Summary
Endothelial dysfunction leading to vascular leak is the hallmark of severe dengue. Vascular leak typically becomes clinically evident 3–6 days after the onset of illness, which is known as the critical phase. This critical phase follows the period of peak viraemia, and lasts for 24–48 hr and usually shows rapid and complete reversal, suggesting that it is likely to occur as a result of inflammatory mediators, rather than infection of the endothelium. Cytokines such as tumour necrosis factor‐α, which are known to be elevated in the critical phase of dengue, are likely to be contributing factors. Dengue NS1, a soluble viral protein, has also been shown to disrupt the endothelial glycocalyx and thus contribute to vascular leak, although there appears to be a discordance between the timing of NS1 antigenaemia and occurrence of vascular leak. In addition, many inflammatory lipid mediators are elevated in acute dengue viral infection such as platelet activating factor (PAF) and leukotrienes. Furthermore, many other inflammatory mediators such as vascular endothelial growth factor and angiopoietin‐2 have been shown to be elevated in patients with dengue haemorrhagic fever, exerting their action in part by inducing the activity of phospholipases, which have diverse inflammatory effects including generation of PAF. Platelets have also been shown to significantly contribute to endothelial dysfunction by production of interleukin‐1β through activation of the NLRP3 inflammasome and also by inducing production of inflammatory cytokines by monocytes. Drugs that block down‐stream immunological mediator pathways such as PAF may also be beneficial in the treatment of severe disease.
Keywords: dengue, lipid mediators, NS1 antigen, platelet activating factor, vascular leak
Introduction
Dengue viral infections represent one of the most rapidly emerging mosquito‐borne infections in the world, spreading to many geographical regions and causing almost 100 million apparent dengue infections each year.1 From 2005 to 2015, although the mortality rates due to many infectious diseases decreased, the deaths due to dengue increased by 48·7%, resulting in an estimated 18 400 deaths in 2015.2 Although there is now a dengue vaccine, which is licensed to be used in individuals over 9 years of age in several countries, it is only recommended in countries with high rates of dengue seroprevalence, due to its varied efficacy.3 Intense monitoring with meticulous fluid control is currently the only option in the management of acute dengue viral infection, as specific treatments for dengue are not yet available.
Although infection with the dengue virus (DENV) is associated with a self‐limiting illness in the majority of individuals, it can cause severe clinical disease manifestations such as dengue haemorrhagic fever (DHF) and organ involvement in a significant proportion of individuals.4, 5 Patients with dengue may progress through three clinical phases known as the febrile phase, the critical phase and the recovery phase.6 Once infected with DENV, after an initial incubation period of typically 3–7 days, the infection manifests with a sudden onset of high fever, accompanied by high viraemia, which is known as the febrile phase.6 Some individuals proceed to the critical phase, which lasts for 24–48 hours and is associated with plasma leakage; whereas others directly proceed to the recovery phase without developing plasma leakage.7 Severe dengue is associated with a transient increase in vascular permeability due to endothelial dysfunction in the critical phase.8 Increase in vascular permeability is associated with vascular leakage, resulting in accumulation of fluid in pleural and peritoneal cavities, and with reduction in blood pressure and pulse pressure, resulting in poor organ perfusion.5, 8 The post‐capillary venules have been shown to be the predominant site, where increased permeability due to vascular leak is observed.9 Changes in the microvascular circulation leading to reduced blood flow and perfusion have been shown to occur in patients who develop plasma leakage during the critical phase.10 Many cytokine mediators,11 mast cell products,12, 13 inflammatory lipid mediators14, 15, 16 and the disruption of the function of the endothelial glyocalyx by dengue NS117, 18 have been implicated in vascular leak. These will be discussed in turn.
Cytokines as a cause of vascular leak
Many pro‐inflammatory and immunosuppressive cytokines and chemokines are elevated in patients with dengue viral infection.19, 20, 21, 22 These pro‐inflammatory cytokines were thought to be produced by highly cross‐reactive T cells, which expand in secondary dengue infection, but are less effective in clearing the infecting virus serotype.23, 24, 25 However, subsequent studies have shown that DENV‐specific T cells are present in low frequency or undetectable during the leakage phase of acute dengue,26, 27 and produce minimal amounts of these cytokines.19 More recent studies suggest that DENV‐specific T cells could in fact be protective.28, 29 Instead it is more likely that other haematopoietic cells that are directly infected with the DENV such as dendritic cells, monocytes and macrophages are the predominant source of these cytokines through activation of innate immune pathways.30, 31 Although many types of pro‐inflammatory and immunosuppressive cytokines are seen at higher levels in patients with DHF when compared to those with dengue fever (DF),20, 32 the levels of these cytokines are different during the febrile and critical phases.4, 11, 30 While immunosuppressive cytokines such as interleukin‐10 (IL‐10) decrease during the critical phase, cytokines that have been associated with vascular leak such as tumour necrosis factor‐α (TNF‐α), tend to increase.4, 30 Although TNF‐α and many other cytokines are also increased in severe forms of influenza virus infection, the levels of some of these cytokines are typically lower than those detected in patients with DHF.33, 34 For instance, the median levels of TNF‐α in patients with severe influenza infection were below 5 pg/ml,33, 34 whereas the median levels of those with DHF have been reported to be well over 10 pg/ml during the critical phase, with levels > 40 pg/ml in some patients.19, 30, 32 Therefore, although vascular leak is not seen in infections such as influenza, which can be associated with a cytokine storm, the levels of many cytokines such as TNF‐α can be much higher in dengue infection, which may contribute to vascular leak.
In vitro experiments have shown that sera from patients with DHF do disrupt the endothelial monolayer, resulting in morphological changes.11 In dengue mouse models, use of TNF‐α blocking antibodies was associated with improved survival and reduction in liver involvement,35 suggesting that cytokines such as TNF‐α could indeed be contributing to the vascular leak in acute dengue (Fig. 1). Interleukin‐2 used in high doses has been shown to cause vascular leak syndrome in patients with renal carcinoma and melanoma.36 However, the contribution of IL‐2 in causing vascular leak in dengue is debatable, as some studies have shown that the IL‐2 is elevated,37 whereas others have shown that IL‐2 is actually reduced.19, 20
Figure 1.
