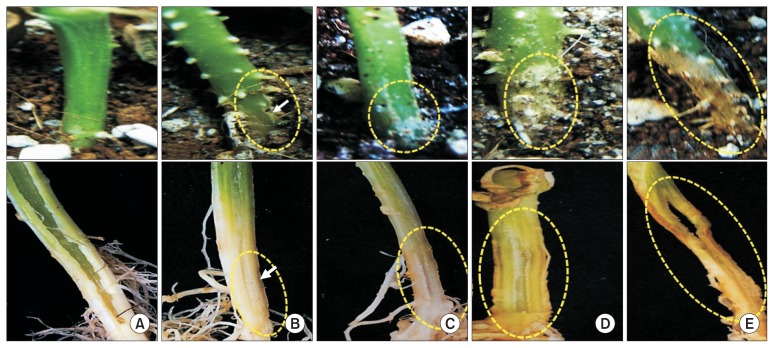
In the inoculation test of the root-knot nematode, all cucurbitaceous plants tested were susceptible to the nematode, forming abundant large root-knot galls with no vascular wilt or rot symptoms occurred in all cucurbitaceous vegetables examined (Table 4, Fig. 6). Root-knot galls were formed most prominently in oriental melon (GI of 4.3) and eggmasses were most abundantly formed in watermelon (EI of 4.0), respectively; however, there were no significant differences in gall and eggmass formations among the other crops examined (Table 4). Among plant growth parameters, root weights of all vegetable crops increased significantly by the nematode infection probably due to the increased root weights by the severe galling, but other growth parameters such as shoot length and weight were little affected by the nematode infection except for the shoot length increased in shintosa infected with the nematode compared to the control plants, which resulted from the tender stem growth probably affected by the nematode infection (Table 3).
Table 4.
Effects of root-knot nematode (RKN) infection on development of wilt symptoms (WS), the plant growths, and the formation of root-knot galls and eggmasses on the cucurbitaceous vegetables 4 weeks after the nematode inoculation
| Plant | GI* | EI† | WS (DI)‡ | Shoot-height | Shoot-weight | Root-weight | |||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| Control | RKN | Control | RKN | Control | RKN | ||||
| Oriental melon | 4.3 ± 1.2a§ | 3.5 ± 0.6c | 0.0 ± 0.0 | 63.9 ± 14.7X|| | 72.4 ± 5.9X | 24.5 ± 3.5X | 28.0 ± 4.1X | 2.7 ± 0.6Y | 5.0 ± 0.7X |
| Watermelon | 2.0 ± 0.0b | 5.0 ± 0.0a | 0.0 ± 0.0 | 100.3 ± 7.0X | 94.7 ± 12.4X | 16.0 ± 1.6Y | 16.5 ± 1.6X | 0.8 ± 0.2Y | 1.9 ± 0.2X |
| Shintosa | 3.0 ± 0.0b | 4.0 ± 0.0bc | 0.0 ± 0.0 | 21.7 ± 5.0Y | 36.5 ± 6.1X | 26.4 ± 4.4X | 25.3 ± 4.3X | 4.5 ± 0.7Y | 6.7 ± 1.2X |
| Cucumber | 3.0 ± 0.0b | 4.3 ± 0.5b | 0.0 ± 0.0 | 67.8 ± 6.2X | 72.5 ± 6.6X | 25.5 ± 2.7X | 24.7 ± 3.0Y | 2.6 ± 0.3Y | 4.8 ± 0.3X |
Gall index (GI): 0–5 rating scale according to the percentage of galled tissue; 0 = 0–10% of galled roots; 1 = 11–20%; 2 = 21–50%; 3 = 51–80%; 4 = 81–90%; 5 = 91–100% (Baker, 1985).
Eggmass index (EI) was assigned to each count using a rating of 1 = no masses; 2 = 1–3 egg masses; 3 = 4–10 egg masses; 5 = 31–100 egg masses; 6 > = 100 egg masses per root system (Roberts et al., 1990).
