Summary
Rationale: Ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, decreases sweat chloride concentration, and improves pulmonary function in 6% of cystic fibrosis (CF) patients with specific CFTR mutations. Ivacaftor increases chloride transport in many other CFTR mutations in non-human cells, if CFTR is in the epithelium. Some CF patients have CFTR in the epithelium with residual CFTR function. The effect of ivacaftor in these patients is unknown. Methods: This was a series of randomized, crossover N-of-1 trials of ivacaftor and placebo in CF patients ≥8 years old with potential residual CFTR function (intermediate sweat chloride concentration, pancreatic sufficient, or mild bronchiectasis on chest CT). Human nasal epithelium (HNE) was obtained via nasal brushing and cultured. Sweat chloride concentration change was the in vivo outcome. Chloride current change in HNE cultures with ivacaftor was the in vitro outcome. Results: Three subjects had decreased sweat chloride concentration (−14.8 to −40.8 mmol/L, P<0.01). Two subjects had unchanged sweat chloride concentration. Two subjects had increased sweat chloride concentration (+23.8 and +27.3 mmol/L, P<0.001); both were heterozygous for A455E and pancreatic sufficient. Only subjects with decreased sweat chloride concentration had increased chloride current in HNE cultures. Conclusions: Some CF patients with residual CFTR function have decreased sweat chloride concentration with ivacaftor. Increased chloride current in HNE cultures among subjects with decreased sweat chloride concentrations may predict clinical response to ivacaftor. Ivacaftor can increase sweat chloride concentration in certain mutations with unclear clinical effect.
Keywords: cystic fibrosis, ivacaftor, CFTR modulators, N-of-1 studies, personalized medicine, sweat chloride concentration, CFTR, human nasal epithelium
INTRODUCTION
The prognosis of cystic fibrosis (CF) has significantly changed for some patients with the emergence of a new class of drugs that target the underlying cause of CF: Defective or absent CF transmembrane conductance regulator (CFTR). Ivacaftor, a CFTR potentiator, increases epithelial chloride transport through CFTR.1,2 Lumacaftor, a CFTR corrector, chaperones the folding and insertion of CFTR into the epithelium.3,4 Ivacaftor and ivacaftor combined with lumacaftor result in decreased sweat chloride concentration, improved pulmonary function, fewer pulmonary exacerbations, and improved mortality,5–9 but were tested and FDA-approved for only a few CFTR mutations. Ivacaftor is approved for R117H and nine CFTR class III mutations, which encompasses 6% of CF patients in the US.10–12 Lumacaftor is approved only for F508del homozygotes, which is 46% of CF patients in the US.13 Almost half of CF patients do not have an indication for nor access to CFTR modulators14; some might benefit and are being left behind.
Randomized controlled trials (RCTs) are the standard for clinical trials testing efficacy of new drugs in clinical trials. However, RCTs require a large homogeneous population to predict drug efficacy. There are over 2,000 CFTR mutations, many of them rare, with millions of potential combinations13; therefore, it is impossible to conduct RCTs of ivacaftor for every mutation.15
The N-of-1 study design allows for rigorous investigation into treatment effectiveness of CFTR modulators in individual subjects with any combination of CFTR mutations, including rare or de novo mutations. It is a design that helps close the gap between large population-based evidence and clinical practice. It will likely play an important role in the development of evidence-based personalized medicine. Since each subject serves as his or her own control, the N-of-1 trial design allows for broad inclusion criteria and diverse subjects, unlike traditional RCTs that require a homogenous study population.16–18 In N-of-1 studies, each subject is considered a separate trial as each subject receives both drug and placebo, and because of this, it is superior to a traditional case series report.16–18
Little is known about the effect of CFTR modulators on the majority of CFTR mutations.15 While many mutations result in no CFTR protein in the epithelium, some mutations result in production and insertion of CFTR protein in the epithelium, resulting in residual CFTR function. Residual CFTR function can manifest clinically as an intermediate sweat chloride concentration,19,20 pancreatic sufficiency,20,21 or less severe pulmonary disease.20,22–24 In non-human cells, ivacaftor increases CFTR channel open time and chloride transport in CFTR in many CFTR mutations, if there is CFTR protein in the epithelium.2,14 Ivacaftor decreases sweat chloride concentration with clinical benefits in patients with R117H and P67L, mutations that result in residual CFTR chloride transport.10,25,26 There are potentially patients with residual CFTR function who would respond to ivacaftor, based on the effect of ivacaftor in these previous studies.
