Summary
Natural killer (NK) cell‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) is of considerable interest in viral infection. However, little is known about NK‐ADCC responses in chronic hepatitis C virus (HCV) infection. In this study, impaired non‐specific antibody‐dependent CD56+ NK cell responses were observed in chronic HCV infection, as shown by decreased degranulation (extracellular CD107a expression) and interferon (IFN)‐γ production in response to antibody‐bound P815 cells. A peptide pool composed of epitopes recognized by anti‐HCV‐E1/E2 antibodies could induce pronounced HCV‐specific antibody‐dependent NK cell responses in sera from approximately half the chronic HCV carriers. Additionally, HCV‐specific epitopes with the capacity to induce robust NK‐ADCC activity were identified. Five linear NK‐ADCC epitopes (aa211‐aa217, aa384‐aa391, aa464‐aa475, aa544‐aa551 and aa648‐aa659 of the HCV envelope) were identified and do not overlap with putative linear neutralizing epitopes. This study revealed the dysfunctional characteristics of antibody‐dependent CD56+ NK cell responses in chronic HCV carriers. The key non‐neutralizing NK‐ADCC epitopes identified in this study may act as new targets for immunological intervention.
Keywords: antibody‐dependent cellular cytotoxicity, epitope, hepatitis C virus, natural killer cell, non‐neutralizing antibody
Introduction
Chronic hepatitis C virus (HCV) infection is characterized by the persistence of detectable circulating HCV RNA and anti‐HCV antibodies. However, these antibodies fail to control HCV infection, as the production of neutralizing antibodies usually lags behind the evolution of HCV E1/E2 quasispecies within infected patients 1. Some studies indicated that the extent of anti‐HCV responses mediated by NK cells was associated with the outcome of HCV acute infection 2, 3. In addition to natural cytotoxicity, NK cell activity triggered by an imbalance in signals received by activating and inhibitory receptors expressed on NK cells, NK cell‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) may also be involved in immune protection in HCV infection. NK‐ADCC is elicited by activation of FcγRIII (CD16) on NK cells by the Fc portion of an immunoglobulin (Ig)G antibody bound to an antigen. After engagement, the activated NK cells promptly degranulate and release perforin and granzymes, which results in the lysis of target cells. Activated NK cells also release immune modulatory cytokines such as interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α 4. NK‐ADCC was shown to be associated with disease progression in human immunodeficiency virus (HIV)‐infected patients and simian immunodeficiency virus (SIV)‐infected macaques, and was reported as a major contributor in preventing HIV infection in the RV144 clinical trial 5, 6. The induction of NK‐ADCC responses has been proposed as an alternative strategy for HIV and HCV vaccine development 7. Nattermann et al. demonstrated that anti‐HCV E2 antibodies could mediate ADCC in approximately 50% of HCV‐infected subjects, whether patients with acute, self‐limited or chronic HCV infection 8. However, it is still unclear whether or not the capacity of antibody‐dependent NK cell responses is impaired by chronic HCV infection. Also, we do not know whether the antibodies are capable of mediating NK‐ADCC overlap or are completely distinct from the neutralizing antibodies produced during HCV infection.
In this study, we demonstrated that ex‐vivo non‐specific antibody‐dependent CD56+ NK cell responses induced by antibody‐coated P815 cells were functionally impaired in chronic HCV infection. In addition, linear epitopes located in the HCV‐E1/E2 protein that could mediate robust NK‐ADCC were identified and compared with putative neutralizing epitopes.
Materials and methods
Study subjects
A total of 31 chronic HCV carriers and 49 healthy controls were recruited from a village in central China 9. Chronic HCV infection was identified by anti‐HCV responses and detection of HCV RNA. The clinical and laboratory characteristics of the study subjects are summarized in Table 1. Plasma HCV antibodies were detected using the Architect anti‐HCV assay (Abbott GmbH & Co KG, Wiesbaden, Germany) and confirmed by the HCV‐recombinant immunoblot assay (RIBA) assay (Wantai Biological Pharmacy, Beijing, China). HCV RNA was detected using the Abbott real‐time HCV amplification kit (Abbott Molecular, Des Plaines, IL, USA), according to the manufacturer's instructions. None of the hepatitis C patients received any form of anti‐HCV therapy, and all participants were negative for hepatitis A virus (HAV), hepatitis B virus (HBV), HIV and tuberculosis (TB). Plasma and peripheral blood mononuclear cells (PBMCs) were separated from ethylendiamine tetraacetic acid (EDTA) anti‐coagulated whole blood specimens and stored at −80°C and −180°C, respectively. The study protocol was approved by the institutional review authorities of Peking University Health Science Center. Informed consent was obtained from each patient enrolled in the study.
Table 1.
Characteristics of 31 chronic hepatitis C virus (HCV) carriers and 49 healthy controls
| Characteristics | Chronic HCV | Healthy |
|---|---|---|
| Number | 31 | 49 |
| Female (%)* | 18 (58·1) | 30 (61·2) |
| Age (years) † | 48 (62 − 33) | 46 (58 − 34) |
| BMI † | 23·2 (25·2 − 20·4) | 22·8 (25·0 − 20·6) |
| Clinical data | ||
| anti‐HCV S/CO value † | 14·12 (10·3 − 16·2) | Negative |
| HCV RNA (log10 IU/ml) † | 6·43 (6·60 − 5·91) | Negative |
| HCV genotype | ||
| 1b | 21 | n.a. |
| 2a | 8 | n.a. |
| others | 0 | n.a. |
| ALT (IU/l) † | 48 (128 − 17) | 28 (34 − 15) |
| AST (IU/l) † | 44 (109 − 18) | 26 (33 − 14) |
| Total protein (g/l) † | 78·2 (85·2 − 70·6) | 76·8 (82·4 − 72·4) |
| Albumin (g/l) † | 44·0 (51·3 − 36·5) | 45·6 (53·0 − 38·7) |
| Total bilirubin (μmol/l) † | 14·1 (17·2 − 11·2) | 11·8 (15·4 − 7·5) |
| Direct bilirubin (μmol/l) † | 4·3 (5·9 − 3·0) | 4.0 (5.9 − 2.7) |
*Number of cases (%). †Mean (range). BMI = body mass index; n.a. = not available; S/CO = signal/cut‐off; ALT = alanine aminotransferase; AST = aspartate aminotransferase.