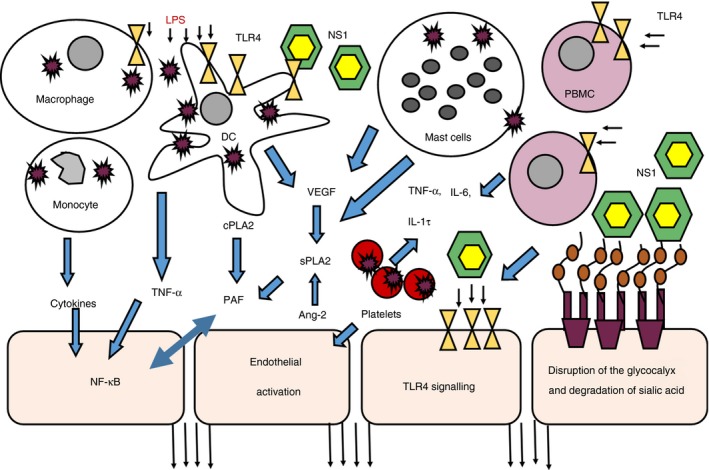
Model of endothelial dysfunction in dengue infection. Cytokines and tumour necrosis factor‐α (TNF‐α) produced by dengue virus (DENV) ‐infected monocytes, dendritic cells (DCs) and macrophages possibly cause endothelial activation and also activate nuclear factor‐κB (NF‐κB) to induce platelet‐activating factor (PAF) production. LPS present in the blood by possible microbial translocation amplifies this effect and also induces PAF production from these cells. Vascular endothelial growth factor (VEGF) produced by mast cells and other cells act on secretory phospholipase A2 (sPLA2) to induce production of PAF and mast cells are also a possible source of sPLA2, which leads to PAF production. Platelets, which are also directly infected by the DENV, produce many cytokines by forming platelet monocyte aggregates. Platelet‐derived microparticles, are an important source of interleukin‐1β (IL‐1β), which are produced by activation of the NLRP3 inflammasome. NS1 could also act through Toll‐like receptor 4 (TLR4) inducing phospholipases to generate PAF from phospholipids and also induce production of many inflammatory cytokines, which contribute to the ‘cytokine storm’. Ang‐2 produced by endothelial activation also in turn activated sPLA2 to induce PAF production. Dengue NS1 hexamers bind to the endothelial glycocalyx, resulting in loss of sialic acid. NS1 also leads to the cleavage of heparan sulphate from the endothelial glycocalyx.
Mast cell products associated with vascular leak
The importance of mast cells and their products such as cytokines and vascular endothelial growth factor (VEGF) in the pathogenesis of dengue is emerging.12, 38, 39 Levels of VEGF, chymase and tryptase were found to be higher in patients with dengue, especially in those with plasma leakage.12, 38 One early study showed that 24‐hr urine histamine content was elevated in patients with DHF40 and also that in murine models the increased permeability of the blood–brain barrier was significantly reduced by antihistamines in a dose‐dependent manner.41 In vitro studies have shown that mast cells are directly infected with the DENV and also that antibody‐dependent enhancement leads to increased mast cell degranulation in both in vitro and in vivo dengue mouse models.38, 42 Although mast cells are infected with the virus, the virus titres needed to infect mast cells have been shown to be several fold higher than other DENV permissive cells such as monocytes and dendritic cells.43 Therefore, DENV‐specific antibodies are thought to play a significant role in secondary dengue infections, by forming immune complexes with the DENV and thereby enhancing infection of mast cells via Fcγ receptors.38 This higher infection of mast cells is thought to lead to increased degranulation during secondary dengue viral infections, thereby leading to enhanced endothelial activation and vascular leakage.43
Although we and others have found that levels of mast cell tryptase, which is a protease specific to mast cells, are elevated in patients with DHF when compared with those with DF, their levels are still usually within the normal range.12, 16 Interestingly, the levels of tryptase and the activity of secretory phospholipases A2 (sPLA2) do associate with the degree of viraemia. However, the levels and the changes of serum mast cell tryptase in patients with primary or secondary dengue infection are similar. Others have found that although mast cell products such as VEGF are higher in patients with more severe forms of dengue, there is no difference in the levels of VEGF in those with primary and secondary dengue.44 As dengue viral infection is characterized by rapidly changing clinical and laboratory parameters, it would be important to evaluate several mast cell proteases and other mast cell mediators longitudinally during the course of illness in patients with DF and DHF and also in those with primary and secondary dengue viral infection. As VEGF is also produced by macrophages, endothelial cells, T cells and a variety of other cell types,45 the role of mast cells as a source of VEGF in dengue, and their contribution to disease pathogenesis should be further evaluated. However, as early studies showed that histamine released from mast cells could be playing a role in vascular leakage,40, 41 it would be important to also further investigate this mediator.
Lipid mediators
Platelet‐activating factor (PAF) is a phospholipid that has many physiological functions and exerts its actions in part by binding to a specific receptor known as PAF receptor.46 It is a potent inducer of increased vascular permeability in sepsis and anaphylaxis47 and has been shown to activate nuclear factor‐κB (NF‐κB), resulting in the expression of many inflammatory cytokines such as TNF‐α and IL‐1β.48, 49, 50 PAF is rapidly released by many cells such as endothelial cells, leucocytes, mast cells, macrophages and monocytes by the action of both secretory phospholipases and cytosolic phospholipases on phospholipids.51, 52 The PAF receptor is a G‐protein‐linked receptor and its expression is regulated by inflammatory responses and also by PAF itself.46 PAF has been shown to be associated with vascular leak in murine models of dengue viral infection, which can be reversed by PAF inhibitors.53 We recently showed that PAF was significantly elevated in patients with DHF, especially during the critical phase.14 Reduction in the expression of the tight junction protein ZO‐1 and reduction in trans‐endothelial electrical resistance induced by sera from patients with acute dengue was significantly blocked by the use of PAF receptor blockade,14 suggesting that PAF is an important mediator of increased endothelial permeability in acute dengue.
Phospholipase A2s comprise a group of enzymes generating inflammatory free fatty acids including PAF precursors and also lysophospholipids; and play a major role in the systemic inflammatory response.54, 55 The two major groups of phospholipase A2, which are the cytoplasmic phospholipase A2 and secretory sPLA2 are known to generate and regulate PAF.52, 56 We found that the sPLA2 activity was markedly higher in patients who developed vascular leak when compared with those with DF, very early during the course of illness.16 VEGF, which has been shown to be elevated in those with DHF, is also known to induce the production of sPLA2s56 (Fig. 1). It has been shown that the increase in vascular permeability due to VEGF was completely abolished by PAF receptor blockers in vivo in rodent models.57 Therefore, VEGF could be contributing to increasing endothelial permeability through PAF.
Among the many subtypes of sPLA2s, group IIA predominates in serum and its activity has been shown to be induced by many inflammatory stimuli and bacterial products such as lipopolysaccharide (LPS).52, 58 Microbial translocation has been shown to occur in dengue and higher LPS levels have been seen in those with vascular leakage when compared with those with DF.59, 60 We found that LPS acts synergistically with the DENV to induce production of PAF and many other inflammatory cytokines such as TNF‐α and IL‐6,30 thereby suggesting that LPS could contribute to the increased vascular permeability in acute dengue by inducing PAF, possibly through increasing activity of cPLA2 and sPLA2 (Fig. 1). As dengue NS1 has also been shown to act through Toll‐like receptor 4 (TLR4) and induce cytokine production,18 it is possible that NS1 could contribute to the increase in sPLA2 activity through TLR4.