We hypothesized that some patients with residual CFTR function would respond to ivacaftor. Rather than target specific CFTR mutations we randomly enrolled patients with potential residual CFTR function (intermediate sweat chloride concentration, pancreatic sufficient, or mild bronchiectasis). Using the N-of-1 study design, we investigated both the in vivo and in vitro effects of ivacaftor in CF patients with potential residual CFTR function. Sweat chloride concentration and pulmonary function change were the in vivo outcome. Chloride current change in the subject’s nasal epithelia (HNE) cultures was the in vitro outcome.
METHODS
Study Design
This was a series of N-of-1 studies of ivacaftor that utilized a double-blinded, randomized, crossover design. In N-of-1 studies, each subject served as his or her own control.16–18 The institutional review board (IRB) at the University of California, San Francisco approved the study (12-09712). The study was conducted in accordance with the Declaration of Helsinki. Written, age-appropriate informed consent/assent was obtained from all subjects or their legal guardians. Subjects completed the trial between January and July 2014.
Study Population
This study included clinically stable subjects age 16 years and older with CF and potential residual CFTR function. Potential residual CFTR function was defined as either history of pancreatic sufficiency, intermediate sweat chloride concentration (40–80 mmol/L), or mild bronchiectasis on chest CT in association with two severe CFTR mutations. Exclusion criteria included CFTR class III mutations, R117H mutation, and F508del homozygotes. Subjects continued on all pre-study medications, except for those contraindicated with ivacaftor (CYP3A inducers and inhibitors).
Intervention
Eligible subjects were randomized to ivacaftor 150 mg by mouth twice a day for 14 days followed by placebo for 14 days, or vice versa. Randomization was based on a computer-generated schedule produced by our research pharmacy, which was concealed from study personnel until study completion. Ivacaftor was purchased at full retail cost and encapsulated with sucrose to match the sucrose-filled placebo capsules. Prior to beginning study drug, there was a 2-week run-in period to ensure clinical stability, assessed by modified Fuchs criteria27 and pulmonary function. There was a washout period of a minimum of 14 days between study drug cycles to account for carryover effect. The washout period was extended to 6 weeks for subjects on alternating cycles of inhaled antibiotics to coordinate the study drug cycle with the inhaled antibiotic cycle.
Interim history, vital signs, physical exam, and laboratory tests (sweat chloride test, spirometry, complete blood count, liver function tests, and pregnancy test in all female subjects) were completed at each study visit. Adherence was estimated by pill count and subject report.
In Vivo Outcomes
The primary outcome measure was the absolute change in sweat chloride concentration from baseline. Sweat chloride concentration was measured by an approved standardized method.28 The secondary outcome measure was the change in forced expiratory volume in one second (FEV1) percent predicted. Spirometry was performed according to American Thoracic Society standards at the beginning and end of each intervention period.29 FEV1 percent predicted was calculated using Eigen–Wang–NHANES III equations.30
In Vitro Outcomes
Nasal brushing of the nasal turbinates was performed to obtain human nasal epithelium (HNE) from each subject. HNE cells were isolated and cultured for 21–28 days on 12 mm Snapwell cell culture inserts (Corning Inc., Corning, NY) to insure full differentiation. HNE cultures were mounted to water-jacketed EasyMount Ussing chambers (Physiologic Instruments, San Diego, CA). Transepithelial chloride current was measured using a 4-electrode voltage clamp after acute exposure to ivacaftor (1 μM; apical). Ivacaftor was tested in presence of amiloride (100 μM; apical) to block sodium absorption and forskolin (20 μM; serosal) to activate cAMP-dependent CFTR chloride transport. Patient-specific CFTR chloride currents were quantified with 50 μM CFTRinh172 (CF Foundation Therapeutics, Inc., Bethesda, MD). Ivacaftor was purchased from Sell-eckchem.com. Protocol details are in the e-Appendix 1.