Evaluation of the non‐specific antibody‐dependent NK cell responses by intracellular cytokine staining
A novel non‐specific ADCC assay based on intracellular cytokine staining (ICS) was used to detect ADCC responses by circulating CD56+ NK cells 10. Briefly, 1 × 105 P815 cells (a mouse leukaemic cell line) were treated with medium or with a 1 : 100 dilution of polyclonal rabbit anti‐mouse lymphocyte serum (Accurate Chemical and Scientific Corp., Westbury, NY, USA) for 1 h at 37°C/5% CO2 in a volume of 200 μl of R10 medium (RPMI‐1640 medium supplemented with 10% fetal bovine serum (FBS), 2 mmol L‐glutamine, 100 U/ml penicillin and 100 mg/ml streptomycin), and then washed twice with ice‐cold R10 medium; 1 × 106 peripheral blood mononuclear cells (PBMCs) were stimulated with R10 medium alone, uncoated P815 cells, antibody ‐coated P815 cells or phorbol myristate acetate (PMA) plus ionomycin (positive control) (Sigma‐Aldrich, St Louis, MO, USA). Cells were cultured with CD107a‐phycoerythrin‐cyanin 5 (PE‐Cy5) (clone H4A3; BD Biosciences, San Jose, CA, USA), Golgi‐Stop (BD Biosciences) and brefeldin A (Sigma‐Aldrich) for 6 h at 37°C/5% CO2. After culture, PBMCs were stained with CD3‐eFluor 450 (clone 17A2; eBioscience; San Diego, CA, USA), CD16‐allophycocyanin (APC)‐Cy7 (clone 3G8; BD Biosciences) and CD56‐PE‐Cy7 (clone B159; BD Biosciences). Then, cells were permeabilized using 0·25% saponin (Thermo Fisher Scientific; Waltham, MA, USA), and ICS was carried out with IFN‐γ fluorescein isothiocyanate (FITC) (clone 25723.11; BD Biosciences) and TNF‐α‐APC (clone 6401.1111; BD Biosciences). After staining, cells were washed in phosphate‐buffered saline (PBS) and fixed with 2% paraformaldehyde (PFA). All data were acquired on a BD FACS Fortessa (BD Biosciences) and analysed using FlowJo software (Treestar, Ashland, OR, USA).
NK cell purification
Untouched NK cells were enriched from PBMCs using an NK cell isolation kit (Miltenyi Biotec, Auburn, CA, USA). In brief, NK cells were negatively isolated by depleting non‐NK cells (i.e. T cells, B cells, stem cells, dendritic cells, monocytes, granulocytes and erythroid cells) using a cocktail of biotin‐conjugated antibodies, followed by streptavidin‐coated microbeads. Isolation of highly pure NK cells was achieved by depletion of magnetically labelled cells. The purity of NK cells obtained in this fashion was consistently greater than 95%. Isolated 1 × 105 NK cells were co‐cultured with uncoated or 1 × 104 antibody‐coated P815 cells at 37°C/5% CO2 for 24 h, and cell‐free supernatants (NK‐ADCC supernatants) were collected for enzyme‐linked immunosorbent assay (ELISA), as described below.
ELISA
The levels of IFN‐γ, TNF‐α, transforming growth factor (TGF)‐β and interleukin (IL)‐10 in NK‐ADCC supernatants were analysed using Ready‐SET‐Go ELISA kits according to the manufacturer's instructions (eBioscience). The sensitivities of the ELISAs were 4 pg/ml for IFN‐γ, 4 pg/ml for TNF‐α, 8 pg/ml for TGF‐β and 2 pg/ml for IL‐10. Granzyme B was detected using a Platinum ELISA kit (eBioscience) with a sensitivity of 0·2 pg/ml.
Peptide recognition of HCV‐E1/E2 antibodies
All HCV‐E1/E2 antibody‐specific peptides employed in this study were designed according to the sequences of published epitopes located in the HCV E1/E2 region, with slight modifications based on the sequence characteristics of HCV genotypes 1b and 2a, which are the predominant circulating genotypes in mainland China. Peptide sequences are presented in Table 2. In particular, epitopes 1 11, 5 12, 13, 7 14, 8 15, 16, 10 17 and 12 18, 19 are overlapped with six putative linear neutralizing epitopes, and the other 11 epitopes (2, 3, 4, 6, 9, 11, 13, 14, 15, 16 and 17) 20, 21, 22, 23, 24, 25 are targets recognized by well‐characterized anti‐HCV E1/E2 monoclonal antibodies. All solid‐phase peptides were synthesized to 95% purity by Bio‐Scientific Co. (Shanghai, China). Each peptide was dissolved to a concentration of 1 mg/ml in RPMI‐1640 containing 10% dimethyl sulphoxide (DMSO), and stock solutions were diluted further in RPMI‐1640 to a working concentration of 1 μg/ml.
Table 2.
Peptide characteristics
| Peptide no. | Sequence | Location in H77* | Protein |
|---|---|---|---|
| 1 | YEVRNVSGIYHVTNDCSNS | 192 − 210 | E1 |
| 2 | SSGLYHVTNDC | 197 − 207 | E1 |
| 3 | SIVYEAA | 211 − 217 | E1 |
| 4 | RHWTTQGCNC | 297 − 306 | E1 |
| 5 | SGHRMAWDMMMNWSPTT | 314 − 330 | E1 |
| 6 | ETHVTGGS | 384 − 391 | E2 |
| 7 | TAGLVGLLTPGA | 396 − 407 | E2 |
| 8 | SQKIQLVNTNGSWHIN | 408 − 423 | E2 |
| 9 | GSWHINRTALNCND | 418 − 431 | E2 |
| 10 | SLNTGWLAGLFYQHKF | 432 − 447 | E2 |
| 11 | DFDQGWGPISYA | 464 − 475 | E2 |
| 12 | IVPAKSVCGPVYCFTPSPVV | 496 − 515 | E2 |
| 13 | SGAPTYSWGA | 522 − 531 | E2 |
| 14 | PPLGNWFG | 544 − 551 | E2 |
| 15 | YRLWHYPCT | 613 − 621 | E2 |
| 16 | LDAACNWTRGERCD | 640 − 653 | E2 |
| 17 | RGERCDLEDRDR | 648 − 659 | E2 |
A total of 17 linear peptides were reported to be recognized by defined anti‐hepatitis C virus (HCV) E1/E2 monoclonal antibodies. Underlined epitopes 1 11, 5 12, 13, 7 14, 8 15, 16, 10 17 and 12 18, 19 are overlapped with six putative linear neutralizing epitopes, and the other 11 epitopes (2, 3, 4, 6, 9, 11, 13, 14, 15, 16 and 17) are targets recognized by well‐characterized anti‐HCV E1/E2 monoclonal antibodies 20, 21, 22, 23, 24, 25. *The first and final positions are numbered with reference to the consensus sequence of the polyprotein of HCV strain H77 (Genbank accession number AB009606).