Wilt symptom development as measured by wilt disease severity index (DI) 0 to 5; 0 = no symptom; 1 = underground stem yellow-brownish discolored; 2 = < 30% aboveground stem brownish discolored; 3 = stem bottom region decayed; 4 = stem darkly discolored and split; 5 = whole plant dead; modified from Bletsos (2005).
Means with the same letters (a, b, c) are not significantly different (P ≤ 0.05) within a column by least significant difference (LSD) test.
Means with the same letters (X, Y) are not significantly different (P ≤ 0.05) between control and RKN in the same plant by LSD test.
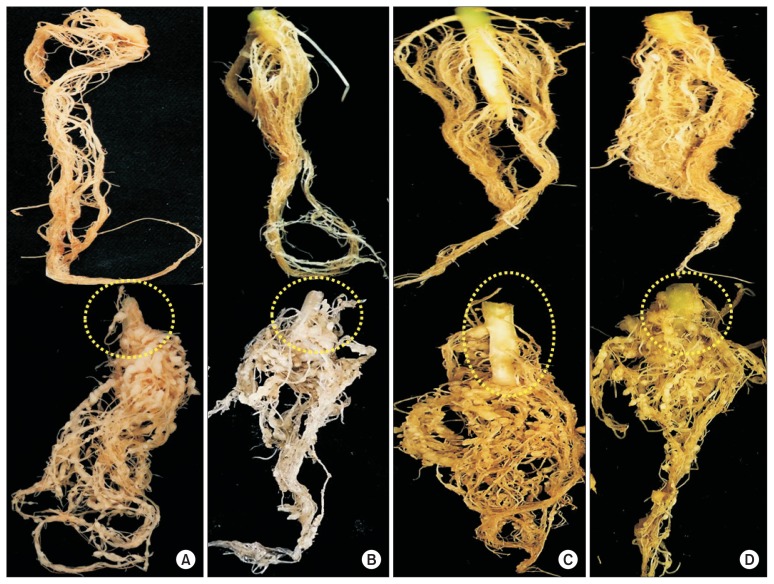
Fig. 6.
Formation of root-knot galls on cucurbitaceous crops (below) compared to their healthy non-infected roots (upper) on oriental melon (A), watermelon (B), shintosa (C), and cucumber (D) at 4 weeks after Meloidogyne incognita inoculation. Severe root galls were formed on all the crop roots inoculated with the root-knot nematode, on which no basal stem and root rots or vascular discoloration occurred (yellow circles).
Discussion
Twenty-seven Fusarium isolates obtained from 216 plant and soil samples in greenhouse-grown oriental melon were identified by morphological and molecular characteristics into 6 Fusarium species. Among these, F. oxysporum, F. commune and F. proliferatum were more prevalent than the other Fusarium species examined, suggesting these may be major Fusarium species in the greenhouse-grown oriental melons with wilt symptoms. Among the major Fusarium species, F. oxysporum has been known as causal pathogens of the fusarium wilts in the cucurbitaceous vegetables, for which different forma speciales induce the fusarium wilts in different crop species; F. oxysporum f. sp. melonis in oriental melon, F. oxysporum f. sp. niveum in watermelon, and F. oxysporum f. sp. cucumerinum (niveum) in cucumber, respectively (KSPP, 2009). In our study, most F. oxysporum isolates showed significantly higher virulence on oriental melon and watermelon as the isolates of FOM and FON; however, these isolates were differentiated in their virulence on shintosa and cucumber, showing either FOM-type with a low virulence on these crops or FON-type with a high virulence on these crops. This suggests the most abundant forma speciales of F. oxysporum distributing in oriental melon greenhouses may be FOM or FON type with high virulence on the oriental melon. However, no F. oxysporum isolates from the oriental melon greenhouses showed any significant virulence (DI ≥ 1.0) on shintosa that has been used widely as a root-stock resistant to the fusarium wilt (Lee, 1994). All of these indicate that no or little significant population changes of F. oxysporum isolates significantly virulent to shintosa might have occurred even in the continuous oriental melon-cropping areas of Seongju and Yeoju, suggesting the current prevalence of the fusarium wilt in the oriental melon greenhouses may not be due to the breakdown of the resistance in shintosa used as rootstock for grafting of the oriental melon by the occurrence of new virulent pathogen races. However, the increased virulence of the F. oxysporum isolates on the oriental melon may be derived from the pathogen adaptation to continuous cropping system (CCS) of the oriental melon, as microbial populations pathogenic to the crop in CCS increase, accompanying the decrease of beneficial microorganisms (Chen et al., 2011). This suggests the oriental melons growing in greenhouses with CCS are exposed to the increased disease pressure due to the increased pathogen populations, resulting in the severe disease development especially when the grafting to the rootstock resistant to the fusarium wilt has not been practiced.