Statistical Analysis
Paired Student’s t-test and fixed effects linear regression models were used to compare continuous variables. In vitro transepithelial current change was analyzed with paired t-test using SigmaPlot, Systat (San Jose, CA). Statistical analysis was performed using STATA 12.1 (College Station, TX). A P-value of 0.05 was the cutoff for statistical significance.
RESULTS
A total of ten subjects were enrolled and seven subjects completed the study between January and July 2014 (Fig. 1). Mean adherence was 98.2% on ivacaftor and 99.6% on placebo. Adverse events included one episode each of epistaxis, injected conjunctiva, and ileus. Three subjects dropped out due to respiratory illness. Baseline subject characteristics are shown in Table 1.
Fig. 1.
Flowchart of study enrollment and completion.
TABLE 1.
Subject Characteristics
| Subject | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
|---|---|---|---|---|---|---|---|
| CFTR genotype | F508del | G542X | F508del | R334W | 1717-1G->A | 1717-1G->A | F508del |
| 1154insTC | 3849 +10kbC->T | Y563N | 681delC | G85E | A455E | A455E | |
| Age (years) | 20 | 59 | 16 | 25 | 27 | 20 | 19 |
| Sex | Male | Male | Female | Male | Female | Female | Female |
| Race/ethnicity | White | White | White | Persian | Latino | White | White |
| Pancreatic sufficient | No | Yes | Yes | Yes | No | Yes | Yes |
| Sweat chloride concentration (mmol/L) | 134 | 56 | 108 | 109 | 106 | 78 | 66 |
| Baseline FVC (%) | N/A | 69% | 71% | 58% | 101% | 92% | 116% |
| Baseline FEV1 (%) | N/A | 50% | 50% | 54% | 77% | 67% | 115% |
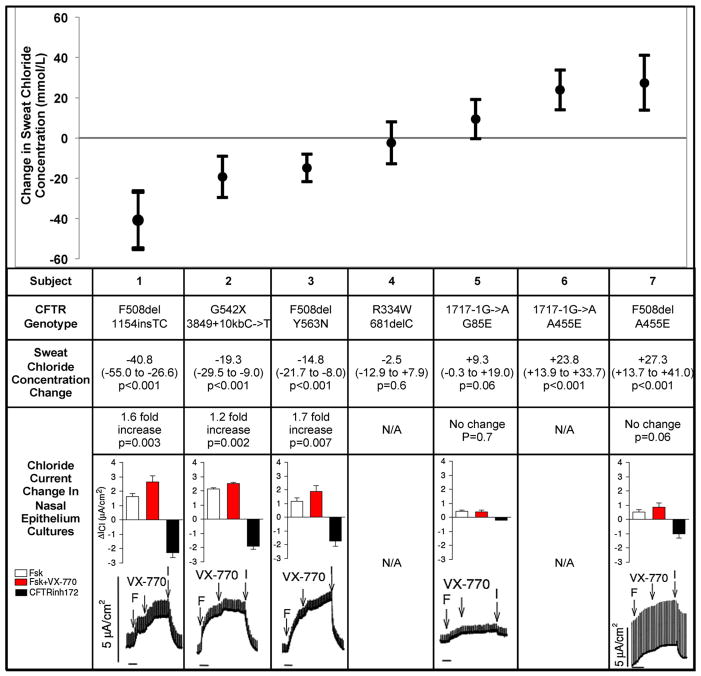
Three subjects had decreased sweat chloride concentration with ivacaftor; response ranged from −14.8 mmol/L to −40.8 mmol/L (Fig. 2). The HNE cultures of all three subjects had a significant increase in chloride current with acute ivacaftor exposure. Of these three subjects, two of the subjects had pancreatic sufficiency as a sign of potential residual CFTR function; the other subject had mild bronchiectasis on chest CT.
Fig. 2.
