Summary
Neovascularization and jeopardized immunity has been critically emphasized for the establishment of malignant progression. Lectins are the diverse class of carbohydrate interacting proteins, having great potential as immunopotentiating and anti‐cancer agents. The present investigation sought to demonstrate the anti‐proliferative activity of Dolichos lablab lectin (DLL) encompassing immunomodulatory attributes. DLL specific to glucose and mannose carbohydrate moieties has been purified to homogeneity from the common dietary legume D. lablab. Results elucidated that DLL agglutinated blood cells non‐specifically and displayed striking mitogenicity to human and murine lymphocytes in vitro with interleukin (IL)‐2 production. The DLL‐conditioned medium exerted cytotoxicity towards malignant cells and neoangiogenesis in vitro. Similarly, in‐vivo anti‐tumour investigation of DLL elucidated the regressed proliferation of ascitic and solid tumour cells, which was paralleled with blockade of tumour neovasculature. DLL‐treated mice showed an up‐regulated immunoregulatory cytokine IL‐2 in contrast to severely declined levels in control mice. Mechanistic validation revealed that DLL has abrogated the microvessel formation by weakening the proangiogenic signals, specifically nuclear factor kappa B (NF‐κB), hypoxia inducible factor 1α (HIF‐1 α), matrix metalloproteinase (MMP)‐2 and 9 and vascular endothelial growth factor (VEGF) in malignant cells leading to tumour regression. In summary, it is evident that the dietary lectin DLL potentially dampens the malignant establishment by mitigating neoangiogenesis and immune shutdown. For the first time, to our knowledge, this study illustrates the critical role of DLL as an immunostimulatory and anti‐angiogenic molecule in cancer therapeutics.
Keywords: anti‐tumour, Dolichos lablab lectin, immunomodulation, mitogenecity, tumour neovasculature
Introduction
Tumours foster a tolerant microenvironment and the activation of plethoric immunosuppressive mechanisms, which may act in concert to counteract effective immune responses. The immunosuppressive tumour immune microenvironment evades T cell responses, either to avoid immune recognition or to disable effector T cells 1, 2. This strategy by malignant cells to breach the immune surveillance mechanism of the host has been linked most decisively to cancer progression 3. Hence, dampening the tumour immunosuppressive microenvironment by stimulating the innate anti‐tumour immunity may be an effective approach to cancer treatment. Immunotherapy aimed at harnessing endogenous anti‐tumour immunity by modifying immune regulatory mechanisms has shown promise in multiple tumour types 2. In compliance with this, recent findings in 2014 indicated that a complete immunological manoeuvre through the manipulation of immune system has represented an effective and reproducible outcome successfully, leading to the regression of large tumours in humans 4. These observations and experimental understanding has not only deviated from our fundamental view of cancer therapeutics but has also propelled forward the renaissance of immunomodulation in eliciting anti‐tumour immune responses, thereby enabling immune‐based therapies to join the mainstream of cancer treatment.
Tumour neovasculature, the blood vessels produced within malignant growth by chronically activated angiogenesis and an unbalanced mix of proangiogenic signals, are known to be highly indispensable, even at the microscopic premalignant phase of neoplastic progression 5, 6, 7. Intensive basic research and clinical practice have accentuated the important correlation between immunomodulation and angiogenesis 8. Substantial evidence indicates that immunological cells such as T cells and certain immunoregulatory cytokines have shown the capacity to enhance immune response sufficiently to induce cancer remissions and inhibit tumour angiogenesis 9, 10, 11. This suggests that a combinatorial approach encompassing anti‐angiogenic and immunostimulatory perspectives has significant implications in the design of dynamic immunotherapeutic strategies for treating malignancies 11.
Lectins are the ubiquitous and diverse class of haemagglutinating proteins of non‐immune origin that bind stereospecifically to cell surface glycan moieties 12, 13, 14. The lectin interactions on immune cell surfaces trigger signal transduction and the production of various beneficial cytokines, due to which these proteins are taking the forefront in biological research 14, 15. The presence of haemagglutinin in the dietary bean Dolichos lablab (common name: Hyacinth bean) has been known since 1964 14, 16. D. lablab lectin (DLL), of the leguminoseae category, belongs to the tribe Phaseoleae, which is demonstrated to be unique in its specificity for the monosaccharides glucose and mannose, unlike the other members of its tribe. Although DLL shares many biological activities with other legume lectins, especially with a prototype legume lectin concanavalin A (Con A), the complete primary sequencing of DLL elucidated several distinguishing features from those of other legume lectins 12, 13, 14. Indispensable evidence from as early as the 1980s reported the mitogenic properties of mannoside‐specific DLL towards lymphocytes 17, but even after three decades there have been no significant translational investigations to date, in spite of its immunomodulatory and therapeutic possibilities. As legume lectins have received remarkable attention due to their potent anti‐neoplastic activity 18, in this present study, for the first time to our knowledge, we sought to investigate the pharmacological potential of immunostimulatory D. lablab lectin directed to glucose and mannose in evoking the anti‐tumour response in murine models targeting critical in‐vivo tumour angiogenic parameters. This current study, envisaging a dietary and natural immunomodulator, promises a future scenario in overcoming immunosuppression and promoting tumour regression.
Materials and methods
Materials
The materials used were lung adenocarcinoma (A549), cervical carcinoma (SiHa, CaSki) and Ehrlich ascites carcinoma (EAC) from the National Centre for Cell Science (NCCS), Pune, India; squamous cell carcinoma (A388) from the National Centre for Biological Science (NCBS), Bengaluru, India and murine Dalton's ascites lymphoma (DLA) cells, a kind gift from Dr Sathees C. Raghavan, Indian Institute of Science (IISc), Bengaluru, India; and human umbilical vascular endothelial cells (HUVEC), endothelial cell growth media (EGM), sodium bicarbonate (NaHCO3), cyanogen bromide (CNBr) activated Sepharose 4B, matrigel [extracellular matrix (ECM) gel], hydron polymer poly‐hydroxyethyl‐methacrylate (poly‐HEMA), Ficoll histopaque, 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromide (MTT), anti‐vascular endothelial growth factor (VEGF) and anti‐mouse/rabbit immunoglobulin (Ig)G antibodies, protease inhibitor cocktail and Freund's adjuvants were obtained from Sigma‐Aldrich (St Louis, MO, USA), anti‐IL‐2, anti‐hypoxia inducible factor 1α (HIF‐1α); anti‐MMP‐2 and 9 antibodies from Santa Cruz Laboratories (Santa Cruz, CA, USA); anti‐nuclear factor kappa B (NF‐κB), anti‐IκB and anti‐β‐actin from BD Biosciences (San Jose, CA, USA); RPMI medium, antibiotic–anti‐micotic solution, fetal bovine serum (FBS) from Invitrogen (Carlsbad, CA, USA); anti‐CD‐31 antibody and immunostaining kit from Leica Biosystems (Wetzlar, Germany). Schiff's fuchsin‐sulphite reagent, ovalbumin, α‐methyl mannoside (αMM) and other carbohydrates were from HiMedia Laboratories, India. Prestained protein marker was obtained from New England Biolabs (Ipswich, MA, USA); cell culture plastic wares were from Eppendorf (Hamburg, Germany); all the other chemicals used were of analytical grade. Fertilized hen's eggs were procured from a local farmhouse in Shivamogga, India. Photographs were taken using a Sony Steady Shot DSC‐W610 camera. All the angiogenesis images were analysed by ImageJ software (NIH, Bethesda, MD, USA).
Purification and characterization of D. lablab lectin (DLL)
D. lablab lectin (DLL) was purified from the seeds of D. lablab by affinity chromatography, as described previously by Mo et al., with slight modifications 19. Briefly, the dried bean flour of D. lablab was extracted at 4ºC with 10 mM phosphate‐buffered saline (PBS) pH 7·4. After subjecting to ammonium sulphate precipitation (20–60% saturation), the filtrate was dialysed and the clear dialysate was applied onto the ovalbumin‐conjugated Sepharose 4B column. The affinity‐adsorbed lectin was eluted specifically with 0·2 M α‐methyl mannoside in PBS at a flow rate of 15 ml/h. The pure lectin fractions were pooled, dialyzed extensively against PBS and analysed by reducing 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE). The gels were then subjected to Coomassie brilliant blue and periodic acid‐Schiff (PAS) staining for protein band visualization and glycoprotein analysis, respectively 20. The bands were documented using the Bio‐Rad Gel DocumentationTM XR + imaging system. Polyclonal antibodies against purified DLL were raised in New Zealand White (NZW) rabbits in accordance with standardized protocols 21. The immunoreactivity of rabbit polyclonal anti‐serum was assessed by immunoblotting and double immunoprecipitation assays.
