Summary
Endogenous retroviruses (HERV) are believed to be pathogenic in several autoimmune diseases. Among them, HERV‐K viruses have been reported recently to be involved in the pathogenesis of rheumatoid arthritis (RA). In this study we have explored the role of humoral immune response against HERV‐K as a potential pathogenetic mechanism in RA. Four different peptides from the extracellular portion of the env protein of HERV‐K (env‐su19–37, env‐su109–126, env‐su164–186, env‐su209–226) were selected by bioinformatic analysis on the basis of their putative immunogenicity. Indirect enzyme‐linked immunosorbent assay (ELISA) was then carried out to quantify antibodies against those peptides on blood samples of 70 consecutive RA patients and 71 healthy controls (HC). Differences between the two groups were analysed using the Mann–Whitney test. Potential correlations between RA laboratory, clinical descriptors and immunoglobulin (Ig)G levels were explored by bivariate regression analysis. Serum autoantibodies against one of four tested peptides of HERV‐K (env‐su19–37) were significantly higher in RA than in HC (19 versus 3%, P = 0·0025). Subgroup analysis showed no association between anti‐HERV‐K peptide humoral response and clinical, serological and clinimetric RA disease descriptors. Serum from RA patients in our series reacted significantly against HERV‐K env‐su19–37 peptide in comparison to the general population suggesting a role for the HERV‐K‐ related, secondary antigenic‐driven immune response in the pathogenesis of RA. Further studies are needed to confirm these results and to explore the role of this HERV‐K surface peptide as a potential therapeutic target.
Keywords: Herv‐K, humoral response, molecular mimicry, peptides, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is one of the most common autoimmune diseases. RA mainly affects joints leading to articular damage, disability and substantial morbidity. The aetiology of RA remains unknown, although the disease appears to be the result of a complex interaction between host genetic and environmental factors 1. Among environmental factors, a role for viruses has been long postulated and endogenous retrovirus (HERV) is one potential candidate in this context.
HERVs endogenated through infection of germline cells give rise to viable progeny in a symbiotic relationship with the host 2. Some HERVs confer biological benefits to humans 3, while other HERVs, notably HERV‐K, may be deleterious to the host, retaining the capacity to replicate under select conditions and, consequently, the potential to trigger host innate and adaptive immune responses 2, 4.
HERV‐K (HML‐2) is one of the most transcriptionally active in the endogenous viral family, and its members maintain open reading frames (ORF) in the genes gag, pol and env that are able to manufacture retroviral proteins and particles, and possibly complete virions 5. HERV‐K is divided into virus types 1 and 2. Type 1 virus encodes the oncogenic protein NP9, while type 2 virus produces Rec and Env proteins, both of which have been implicated in tumorigenesis 6. The hypothesis of a potential pathogenic link between HERV‐K and RA is based on several observations descending from both polymerase chain reaction (PCR)‐based and serological studies 7. The prevalence of insertionally polymorphic HERV‐K was increased significantly in the RA population 8. Moreover, a significantly increased expression has been demonstrated of HERV‐K genes and transcription of viral proteins in peripheral blood, synovial fluid and synoviocytes of RA patients compared to healthy subjects 6, 9. In addition, common amino acid sequences between HERV‐K and host antigens have been reported in RA, suggesting that a mechanism of molecular mimicry may play a role in disease pathogenesis 10, 11.
Considering this background, we performed an in‐silico analysis to predict key antigenic regions of HERV‐K on the basis of their putative ability in igniting an RA‐specific humoral response. Candidate epitopes were then synthesized as short peptides, and an enzyme‐linked immunosorbent assay (ELISA) was used to test the reactivity of RA and control serum to HERV‐K peptides.
Materials and methods
Ethics approval
The study was approved by the Ethics Committee of ASL‐1 Sassari ASL (Prot. 1192/L, 2014). The patients and volunteers gave written informed consent.
Subjects
Seventy consecutive unselected Sardinian patients with RA diagnosed according to the American College of Rheumatology criteria, referred to the out‐patient clinic of the Rheumatology Unit at the Department of Clinical and Experimental Medicine of the University of Sassari (Italy), were recruited between March and April 2014 in this cross‐sectional case–control study. Seventy‐one healthy sex‐ and age‐matched subjects from the local blood donor bank were enrolled into the study as healthy controls (HC). All patients and controls lived in Sardinia. For each RA patient enrolled into the study, data regarding disease duration, disease activity, functional state and ongoing treatment were registered systematically. Disease activity was assessed using the Disease Activity Score‐28 (DAS‐28) and functional class by Health Assessment Questionnaire (HAQ). Demographic, clinical and laboratory characteristics of the participants are summarized in Table 1.
Table 1.
