Abstract
The spent mushroom substrate (SMS) of Lentinula edodes that was derived from sawdust bag cultivation was used as materials for controlling Phytophthora blight disease of pepper. Water extract from SMS (WESMS) of L. edodes inhibited mycelial growth of Phytophthora capsici, suppressed Phytophthora blight disease of pepper seedlings by 65% and promoted growth of the plant over 30%. In high performance liquid chromatography (HPLC) analysis, oxalic acid was detected as the main organic acid compound in WESMS and inhibited the fungal mycelium at a minimum concentration of 200 mg/l. In quantitative real-time PCR, the transcriptional expression of CaBPR1 (PR protein 1), CaBGLU (β-1,3-glucanase), CaPR-4 (PR protein 4), and CaPR-10 (PR protein 10) were significantly enhanced on WESMS and DL-β-aminobutyric acid (BABA) treated pepper leaves. In addition, the salicylic acid content was also increased 4 to 6 folds in the WESMS and BABA treated pepper leaves compared to water treated leaf sample. These findings suggest that WESMS of L. edodes suppress Phytophthora blight disease of pepper through multiple effects including antifungal activity, plant growth promotion, and defense gene induction.
Keywords: defense response, Lentinula edodes, pepper, Phytophthora blight disease, spent mushroom substrate
Phytophthora blight disease, caused by Phytophthora capsici, is the most chronic disease in pepper in Korea. In 2003, the disease brought dramatic reduction in both yield and quality of 69–82% in chief producing district of pepper in Korea (Kim, 2004). P. capsici is soilborne plant pathogen and can infect all parts of the host plants by splashing from the infected soil. Once the disease is occurred in the field and greenhouse, the disease is difficult to control because the pathogen is rapidly dispersed through furrow of pepper field. Management of the disease has mostly relied on cultural practices, crop rotation and chemical fungicides such as metalaxyl and captafol (Hwang and Kim, 1995). Although the fungicides have showed powerful protective effect against the disease, their overuse has also led to environment pollution, reduction of useful microorganisms in rhizosphere and emergence of new isolates resistant to the fungicides (Parra and Ristaino, 2001). Therefore, alternative methods using plant extract, compost water extracts and microbial fungicides have been developed to biologically control plant disease (Sang et al., 2010, 2013; Stephan et al., 2005).
It has been known that different mushroom species produce a large number of antimicrobial compounds against bacteria and fungi (Alves et al., 2012; Poucheret et al., 2006; Suay et al., 2000). They have mostly been studied on antimicrobial activity against a range of human pathogens (Kwak et al., 2015; Wong et al., 2010). In agricultural application, an antifungal composition extracted from mycerial culture of mushroom was utilized to suppress plant pathogens (Inagaki and Yamaguchi, 2009). However, the high cost is required for installing and maintaining massive culturing facilities and equipment for its practical application in agriculture. Different edible mushroom including oyster, button, shiitake and winter mushrooms have increasingly been cultivated worldwide. In Korea, the production of the edible mushrooms was estimated to be about 620,000 tons (Kwak et al., 2015) and it is estimated that by-product, spent mushroom substrate (SMS) over two million ton is being produced. The SMS is packed with mushroom mycelia and residues of fruiting body and substrate. Also, it was known that SMS retains a variety of bioactive substances such as extracellular enzymes, antimicrobial compounds, and secondary metabolites (Kwak et al., 2015; Suess and Curtis, 2006). Potential value of the SMS has been assessed in production of bulk enzymes, plant disease control, bioremidation, fertilizer and animal feeding (Suess and Curtis, 2006). SMS resembles to a cultural type of fungi, solid state fermentation (SSF) that was used to produce high activity of useful substance such as antibiotics (Subramaniyam and Vimala, 2012).
In Korea, yield of L. edodes (Shiitake mushroom in Japan) was estimated to be about 37,000 ton a year. The shiitake mushroom (Pyogo mushroom in Korea) has mainly been cultivated on tree logs. However, recently, bag cultivation using sawdust is rapidly increasing yearly due to its high yield and easily process (Jang et al., 2012) and currently, the sawdust cultivation of Pyogo mushroom is approaching to almost 50% of the cultivation in Korea. The mushroom growers faced on disposal problem of the SMS and required the available measures to recycle it without environmental hazard.
Antimicrobial compounds, lentinamicin (octa-2,3-diene-5,7 diyne-1-ol), β-ethyl phenyl alcohol and lentin, an antifungal protein was isolated from liquid cultures of L. edodes include (Komemushi et al., 1996; Ngai and Ng, 2003). It was reported lentin inhibits mycerial growth of different fungal species such as Physalospora piricola, Botrytis cinerea, and Mycosphaerella arachidicola (Ngai and Ng, 2003). In some reports, water extracts of SMSs from different mushroom species have been used to suppress plant diseases Phytophthora blight, Pythium dampening-off, apple scab, and cucumber anthracnose caused by fungal pathogens (Inagaki and Yamaguchi, 2009; Pacumbaba et al., 1999; Parada et al., 2011; Shibuya and Minami, 2001). Hence, it was expected that the SMS of L. edodes can potentially be used as an available material for suppressing Phytophthora blight disease of pepper.
In plant-pathogen interactions, elicitors that induce defense response in plants are released from cell walls of the mycelia of fungal pathogens (Sang et al., 2010; Sunwoo et al., 1996; Repka et al., 2001). Once host plant recognizes an elicitor, activated mediator such as salicylic acid (SA) induces the expression of defense genes and finally develops to enhance plant resistance. Interestingly, previous reports showed water extract from heat treated SMS enhances protective effect against fungal disease on cucumber and induces expression of defense genes (Parada et al., 2011, 2012). In addition, an elicitor that is effective to control cucumber seedling against Collectotrichum lagenarium was partially purified from basidiocarps of L. edodes (Nagai et al., 2002).
In this study, we investigated the protective effect of water extract from SMS (WESMS) of L. edodes against Phytophthora blight disease of pepper and identified the potential antifungal compound in the extract composition. Furthermore, we examined the plant’s defense response on resistance gene expression of pepper when treated with water extract of SMS.
