Abstract
Purpose
To characterize the natural history of rod-mediated dark adaptation (RMDA) over 2 years in eyes with intermediate age-related macular degeneration (AMD). This information will be useful in understanding the potential of RMDA to serve as a functional endpoint in proof-of-concept studies and clinical trials on intermediate AMD.
Methods
RMDA was measured in eyes with intermediate AMD at baseline and follow-up visits over 2 years at 6, 12, 18, and 24 months. A computerized dark adaptometer measured sensitivity for targets centered at 11° on the superior vertical meridian of the retina. Rod intercept time (RIT) characterized the speed of dark adaptation and was defined as the duration (in minutes) required for sensitivity to reach a criterion level of 3.0 log units of attenuation of the stimulus.
Results
Mean change in RIT over 24 months for 30 eyes was 10.5 minutes (standard deviation 19.4), p < 0.0001; 73.3% of eyes had a RIT increase >1 minute, 56.7% had an increase >3 minutes, and 36.7% had an increase >6 minutes; for 26.7% RIT was unchanged (0- to 1-minute increase) or decreased. Greater increase in RIT over 24 months was associated with smoking.
Conclusions
RMDA slows in intermediate AMD over 2 years in most eyes. There was wide variability in RIT at both baseline and in the extent to which it increased over 24 months. A major risk factor for AMD, smoking, exacerbated RMDA slowing.
Translational Relevance
RMDA as assessed by RIT may be useful as a functional endpoint in proof-of-concept studies and clinical trials on intermediate AMD with 2-year designs.
Keywords: age-related macular degeneration, endpoint, dark adaptation
Introduction
As strategies for arresting the progression of intermediate age-related macular degeneration (AMD) are developed, there is a need for functional endpoint measures to evaluate these novel interventions in translational proof-of-concept studies and ultimately in larger, confirmatory clinical trials. A major barrier to developing treatments to slow or halt progression of intermediate AMD to advanced disease is the lack of valid and responsive functional endpoints. Visual acuity under photopic conditions has been a useful outcome in clinical trials of agents to prevent or arrest the spread of choroidal neovascularization (CNV), yet acuity decreases only minimally or not at all during intermediate AMD in most patients.
Previous work collectively suggests that rod-mediated dark adaptation (RMDA) is a promising candidate as a functional endpoint measure for evaluating interventions to slow intermediate AMD progression. RMDA refers to the time course of recovery of rod-mediated light sensitivity following a very bright light that bleaches the photopigment.1 Cross-sectional studies have established that slowed RMDA is associated with AMD,2–4 with longer delays observed at increasing AMD severity.5,6 The presence of reticular pseudodrusen7,8 in AMD eyes (more recently referred to as subretinal drusenoid deposits, SDD9,10) increases the risk for progression to advanced AMD, and thus it is noteworthy that SDD are associated with an exacerbated slowing in RMDA as compared with eyes without these lesions.6,11 Delayed RMDA is a functional biomarker for incident AMD, with its presence doubling risk of having AMD 3 years later.12 There is also substantial biological plausibility for RMDA's relevance to AMD incidence and progression. RMDA is the visual functional manifestation of the retinoid cycle,13,14 which refers to the process of eliminating products of light absorption from photoreceptor outer segments, recycling of the released retinoid to its original form (11-cis-retinal), and regenerating the visual photopigment opsin. The retinoid cycle's physiologic processes depend on good metabolic exchange between the choroid and photoreceptors, which can be negatively impacted by retinal structural changes in aging and AMD. For example the choroid, the blood supply to the outer retina, thins during the aging process and in AMD.15–17 Retinal pigment epithelial cells in AMD exhibit variable cell morphology, layer thickening, and redistribution and loss of lipofuscin.18–20 Drusen and SDD, the characteristic lipid- and protein-containing depositions of AMD, can cause a diffusion barrier, thus, slowing the transfer of nutrients between the choroid and photoreceptors, consistent with a slowing in RMDA in AMD.21 These changes in chorioretinal anatomy in AMD support the biological plausibility that RMDA would be deleteriously impacted by AMD.
The natural history of an endpoint is critical information in designing clinical trials. In order for a functional measure to be adopted in a study design, it is important to understand the measure's natural history, that is, to what extent does the functional measure change, if at all, over time during the course of intermediate AMD. The expected change in a nonintervention group would be based on the RMDA's natural history over that time period and compared against the hypothesized change in RMDA in a treatment arm. Little information is available on the natural history of RMDA during intermediate AMD, and thus this study is designed to assess RMDA in eyes with intermediate AMD over 2 years.
Methods
This research was approved by the institutional review board of the University of Alabama at Birmingham (UAB) and followed the tenets of the Declaration of Helsinki. Participants were recruited from the retina service of the Callahan Eye Hospital Clinics at UAB, Birmingham, AL and provided written informed consent after the nature and purpose of the study were described. Enrollees were ≥ 50 years old and had intermediate AMD in at least one eye (designated as the study eye), defined as the presence of multiple large confluent drusen covering ≥ 0.5 disk area, with or without increased or decreased pigmentation of the retinal pigment epithelium. The study eye was required to have best-corrected visual acuity of 20/100 or better. The fellow eye was required to have intermediate AMD, geographic atrophy (GA), or CNV. Determination of AMD status was based on three field, digital color fundus photography on the day of testing (450 plus camera; Carl Zeiss Meditec, Dublin, CA). Photographs were evaluated by a trained and experienced grader masked to all other participant characteristics. Exclusion criteria for the study eye included other retinal diseases, glaucoma, diabetes, cataract surgery within 3 months of the enrollment date, significant lens opacity, and previous vitreous or retinal surgery, laser photocoagulation, and intravitreal drug delivery. Exclusion criteria for the fellow eye were identical to those of the study eye except previous vitreous or retinal surgery, laser photocoagulation, intravitreal drug delivery, and recent cataract surgery were allowable. Persons who were unable to perform a dark adaptometry task were excluded, as were persons with neurological conditions that can impair vision and conditions that would impair the ability to follow simple instructions.