Identification of HCV E1/E2‐specific NK‐ADCC in chronic HCV‐infected subjects
In order to confirm whether HCV membrane protein could induce an NK‐ADCC response, huh7·5‐based HCV replicon cells (HCV‐Con I‐Rep), which were transfected stably with a plasmid pNNeo/3‐5 BRG (kindly donated by Dr Stanley M. Lemon, University of Texas Medical Branch at Galveston) containing HCV‐1b subgenome, were cultured on 48‐well plates. When cell confluence reached 80%, cells were washed three times with 1 × PBS and incubated with heat‐inactivated sera from chronic HCV carriers (all were HCV‐1b genotype) or healthy donors (diluted 1 : 200 in PBS) for 2 h at 37°C. After that, the plates were washed five times by PBS to remove unbinding antibodies; 1 × 105 purified NK cells from autologous individual were added to each well. After 24 h incubation, cell‐free supernatants were collected for IFN‐γ detection by Ready‐SET‐Go ELISA kit (eBioscience) with a sensitivity of 4 pg/ml.
We then tested whether HCV E1/E2 linear epitopes could induce an NK‐ADCC response; 96‐well ELISA plates (Nunc MaxiSorp; eBioscience) were precoated with 17 different HCV‐E1/E2 epitope peptides pool (600 ng/well) and incubated with heat‐inactivated sera from chronic HCV carriers or healthy donors (diluted 1 : 200 in PBS) for 2 h at 37°C. The remainder of the experimental procedure was similar to the above description. The five serum samples that induced the highest NK‐ADCC were used for follow‐up experiments.
Screening of HCV E1/E2‐specific NK‐ADCC linear epitopes
For screening of HCV E1/E2‐specific NK‐ADCC linear epitopes, 96‐well ELISA plates (Nunc MaxiSorp; eBioscience) were precoated individually with the 17 HCV‐E1E2 epitope peptides (600 ng/well) and incubated with heat‐inactivated sera samples (diluted 1 : 200 in PBS) from five chronic HCV carriers with robust NK‐ADCC responses (as determined in the above experiment) for 2 h at 37°C. The plates were washed five times with PBS and 1 × 105 purified NK cells from a constant healthy individual were added to each well. After 24 h, cell‐free supernatants were collected for IFN‐γ detection by Ready‐SET‐Go ELISA kit (eBioscience).
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 5.0 software (GraphPad Software Inc., San Diego, CA, USA). Comparisons between groups were performed using the Mann–Whitney U‐test or a non‐parametric t‐test, as necessary. Spearman's correlation test was used to evaluate correlations between groups. All P‐values were two‐tailed and considered significant when less than 0·05.
Results
Non‐specific antibody‐dependent NK cell responses were impaired significantly in chronic HCV infection
To investigate the characteristics of non‐specific antibody‐dependent NK cell responses, PBMCs were stimulated with antibody‐coated P815 cells and the expression of CD107a, TNF‐α and IFN‐γ was detected by flow cytometry. As shown in Supporting information, Fig. S1a, CD56+ NK cells were gated from live CD3‐CD56+ lymphocytes. Compared to NK cells stimulated with uncoated P815 cells, CD16 expression decreased dramatically on NK cells stimulated with antibody‐coated P815 cells, while the expression of CD107a, IFN‐γ and TNF‐α increased significantly, indicating the induction of antibody‐dependent NK cell responses (Fig. 1a). Samples incubated with PMA plus ionomycin were used as positive controls.
Figure 1.
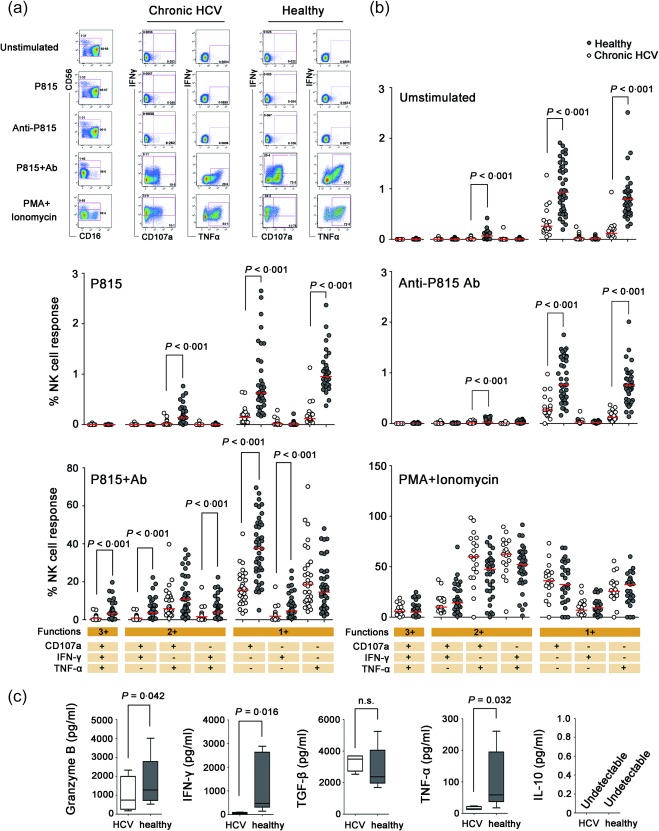
Impaired CD56+ natural killer‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) responses in chronic hepatitis C virus (HCV) carriers. (a) Gating strategies for CD16, CD107a, interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α on CD3–CD56+ NK cells stimulated with medium alone (unstimulated), P815 cells (P815), anti‐P815 antibody (anti‐P815), antibody‐coated P815 cells (P815 + antibody) and phorbol myristate acetate (PMA) plus ionomycin. Representative results from one chronic HCV‐infected patient and one healthy individual are shown. (b) Degranulation (CD107a) and cytokine secretion (IFN‐γ and TNF‐α) in CD56+ NK cells on unstimulated condition or activated with antibody‐coated P815 cells in 31 chronic HCV‐infected patients and 49 healthy controls. The percentages of single‐, double‐ and triple‐positive CD56+ NK cells are shown, and the red bars indicate the median values. Comparisons between groups were performed using the Mann–Whitney U‐test. (c) Secreted cytokines were quantified in NK‐ADCC supernatants by enzyme‐linked immunosorbent assay (ELISA). Purified NK cells from HCV carriers (n = 5) and healthy donors (n = 5) were stimulated with antibody‐coated P815 cells for 24 h, and supernatants were collected to test the levels of granzyme B, IFN‐γ, TNF‐α, transforming growth factor (TGF)‐β and IL‐10 by ELISA. Data are shown as the median cytokine concentration and the interquartile range. Statistical analyses were performed by non‐parametric t‐test. All P‐values are two‐tailed and were considered significant when less than 0·05. [Colour figure can be viewed at wileyonlinelibrary.com].