Materials and Methods
WESMS of L. edodes
SMS of L. edodes (variety, Chamarang) were obtained from the Mushroom Research Institute (Gwangju, Gyeonggi Province, Korea). The liquid spawn (10 ml) of the shiitake mushroom was inoculated in bags containing 1.5 kg oak sawdust substrate supplemented with 20% rice bran and incubated at 25°C for 60 days. After the mycelium colonization was completed, the bags were exposed to daylight to promote mushroom formation at 18–20°C. After the fruiting bodies were harvested with 4 cycles, SMS was used for this experiment. The SMS was mixed with water (1:3 ratio w/v) and heated at 90°C for 2 h. The mixture was then filtered through two layers of Miracloth (Calbiochem, La Jolla, CA, USA), and centrifuged for 10 min at 10,000 rpm and the supernatant was used as the water extract of SMS (WESMS). Water extract from natural mushroom substrate (WENMS) that was not inoculated with spawn of L. edodes was used as control sample.
Antimicrobial activity
P. capsici KACC40180 was obtained from Korea Agricultural Culture Collection in National Institute of Agricultural Sciences, Rural Development Administration, Wanju, Korea. The effect of WESMS on the mycelial growth of P. capsici was determined on potato dextrose agar (PDA) media. PDA powder was added to the WESMS. After autoclaving, 4-day-old mycelial plugs (5 mm diameter) of P. capsici grown on V8 medium were used to inoculate 20 ml of the PDA-WESMS. This was then incubated at 25°C for 7 days. For the control, WENSM replaced the WESMS in the method detailed above. To evaluate the antifungal activity of oxalic acid, final concentration of oxalic acid (Sigma-Aldrich, St. Louis, MO, USA) were adjusted to ensure a range between 100–400 mg/l in PDA. The mycelial plugs (5 mm) were inoculated on the oxalic acid-PDA media, and the mycelial growth rate was checked after 7 days.
Analysis of oxalic acid
WESMS was used to analyses content of oxalic acid. High performance liquid chromatography (HPLC) analysis was conducted on Aglilent 1100 series, which consisted of a variable wavelength detector and a Kinetex c18 (250 × 4.6 mm, particle size of 5 μm; Phenomenex, Torrance, CA, USA). The temperature was maintained at 24°C and the flow rate at 0.5 ml/min. The mobile phase used was 20 mM/l phosphoric acid. Calibration curves were linear (R2 > 0.999) over the concentration range of 10–500 mg/kg with acceptable accuracy and precision.
Protective effect on Phytophthora blight disease of WESMS
Five-week-old seedlings of pepper “Bugwang” grown on soil bed in pots (9 cm) were used for this experiment. The pepper seedlings were irrigated three times with WESMS (20 ml) of L. edodes at an interval of three days. DL-β-aminobutyric acid (BABA, 0.5%) (Sigma-Aldrich) and sterile distilled water was treated on the plants as positive and negative controls. Zoospores of P. capsici were used as inoculum for infecting the pepper. To produce zoospores from the fungus, mycelium of the fungus was grown on V8 agar media (20% V8 juice, 0.03% CaCO3, and 2% agar) maintained at 24°C with a 12 h light-and-dark cycle and cool white fluorescent irradiation for 7 days. For the release of zoospores from the sporangia, agar discs (1.0 cm2) was cut from the edge of a colony that has been grown on V8 juice agar and incubated the discs in a shallow layer of distilled water in a Petri dish, at 28°C for 4 days, under continuous fluorescent light to induce formation of sporangia. After placing the plate on ice for 20 min, zoospores released from sporangia for incubating at the room temperature for 30 min.
The concentration of the zoospore suspension was adjusted to 105 spores/ml with a hemocycrometer (Morris and Nicholls, 1978). The zoospore suspension of 1 ml was inoculated on soil bed in pepper seedling pots. After incubation in greenhouse at 24°C for 5 days, disease symptoms were evaluated based on a 0–5 scale where, 0 = no visible disease symptoms; 1 = slightly wilted leaves with brownish lesions beginning to appear on the stems; 2 = 30–50% of plant diseased; 3 = 51–70% of plant diseased; 4 = 71–90% of plant diseased; and 5 = dead plant. The disease severity was calculated using the formula of Sunwoo et al. (1996). Nine plants were used for each treatment with three replicates.
RT-PCR and quantitative real-time PCR analysis
The experiment was done to qualify expression induction of defense genes on pepper treated with WESMS. Primers targeting defense genes in pepper used in RT-PCR and qRT-PCR were listed in Table 1 (Sang et al., 2010; Yang et al., 2011). Pepper seedling grown in greenhouse for four weeks were treated with WESMS, 0.5% BABA and water by foliar spray and soil-irrigation. The leaves were collected at 0, 48, 96, and 120 h after the treatments. Samples were snap frozen in liquid nitrogen and stored at −80°C until required for examination. Total RNA was isolated using TRIzol-Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions, and then treated with RNAse-free-DNase (Qiagen, Valencia, CA, USA). One microgram of DNA-free RNA was used for first-strand cDNA synthesis using the SuperScript III First-strand Synthesis system for RT-PCR (Invitrogen) and following the manufacturer’s instructions. The total RNA was amplified with the RT-PCR primers listed in Table 1 under the following PCR conditions: 1 cycle of 1 min at 94°C; 25 cycles of 30 s at 94°C, 30 s at 60°C, 1 min at 72°C; and a final extension cycle of 10 min at 72°C. PCR products were visualized in 1.5% agarose gel by staining with ethidium bromide. For qRT-PCR, PCR reaction was performed in a LightCycler® 96 System (Roche, Welwyn Garden, UK). The reaction mixture (20 μl) consisted of 2× SYBR Green 1 master mixtures (Roche), cDNA and 10 pmol of each qRT-PCR primer. PCR profile was as follows: 10 min for initial polymerase activation, followed by 40 cycles of 95°C for 15 s, 58°C for 30 s and 72°C for 30 s. The relative gene expression was calibrated and normalized to level of qActin mRNA by the software provided from the PCR system.
Table 1.