Interestingly, there is a striking pattern in PAF levels in patients with dengue, with PAF levels being high in the morning and almost undetectable in early afternoon. This pattern was more prominently observed in those with DHF.14, 16 As changes in PAF levels have not been evaluated twice a day in other disease conditions such as sepsis and anaphylaxis, where high PAF levels are seen, it is not clear if this phenomenon is observed only in dengue viral infection. However, it has been shown that pro‐inflammatory cytokines such as TNF‐α, IL‐1β and interferon‐γ, induce PAF production by monocytes in a bi‐phasic manner.49 PAF is known to activate NF‐κB, which regulates the production of many inflammatory cytokines. Therefore, activation of NF‐κB by PAF could in turn lead to production of TNF‐α and IL‐1β, further leading to more PAF production.61 Indeed, we have observed that the kinetics of changes in TNF‐α and IL‐1β follow the same patterns as PAF in acute dengue.30
Sphingosine ‐1‐phosphate (S1P) is a signalling sphingolipid that enhances endothelial barrier integrity62, 63 and also counteracts the effects of VEGF on the vascular endothelium.62 It has been shown to oppose the actions of PAF in murine models by inhibiting the extent of vascular leak and impairing the inflammatory response driven by PAF.64, 65 We and others found that blood S1P levels were significantly lower in those with DHF, especially during the critical phase.15, 66 Platelets are an important source of S1P63 and we found that serum S1P levels significantly correlated with platelet counts in acute dengue.15 Therefore, reduction in platelet counts could contribute to a decrease in S1P levels, especially in the critical phase of dengue, leading to increase in the endothelial permeability.
Leukotrienes are generated by the action of lipoxygenase enzymes on arachidonic acid substrates, and include LTB4, LTC4, LTD4 and LTE4.67 Apart from being pro‐inflammatory mediators and potent chemoattractants, some are also known to increase vascular permeability and induce vascular leak.67 Increased activity of LTB4 has been observed in patients with acute dengue viral infection, and exposure of neutrophils to the DENV induced the production of LTB4.68 In addition, in experimental dengue mouse models, treatment with leukotriene receptor antagonists resulted in a significant reduction in plasma leakage in dengue‐infected mice,39 suggesting that leukotrienes may be playing an important role in fluid leakage in dengue.
Increase in endothelial permeability induced by dengue NS1
The NS1 protein of the DENV is a secretory lipoprotein that plays an essential role in viral replication intracellularly69 and causes many effects on the immune system in secretory forms.17, 18, 70, 71 It is present intracellularly as a monomer, on the cell membrane as a dimer and as a hexamer with a lipid rich core in the secretory form.72 Higher NS1 levels have been shown to associate with DHF73 and the NS1 antigen has been shown to persist for a longer duration in those with vascular leak.70, 74 DENV NS1 is known to interact with the complement system,75 and activates many innate immune cells to produce inflammatory cytokines by acting through TLR4;18 and also induces immunosuppressive cytokines such as IL‐10 from monocytes, thereby potentially contributing to disease pathogenesis.70 NS1 was shown to induce a dose‐dependent increase in the production of a number of pro‐inflammatory cytokines such as IL‐6, IL‐1β, TNF‐α from peripheral blood mononuclear cells, acting through TLR4, which is likely to contribute to the ‘cytokine storm’ associated with dengue.18 However, in vitro experiments carried out endothelial monolayers and in dengue mouse models suggest that NS1 could also play a role on the vascular endothelium and so induce vascular leak (Fig. 1).
In dengue mouse models, DENV NS1 of all serotypes increased the endothelial permeability in a dose‐dependent manner, which was abrogated by anti‐NS1 antibodies.17 Subsequent experiments showed that NS1 also disrupted the endothelial glycocalyx layer in human pulmonary vascular endothelial cells in a dose‐dependent manner and that exposure to NS1 resulted in loss of sialic acid from the cell surface.76 In addition, NS1 also induced shedding of the heparan sulphate proteoglycans, which contributed to disruption of the endothelial glycocalyx layer.76 Binding of NS1 to endothelial monolayers in vitro was maximal between 3 and 12 hr and returned to normal 48 hr after treatment with NS1.76
Studies carried out on patients with dengue have shown varied results regarding the presence of NS1 antigenaemia, its persistence and relation to clinical disease severity. Although we have found that patients who are likely to have NS1 antigenaemia beyond day 5 of illness, are likely to have more severe forms of dengue,74 some studies have found that NS1 antigen levels, especially during days 4–8 of illness, were lower in patients with more severe forms of illness.77 NS1 persistence and NS1 antigen levels found in acute infection have been shown to depend on the infecting DENV serotype, as those who are infected with DENV‐1 were found to have higher NS1 antigen levels and NS1 persistence beyond day 5 of illness.77, 78 A large study in Vietnam has shown that NS1 antigen levels were similar in primary and secondary infection and also in those with DF and DHF.78 As NS1 antigen levels appear to be similar in those who develop vascular leak and in those who do not, the contribution of NS1 to vascular leak should be further evaluated. Similar to observations in humans, where vascular leak becomes clinically detectable around day 4–7 of illness,7 mice injected with NS1 developed vascular leak approximately 3 days later.17 Therefore, vascular leak often appears to occur at a point in the illness when the NS1 antigen levels are often lower. Therefore, although NS1 induced endothelial permeability in endothelial cell monolayers, which was maximal during the first 24 hr, the relevance of this in vivo in patients with dengue should be further evaluated due to the discordance of the timing of vascular leak and NS1 antigenaemia.
Endothelial infection and production of inflammatory mediators
In vitro studies have shown that endothelial cells can be directly infected by the DENV and produce many inflammatory mediators that lead to endothelial dysfunction.79, 80, 81 In dengue mouse models, marked apoptosis of endothelial cells has been observed, which is thought to be due to direct infection of the cells and also due to TNF‐α.82, 83 It has also been shown using in vitro models that sera of patients with acute dengue resulted in endothelial cell apoptosis and lysis, which have been attributed to anti‐NS1 specific antibodies causing direct damage through nitric oxide and complement‐mediated damage.84, 85 Vectors that expressed DENV proteases were also shown to induce endothelial cell apoptosis in dengue mouse models, by inducing NF‐κB.86 However, autopsy studies have failed to show that endothelial cells are infected by the virus during dengue viral infection and have not demonstrated any endothelial damage.87, 88 Moreover, antigen–antibody complexes or complement deposition were not seen in any of these cases.87 The increase in endothelial permeability is transient in dengue, with the clinically evident fluid leakage phase lasting for 24–48 hr and the vascular leak completely ceasing following this phase. Therefore, as shown in autopsy studies in patients with dengue infection, it is questionable if significant direct endothelial infection by the DENV actually occurs in vivo.
Excessive endothelial activation has also shown to be an important cause of vascular leak through activation of NF‐κB.9 Excessive endothelial activation leads to the production of many inflammatory cytokines that lead to increased vascular permeability and inhibition of NF‐κB in sepsis mouse models, reduced vascular leak, reduced multi‐organ failure and lower survival.9 Therefore, rather than direct infection of the endothelium leading to endothelial apoptosis and dysfunction, it is more likely that the DENV could be causing widespread activation of the endothelium through NF‐κB, which contributes to vascular leak.