Subsequently, antibody‐dependent NK cell responses in 31 chronic HCV carriers was characterized and compared with healthy controls. As shown in Fig. 1b, the frequency of NK cells expressing CD107a or IFN‐γ after stimulation with antibody‐coated P815 cells was reduced significantly in chronic HCV carriers compared with healthy controls (each P < 0·001). After stimulation with antibody‐coated P815 cells, CD107a/IFN‐γ/TNF‐α triple‐positive NK cells and IFN‐γ/TNF‐α or CD107a/IFN‐γ double‐positive NK cells were reduced dramatically in HCV‐infected individuals compared to healthy controls (each P < 0·001). These results indicated that antibody‐dependent NK cell responses were impaired in chronic HCV infection, although the frequency of total CD56+ NK cells in circulating lymphocytes was increased in chronic HCV carriers (Supporting information, Fig. S1b, P = 0·046). The reason might ascribed partially to the impaired spontaneous cytotoxity of NK cells, as the frequency of CD107a, TNF‐α– and CD107a/TNF‐α‐positive NK cells were decreased significantly in chronic HCV infection when cultured with media alone or anti‐P815 antibody alone (P < 0·001, Fig. 1b).
To verify the results of the ICS, purified NK cells (five subjects per group) were stimulated with antibody‐coated P815 cells for 24 h and culture supernatants were harvested for cytokine detection. Granzyme B, IFN‐γ, TNF‐α, TGF‐β and IL‐10 levels were determined by ELISA and compared between chronic HCV carriers and healthy controls. Similar to the results by ICS, levels of granzyme B (P = 0·042), IFN‐γ (P = 0·016) and TNF‐α (P = 0·032) were significantly lower in supernatants from HCV‐infected carriers than in healthy controls, while there was no difference in TGF‐β levels (Fig. 1c). In addition, IL‐10 production was undetectable in supernatants from either HCV‐infected subjects or healthy individuals (Fig. 1c).
NK‐ADCC responses were higher in chronic HCV carriers with abnormal serum alanine aminotransferase (ALT)
We then identified the relationship between ALT level and NK‐ADCC response; 31 chronic HCV carriers were separated into ALT normal (ALT ≤ 40 IU/l) and abnormal groups (ALT > 40 IU/l). As shown in Fig. 2, the percentage of CD107a+ CD56+ NK cells was significantly higher in patients with abnormal ALT than patients with normal ALT (P = 0·039). A similar trend was observed for the percentage of IFN‐γ‐producing NK cells, although it did not reach a significant difference (P = 0·082, Fig. 2). It is possible that the stronger NK‐ADCC response induced more HCV‐infected target cell lysis and resulted in increase of ALT production. However, no correlations between NK‐ADCC responses and HCV loads or non‐invasive markers of fibrosis (APRI/FIB‐4) were found in the study (data not shown).
Figure 2.
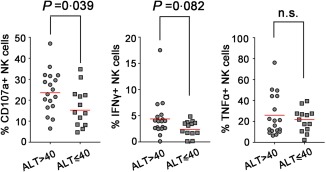
Natural killer‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) responses were higher in chronic hepatitis C virus (HCV) carriers with abnormal serum alanine aminotransferase (ALT). Thirty‐one chronic HCV carriers were divided into two groups according to serum ALT level (higher or not than 40 IU/l). The percentage of CD107a, interferon (IFN)‐γ and tumour necrosis factor (TNF)‐α‐positive NK cells were compared between the ALT normal group (ALT ≤ 40 IU/l) and the abnormal group (ALT > 40 IU/l). Statistical analyses were performed by non‐parametric t test. All P‐values were two‐tailed and considered significant when lower than 0·05. [Colour figure can be viewed at wileyonlinelibrary.com].
Non‐specific antibody‐dependent NK cell responses were correlated with the loss of CD16 on NK cells
We found that the potency of the antibody‐dependent NK cell responses (reflected in the frequency of CD107a+ and IFN‐γ+ NK cells) correlated directly with decreased expression of FcγRIIIa (CD16) on NK cells in both chronic HCV patients and controls [for CD107a, P < 0·001 for chronic HCV patients and P = 0·038 for healthy controls (Fig. 3a); for IFN‐γ, P< 0·001 for chronic HCV patients and P = 0·009 for healthy controls (Fig. 3b)]. Additionally, the cytotoxicity receptors NKp46 (P = 0·006 for total NK and CD56dim subset) and NKG2D (P < 0·001 for total, CD56dim and CD56bright NK cells) were down‐regulated on NK cells in chronic HCV carriers (Supporting information, Fig. S1c) 26, 27, 28.
Figure 3.