Primers used for reverse transcription and quantitative real-time PCR
| Primer | Sequence | Reference |
|---|---|---|
| For RT-PCR | ||
| CaPR4 | F: 5′-GGCGCAGAGTGCTACGAAC-3′ R: 5′-AGTGTCCAATTGGTTAAACACG-3′ |
Sang et al., 2010 |
| CaPR10 | F: 5′-CTTACTGACAAGTCCACAGCCT-3′ R: 5′-GCAGAAGCTTCAAATTTGCC-3′ |
Sang et al., 2010 |
| 18S rRNA | F: 5′-CGGTCCGCCTATGGTGAGCACCGGTCG-3′ R: 5′-TTCTTGGATTTATGAAAGACGAACAACTGC-3′ |
Sang et al., 2010 |
| For qRT-PCR | ||
| CaPR1 | F: 5′-ACTTGCAATTATGATCCACC-3′ R: 5′-ACTCCAGTTACTGCACCATT-3′ |
Yang et al., 2011 |
| CaBGLU | F: 5′-TAAAAGGGGAAGTCCAAGAAGG-3′ R: 5′-TCAGCAAAAATGTCCAAAAATC-3′ |
Yang et al., 2011 |
| CaPR4 | F: 5′-AACTGGGATTTGAGAACTGCCAGC-3′ R: 5′-ATCCAAGGTACATATAGAGCTTCC-3′ |
Yang et al., 2011 |
| CaPR10 | F: 5′-ATGTTGAAGGTGATGGTGGTGCTG-3′ R: 5′-TCCCTTAGAAGAACTGATACAACC-3′ |
Yang et al., 2011 |
| CaActin | F: 5′-TTGGACTCTGGTGATGGTGTG-3′ R: 5′-AACATGGTTGAGCCACCACTG-3′ |
Yang et al., 2011 |
F, forward; R, reverse.
Extraction and measurement of SA from pepper leaves
For determining the total SA, one gram of pepper leaves was frozen in liquid nitrogen, ground in a mortar, and mixed in 3 ml of 90% methanol. The mixture was centrifuged for 15 min at 4°C at 12,000g and the supernatant was collected. The supernatants were evaporated at 40°C under vacuum. The residues were resuspended in a mixture of 100 μl of 5% trichloroacetic acid (Sigma-Aldrich) and 1 ml of 99.8% methanol (w/v). The volume of each extract was adjusted with distilled water to 5 ml, and centrifuged at 8,000g for 10 min. The supernatants of the extracts were used for measuring SA content. SA content was detected on liquid chromatograph mass spectrometer (LCMS8050; Shimadzu, Kyoto, Japan) on Kinetex c18 column (2.6 μm, 100 × 2.1 mm; Phenomenex). Calibration curves were linear (R2 > 0.99) over the concentration range of 10–500 ng/ml with acceptable accuracy and precision.
Results and Discussion
Antifungal activity of L. edodes WESMS against P. capsici
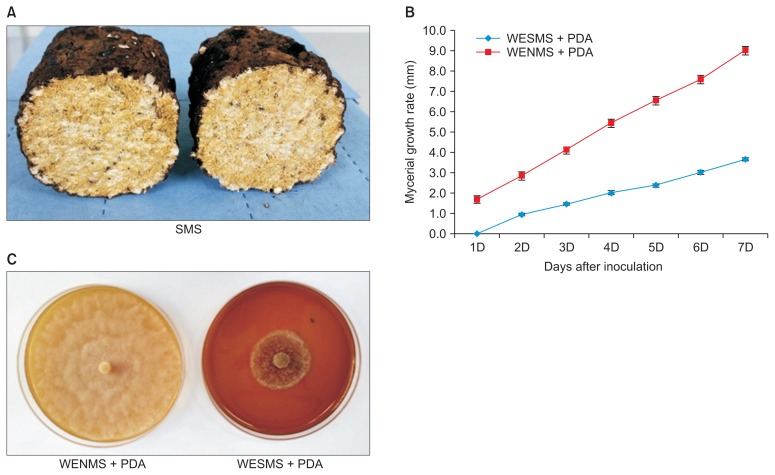
Fig. 1A shows form of SMS of L. edodes used in this study. The SMS includes mushroom mycelia and substrate residues on macroscopic observation. The SMS and water (1:3 w/v) was mixed and heated at 80°C. The mixture was filtrated on Miracloth and centrifuged for removing the residues in the water and resultant water extract was used for this experiment. The growth of P. capsici was tested on PDA supplemented with WESMS of L. edodes. The mycelial growth rate was investigated for 1–7 days after inoculation of mycelial plugs (Fig. 1B). The WESMS inhibited mycelial growth over 65% on the 7th day after inoculation of P. capsici (Fig. 1C). On the other hand, the fungi was fully grown PDA containing WENMS that did not inoculate with spawn of L. edodes. WESMS of mushroom species such as Lyophyllum decastes have been evaluated for their antifungal properties against cucumber anthracnose fungi, Colletotrichum orbiculare (Parada et al., 2011). Fruiting bodies and mycelia of L. edodes have been the main source of clinical effects against human related microbes including bacteria and fungi (Poucheret et al., 2006). Recently, mycelial culture filtrate of L. edodes has been reported to suppress the growth of phytopathogenic fungi and bacteria in agricultural plants (Bender et al., 2003). In their study, the culture filtrates of L. edodes were able to inhibit spore germination of Colletotrichum higginsianum completely, but did not have an effect on that of P. capsici. In contrast, L. edodes WESMS of this study clearly inhibited mycelial growth of P. capsici, speculating that additional compounds associated with antifungal activity can be produced in WESMS composition.
Fig. 1.
Inhibition of mycerial growth of Phytophthora capsici on water extract from spent mushroom substrate (SMS) of Lentinula edodes. The mycerial plugs were inoculated on potato dextrose agar (PDA) media containing water extracts from spent mushroom substrate (WESMS) and nature mushroom substrate (WENMS) of L. edodes. (A) Form of L. edodes SMS obtained from mushroom farm. (B) Mycerial growth rate on days after inoculation of P. capsici. (C) Mycerial inhibition on WENMS + PDA and WESMS + PDA media after 7 days of inoculation.