Angiopoietin and vascular leak
Angiopoietin 1 (ang‐1) and 2 (ang‐2) act through their tyrosine kinase receptor to maintain endothelial integrity. Although ang‐1 maintains endothelial barrier integrity, ang‐2 has an opposing effect and increases endothelial permeability.89 Ang‐2, which is exclusively produced by the endothelium, was found to be higher in those with severe plasma leakage, whereas ang‐1 levels were lower.89, 90 However, a more recent study that evaluated the disturbances in the microcirculation at several time‐points in clinical disease along with vascular endothelial activation markers found that although ang‐2 was elevated in those with plasma leakage, the ang‐2/ang‐1 ratios and plasma vascular cell adhesion molecule 1 levels were similar in those who developed plasma leakage, when compared with those who did not.10
Both ang‐1 and ang‐2 are important regulators of PAF production by the endothelium. The two angiopoietins were shown to induce PAF very rapidly in bovine endothelial cell lines in a bi‐phasic manner.91 The induction of PAF production by endothelial cell lines was abolished by the use of sPLA2 inhibitors, implying that ang‐1 and ang‐2 stimulated PAF production through activation of sPLA2.91 As we found that sPLA2 activity was significantly higher in those who developed plasma leakage16 and as sPLA2s type V is known to induce PAF production by endothelial cells,91 endothelial activation leading to increased production of ang‐2 could also be acting through sPLA2 to induce PAF (Fig. 1).
Platelets and vascular leak
Platelets in patients with dengue viral infection have been shown to express P‐selectin, which is an adhesion molecule that facilitates platelet binding to leucocytes.92 Platelets are known to form platelet–monocyte aggregates that correlate with the presence of thrombocytopenia, and these complexes significantly associate with vascular leak.92 Such platelet–monocyte interactions induce cytokine production by monocytes93, 94 and such platelet–monocyte interactions in dengue induce production of IL‐1β, IL‐8 and IL‐10 by monocytes.92 We and others have found that IL‐10, IL‐1β, monocyte chemoattractant protein 1 and IL‐8 levels were associated with severe dengue22, 95 and that monocytes were likely to be the predominant source of IL‐10.96 Apart from interaction with monocytes, platelets are also known to contribute to vascular permeability due to production of IL‐1β by platelet microparticles.97
High IL‐1β has been demonstrated in patients with severe dengue and is thought to associate with increase in vascular permeability.30, 97, 98 Platelet‐derived microparticles were shown to be an important source of IL‐1β during dengue infection, which is thought to be generated by activation of the NLRP3 inflammasome.97 Microparticles derived from platelets were shown to be enriched with the NLRP3 inflammasome in patients with dengue and reactive oxygen species have been shown to be responsible for the inflammasome activation.97 As the proportion of IL‐1β‐enriched platelets and microparticles was significantly higher in patients who developed vascular leak, IL‐1β could also be contributing to increased vascular permeability. Numerous studies have shown that platelets and megakaryocytes can be infected in vitro and also that active infection of platelets has been shown to occur in patients with acute dengue.99, 100, 101, 102 Such infection of platelets may lead to thrombocytopenia and cytokine production, and so potentially contribute to severe clinical disease.103
Apart from IL‐1β, it has been shown that serotonin released from microparticles of activated platelets also leads to vascular leak.104 Although red‐cell‐derived microparticles were elevated in patients with DHF and correlated with disease severity, platelet‐derived microparticles were found to be significantly reduced.105 Serum serotonin levels were found to be significantly lower in patients with acute dengue, especially in those with DHF.106 Therefore, it is unlikely that serotonin is playing a significant role in the vascular leak in patients with dengue.
Other mediators of vascular leak
Nitric oxide produced by the activated endothelium has been shown to modulate vascular permeability.107 The presence of endothelium‐derived nitric oxide was found to be higher in patients with DHF in the febrile phase, when compared with those with DF, although no changes were observed when these patients were followed longitudinally during illness.108 In another study, it was also shown that platelet‐derived nitric oxide was elevated in patients with DHF, and it was suggested that this led to vascular leak and haemorrhagic manifestations.109 However, the activity of nitric oxide appears to be crucial for antiviral cytokines to exert their effects, as nitric oxide synthase‐2 knockout mice, had increased viral loads, more severe disease and lethality when infected with the DENV.110 As nitric oxide also appears to play a protective role in acute dengue viral infection, its role in causing possible disease pathogenesis should be further investigated.
Other mediators that are known to cause vascular leak include bradykinins, complement proteins C3a and C5a, IL‐33, fibrin products, prostaglandins E2, F2a and D2.111, 112, 113 Patients with DHF were shown to have higher levels of C3a, C4a and C5a when compared with patients with DF, along with higher levels of factor D and lower levels of factor H.114 As the levels of C1q were normal in patients with DF and DHF, it has been suggested that a dysregulation of the alternative pathway of complement activation could contribute to complement activation.114 However, the role of complement components in mediating vascular leak in acute dengue has not been extensively studied and therefore, it is difficult to currently conclude on their role in increasing vascular permeability. However, complement components of both classical and alternative pathways have also been shown to cause liver damage in autopsies of children who died of DHF, suggesting that activation of complement could be playing a role in dengue.87 We have evaluated IL‐33 levels in patients with DHF and DF along with bradykinin at different phases of the illness. We did not observe any changes in either IL‐33 or bradykinin levels in patients with acute dengue, during any of the phases when compared with healthy volunteers (Malavige, unpublished). The role of prostaglandins in acute dengue has not been extensively investigated so far and it would be interesting to evaluate whether these mediators also play a role in the disease pathogenesis.
Conclusions
Endothelial dysfunction leading to increased vascular permeability is a hallmark of severe dengue, leading to leakage of fluid into pleural and peritoneal cavities and shock. Although cytokines such as TNF‐α, which are highly elevated in dengue, and are likely to result in increased vascular permeability, the roles of DENV‐NS1 antigen and lipid mediators such as PAF in causing vascular leak are emerging. It may be that in practice, there are several pathways that co‐contribute to vascular leak, but by understanding key mechanisms there may be opportunities for intervention. Therefore, although dengue vaccines that elicit neutralizing antibodies to DENV‐NS1 are likely to be helpful in reducing disease pathogenesis due to NS1, drugs that block PAF receptors or the pathways in which PAF is generated may be helpful in the treatment of acute illness. However, there are many other mediators that cause vascular leak which have not been investigated in patients with dengue and it would be important to further evaluate their role to develop therapeutics for treatment of disease.
Disclosures
No conflicts of interest.
Acknowledgements
We are grateful to the National Science Foundation, Sri Lanka (RPHS/2016/D06) and the MRC, UK and NIHR Biomedical Research Centre for the funding.
