Summary
Oral tolerance refers to the specific inhibition of immune responsiveness to T‐cell‐dependent antigens contacted through the oral route before parenteral immunization. Oral tolerance to one protein does not inhibit immune responses to other unrelated proteins, but parenteral injection of tolerated antigens plus adjuvant into tolerant, but not normal, mice inhibits immune responses to antigens injected concomitantly or soon thereafter. The inhibitory effect triggered by parenteral injection of tolerated proteins is known as bystander suppression or indirect effects of oral tolerance. Intraperitoneal injection of ovalbumin (OVA) plus alum adjuvant in OVA‐tolerant mice soon before skin injury inhibits inflammation and improves cutaneous wound healing. However, as OVA is not a regular component of mouse chow, we tested whether indirect effects could be triggered by zein, the main protein of corn that is regularly present in mouse chow. We show that intraperitoneal injection of a single dose (10 μg) of zein plus alum adjuvant soon before skin injury in mice reduces leucocyte infiltration but increase the number of T cells and the expression of resistin‐like molecule‐α (a marker of alternatively activated macrophages) in the wound bed, increases the expression of transforming growth factor‐β 3 in the newly formed epidermis and reduces cutaneous scar formation. These results suggest that indirect effects of oral tolerance triggered by parenteral injection of regular dietary components may be further explored as one alternative way to promote scarless wound healing.
Keywords: cutaneous scar, immunological tolerance, inflammation, mucosa, skin wound healing
Abbreviations
- Al(OH)3
aluminium hydroxide
- H&E
haematoxylin and eosin
- IL
interleukin
- OVA
ovalbumin
- RELM‐α
resistin‐like molecule α
- SMA
smooth muscle actin
- TGF
transforming growth factor
Introduction
The absorption of intact or partially digested proteins through the intestinal mucosa is a common occurrence after meals and may trigger anaphylactic reactions.1 However, the most common immunological consequence of protein absorption by the oral route is oral tolerance or stabilization of specific serum antibody levels.2, 3 At the beginning of the twentieth century it was observed that animals previously fed with diets containing corn failed to display anaphylactic symptoms upon injections of corn proteins.4 Similar observations were also reported with milk proteins.5 Oral tolerance is a specific T‐cell‐dependent phenomenon and ingestion of one protein does not block immunization to unrelated proteins.6 However, the re‐exposure to orally tolerated proteins through the parenteral route inhibits immunization to other, unrelated, proteins injected together or soon thereafter,7, 8, 9, 10 and blocks non‐specific inflammation.11 The inhibitory phenomenon triggered by parenteral injection of tolerated proteins is known as indirect effects of oral tolerance or bystander suppression.8, 12 Indirect effects of intraperitoneal injection of zein block concomitantly immunization with ovalbumin (OVA) + Al(OH)3 in naive mice.10 In addition, parenteral injection of OVA plus adjuvant into OVA‐tolerant mice inhibits acute inflammatory reactions, such as paw oedema induced by carrageenan11 and also blocks granuloma formation around Schistosoma mansoni eggs.13, 14 Furthermore, intraperitoneal injection of OVA into OVA‐tolerant mice, minutes before skin wound, reduces leucocyte infiltration in the wound bed and results in scarless wound healing.15, 16
Scar formation normally occurs after skin wound in adult mammals but complete regeneration of skin is a frequent outcome after injury in fetal mammals.17, 18, 19 Skin regeneration in fetal mammals has been associated with a small inflammatory infiltrate, increase in transforming growth factor‐β 3 (TGF‐β 3) level and differential expression of extracellular matrix components.17, 19, 20, 21 In adult mammals, scarless healing is associated with mild inflammation after injury and the increase in the levels of growth factors or cytokines associated with alternatively activated macrophages.21, 22, 23 On the other hand, excessive inflammation may result in a hypertrophic scar or in chronic ulcers.22, 24 Unfortunately, clinical problems associated with impaired wound healing and excessive scarring are still difficult to manage.19 The search for new strategies to deal with these important clinical problems needs to be encouraged.
We previously showed that intraperitoneal injection of OVA in OVA‐orally tolerant mice, minutes before skin wound, promotes scarless wound healing associated with improvement in the pattern of collagen deposition in the neodermis and reduced number of mast cells and granulocytes in the wound bed.15 Because corn is regularly used as a dietary component, we examined whether injection of zein promotes scarless wound healing.
Materials and methods
Animals
Eight‐week‐old male C57BL/6 mice were obtained from Universidade Federal de Minas Gerais, Brazil. The animal care and handling procedures were in accordance with the guidelines of the Institutional Animal Care and Use Committee, and the study received approval from the local animal ethics committee (Animal Ethics Review Board – Comitê de Ética em Experimentação Animal/Universidade Federal de Minas Gerais). All mice were fed a standard rodent chow that contained corn in its composition (Nuvilab CR‐ 1, Nuvital Nutrientes S/A, São Paulo, Brazil). Each group contained six mice per time‐point.
Parenteral injection of dietary protein
Purified zein (Sigma, St Louis, MO) was used for intraperitoneal immunization. The experimental group (zein group) received one intraperitoneal injection of 0·20 ml of 10 μg zein plus 1·6 mg Al(OH)3 immediately before the wounding procedure. One control group received 1·6 mg Al(OH)3 and the other control group received saline by intraperitoneal injection.
Wounding
Mice were anaesthetized with ketamine (97 mg/kg) and xylazine (15 mg/kg) and their dorsal thoracic skin was shaved and cleaned with 70% ethanol before wounding. Two circular through‐and‐through full‐thickness excisional wounds (each with 6·5‐mm diameter, 0·33‐cm2 area) were made by picking up a fold of skin and using a biopsy punch, resulting in one wound on each side of the midline. The wounds were left unsutured and without dressing. All animals were housed individually to prevent traumatic damage to the wounds by other mice.