Agglutination and carbohydrate inhibition assay
Haemagglutination assay (HA) was carried out using trypsinized 2% chick, rabbit and human blood (types A, B, AB and O) erythrocyte suspension, as described previously 20. Informed consent was obtained from all healthy human volunteers (age range = 20–30 years) for obtaining peripheral venous blood. Briefly, 100 μl of erythrocytes suspension was added to an equal volume of serially diluted protein solution in a concavity agglutination plate, mixed gently and incubated at 37°C for 1 h to visualize the agglutination. Carbohydrate‐mediated HA inhibition was assessed by performing haemagglutination assay in the preincubated DLL with serially diluted sugars and glycoproteins 20.
Leucoagglutination assay was carried out using human peripheral blood lymphocytes (PBLs) that were separated using Ficoll‐Hypaque and density gradient centrifugation, as described previously 21. The separated lymphocytes (100 μl) in 10 mM PBS were then added to an equal volume of DLL in a concavity agglutination plate, mixed gently and incubated at 37°C for 1 h. Cell agglutinations were visualized using optical light microscopy and photographed.
Lymphocyte mitogenicity assay
Human PBLs, murine splenocytes and thymocytes were isolated and cultured in complete RPMI medium in a humidified environment at 37°C 21. The human and murine lymphocytes were cultured separately in flat‐bottomed 96‐well microtitre plates (200 μl of 2·5 × 106 cells/ml) in a CO2 incubator at 37°C with 5% CO2. After 3 h incubation, the cells were treated with crude DLL extracts, DLL (0, 5 and 10 μg/ml of media) and reference mitogen Con A (0, 5 and 10 μg/ml) for 72 h and MTT assay was performed. Supernatants from 24 h DLL (10 μg/ml)‐stimulated cultures were collected and used as conditioned media (DLL‐CM) for further studies 17.
Cell culture and in‐vitro treatment
The cells A549, A388, SiHa, CaSki, DLA and EAC were grown in RPMI medium supplemented with 10% FBS, antibiotic–micotics and NaHCO3 (0·37%) and incubated at 37°C in 5% CO2 with 98% humidity. The anti‐proliferative effect of DLL (0–50 µg/ml) and DLL‐CM (0–50%) for 48 h was evaluated by MTT, trypan blue and lactate dehydrogenase (LDH) release assays and IC50 values were calculated. For every experiment 5‐fluorouracil was used as a positive control. Each experiment was repeated a minimum of three times independently and analysed with an appropriate vehicle and positive control 22.
Chorioallontoic membrane (CAM) assay
DLL‐ and DLL‐CM‐mediated angiogenic responsiveness to the recombinant VEGF165 (rVEGF165) induced in‐vivo CAM angiogenesis were studied as described previously 23. Of note, DLL (10 μg/CAM) or DLL‐CM (10 μg/CAM) were added to the growing fertilized eggs (5th day) and changes in the vascularization patterns were examined and photographed.
Rat aortic ring assay
The effect of DLL and DLL‐CM on neovessel sprouting was studied by culturing aortic explants in matrigel as per the previously described methods, with slight modifications 24. Briefly, transverse sectioned thoracic aortas with 1 mm diameter were cultured in matrigel immersed with RPMI media in a humidified environment at 37°C for 2 weeks. DLL (10 μg/ml) or DLL‐CM (30%) was added directly to the culture media and allowed to diffuse into the gel. Cultures were investigated by inverted light microscopy (Olympus) at ×10 optical magnification.
Capillary tube formation assay
Functional analysis of DLL and DLL‐CM on endothelial cells for vascular tube formation was performed following previous methods, with slight modifications 25. Briefly, 100 μl of 2 × 104 endothelial cells in EGM were seeded onto matrigel‐coated 96‐well plates followed by treatment with or without rVEGF165, DLL (10 μg/ml) and DLL‐CM (30%). After 4–6 h of incubation at 37°C with 5% CO2, the cells were assessed for formation of capillary‐like structures using an inverted light microscope (Olympus) at ×10 optical magnification.
Transwell invasion assay
The cell invasion inhibitory effects of DLL and DLL‐CM were investigated by Transwell invasion assay 26. Briefly, the harvested A549 cells (3 × 104) in 200 μl of serum‐free RPMI medium with or without DLL (10 µg/ml) and DLL‐CM (30%), respectively, were added to the matrigel‐coated upper chamber; 0·6 ml of RPMI with 20% FBS was added to the lower compartment and the Transwell‐containing plates were incubated at 37°C and 5% CO2 for 8 h. The invaded cells were stained with 0.1% crystal violet and were captured using a photomicroscope at ×40 resolution.
In‐vivo studies
Animals
BALB/c mice, Swiss albino mice aged 6–7 weeks, weighing 25–30 g, and Swiss albino Wistar rats aged 7–8 weeks, weighing 125–150 g, were housed under standard laboratory conditions and fed with commercial rodent meal and water ad libitum. All the animal experiments were approved by the Institutional Animal Ethics Committee (IAEC), National College of Pharmacy, Shimoga, India, in accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) guidelines for laboratory animal facility (NCP/IAEC/CL/101/05/2012‐13).
Animal tumour development and treatment
DLA cells were cultured by intraperitoneal (i.p.) transplantation to develop ascites tumour. After the fourth day of tumour growth, the mice were administered with three doses of DLL [0, 20 and 30 mg/kg body weight (b.w.) i.p., n = 6] on every alternative day. Ascitic tumour parameters were noted. Solid lymphoma (DLS) was developed in mice by injecting the cells subcutaneously (s.c.) in the right hindlimb according to previously described methods 23. After the visible development of the tumour, mice were administered with six doses of DLL (0, 20 and 30 mg/kg b.w. i.p., n = 6) on alternate days and tumour progression was monitored. The number of animals that survived and the duration after the treatment regimen were documented in a separate analysis. Toxicological parameters were examined by measuring the hepatic catalase and lipid peroxidation 27. To assess the physiological effect of DLL in normal mice, the serum of DLL‐treated animals were subjected to liver and kidney function tests by measuring the levels of alkaline phosphatase (ALP), creatinine and urea 23.
MVD evaluation in peritoneum
Microvessel density (MVD) was assessed in the peritoneal lining of control and treated ascitic lymphomas, as reported previously 28. Briefly, after the treatment regimen as described above, the response of DLL to the tumour‐induced neovessel formation in peritoneum lining was observed and documented. Further, peritoneal tissue sections were processed for haematoxylin and eosin (H&E) staining and the MVD/high‐power field (HPF) was quantified manually.
ELISA
The in‐vitro and in‐vivo secretion of IL‐2 levels and serum VEGF‐A were quantified by enzyme‐linked immunosorbent assay (ELISA). In brief, 100 μl of conditioned media or serum were coated in coating buffer at 4°C incubated with anti‐IL‐2 or anti‐VEGF‐A, followed by reincubation with ALP‐conjugated secondary antibody. IL‐2 and VEGF‐A was quantified by measuring absorbance at 405 nm by using p‐nitrophenyl phosphate (PNPP) as substrate.
Matrigel plug assay
BALB/c mice were implanted with 0·5 ml of matrigel + rVEGF165 (10 ng/plug) mixture with or without DLL (10 μg/plug) in the right flank. After 7 days following the implantation procedure, matrigel implants were harvested and liquefied by incubation at 4°C overnight in 300 μl PBS. Further, the angiogenesis was quantified by estimating haemoglobin content in implants, as per previous reports 29.
Corneal neovascularization assay
The rat corneal micropocket assay was performed in Swiss albino Wistar rats, as described previously with slight modifications 30. Briefly after anaesthetizing the animal, micropockets were created using the slit knife in cornea. Hydron pellets containing 10 ng/pellet of either rVEGF165 plus or minus DLL (10 µg) were implanted into corneal pockets. After 6–8 days the eyes were examined for neovasculature and photographed. The corneas were then dissected and subjected to histopathological assessment.
Protein extracts and Western blotting
Protein extracts were obtained from DLA and DLS tumour tissues (in vivo) for Western blot analysis. The proteins (30 μg) were resolved by SDS‐PAGE and transferred onto nitrocellulose membrane. After blocking with 3% BSA in Tris‐buffered saline Tween 20 (TBST) (150 mM NaCl, 10 mM Tris and 0.025% Tween 20 in dH2O), immunoblot analysis was performed for HIF‐1α, VEGF‐A, MMP‐2, MMP‐9, NF‐κB, inhibitory kappa B (I‐κB) and β‐actin proteins using appropriate primary/secondary antibodies in blocking buffer three times independently, as per the manufacturer's recommendations. Results were repeated to the control by using ImageJ software.
Gelatin zymogram
DLA ascitic secretions were subjected to gelatin zymography to quantify gelatinase activity of MMP‐2 and MMP‐9. Briefly, proteins of the whole cell lysate were resolved in 8% SDS‐PAGE gels containing 0·1% (w/v) gelatin and zymogram was developed, as reported previously 28, 31. Inhibition of gelatin lysis zones were documented using the Bio‐Rad Gel DocumentationTM XR+ Imaging System.