Sweat chloride concentration and nasal epithelium chloride current change with ivacaftor. This figure compares subjects’ sweat chloride concentration changes and chloride current changes in cultured nasal cells with ivacaftor. Subjects are listed in order of sweat chloride concentration change (mmol/L) with ivacaftor from greatest decrease to greatest increase. The CFTR mutations for each subject are shown. Baseline cAMP-dependent CFTR chloride current (μA/cm2) with forskolin (20 μM) was measured for individual subjects in the cultured nasal cells (white column). The CFTR chloride current change with acute exposure to ivacaftor (1 μM) and forskolin was measured (red column). The black column shows the CFTR chloride current inhibition with CFTRinh172 (50 μM). The Ussing chamber tracings of CFTR chloride current with forskolin (F), ivacaftor (VX-770), and CFTRinh172 (I) are shown for each subject.
Sweat chloride concentration increased with ivacaftor in two subjects; response ranged from +23.8 to +27.3. One subject’s HNE culture did not have a significant change in chloride current with acute ivacaftor exposure. The other subject did not have viable HNE cultures despite multiple nasal brushings attempts. Both subjects had pancreatic sufficiency as a sign of potential residual CFTR function.
Two subjects did not have a significant change in sweat chloride concentration with ivacaftor. One subject’s HNE culture did not have significant chloride change with ivacaftor. The other subject withdrew consent to nasal brushing due to concern for discomfort. One subject was pancreatic sufficient and the other had intermediate sweat chloride concentration as a sign of potential residual CFTR function.
All subjects with decreased sweat chloride concentrations also had significant increases in chloride current with acute ivacaftor exposure. The subjects that either had no change or increased sweat chloride concentration on sweat testing had no significant change in chloride current in the HNE cultures.
None of the subjects had a change in pulmonary function during a 6-week study period regardless of the sweat chloride concentration change with ivacaftor treatment.
DISCUSSION
This series of randomized, double-blinded, placebo-controlled N-of-1 trials investigated the in vivo and in vitro effects of ivacaftor, a CFTR potentiator, in CF patients with potential residual CFTR function, rather than targeting specific CFTR mutations as in traditional randomized control trials. Three subjects had a decrease in sweat chloride concentration with ivacaftor treatment while, unexpectedly, two subjects had increase in sweat chloride concentration. The HNE cultures with significant increases in chloride current were observed from the three subjects with decreases in sweat chloride concentrations. This study expands upon in vitro studies showing that ivacaftor potentiates CFTR protein in the epithelium from some residual function CFTR mutations.1,2,14
The sweat chloride concentration decreases observed in our subjects are similar or greater than observed in previous CFTR modulator studies. Subject 1 had a decrease in sweat chloride concentration of −40 mmol/L, which is similar to the decrease in sweat chloride concentration observed in ivacaftor trials in class III CFTR mutations.5,6,8,11 Subject 1’s baseline sweat chloride concentration was 134 mmol/L, as is seen in some CF patients24, and it is not known if even with a 40 mmol/L decrease whether ivacaftor would confer a long-term clinical benefit. The sweat chloride concentration decrease in Subjects 2 and 3 (−19.3 and −14.8 mmol/L) was slightly less than the mean decrease observed in trials of ivacaftor in R117H mutations.10,12 All three subjects had a sweat chloride concentration decrease that was greater than that observed in trials of lumacaftor–ivacaftor in patients with F508del/F508del.31,32
The subjects with decreased sweat chloride concentrations and increased chloride current in HNE cultures carry particular CFTR genotypes that may explain the response to ivacaftor. Subject 1’s CFTR mutations are F508del and 1154InsTC, which are both severe mutations that classically do not produce mature CFTR protein. F508del is a missense mutation that leads to misfolded protein, impaired trafficking, and dysfunctional channel gating.33 However, about 10–15% of F508del homozygotes have some mature CFTR protein with residual CFTR function and ivacaftor potentiates CFTR protein from F508del when in the epithelium,2,34 although this has not yet been investigated clinically in humans. Subject 1’s observed response to ivacaftor may be due to a similar phenomenon. The 1154InsTC mutation shifts the reading frame to introduce a termination codon at amino acid residue 369 and is classified as a class 1 mutation.35 The truncated protein is thought to not be functional. Our functional data showed a significant stimulatory effect by VX-770 that was larger than in cultures homozygous for F508del CFTR (data not shown) suggesting that there may have been read-through of the 1154InsTC mutation. However, to our knowledge, there are no reports that quantified the amount of read-through of this mutation. Subject 2’s CFTR mutations are G542X, a nonsense mutation with no CFTR protein produced,36 and mutation 3849 +10kbC- >T, with a partially active splice site that results in both normally and aberrantly spliced CFTR RNA.37 Ivacaftor increases chloride current and channel open probability of normal CFTR protein in Fischer Rat Thyroid cells.1 The decreased sweat chloride concentration in Subject 2 is likely due to ivacaftor potentiating the fraction of normal CFTR protein produced from 3849 +10kbC- >T. Subject 3 has CFTR mutations F508del and Y563N, a rare missense mutation that disrupts CFTR folding, but does produce some membrane expression of mutant CFTR protein.38 Subject 3’s response may involve ivacaftor potentiating CFTR protein from Y563N or from F508del, as described above with Subject 1.