Macroscopic analysis
Wounds or the healed area were photographed with an in‐picture ruler for scale using a digital camera (Sony DSC‐F717, Japan) immediately after or at 1, 3, 5, 7 and 40 days post‐wounding. The images were imported into image analysis software (image tool 3·0 http://compdent.uthscsa.edu/dig/itdesc.html) and wound outlines were manually traced for calculation of wound area and scar area.
Histology
Mice were killed by lethal doses of anaesthetics at 7 or 40 days, shaved when necessary, and the skin with the lesions was dissected. One of the lesions was fixed in Carson's modified Millonig's phosphate‐buffered formalin for 24 hr, perpendicularly sectioned in its half and the separate pieces were dehydrated in ethanol and embedded in paraffin for histological studies following standard protocols. Serial 5‐μm transverse sections from the middle of the wound were stained with haematoxylin & eosin (H&E), toluidine blue or Gomori's trichrome. Each group contained six mice per time‐point and we analysed one section per wound, per mouse, per time‐point, resulting in six sections per time‐point. Digital images of tissues were obtained using an automatic digital slide scanner (Pannoramic MIDI, 3DHISTECH Ltd., Budapest, Hungary).
Morphometry
Histological sections were examined under light microscopy using an intersection grid placed at the ocular lens, at high magnification (1000×). Fibroblasts and leucocytes were identified by their characteristic morphology in H&E‐stained sections: the nuclei of fibroblasts are elongated and can be quite condensed. Most of the leucocytes are rounded cells with variable nuclei forms. Mast cells were identified after toluidine blue staining. Leucocytes, fibroblasts and mast cells were counted in 10 fields of 10 000 μm2 within the wound healing area of one section per mouse and the results from six sections per group were expressed as the mean ± SEM. Each microvessel was defined as a lumen surrounded by a rim of endothelial cells, clearly separated from adjacent microvessels. The numbers of vessels were determined in 12 fields of 10 000 μm2, within the wound healing area of one section per mouse and the results from six sections per group were expressed as the mean ± SEM.
Immunostaining and confocal microscopy
One of the lesions was dissected, washed in PBS, placed over Waterman paper to avoid folds and fixed and cryosubstituted in −80° solution containing 80% methanol and 20% dimethyl sulphoxide for 6 days, transferred to −20° for 1 day, and then brought to room temperature. Samples were rinsed three times in absolute ethanol, twice in xylene and embedded in paraplast. Five‐micrometre transverse sections from the middle of the wound were mounted on slides, deparaffinized, rehydrated and incubated in blocking solution (1% BSA and 0·1% Tween 20 in PBS) at room temperature for 1 hr. Immunofluorescence‐labelling and quantitative confocal microscopy were used to investigate the distribution and quantity of leucocytes (CD45+), T cells (CD3+), B cells (CD45R+), macrophages (F4/80+), alternatively activated macrophages [resistin‐like molecule‐α (RELM‐α)+], myofibroblasts [α‐smooth muscle actin (SMA)+] and TGF‐β 3. Sections were incubated overnight at 4° with the following primary antibodies: allophycocyanin‐conjugated rat anti‐CD45 (BD Biosciences, bdbiosciences.com), Alexa Fluor® 647 anti‐mouse F4/80 (Invitrogen, Carlsbad, CA), unlabelled rat anti‐mouse‐CD3 (BD Pharmingen™, Franklin Lakes, NJ), unlabelled rat anti‐CD45R (Sigma‐Aldrich, St Louis, MO), unlabelled rabbit anti‐RELM‐α (Abcam, Cambridge, MA), unlabelled mouse anti‐α‐SMA (Sigma‐Aldrich) and unlabelled rabbit anti‐TGF‐β 3 (Santa Cruz Biotechnology, Inc., Santa Cruz CA). After five rinses in PBS, when necessary, sections were incubated for 1 hr at room temperature in the dark with the following secondary antibodies: Alexa Fluor® 488 goat anti‐mouse IgG2a (γ2a), Alexa Fluor® 488 goat anti‐rat IgG (H+L) or Alexa Fluor® 488 goat anti‐rabbit IgG (H+L) (Molecular Probes, Eugene, OR). After five rinses in PBS, sections were mounted in a mixture of 10% 1·0 m Tris–HCl, pH 9·0 and 90% glycerol and viewed using a laser scanning confocal microscope (Zeiss 510META; Carl Zeiss AG, Oberkochen, Germany). Optimal confocal settings (aperture, gain and laser power) were determined at the beginning of each imaging session and then held constant during the analysis of all samples. Nuclei were labelled with 4′6‐diamidino‐2‐phenylindole dihydrochloride (DAPI) (Molecular Probes, Carlsbad, CA). Images were captured at 12 bits and analysed in the grey‐scale range of 0–255 with image tool 3·0 software (http://compdent.uthscsa.edu/dig/itdesc.html). Fluorescence intensity was recorded as the sum of grey values of all pixels divided by the area (in μm2) × 10−3. Background fluorescence was measured in each sample and subtracted from the values obtained for the fluorescence intensity. We captured five images per section in different areas of wound bed.
Cytokine analysis in skin extracts
For cytokine analysis, the experimental group treated as described above (zein group) was compared with the control saline group (n = 6 mice per group, per time‐point). The areas of skin containing the lesion were collected at 12 hr, 1 day, 3 days and 7 days after lesion, homogenized, centrifuged and stored at −80° until they were processed for cytokine analyses. We used the kit Mouse Th1/Th2 10plex (Mouse, Ready‐to‐Use Flow Cytomix Multiplex, eBioscience Inc., San Diego, CA) for quantitative detection of mouse granulocyte–macrophage colony‐stimulating factor, interferon‐γ, interleukin‐1α (IL‐1α), IL‐2, IL‐4, IL‐5, IL‐6, IL‐10, IL‐17 and tumour necrosis factor‐α, according to the manufacturer's protocol and read in an FL3 channel FACSCalibur™ flow cytometer (BD Bisociences). Level of TGF‐β was measured using an immunoassay kit from R&D Systems (Minneapolis, MN), following the manufacturer's protocol. Absorbance was measured at 492 nm using an ELISA reader (Bio‐Rad Model 450).