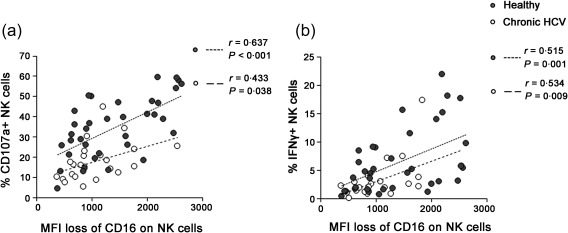
Non‐specific antibody‐dependent natural killer (NK) cell responses were correlated with the loss of CD16 on NK cells. the potency of the antibody‐dependent NK cell responses were calculated as the frequencies of CD107a+ (a) or interferon (IFN)‐γ+ (b) NK cells incubating with antibody‐bound P815 cells minus the percentage of these cells incubating with P815 cells alone. The Pearson's correlation test was used to evaluate correlations between groups. Loss of CD16+ cells on CD56+ NK cells indicated CD16 mean fluorescence intensity (MFI) of these cells stimulated with P815 cells alone minus CD16 MFI following stimulation with antibody‐bound P815 cells. All P‐values are two‐tailed and were considered significant when less than 0·05.
CD56dim NK cells, but not CD56bright NK cells, contribute mainly to NK‐ADCC activation
In order to determine which NK cell subsets, CD56dim or CD56bright, hold the main capacity to induced NK‐ADCC activation, we compared the three functions (CD107a/IFN‐γ/TNF‐α) profile between CD56dim and CD56bright subsets activation following stimulation with antibody‐coated P815 cells. The result showed that three single functions, triple and double combinations of functions, were all higher in the activated CD56dim NK cells (all P < 0·001) (Fig. 4), indicating that the CD56dim subset contributed mainly to NK‐ADCC activation.
Figure 4.
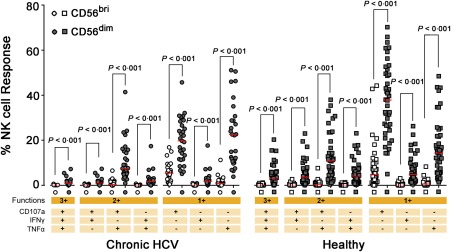
Comparision of natural killer‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) responses mediated by CD56dim NK cells and CD56bri NK subset. The functional profile of CD56dim and CD56bri subsets stimulated with antibody‐coated p815 cells was shown. The percentages of CD107a/interferon (IFN)‐γ/tumour necrosis factor (TNF)‐α single‐, double‐ or triple‐positive NK cells in the respective subset were shown and the red bar indicated median value. Comparisons between groups were performed using Mann–Whitney U‐test. All P‐values were two‐tailed and considered significant when lower than 0·05. [Colour figure can be viewed at wileyonlinelibrary.com].
Identification of HCV‐specific antibody‐dependent NK cell responses in chronic HCV‐infected subjects in vitro
To study whether HCV membrane protein could induce an NK‐ADCC response, huh7·5‐based HCV replicon cells (HCV‐Con I‐Rep) were used to bind anti‐HCV sera to activate autologous NK cells. The result showed that significantly higher IFN‐γ concentrations were detected in the group bound with positive anti‐HCV sera than the group inoculated with healthy sera (P < 0·001) (Fig. 5a). To confirm HCV‐specific antibody‐dependent NK cell responses, 17 linear peptides (Table 2) recognized by defined anti‐HCV E1/E2 monoclonal antibodies were pooled and used to bind anti‐HCV sera to activate autologous NK cells. As shown in Fig. 5b, the peptide pool induced HCV‐specific NK‐ADCC responses successfully in 18 of 31 (58·1%) patients.
Figure 5.
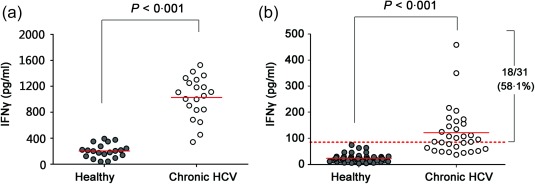
Identification of hepatitis C virus (HCV)‐specific natural killer‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) functions in chronic HCV‐infected subjects in vitro. (a) Huh7·5‐based HCV replicon cells (HCV‐Con‐Rep) were cultured on 48‐well plate to 80% confluence and were incubated with heat‐inactivated sera from 20 chronic HCV carriers (all were HCV‐1b genotype) or 20 healthy donors to form antigen–antibody complexes. Purified autologous NK cells were used as effector cells. Interferon (IFN)‐γ levels in the culture supernatants were determined by enzyme‐linked immunosorbent assay (ELISA). (b) Seventeen peptides representing epitopes known to be recognized by anti‐HCV antibodies were pooled and precoated onto 96‐well plates. Serum samples from 31 HCV patients and 49 healthy individuals were added subsequently. Purified autologous NK cells were used as effector cells and then IFN‐γ in the supernatants were detected. The dotted red line indicates the average IFN‐γ level of the healthy controls plus three times the standard deviation (mean ± 3 s.d.). Comparisons between groups were performed using the Mann–Whitney U‐test. All P‐values are two‐tailed and were considered significant when less than 0·05. [Colour figure can be viewed at wileyonlinelibrary.com].
Screening of linear epitopes capable of mediating robust antibody‐dependent NK responses
To determine the specific epitopes capable of mediating robust antibody‐dependent NK responses, each of the 17 peptides was bound to sera from HCV carriers with robust antibody‐dependent NK responses (as determined in Fig. 5b), and the antigen–antibody complexes were used to activate purified NK cells from two different healthy donors (donors A and B) (with the exception that case 271 was tested only against donor A due to the limited volume of serum available). As shown in Fig. 6, peptides were bound to five different HCV sera (HCV‐1b for cases 37 and 121 and HCV‐2a for cases 204, 152 and 271). Among the 17 peptides, peptides 3, 6, 11, 14 and 17 induced higher NK responses than the other 12 peptides in both healthy donors, suggesting the conservation of specific NK‐ADCC epitopes.
Figure 6.