Immunohistochemical (IHC) analysis
Immunological detection of CD31, HIF‐1α, MMP‐2, MMP‐9 and NF‐κB in the formalin‐fixed sections of the peritoneum and solid tumour tissues of the control and DLL‐treated animals was performed using the specific antibodies, as per the manufacturer's instructions. Observations were made using the EVOS imaging system (Life Technologies, Paisley, UK) under ×20 brightfield objective and the changes in intensity of the antibody staining were evaluated 32.
Statistics
The data were analysed and graphs were made using MS Excel version 8.1. Statistical significance was evaluated by one‐way analysis of variance (anova) followed by Student's t‐test. Values were expressed as mean ± standard error (s.e.m.). Values of *P< 0·05 and **P < 0·01 were considered statistically significant.
Results
Purified DLL specific to glucose/mannose agglutinates haemocytes and leucocytes
D. lablab lectin was purified to the electrophoretic homogeneity from the crude D. lablab bean extract (Supporting information, Fig. S1a). Molecular mass estimation by reducing SDS‐PAGE revealed that purified DLL resolved into five bands comprising α (band 1–4) and β (band 5) subunits, with apparent molecular weight ranging from 10 to 22 kDa (Fig. 1a), similar to previous reports 19. The chromatographic component DLL was tested for the haemagglutination activity which agglutinated chick, rabbit and human erythrocytes irrespective of blood type (Fig. 1b). Leucoagglutination assay deduced a clear agglutination of the cells mediated by DLL in the microscopic visualization (Fig. 1c,d). The minimal concentration for DLL to provide a visible haemagglutination was observed to be ∼3 μg in human RBC suspension (Supporting information, Fig. S1b). DLL was found to be positive to PAS staining, confirming the glycoprotein nature of the lectin (Supporting information, Fig. S1c). Rabbit anti‐DLL antibodies showed strong immunoreactivity to DLL as analysed by immunoblot and the double immunodiffusion technique (Supporting information, Fig. S1d,e). DLL haemagglutination was inhibited specifically by the monosaccharides D‐mannose and D‐glucose, with inhibitory concentration of ∼6 mM (data not shown).
Figure 1.
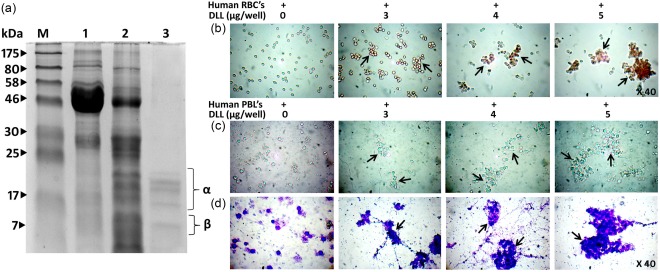
Purified Dolichos lablab lectin (DLL) exerts potent erythro‐ and leucoagglutinating activity. (a) Molecular mass determination of purified D. lablab lectin by sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) under reducing conditions. Lane profile: M = marker, 1 = crude D. lablab extracts (3%), 2 = ammonium sulphate precipitated (20–60%) DLL extracts, 3 = purified Glc/Man‐specific DLL. Lane 3 shows purified protein fractions with five subunits with apparent masses ranging from 10 to 22 kDa. The lectin activity of the purified DLL was examined by its cell agglutinating characteristics employing human red blood cells (RBCs) and leucocytes. (b) DLL potently agglutinates 2% trypsinized human RBCs in sharp contrast to the standard untreated with distinguished separate RBCs. (c) DLL displays leucocyte agglutinating behaviour in a concentration‐dependent manner. The leucocytes were separated through density gradient centrifugation, as described in the Materials and methods section. The illustrations are true‐colour microscopic photographs at ×40 optical resolution. (d) Leucocytes visualized post‐DLL treatment and leucocyte staining at ×40 resolution exhibit significant agglutinating pattern that is conspicuously distinctive to that of control. The dark arrows indicate the agglutinated cells. The results are representative of three independent experiments. [Colour figure can be viewed at wileyonlinelibrary.com].
DLL induces potent mitogenicity in human and murine lymphocytes with IL‐2 synthesis
Human PBLs, murine splenocytes and thymocytes were subjected to DLL stimulation to investigate the lectins’ immunoproliferative properties (Fig. 2a–c). The purified DLL (5 and 10 μg/ml) triggered a potent and significant mitogenic response in both human (Fig. 2d) and murine lymphocytes (Fig. 2e,f) in a dose‐dependent manner, which is more strikingly equivalent to Con A. Upon treatment with DLL, formation of aberrant lymphocyte clusters were observed, which is reported to be one of the prerequisite events in the lymphocyte stimulation (Fig. 2a). As can be seen in Fig. 2a–c, microscopic evaluation reveals a varied and distinctive pattern of cell cluster formation in human and murine lymphocytes by DLL. The proliferative index for control was taken as 1·0 and others are represented as fold increase or decrease over the control. Supernatants from the DLL‐stimulated cell cultures were subjected to the estimation of IL‐2 secretions. Similar to the previous report 17, DLL‐stimulated lymphocytes showed increased IL‐2 synthesis in optimal concentrations (Fig. 2g).
Figure 2.
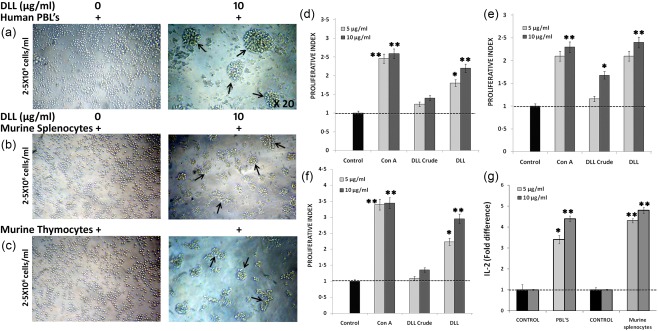
Immunostimulatory activity of Dolichos lablab lectin (DLL) on human and murine lymphocytes in vitro. To investigate the mitogenic attribute of DLL, in‐vitro cultured lymphocytes were treated with varying lectin concentrations and subjected to 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromide (MTT) proliferation assay, as told in the Methodology section. (a) Representative visual depictions of the purified DLL‐induced mitogenicity on human peripheral blood lymphocytes (2·5 × 106 cells/ml) at 10 μg/ml concentration. The cell cluster formation, a prerequisite event in the cellular proliferation, could be observed in DLL, whereas untreated cells appear distinctively separated. Similar cell clusters were also observed in (b) murine splenocytes (c) and thymocytes. The true‐coloured and unstained images were observed through brightfield inverted microscope (Olympus) at ×20 optical magnification. The proliferative effect of DLL was expressed in terms of proliferative index. The index for control (non‐stimulated) cells was taken as 1·0 and for others it is calculated as a ratio of absorbance at 570 nm of the test sample to that of the control. Graphs indicating the proliferative index of DLL on human peripheral blood lymphocytes (PBLs) (d), splenocytes (e) and thymocytes (f) in vitro. (g) Effect of DLL on ex‐vivo interleukin (IL)‐2 production by human PBL and murine splenocytes. Results are the means of three determinations, each conducted in triplicate. Statistically significant values are *P < 0·05; ** P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com].
DLL‐CM displays cytotoxic and anti‐angiogenic attribute in vitro
Compelling evidence from various experiments has indicated that T cells are essential for an anti‐proliferative and anti‐angiogenic immune response. As it is known that glucose/mannose‐specific DLL is a potent T cell mitogen 17, we attempted to understand the anti‐proliferative and neovessel inhibitory effect of DLL‐CM and DLL in standard in‐vitro assay systems. In‐vitro cytotoxicity activity of DLL‐CM, as verified by cytotoxic assays, elucidated a decreased cell viability of A549, A388, SiHa, CaSki, DLA and EAC cells, where DLL alone did not show significant cytotoxicity or sensitivity, as shown in Table 1. With respect to the effect of DLL and DLL‐CM on angiogenesis, results inferred that MVD counts in ex‐ovo CAM and rat aortic angiogenesis influenced by rVEGF165 was regressed markedly by the conditioned medium (68·8 and 77·1%, respectively) (Fig. 3a,b). Treatment with DLL displayed 28·4 and 22·7% inhibition of CAM vessel density and aortic sprouting, respectively, which is apparently 2·4‐ and 3·5‐fold less than the effect of conditioned medium (Fig. 3d,e). To further affirm the anti‐angiogenic activity, DLL‐CM was verified by tube formation assay, which indicated a decreased tube‐forming efficiency by 71·3%, whereas DLL alone showed very negligible inhibition (Fig. 3c,f).
Table 1.