Statistical analysis
The statistical significance of differences between groups was determined using one‐way analysis of variance, followed by the Student–Newman–Keuls test, using graphpad prism (GraphPad Software, San Diego, CA). Values of P ≤ 0·05 were considered significant. The results are expressed as mean ± SEM.
Results
The effect of intraperitoneal injection of zein + Al(OH)3 upon skin wound healing was evaluated macroscopically and microscopically. Macroscopic qualitative analysis showed reduced oedema around lesions and reduced scabs in the zein group compared with both saline and adjuvant control groups (Fig. 1a). In the first 5 days, there was no significant difference in the wound area between groups, but at day 7 the wound area in mice injected with zein was smaller than the wound area in mice injected with adjuvant (Fig. 1b).
Figure 1.
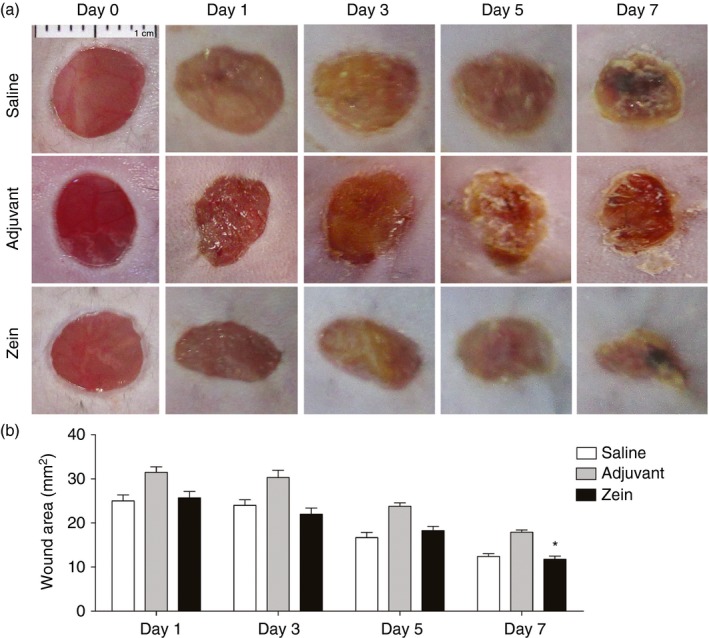
Intraperitoneal injection of zein soon before skin injury reduces scab. (a) Macroscopic appearance of wounds immediately after and at days 1, 3, 5 and 7 after skin injury in mice injected with saline, adjuvant or zein + adjuvant immediately before wounds. (b) Wound healing area at days 1, 3, 5 and 7 after skin injury. Data represent mean ± SEM. *P ≤ 0·05 compared with adjuvant group; n = 6 mice, 12 wounds. [Colour figure can be viewed at wileyonlinelibrary.com]
Qualitative histological analysis of H&E‐stained section at day 7 after wounding suggested that injection of zein reduced the wound granulation tissue and the inflammatory infiltrate in the wound bed (Fig. 2a). Besides, in control groups, a higher amount of inflammatory cells was present in areas adjacent to the injured area (Fig. 2a).
Figure 2.
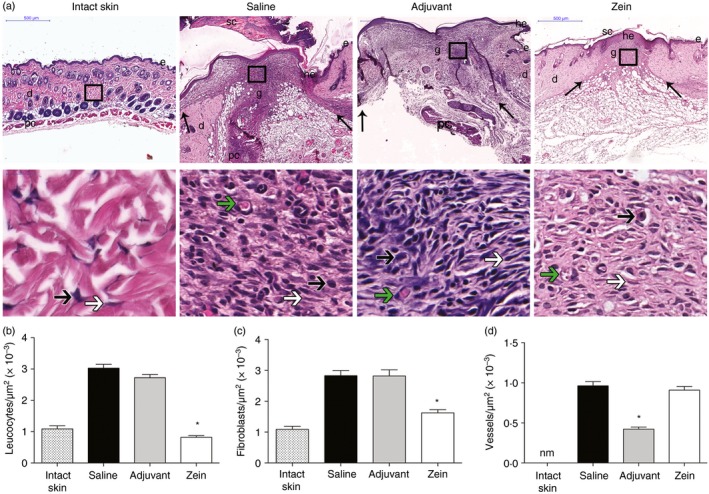
Injection of zein before skin injury reduces granulation tissue and preserves angiogenesis. (a) Haematoxylin & eosin staining of intact skin and wounds at day 7 after skin injury in mice injected with saline, adjuvant or zein + adjuvant. The arrows in upper panels indicate the limits of granulation tissue area and small letters represent: d, dermis; e, epidermis; g, granulation tissue; he, hypertrophic epidermis; sc, scab; pc, panniculus carnosus. Boxes in the upper panels indicate the area where the regions are shown in higher magnification to illustrate leucocytes (black arrows), fibroblasts (white arrows) and microvessels (green arrows). Scale bars: upper panels, 500 µm; lower panels, 20 μm. Morphometric analysis of leucocytes (b), fibroblasts (c) and microvessels (d). Data represent mean ± SEM *P ≤ 0·05 compared with saline group, n = 6. [Colour figure can be viewed at wileyonlinelibrary.com]
Morphometric analysis of sections stained with H&E showed reduced numbers of leucocytes (Fig. 2b) and of fibroblasts (Fig. 2c) in the wound bed of the zein group compared with the saline and adjuvant control groups. No difference was found in the number of vessels in the granulation tissue area of mice injected with zein compared with mice injected with saline. However, mice injected with only adjuvant before the wound showed a lower number of vessels than mice injected with saline (Fig. 2d).