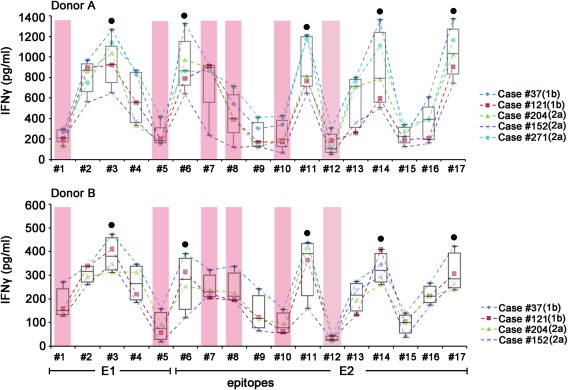
Screening of linear epitopes within the hepatitis C virus (HCV) E1/E2 region for their ability to induce natural killer‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC). Individual synthetic HCV‐E1E2 peptides were precoated onto 96‐well plates and incubated with serum samples from five different chronic HCV carriers (genotype 1b for 37 and 121; genotype 2a for 204, 152 and 271). Purified NK cells from two healthy donors (donor A for the upper panel and donor B for the lower panel) were used as effector cells. Interferon (IFN)‐γ levels in cell‐free supernatants were determined by enzyme‐linked immunosorbent assay (ELISA). HCV patient no. 271 was not tested with donor B NK cells due to the limited amount of serum available. Peptides 3, 6, 11, 14 and 17 (indicated by black dots) induced higher NK‐ADCC responses than the other 12 peptides in both donors. Six putative neutralizing epitopes overlapped with peptides 1, 5, 7, 8, 10 and 12 are indicated by pink columns. The median and the interquartile ranges are shown for each peptide. [Colour figure can be viewed at wileyonlinelibrary.com].
As shown in Table 2 and Fig. 7, epitopes aa192–202 and 313–326 in the E1 region, epitope aa396‐407 in the HVR1 region and epitopes aa412–423 and aa496–515 in the E2 region are putative linear neutralizing epitopes. In addition, epitope aa434–446 can be recognized by some antibodies and is also a potential neutralizing epitope 29. Among the 17 HCV E1/E2‐derived peptides tested in this study, 1, 5, 7, 8, 10 and 12 overlap with the putative linear neutralizing epitopes. None of the NK‐ADCC peptides (3, 6, 11, 14 and 17) overlapped with those putative neutralizing epitopes. These data indicate that NK‐ADCC epitopes may be distinct from neutralizing epitopes.
Figure 7.
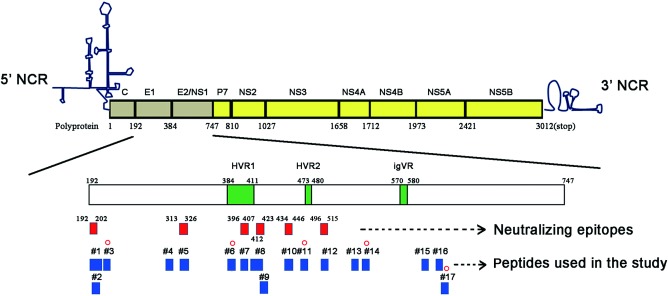
Distribution of five defined linear natural killer‐mediated antibody‐dependent cellular cytotoxicity (NK‐ADCC) epitopes on hepatitis C virus (HCV)‐E1/E2. The position of HVR1 (aa384‐aa411), HVR2 (aa473‐aa480) and igVR (aa570‐aa580) in the open reading frame (ORF) of E1/E2 protein (aa192–aa747) are shown by green rectangles. Six putative neutralizing epitopes (aa192–aa202, aa313–aa326, aa396–407, aa412–aa423, aa434–aa446, and aa496–515) are indicated by red rectangles. The 17 linear epitope peptides used in this study are indicated by blue rectangles. NK‐ADCC‐specific epitopes (3, 6, 11, 14 and 17) identified in this study are marked with red circles. HVR = hypervariable region; igVR = intergenotypic variable region; NCR = non‐coding region. [Colour figure can be viewed at wileyonlinelibrary.com].
Discussion
Because NK cells are enriched in human liver 30, 31 and NK‐related immune factors can predict viral clearance after acute HCV infection 3, 31, 32, 33, NK cell‐mediated immunity is thought to play a pivotal role in host defence against HCV infection. Recently, Grebely et al. demonstrated that spontaneous clearance of HCV occurred most often after the induction of an anti‐HCV humoral immune response, indicating that antibody‐mediated immune responses such as NK‐ADCC might contribute to the spontaneous clearance of HCV 34. Unlike neutralizing antibodies, which directly prevent viral infection, NK‐ADCC antibodies eliminate HCV‐infected cells by triggering the release of cytotoxic substances from NK cells, including granzyme, perforin and cytokines 35. Whether or not NK‐ADCC and NK cell natural cytotoxicity are impaired in chronic HCV infection is still controversial. NK natural cytotoxicity has been reported to be diminished 30, 36, unaffected or even increased 33, 37, 38, 39, 40 in chronic HCV infection. However, little is known regarding antibody‐dependent NK cell responses in chronic HCV infection. Liu et al. reported impaired non‐specific NK‐ADCC in HIV‐1 infection using P815 cells (a mouse mastocytoma cell line) as target cells, which avoids the interference caused by activation of killer activation receptors (KARs) or killer inhibitory receptors (KIRs) on NK cells 41. Alter et al. found that the percentage of NK cells expressing CD107a after activation with plate‐coated P815‐antibody complex was decreased in chronic HCV patients compared to healthy controls, but a statistical difference was not found due to the limited number of cases examined 3.
In the present study, we found that the capacity of circulating NK cells to degranulate and produce IFN‐γ following engagement of CD16 with antibody‐bound P815 cells was impaired significantly in chronic HCV carriers. Impaired spontaneous cytotoxicity of NK cells and altered NKp46 and CD16 expression might contribute separately to the deficient NK‐ADCC responses in chronic HCV patients. NK cell‐mediated ADCC was reported to be decreased in individuals with persistent HIV infection, but increased in HIV elite controllers 42. However, it is hard to evaluate HCV‐specific NK‐ADCC in HCV resolvers due to the rapid decay of HCV‐E1/E2 antibody responses in these individuals 43. Consistent with this, we have tested non‐specific antibody‐dependent NK cell responses induced by HCV E1/E2 peptides in HCV spontaneous resolvers within 5 − 10 years after HCV clearance, and no NK‐ADCC responses were found in these patients (data not shown).
In addition, five linear epitopes (3, 6, 11, 14 and 17) that could induce robust antibody‐dependent NK cell responses were identified. These epitopes did not overlap with putative linear neutralizing epitopes except for 17, which is located in 648 − 659 of the E2 region and overlaps partially with a conformational epitope that has been reported to induce a broad neutralizing activity towards diverse HCV genotypes in a small animal model 44. Overall, this observation indicates that the antibodies induced by neutralizing epitopes of HCV E1/E2 interfere with the ability of viruses to recognize susceptible host cells, but have little ability to induce NK cell‐mediated ADCC.