The Fusarium species other than F. oxysporum were isolated from the oriental melon greenhouses in this study. In the other study, various Fusarium species including F. oxysporum were isolated from cucurbits plantation in Iran, which cause root and stem rots of the cucurbits (Chehri et al., 2011). Several Fusarium species other than F. oxysporum are also recorded as pathogens (such as fruit rot) in the oriental melon (KSPP, 2009). In this study, most F. proliferatum isolates showed significantly higher pathogenicity on the oriental melon than any other Fusarium species, inducing severe wilt symptoms (even plant death) caused by severe stem rotting, but not by the vascular destruction inducing authentic fusarium wilts. These suggest that all Fusarium isolates in this study might not have been derived from the soils and plant tissues with authentic vascular wilt symptoms, but superficial wilt symptoms derived from root and/or stem rots which affect adversely on the absorption and translocation of water and nutritional substances (Agrios, 2004). Considering these aspects, misidentification of fusarium wilt symptoms may not be excluded from the growers’ understanding on the prevalent disease situations in the oriental melon greenhouses.
F. oxysporum is a genetically heterogeneous polytypic morphospecies (O’Donnel and Cigelnik, 1997; Waalwijk et al., 1996), and the taxon has been regarded as a species complex whose strains are widely distributed in soil and also found in wide range of aquatic ecosystems (Gordon and Martyn, 1997; Palmero et al., 2009; Swathi et al., 2013). Also the F. oxysporum isolates distributed in the oriental melon in the present study showed the high morphological variations and high diversifications within the same clade of F. oxysporum in the phylogenetic analysis based on EF-1α gene sequences. However, there was no significant difference in the degree of virulence among the four subclades (subclade 1 [F10, F21, FOM, FON], subclade 2 [F4, F19, F23], subclade 3 [F20], and subclade 4 [F8, F9]) showing their average DIs of 0.93, 1.43. 1.00, and 0.95, respectively. These results suggest morphological, genetic and pathological variations in F. oxysporum complex might have been derived hardly from common components of selection pressure that drives variations of pathogen’s characteristics (Wachter and Hill, 2016). These variations may be driven by the relationships of the pathogens with host plants for the changes of pathological characteristics and by environmental and soil factors influencing their survival, growth and reproduction for the changes of morphological and genetic characteristics.
In our study, all cucurbitaceous vegetables were susceptible to the root-knot nematode, M. incognita, forming extensively large root-knot galls, which is one of pathological features characteristic to the symptoms formed on vegetable crops infected with M. incognita (Sardanelli et al., 1983). It is also noticed in our previous studies that remarkably large galls are formed in susceptible carrot lines infected with M. incognita (Seo et al., 2014, 2015). Above-ground symptoms caused by this root-knot nematodes are stunting and yellowing, and sudden wilting especially in hot and dry conditions in summer (Sardanelli et al., 1983). However, the wilting symptoms caused by the root-knot nematode are derived from the increased transpiration relative to water-uptake of the root, especially in hot and dry weather conditions, but not derived from vascular tissue decays, which is distinguishable from the authentic fusarium wilt symptoms. In our study, no wilt symptoms were developed even by the severe root-knot nematode infection, suggesting that the increased nematode populations by the continuous cropping of the oriental melon in greenhouses alone may not be responsible for the current prevalence of the fusarium wilt in the oriental melon.