The study protocol consisted of a baseline visit and follow-up visits over 2 years at 6, 12, 18, and 24 months. Information on participant demographic characteristics, smoking status, and a family history of AMD (first degree relatives) was obtained at baseline through interview. Best-corrected visual acuity was measured at all visits using the electronic visual acuity (EVA) tester22 and expressed as the logarithm of the minimum angle resolvable (logMAR). At baseline and all follow-up visits color fundus photography was carried out on both eyes as described above, and the grader evaluated photographs to determine AMD severity at each visit.
RMDA in the study eye was assessed by the AdaptDx, a computerized dark adaptometer (MacuLogix, Middletown, PA). Following pupillary dilation with 1% tropicamide and 2.5% phenylephrine hydrochloride, the participant's head was positioned in the forehead chinrest. Trial lenses were used to refract the eye for the test distance as needed, and the fellow eye was covered with an opaque eye patch. An infrared camera provided a view of the eye for the examiner, who facilitated the positioning of the eye with respect to the red fixation light using a reticule superimposed on the eye's image. Testing began with a partial photo-bleach flash (0.8-ms duration, 1.8 × 104 scot cd/m2 s intensity, an equivalent 76% bleach23) subtending 4° and centered at 11° on the inferior vertical meridian (i.e., superior vertical meridian on the retina). This was also the location of the test target. Threshold measurement for a 2° diameter, 500-nm circular target began 15 seconds after the bleaching flash. Log thresholds were expressed as sensitivity (reciprocal of threshold) in decibel units as a function of time from bleach offset. The participant was instructed to maintain focus on the fixation light and to press a button when a flashing target first became visible within the bleached area. Threshold was estimated using a 3-down/1-up modified staircase estimate procedure and continued at 30-second intervals for 40 minutes or until the rod-intercept time (RIT) could be computed. The rate of RMDA is defined by RIT, the duration (in minutes) required for sensitivity to reach a criterion level of 5.0 × 10−3 scotopic cd/m2 (3.0 log units of attenuation of the stimulus); this sensitivity level is located in the latter half of the second component of rod recovery.13 The AdaptDx's software computes the RIT automatically, or indicates that it cannot be computed if thresholds do not reach 3.0 log units of attenuation within the 40-minute period (i.e., when the eye's sensitivity recovery is very slow). In this latter case, the software outputs that the RIT is “indeterminate.” Test–retest reproducibility of RIT has been previously established and is high (r = 0.95).6
At the 24-month visit, both the study and fellow eyes were examined for the presence of SDD based on evaluation of multimodal imaging at the 24-month visit. In addition to color fundus photographs as described above, we also obtained infrared reflectance (IR) and 488-nm excitation autofluorescence (AF) images, and spectral-domain optical coherence tomography (SD-OCT) volumes of the macula. SD-OCT, IR, and AF images were captured on the Spectralis HRA + OCT (Heidelberg Engineering, Heidelberg, Germany). B-scans of the macula volumes were horizontally oriented and centered over the fovea across an area of 20° × 15° (5.7 × 4.2 mm), as reported by the software. Automatic real-time averaging was set between 8 and 18. The SDD identification process has been described in detail elsewhere24,25 and is summarized here. The grader was masked to all other participant characteristics. To assess for the presence of SDD in SD-OCT, IR, and AF images, we used Heidelberg Eye Explorer (HEYEX version 1.6.4.0 with Spectralis Viewing Module 5.3.2.0; Heidelberg Engineering). To assess color fundus photographs we used OphthaVision (version 3.50; Escalon Medical Corp., Ardmore, PA). SD-OCT was graded for presence of SDD first, followed by the grading of the three en face imaging modalities. Our criteria for SDD at the eye level required identification on ≥ 1 en face modality and OCT or on ≥2 en face modalities in the absence of OCT findings (called strict criteria).24
Statistical Analysis
The Kruskal-Wallis test was used to compare RIT and change in RIT between groups. Spearman's correlation was used to test the association between change in RIT and VA. P values of ≤ 0.05 were considered statistically significant. A paired t-test was used to compare the change in RIT between baseline and 24 months.
Results
Table 1 presents demographic and baseline characteristics of the 30 persons enrolled in the study. Over 80% of participants were age ≥ 70 years old, with approximately equal numbers of men and women. All participants were white of non-Hispanic origin, and 63.3% (19 of 30) were current or former smokers. Half (15 of 30) the participants reported they had a family history of AMD. By design as described in Methods, at baseline all participants had intermediate AMD in the eye to be tested for dark adaptation. The fellow eye had intermediate AMD for nine participants and advanced AMD for 21 participants; of the fellow advanced AMD eyes, 16 had CNV, two had GA, and three had both CNV and GA. At baseline 20 eyes were pseudophakic and eight eyes were phakic and remained so throughout the 24-month follow-up. Two eyes were phakic at baseline but became pseudophakic during the 2-year follow-up. All five visits over 24-months were completed for 28 of 30 participants; one participant missed the 18-month visit because of illness, and one participant was fatigued at the 24-month visit so could not complete dark adaptation testing. Figure 1 shows examples of dark adaptation plots for eyes at baseline illustrating very fast to very slow recovery of sensitivity. At baseline, RIT averaged 20.3 minutes (standard deviation [SD] 14.3) and ranged widely from 6.8 to 54.2 minutes. At 24-months RIT averaged 30.8 minutes (SD 29.9) and ranged widely from 8.8 to 124.4 minutes.
Table 1.