We used immunofluorescence with anti‐CD45 (Fig. 3a,d) and anti‐α‐SMA (Fig. 3b,e) to evaluate the presence of leucocytes and myofibroblasts in the wound bed. In mice injected with zein there was reduced expression of CD45 and α‐SMA in the wound bed, compared with mice injected with saline or adjuvant. Mast cells were found in small numbers in mice injected with zein (Fig. 3c,f). These results indicated that injection of zein soon before the skin injury produces an anti‐inflammatory effect and impairs the differentiation of myofibroblasts.
Figure 3.
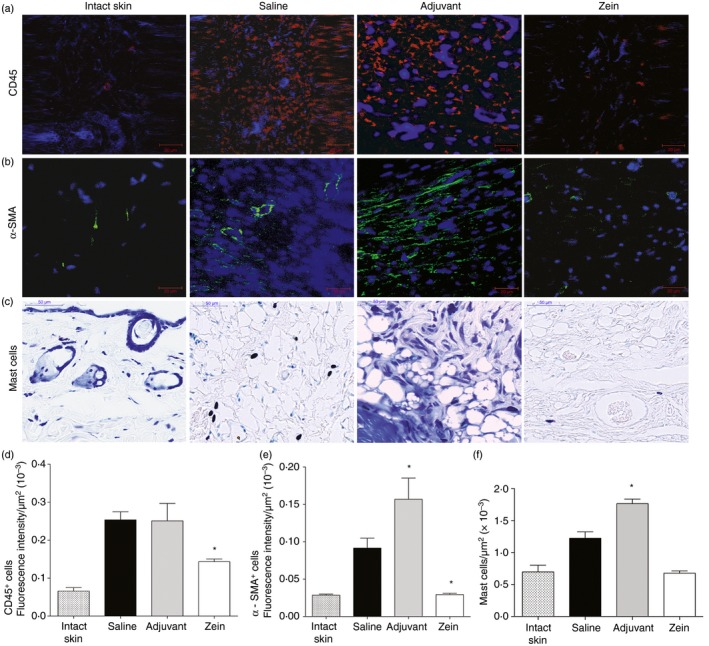
Injection of zein before skin injury reduces the expression of CD45 (leucocytes), α‐smooth muscle actin (SMA) (myofibroblasts) and the number of mast cells in the wound bed. (a,b) Immunostaining of intact skin and wounds, at day 7 after skin injury, with anti‐CD45 or with anti‐α‐SMA; nuclear counterstaining with 4′6‐diamidino‐2‐phenylindol (blue); (c) toluidine blue‐stained sections. The wounds are from mice injected with saline, adjuvant or zein + adjuvant minutes before skin injury. Data represent mean ± SEM of fluorescence intensity (in μm2 × 10−3) in sections immunostained with CD45 (d) or α‐SMA (e); and morphometric analysis of mast cells (f). *P ≤ 0·05 compared with saline group, n = 6. [Colour figure can be viewed at wileyonlinelibrary.com]
We compared the levels of 11 different cytokines in the wound bed at 12 hr, 1 day, 3 days and 7 days after lesion in mice injected with saline or zein (Fig. 4). No significant difference in IL‐10, interferon‐γ, tumour necrosis factor‐α, granulocyte–macrophage colony‐stimulating factor and IL‐5 was observed between groups at all times analysed. However, in mice injected with zein, IL‐6 and IL‐2 were decreased at 12 hr after injury; at day 1 after injury and zein injection, IL‐1α, IL‐4 and IL‐17 were increased; and at day 3 after injury and zein injection, IL‐17 was reduced and TGF‐β 1 was increased.
Figure 4.
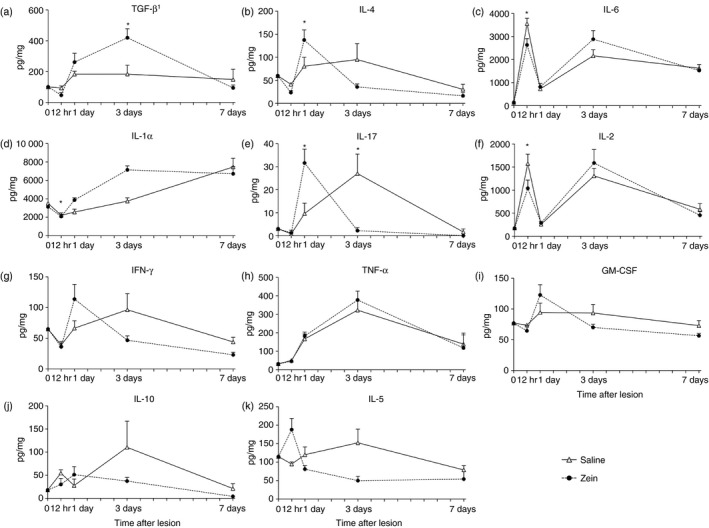
Kinetic analysis of cytokines in skin wound bed. Cytokines were analysed in intact skin (day 0) and in wounds from mice injected with saline (open triangle) or mice injected with zein (black circle), at 12 hr, and days 1, 3 and 7 after skin injury. Data represent mean ± SEM (six mice/group/time point). *P ≤ 0·05 compared with saline group at the same time after skin injury.
Using immunofluorescence, we compared the wounds of mice injected with zein with those of control groups for the presence of alternatively activated macrophages (RELM‐α +), T lymphocytes (CD3+), B lymphocytes (CD45R+) and TGF‐β 3. Figure 5 shows that injection of zein resulted in increased expression of RELM‐α, increased expression of CD3 and increased expression of TGF‐β 3 in the wound bed. B lymphocytes were not found in the wound bed of any group (not shown).