References
- 1. Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL et al The global distribution and burden of dengue. Nature 2013; 496:504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mortality GBD , Causes of Death C . Global, regional, and national life expectancy, all‐cause mortality, and cause‐specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388:1459–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deen J. The dengue vaccine dilemma: balancing the individual and population risks and benefits. PLoS Med 2016; 13:e1002182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fernando S, Wijewickrama A, Gomes L, Punchihewa CT, Madusanka SD, Dissanayake H et al Patterns and causes of liver involvement in acute dengue infection. BMC Infect Dis 2016; 16:319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lee TH, Lee LK, Lye DC, Leo YS. Current management of severe dengue infection. Expert Rev Anti Infect Ther 2017; 15:67–78. [DOI] [PubMed] [Google Scholar]
- 6. WHO , editor. Comprehensive Guidelines for Prevention and Control of Dengue Fever and Dengue Haemorrhagic Fever. New Delhi, India: SEARO, World Health Organization, 2011. [Google Scholar]
- 7. Malavige GN, Ogg GS. T cell responses in dengue viral infections. J Clin Virol 2013; 58:605–11. [DOI] [PubMed] [Google Scholar]
- 8. Yacoub S, Wertheim H, Simmons CP, Screaton G, Wills B. Microvascular and endothelial function for risk prediction in dengue: an observational study. Lancet 2015; 385(Suppl 1):S102. [DOI] [PubMed] [Google Scholar]
- 9. Steinberg BE, Goldenberg NM, Lee WL. Do viral infections mimic bacterial sepsis? The role of microvascular permeability: a review of mechanisms and methods. Antiviral Res 2012; 93:2–15. [DOI] [PubMed] [Google Scholar]
- 10. Yacoub S, Lam PK, le Vu HM, Le TL, Ha NT, Toan TT et al Association of microvascular function and endothelial biomarkers with clinical outcome in dengue: an observational study. J Infect Dis 2016; 214:697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Appanna R, Wang SM, Ponnampalavanar SA, Lum LC, Sekaran SD. Cytokine factors present in dengue patient sera induces alterations of junctional proteins in human endothelial cells. Am J Trop Med Hyg 2012; 87:936–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Furuta T, Murao LA, Lan NT, Huy NT, Huong VT, Thuy TT et al Association of mast cell‐derived VEGF and proteases in Dengue shock syndrome. PLoS Negl Trop Dis 2012; 6:e1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Srikiatkhachorn A, Ajariyakhajorn C, Endy TP, Kalayanarooj S, Libraty DH, Green S et al Virus‐induced decline in soluble vascular endothelial growth receptor 2 is associated with plasma leakage in dengue hemorrhagic fever. J Virol 2007; 81:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jeewandara C, Gomes L, Wickramasinghe N, Gutowska‐Owsiak D, Waithe D, Paranavitane SA et al Platelet activating factor contributes to vascular leak in acute dengue infection. PLoS Negl Trop Dis 2015; 9:e0003459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gomes L, Fernando S, Fernando RH, Wickramasinghe N, Shyamali NL, Ogg GS et al Sphingosine 1‐phosphate in acute dengue infection. PLoS One 2014; 9:e113394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jeewandara C, Gomes L, Udari S, Paranavitane SA, Shyamali NLA, Ogg GS et al Secretory phospholipase A2 in the pathogenesis of acute dengue infection. Immun Inflamm Dis 2016; 5:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Beatty PR, Puerta‐Guardo H, Killingbeck SS, Glasner DR, Hopkins K, Harris E. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 2015; 7:304ra141. [DOI] [PubMed] [Google Scholar]
- 18. Modhiran N, Watterson D, Muller DA, Panetta AK, Sester DP, Liu L et al Dengue virus NS1 protein activates cells via Toll‐like receptor 4 and disrupts endothelial cell monolayer integrity. Sci Transl Med 2015; 7:304ra142. [DOI] [PubMed] [Google Scholar]
- 19. Malavige GN, Huang LC, Salimi M, Gomes L, Jayaratne SD, Ogg GS. Cellular and cytokine correlates of severe dengue infection. PLoS One 2012; 7:e50387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Butthep P, Chunhakan S, Yoksan S, Tangnararatchakit K, Chuansumrit A. Alteration of cytokines and chemokines during febrile episodes associated with endothelial cell damage and plasma leakage in dengue hemorrhagic fever. Pediatr Infect Dis J 2012; 31:e232–8. [DOI] [PubMed] [Google Scholar]
- 21. Bozza FA, Cruz OG, Zagne SM, Azeredo EL, Nogueira RM, Assis EF et al Multiplex cytokine profile from dengue patients: MIP‐1β and IFN‐γ as predictive factors for severity. BMC Infect Dis 2008; 8:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Malavige GN, Gomes L, Alles L, Chang T, Salimi M, Fernando S et al Serum IL‐10 as a marker of severe dengue infection. BMC Infect Dis 2013; 13:341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dong T, Moran E, Vinh Chau N, Simmons C, Luhn K, Peng Y et al High pro‐inflammatory cytokine secretion and loss of high avidity cross‐reactive cytotoxic T‐cells during the course of secondary dengue virus infection. PLoS One 2007; 2:e1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mongkolsapaya J, Dejnirattisai W, Xu XN, Vasanawathana S, Tangthawornchaikul N, Chairunsri A et al Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 2003; 9:921–7. [DOI] [PubMed] [Google Scholar]
- 25. Moran E, Simmons C, Vinh Chau N, Luhn K, Wills B, Dung NP et al Preservation of a critical epitope core region is associated with the high degree of flaviviral cross‐reactivity exhibited by a dengue‐specific CD4+ T cell clone. Eur J Immunol 2008; 38:1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mongkolsapaya J, Duangchinda T, Dejnirattisai W, Vasanawathana S, Avirutnan P, Jairungsri A et al T cell responses in dengue hemorrhagic fever: are cross‐reactive T cells suboptimal? J Immunol 2006; 176:3821–9. [DOI] [PubMed] [Google Scholar]
- 27. Chau TN, Quyen NT, Thuy TT, Tuan NM, Hoang DM, Dung NT et al Dengue in Vietnamese infants – results of infection‐enhancement assays correlate with age‐related disease epidemiology, and cellular immune responses correlate with disease severity. J Infect Dis 2008; 198:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weiskopf D, Bangs DJ, Sidney J, Kolla RV, De Silva AD, de Silva AM et al Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci USA 2015; 112:E4256–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weiskopf D, Cerpas C, Angelo MA, Bangs DJ, Sidney J, Paul S et al Human CD8+ T‐cell responses against the 4 dengue virus serotypes are associated with distinct patterns of protein targets. J Infect Dis 2015; 212:1743–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kamaladasa A, Gomes L, Jeewandara C, Shyamali NL, Ogg GS, Malavige GN. Lipopolysaccharide acts synergistically with the dengue virus to induce monocyte production of platelet activating factor and other inflammatory mediators. Antiviral Res 2016; 133:183–90. [DOI] [PubMed] [Google Scholar]