Demographic and Baseline Characteristics of the Sample (N = 30)
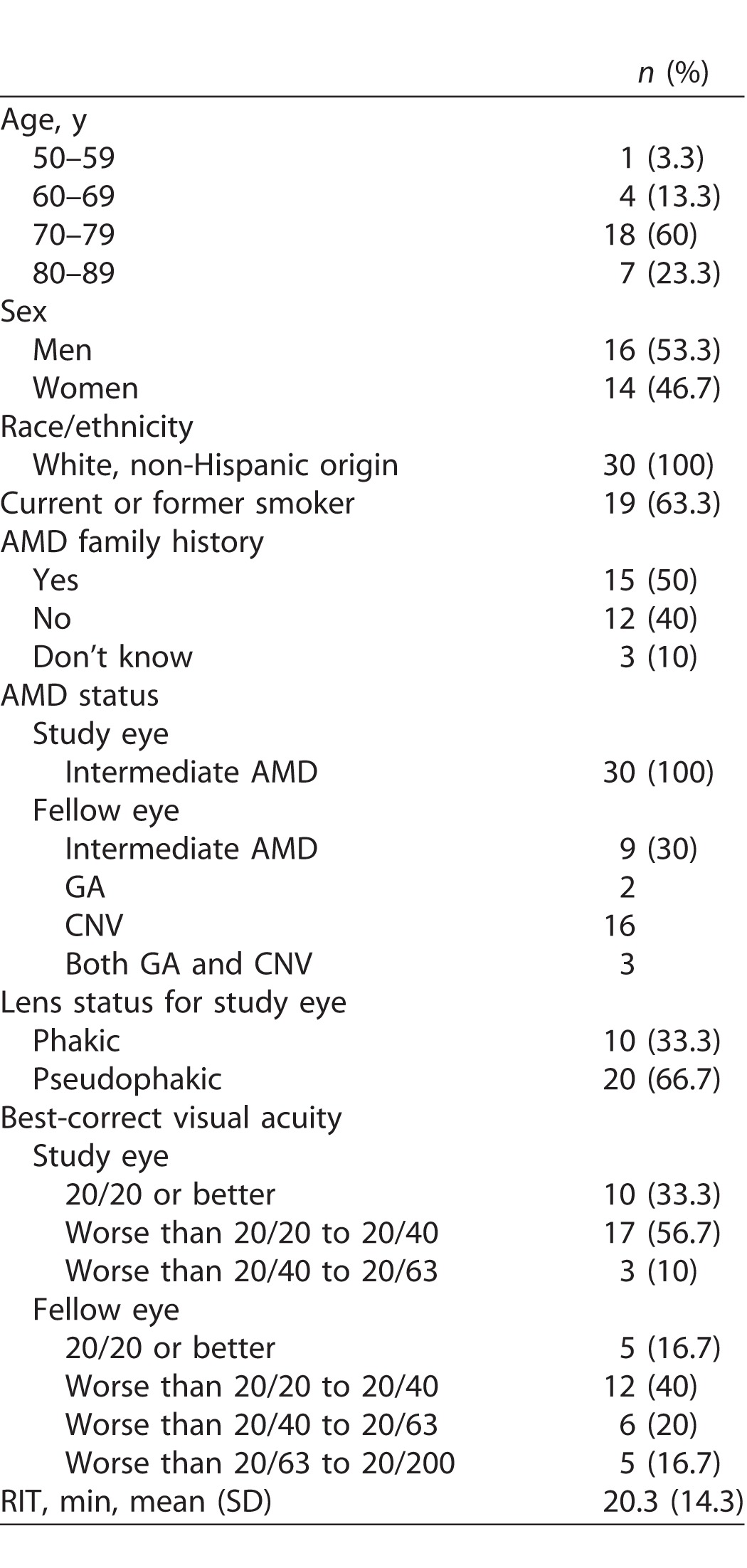
Figure 1.
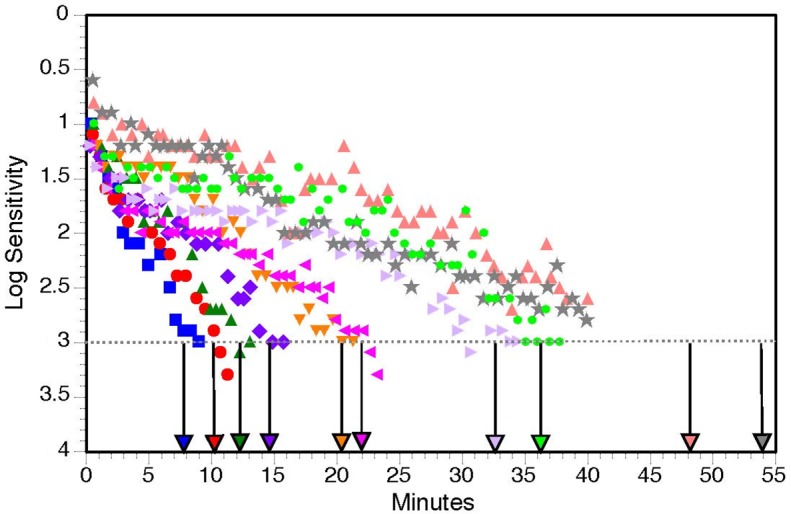
Examples of dark adaptation plots for participants at baseline illustrating very fast recovery to very slow recovery of sensitivity. Color-coded vertical arrows above the abscissa indicate RIT computed by the AdaptDx for that participant.
Mean change in RIT over 24 months across all eyes was 10.5 minutes (SD 19.4), p < 0.0001, signifying that RMDA slowed on average. We also examined RIT change separately for the 23 eyes where the AdaptDx software automatically computed the RIT at all visits; mean change in RIT from baseline to 24 months (RIT24months − RITbaseline) was 5.1 minutes (SD 5.5, minimum −1.4, maximum 18.3), p < 0.0001. For the remaining seven eyes the AdaptDx software indicated that RIT was “indeterminate” for one or more visits (for these visits we estimated RIT using nonlinear regression as described previously26). Mean change in RIT for these eyes from baseline to 24 months (RIT24months − RITbaseline) was 28.4 minutes (SD 35.7, minimum −9.1, maximum 80.8), P equal to 0.1563.