Demographic, clinical and laboratory features of rheumatoid arthritis (RA) patients and healthy controls (HC)
| RA | HC | |
|---|---|---|
| n = 70 | n = 71 | |
| Age, years | 57 (18) | 58 (17) |
| Female sex, n (%) | 58 (82.9) | 57 (80) |
| HAQ (0–3) | 0.87 (1.18) | – |
| DAS‐28 | 3.24 (2.59) | – |
| CRP, mg/dl | 0.49 (0.8) | – |
| ESR, mm/h | 17 (26.5) | – |
| Steroids, n (%) | 32 (45.7) | – |
| DMARDs, n (%) | 43 (61.4) | – |
| Anti‐TNF, n (%) | 22 (31.4) | – |
| Tocilizumab, n (%) | 12 (17.1) | – |
Continuous data are expressed as median (IQR). HAQ = Health Assessment Questionnaire; DAS‐28 = Disease Activity Score‐28; CRP = C‐reactive protein; ESR = erythrocyte sedimentation rate; DMARDs = disease modifying anti‐rheumatic drugs; anti‐TNF = anti‐tumour necrosis factor.
Blood cell separation
Serum was isolated from heparinized peripheral blood samples by layering on Ficoll/Hypaque, as described elsewhere 12.
Peptides
In‐silico analysis performed using IEDB Analysis Resource software, in particular the Antibody Epitope Prediction, Bepipred linear epitope prediction software (http://www.iedb.org/home_v3.php), allowed us to identify different antigenic peptides derived from HERV‐K env surface protein (UniProtKB Accession number: Q69384); four were selected for further analysis: HERV‐K env19–37 (VWVPGPTDDRCPAKPEEEG), HERV‐K env109–126 (RPKGKTCPKEIPKGSKNT), ERV‐K env164–186 (SGQTQSCPSAQVSPAVDSDLTES) and HERV‐K env205–226 (EKGISTPRPKIISPVSGPEHPE). All peptides were synthesized commercially (LifeTein, South Plainfield, NJ, USA) with a purity > 90% and kept frozen in single‐use aliquots [10 mM] at −80°C.
ELISA
Indirect ELISA was carried out to detect specific antibodies against all synthetic peptides (assayed at 10 µg/ml) included into the study, as reported previously 2, 10 Ninety‐six‐well Nunc immunoplates were coated overnight at 4°C with 10 µg/ml of peptides diluted in 0·05 M carbonate–bicarbonate buffer, pH 9·5 (Sigma, St Louis, MO, USA). Plates were then blocked for 1 h at room temperature with 5% non‐fat dried milk (Sigma) and washed twice with phosphate‐buffered saline (PBS) containing 0·05% Tween‐20 (PBS‐T). Cerebrospinal fluid (CSF) samples were subsequently added at a 1 : 2 dilution in PBS‐T for 2 h at room temperature, while sera were added at a 1 : 100 dilution. After five washes in PBS‐T, 100 µl of alkaline phosphatase‐conjugated goat anti‐human immunoglobulin G polyclonal antibody (1 : 1000; Sigma) was added for 1 h at room temperature. Plates were washed again five times in PBS‐T and para nitrophenylphosphate (Sigma) added as substrate for alkaline phosphatase. Plates were incubated at 37°C in the dark for 3–6 min and the absorbance at 405 nm read on a VERSATunable Max microplate reader (Molecular Devices, Sunnyvale, CA, USA). Negative control wells were obtained by incubation of immobilized peptides with secondary antibody alone, and their mean values subtracted from all samples. Positive control sera were also included in all experiments. Results are expressed as means of triplicate 405 nm optical density (OD) values.
Statistical analysis
The analysis was performed using GraphPad Prism version 6.0 software. The Mann–Whitney test was performed for non‐parametric comparisons. A value of P < 0·05 was considered significant. Comparisons between RA patients and HC groups were performed using Fisher's exact test for non‐parametric data, while receiver‐operating characteristic (ROC) curves were utilized to evaluate the accuracy of the experiments. The optimal cut‐off values were chosen according to ROC analysis, setting specificity at > 90% for all serum and CSF samples measured.
Results
ELISA
Antibodies against HERV‐K env peptides were tested in serum of RA and HC samples.
HERV‐K env‐su19–37 antibodies were above positivity in the sera of 19% RA patients and in only 3% of HCs (AUC = 0·84, RA versus HC P < 0·0025) (Fig. 1a). Regarding HERV‐K env‐su109–126 peptide, 9% of RA patients and 3% of HC were positive for antibodies in serum (P = 0·165, AUC = 0·71) (Fig. 1b).
Figure 1.