IC50 value of Dolichos lablab lectin (DLL) and DLL‐conditioned medium (CM) against various human and mouse cancer cell lines
| DLL (µg) | DLL‐CM(µg) | 5‐Fluorouracil (µg) | |
|---|---|---|---|
| Cell line | IC50 value | IC50 value | IC50 value |
| A549 | 34·7 ± 2·9 | 4·2 ± 0·6* | 3·1 ± 0·5* |
| A388 | 36·1 ± 3·7 | 5·3 ± 0·4** | 4·7 ± 0·8 |
| SiHa | 38·3 ± 3·3 | 5·7 ± 0·3** | 3·7 ± 0·6* |
| CaSki | 37·7 ± 3·1 | 6·1 ± 0·5* | 4·4 ± 0·4** |
| DLA | 38·6 ± 1·5 | 6·4 ± 0·6* | 2·3 ± 0·3** |
| EAC | 44·3 ± 3·5 | 7·3 ± 0·5* | 4·0 ± 0·2** |
5‐Fluorouracil is used a positive control. Cytotoxicity was measured by 3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐diphenyltetrazoliumbromide (MTT), Trypan blue and lactate dehydrogenase (LDH) release assays against each cell line in three independent (n = 3) and values represent IC50 by MTT. Dimethylsulphoxide (DMSO) is used as a vehicle control, which showed very negligible cytotoxicity. Values are indicated by mean ± standard error (s.e.) and statistically significant values are expressed as *P < 0·05 and **P < 0·01.
Figure 3.
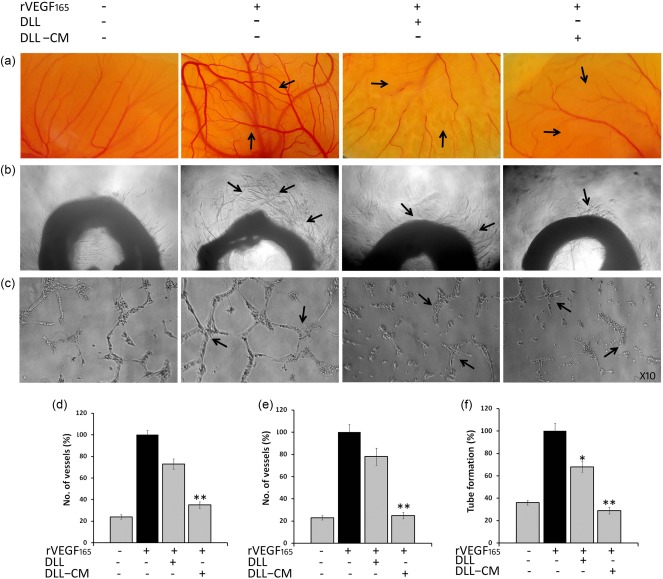
Anti‐angiogenic activity of Dolichos lablab lectin‐conditioned medium (DLL‐CM) in in‐vitro angiogenic assay models. Indirect angioinhibitory effect of DLL on various angiogenic systems in vitro. DLL‐CM was the supernatant of DLL exposed to murine splenocytes after 24 h incubation. DLL‐CM exhibits regressed angiogenesis in (a) ex‐vivo chorioallontoic membrane (CAM) and (b) rat aortic angiogenesis assay. DLL directly exhibited only a minimal angiolytic effect. (c) DLL‐CM exposed to human umbilical vein endothelial cells (HUVEC) cells inhibits the tube‐forming efficiency. Pictographical representation of (d) ex‐vivo CAM, (e) aortic ring angiogenesis and (f) tube formation assay. Data were represented as mean ± standard error of the mean (s.e.m.) of three independent experiments. Statistically significant values are expressed as *P < 0·05 and **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com].
DLL impedes proliferation of murine ascitic and solid lymphoma tumour in vivo through immunomodulation
IL‐2 is an immunoregulatory cytokine possessing anti‐tumour activity 4. Our observations and previous report indicate 17 that DLL stimulates the immune system through the secretion of IL‐2 (Fig. 2g). This has prompted us to investigate the effect of lectins in mitigating the progression of malignant cells in in‐vivo murine ascitic and solid lymphoma tumour models. Results inferred that, in ascites tumour, administration of DLL resulted in significant diminution of tumour growth by 71·8 and 82·6% in varying concentrations (Supporting information, Fig. S2a). Decisive evidence has indicated that DLL treatment has impacted negatively the various tumorigenic parameters, such as cell count and ascites secretion, in a dose‐dependent manner (Supporting information, Fig. S2b,c). Moreover, survival analysis after the treatment regimen showed an approximate 2·1–2·5‐fold prolonged lifespan, although a moderate recurrence (∼10%) was observed, as analysed by body weight index (Supporting information, Fig. S2d).
The tumour inhibitory potential of DLL was re‐evaluated further in Dalton's lymphoma solid tumour in mice. Following six consecutive DLL administrations on alternate days, there was considerable dose‐dependent growth inhibition. The dissected tumour in the control weighed 17 g, whereas in treated mice it was 2·61–3·12‐fold less than that of control at varying concentrations, thereby providing a clear perspective on the regression of tumour growth (Fig. 4a–e). As in the ascites model study, DLL was able to extend the animals’ lifespan from 45 to 98 and > 120 days (Fig. 4f). However, oral administration of DLL after tumour onset did not produce the same tumour inhibitory results (data not shown). As DLL‐CM exerted cytotoxicity in vitro (Table 1), the immunomodulatory implications of DLL in endogenous systems were evaluated. Interestingly, concurrent to the in‐vitro conditions, DLL up‐regulated the serum IL‐2 by twofold in tumour mice to that of control (Fig. 4i). Furthermore, histopathological and gross examinations of internal organs showed no toxicity after DLL treatment (Supporting information, Fig. S3a–c). Biochemical evaluation of liver enzymes revealed a decreased lipid peroxidation and elevated catalase activity upon DLL treatment (Fig. 4g,h). Serological and haematological examination produced normal values after DLL treatment in normal non‐tumour‐bearing mice, indicating limited secondary complications (data not shown).
Figure 4.
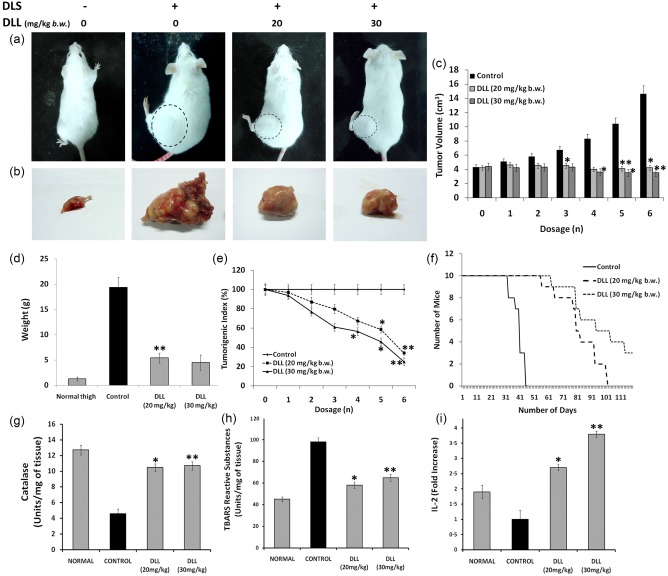
Dolichos lablab lectin (DLL) mitigates the proliferation of murine Dalton's lymphoma solid tumour in‐vivo with up‐regulation of IL‐2 levels. Solid tumour was induced by injecting Dalton's lymphoma cells (1 × 106 cells/mouse) in hindlimbs of Swiss albino mice. After the palpable development of tumour, six doses of DLL (20 and 30 mg/kg body weight b.w.) were administered intraperitoneally (i.p.) to tumour‐bearing mice on every alternative day. (a) Tumour morphology of the DLS‐induced mice with and without DLL treatment. The circular dotted lines represent the extent of tumour growth. (b) Gross anatomical appearance of the excised DLS tumours. Note the extensive vascularization surrounding the tumour tissue of the untreated control. (c) The effect of DLL on DLS tumour progression at different time‐points. (d) Weight of the excised normal thigh, control and DLL‐treated tumours (g). (e) Tumorigenic index elucidating the dose‐dependent decrease of tumour progression post‐DLL regimen. (f) Kaplan–Meier survival curve of the animals treated with DLL. Effect of DLL in the endogenous hepatic anti‐oxidant system (d) pronounced catalase enzymes levels in DLL‐treated mice. Note control with severely declined enzyme content. (e) Decreased lipid peroxidation in DLL‐treated mice, as assessed by the thiobarbituric reactive substances (TBARS) method. (f) Fold difference in the serum interleukin (IL)‐2 levels of DLL‐treated animals compared to control. The control animal shows declined levels of IL‐2, whereas DLL at 20 and 30 mg/kg b.w. via i.p. administration shows pronounced IL‐2 levels. Results are the means of three determinations, each conducted in triplicate. Statistically significant values are *P < 0·05; ** P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com].