Table 2 shows for each individual tested eye, visual acuity (logMAR) at baseline, 12 months, and 24 months and the extent to which they changed over time. Worsening of acuity over 24 months was not associated with an increased RIT over 24 months (r = 0.036, P = 0.849). Mean acuity change (worsening) in acuity over 24 months across all participants was 0.08, approximately one line on the Early Treatment Diabetic Retinopahty Study (ETDRS) chart (SD 0.12). Only 7 of 30 eyes (23.3%) worsened by two to three lines. The vast majority of eyes (23 of 30 eyes, 76.7%) worsened only by one line (0.1 logMAR), stayed the same, or improved over the 24 months. With respect to change in visual acuity over 12 months, 28 of 30 eyes (93.3%) worsened by 0.1 logMAR (one line), stayed the same, or improved. Two of 30 eyes (6.7%) worsened by 0.3 logMAR (3 lines).
Table 2.
For the Test Eye in Individual Participants, Changes in Visual Acuity and Rod-Mediated Dark Adaptation over 12 and 24 Months
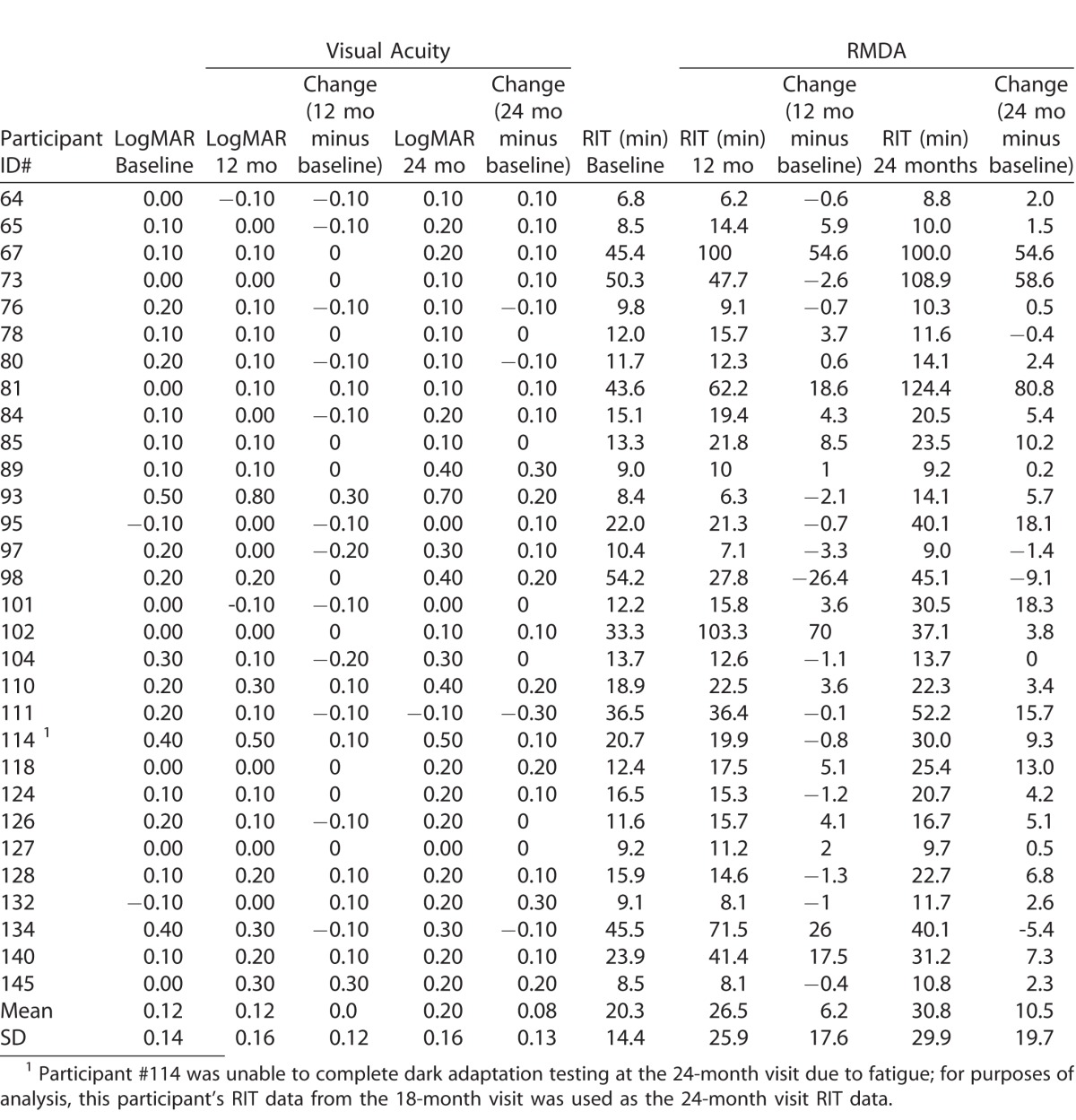
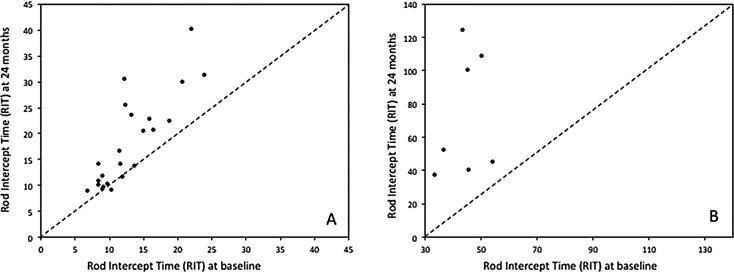
Figure 2 is a scatterplot showing the relationship between RIT at baseline and the 24-month follow-up. Panel A displays eyes where the AdaptDx automatically computed the RIT at all visits, and Panel B displays eyes where the device indicated that RIT was indeterminate (we estimated RIT using nonlinear regression as described previously26). For the vast majority of eyes, RIT was greater at the 24-month follow-up compared with baseline, as revealed by most points being above the diagnonal. As indicated in Table 2, 22 of 30 eyes (73.3%) had an increase in RIT > 1 minute, 17 of 30 eyes (56.7%) had an increase in RIT > 3 minutes, and 11 of 30 eyes (36.7%) had an increase in RIT > 6 minutes. Eight of 30 eyes (26.7%) had a RIT that was unchanged (defined as a 0- or ≤1-minute increase) or decreased. Three participants displayed extreme slowing in rod-mediated dark adaptation over 24 months (increase in RIT > 50 minutes), which coincided with their having very high RIT at baseline (>40 minutes). At the 12-month visit, 13 of 30 eyes had a RIT > 3 minutes. Seventeen of 30 eyes either had no change in RIT or RIT increased by < 3 minutes or RIT decreased.