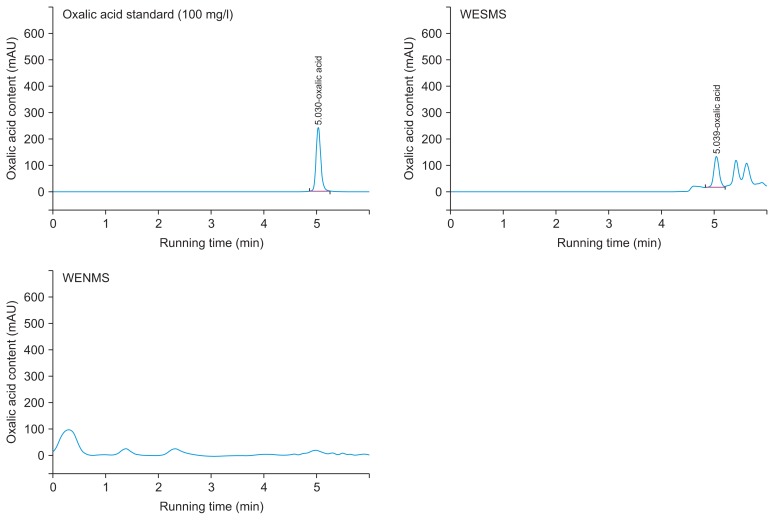
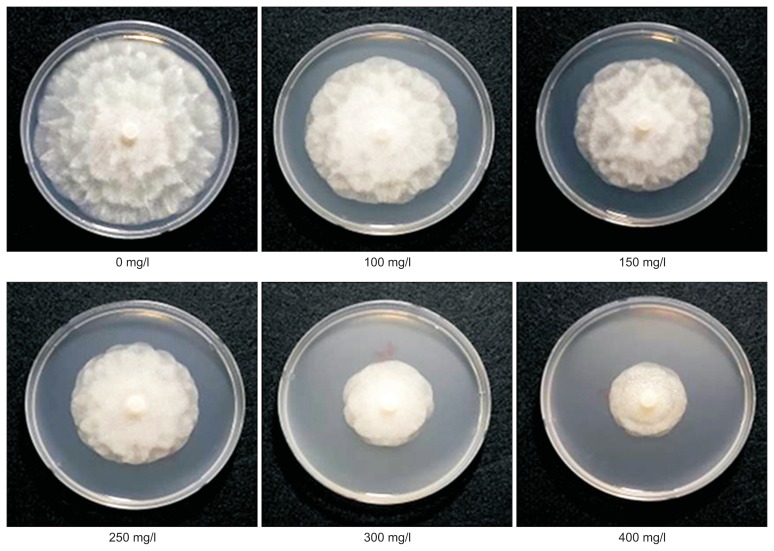
Furthermore, to identify the antifungal compound, the L. edodes WESMS was subjected to octadecyl-silica column chromatography and was eluted with different concentrations from 5% to 100% methanol (data not shown). None of the methanol elute inhibited the mycelial growth of P. capsici, but the fraction that passed through the column (referred hereafter as passed fraction) showed ability to inhibit mycelial growth when compared to the original WESMS. The pH of the passed fraction was 3.9, and it was speculated that an organic acid was causing such low pH characteristics. Thus, an organic acid analysis using WESMS was conducted on HPLC. As shown in Fig. 2, a peak indicating oxalic acid was detected in WESMS with a concentration of 65 mg/l, but not in WENSM, suggesting that L. edodes produces oxalic acid as main organic acid in mushroom substrate during the mycelial growth or fruiting body stages. We further investigated if oxalic acid has antifungal activity against P. capsici. Mycelial plugs of P. capsici were inoculated on PDA media that contain different concentrations (100, 150, 200, 250, 300, 350, and 400 mg/l) of commercial oxalic acid (Sigma-Aldrich). The PDA media containing oxalic acid was seen to inhibit mycelial growth by 20% to 70% depending on the concentration of the oxalic acid (Fig. 3). Oxalic acid isolated from L. edodes has been shown to exhibit antimicrobial activity against bacterial species, Bacillus cereus, Staphylococcus aureus, and Streptococcus faecalis (Bender et al., 2003; Poucheret et al., 2006). As far as we know, there is no report on the antifungal activity of oxalic acid from mushroom species. Secondary metabolites of three types that inhibit zoospore germination of P. capsici were identified from culture filtrate of Clitocybe nuda (Chen et al., 2012). Of them, active compound, indole-3-carbaldehyde inhibits the spore germination of the pathogen at minimum concentration of 500 mg/l. Compared to the compound, oxalic acid of current study completely inhibited zoospore germination of P. capsici at concentration less than 200 mg/l (data not shown), showing oxalic acid is higher antifungal activity than the reported compound. Interestingly, it has been known that oxalic acid from Sclerotinia is an essential determinant in pathogenicity (Cessna et al., 2000). The oxalic acid produced from plant pathogenic fungi provide effective condition of low pH to give the maximal activities of fungal enzymes that degrade cell wall of the host plant. Also, it was proposed the oxalate anion chelate to cell wall Ca2+ and consequently hampers the function of Ca2+ dependent defense responses. In the natural environment, it is likely that L. edodes invades woods and produce lignocellulytic enzymes such as laccase, cellulase, and xylanase to degrade the cell walls of the host plant and the resulting product, glucose is used in energy metabolism (Elisashvili et al., 2008). Optimal enzyme activity of a laccase (EC 1.10.3.2), Lcc 1 of L. edodes was observed at pH 4.0 (Nagai et al., 2002). In addition, xylanase that was purified from waste oak logs of L. edodes (Lee et al., 2007) showed optimal enzymatic activity at pH 4.0. As mentioned above, WESMS of L. edodes was about pH 3.9 that is similar to optimal pH conditions of the reported both enzymes. Thus, it was assumed that oxalic acid in SMS of L. edodes plays critical role in providing optimal activity for the lignocellulytic enzymes and finally offering beneficial condition in microbial interaction between host and mushroom.
Fig. 2.
High performance liquid chromatography (HPLC) profile of oxalic acid in water extract from spent mushroom substrate (WESMS) and nature mushroom substrate (WENMS) of Lentinula edodes.
Fig. 3.
Mycerial growth inhibition of Phytophthora capsici on potato dextrose agar media containing different oxalic acid concentrations.