Unexpectedly, two subjects had a significant increase in sweat chloride concentration with ivacaftor. There was no change in pulmonary function with ivacaftor treatment in either of these subjects. Both subjects were heterozygous for mutation A455E. Subject 7’s chloride current in the cultured cells increased with ivacaftor stimulation, although it was less than 5% of normal CFTR chloride transport and did not reach statistical significance (P = 0.06). Acute ivacaftor exposure was shown to increase A455E-CFTR chloride current when recombinantly expressed in Fischer Rat Thyroid cells.14 The chloride current increase we observed in the subject’s cultured cells was likely the effect of ivacaftor stimulating A455E CFTR protein, however, the response was less robust than what was observed in Fischer Rat Thyroid cells. A455E is a class V CFTR mutation that produces CFTR protein with normal function, but turns over rapidly in the cell membrane,39 resulting in only 11.5% of normal CFTR activity.2,38 In vitro studies show that ivacaftor destabilizes CFTR protein from CFTR mutation F508del, resulting in decreased amount of mature CFTR protein in the epithelium.40,41 Since A455E CFTR protein is unstable at baseline, ivacaftor may further destabilize the protein, resulting in decreased mature CFTR in the cell membrane, as it does in F508del. This destabilization effect on CFTR may explain the subjects’ increased sweat chloride concentration and less than robust chloride current increase in cultured cells. Further studies of ivacaftor in patients with an A445E mutation are needed to know if this potentially adverse effect of ivacaftor is consistently associated with this genotype and how it compares to the in vivo response.
There were no pulmonary function changes in any subject regardless of sweat chloride concentration change with ivacaftor. This was not unexpected given the short course of ivacaftor. With a longer course of ivacaftor the subjects may have pulmonary function improvement or stabilization over time as seen in G551D patients.9 After the completion of the clinical trial, the three subjects who responded with decreased sweat chloride concentrations were prescribed ivacaftor by their primary pulmonologists. All three subjects have reported clinical improvement with ivacaftor over the last 2 years. Prior to starting ivacaftor, Subject 1 had yearly pulmonary exacerbations requiring IV antibiotics. In the 2 years on ivacaftor, Subject 1 has not required IV antibiotics, no longer produces mucus, and has less frequent cough. Prior to starting ivacaftor, Subject 2 had yearly pulmonary exacerbations and Subject 3 had pulmonary exacerbations about every 3 months. Both Subject 2 and Subject 3 have not had a pulmonary exacerbation, have decreased cough, and no decline in lung function since starting ivacaftor 2 years ago. Additionally, Subject 2 was unable to gain weight prior to starting ivacaftor but his BMI is now increased from 21.6 to 23.