Figure 2.
Scatterplots showing the relationship between RIT at baseline and RIT at 24 months. Panel A shows data for eyes for which the AdaptDx software computed RIT automatically. Panel B shows data for eyes where recovery of sensitivity was so slow that the AdaptDx did not compute RIT. For these eyes we used nonlinear regression to estimate RIT, as described previously.26 Both panels show that for the vast majority of eyes, RIT at 24 months was greater than at baseline, signifying that the speed of RMDA had slowed over 24 months.
By 24 months, 9 of 30 (30%) tested eyes converted from intermediate AMD to advanced AMD, with two eyes developing both CNV and GA, four eyes GA only, and four eyes CNV only. Eyes with advanced AMD at 24 months did not have significantly longer RIT at baseline as compared with those with intermediate AMD at 24 months (mean [M] 24.8, SD 16.3 versus M 18.4, SD 13.4, P = 0.1839). At 24 months 25 of 30 test eyes had SDD, with the balance showing no signs of SDD. Eyes with SDD at 24 months had on average higher RIT at baseline than did eyes without SDD at 24 months; however, this finding did not reach statistical significance (eyes with SDD, mean RIT = 22.3 minutes, SD 15.0; eyes with no SDD, mean RIT 10.3 minutes, SD 2.3, P = 0.071).
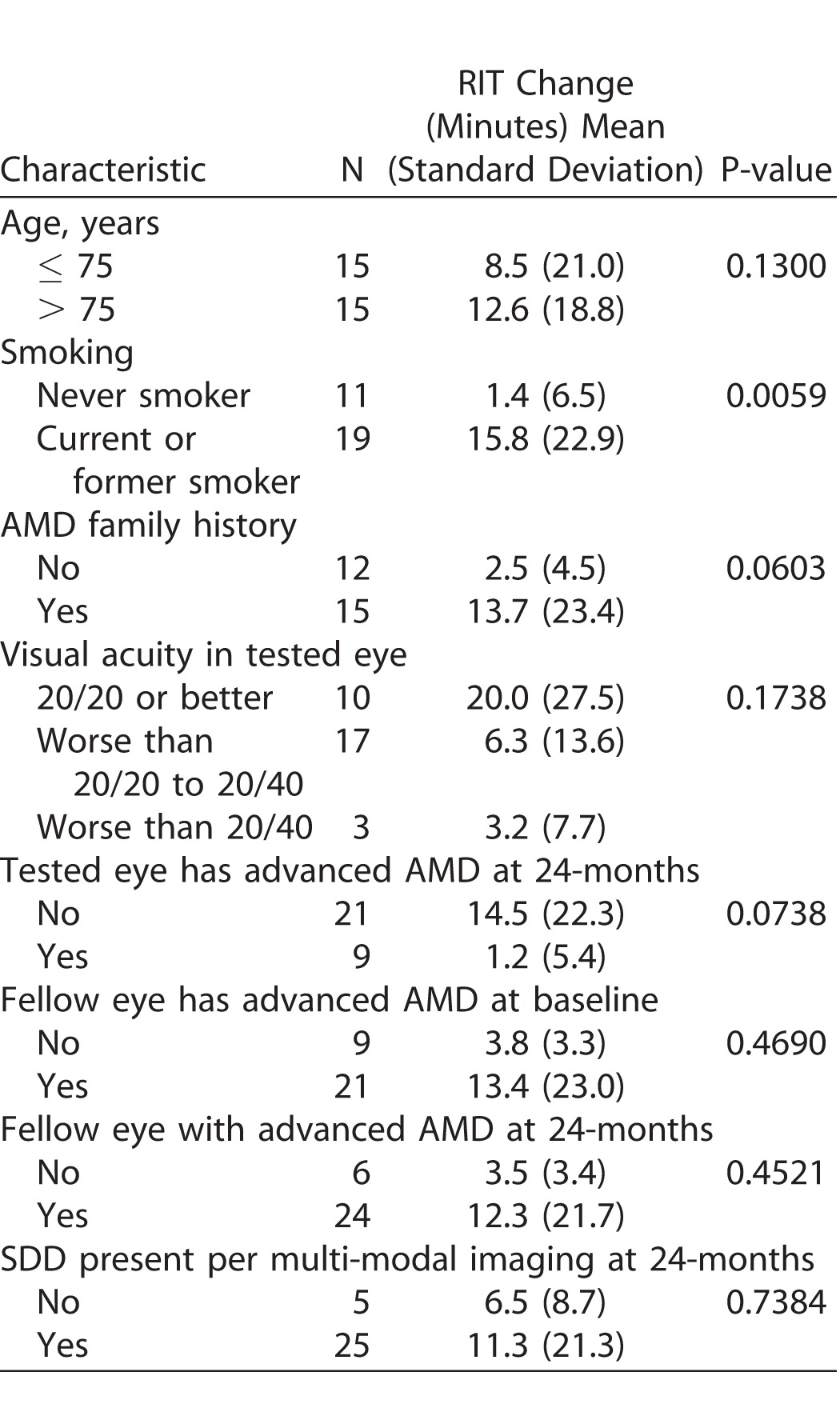
Table 3 examines characteristics associated with change in RIT (slowing in the rate of RMDA) from baseline to 24 months. Greater slowing in dark adaptation over 24 months (larger RIT increase) was associated with being a current or former smoker (P = 0.0059) and having a family history of AMD (P = 0.060), although the latter was of borderline statistical significance. Advanced age, visual acuity at baseline, advanced AMD status at baseline or follow-up for the test or fellow eyes, and SDD presence in the test eye at 24 months were not associated with change in RIT.