Although we have identified five potential NK‐ADCC epitopes in the present study, their ability to mediate NK‐ADCC responses against different HCV genotypes remains to be determined. Also, whether CD56–CD16+ NK cells could mediate ADCC and whether their functions were impaired are still undefined. In conclusion, our results may be applicable in the development of novel immunological intervention strategies designed to elicit robust NK‐ADCC responses against HCV infection.
Disclosure
None.
Author contributions
L. L., M. J., J. W. and X. F. performed the experiments, L. Z. and Z. X. analysed data, H. L. edited the manuscript, T. S. designed the study, L. L. and M. J. wrote the paper.
Supporting information
Fig. S1. Distribution and phenotypical patterns of circulating CD56+ natural killer (NK) cells in chronic hepatitis C virus (HCV) carriers. (a) Gating strategies for CD56bright and CD56dim NK subsets in peripheral blood mononuclear cells (PBMCs). (b) The frequencies of total CD56+ NK cells within PBMCs (b) and the frequencies of CD56bright and CD56dim NK cells within total CD56+ NK cells (c) were calculated. Statistical analyses were performed by non‐parametric t‐test. All P‐values are two‐tailed and were considered significant when less than 0·05. (c) The percentages of total CD56+ NK cells, CD56bright and CD56dim NK subsets expressing CD158, CD161, NKG2A, NKp46, NKG2C and NKG2D were determined by flow cytometry and are shown as the medians and interquartile ranges. Statistical analyses were performed by non‐parametric t‐test. All P‐values are two‐tailed and were considered significant when lower than 0·05.
Acknowledgements
We thank all participants recruited in this study and appreciate staff in Shangcai Center for Disease Control and Prevention for helping to collect blood samples. This work was supported by grants from the National Natural Science Foundation of China (81271826), the National Science and Technology Major Project for Infectious Diseases (2014ZX10001001‐002‐004) and State Key Laboratory of Infectious Disease Prevention and Control (2015SKLID506).
References
- 1. Von Hahn T, Yoon JC, Alter H et al Hepatitis C virus continuously escapes from neutralizing antibody and T‐cell responses during chronic infection in vivo . Gastroenterology 2007; 132:667–78. [DOI] [PubMed] [Google Scholar]
- 2. Kokordelis P, Kramer B, Korner C et al An effective interferon‐gamma‐mediated inhibition of hepatitis C virus replication by natural killer cells is associated with spontaneous clearance of acute hepatitis C in human immunodeficiency virus‐positive patients. Hepatology 2014; 59:814–27. [DOI] [PubMed] [Google Scholar]
- 3. Alter G, Jost S, Rihn S et al Reduced frequencies of NKp30+NKp46+, CD161+, and NKG2D+ NK cells in acute HCV infection may predict viral clearance. J Hepatol 2011; 55:278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yamada T, Watanabe N, Nakamura T et al Antibody‐dependent cellular cytotoxicity via humoral immune epitope of Nef protein expressed on cell surface. J Immunol 2004; 172:2401–6. [DOI] [PubMed] [Google Scholar]
- 5. McElrath MJ, Haynes BF. Induction of immunity to human immunodeficiency virus type‐1 by vaccination. Immunity 2010; 33:542–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haynes BF, Gilbert PB, McElrath MJ et al Immune‐correlates analysis of an HIV‐1 vaccine efficacy trial. N Engl J Med 2012; 366:1275–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rerks‐Ngarm S, Pitisuttithum P, Nitayaphan S et al Vaccination with ALVAC and AIDSVAX to prevent HIV‐1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
- 8. Nattermann J, Schneiders AM, Leifeld L et al Serum antibodies against the hepatitis C virus E2 protein mediate antibody‐dependent cellular cytotoxicity (ADCC). J Hepatol 2005; 42:499–504. [DOI] [PubMed] [Google Scholar]
- 9. Liu Y, Lv Q, Gao J et al Coinfection with HIV‐1 alleviates iron accumulation in patients with chronic hepatitis C virus infection. PLOS ONE 2014; 9:e98039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wren LH, Chung AW, Isitman G et al Specific antibody‐dependent cellular cytotoxicity responses associated with slow progression of HIV infection. Immunology 2013; 138:116–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Keck ZY, Sung VM, Perkins S et al Human monoclonal antibody to hepatitis C virus E1 glycoprotein that blocks virus attachment and viral infectivity. J Virol 2004; 78:7257–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haberstroh A, Schnober EK, Zeisel MB et al Neutralizing host responses in hepatitis C virus infection target viral entry at postbinding steps and membrane fusion. Gastroenterology 2008; 135:1719–28.e1. [DOI] [PubMed] [Google Scholar]
- 13. Meunier JC, Russell RS, Goossens V et al Isolation and characterization of broadly neutralizing human monoclonal antibodies to the e1 glycoprotein of hepatitis C virus. J Virol 2008; 82:966–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Guan M, Wang W, Liu X et al Three different functional microdomains in the hepatitis C virus hypervariable region 1 (HVR1) mediate entry and immune evasion. J Biol Chem 2012; 287:35631–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Broering TJ, Garrity KA, Boatright NK et al Identification and characterization of broadly neutralizing human monoclonal antibodies directed against the E2 envelope glycoprotein of hepatitis C virus. J Virol 2009; 83:12473–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hsu M, Zhang J, Flint M et al Hepatitis C virus glycoproteins mediate pH‐dependent cell entry of pseudotyped retroviral particles. Proc Natl Acad Sci USA 2003; 100:7271–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tarr AW, Urbanowicz RA, Jayaraj D et al Naturally occurring antibodies that recognize linear epitopes in the amino terminus of the hepatitis C virus E2 protein confer noninterfering, additive neutralization. J Virol 2012; 86:2739–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Krey T, d'Alayer J, Kikuti CM et al The disulfide bonds in glycoprotein E2 of hepatitis C virus reveal the tertiary organization of the molecule. PLoS Pathog 2010; 6:e1000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kachko A, Kochneva G, Sivolobova G et al New neutralizing antibody epitopes in hepatitis C virus envelope glycoproteins are revealed by dissecting peptide recognition profiles. Vaccine 2011; 30:69–77. [DOI] [PubMed] [Google Scholar]