DLL deters tumour‐reliant and independent neovascularization in rodents
Tumour development induces an early angiogenesis response, which is a critical and rate‐limiting event in tumour progression 5, 6. Hence, modalities blocking neovascular sprout are of particular interest in oncolytic strategies. Because we observed DLL‐CM inhibiting angiogenesis in vitro (Fig. 3), DLL was investigated for its deterrent effect on tumour‐induced angiogenesis in murine ascites and solid lymphoma. Visible observation demonstrated that DLL decreased the development of ascitic lymphoma‐induced peritoneal angiogenesis considerably by 1·.21–1·64‐fold compared to the control (Fig. 5a). Measurement of microvascular density (MVD) by H&E and anti‐CD31 immunostained histological sections again validated that DLL restricted the MVD/HPF to 28·8 ± 1·2 and 24·32 ± 2·6 in 20 and 30 mg/kg concentrations, respectively, compared to the untreated mice (57 ± 6·2, Fig. 5b,c). Similarly, the implication of DLL in blocking the tumour vessel density was studied further by employing Dalton's lymphoma solid tumours, which were subjected to histological and IHC studies. The results show that DLL lowered the MVD/HPF drastically, by ∼56 and ∼72% at 20 mg/kg b.w. and 30 mg/kg b.w., respectively, to that of control (Fig. 5d,e). It should be noted that the decreased tumour MVD concurred with the regressed tumour volume and prolonged survival in the DLL group (Fig. 5f).
Figure 5.
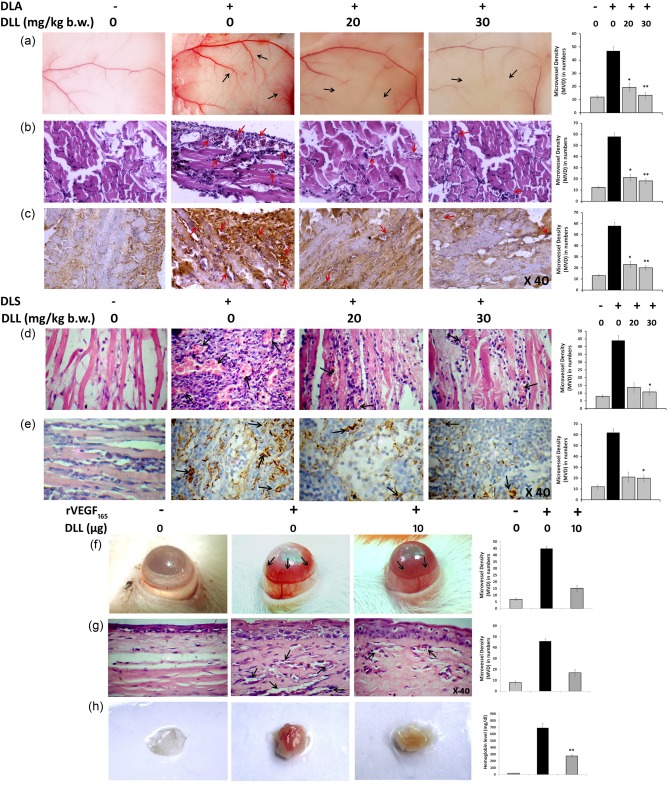
Dolichos lablab lectin (DLL) abrogates tumoral and non‐tumoral angiogenesis in vivo. The effect of DLL in the tumour‐induced angiogenesis was evaluated by murine ascites and solid tumour models in vivo, treated with DLL 20 and 30 mg/kg body weight intraperitoneally (i.p.). (a) The peritoneal lining of mice showing the visible suppression of peritoneal microvessel in DLL‐treated mice. (b) Haematoxylin and eosin (H&E)‐stained peritoneum sections showing the decreased vascular count (c). CD31 immunohistochemistry (IHC) of the peritoneal sections depicting hypervascularization (intensive brownish staining) in the control section in sharp contrast to the DLL. The reticence of tumour angiogenesis was confirmed by (d) H&E and (e) IHC (CD31) photomicrographs of solid tumour sections depicting a significant reduction in vessel density. The effect of DLL on corneal angiogenesis and matrigel plug model systems was studied by inducing angiogenesis with recombinant vascular endothelial factor (rVEGF)165 and treatment with DLL. (f) DLL regresses rat corneal angiogenesis. (g) Micrographs of H&E‐stained rat corneal sections confirming the reduced microvascular density/high‐power field (MVD/HPF) upon DLL treatment. The black arrows indicate the extent of vascularization. The representative MVD counts were provided adjacent to every corresponding image. (h) Matrigel plug assay depicting control with aberrant vascularization in contrast to the DLL‐treated mice. Pictograph depicting the level of haemoglobin content in matrigel implants is provided adjacently. Results are the means of three independent experiments. Statistically significant values are *P < 0·05; ** P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com].
To examine the angiopreventive effect of DLL in non‐tumour systems, lectin was subjected to rVEGF165‐induced angiogenesis in vivo. The results indicated that DLL arrested the corneal angiogenesis substantially in rats (Fig. 5f), with the MVD count reduced by 1·43‐fold compared to the rVEGF165‐treated control, and this was confirmed further by histopathological examination of corneal sections (Fig. 5g). Matrigel plug assay also displayed a similar 1·35‐fold reduction in rVEGF165‐mediated vasculature in the DLL group compared to the control, as verified visibly and by haemoglobin estimation (Fig. 5h).
DLL abrogates neovasculogenesis through altering the angiogenic signalling cascade in malignant cells
As the angiogenic capacity of tumour cells is largely attributable to the intrinsic genetic alterations that contribute to the establishment of the malignant phenotype 33, DLL‐treated cells were subjected to specific gene expression studies. DLL down‐regulated various pro‐angiogenic gene expressions, such as HIF‐1α, VEGF‐A, NF‐κB, I‐κB, MMP‐2 and MMP‐9 in both in‐vivo ascitic and solid tumours compared to that of untreated tumours, as validated by Western blot (Fig. 6a,b). Serological determination of secreted VEGF showed abridged levels in contrast to control (Fig. 6c). In corroboration with these observations, gelatin zymography revealed a decreased level of MMP‐2 and MMP‐9 in DLL‐treated ascitic secretions (Fig. 6d). Counteraction of MMP‐2 and MMP‐9 expression resulted in the inhibition of invasive potency of A549 cells (Fig. 6e). Immunohistochemical examination for the detection of HIF‐1α, NF‐κB, MMP‐2 and MMP‐9 corroborated the immunoblot, gelatin zymogram and invasion assay results further (Fig. 6f–j).
Figure 6.
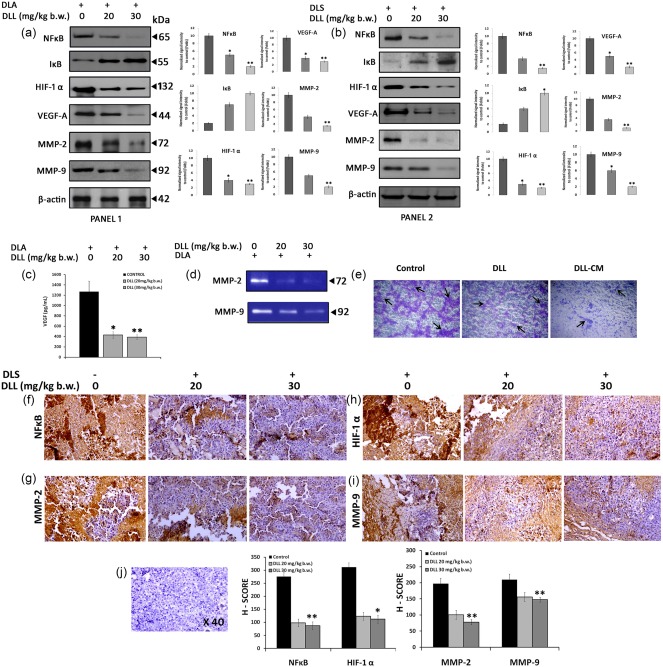
Translational down‐regulation of tumoral angiogenic gene expression by Dolichos lablab lectin (DLL). Immunoblots, gelatin zymography and immunohistochemistry (IHC) were carried out using in‐vivo tumour cells of both Dalton's ascites lymphoma (DLA) and Dalton's lymphoma solid tumour (DLS) with or without DLL treatment in two different concentrations. (a,b) Immunoblots showing altered translational expression of proangiogenic genes such as nuclear factor kappa B (NF‐κB), inhibitory kappa B (I‐κB), hypoxia inducible factor 1α (HIF‐1α), vascular endothelial growth factor (VEGF)‐A, matrix metalloproteinase (MMP)‐2 and 9 in ascitic (panel 1) and solid tumour (panel 2). The graphs indicate the densitometric values of corresponding Western blot data. (c) Reduction of secreted serum VEGF levels in DLL‐treated DLA mice in vivo. (d) Gelatin zymography showing reduced gelatinolytic activity in DLL‐treated mice. (e) DLL‐conditioned medium (DLL‐CM) inhibits the migration of A549 cell in vitro. IHV should be replaced with IHC detection of DLL‐induced altered gene expression. (f,i) IHC detection of proangiogenic gene expression in solid tumours in vivo after DLL treatment. The intense brown staining indicates the respective protein expression. Representative graphs demonstrating the gene expression corresponding to the IHC data are given below. (j) Representative counter haematoxylin‐stained section. Data are represented as mean ± standard error of the mean (s.e.m.) of three independent experiments. Statistically significant values are expressed as *P < 0·05 and **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com].