Conclusively, our present study suggests the current prevalence of the fusarium wilts in the oriental melon greenhouses with CCS may not be derived from the qualitative changes of inoculum potentials (pathogen’s virulence to the root-stock plant such as shintosa). Also the root-knot nematodes, which have been enormously increased in inoculum density in such CCS, may have contributed little to the prevalence of the oriental melon fusarium wilts as the severe root-knot nematode infection rarely induced the fusarium wilt symptoms in the present study. As indicated previously, the prevalence of wilt symptoms in the oriental melon greenhouses may be attributed to the fusarium diseases caused by Fusarium species other than F. oxysporum such as F. proliferatum, causing severe diseases with seeming wilt symptoms due to root and/or stem rots but not authentic vascular dysfunctions. However, it is well known the fungal diseases become severer by the co-infection of the root-knot nematodes that predispose plant to infection by soil-borne disease fungi (Armstrong et al., 1976; Sumner and Johnson, 1973) and induce complex diseases (Atkinson, 1892; Son et al., 2008, 2009), on which more studies need to be done to reveal the reasons for the current prevalence of the oriental melon fusarium wilt for certain.
References
- Agrios GN. Plant pathology. 5th ed. Academic Press; San Diego, CA, USA: 2004. p. 922. [Google Scholar]
- Armstrong JM, Jatala P, Jensen HJ. Station Bulletin. Agricultural Experiment Station, Oregon State University; Corvallis, OR, USA: 1976. Bibliography of nematode interactions with other organisms in plant disease complexes; p. 623. [Google Scholar]
- Atkinson GF. Some diseases of cotton. Alabama Polytech Inst Agric Exp Stn Bull. 1892;41:61–65. [Google Scholar]
- Baker KR. Nematode extractions and bioassays. In: Baker KR, Carter CC, Sasser JN, editors. An advanced treatise on meloidogyne. Vol. II. Methodology. Department of Plant Pathology, North Carolina State University; Raleigh, NC, USA: 1985. pp. 19–35. [Google Scholar]
- Banihashemi Z, deZeeuw DJ. The behavior of Fusarium oxysporum f. sp. melonis in the presence and absence of host plants. Phytopathology. 1975;65:1212–1217. doi: 10.1094/Phyto-65-1212. [DOI] [Google Scholar]
- Bletsos FA. Use of grafting and calcium cyanamide as alternatives to methyl bromide soil fumigation and their effects on growth, yield, quality and fusarium wilt control in melon. J Phytopathol. 2005;153:155–161. doi: 10.1111/j.1439-0434.2005.00945.x. [DOI] [Google Scholar]
- Byeon IS, Suh SY, Lee YS, Chung JB. Effect of double-cropping systems on nematode population in plastic film house soils of oriental melon cultivation. Korean J Environ Agric. 2014;33:17–23. doi: 10.5338/KJEA.2014.33.1.17. (in Korean) [DOI] [Google Scholar]
- Cafri D, Katan J, Katan T. Cross-pathogenicity between formae speciales of Fusarium oxysporum, the pathogens of cucumber and melon. J Phytopathol. 2005;153:615–622. doi: 10.1111/j.1439-0434.2005.01029.x. [DOI] [Google Scholar]