Table 3.
Characteristics Associated with Change in RIT over 24 Months
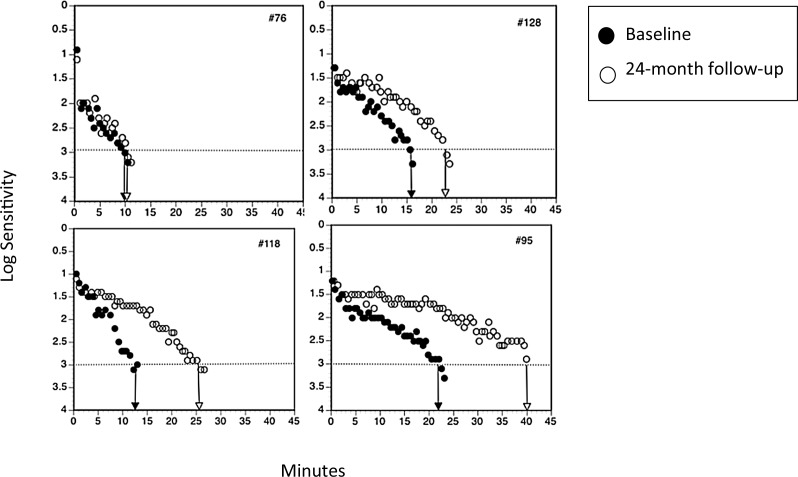
To illustrate different patterns of change in RIT over 24 months, Figure 3 depicts examples of dark adaptation plots at baseline and 24 months for four participants. Panel A shows a participant that had fast dark adaptation at baseline, which was unchanged 24 months later. Panels B, C, and D show examples of slowing in dark adaptation (increased RIT) by the 24-month visit.
Figure 3.
Representative examples of dark adaptation plots for four participants at baseline and 24 months. Panel A shows a participant with fast dark adaptation at baseline, which was unchanged 24 months later. Panels B, C, and D show examples of worsening dark adaptation delays (increased RIT) by the 24-month visit.
Discussion
In considering possible endpoints for clinical trial design in intermediate AMD, it is important to clarify what constitutes a clinically (practically) significant change in a measure. The US Food and Drug Administration currently views a clinically significant change in visual acuity as a three line difference in visual acuity on the ETDRS chart (a 0.3 logMAR change); a smaller difference such as a two line difference might be acceptable if the degree of risk is correspondingly smaller.27 By this standard, it is clear from our study that visual acuity in intermediate AMD does not meet clinical significance as a functional endpoint in a 12- or 24-month study design. Only 2 of 30 eyes exhibited a three line decline in visual acuity over 24-months, with the vast majority of eyes showing only a one line or no decline. In contrast, RMDA slowed by at least 1 minute (i.e., RIT increased) in approximately three-quarters of eyes with intermediate AMD over a 2-year period, and slowed by 3 or more minutes in 56.7% of eyes. There was wide variability in the extent to which RMDA slowed across eyes, ranging from no slowing to dramatic slowing over 2 years. Yet this variability is not surprising given the large body of work demonstrating that AMD has a broad ranging visual functional phenotype even within the same disease severity levels.28 It is also noteworthy that eyes with substantial slowing at baseline tended to show the most extreme delays at 24 months.
A question that arises is how should a clinically significant change in RIT be defined. All approaches to answering this question involve some arbitrariness. First, it is important to acknowledge that statistical significance is not commensurate with clinical significance. One approach to establishing clinical significance is to examine how RIT relates to the performance of an everyday visual task under low luminance conditions, such as night driving or searching for an object under poor illumination. However, such data are not yet available. Even if one could establish a strong association between, for example, the number of lane deviations during night driving and RIT, one is still left with the challenge of identifying how many lane deviations (and its associated RIT) constitute a problem of clinical or practical significance. Clinical significance could also be revealed by understanding the relationship between RIT in eyes with intermediate AMD and the risk of having advanced AMD at some future time point. If increasingly large RITs are associated with increasingly large risks for incident advanced AMD, this RIT/risk table could guide what is viewed as clinically significant. Unfortunately, currently there are no prospective studies on RMDA and intermediate AMD of sufficient sample size to address this issue. Another approach would be to establish a relationship between structural characteristics of intermediate AMD known to be central to its pathological course and proposed functional endpoints such as RMDA, a rapidly growing area of interest.6,11,29,30
Adding credibility to RIT as a promising functional endpoint for intermediate AMD is its relationship to established risk factors for AMD. Smoking incurs a 3- to 5-fold increased risk for AMD.31–35 We found that current or previous smokers experienced larger RMDA delays (greater RIT increase) over 2 years as compared with nonsmokers. In addition, those who reported a family history of AMD tended to exhibit greater RIT increases over 2 years, although this finding had borderline significance and awaits confirmation in larger sample studies. Further work is needed to identify patient characteristics that are high risk for significant reduction in RIT over time.
This exploratory study's primary purpose was to evaluate the natural history of RMDA over 24 months, yet it also provides the opportunity to examine the extent of change over 12 months. Shorter follow-up periods in proof-of-concept studies and large, confirmatory clinical trials are often preferred because they more efficiently (in terms of time and financial resources) answer research questions of interest. Changes in RIT over 12 months were modest to nonexistent in eyes with intermediate AMD, consistent with an earlier report.36 Thus, our data do not support the promise of RMDA as an endpoint for study designs limited to 12 months.