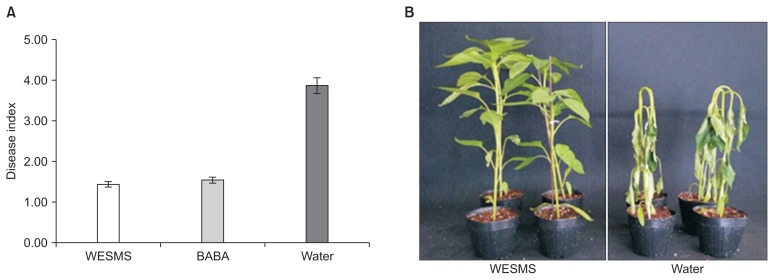
Suppression of Phytophthora blight disease of pepper by L. edodes WESMS
In order to investigate whether the protective effect of L. edodes against Phytophthora blight disease of pepper, pepper seedlings were treated with WESMS. The water and BABA was treated as negative and positive controls. As shown in Fig. 4A, pepper plants treated with WESMS and BABA exhibited protective effect of 64% and 62% when compared to water treated pepper plants, whereas water treated the pepper seedlings were severely wilted with disease index 3.8–4.0, showing typical Phytophthora blight symptom. It was speculated that antifungal compounds such as oxalic acid in L. edodes WESMS act in suppressing the disease by inhibiting the growth of the pathogen. Thus, oxalic acid (400 mg/l) that showed highly antifungal activity was used to treat pepper seedling to determine its ability to suppress the Phytophthora blight disease. The treatment did show protective effect against Phytophthora blight disease of pepper seedling comparing to WESMS (data not shown). As mentioned above, in vitro the oxalic acid content of 65 mg/l in WESMS did not inhibit mycelial growth, and thus it was considered that WESMS composition includes additional compounds that aided to suppress the disease. In a previous study, culture filtrates of Coprinus comatus and C. nuda showed that protective effects more than 80% differed from the 10% of the control, while the culture filtrate of L. edodes did show low protective effect less than 20% (Chen and Huang, 2010). It was reported that SSF exhibited higher activity of diverse bioactive substances including antibiotics than that of submerged fermentation of liquid cultural type (Subramaniyam and Vimala, 2012; Vikineswary et al., 1997). Therefore, the higher protective effect in SMS of L. edodes than that in its culture filtrate may be attributed to the similar cultural type to SSF. It was also reported that WESMS of the edible mushrooms L. decastes and Pleurotus eryngii suppresses fungal diseases, powdery mildew, and anthracnose in cucumber plant, although they did not exhibit direct antifungal activity on the fungal mycellium (Parada et al., 2012), suggesting that the WESMS possess additional substance promoting plant healthy and defense response that can ultimately contribute to suppress the plant disease.
Fig. 4.
Protective effect of water extract from spent mushroom substrate (WESMS) of Lentinula edodes against Phytophthora blight disease of pepper. Phytophthora capsici was inoculated on pepper after treatment with WESMS of L. edodes. The diseases index was checked on pepper for 8 day after inoculation (A) and disease severity was observed after 7 days of inoculation (B). Value represents the mean disease index ± standard deviations of nine replicates for each treatment. The experiment was repeated three times.
In following experiment, we investigated if L. edodes WESMS promotes pepper growth by measuring plant height, leaf number, and fresh weights of roots and shoots. L. edodes WESMS-treatment promoted the growth of pepper height by 40% and 24% compared to water- and WENMS-treatment (Table 2). Particularly, the highest mean number of 12 leaves in WESMS-treated plants is significant on nine leaves and seven leaves of WENSM and water treatments. Moreover, the WESMS treatment also increased the fresh weight of root and shoot from 60% to 85%. The results suggest that WESMS of L. edodes is beneficial to promote growth of pepper plants. Similarly to our result, there are several reports that SMSs from Agaricus bisporus, Hericium erinaceus and Pleurotus ostreatus are effective to the growth promotions of pea, pepper and tomato plants (Ahlawat et al., 2011; Kwak et al., 2015; Roy et al., 2015).
Table 2.
Effect on tomato growth promotion of water extract from spent mushroom substrates of Lentinula edodes
| Treated | Plant height (cm) | Leaf length (cm) | Leaf width (cm) | No. of leaves | Fresh weight (g) | |
|---|---|---|---|---|---|---|
|
| ||||||
| Shoot | Root | |||||
| WESMS | 25.98 ± 1.96a | 9.03 ± 0.71a | 4.49 ± 0.38a | 12.20 ± 2.17a* | 0.98 ± 0.16a | 0.21 ± 0.05a |
| WENMS | 19.40 ± 1.08c | 7.34 ± 0.76bc | 3.63 ± 0.35bc | 9.20 ± 1.92bc | 0.62 ± 0.08b | 0.10 ± 0.03b |
| Water | 15.60 ± 0.86d | 6.95 ± 1.17c | 3.29 ± 0.52c | 7.00 ± 1.22c | 0.31 ± 0.08c | 0.03 ± 0.02b |
WESMS, water extract of spent mushroom substrate; WENMS, water extract of natural mushroom substrate without inoculum.
The different letters are significantly (P < 0.05) different according to Duncan’s multiple test.
Effect of WESMS on expression induction of defense genes in pepper
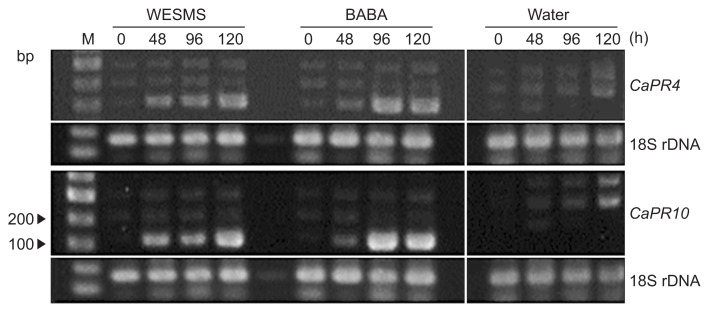
It was reported compost water extract induce the expression of defense genes in pepper and expecially, expression signal was significantly detected on CaPR4 and CaPR10 from 48 h after the water extract treatment (Sang and Kim, 2011). Thus, the expression level of the defense genes in pepper was checked on different time (0, 48, 96, and 120 h) after WESMS, BABA and water treatments. Total RNA was extracted from the treated pepper leaves and subjected to RT-PCR targeting on defense genes, CaPR4 and CaPR10. The RT-PCR bands were observed in WESMS and BABA treated RNA samples after treatments of 48, 96, and 120 h, but not in water (Fig. 5). The defense genes were expressed between 48 h and 120 h after WESMS and BABA treatments. It was observed that the highest expression level of the defense genes is maximally induced after 96 h of the treatments.
Fig. 5.
Expression level of defense genes in pepper on different hours after treatments of water extract from spent mushroom substrate (WESMS) of Lentinula edodes. L. edodes WESMS, DL-β-amino butyric acid (BABA, positive control) and sterile distilled water (negative control) was treated on pepper roots and leaves and different hours (0, 48, 96, and 120 h) were given after the treatment. Total RNA was extracted from leaf samples of the treated peppers and subjected to RT-PCR using primers targeting CaPR4, CaPR10 and 18S rDNA (positive control). The PCR products were visualized on agarose gel (1.5%) by staining with ethidium bromide. M, 100-bp DNA ladder.