- Chehri K, Salleh B, Yli-Mattila T, Reddy KR, Abbasi S. Molecular characterization of pathogenic Fusarium species in cucurbit plants from Kermanshah province, Iran. Saudi J Biol Sci. 2011;18:341–351. doi: 10.1016/j.sjbs.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Yang X, Raza W, Li J, Liu Y, Qiu M, Shen Q. Trichoderma harzianum SQR-T037 rapidly degrades allelochemicals in rhizospheres of continuously cropped cucumbers. Appl Microbiol Biotechnol. 2011;89:1653–1663. doi: 10.1007/s00253-010-2948-x. [DOI] [PubMed] [Google Scholar]
- Cho MR, Lee BC, Kim DS, Jeon HY, Yiem MS, Lee JO. Distribution of plant-parasitic nematodes in fruit vegetable production areas in Korea and identification of root-knot nematodes by enzyme phenotypes. Korean J Appl Entomol. 2000;39:123–129. [Google Scholar]
- da Silva FP, Vechiato MH, Harakava R. EF-1α gene and IGS rDNA sequencing of Fusarium oxysporum f. sp. vasinfectum and F. oxysporum f. sp. phaseoli reveals polyphyletic origin of strains. Trop Plant Pathol. 2014;39:64–73. doi: 10.1590/S1982-56762014000100008. [DOI] [Google Scholar]
- Fisher NL, Burgess LW, Toussoun TA, Nelson PE. Carnation leaves as a substrate and for preserving cultures of Fusarium species. Phytopathology. 1982;72:151–153. doi: 10.1094/Phyto-72-151. [DOI] [Google Scholar]
- Freeman S, Zveibil A, Vintal H, Maymon M. Isolation of nonpathogenic mutants of Fusarium oxysporum f. sp. melonis for biological control of Fusarium wilt in cucurbits. Phytopathology. 2002;92:164–168. doi: 10.1094/PHYTO.2002.92.2.164. [DOI] [PubMed] [Google Scholar]
- Geiser DM, del Mar Jiménez-Gasco M, Kang S, Makalowska I, Veeraraghavan N, Ward TJ, Zhang N, Kuldau GA, O’Donnell K. FUSARIUM-ID v.1.0: a DNA sequence database for identifying Fusarium. Eur J Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- Gordon TR, Martyn RD. The evolutionary biology of Fusarium oxysporum. Annu Rev Phytopathol. 1997;35:111–128. doi: 10.1146/annurev.phyto.35.1.111. [DOI] [PubMed] [Google Scholar]
- Kim DG. Distribution and population dynamics of Meloidogyne arenaria on oriental melon (Cucumis melo L.) under greenhouse conditions in Korea. Russian J Nematol. 2001a;9:61–68. [Google Scholar]
- Kim DG. Occurrence of root-knot nematodes on fruit vegetables under greenhouse conditions in Korea. Res Plant Dis. 2001b;7:69–79. (in Korean) [Google Scholar]
- Kim DG, Choi SK. Effects of incorporation method of nematicides on reproduction of Meloidogyne arenaria. Korean J Appl Entomol. 2001;40:89–95. (in Korean) [Google Scholar]
- Kim DG, Kim SH, Lee JH. Observation of root-knot nematodes in the root gall formed on oriental melon. Plant Pathol J. 2005;21:73–76. doi: 10.5423/PPJ.2005.21.1.073. [DOI] [Google Scholar]
- Kim DG, Yeon IK. Development of Meloidogyne arenaria on oriental melon (Cucumis melo L.) in relation to degree-day accumulation under greenhouse conditions. Plant Pathol J. 2001;17:159–163. [Google Scholar]
- Kim JW, Kim HJ. Fusarium fruit rot of postharvest oriental melon (Cucumis melo L. var. makuwa Mak.) caused by Fusarium spp. Res Plant Dis. 2004;10:260–267. doi: 10.5423/RPD.2004.10.4.260. (in Korean) [DOI] [Google Scholar]
- Kinloch RA, Hinson K. The Florida program for evaluating soybean (Glycine max (L.) Merr.) genotypes for susceptibility to root-knot nematode disease. Proc Soil Crop Sci Fla. 1972;32:173–176. [Google Scholar]
- Komada H. Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Prot Res. 1975;8:114–125. [Google Scholar]