Discussion
The legume lectin family is well known for its far‐ranging biological functions 18. The unique glycan‐interacting potential of lectins on the immune cell surfaces ensues positive amplification of the immune system, thereby rendering them invaluable as tools in oncological and biomedical research 14, 15. In line with previous reports 19, we have purified a well‐known glucose/mannose‐specific D. lablab (DLL) lectin from the common dietary bean D. lablab of the leguminoseae family. The complete amino acid sequence of the lectin and its mitogenic attribute was reported long ago 14 but, despite its well‐characterized and immunostimulatory properties there were only negligible reports promulgating its pharmacological implications. It is apparent that this immunomodulatory drug class has salience in oncotherapeutics, due to the notion that survival of malignant cells is dependent upon the microenvironment and evasion of the host's anti‐tumour immune response 34. In our study, screening against multiple cancer cells revealed that DLL failed to produce a significant inhibitory activity. Interestingly, DLL‐CM, which expresses significant IL‐2 levels, exhibited an anti‐proliferative effect (Table 1). The immunomodulatory implication of DLL prompted us to expand our investigation into Dalton's lymphoma, a reliable in‐vivo tumour model which induces potential lethal effects in mice. Results demonstrated that DLL effectively regressed pivotal tumour parameters of DLA/DLS in vivo with an extended life span (Supporting information, Figs S2 and S4).
There is a well‐recognized relationship between anti‐tumour activity and immunomodulation 35. T cells are a central component of an adaptive immune system that is indispensable for immune system maintenance and immune surveillance to eliminate transformed cells 36. However, chronic malignancy results in T cell exhaustion, with a major decline in IL‐2 synthesis that leads to debilitating consequences regarding anti‐tumour immunity 36, 37, 38. As DLL is a potent T cell mitogen 17, tumour inhibitory activity of the lectin (Supporting information, Figs S2 and S4) in our study suggests that the protein abrogates immunosuppression and T cell exhaustion in tumour mice. Our experimental evidence has indicated that DLL up‐regulated IL‐2 levels significantly in tumour mice compared to that of severely decreased levels in untreated mice (Fig. 4i). This corresponds clearly with the regressed tumour growth in ascitic (Supporting information, Fig. S2) and solid tumour (Fig. 4), thereby indicating that DLL has probably reversed the T cell exhaustion, consequentially breaking the IL‐2 shutdown and eliciting an anti‐tumour response.
Transformed cells do not become tumorigenic unless they acquire angiogenic potential 39. Tumour neovasculaturization, one of the integral operations in malignant expansion, is marked by precocious capillary sprouting with convoluted, distorted and excessive vascular branching 5, 6, 40. It is well known that Dalton's lymphoma induces massive peritoneal microvessel and vascular pathologies leading to invasiveness. Our observations showed that DL ascitic tumours and DL solid tumours showed enormous blood vessels which appeared to be highly disorganized and chaotic (Fig. 5a–e). Convincing observations from our study have clarified that the DLL regimen potently regressed the tumour‐induced neovasculature, which correlates positively with a reduced tumorigenic index (Fig. 4e). Furthermore, earlier findings have provided evidence that among diverse roles, IL‐2 displays anti‐angiogenic and tumour‐regressive properties 41. Collectively, our results demonstrate that elevated IL‐2 cytokine has been attributed to the DLL‐mediated anti‐angiogenesis (Fig. 5) and tumour regression in vivo (Supporting information, Figs S2 and S4). Relative evidence could be observed from the angiopreventive and anti‐proliferative effect of DLL‐CM, with high IL‐2 levels in in‐vitro angiogenesis (Fig. 3a–f) and cytotoxicity assay systems (Table 1), respectively. These observations explain that DLL has abrogated the tumour progression possibly through altering the immunological and angiogenic parameters.
Compelling reports provide a potential link between NF‐κB activation and oncogenesis, which functions as a transcriptional regulator in a variety of malignancies as evidenced through the identification of cancer‐specific, cancer‐relevant gene targets controlled by NF‐κB 1, 42. In line with this, our experiments have demonstrated that both DL ascitic and solid tumours have shown elevated NF‐κB levels with aberrant cellular proliferation and invasiveness (Fig. 6a,b), but administration with DLL was able to reduce NF‐κB expression substantially in tumour mice, which explains the potential role of lectin in altering the pro‐oncogenic transcription factor, thus showing visible impairment in tumour progression (Fig. 4a,b). One of the prominent features of the tumour microenvironment is the low oxygen tension or hypoxia which promptly induces HIF‐1‐mediated vascular switch, a prerequisite event for most malignancies and solid tumours to develop from a quiescent tumour into a more aggressive form 42, 43, 44, 45. Our studies suggest that DLL treatment lowered the expression of the oxygen sensor HIF‐1, a key regulatory factor involved in oncogenic conversion, and promoting neoangiogenesis in mice (Fig. 6a,b). Reports show that constitutive HIF‐1α and NF‐κB activation in cancer cells has been shown to trigger autocrine of angiogenic chemokines, mainly VEGF, a pivotal factor for mitogenesis and capillary permeability of endothelial cells 42, 43, 44, 45, 46, 47. Our experimental observations showed that DLL reduced VEGF expression, along with MMP‐2 and MMP‐9, thereby confirming the down‐regulated proangiogenic signalling cascade in vivo (Fig. 6). In support of this, decreased MMP expression is reflected in the retarded invasiveness of cancer cells (Fig. 6e). Significantly, DLL retarded the tube formation of functional endothelial cells (HUVECs) and rVEGF165‐induced neovessel formation in vitro (Fig. 3). Moreover, DLL potentially reduced the peroxidized lipids in tumour mice (Fig. 4h), which have been highly implicated in tumour angiogenesis and release of HIF‐1α into the tumour microenvironment 48, 49. Together, this evidence highlights DLL's potential in neovascularization inhibition.
Taken together, decisive observations demonstrate that dietary lectin DLL exerts potent anti‐proliferative activity towards malignant cells. Immunopotentiating DLL impedes tumour progression specifically by antagonizing the neoangiogenesis formation in murine Dalton's lymphoma ascitic and solid tumours. This study highlights the critical role of dietary protein as an immunostimulatory and anti‐angiogenic molecule targeting cancer cell progression (Fig. 7). More in‐depth investigations expediting molecular insights into tumour immunomodulation of this leguminous lectin could prove vital in making breakthrough improvements in cancer therapeutics.
Figure 7.
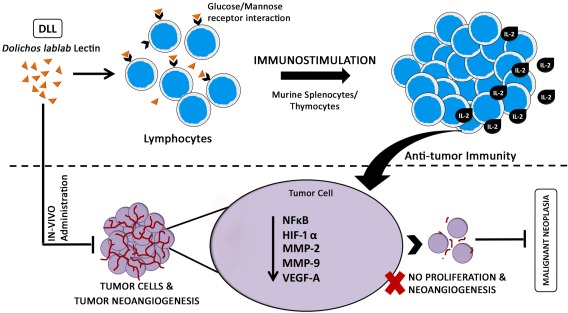
Schematic demonstration of glc/man interacting Dolichos lablab lectin (DLL) induced anti‐tumour effect. Hypothetical model diagram depicting the possible mechanism of action of immunostimulatory DLL in eliciting the potent anti‐neoplastic and anti‐angiogenic response in in‐vivo murine Dalton's lymphoma tumour. [Colour figure can be viewed at wileyonlinelibrary.com].
Disclosure
The authors declare that there are no conflicts of interest.