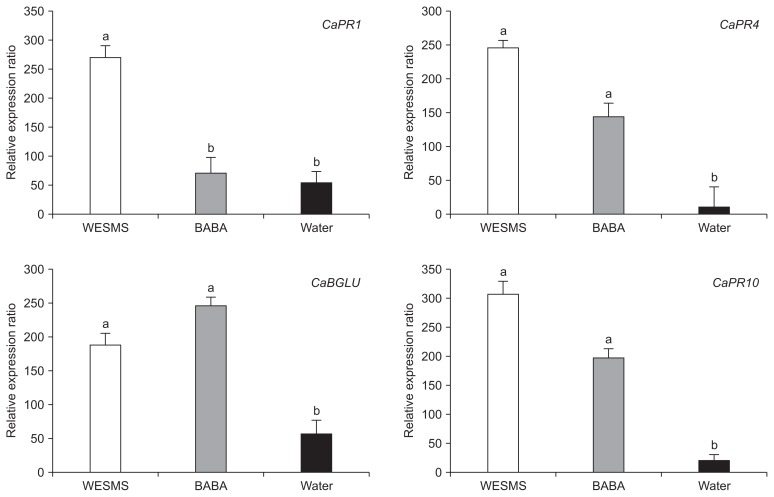
To determine the expression quantity of defense genes, each total RNA was extracted from the leaf samples of WESMS, BABA, and water treated pepper plants for 96 h and was subjected to qRT-PCR. As shown in Fig. 6, the expression of the PR genes CABPR1 (PR protein 1), CaBGLU (β-1,3-glucanase), CaPR-4 (PR protein 4), or CaPR-10 (PR protein 10) was significantly enhanced (Fig. 6) by WESMS and BABA (positive control) compared to water. It is known that the induction of defense-related genes such as CaPR1 and CaBGLU was essential for systemic acquired resistance (SAR) in pepper plants (Kim and Hwang, 2000; Kim et al., 1989). The defense genes are known to be triggered through a SA-dependent signaling pathway (Cameron et al., 1999), reflecting the induction of SA accumulation by WESMS treatment. To estimate whether the WESMS-treated pepper could be correlated with an accumulation of SA, the content of total SA in pepper leaves was determined using a liquid chromatograph mass spectrometer. Increased SA levels of 4- to 6-fold were found in the leaves 96 h after treatment with WESMS and BABA, in comparison to that with water (Table 3). From the result, it is presumable that WESMS are associated with SAR mediated by a SA dependent process (Walters and Fountaine, 2009).
Fig. 6.
Quantitative real-time PCR (qRT-PCR) analysis of defense genes in pepper plants by treatment of water extract from spent mushroom substrate (WESMS) of Lentinula edodes. The total RNA was extracted from pepper leaves at 96 h after water, DL-β-aminobutyric acid (BABA, 0.5%) and WESMS treatments and subjected to qRT-PCR using primers (Table 1) targeting defense genes in pepper. Each gene expression was normalized to reference gene, CaActin. Expression value is the average of three replications and the bar indicates the standard deviation. Different letters indicate significant differences between treatments (P < 0.05 according to Duncan’s multiple test).
Table 3.
Salicylic acid content in peppers treated by water extract from spent mushroom substrate of Lentinula edodes
| Treated | Salicylic acid content (ng/ml) on hours | ||
|---|---|---|---|
|
| |||
| 48 h | 96 h | 120 h | |
| WESMS | 12.77 ± 1.34 | 12.79 ± 1.65 | 18.68 ± 0.41 |
| BABA | 26.06 ± 1.62 | 31.62 ± 1.92 | 14.85 ± 1.90 |
| Water | 5.24 ± 1.27 | 3.49 ± 0.71 | 5.97 ± 1.73 |
Values are presented as means ± standard deviations of three replicates.
WESMS, water extract of spent mushroom substrate; BABA, DL-β-aminobutyric acid.
In addition, induced systemic resistance (ISR) has been defined as a result of colonization of plant roots by certain strains of plant growth-promoting rhizobacteria (PGPR) and is mediated by a jasmonate (JA)-and-ethylene (ET)-sensitive pathway (Walters and Fountaine, 2009; Walters et al., 2005). In pepper plant, it is known that CaPR4 and CaPR10 are respectively related to ET/JA and SA/ET/JA responsive signaling (Park et al., 2001, 2004; Yang et al., 2009), which are responsible for the ISR defense mechanism of plant resistance. In this study, it was also observed that WESMS treatment elicited an upregulation of transcriptional expression of CaPR4 and CaPR10, to a level greater than about 10 times that recorded when treated with water (Fig. 6).
It was demonstrated that the water extract from autoclaved SMS of an edible mushroom, L. decastes could control cucumber anthracnose disease and enhance the state of SAR inducing defense genes such as chitinase and β-1,3-glucanase (Parada et al., 2011). Molecular evidence of defense genes demonstrated that root treatment of WESMS tested in this study was effective in establishing the ISR in leaves of pepper plants. Fungal culture fluids and the fraction that is heat-released from mycelial cell walls have been used widely as elicitors (Parker et al., 1991; Repka et al., 2001). Further, carbohydrate and protein elicitors that induce defense mechanism in plants are released from the mycelia of fungal pathogens (Shibuya and Minami, 2001; Walters et al., 2005). The SMS mushroom mycelia seem to be a viable and abundant source of the elicitors. In the present study, heat-treated WESMS of L. edodes activated defense genes related to SAR and ISR. In mushrooms, elicitors from fruiting bodies of L. edodes have partially been purified to control cucumber anthracnose (Di Piero et al., 2006). Hence, it was reasonably assumed the elicitor can be released from mycelial residues in the SMS on heat-treated extraction steps. However, the components of the elicitor that act in the defense mechanisms of WESMS should be identified from the WESMS of L. edodes in further investigations.
Overall, this study showed that L. edodes WESMS is effective for suppressing the Phytophthora blight disease of pepper. The protective effect in WESMS treated pepper might be associated with multiple functions including antifungal activity, defense gene induction, and plant growth promotion. Therefore, use of L. edodes WESMS may provide a new eco-friendly technology for disease control against Phytophthora blight of pepper recycling agricultural waste of SMS. Nevertheless, applications of L. edodes WESMS should be evaluated for disease control at the field and greenhouse scales through more studies.
Acknowledgments
This research is supported by Agenda program (Grant No. PJ009969) of Rural Development Administration and Research village program of Small and Medium Business Administration (Grant No. 2016-210).