- 20. Clayton RF, Owsianka A, Aitken J et al Analysis of antigenicity and topology of E2 glycoprotein present on recombinant hepatitis C virus‐like particles. J Virol 2002; 76 :72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Owsianka A, Clayton RF, Loomis‐Price LD et al Functional analysis of hepatitis C virus E2 glycoproteins and virus‐like particles reveals structural dissimilarities between different forms of E2. J Gen Virol 2001; 82:1877–83. [DOI] [PubMed] [Google Scholar]
- 22. Owsianka A, Tarr AW, Juttla VS et al Monoclonal antibody AP33 defines a broadly neutralizing epitope on the hepatitis C virus E2 envelope glycoprotein. J Virol 2005; 79:11095–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Koutsoudakis G, Dragun J, Perez‐Del‐Pulgar S et al Interplay between basic residues of hepatitis C virus glycoprotein E2 with viral receptors, neutralizing antibodies and lipoproteins. PLOS ONE 2012; 7:e52651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Flint M, Maidens C, Loomis‐Price LD et al Characterization of hepatitis C virus E2 glycoprotein interaction with a putative cellular receptor, CD81. J Virol 1999; 73:6235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ndongo N, Rechoum Y, De Sequeira S et al Inhibition of the binding of HCV serum particles to human hepatocytes by E1E2‐specific D32.10 monoclonal antibody. J Med Virol 2009; 81:1726–33. [DOI] [PubMed] [Google Scholar]
- 26. Nattermann J, Feldmann G, Ahlenstiel G et al Surface expression and cytolytic function of natural killer cell receptors is altered in chronic hepatitis C. Gut 2006; 55:869–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bozzano F, Picciotto A, Costa P et al Activating NK cell receptor expression/function (NKp30, NKp46, DNAM‐1) during chronic viraemic HCV infection is associated with the outcome of combined treatment. Eur J Immunol 2011; 41:2905–14. [DOI] [PubMed] [Google Scholar]
- 28. Pembroke T, Christian A, Jones E et al The paradox of NKp46+ natural killer cells: drivers of severe hepatitis C virus‐induced pathology but in‐vivo resistance to interferon alpha treatment. Gut 2014; 63:515–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wahid A, Dubuisson J. Virus‐neutralizing antibodies to hepatitis C virus. J Viral Hepatol 2013; 20:369–76. [DOI] [PubMed] [Google Scholar]
- 30. Corado J, Toro F, Rivera H et al Impairment of natural killer (NK) cytotoxic activity in hepatitis C virus (HCV) infection. Clin Exp Immunol 1997; 109:451–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cheent K, Khakoo SI. Natural killer cells and hepatitis C: action and reaction. Gut 2011; 60:268–78. [DOI] [PubMed] [Google Scholar]
- 32. Khakoo SI, Thio CL, Martin MP et al HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 2004; 305:872–4. [DOI] [PubMed] [Google Scholar]
- 33. Golden‐Mason L, Madrigal‐Estebas L, McGrath E et al Altered natural killer cell subset distributions in resolved and persistent hepatitis C virus infection following single source exposure. Gut 2008; 57:1121–8. [DOI] [PubMed] [Google Scholar]
- 34. Grebely J, Page K, Sacks‐Davis R et al The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatology 2014; 59:109–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pollara J, Bonsignori M, Moody MA et al Epitope specificity of human immunodeficiency virus‐1 antibody dependent cellular cytotoxicity (ADCC) responses. Curr HIV Res 2013; 11:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Par G, Rukavina D, Podack ER et al Decrease in CD3‐negative‐CD8dim(+) and Vdelta2/Vgamma9 TcR+ peripheral blood lymphocyte counts, low perforin expression and the impairment of natural killer cell activity is associated with chronic hepatitis C virus infection. J Hepatol 2002; 37:514–22. [DOI] [PubMed] [Google Scholar]
- 37. Morishima C, Paschal DM, Wang CC et al Decreased NK cell frequency in chronic hepatitis C does not affect ex vivo cytolytic killing. Hepatology 2006; 43:573–80. [DOI] [PubMed] [Google Scholar]
- 38. Oliviero B, Varchetta S, Paudice E et al Natural killer cell functional dichotomy in chronic hepatitis B and chronic hepatitis C virus infections. Gastroenterology 2009; 137:1151–60, 1160 e1–7. [DOI] [PubMed] [Google Scholar]
- 39. Ahlenstiel G, Titerence RH, Koh C et al Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon‐alfa‐dependent manner. Gastroenterology 2010; 138:325–35 e1‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoon JC, Shiina M, Ahlenstiel G et al Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology 2009; 49:12–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liu Q, Sun Y, Rihn S et al Matrix metalloprotease inhibitors restore impaired NK cell‐mediated antibody‐dependent cellular cytotoxicity in human immunodeficiency virus type 1 infection. J Virol 2009; 83:8705–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jia M, Li D, He X et al Impaired natural killer cell‐induced antibody‐dependent cell‐mediated cytotoxicity is associated with human immunodeficiency virus‐1 disease progression. Clin Exp Immunol 2013; 171:107–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Takaki A, Wiese M, Maertens G et al Cellular immune responses persist and humoral responses decrease two decades after recovery from a single‐source outbreak of hepatitis C. Nat Med 2000; 6:578–82. [DOI] [PubMed] [Google Scholar]
- 44. Giang E, Dorner M, Prentoe JC et al Human broadly neutralizing antibodies to the envelope glycoprotein complex of hepatitis C virus. Proc Natl Acad Sci USA 2012; 109:6205–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Distribution and phenotypical patterns of circulating CD56+ natural killer (NK) cells in chronic hepatitis C virus (HCV) carriers. (a) Gating strategies for CD56bright and CD56dim NK subsets in peripheral blood mononuclear cells (PBMCs). (b) The frequencies of total CD56+ NK cells within PBMCs (b) and the frequencies of CD56bright and CD56dim NK cells within total CD56+ NK cells (c) were calculated. Statistical analyses were performed by non‐parametric t‐test. All P‐values are two‐tailed and were considered significant when less than 0·05. (c) The percentages of total CD56+ NK cells, CD56bright and CD56dim NK subsets expressing CD158, CD161, NKG2A, NKp46, NKG2C and NKG2D were determined by flow cytometry and are shown as the medians and interquartile ranges. Statistical analyses were performed by non‐parametric t‐test. All P‐values are two‐tailed and were considered significant when lower than 0·05.