Figure 5.
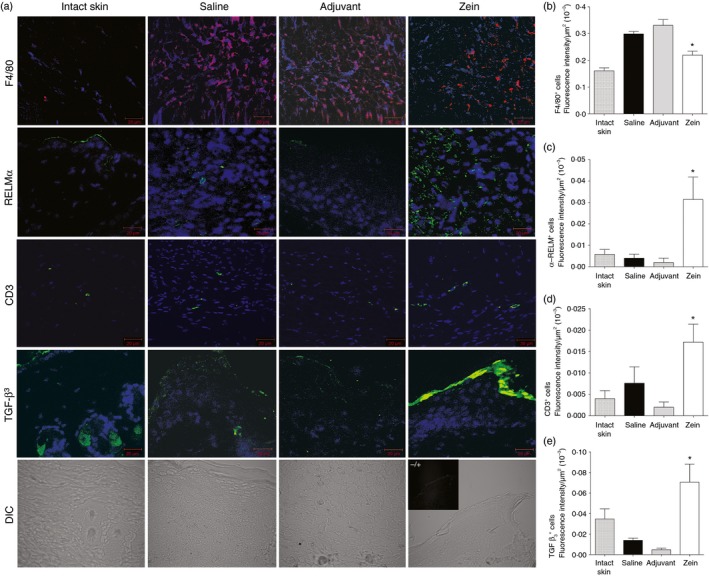
Injection of zein before skin injury increases the expression of resistin‐like molecule α (RELM‐α; alternatively activated macrophages), CD3 and transforming growth factor β 3 (TGF‐β 3) in the wound bed. (a) Immunostaining of intact skin and wounds, at day 7 after skin injury, with specific antibodies and nuclear counterstaining with 4′6‐diamidino‐2‐phenylindol (blue). Photomicrographs in the bottom line illustrate typical sections of each group imaged using differential interference contrast (DIC) and one insert with a picture of control staining lacking the primary antibody. Data represent mean ± SEM of fluorescence intensity (in μm2 × 10−3) in sections immunostained with anti‐F4/80 (b), anti‐RELM‐α (c), anti‐CD3 (d) or anti‐TGF‐β 3 (e). *P ≤ 0·05 compared with saline group, n = 6. [Colour figure can be viewed at wileyonlinelibrary.com]
The healed areas were compared at day 40 after lesion, to analyse the effect of zein injection in the remodelling phase of wound healing. Figure 6 shows that mice injected with zein have improved scarring compared with control mice that received saline or adjuvant. In mice injected with adjuvant without zein the scar area was more prominent than in mice of the other groups (Fig. 6a,b). The pattern of collagen organization in the reduced scar of mice injected with zein was more similar to the pattern of collagen found in intact skin (Fig. 6c).
Figure 6.
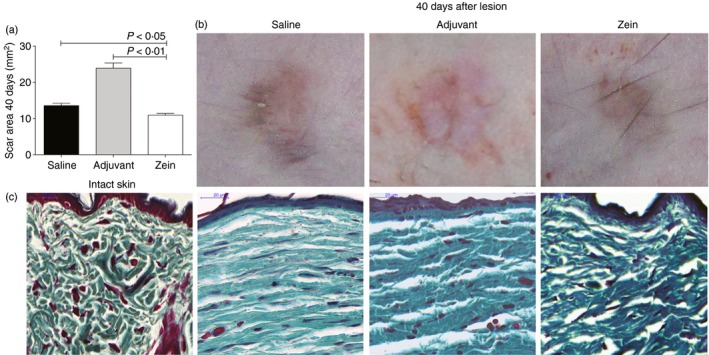
Zein reduces cutaneous scarring and improves extracellular matrix remodelling. (a) Scar area and (b) representative pictures of dorsal skin 40 days after skin injury in control mice (saline and adjuvant groups) and in mice injected with zein. (c) Sections of intact skin or wounds harvested 40 days after skin injury stained with Gomori's trichrome. Scale bar 20 μm. n = 6. [Colour figure can be viewed at wileyonlinelibrary.com].
Discussion
The role of different types of inflammatory cells in wound repair and in regeneration is not totally clear. Inflammation is beneficial to some aspects of the healing process but if in excess it may hinder the healing or generate hypertrophic scars.17, 25, 26
Previous work has shown that parenteral immunization with OVA in mice that received OVA by the oral route for 3 consecutive days, inhibits inflammatory reactions to unrelated antigens,11, 13 reduces the inflammation during skin wound healing and produces scarless healing.15, 16 Herein, we show similar results after injection of zein, an abundant protein of corn that is present in the regular mouse chow. The mice used in the experiments reported here were fed with rodent chow containing zein, from weaning. Moreover, zein was present in the maternal diet and may have influenced the ontogeny of the pups’ immune system, as it occurs with other food proteins.27 The form of exposure to zein, therefore, lacks the episodic character of previous experimental models using OVA.15, 16 However, injection of either OVA15 or zein (present results) before excisional injury in mice that were previously exposed to these proteins by the oral route reduced inflammatory cell infiltration in the wound healing area and resulted in scarless healing with collagen fibres in the neodermis organized in a pattern more similar to the intact skin. Additionally, the macroscopic appearance of the scar was slightly better than that of the control wound.
Recent studies using different types of mast‐cell‐deficient mice concluded that mast cells do not play a significant non‐redundant role in skin wound healing and fibrosis.28, 29 However, similarly to what was found after OVA injection in OVA‐tolerant mice,15, 16 in mice fed with zein the number of mast cells is reduced by injection of zein before skin injury. We cannot exclude the possibility that the reduced number of mast cells in the wound‐healing area of mice injected with the tolerated protein release mediators or cytokines that differently influence the wound healing process.
