Abstract
A study finds that deficits in touch-sensing somatosensory neurons contribute to social interaction and anxiety phenotypes in mouse models of autism and Rett syndrome. These findings suggest that some core symptoms of autism might originate from aberrant development or function of the peripheral nervous system.
Autism spectrum disorder (ASD) is characterized by social interaction and communication difficulties as well as repetitive or restricted behavior. However, a growing number of reports suggest that sensory processing is also affected in a majority of patients with ASD (Baranek, 2002). To date, preclinical autism research has shown a brain bias, with an almost exclusive focus on the central nervous system. However, a new study by David Ginty’s group presents compelling evidence that some ASD-related deficits may be caused by faulty wiring in the peripheral nervous system (Orefice et al., 2016).
Ginty’s lab has a history of conducting ground-breaking research on the development and function of the peripheral nervous system. In this new study, Orefice et al. probed for differences in touch sensitivity between wild-type mice, mouse models of autism, and Mecp2 mutants that model Rett syndrome using a texture-specific novel object recognition test (NORT) (Orefice et al., 2016). They found that these mouse models failed to distinguish between smooth and rough-textured objects, whereas wild-type controls could readily recognize a novel textured object. These data suggest that global whole-body deletion of several autism-related genes leads to impairment of touch sensitivity.
To further explore differences in touch sensitivity Orefice et al. puffed air onto the back skin of mice and evaluated whether this brief “prepulse” inhibited a subsequent startle response to a loud sound. They found the air puff prepulse was more effective at inhibiting the startle response in mutants when compared to controls, suggesting heightened sensitivity to gentle tactile stimuli. Indeed, further testing revealed that mutant mice were hypersensitive to the air puff alone, eliciting an exaggerated startle response. In contrast, several ASD mutants were indistinguishable from controls in both a visual NORT task and response to auditory prepulses, suggesting that these animals have abnormal tactile perception rather than more global sensory processing deficits.
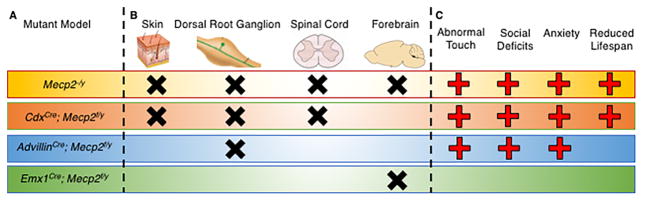
While many individuals with ASD are hypersensitive to tactile and other sensory stimuli, it is unclear if these exaggerated responses reflect problems with the peripheral or central nervous system. To explore the mechanistic basis for tactile deficits in the Mecp2 mutant mice, Orefice et al. deleted Mecp2 in different body areas, namely from forebrain excitatory neurons (using Emx1cre); all cells below cervical spinal level 2, which includes the spinal cord and peripheral nervous system (using Cdx2Cre); or sensory ganglia, including trigeminal and dorsal root ganglia (DRG; using AdvillinCre) (Figure 1). Sensory testing revealed that loss of Mecp2 in the somatosensory system alone causes the tactile hypersensitivity, tactile discrimination deficits (tactile NORT) and hypersensitivity to gentle touch stimuli seen in the global Mecp2 mutants. Surprisingly, Mecp2 deletion in forebrain excitatory neurons did not affect performance in these tactile assays, ruling out forebrain neurons in mutant-specific tactile hypersensitivity and discrimination deficits. To determine whether Mecp2 expression is necessary for the continued functioning of adult somatosensory neurons, the authors inducibly knocked out Mecp2 in DRG neurons of juvenile mice and found that these animals recapitulated tactile behavioral deficits characterized in constitutive knockout models.
Figure 1. Deletion of Mecp2 in peripheral sensory ganglia is sufficient to produce some autism-related phenotypes.
A) Mecp2 mutation in B) the indicated tissues (black X) caused C) behavioral changes relative to wild-type mice (red plus sign). “Tactile deficits” denote problems with tactile discrimination, tactile prepulse inhibition, and hypersensitivity to gentle tactile stimuli. “Social deficits” include deficits in sociability, social novelty, and social approach. “Anxiety” signifies reduced exploration in an open field and failure to habituate to repeat presentations of a startling tone.
To ascertain why tactile responses might be enhanced in Mecp2 mutants, Ginty’s group characterized GABAergic signaling in Mecp2 mutant mice as well as GABAA β3 subunit (Gabrb3) heterozygous knockout mice. Gabrb3 mutant mice were previously found to show autism-like behaviors (DeLorey et al., 2011), and Gabrb3 was previously found to be reduced in Mecp2 deficient mice (Samaco et al., 2005). Immunohistochemical comparison revealed that Mecp2 mutant and Gabrb3 mutant lines showed deficient GABRB3-containing GABAA receptors in sensory axon terminals in the spinal cord. This observation suggests that loss of presynaptic inhibition (PSI) of LTMR inputs in the spinal cord (SC) might drive tactile hypersensitivity. Subsequent electrophysiological recordings of SC slices confirmed this possibility by showing an increased probability of presynaptic excitatory neurotransmitter release in LTMR afferents. These data, along with in vivo recording, indicate that downregulation of inhibitory GABAA receptors on somatosensory LTMR afferents and a loss in PSI might produce increased light touch sensitivity in Mecp2 and Gabrb3 mutant mice.