- Kornerup A, Wanscher JH. Methuen handbook of colour. 3rd ed. Methuen Publishing; London, UK: 1978. [Google Scholar]
- [KSPP] Korean Society of Plant Pathology. List of plant diseases in Korea. 5th ed. KSPP; Suwon, Korea: 2009. [Google Scholar]
- Kwon TY, Jung KC, Sim YG, Choi BS, Park SD. Cultural and chemical control of root-knot nematodes, Meloidogyne sp. on oriental melon in plastic film house. RDA J Crop Prot. 1998;40:96–101. [Google Scholar]
- Lee JM. Cultivation of grafted vegetables I: current status, grafting methods, and benefits. HortScience. 1994;29:235–239. [Google Scholar]
- Lee JM, Oda M. Grafting of herbaceous vegetable and ornamental crops. Hortic Rev. 2003;28:61–124. [Google Scholar]
- Lee WJ, Lee JH, Jang KS, Choi YH, Kim HT, Choi GJ. Development of efficient screening methods for melon plants resistant to Fusarium oxysporum f. sp. melonis. Korean J Hortic Sci Technol. 2015;33:70–82. doi: 10.7235/hort.2015.14101. [DOI] [Google Scholar]
- Leslie JF, Summerell BA. The Fusarium laboratory manual. Blackwell Publishing; Ames, IA, USA: 2006. [DOI] [Google Scholar]
- [MAFRA] Ministry of Agriculture, Food, and Rural Affairs, Republic of Korea. Agriculture, food, and rural affairs statistical yearbook, 2015. MAFRA; Sejong, Korea: 2015. [Google Scholar]
- Marasas WFO, Rheeder JP, Lamprecht SC, Zeller KA, Leslie JF. Fusarium andiyazi sp. nov., a new species from sorghum. Mycologia. 2001;93:1203–1210. doi: 10.2307/3761681. [DOI] [Google Scholar]
- Matsumoto Y, Ogawara T, Miyagi M, Watanabe N, Kuboyama T. Response of wild Cucumis species to inoculation with Fusarium oxysporum f. sp. melonis race 1.2y. J Jpn Soc Hortic Sci. 2011;80:414–419. doi: 10.2503/jjshs1.80.414. [DOI] [Google Scholar]
- O’Donnel K, Cigelnik E. Two divergent intragenomic rDNA ITS2 types within a monophyletic lineage of the fungus Fusarium are nonorthologous. Mol Phylogenet Evol. 1997;7:103–116. doi: 10.1006/mpev.1996.0376. [DOI] [PubMed] [Google Scholar]
- Owen JH. Fusarium wilt of cucumber. Phytopathology. 1955;45:435–439. [Google Scholar]
- Palmero D, Iglesias C, de Cara M, Lomas T, Santos M, Tello JC. Species of Fusarium isolated from river and sea water of southeastern Spain and pathogenicity on four plant species. Plant Dis. 2009;93:377–385. doi: 10.1094/PDIS-93-4-0377. [DOI] [PubMed] [Google Scholar]
- Park SD, Kwon TY, Choi BS, Lee WS, Choi YE. Studies on integrated control against root-knot nematode of fruit vegetable (oriental melon and cucumber) in vinyl house. Korean J Appl Entomol. 1995;34:75–81. (in Korean) [Google Scholar]
- Rhoades HL. Effects of Indigofera hirsuta on Belonolaimus longicaudatus, Meloidogyne incognita, and M. javanica and subsequent crop yield. Plant Dis Report. 1976;60:384–386. [Google Scholar]
- Roberts PA, Dalmasso A, Cap GB, Castagnone-Sereno P. Resistance in Lycopersicon peruvianum to isolates of Mi gene-compatible Meloidogyne populations. J Nematol. 1990;22:585–589. [PMC free article] [PubMed] [Google Scholar]
- Sardanelli S, Krusberg LR, Kantzes JG. Fact Sheet 381. University of Maryland Extension, University of Maryland; College Park, MD, USA: 1983. Plant parasitic nematodes in Maryland. [Google Scholar]