References
- Ahlawat OP, Manikandan K, Sagar MP, Raj D, Gupta P, Vijay B. Effect of composted button mushroom spent substrate on yield, quality and disease incidence of Pea (Pisum sativum) Mushroom Res. 2011;20:87–94. [Google Scholar]
- Alves MJ, Ferreira IC, Dias J, Teixeira V, Martins A, Pintado M. A review on antimicrobial activity of mushroom (Basidiomycetes) extracts and isolated compounds. Planta Med. 2012;78:1707–1718. doi: 10.1055/s-0032-1315370. [DOI] [PubMed] [Google Scholar]
- Bender S, Dumitrache-Anghel CN, Backhaus J, Christie G, Cross RF, Lonergan GT, Baker WL. A case for caution in assessing the antibiotic activity of extracts of culinary-medicinal Shiitake mushroom [Lentinus edodes (Berk.) Singer] (Agaricomycetideae) Int J Med Mushrooms. 2003;5:31–35. doi: 10.1615/InterJMedicMush.v5.i1.40. [DOI] [Google Scholar]
- Cameron RK, Paiva NL, Lamb CJ, Dixon RA. Accumulation of salicylic acid and PR-1 gene transcripts in relation to the systemic acquired resistance (SAR) response induced by Pseudomonas syringae pv. tomato in Arabidopsis. Physiol Mol Plant Pathol. 1999;55:121–130. doi: 10.1006/pmpp.1999.0214. [DOI] [Google Scholar]
- Cessna SG, Sears VE, Dickman MB, Low PS. Oxalic acid, a pathogenicity factor for Sclerotinia sclerotiorum, suppresses the oxidative burst of the host plant. Plant Cell. 2000;12:2191–2200. doi: 10.1105/tpc.12.11.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JT, Huang JW. Antimicrobial activity of edible mushroom culture filtrates on plant pathogens. Plant Pathol Bull. 2010;19:261–270. [Google Scholar]
- Chen JT, Su HJ, Huang JW. Isolation and identification of secondary metabolites of Clitocybe nuda responsible for inhibition of zoospore germination of Phytophthora capsici. J Agric Food Chem. 2012;60:7341–7344. doi: 10.1021/jf301570y. [DOI] [PubMed] [Google Scholar]
- Di Piero RM, Wulff NA, Pascholati SF. Partial purification of elicitor from Lentinula edodes basidiocarps protecting cucumber seedling against Collectotrichum lagenarium. Braz J Microbiol. 2006;37:175–180. doi: 10.1590/S1517-83822006000200015. [DOI] [Google Scholar]
- Elisashvili V, Penninckx M, Kachlishvili E, Tsiklauri N, Metreveli E, Kharziani T, Kvesitadze G. Lentinus edodes and Pleurotus species lignocellulolytic enzymes activity in submerged and solid-state fermentation of lignocellulosic wastes of different composition. Bioresour Technol. 2008;99:457–462. doi: 10.1016/j.biortech.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Hwang BK, Kim CH. Phytophthora blight of pepper and its control in Korea. Plant Dis. 1995;79:221–227. doi: 10.1094/PD-79-0221. [DOI] [Google Scholar]
- Inagaki R, Yamaguchi A. Spent substrate of shiitake (Lentinula edodes) inhibits symptoms of anthracnose in cucumber. Mushroom Sci Biotechnol. 2009;17:113–115. [Google Scholar]
- Jang CS, Min KT, Kim ME. Monthly shiitake observation. Korea Economic Research Institute; Seoul, Korea: 2012. pp. 1–4. [Google Scholar]
- Kim CH. Review of disease incidence of major crops in 2003. Res Plant Dis. 2004;10:1–7. doi: 10.5423/RPD.2004.10.1.001. [DOI] [Google Scholar]
- Kim YJ, Hwang BK. Pepper gene encoding a basic pathogenesis-related 1 protein is pathogen and ethylene inducible. Physiol Plant. 2000;108:51–60. doi: 10.1034/j.1399-3054.2000.108001051.x. [DOI] [Google Scholar]
- Kim YJ, Hwang BK, Park KW. Expression of age-related resistance in pepper plants infected with Phytophthora capsici. Plant Dis. 1989;73:745–747. doi: 10.1094/PD-73-0745. [DOI] [Google Scholar]
- Komemushi S, Yamamoto Y, Fujita T. Purification and identification of antimicrobial substances produced by Lentinus edodes. J Antibacter Antifung Agents. 1996;24:21–25. [Google Scholar]
- Kwak AM, Kang DS, Lee SY, Kang HW. Effect of spent mushroom substrates on Phytophthora blight disease and growth promotion of pepper. J Mushrooms. 2015;13:16–20. doi: 10.14480/JM.2015.13.1.16. [DOI] [Google Scholar]
- Lee JW, Gwak KS, Kim SI, Kim M, Cho DH, Choi IG. Characterization of xylanase from Lentinus edodes M290 cultured on waste mushroom logs. J Microbiol Biotechnol. 2007;17:1811–1817. [PubMed] [Google Scholar]
- Morris SC, Nicholls PJ. An evaluation of optical density to estimate fungal spore concentrations in water suspensions. Phytopathology. 1978;68:1240–1242. doi: 10.1094/Phyto-68-1240. [DOI] [Google Scholar]
- Nagai M, Sato T, Watanabe H, Saito K, Kawata M, Enei H. Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl Microbiol Biotechnol. 2002;60:327–335. doi: 10.1007/s00253-002-1109-2. [DOI] [PubMed] [Google Scholar]
- Ngai PH, Ng TB. Lentin, a novel and potent antifungal protein from shitake mushroom with inhibitory effects on activity of human immunodeficiency virus-1 reverse transcriptase and proliferation of leukemia cells. Life Sci. 2003;73:3363–3374. doi: 10.1016/j.lfs.2003.06.023. [DOI] [PubMed] [Google Scholar]
- Pacumbaba RP, Beyl CA, Pacumbaba RO. Shiitake mycelial leachate suppresses growth of some bacterial species and symptoms of bacterial wilt of tomato and lima bean in vitro. Plant Dis. 1999;83:20–23. doi: 10.1094/PDIS.1999.83.1.20. [DOI] [PubMed] [Google Scholar]
- Parada RY, Murakami S, Shimomura N, Egusa M, Otani H. Autoclaved spent substrate of hatakeshimeji mushroom (Lyophyllum decastes Sing) and its water extract protect cucumber from anthracnose. Crop Protect. 2011;30:443–450. doi: 10.1016/j.cropro.2010.11.021. [DOI] [Google Scholar]