B lymphocytes (CD45R+) were not detected in our study and these cells were not detected in the wounded skin by other authors30 who used the same wound healing model as us. As suggested by Nishio et al.,30 it is possible that B cells do not directly participate in the healing process, but may be indirectly taking part in this process through their secreted products. Studies in human dermal wound healing also found low numbers of B cells in the inflammatory infiltrate.31, 32 On the other hand, the number of T lymphocytes increases after injection of tolerated proteins (zein, present results, or OVA15). Using immunohistochemistry, we think it will be hard to assess the profile of T lymphocytes infiltrated into the wound bed because they are at a low frequency. The use of flow cytometry to identify lymphocyte subtypes should be considered in future studies.
In mice orally tolerant to OVA and injected with OVA before skin injury, it was shown that the number of M2 macrophages tended to be higher than in control mice, but that difference was not statistically significant.15 However, injection of zein produced a significant increase in M2 macrophages. It is possible that the tolerance induced by zein, which derives from the prolonged ingestion of this protein, produced a rapid and stronger trophic effect on cells involved in the healing process than the effect produced by tolerance to OVA. The differences in strategies to induce oral tolerance, i.e. in the form of administration of antigen, either by gavage or continuous feeding, and variations in the dose of antigens, have been shown to influence the immune parameters analysed.33, 34 So, it is not easy to compare the cytokine profiles that result from zein injection with that produced by OVA injection before wounding in tolerant mice. Yet, similarly to the injection of zein, the injection of OVA in mice orally tolerant to OVA produced scarless healing that was not associated with a simple shift in T helper type 1/type 2 cytokines.15
The injection of zein significantly decreased the expression of IL‐2 and IL‐6 at 12 hr after injury; it increased the expression of IL‐4, and IL‐17 at 24 hr after injury and, at day 3 after injury, zein injection increased the expression of TGF‐β 1 and decreased the expression of IL‐17. Taken together with the morphological analysis of wound healing at day 7 after injury, our results suggest that zein injection turned the wound healing medium into a more trophic one with a more alternatively activated macrophages and increased expression of TGF‐β 3.
In animals injected with zein, IL‐4 increases in the healing area at the first day of wounding. Interleukin‐4 strongly activates type‐2 macrophages that produce anti‐inflammatory cytokines such as IL‐10 and TGF‐β 1.35, 36 There was no significant difference in IL‐10 levels but TGF‐β 1 was transiently increased at day 3 after injury. Interleukin‐17 is also increased on the first day. The main producers of IL‐17 in the skin are γδ T cells,37 that also secrete keratinocyte growth factors and can enhance the proliferation of keratinocytes after injury.38 The rapid and transient increase in IL‐17 may be involved in improved wound healing in animals that received the injection of zein before wounding. These results suggest that injection of zein before injury produces a faster increase in inflammatory cytokines rapidly followed by increase in trophic cytokines.
The transient increase in TGF‐β 1 at day 3 after wounding in the zein‐treated mice may be related to improved wound healing because the literature reports that TGF‐β 1 triggers the expression of the integrins required for keratinocyte migration through the fibronectin‐rich temporary matrix.39, 40 Before re‐epithelialization, TGF‐β 1 acts as a wound‐healing promoting factor, but if in excess it may lead to hypertrophic scarring and keloid as fibroblasts are activated to differentiate into myofibroblasts mainly by interaction with TGF‐β 1 and TGF‐β 2.41, 42
Despite the transient increase in TGF‐β 1 in mice injected with zein, this treatment impairs the increase of myofibroblasts in the wound bed. Decrease in myofibroblasts and increase in TGF‐β 3 are characteristic of fetal skin wound regeneration.
In general, there is no significant expression of TGF‐β 3 during wound healing in adult mammals but exogenous addition of this TGF‐β isoform in the healing skin wounds of adult rodents reduces cutaneous scarring.43 On the other hand, in mammalian fetuses, which are able to regenerate skin structures, TGF‐β 3 is found in high concentrations during wound healing.17, 18 It is interesting that, upon parenteral injection of zein, the expression of TGF‐β 3 in keratinocytes of the neo‐epidermis is much higher than in control groups.
Transforming growth factor‐β 3 is also important to promote angiogenesis. Shah et al., showed that wounds in adult rats treated with TGF‐β 3 presented increased angiogenesis compared with control wounds.43 In our study, angiogenesis in the wound bed of mice treated with zein plus adjuvant was not different from control wounds in mice injected with saline but, in zein‐treated mice the wounds were more vascular than wounds in mice treated with only adjuvant.
Recent studies have revealed the heterogeneous population of macrophage that contributes to cutaneous wound healing.44, 45, 46 These cells have plastic phenotypes and their actions vary according to the context where they are inserted and the stimulus that triggered their differentiation.45 So, the phenotype of macrophages may vary during the wound healing process, where the inflammatory phase is richer in M1 macrophages and the granulation phase is richer in alternatively activated (M2) macrophages.22 The increase in M2 macrophages in mice injected with zein is consistent with the higher amount in IL‐4, one of the cytokines that triggers the differential activation of macrophages engaged in wound healing.36
The mechanisms of the anti‐inflammatory effects triggered by the injection of tolerated antigens are unknown. The most popular explanation, called innocent bystander effect8 was contradicted by several of our previous experiments.12 Traditionally seen as specific inhibition of immune responsiveness, oral tolerance is actually an expression of a steady state in immune responsiveness.2, 3 Tolerance to self‐components in normal animals occurs despite the presence of small amounts of autoantibodies, but these antibodies remain stable in the presence of their respective specific self‐components.47 Similar to ‘self tolerance’, higher lymphocyte activity and cytokine production occurs in orally tolerant mice alongside specific inhibition.2 So, the effects triggered by the injection of tolerated antigens may be an aspect, still not understood, of the robust physiological stability of antibody formation to dietary proteins, similar to the steady‐state displayed in the formation of autoantibodies, called ‘self tolerance’.