- Schroers HJ, O’Donnell K, Lamprecht SC, Kammeyer PL, Johnson S, Sutton DA, Rinaldi MG, Geiser DM, Summerbell RC. Taxonomy and phylogeny of the Fusarium dimerum species group. Mycologia. 2009;101:44–70. doi: 10.3852/08-002. [DOI] [PubMed] [Google Scholar]
- Seo Y, Kim YS, Park Y, Kim YH. Comparisons of pathological responses in carrot to root-knot nematodes. Plant Pathol J. 2015;31:441–445. doi: 10.5423/PPJ.NT.06.2015.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo Y, Park J, Kim YS, Park Y, Kim YH. Screening and histopathological characterization of Korean carrot lines for resistance to the root-knot nematode Meloidogyne incognita. Plant Pathol J. 2014;30:75–81. doi: 10.5423/PPJ.OA.08.2013.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skovgaard K, Rosendahl S, O’Donnell K, Nirenberg HI. Fusarium commune is a new species identified by morphological and molecular phylogenetic data. Mycologia. 2003;95:630–636. doi: 10.1080/15572536.2004.11833067. [DOI] [PubMed] [Google Scholar]
- Son SH, Khan Z, Kim SG, Kim YH. Effects of seed treatment with rhizobacterium, Paenibacillus species on management of root-knot nematode-Fusarium wilt fungus disease complex in tomato plants. Russ J Nematol. 2008;16:97–105. [Google Scholar]
- Son SH, Khan Z, Kim SG, Kim YH. Plant growth-promoting rhizobacteria, Paenibacillus polymyxa and Paenibacillus lentimorbus suppress disease complex caused by root-knot nematode and fusarium wilt fungus. J Appl Microbiol. 2009;107:524–532. doi: 10.1111/j.1365-2672.2009.04238.x. [DOI] [PubMed] [Google Scholar]
- Southey JF. Laboratory methods for work with plant and soil nematodes. Ministry of Agriculture Fisheries and Food, Her Majesty’s Stationery Office; London, UK: 1986. [Google Scholar]
- Sumner DR, Johnson AW. Effect of root-knot nematodes on Fusarium wilt of watermelon. Phytopathology. 1973;63:857–861. doi: 10.1094/Phyto-63-857. [DOI] [Google Scholar]
- Swathi J, Sowjanya KM, Narendra K, Satya AK. Bioactivity assay of an isolated marine Fusarium sp. Int J Bio-Sci Bio-Technol. 2013;5:179–186. doi: 10.14257/ijbsbt.2013.5.5.19. [DOI] [Google Scholar]
- Thompson D, Henry R. Single-step protocol for preparation of plant tissue for analysis by PCR. Biotechniques. 1995;19:394–397. 400. [PubMed] [Google Scholar]
- Waalwijk C, de Koning JRA, Baayen RP, Gams W. Discordant groupings of Fusarium spp. from sections Elegans, Liseola and Dlaminia based on ribosomal ITS1 and ITS2 sequences. Mycologia. 1996;88:361–368. doi: 10.2307/3760877. [DOI] [Google Scholar]
- Wachter J, Hill S. Positive selection pressure drives variation on the surface-exposed variable proteins of the pathogenic Neisseria. PLoS One. 2016;11:e0161348. doi: 10.1371/journal.pone.0161348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White TJ, Bruns T, Lee S, Taylor J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ, editors. PCR protocols: a guide to methods and applications. Academic Press; San Diego, CA, USA: 1990. pp. 315–322. [Google Scholar]
- Zhou XG, Everts KL. Characterization of a regional population of Fusarium oxysporum f. sp. niveum by race, cross pathogenicity, and vegetative compatibility. Phytopathology. 2007;97:461–469. doi: 10.1094/PHYTO-97-4-0461. [DOI] [PubMed] [Google Scholar]