- Parada RY, Murakami S, Shimomura N, Otani H. Suppression of fungal and bacterial diseases of cucumber plants by using the spent mushroom substrate of Lyophyllum decastes and Pleurotus eryngii. J Phytopathol. 2012;160:390–396. doi: 10.1111/j.1439-0434.2012.01916.x. [DOI] [Google Scholar]
- Park CJ, Kim KJ, Shin R, Park JM, Shin YC, Paek KH. Pathogenesis-related protein 10 isolated from hot pepper functions as a ribonuclease in an antiviral pathway. Plant J. 2004;37:186–198. doi: 10.1046/j.1365-313X.2003.01951.x. [DOI] [PubMed] [Google Scholar]
- Park CJ, Shin R, Park JM, Lee GJ, Yoo TH, Paek KH. A hot pepper cDNA encoding a pathogenesis-related protein 4 is induced during the resistance response to tobacco mosaic virus. Mol Cells. 2001;11:122–127. [PubMed] [Google Scholar]
- Parker JE, Schulte W, Hahlbrock K, Scheel D. An extracellular glycoprotein from Phytophthora megasperma f. sp. glycinea elicits phytoalexin synthesis in cultured parsley cells and protoplasts. Mol Plant-Microbe Interact. 1991;4:19–27. doi: 10.1094/MPMI-4-019. [DOI] [Google Scholar]
- Parra G, Ristaino JB. Resistance to mefenoxam and metalaxyl among field isolates of Phytophthora capsici causing Phytophthora blight of bell pepper. Plant Dis. 2001;85:1069–1075. doi: 10.1094/PDIS.2001.85.10.1069. [DOI] [PubMed] [Google Scholar]
- Poucheret P, Fons F, Rapior S. Biological and pharmacological activity of higher fungi: 20-year retrospective analysis. Mycologie. 2006;27:311–333. [Google Scholar]
- Repka V, Fischerova I, Silharova K. Biological activity of elicitor released from mycelium of grapevine isolate of the necrotrophic fungus Botrytis cinerea. Vitis. 2001;40:205–212. [Google Scholar]
- Roy S, Barman S, Chakraborty U, Chakraborty B. Evaluation of spent mushroom substrate as biofertilizer for growth improvement of Capsicum annuum L. J Appl Biol Biotechnol. 2015;3:022–027. doi: 10.7324/JABB.2015.3305. [DOI] [Google Scholar]
- Sang MK, Kim JG, Kim KD. Biocontrol activity and induction of systemic resistance in pepper by compost water extracts against Phytophthora capsici. Phytopathology. 2010;100:774–783. doi: 10.1094/PHYTO-100-8-0774. [DOI] [PubMed] [Google Scholar]
- Sang MK, Kim KD. Biocontrol activity and primed systemic resistance by compost water extracts against anthracnoses of pepper and cucumber. Phytopathology. 2011;101:732–740. doi: 10.1094/PHYTO-10-10-0287. [DOI] [PubMed] [Google Scholar]
- Sang MK, Shrestha A, Kim DY, Park K, Pak CH, Kim KD. Biocontrol of Phytophthora blight and anthracnose in pepper by sequentially selected antagonistic rhizobacteria against Phytophthora capsici. Plant Pathol J. 2013;29:154–167. doi: 10.5423/PPJ.OA.07.2012.0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya N, Minami E. Oligosaccharide signaling for defense responses in plant. Physiol Mol Plant Pathol. 2001;59:223–233. doi: 10.1006/pmpp.2001.0364. [DOI] [Google Scholar]
- Stephan D, Schmitt A, Martins Cavalho S, Seddon B, Koch E. Eur J Plant Pathol. Vol. 112. British Columbia Mushroom Industry; Columbia, UK: 2005. Evaluation of biocontrol preparations and plant extracts for the control of Phytophthora infestans on potato leaves; pp. 235–246.pp. 101 [DOI] [Google Scholar]
- Suay I, Arenal F, Asensio FJ, Basilio A, Cabello MA, Díez MT, García JB, del Val AG, Gorrochategui J, Hernández P, Peláez F, Vicente MF. Screening of basidiomycetes for antimicrobial activities. Antonie Leeuwenhoek. 2000;78:129–139. doi: 10.1023/A:1026552024021. [DOI] [PubMed] [Google Scholar]
- Subramaniyam R, Vimala R. Solid state and submerged fermentation for the production of bioactive substances: a comparative study. Int J Sci Nat. 2012;3:480–486. [Google Scholar]
- Suess A, Curtis J. Report: value-added strategies for spent mushroom substrate in BC. British Columbia Mushroom Industry; Columbia, UK: 2006. p. 101. [Google Scholar]
- Sunwoo JY, Lee YK, Hwang BK. Induced resistance against Phytophthora capsici in pepper plants in response to DL-β-amino-n-butyric acid. Eur J Plant Pathol. 1996;102:663–670. doi: 10.1007/BF01877247. [DOI] [Google Scholar]
- Vikineswary S, Kumaran KS, Ling SK, Dinesh N, Shim YL. Solid substrate fermentation of agroresidues for value added products: the Malaysian experience. Global Environ Biotechnol. 1997;3:301–305. doi: 10.1007/978-94-017-1711-3_25. [DOI] [Google Scholar]
- Walters D, Walsh D, Newton A, Lyon G. Induced resistance for plant disease control: maximizing the efficacy of resistance elicitors. Phytopathology. 2005;95:1368–1373. doi: 10.1094/PHYTO-95-1368. [DOI] [PubMed] [Google Scholar]
- Walters DR, Fountaine JM. Practical application of induced resistance to plant diseases: an appraisal of effectiveness under field conditions. J Agric Sci. 2009;147:523–535. doi: 10.1017/S0021859609008806. [DOI] [Google Scholar]
- Wong JH, Ng TB, Cheung RC, Ye XJ, Wang HX, Lam SK, Lin P, Chan YS, Fang EF, Ngai PH, Xia LX, Ye XY, Jiang Y, Liu F. Proteins with antifungal properties and other medicinal applications from plants and mushrooms. Appl Microbiol Biotechnol. 2010;87:1221–1235. doi: 10.1007/s00253-010-2690-4. [DOI] [PubMed] [Google Scholar]
- Yang JW, Yi HS, Kim HK, Lee BY, Lee SH, Ghim SY, Ryu CM. Whitefly infestation of pepper plants elicits defence responses against bacterial pathogens in leaves and roots and changes the below-ground micro-flora. J Ecol. 2011;99:46–56. doi: 10.1111/j.1365-2745.2010.01756.x. [DOI] [Google Scholar]
- Yang JW, Yu SH, Ryu CM. Priming of defense-related genes confers root-colonizing bacilli-elicited induced systemic resistance in pepper. Plant Pathol J. 2009;25:389–399. doi: 10.5423/PPJ.2009.25.4.389. [DOI] [Google Scholar]