In conclusion, we show that intraperitoneal immunization with a dietary protein (zein) before skin injury reduces the cutaneous scarring, without retarding the healing process. Other routes for parenteral administration of the tolerated protein will need to be tested aimed at future clinical applications. However, our results suggest that parenteral injection of tolerated proteins may be explored as an alternative way of intervening in the inflammatory phase of wound healing, which has consequences for the subsequent phases. This is a promising strategy, mainly in medical conditions where surgical interventions can be programmed. In addition, parenteral injection of tolerated protein may also be explored as a model to study the interactions of leucocytes, keratinocytes and fibroblasts that result in scarless healing.
Acknowledgements
Research grants were provided by Fundação de Amparo a Pesquisa de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, Brazil) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). Cláudia R Carvalho received a fellowship from CNPq. Digital images of tissues were obtained using an automatic digital slide scanner and confocal microscope at the Centre of Acquisition and Processing of Images, Universidade Federal de Minas Gerais, Brazil.
Author contribution
T.A.C. performed the experiments, analysed the data and wrote the paper. K.S.S. helped in wounding experiments and histopathological analysis. R.A.C. co‐supervised the study and contributed to analysis of immunohistochemistry data. N.M.V. contributed to writing the paper and is senior author. C.R.C. designed and supervised the research and wrote the paper.
Disclosures
The authors declared no conflict of interest.
References
- 1. Moran TP, Burks AW. Is clinical tolerance possible after allergen immunotherapy? Curr Allergy Asthma Rep 2015; 15:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Castro‐Junior AB, Horta BC, Gomes‐Santos AC, Cunha AP, Silva Steinberg R, Nascimento DS et al Oral tolerance correlates with high levels of lymphocyte activity. Cell Immunol 2012; 280:171–81. [DOI] [PubMed] [Google Scholar]
- 3. Verdolin BA, Ficker SM, Faria AM, Vaz NM, Carvalho CR. Stabilization of serum antibody responses triggered by initial mucosal contact with the antigen independently of oral tolerance induction. Braz J Med Biol Res 2001; 34:211–9. [DOI] [PubMed] [Google Scholar]
- 4. Wells HG, Osborne TB. The biological reactions of the vegetable proteins. J Infect Dis 1911; 8:66–124. [Google Scholar]
- 5. Besredka A. De l'anaphylaxie. Sixiéme memoire de l'anaphylaxie lactique. Ann Inst Pasteur 1909; 23:166–74. [Google Scholar]
- 6. Hanson DG, Vaz NM, Maia LCS, Hornbrook MM, Lynch JL, Roy CA. Inhibition of specific immune responses by feeding protein antigens. Int Arch Allergy Appl Immunol 1977; 55:526–32. [DOI] [PubMed] [Google Scholar]
- 7. Vaz NM, Hanson DG, Maia LCS, Lynch JL. Cross‐suppression of specific immune responses after oral tolerance. Mem Inst Oswaldo Cruz 1981; 76:83–91. [DOI] [PubMed] [Google Scholar]
- 8. Miller A, Lider O, Weiner HL. Antigen driven bystander suppression after oral administration of antigen. J Exp Med 1991; 174:791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Carvalho CR, Verdolin BA, de Souza AV, Vaz NM. Indirect effects of oral tolerance in mice. Scand J Immunol 1994; 39:533–8. [DOI] [PubMed] [Google Scholar]
- 10. Carvalho CR, Vaz NM. Indirect effects are independent of the way of tolerance induction. Scand J Immunol 1996; 43:613–8. [DOI] [PubMed] [Google Scholar]
- 11. Ramos GC, Rodrigues CM, Azevedo GM Jr, Pinho V, Carvalho CR, Vaz NM. Cell‐mediated immune response to unrelated proteins and unspecific inflammation blocked by orally tolerated proteins. Immunology 2009; 126:354–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Carvalho CR, Verdolin BA, Vaz NM. Indirect effects of oral tolerance cannot be ascribed to bystander suppression. Scand J Immunol 1997; 45:276–81. [DOI] [PubMed] [Google Scholar]
- 13. Azevedo GM Jr, Costa RA, Resende MA, Rodrigues CM, Vaz NM, Carvalho CR. Indirect effects of oral tolerance inhibit pulmonary granulomas to Schistosoma mansoni eggs. Clin Dev Immunol 2012; 2012:293625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carvalho CR, Lenzi HL, Correa‐Oliveira R, Vaz NM. Indirect effects of oral tolerance to ovalbumin interfere with the immune responses triggered by Schistosoma mansoni eggs. Braz J Med Biol Res 2002; 35:1195–9. [DOI] [PubMed] [Google Scholar]
- 15. Costa RA, Matos LB, Cantaruti TA, de Souza KS, Vaz NM, Carvalho CR. Systemic effects of oral tolerance reduce the cutaneous scarring. Immunobiology 2016; 221:475–85. [DOI] [PubMed] [Google Scholar]
- 16. Costa RA, Ruiz‐de‐Souza V, Azevedo GM Jr, Gava E, Kitten GT, Vaz NM et al Indirect effects of oral tolerance improve wound healing in skin. Wound Repair Regen 2011; 19:487–97. [DOI] [PubMed] [Google Scholar]
- 17. Ferguson MW, O'Kane S. Scar‐free healing: from embryonic mechanisms to adult therapeutic intervention. Philos Trans R Soc Lond B Biol Sci 2004; 359:839–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rolfe KJ, Grobbelaar AO. A review of fetal scarless healing. ISRN Dermatol 2012; 2012:698034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leavitt T, Hu MS, Marshall CD, Barnes LA, Lorenz HP, Longaker MT. Scarless wound healing: finding the right cells and signals. Cell Tissue Res 2016; 365:483–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lichtman MK, Otero‐Vinas M, Falanga V. Transforming growth factor β (TGF‐β) isoforms in wound healing and fibrosis. Wound Repair Regen 2016; 24:215–22. [DOI] [PubMed] [Google Scholar]