Strengths of this study are as follows. To our knowledge this is the first study to examine the natural history of RMDA over 2 years in well-characterized eyes with intermediate AMD. RMDA was measured with a technique having high test–retest reproducibility in patients with intermediate AMD,6 although future work should examine how to further enhance reproducibility. We have linked larger increases in RIT to a major risk factor for AMD, namely smoking. Limitations must also be acknowledged. The sample size was relatively small. While a sample size of 30 participants allowed us to obtain information on the diversity of RIT changes over time in intermediate AMD, it was most likely insufficient for understanding factors associated with greater RIT change over time in this population. The presence of SDD is known to increase the risk for exacerbated visual dysfunction and advanced AMD6,11,37–39; however, multimodal imaging used to identify SDD was only performed at the 24-month visit, but not at baseline. A limitation of the device used to measure dark adaptation in this study is that it currently does not estimate RIT for eyes with extremely slow dark adaptation (Reviewed in Ref. 6), which was not uncommon; however, in these cases we used nonlinear regression to estimate RIT per our earlier work.26 All participants were white of non-Hispanic origin, and thus the generalizability of our findings to other races/ethnicities remains to be determined.
In conclusion, this study suggests that RMDA slows in intermediate AMD over 24 months in most eyes. For approximately three-quarters of eyes the increase in RIT was greater than 1 minute, for half it was greater than 3 minutes, and for one-quarter of eyes it was greater than 6 minutes. There was wide variability in RIT at both baseline and in the extent to which it increased over 24 months. Smoking, a major risk factor for AMD, was associated with a greater increase in RIT over 2 years. Increase in RIT over 12 months for over half of eyes was modest (≤3 minutes) or nonexistent. Our results support RMDA assessed by RIT as having promise as a functional endpoint in proof-of-concept studies and confirmatory clinical trials on intermediate AMD with 2-year designs. Future work should be targeted at clarifying the factors that impact RIT increases over time in intermediate AMD and at identifying a definition of clinically significant changes in RIT that the scientific and regulatory communities could widely agree upon.
Acknowledgments
Supported by grants from Genentech, the National Institutes of Health (R01-AG04212), the EyeSight Foundation of Alabama, the Dorsett Davis Discovery Fund, the Alfreda J. Schueler Trust, and Research to Prevent Blindness, Inc.
Disclosure: C. Owsley, patent holder on the apparatus used to measure dark adaptation in this study (P); M. Clark, None; G. McGwin Jr., None
References
- 1. Barlow HB. Dark and light adaptation: psychophysics. : Jameson D,, Hurvich LM. (eds), Handbook of Sensory Physiology: Visual Psychophysics. New York, New York: Springer-Verlag; 1972: 1–55. [Google Scholar]
- 2. Owsley C,, Jackson GR,, White MF,, Feist R,, Edwards D. Delays in rod-mediated dark adaptation in early age-related maculopathy. Ophthalmology. 2001; 108: 1196–1202. [DOI] [PubMed] [Google Scholar]
- 3. Steinmetz RL,, Haimovici R,, Jubb C,, Fitzke FW,, Bird AC. Symptomatic abnormalities of dark adaptation in patients with age-related Bruch's membrane change. Br J Ophthalmol. 1993; 77: 549–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dimitrov PN,, Guymer RH,, Zele AJ,, Anderson AJ,, Vingrys AJ. Measuring rod and cone dynamics in age-related maculopathy. Invest Ophthalmol Vis Sci. 2008; 49: 55–65. [DOI] [PubMed] [Google Scholar]
- 5. Owsley C,, McGwin G,, Jackson G,, Kallies K,, Clark M. Cone- and rod-mediated dark adaptation impairment in age-related maculopathy. Ophthalmology. 2007; 114: 1728–1735. [DOI] [PubMed] [Google Scholar]
- 6. Flamendorf J,, Agrón E,, Wong WT,, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015; 122: 2053–2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mimoun G,, Soubrane G,, Coscas G. Macular drusen [in French]. J Fr Ophtalmol. 1990; 13: 511–530. [PubMed] [Google Scholar]
- 8. Arnold JJ,, Sarks SH,, C KM,, Sarks JP, Reticular pseudodrusen. A risk factor in age-related maculopathy. Retina. 1995; 15: 183–191. [PubMed] [Google Scholar]
- 9. Rudolf M,, Malek G,, Messinger JD,, Wang L,, Clark ME,, Curcio CA. Sub-retinal drusenoid deposits in human retina: organization and composition. Exp Eye Res. 2008; 87: 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zweifel SA,, Spaide RF,, Curcio CA,, Malek G,, Imamura Y. Reticular pseudodrusen are subretinal drusenoid deposits. Ophthalmology. 2010; 117: 303–312. [DOI] [PubMed] [Google Scholar]
- 11. Neely D,, Zarubina AV,, Clark ME,, et al. Association between visual function and subretinal drusenoid deposits in normal and early age-related macular degeneration eyes. Retina. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owsley C,, McGwin G, Jr,, Clark ME,, et al. Delayed rod-mediated dark adaptation is a functional biomarker for incident early age-related macular degeneration. Ophthalmology. 2016; 123: 344–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lamb TD,, Pugh ENJ. Dark adaptation and the retinoid cycle of vision. Prog Retin Eye Res. 2004; 23: 307–380. [DOI] [PubMed] [Google Scholar]
- 14. Lamb TD,, Pugh EN. Phototransduction, dark adaptation, and rhodopsin regeneration. Invest Ophthalmol Vis Sci. 2006; 47: 5138–5152. [DOI] [PubMed] [Google Scholar]