Author contributions
V. V. performed experiments and wrote the manuscript. P. T. and V. B. R. provided experimental assistance and V. K. provided research advice. B. T. P. and S. N. P assisted in the experimental design and data analysis.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Purification of Dolichos lablab lectin (DLL) by ovalbumin‐Sepharose 4B affinity chromatography. (a) Chromatograph of DLL purification on an ovalbumin‐Sepharose 4B column. DLL specifically eluted by α‐methyl mannoside, protein elutions monitored at 280 nm. The arrows indicate the point of elution. (b) Haemagglutination assay employing the serially diluted purified DLL starting with 400 µg concentration in concavity assay plate. The titre value of DLL is ∼3 μg, which represents the minimal concentration of the extracts required to produce visible agglutination at the highest dilution. (c) Periodic acid‐Schiff (PAS) staining of the purified DLL resolved on 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) to detect the glycoprotein nature of the proteins. Lane profile: M = marker, 1 = purified DLL, 2 = ovalbumin, 3 = bovine serum albumin. (d) Immunoblot analysis of rabbit anti‐DLL polyclonal antibodies. DLL resolved on 12% SDS‐PAGE was transferred to polyvinylidene difluoride (PVDF) membrane and probed with anti‐serum (1 : 500). Load concentration for all the gels: 20 μg/well. (e) Double immunodiffusion to analyse the immunoprecipitation reaction of anti‐DLL antibodies towards crude DLL extract and purified DLL. Antigen/anti‐sera load volume: 10 μl/well. 1 = anti‐DLL antibody, 2 = purified DLL, 3 = crude DLL. A clear ‘v’‐shaped precipitin line indicating the pattern of immunologically identical proteins from the crude and purified DLL.
Fig. S2. Dolichos lablab lectin (DLL) exerts anti‐tumour potential on murine Dalton's lymphoma ascites tumour in vivo. Anti‐neoplasmic property of DLL was investigated in Dalton's lymphoma ascites (DLA) in‐vivo tumour model. Ascites tumour was induced in Swiss albino mice by injecting DLA cells (5 × 106 cells/mouse) intraperitoneally (i.p.). Treatment regimen included three doses of DLL on alternative days, administered in the concentration range of 20 and 30 mg/kg body weight (i.p.) followed by the evaluation of tumour parameters. (a) Regressed tumorigenic index in a dose‐ and concentration‐dependent manner indicative of the percentage reduction in the tumour growth, (b) contracted‐tumour cell population (c) and decrease in ascites secretion. All the parameters were evaluated in comparison to the respective control tumoral‐bearing animal. (d) Kaplan–Meier graph showing the prolonged lifespan of DLL‐treated animals. Results are the means of three determinations, each conducted in triplicate. Statistically significant values are *P < 0·05; ** P < 0·01.
Fig. S3. Dolichos lablab lectin (DLL) exhibits minimal adverse secondary complication on internal organs. To investigate the toxicological profiles of DLL in the internal organs, paraffin‐embedded liver and spleen sections of the normal and experimental animals were stained with haematoxylin and eosin (H&E) followed by evaluation under light microscopy. Images depicting the representative histological sections of liver (a) and spleen (b) at ×40 optical magnification. (c) Weight of liver and spleen.
Acknowledgements
B. T. P. gratefully acknowledges the grant extended by DBT (6242‐P37/RGCB/PMD/DBT/PBKR/2015), UGC (F. no. 41‐507/2012 (SR) and VGST (VGST/GRD231/CISEE/2013‐14). V. V. kindly acknowledges the fellowship received from University Grants Commission (UGC), Government of India [F. no.41‐1260/2012 (SR)]. S. N. P. kindly thanks the grant from UGC, Government of India [F. no.41‐1260/2012 (SR)].
Contributor Information
S. N. Pramod, Email: snpramod20@yahoo.com
B. T. Prabhakar, Email: pbtssc@gmail.com.
References
- 1. Rabinovich GA, Gabrilovich D, Sotomayor EM. Immunosuppressive strategies that are mediated by tumor cells. Annu Rev Immunol 2007; 25:267–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ghirelli C, Hagemann T. Targeting immunosuppression for cancer therapy. J Clin Invest 2013; 123:2355–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deepak P, Kumar S Jr, Kishore D, Acharya A. IL‐13 from Th2‐type cells suppresses induction of antigen‐specific Th1 immunity in a T‐cell lymphoma. Int Immunol 2010; 22:53–63. [DOI] [PubMed] [Google Scholar]
- 4. Rosenberg SA. IL‐2: the first effective immunotherapy for human cancer. J Immunol 2014; 192:5451–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Douglas H, Weinberg AR. Hallmarks of cancer: the next generation. Cell 2011; 144:646–74. [DOI] [PubMed] [Google Scholar]
- 6. Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst 1990; 82:4–6. [DOI] [PubMed] [Google Scholar]
- 7. Folkman J. Angiogenesis: an organizing principle for drug discovery? Nat Rev Drug Discov 2007; 6:273–86. [DOI] [PubMed] [Google Scholar]
- 8. Heine A, Held SA, Bringmann A, Holderried TA, Brossart P. Immunomodulatory effects of anti‐angiogenic drugs. Leukemia 2011; 25:899–905. [DOI] [PubMed] [Google Scholar]
- 9. Dickerson EB, Akhtar N, Steinberg H et al Enhancement of the antiangiogenic activity of interleukin‐12 by peptide targeted delivery of the cytokine to alphavbeta3 integrin. Mol Cancer Res 2004; 2:663–73. [PubMed] [Google Scholar]
- 10. Hayakawa Y, Takeda K, Yagita H et al IFN‐γ‐mediated inhibition of tumor angiogenesis by natural killer T‐cell ligand, α‐galactosylceramide. Blood 2002; 100:1728–33. [PubMed] [Google Scholar]
- 11. Beatty G, Paterson Y. IFN‐gamma‐dependent inhibition of tumor angiogenesis by tumor‐infiltrating CD4+ T cells requires tumor responsiveness to IFN‐gamma. J Immunol 2001; 166:2276–82. [DOI] [PubMed] [Google Scholar]
- 12. Lis H, Sharon N. Lectins: carbohydrate specific proteins that mediate cellular recognition. Chem Rev 1998; 98:637–74. [DOI] [PubMed] [Google Scholar]
- 13. Sharon N, Lis H. Legume lectins – a large family of homologous proteins. FASEB J 1990; 4:3198–208. [DOI] [PubMed] [Google Scholar]
- 14. Gowda LR, Savithri HS, Rao DR. The complete primary structure of a unique mannose/glucose‐specific lectin from field bean (Dolichos lab lab). J Biol Chem 1994; 269:18789–93. [PubMed] [Google Scholar]
- 15. Souza MA, Carvalho FC, Ruas LP, Ricci‐Azevedo R, Roque‐Barreira MC. The immunomodulatory effect of plant lectins: a review with emphasis on ArtinM properties. Glycoconj J 2013; 30:641–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Güran A, Tichá M, Filka K, Kocourek J. Isolation and properties of a lectin from the seeds of the Indian bean or lablab (Dolichos lablab L.). Biochem J 1983; 209:653–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Favero J, Miquel F, Dornand J, Mani JC. Determination of mitogenic properties and lymphocyte target sites of Dolichos lablab lectin (DLA): comparative study with concanavalin A and galactose oxidase cell surface receptors. Cell Immunol 1988; 112:302–14. [DOI] [PubMed] [Google Scholar]
- 18. Li WW, Yu JY, Xu HL, Bao JK. Concanavalin A: a potential anti neoplastic agent targeting apoptosis, autophagy and antiangiogenesis for cancer therapeutics. Biochem Biophys Res Commun 2011; 414:282–6. [DOI] [PubMed] [Google Scholar]
- 19. Mo H, Meah Y, Moore JG, Goldstein IJ. Purification and characterization of Dolichos lablab lectin. Glycobiology 1999; 9:173–9. [DOI] [PubMed] [Google Scholar]
- 20. Pramod SN, Venkatesh YP. Utility of pentose colorimetric assay for the purification of potato lectin, an arabinose‐rich glycoprotein. Glycoconj J 2006; 23:481–8. [DOI] [PubMed] [Google Scholar]
- 21. Clement F, Pramod SN, Venkatesh YP. Identity of the immunomodulatory proteins from garlic (Allium sativum) with the major garlic lectins or agglutinins. Int Immunopharmacol 2010; 10:316–24. [DOI] [PubMed] [Google Scholar]
- 22. Al‐Ghorbani M, Vigneshwaran V, Ranganatha VL, Prabhakar BT, Khanum SA. Synthesis of oxadiazole–morpholine derivatives and manifestation of the repressed CD31 microvessel density (MVD) as tumoral angiogenic parameters in Dalton's lymphoma. Bioorg Chem 2015; 60:136–46. [DOI] [PubMed] [Google Scholar]
- 23. Vijay Avin BR, Thirusangu P, Ranganatha VL, Firdouse A, Prabhakar BT, Khanum SA. Synthesis and tumor inhibitory activity of novel coumarin analogs targeting angiogenesis and apoptosis. Eur J Med Chem 2014; 75:211–21. [DOI] [PubMed] [Google Scholar]