- 31. Duran A, Valero N, Mosquera J, Delgado L, Alvarez‐Mon M, Torres M. Role of the myeloid differentiation primary response (MYD88) and TIR‐domain‐containing adapter‐inducing interferon‐β (TRIF) pathways in dengue. Life Sci 2016; 162:33–40. [DOI] [PubMed] [Google Scholar]
- 32. Priyadarshini D, Gadia RR, Tripathy A, Gurukumar KR, Bhagat A, Patwardhan S et al Clinical findings and pro‐inflammatory cytokines in dengue patients in Western India: a facility‐based study. PLoS One 2010; 5:e8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hagau N, Slavcovici A, Gonganau DN, Oltean S, Dirzu DS, Brezoszki ES et al Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care 2010; 14:R203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. To KK, Hung IF, Li IW, Lee KL, Koo CK, Yan WW et al Delayed clearance of viral load and marked cytokine activation in severe cases of pandemic H1N1 2009 influenza virus infection. Clin Infect Dis 2010; 50:850–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Phanthanawiboon S, Limkittikul K, Sakai Y, Takakura N, Saijo M, Kurosu T. Acute systemic infection with dengue virus leads to vascular leakage and death through tumor necrosis factor‐α and Tie2/angiopoietin signaling in mice lacking type I and II interferon receptors. PLoS One 2016; 11:e0148564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gallagher DC, Bhatt RS, Parikh SM, Patel P, Seery V, McDermott DF et al Angiopoietin 2 is a potential mediator of high‐dose interleukin 2‐induced vascular leak. Clin Cancer Res 2007; 13:2115–20. [DOI] [PubMed] [Google Scholar]
- 37. Kurane I, Innis BL, Nimmannitya S, Nisalak A, Meager A, Janus J et al Activation of T lymphocytes in dengue virus infections. High levels of soluble interleukin 2 receptor, soluble CD4, soluble CD8, interleukin 2, and interferon‐γ in sera of children with dengue. J Clin Investig 1991; 88:1473–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Syenina A, Jagaraj CJ, Aman SA, Sridharan A, St John AL. Dengue vascular leakage is augmented by mast cell degranulation mediated by immunoglobulin Fcγ receptors. Elife 2015; doi: 10.7554/eLife.05291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. St John ALR, Rathore AP, Raghavan B, Ng ML, Abraham SN. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus‐induced vascular leakage. Elife 2013; 2:e00481. doi: 10.7554/eLife.00481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tuchinda M, Dhorranintra B, Tuchinda P. Histamine content in 24‐hour urine in patients with dengue haemorrhagic fever. Southeast Asian J Trop Med Public Health 1977; 8:80–3. [PubMed] [Google Scholar]
- 41. Chaturvedi UC, Dhawan R, Khanna M, Mathur A. Breakdown of the blood–brain barrier during dengue virus infection of mice. J Gen Virol 1991; 72:859–66. [DOI] [PubMed] [Google Scholar]
- 42. Brown MG, Hermann LL, Issekutz AC, Marshall JS, Rowter D, Al‐Afif A et al Dengue virus infection of mast cells triggers endothelial cell activation. J Virol 2011; 85:1145–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. St John AL. Influence of mast cells on dengue protective immunity and immune pathology. PLoS Pathog 2013; 9:e1003783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Thakur P, Chakravarti A, Aggarwal S, Uppal B, Bhalla P. Elevated levels of vascular endothelial growth factor in adults with severe dengue infection. Virusdisease 2016; 27:48–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005; 109:227–41. [DOI] [PubMed] [Google Scholar]
- 46. Prescott SM, Zimmerman GA, Stafforini DM, McIntyre TM. Platelet‐activating factor and related lipid mediators. Annu Rev Biochem 2000; 69:419–45. [DOI] [PubMed] [Google Scholar]
- 47. Vadas P, Gold M, Perelman B, Liss GM, Lack G, Blyth T et al Platelet‐activating factor, PAF acetylhydrolase, and severe anaphylaxis. N Engl J Med 2008; 358:28–35. [DOI] [PubMed] [Google Scholar]
- 48. Han SJ, Ko HM, Choi JH, Seo KH, Lee HS, Choi EK et al Molecular mechanisms for lipopolysaccharide‐induced biphasic activation of nuclear factor‐κB (NF‐κB). J Biol Chem 2002; 277:44715–21. [DOI] [PubMed] [Google Scholar]
- 49. Valone FH, Epstein LB. Biphasic platelet‐activating factor synthesis by human monocytes stimulated with IL‐1β, tumor necrosis factor, or IFN‐γ . J Immunol 1988; 141:3945–50. [PubMed] [Google Scholar]
- 50. Im SY, Han SJ, Ko HM, Choi JH, Chun SB, Lee DG et al Involvement of nuclear factor‐κB in platelet‐activating factor‐mediated tumor necrosis factor‐α expression. Eur J Immunol 1997; 27:2800–4. [DOI] [PubMed] [Google Scholar]
- 51. Walterscheid JP, Ullrich SE, Nghiem DX. Platelet‐activating factor, a molecular sensor for cellular damage, activates systemic immune suppression. J Exp Med 2002; 195:171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Murakami M, Sato H, Miki Y, Yamamoto K, Taketomi Y. A new era of secreted phospholipase A(2). J Lipid Res 2015; 56:1248–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Souza DG, Fagundes CT, Sousa LP, Amaral FA, Souza RS, Souza AL et al Essential role of platelet‐activating factor receptor in the pathogenesis of Dengue virus infection. Proc Natl Acad Sci USA 2009; 106:14138–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lambeau G, Gelb MH. Biochemistry and physiology of mammalian secreted phospholipases A2. Annu Rev Biochem 2008; 77:495–520. [DOI] [PubMed] [Google Scholar]
- 55. Rosenson RS, Gelb MH. Secretory phospholipase A2: a multifaceted family of proatherogenic enzymes. Curr Cardiol Rep 2009; 11:445–51. [DOI] [PubMed] [Google Scholar]
- 56. Bernatchez PN, Winstead MV, Dennis EA, Sirois MG. VEGF stimulation of endothelial cell PAF synthesis is mediated by group V 14 kDa secretory phospholipase A2. Br J Pharmacol 2001; 134:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sirois MG, Edelman ER. VEGF effect on vascular permeability is mediated by synthesis of platelet‐activating factor. Am J Physiol 1997; 272:H2746–56. [DOI] [PubMed] [Google Scholar]
- 58. Corke C, Glenister K, Watson T. Circulating secretory phospholipase A2 in critical illness – the importance of the intestine. Crit Care Resusc 2001; 3:244–9. [PubMed] [Google Scholar]