- 15. Wakatsuki Y,, Shinojima A,, Kawamura A,, Yuzawa M. Correlation of aging and segmental choroidal thickness measurement using swept source optical coherence tomography in health eyes. PLoS One. 2015; 10: e0144156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sigler EJ,, Randolph JC. Comparison of macular choroidal thickness among patients over age 65 with early atrophic age-related macular degeneration and normals. Invest Ophthalmol Vis Sci. 2013; 54: 6307–6313. [DOI] [PubMed] [Google Scholar]
- 17. Garg A,, Oll M,, Yzer S,, et al. Reticular pseudodrusen in early age-related macular degeneration are associated with choroidal thinning. Invest Ophthalmol Vis Sci. 2013; 54: 7075–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ach T,, Huisingh C,, McGwin G, Jr,, et al. Quantitative autofluorescence and cell density maps of the human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2014; 55: 4832–4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ach T,, Tolstik E,, Messinger JD,, Zarubina AV,, Heintzmann R,, Curcio CA. Lipofuscin redistribution and loss accompanied by cytoskeletal stress in retinal pigment epithelium of eyes with age-related macular degeneration. Invest Ophthalmol Vis Sci. 2015; 56: 3242–3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zanzottera EC,, Ach T,, Huisingh C,, Messinger JD,, Spaide RF,, Curcio CA. Visualizing retinal pigment epithelium phenotypes in the transition to geographic atrophy in age-related macular degeneration. Retina. 2016; 36 suppl 1: S12–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Owsley C,, McGwin G,, Jackson GR,, et al. Effect of short-term, high-dose retinol on dark adaptation in aging and early age-related maculopathy. Invest Ophthalmol Vis Sci. 2006; 47: 1310–1318. [DOI] [PubMed] [Google Scholar]
- 22. Beck RW,, Moke PS,, Turpin AH,, et al. A computerized method of visual acuity testing: adaptation of the early treatment of diabetic retinopathy study testing protocol. Am J Ophthalmol. 2003; 135: 194–205. [DOI] [PubMed] [Google Scholar]
- 23. Pugh EN. Rushton's paradox: rod dark adaptation after flash photolysis. J Physiol (Lond.). 1975; 248: 413–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zarubina AV,, Neely DC,, Clark ME,, et al. Prevalence of subretinal drusenoid deposits in older persons with and without age-related macular degeneration, by multimodal imaging. Ophthalmology. 2016; 123: 1090–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huisingh C,, McGwin G, Jr,, Neely D,, et al. The association between subretinal drusenoid deposits in older adults in normal macular health and incident age-related macular degeneration. Invest Ophthalmol Vis Sci. 2016; 57: 739–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McGwin G, Jr,, Jackson GR,, Owsley C. Using nonlinear regression to estimate parameters of dark adaptation. Behav Res Methods Instrum Comput. 1999; 31: 712–717. [DOI] [PubMed] [Google Scholar]
- 27. Csaky KG,, Richman EA,, Ferris FL., III. Report from the NEI/FDA Ophthalmic Clinical Trial Design and Endpoints Symposium. Invest Ophthalmol Vis Sci. 2008; 49: 479–489. [DOI] [PubMed] [Google Scholar]
- 28. Hogg RE,, Chakravarthy U. Visual function and dysfunction in early and late age-related maculopathy. Prog Ret Eye Res. 2006; 249–276. [DOI] [PubMed] [Google Scholar]
- 29. Sevilla MB,, McGwin G, Jr,, Lad EM,, et al. Relating retinal morphology and function in aging and early to intermediate age-related macular degeneration subjects. Am J Ophthalmol. 2016; 165: 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laíns I,, Miller JB,, Park DH,, et al. Structural changes associated with delayed dark adaptation in age-related macular degeneration. Ophthalmology. 2017; e-pub ahead of print. doi: 10.1016/j.ophtha.2017.03.061. [DOI] [PubMed] [Google Scholar]
- 31. Smith W,, Assink J,, Klein R,, et al. Risk factors for age-related macular degeneration: Pooled findings from three continents. Ophthalmology. 2001; 108: 697–704. [DOI] [PubMed] [Google Scholar]
- 32. Eye Disease Case Control Study Group. Risk factors for neovascular age-related macular degeneration. Arch Ophthamol. 1992; 110: 1701–1708. [DOI] [PubMed] [Google Scholar]
- 33. Delcourt C,, Diaz JL,, Ponton-Sanchez A,, Papoz L; for the POLA Study Group. Smoking and age-related macular degeneration: The POLA study. Arch Ophthalmol. 1998; 116: 1031–1035. [DOI] [PubMed] [Google Scholar]
- 34. Smith W,, Mitchell P,, Leeder SR. Smoking and age-related maculopathy: the Blue Mountains Eye Study. Arch Ophthalmol. 1996; 114: 1518–1523. [DOI] [PubMed] [Google Scholar]
- 35. Klein R,, Klein BE,, Moss SE. Relation of smoking to the incidence of age-related maculopathy. The Beaver Dam Eye Study. Am J Epidemiol. 1998; 147: 103–110. [DOI] [PubMed] [Google Scholar]
- 36. Jackson GR,, Clark ME,, Scott IU,, Walter LE,, Quillen DA,, Brigell MG. Twelve-month natural history of dark adaptation in patients with AMD. Optom Vis Sci. 2014; 91: 925–931. [DOI] [PubMed] [Google Scholar]
- 37. Steinberg JS,, Fitzke FW,, Fimmers R,, Fleckenstein M,, Holz FG,, Schmitz-Valckenberg S. Scotopic and photopic microperimetry in patients with reticular drusen and age-related macular degeneration. JAMA Ophthalmol. 2015; 133: 690–697. [DOI] [PubMed] [Google Scholar]
- 38. Ooto S,, Suzuki M,, Vongkulsiri S,, Sato T,, Spaide RF. Multimodal visual function testing in eyes with nonexudatuve age-related macular degneration. Retina. 2015; 35: 1726–1734. [DOI] [PubMed] [Google Scholar]
- 39. Zhou Q,, Daniel E,, Maguire MG,, et al. Pseudodrusen and incidence of late age-related macular degeneration in fellow eyes in the CAPT Trials. Ophthalmology. 2016; 123: 1530–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]