- 21. Walraven M, Talhout W, Beelen RH, van Egmond M, Ulrich MM. Healthy human second‐trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen 2016; 24:533–41. [DOI] [PubMed] [Google Scholar]
- 22. Willenborg S, Eming SA. Macrophages – sensors and effectors coordinating skin damage and repair. J Dtsch Dermatol Ges 2014; 12:214–21, –23. [DOI] [PubMed] [Google Scholar]
- 23. Larson BJ, Longaker MT, Lorenz HP. Scarless fetal wound healing: a basic science review. Plast Reconstr Surg 2010; 126:1172–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martin P, Leibovich SJ. Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 2005; 15:599–607. [DOI] [PubMed] [Google Scholar]
- 25. Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007; 127:514–25. [DOI] [PubMed] [Google Scholar]
- 26. Martin P. Wound healing – aiming for perfect skin regeneration. Science 1997; 276:75–81. [DOI] [PubMed] [Google Scholar]
- 27. Fusaro AE, de Brito CA, Taniguchi EF, Muniz BP, Victor JR, Orii NM et al Balance between early life tolerance and sensitization in allergy: dependence on the timing and intensity of prenatal and postnatal allergen exposure of the mother. Immunology 2009; 128(1 Suppl):e541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nauta AC, Grova M, Montoro DT, Zimmermann A, Tsai M, Gurtner GC et al Evidence that mast cells are not required for healing of splinted cutaneous excisional wounds in mice. PLoS ONE 2013; 8:e59167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Willenborg S, Eckes B, Brinckmann J, Krieg T, Waisman A, Hartmann K et al Genetic ablation of mast cells redefines the role of mast cells in skin wound healing and bleomycin‐induced fibrosis. J Invest Dermatol 2014; 134:2005–15. [DOI] [PubMed] [Google Scholar]
- 30. Nishio N, Ito S, Suzuki H, Isobe K. Antibodies to wounded tissue enhance cutaneous wound healing. Immunology 2009; 128:369–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Boyce DE, Jones WD, Ruge F, Harding KG, Moore K. The role of lymphocytes in human dermal wound healing. Br J Dermatol 2000; 143:59–65. [DOI] [PubMed] [Google Scholar]
- 32. Loots MA, Lamme EN, Zeegelaar J, Mekkes JR, Bos JD, Middelkoop E. Differences in cellular infiltrate and extracellular matrix of chronic diabetic and venous ulcers versus acute wounds. J Invest Dermatol 1998; 111:850–7. [DOI] [PubMed] [Google Scholar]
- 33. Faria AMC, Garcia G, Rios MJC, Michalaros CL, Vaz NM. Decrease in susceptibility to oral tolerance induction and the occurrence of oral immunization to ovalbumin in 20–38‐week‐old mice. The effect of interval between oral exposures and rate of antigen intake in oral immunization.. Immunology 1993; 78:147–51. [PMC free article] [PubMed] [Google Scholar]
- 34. Oliveira RP, Santiago AF, Ficker SM, Gomes‐Santos AC, Faria AM. Antigen administration by continuous feeding enhances oral tolerance and leads to long‐lasting effects. J Immunol Methods 2015; 421:36–43. [DOI] [PubMed] [Google Scholar]
- 35. Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol 2013; 93:875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008; 8:958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. O'Brien RL, Born WK. Dermal γδ T cells – what have we learned? Cell Immunol 2015; 296:62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Havran WL, Jameson JM. Epidermal T cells and wound healing. J Immunol 2010; 184:5423–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gailit J, Welch MP, Clark RA. TGF‐β1 stimulates expression of keratinocyte integrins during re‐epithelialization of cutaneous wounds. J Invest Dermatol 1994; 103:221–7. [DOI] [PubMed] [Google Scholar]
- 40. Zambruno G, Marchisio PC, Marconi A, Vaschieri C, Melchiori A, Giannetti A et al Transforming growth factor‐β1 modulates β1 and β5 integrin receptors and induces the de novo expression of the αvβ6 heterodimer in normal human keratinocytes: implications for wound healing. J Cell Biol 1995; 129:853–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pakyari M, Farrokhi A, Maharlooei MK, Ghahary A. Critical role of transforming growth factor β in different phases of wound healing. Adv Wound Care (New Rochelle) 2013; 2:215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Desmouliere A. Factors influencing myofibroblast differentiation during wound healing and fibrosis. Cell Biol Int 1995; 19:471–6. [DOI] [PubMed] [Google Scholar]
- 43. Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF‐β1 and TGF‐β2 or exogenous addition of TGF‐β3 to cutaneous rat wounds reduces scarring. J Cell Sci 1995; 108(Pt 3):985–1002. [DOI] [PubMed] [Google Scholar]
- 44. Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W et al Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010; 184:3964–77. [DOI] [PubMed] [Google Scholar]
- 45. Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology 2011; 216:753–62. [DOI] [PubMed] [Google Scholar]
- 46. Okizaki S, Ito Y, Hosono K, Oba K, Ohkubo H, Amano H et al Suppressed recruitment of alternatively activated macrophages reduces TGF‐β1 and impairs wound healing in streptozotocin‐induced diabetic mice. Biomed Pharmacother 2015; 70:317–25. [DOI] [PubMed] [Google Scholar]
- 47. Coutinho A, Kazatchkine MD, Avrameas S. Natural autoantibodies. Curr Opin Immunol 1995; 7:812–8. [DOI] [PubMed] [Google Scholar]