- 24. Kruger EA, Duray PH, Tsokos MG et al Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun 2000; 268:183–91. [DOI] [PubMed] [Google Scholar]
- 25. Arnaoutova I, Kleinman HK. In vitro angiogenesis: endothelial cell tube formation on gelled basement membrane extract. Nat Protoc 2010; 5:628–35. [DOI] [PubMed] [Google Scholar]
- 26. Albini A, Benelli R. The chemoinvasion assay: a method to assess tumor and endothelial cell invasion and its modulation. Nat Protoc 2007; 2:504–11. [DOI] [PubMed] [Google Scholar]
- 27. Isha D, Nitesh K, Manjula SN, Vipan P, Manjunath SM, Pai KSR. Preliminary evaluation of in vitro cytotoxicity and in vivo antitumor activity of Premna herbacea Roxb. in Ehrlich ascites carcinoma model and Dalton's lymphoma ascites model. Exp Toxicol Pathol 2013; 65:235–42. [DOI] [PubMed] [Google Scholar]
- 28. Vijay Avin BR, Prabhu T, Ramesh CK et al New role of lupeol in reticence of angiogenesis, the cellular parameter of neoplastic progression in tumorigenesis models through altered gene expression. Biochem Biophys Res Commun 2014; 448:139–44. [DOI] [PubMed] [Google Scholar]
- 29. Park CC, Morel JC, Amin MA, Connors MA, Harlow LA, Koch AE. Evidence of IL‐18 as a novel angiogenic mediator. J Immunol 2001; 167:1644–53. [DOI] [PubMed] [Google Scholar]
- 30. Shan S, Lockhart AC, Saito WY, Knapp AM, Laderoute KR, Dewhirst MW. The novel tubulin‐binding drug BTO‐956 inhibits R3230AC mammary carcinoma growth and angiogenesis in Fischer 344 rats. Clin Cancer Res 2001; 7:2590–6. [PubMed] [Google Scholar]
- 31. Jayasooriya RG, Lee YG, Kang CH et al Piceatannol inhibits MMP‐9‐dependent invasion of tumor necrosis factor‐α‐stimulated DU145 cells by suppressing the Akt‐mediated nuclear factor‐κB pathway. Oncol Lett 2013; 5:341–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng Y, Ni Y, Huang X, Wang Z, Han W. Overexpression of HIF‐1α indicates a poor prognosis in tongue carcinoma and may be associated with tumour metastasis. Oncol Lett 2013; 5:1285–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mengfeng L. Tumor angiogenesis, antiangiogenic therapy and anti‐antiangiogenesis response In: Kaiser HE, Nasir A. eds. Selected aspects of cancer progression: metastasis, apoptosis and immune response. Netherlands: Springer, 2008:91–102. [Google Scholar]
- 34. Quach H, Ritchie D, Stewart AK et al Mechanism of action of immunomodulatory drugs (IMiDS) in multiple myeloma. Leukemia 2010; 24:22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen JN, Ma CY, Tsai PF, Wang YT, Wu JS. In vitro antitumor and immunomodulatory effects of the protein PCP‐3A from mushroom Pleurotus citrinopileatus . J Agric Food Chem 2010; 58:12117–22. [DOI] [PubMed] [Google Scholar]
- 36. Mumtaz YB, Qiangzhong M, Shazia A, Richard PJ. T cell exhaustion and interleukin 2 downregulation. Cytokine 2015; 71:339–47. [DOI] [PubMed] [Google Scholar]
- 37. Schietinger A, Greenberg PD. Tolerance and exhaustion: defining mechanisms of T cell dysfunction. Trends Immunol 2014; 35:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wherry EJ. T cell exhaustion. Nat Immunol 2011; 12:492–9. [DOI] [PubMed] [Google Scholar]
- 39. Tandle A, Blazer DG, Libutti SK. Antiangiogenic gene therapy of cancer: recent developments. J Transl Med 2004; 2:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhat TA, Singh RP. Tumor angiogenesis – a potential target in cancer chemoprevention. Food Chem Toxicol 2008; 46:1334–45. [DOI] [PubMed] [Google Scholar]
- 41. Sakkoula E, Synetos EP, Maragoudakis ME. Involvement of nitric oxide in the inhibition of angiogenesis by interleukin‐2. Br J Pharmacol 1997; 122:793–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kim HJ, Hawke N, Baldwin AS. NF‐κB and IKK as therapeutic targets in cancer. Cell Death Differ 2006; 13:738–47. [DOI] [PubMed] [Google Scholar]
- 43. Fong GH. Regulation of angiogenesis by oxygen sensing mechanisms. J Mol Med (Berl) 2009; 87:549–60. [DOI] [PubMed] [Google Scholar]
- 44. Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia‐inducible factor 1 is a basic‐helix‐loop‐helix‐PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA 1995; 92:5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thirusangu P, Vigneshwaran V, Prashanth T et al BP‐1T, an antiangiogenic benzophenone‐thiazole pharmacophore, counteracts HIF‐1 signalling through p53/MDM2‐mediated HIF‐1α proteasomal degradation. Angiogenesis 2017; 20:55–71. [DOI] [PubMed] [Google Scholar]
- 46. Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2002; 2:795–803. [DOI] [PubMed] [Google Scholar]
- 47. Belakavadi M, Prabhakar BT, Salimath BP. Butyrate‐induced proapoptotic and antiangiogenic pathways in EAT cells require activation of CAD and downregulation of VEGF. Biochem Biophys Res Commun 2005; 335:993–1001. [DOI] [PubMed] [Google Scholar]
- 48. Thirusangu P, Vigneshwaran V, Vijay Avin BR, Rakesh H, Vikas HM, Prabhakar BT. Scutellarein antagonizes the tumorigenesis by modulating cytokine VEGF mediated neoangiogenesis and DFF‐40 actuated nucleosomal degradation. Biochem Biophys Res Commun 2017; 484:85–92. [DOI] [PubMed] [Google Scholar]
- 49. Kardeh S, Ashkani‐Esfahani S, Alizadeh AM. Paradoxical action of reactive oxygen species in creation and therapy of cancer. Eur J Pharmacol 2014; 15:150–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Purification of Dolichos lablab lectin (DLL) by ovalbumin‐Sepharose 4B affinity chromatography. (a) Chromatograph of DLL purification on an ovalbumin‐Sepharose 4B column. DLL specifically eluted by α‐methyl mannoside, protein elutions monitored at 280 nm. The arrows indicate the point of elution. (b) Haemagglutination assay employing the serially diluted purified DLL starting with 400 µg concentration in concavity assay plate. The titre value of DLL is ∼3 μg, which represents the minimal concentration of the extracts required to produce visible agglutination at the highest dilution. (c) Periodic acid‐Schiff (PAS) staining of the purified DLL resolved on 12% sodium dodecyl sulphate‐polyacrylamide gel electrophoresis (SDS‐PAGE) to detect the glycoprotein nature of the proteins. Lane profile: M = marker, 1 = purified DLL, 2 = ovalbumin, 3 = bovine serum albumin. (d) Immunoblot analysis of rabbit anti‐DLL polyclonal antibodies. DLL resolved on 12% SDS‐PAGE was transferred to polyvinylidene difluoride (PVDF) membrane and probed with anti‐serum (1 : 500). Load concentration for all the gels: 20 μg/well. (e) Double immunodiffusion to analyse the immunoprecipitation reaction of anti‐DLL antibodies towards crude DLL extract and purified DLL. Antigen/anti‐sera load volume: 10 μl/well. 1 = anti‐DLL antibody, 2 = purified DLL, 3 = crude DLL. A clear ‘v’‐shaped precipitin line indicating the pattern of immunologically identical proteins from the crude and purified DLL.
Fig. S2. Dolichos lablab lectin (DLL) exerts anti‐tumour potential on murine Dalton's lymphoma ascites tumour in vivo. Anti‐neoplasmic property of DLL was investigated in Dalton's lymphoma ascites (DLA) in‐vivo tumour model. Ascites tumour was induced in Swiss albino mice by injecting DLA cells (5 × 106 cells/mouse) intraperitoneally (i.p.). Treatment regimen included three doses of DLL on alternative days, administered in the concentration range of 20 and 30 mg/kg body weight (i.p.) followed by the evaluation of tumour parameters. (a) Regressed tumorigenic index in a dose‐ and concentration‐dependent manner indicative of the percentage reduction in the tumour growth, (b) contracted‐tumour cell population (c) and decrease in ascites secretion. All the parameters were evaluated in comparison to the respective control tumoral‐bearing animal. (d) Kaplan–Meier graph showing the prolonged lifespan of DLL‐treated animals. Results are the means of three determinations, each conducted in triplicate. Statistically significant values are *P < 0·05; ** P < 0·01.
Fig. S3. Dolichos lablab lectin (DLL) exhibits minimal adverse secondary complication on internal organs. To investigate the toxicological profiles of DLL in the internal organs, paraffin‐embedded liver and spleen sections of the normal and experimental animals were stained with haematoxylin and eosin (H&E) followed by evaluation under light microscopy. Images depicting the representative histological sections of liver (a) and spleen (b) at ×40 optical magnification. (c) Weight of liver and spleen.