- 59. van de Weg CA, Koraka P, van Gorp EC, Mairuhu AT, Supriatna M, Soemantri A et al Lipopolysaccharide levels are elevated in dengue virus infected patients and correlate with disease severity. J Clin Virol 2012; 53:38–42. [DOI] [PubMed] [Google Scholar]
- 60. van de Weg CA, Pannuti CS, de Araujo ES, van den Ham HJ, Andeweg AC, Boas LS et al Microbial translocation is associated with extensive immune activation in dengue virus infected patients with severe disease. PLoS Negl Trop Dis 2013; 7:e2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Choi IW, Kim YS, Kim DK, Choi JH, Seo KH, Im SY et al Platelet‐activating factor‐mediated NF‐κB dependency of a late anaphylactic reaction. J Exp Med 2003; 198:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Darwish I, Liles WC. Emerging therapeutic strategies to prevent infection‐related microvascular endothelial activation and dysfunction. Virulence 2013; 4:572–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schaphorst KL, Chiang E, Jacobs KN, Zaiman A, Natarajan V, Wigley F et al Role of sphingosine‐1 phosphate in the enhancement of endothelial barrier integrity by platelet‐released products. Am J Physiol Lung Cell Mol Physiol 2003; 285:L258–67. [DOI] [PubMed] [Google Scholar]
- 64. Adamson RH, Sarai RK, Altangerel A, Thirkill TL, Clark JF, Curry FR. Sphingosine‐1‐phosphate modulation of basal permeability and acute inflammatory responses in rat venular microvessels. Cardiovasc Res 2010; 88:344–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Camerer E, Regard JB, Cornelissen I, Srinivasan Y, Duong DN, Palmer D et al Sphingosine‐1‐phosphate in the plasma compartment regulates basal and inflammation‐induced vascular leak in mice. J Clin Investig 2009; 119:1871–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Michels M, Japtok L, Alisjahbana B, Wisaksana R, Sumardi U, Puspita M et al Decreased plasma levels of the endothelial protective sphingosine‐1‐phosphate are associated with dengue‐induced plasma leakage. J Infect 2015; 71:480–7. [DOI] [PubMed] [Google Scholar]
- 67. Bennett M, Gilroy DW. Lipid mediators in inflammation. Microbiol Spectr. 2016; doi: 10.1128/microbiolspec.MCHD‐0035‐2016. [DOI] [PubMed] [Google Scholar]
- 68. Loke WM, Chow AY, Lam Mok Sing K, Lee CY, Halliwell B, Lim EC et al Augmentation of 5‐lipoxygenase activity and expression during dengue serotype‐2 infection. Virol J 2013; 10:322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Mackenzie JM, Jones MK, Young PR. Immunolocalization of the dengue virus nonstructural glycoprotein NS1 suggests a role in viral RNA replication. Virology 1996; 220:232–40. [DOI] [PubMed] [Google Scholar]
- 70. Adikari TN, Gomes L, Wickramasinghe N, Salimi M, Wijesiriwardana N, Kamaladasa A et al Dengue NS1 antigen contributes to disease severity by inducing interleukin (IL)‐10 by monocytes. Clin Exp Immunol 2016; 184:90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chuang YC, Lin J, Lin YS, Wang S, Yeh TM. Dengue virus nonstructural protein 1‐induced antibodies cross‐react with human plasminogen and enhance its activation. J Immunol 2016; 196:1218–26. [DOI] [PubMed] [Google Scholar]
- 72. Muller DA, Young PR. The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 2013; 98:192–208. [DOI] [PubMed] [Google Scholar]
- 73. Libraty DH, Young PR, Pickering D, Endy TP, Kalayanarooj S, Green S et al High circulating levels of the dengue virus nonstructural protein NS1 early in dengue illness correlate with the development of dengue hemorrhagic fever. J Infect Dis 2002; 186:1165–8. [DOI] [PubMed] [Google Scholar]
- 74. Paranavitane SA, Gomes L, Kamaladasa A, Adikari TN, Wickramasinghe N, Jeewandara C et al Dengue NS1 antigen as a marker of severe clinical disease. BMC Infect Dis 2014; 14:570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Avirutnan P, Punyadee N, Noisakran S, Komoltri C, Thiemmeca S, Auethavornanan K et al Vascular leakage in severe dengue virus infections: a potential role for the nonstructural viral protein NS1 and complement. J Infect Dis 2006; 193:1078–88. [DOI] [PubMed] [Google Scholar]
- 76. Puerta‐Guardo H, Glasner DR, Harris E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog 2016; 12:e1005738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Duong V, Ly S, Lorn Try P, Tuiskunen A, Ong S, Chroeung N et al Clinical and virological factors influencing the performance of a NS1 antigen‐capture assay and potential use as a marker of dengue disease severity. PLoS Negl Trop Dis 2011; 5:e1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fox A, Le NM, Simmons CP, Wolbers M, Wertheim HF, Pham TK et al Immunological and viral determinants of dengue severity in hospitalized adults in Ha Noi, Viet Nam. PLoS Negl Trop Dis 2011; 5:e967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Warke RV, Xhaja K, Martin KJ, Fournier MF, Shaw SK, Brizuela N et al Dengue virus induces novel changes in gene expression of human umbilical vein endothelial cells. J Virol 2003; 77:11822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu P, Woda M, Ennis FA, Libraty DH. Dengue virus infection differentially regulates endothelial barrier function over time through type I interferon effects. J Infect Dis 2009; 200:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Huang YH, Lei HY, Liu HS, Lin YS, Liu CC, Yeh TM. Dengue virus infects human endothelial cells and induces IL‐6 and IL‐8 production. Am J Trop Med Hyg 2000; 63:71–5. [DOI] [PubMed] [Google Scholar]
- 82. Yen YT, Chen HC, Lin YD, Shieh CC, Wu‐Hsieh BA. Enhancement by tumor necrosis factor α of dengue virus‐induced endothelial cell production of reactive nitrogen and oxygen species is key to hemorrhage development. J Virol 2008; 82:12312–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen HC, Hofman FM, Kung JT, Lin YD, Wu‐Hsieh BA. Both virus and tumor necrosis factor α are critical for endothelium damage in a mouse model of dengue virus‐induced hemorrhage. J Virol 2007; 81:5518–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin CF, Lei HY, Shiau AL, Liu CC, Liu HS, Yeh TM et al Antibodies from dengue patient sera cross‐react with endothelial cells and induce damage. J Med Virol 2003; 69:82–90. [DOI] [PubMed] [Google Scholar]
- 85. Lin CF, Lei HY, Shiau AL, Liu HS, Yeh TM, Chen SH et al Endothelial cell apoptosis induced by antibodies against dengue virus nonstructural protein 1 via production of nitric oxide. J Immunol 2002; 169:657–64. [DOI] [PubMed] [Google Scholar]
- 86. Lin JC, Lin SC, Chen WY, Yen YT, Lai CW, Tao MH et al Dengue viral protease interaction with NF‐κB inhibitor α/β results in endothelial cell apoptosis and hemorrhage development. J Immunol 2014; 193:1258–67. [DOI] [PubMed] [Google Scholar]