In addition to characterizing tactile deficits, Orefice et al. sought to determine whether deletion of Mecp2 in somatosensory neurons alone could influence measures of anxiety and autism-related behaviors, including nest building, social interaction, and social approach in a tube dominance assay. Surprisingly, constitutive deletion of Mecp2 in forebrain excitatory neurons resulted in normal mouse phenotypes for all of these measures; whereas constitutive deletion of the gene in DRG resulted in marked deficits. Remarkably, using a Mecp2stop/y mouse line to expresses Mecp2 only in DRG in an otherwise Mecp2-null background, the authors showed that somatosensory expression of Mecp2 gene is sufficient to restore normal tactile responses, as well as normalize anxiety and social behaviors. These results strongly implicate peripheral neurons, and loss of Mecp2 in these neurons, as a driver of somatosensory and social deficits. This study has the potential to reshape the long-held assumption that Mecp2 deficiency in the central nervous system contributes to social deficits.
Using a comprehensive array of mouse lines, Ginty’s group provides strong support for the hypothesis that social and anxiety phenotypes in mouse models of autism and Rett syndrome may be due to problems in the peripheral nervous system. The authors’ work points to an important component of early life sensory experience in shaping subsequent developmental outcomes, adding important context to previous social deprivation studies in animals (Harlow and Dodsworth, 1965) and humans (Sheridan et al., 2012). This new study also provides possible mechanistic insights into tactile hyper- and hyposensitivities in autistic individuals (Baranek, 2002; Cascio, 2010). It is increasingly clear that genetic and environmental risks are at the heart of autism pathology. Ginty’s study suggests these risks may affect the development and function of peripheral sensory neurons, and in turn contribute to social and anxiety phenotypes. Future studies are needed to determine if other autism models show abnormalities in sensory processing, including excitatory/inhibitory imbalance in the spinal cord. It is also possible that selectively impairing the peripheral nervous system (e.g., Cavanaugh et al., 2009; McCoy et al., 2013) could affect more complex behaviors like social interactions and anxiety. And in light of these new findings, it will be intriguing to assess the extent to which symptoms associated with autism can be lessened by targeting the peripheral nervous system with drugs or by developing new sensory interventions, especially during early development.
Acknowledgments
This work was supported by The National Institute of Environmental Health Sciences (DP1ES024088; M.J.Z.), the NIH (R01MH093372, R01NS081127; M.J.Z.), and the Rett Syndrome Research Trust (M.J.Z.).
References
- Baranek GT. Efficacy of Sensory and Motor Interventions for Children with Autism. J Autism Dev Disord. 2002;32:397–422. doi: 10.1023/a:1020541906063. [DOI] [PubMed] [Google Scholar]
- Cascio CJ. Somatosensory processing in neurodevelopmental disorders. Journal of Neurodevelopmental Disorders 2010 2:2. 2010;2:62–69. doi: 10.1007/s11689-010-9046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanaugh DJ, Lee H, Lo L, Shields SD, Zylka MJ, Basbaum AI, Anderson DJ. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc Natl Acad Sci USa. 2009;106:9075–9080. doi: 10.1073/pnas.0901507106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLorey TM, Sahbaie P, Hashemi E, Li WW. Somatosensory and sensorimotor consequences associated with the heterozygous disruption of the autism candidate gene, Gabrb3. Behav Brain Res. 2011;216:36–45. doi: 10.1016/j.bbr.2010.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF, Dodsworth RO. Total social isolation in monkeys. 1965 doi: 10.1073/pnas.54.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy ES, Taylor-Blake B, Street SE, Pribisko AL, Zheng J, Zylka MJ. Peptidergic CGRPα Primary Sensory Neurons Encode Heat and Itch and Tonically Suppress Sensitivity to Cold. Neuron. 2013;78:138–151. doi: 10.1016/j.neuron.2013.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orefice LL, Zimmerman AL, Chirila AM, Sleboda SJ, Head JP, Ginty DD. Peripheral mechanosensory neuron dysfunction underlies tactile and behavioral deficits in mouse models of ASD. Cell. 2016;21:738–748. doi: 10.1016/j.cell.2016.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Hogart A, LaSalle JM. Epigenetic overlap in autism-spectrum neurodevelopmental disorders: MECP2 deficiency causes reduced expression of UBE3A and GABRB3. Hum Mole Genet. 2005;14:483–492. doi: 10.1093/hmg/ddi045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Fox NA, Zeanah CH, McLaughlin KA, Nelson CA. Variation in neural development as a result of exposure to institutionalization early in childhood. Proc Natl Acad Sci USa. 2012;109:12927–12932. doi: 10.1073/pnas.1200041109. [DOI] [PMC free article] [PubMed] [Google Scholar]