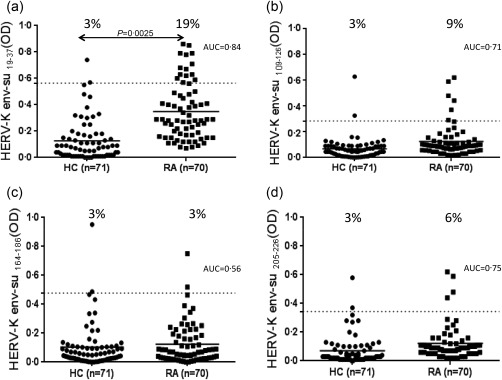
Prevalence of antibodies against peptides derived from endogenous retrovirus (HERV)‐K envelope (env) protein. Seventy rheumatoid arthritis (RA) patients and 71 healthy controls were screened for immunoglobulin (Ig)G antibodies by indirect enzyme‐linked immunosorbent assay (ELISA) against HERV‐K env19–37, (a), HERV‐K env109–126 (b), HERV‐K env164–186 (c) and HERV‐K env205–226 (d) peptides. Scatterplots present median values with interquartile range. Area under receiver‐operating characteristic (ROC) curve (AUC) as well as significant P‐values are displayed.
No statistical differences were found regarding positivity against HERV‐K env‐su164–186 in RA patients (3%) versus HC (3%) (AUC = 0·56, Fig. 1c). HERV‐K env‐su205–226 peptide was recognized in the sera of 6% of RA patients and 3% of HC (P = 0·44, AUC = 0·75) (Fig. 1d). No significant difference was found in the proportion of HERV‐K env‐su19–37 antibody positivity according to disease activity (moderate–severe RA versus low activity and remission RA) and type of immunosuppressive treatment (Supporting information, Table S1). No correlation was found between HERV‐K env‐su19–37, antibody levels and RA descriptors [age, DAS‐28, HAQ, C‐reactive protein (CRP) and erythrocyte sedimentation rate (ESR)] (Supporting information, Table S1). The same results were obtained with the other HERV‐K env‐su peptides tested (data not shown). The serum response of patients and controls towards all four epitopes is shown in Supporting information, Table S2. No patient was positive to all four peptides tested; only four patients were positive to two peptides (HERV‐K109–126 and HERV‐K209–226) (Supporting information, Table S2).
Discussion
HERV‐K mRNA transcripts and IgG anti‐HERV‐K antibodies have been associated with autoimmunity and cancers, as well as neurological conditions (e.g. multiple sclerosis) 2, 13, 14.
For the first time, we searched for a humoral response in patients with RA and controls against selected surface epitopes of HERV‐K env‐su (HERV‐K env‐su19–37, HERV‐K env‐su109–126 and HERV‐K env‐su209–226). Among them, the most interesting peptide was HERV‐K env‐su19–37, which was recognized in the sera of 19% of RA patients and in only 3% of controls.
HERV‐K env‐su19–37 has a high homology with different human antigens, including myosin (with 61% of positive amino acids with sequence EAW76415.1) and netrin (73% of positive amino acids with sequence NP_006172.1). Evidence of a molecular mimicry as a pathogenic mechanism linking HERV‐K to RA was provided by several groups. Freimanis and co‐authors 10 demonstrated a significant humoral response in RA patients against one HERV‐K epitope which shares residues with collagen type II, a common target of pathogenic RA autoantibodies. Similarly, IgGs against antigenic peptide on the matrix segment of HERV‐K10 sharing common sequence with IgG1Fc, a target of rheumatoid factor, were demonstrated in RA patients 11. An intriguing explanation of this result would be that the surface epitope HERV‐K env‐su19–37 may function as an ‘autoantigen’ driving a secondary immunological response.
Unfortunately, we were unable to find any statistically significant correlation between antibody positivity and disease activity, systemic inflammation and type of immunosuppressive treatment. It may be possible that therapy could alter the humoral response. Notably, and also in the series of RA patients described by Nelson and co‐authors, levels of antibodies to HERV‐K antigens showed no correlation with RA disease activity 11.
However, it should be remembered that a number of conditions, both genetic and environmental, may modulate HERV‐K env protein transcription contributing to changes in the amount of immunological response. HERV‐K transcription exhibits a significant increase in the presence of proinflammatory cytokines [tumour necrosis factor (TNF)‐α and interleukin (IL)‐6] in RA with regard to the general population 10. Exogenous viruses such as Epstein–Barr virus (EBV), reported to be linked to RA 15, may activate and stimulate HERV‐K expression 16. Moreover, differential HERV‐K transcription has been shown to be regulated epigenetically by the degree of DNA methylation in RA 17.
In conclusion, we have demonstrated a specific humoral response against a superficial envelope epitope of HERV‐K in RA patients. Results of our study clearly add to the evidence for a possible role of HERV‐K in RA pathogenesis. However, further data need to be provided to support molecular mimicry between HERV‐K and host as one relevant mechanism of disease.