- 87. Aye KS, Charngkaew K, Win N, Wai KZ, Moe K, Punyadee N et al Pathologic highlights of dengue hemorrhagic fever in 13 autopsy cases from Myanmar. Hum Pathol 2014; 45:1221–33. [DOI] [PubMed] [Google Scholar]
- 88. Rathi KR, Arora MM, Sahai K, Tripathi S, Singh SP, Raman DK et al Autopsy findings in fatal dengue haemorrhagic fever – 06 Cases. Med J Armed Forces India 2013; 69:254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Michels M, van der Ven AJ, Djamiatun K, Fijnheer R, de Groot PG, Griffioen AW et al Imbalance of angiopoietin‐1 and angiopoetin‐2 in severe dengue and relationship with thrombocytopenia, endothelial activation, and vascular stability. Am J Trop Med Hyg 2012; 87:943–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. van de Weg CA, Pannuti CS, van den Ham HJ, de Araujo ES, Boas LS, Felix AC et al Serum angiopoietin‐2 and soluble VEGF receptor 2 are surrogate markers for plasma leakage in patients with acute dengue virus infection. J Clin Virol 2014; 60:328–35. [DOI] [PubMed] [Google Scholar]
- 91. Maliba R, Lapointe S, Neagoe PE, Brkovic A, Sirois MG. Angiopoietins‐1 and ‐2 are both capable of mediating endothelial PAF synthesis: intracellular signalling pathways. Cell Signal 2006; 18:1947–57. [DOI] [PubMed] [Google Scholar]
- 92. Hottz ED, Medeiros‐de‐Moraes IM, Vieira‐de‐Abreu A, de Assis EF, Vals‐de‐Souza R, Castro‐Faria‐Neto HC et al Platelet activation and apoptosis modulate monocyte inflammatory responses in dengue. J Immunol 2014; 193:1864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Weyrich AS, Elstad MR, McEver RP, McIntyre TM, Moore KL, Morrissey JH et al Activated platelets signal chemokine synthesis by human monocytes. J Clin Investig 1996; 97:1525–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Dixon DA, Tolley ND, Bemis‐Standoli K, Martinez ML, Weyrich AS, Morrow JD et al Expression of COX‐2 in platelet–monocyte interactions occurs via combinatorial regulation involving adhesion and cytokine signaling. J Clin Investig 2006; 116:2727–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Lee YR, Liu MT, Lei HY, Liu CC, Wu JM, Tung YC et al MCP‐1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J Gen Virol 2006; 87:3623–30. [DOI] [PubMed] [Google Scholar]
- 96. Malavige GNJC, Alles KML, Salimi M, Gomes L, Kamaladasa A, Jayaratne S et al Suppression of virus specific immune responses by IL‐10 in acute dengue infection. PLoS Negl Trop Dis 2013; 7:e2409. doi: 10.1371/journal.pntd.0002409. eCollection 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Hottz ED, Lopes JF, Freitas C, Valls‐de‐Souza R, Oliveira MF, Bozza MT et al Platelets mediate increased endothelium permeability in dengue through NLRP3‐inflammasome activation. Blood 2013; 122:3405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Suharti C, van Gorp EC, Setiati TE, Dolmans WM, Djokomoeljanto RJ, Hack CE et al The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb Haemost 2002; 87:42–6. [PubMed] [Google Scholar]
- 99. Simon AY, Sutherland MR, Pryzdial EL. Dengue virus binding and replication by platelets. Blood 2015; 126:378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Noisakran S, Gibbons RV, Songprakhon P, Jairungsri A, Ajariyakhajorn C, Nisalak A et al Detection of dengue virus in platelets isolated from dengue patients. Southeast Asian J Trop Med Public Health 2009; 40:253–62. [PubMed] [Google Scholar]
- 101. Clark KB, Hsiao HM, Bassit L, Crowe JE Jr, Schinazi RF, Perng GC et al Characterization of dengue virus 2 growth in megakaryocyte‐erythrocyte progenitor cells. Virology 2016; 493:162–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Hsu AY, Wu SR, Tsai JJ, Chen PL, Chen YP, Chen TY et al Infectious dengue vesicles derived from CD61+ cells in acute patient plasma exhibited a diaphanous appearance. Sci Rep 2015; 5:17990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ojha A, Nandi D, Batra H, Singhal R, Annarapu GK, Bhattacharyya S et al Platelet activation determines the severity of thrombocytopenia in dengue infection. Sci Rep 2017; 7:41697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Cloutier N, Pare A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S et al Platelets can enhance vascular permeability. Blood 2012; 120:1334–43. [DOI] [PubMed] [Google Scholar]
- 105. Punyadee N, Mairiang D, Thiemmeca S, Komoltri C, Pan‐Ngum W, Chomanee N et al Microparticles provide a novel biomarker to predict severe clinical outcomes of dengue virus infection. J Virol 2015; 89:1587–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kumar Y, Liang C, Bo Z, Rajapakse JC, Ooi EE, Tannenbaum SR. Serum proteome and cytokine analysis in a longitudinal cohort of adults with primary dengue infection reveals predictive markers of DHF. PLoS Negl Trop Dis 2012; 6:e1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kubes P, Granger DN. Nitric oxide modulates microvascular permeability. Am J Physiol 1992; 262:H611–5. [DOI] [PubMed] [Google Scholar]
- 108. Thein TL, Wong J, Leo YS, Ooi EE, Lye D, Yeo TW. Association between increased vascular nitric oxide bioavailability and progression to dengue hemorrhagic fever in adults. J Infect Dis 2015; 212:711–4. [DOI] [PubMed] [Google Scholar]
- 109. Matsuura C, Moraes TL, Barbosa JB, Moss MB, Siqueira MA, Mann GE et al Nitric oxide activity in platelets of dengue haemorrhagic fever patients: the apparent paradoxical role of ADMA and l‐NMMA. Trans R Soc Trop Med Hyg 2012; 106:174–9. [DOI] [PubMed] [Google Scholar]
- 110. Fagundes CT, Costa VV, Cisalpino D, Amaral FA, Souza PR, Souza RS et al IFN‐γ production depends on IL‐12 and IL‐18 combined action and mediates host resistance to dengue virus infection in a nitric oxide‐dependent manner. PLoS Negl Trop Dis 2011; 5:e1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Choi YS, Choi HJ, Min JK, Pyun BJ, Maeng YS, Park H et al Interleukin‐33 induces angiogenesis and vascular permeability through ST2/TRAF6‐mediated endothelial nitric oxide production. Blood 2009; 114:3117–26. [DOI] [PubMed] [Google Scholar]
- 112. Chizzolini C, Brembilla NC. Prostaglandin E2: igniting the fire. Immunol Cell Biol 2009; 87:510–1. [DOI] [PubMed] [Google Scholar]
- 113. Khan MA, Maasch C, Vater A, Klussmann S, Morser J, Leung LL et al Targeting complement component 5a promotes vascular integrity and limits airway remodeling. Proc Natl Acad Sci USA 2013; 110:6061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Nascimento EJ, Silva AM, Cordeiro MT, Brito CA, Gil LH, Braga‐Neto U et al Alternative complement pathway deregulation is correlated with dengue severity. PLoS One 2009; 4:e6782. [DOI] [PMC free article] [PubMed] [Google Scholar]