Disclosure
No conflicts of interest to declare.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Correlations between anti‐endogenous retrovirus (HERV)‐K su19–37 antibodies and rheumatoid arthritis (RA) descriptors
Table S2. Raw data of serum response of rheumatoid arthritis (RA) patients and HC against endogenous retrovirus (HERV)‐K peptides
Acknowledgements
G. M. conceived and performed the study, analysed the data and contributed to the writing of the manuscript; G. L. E. collected samples from patients, analysed the data and contributed to the writing of the manuscript; E. C. contributed to the analysis of data and writing of the manuscript; S. M., D. C., M. L. C., A. P., M. B., N. M., E. C. and G. B. followed RA patients and collected samples from RA patients; G. P. analysed the data and contributed to the discussion; L. A. S. conceived the study, analysed the data and contributed to the writing of the manuscript. This study received no funding.
References
- 1. McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med 2011; 8:2205–19. [DOI] [PubMed] [Google Scholar]
- 2. Manghera M, Douville RN. Endogenous retrovirus‐K promoter: a landing strip for inflammatory transcription factors?. Retrovirology 2013; 10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dupressoir A, Lavialle C, Heidmann T. From ancestral infectious retroviruses to bona fide cellular genes: role of the captured syncytins in placentation. Placenta 2012; 33:663–71. [DOI] [PubMed] [Google Scholar]
- 4. Kassiotis G, Stoye JP. Immune responses to endogenous retroelements: taking the bad with the good. Nat Rev Immunol 2016; 16:207–19. [DOI] [PubMed] [Google Scholar]
- 5. Bhardwaj N, Montesion M, Roy F et al Differential expression of HERV‐K (HML‐2) proviruses in cells and virions of the teratocarcinoma cell line Tera‐1. Viruses 2015; 7:939–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hohn O, Hanke K, Bannert N. HERV‐K (HML‐2), the best preserved family of HERVs: endogenization, expression, and implications in health and disease. Front Oncol 2013; 3:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Trela M, Nelson PN, Rylance PB. The role of molecular mimicry and other factors in the association of Human Endogenous Retroviruses and autoimmunity. APMIS 2016; 124:88–104. [DOI] [PubMed] [Google Scholar]
- 8. Krzysztalowska‐Wawrzyniak M, Ostanek M, Clark J et al The distribution of human endogenous retrovirus K‐113 in health and autoimmune diseases in Poland. Rheumatology (Oxford) 2011; 50:1310–4. [DOI] [PubMed] [Google Scholar]
- 9. Reynier F, Verjat T, Turrel F et al Increase in human endogenous retrovirus HERV‐K (HML‐2) viral load in active rheumatoid arthritis. Scand J Immunol 2009; 70:295–9. [DOI] [PubMed] [Google Scholar]
- 10. Freimanis G, Hooley P, Ejtehadi HD et al A role for human endogenous retrovirus‐K (HML‐2) in rheumatoid arthritis: investigating mechanisms of pathogenesis. Clin Exp Immunol 2010; 160:340–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nelson PN, Roden D, Nevill A et al Rheumatoid arthritis is associated with IgG antibodies to human endogenous retrovirus gag matrix: a potential pathogenic mechanism of disease? J Rheumatol 2014; 41:1952–60. [DOI] [PubMed] [Google Scholar]
- 12. Erre GL, Cossu D, Masala S et al Mycobacterium tuberculosis lipoarabinomannan antibodies are associated to rheumatoid arthritis in Sardinian patients. Clin Rheumatol 2014; 33:1725–9. [DOI] [PubMed] [Google Scholar]
- 13. Balada E, Vilardell‐Tarrés M, Ordi‐Ros J. Implication of human endogenous retroviruses in the development of autoimmune diseases. Int Rev Immunol 2010; 29:351–70. [DOI] [PubMed] [Google Scholar]
- 14. Tugnet N, Rylance P, Roden D et al Human endogenous retroviruses (HERVs) and autoimmune rheumatic disease: is there a link? Open Rheumatol J 2013; 7:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Erre GL, Mameli G, Cossu D et al Increased Epstein–Barr virus DNA load and antibodies against EBNA1 and EA in Sardinian patients with rheumatoid arthritis. Viral Immunol 2015; 28:385–90. [DOI] [PubMed] [Google Scholar]
- 16. Nelson PN, Carnegie PR, Martin J et al Demystified. Human endogenous retroviruses. Mol Pathol 2003; 56:11–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neidhart M, Rethage J, Kuchen S et al Retrotransposable L1 elements expressed in rheumatoid arthritis synovial tissue: association with genomic DNA hypomethylation and influence on gene expression. Arthritis Rheum 2000; 43:2634–47. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Correlations between anti‐endogenous retrovirus (HERV)‐K su19–37 antibodies and rheumatoid arthritis (RA) descriptors
Table S2. Raw data of serum response of rheumatoid arthritis (RA) patients and HC against endogenous retrovirus (HERV)‐K peptides