Sweat chloride concentration is a functional measure of CFTR activity in the sweat gland epithelia, however, in a non-linear relationship.19 Sweat chloride changes occur within days of ivacaftor, as seen in G551D patients,5,6 so that by the end of 2 weeks a steady state response has been achieved. Decreased sweat chloride concentration at 2 weeks is predictive of long-term improvements in pulmonary function and weight gain in patients with gating mutations.42 However, other studies of ivacaftor in R117H mutation also failed to show an improvement in pulmonary function with long courses of ivacaftor despite improvements in sweat chloride concentration.12 Further research is needed to investigate the correlation between change in sweat chloride concentration and long-term clinical improvements. Assuming the relationship holds, careful N-of-1 studies of ivacaftor and other CFTR modulators are within the capability of all CF clinicians with access to reliable sweat chloride concentration testing. N-of-1 studies using sweat chloride concentration and other biomarkers will likely play an important role in the development of evidence-based personalized medicine in CF, as RCTs are impossible to conduct in subjects with rare or de novo mutations.
Based on our limited experience, cultured nasal cells from CF patients may serve as a screening platform to detect acute response to CFTR modulators and identify candidates for a clinical trial of CFTR modulators. However, this approach may not capture effects due to long-term drug exposure, including destabilization of mature CFTR protein, so that the in vitro and in vivo responses may not always be concordant. We were unable to obtain HNE cultures in two subjects and thus had incomplete in vitro data. Besides using HNE cultures, future N-of-1 studies of CFTR modulators may also consider using individual subjects’ rectal organoid cultures43,44 or patient-specific induced pluripotent stem cells45–47 to predict CFTR modulator clinical efficacy in individual subjects. Due to the high cost of ivacaftor, only a small number of subjects were enrolled in our study. Despite small numbers, our findings may have important clinical implications. Replication of our findings and longer follow-up are important to clarify the clinical effects of ivacaftor in patients with residual CFTR function.
CONCLUSIONS
In conclusion, some CF patients with residual CFTR function and CFTR mutations not currently covered by FDA approval have increased chloride current in HNE cultures and decreased sweat chloride concentration in response to ivacaftor treatment. Acute stimulatory effects of ivacaftor on chloride currents in HNE cultures may predict clinical response to ivacaftor. Inversely, ivacaftor can increase sweat chloride concentration with unknown clinical effect. N-of-1 studies can be used to investigate the effect of CFTR modulators in CF patients with varying phenotypes and genotypes.
Supplementary Material
Acknowledgments
Funding source: Cystic Fibrosis Foundation and private donations, Numbers: RDP-R613, Illek15P0, Illek16G0; NIDDK, Number: P30-DK072517; NIH, Number: DK72517; Elizabeth Nash Foundation; NIGMS, Number: T32-GM007546.
The authors thank the subjects that participated and Diana Dawson for her assistance.
ABBREVIATIONS
- CFTR
cystic fibrosis transmembrane conductance regulator
- FEV1
forced expiratory volume in one second
- HNE
human nasal epithelium
- IRB
institutional review board
- RCT
randomized controlled trial
Footnotes
ClinicalTrials.gov number: NCT01784419.
Notification of Prior Abstract Publication: Part of this work was presented at the North American Cystic Fibrosis Conference in October 2015 in Phoenix, AZ and in October 2016 in Orlando, FL.
Role of sponsors: The sponsors of our study did not contribute to study design, data analysis, or interpretation.
AUTHORS’ CONTRIBUTIONS
MEM takes responsibility for the manuscript content, including data and analysis. NL, DWN, BI, SO, CM, LZ, and WEF contributed substantially to study design, data analysis/interpretation, and writing of manuscript.
Conflicts of Interest
The authors have no conflicts of interest to disclose. The opinions expressed in this article are those of the authors and do not necessarily represent those of the National Institutes of Health, the Cystic Fibrosis Foundation, the Cystic Fibrosis Research, Inc., or the Elizabeth Nash Foundation.
Additional supporting information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Yu H, Burton B, Huang C-J, Worley J, Cao D, Johnson JP, Urrutia A, Joubran J, Seepersaud S, Sussky K. Ivacaftor potentiation of multiple cftr channels with gating mutations. J Cyst Fibros. 2012;11:237–245. doi: 10.1016/j.jcf.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 2.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, Turnbull A, Singh A, Joubran J, Hazlewood A. Rescue of cf airway epithelial cell function in vitro by a cftr potentiator, vx-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Goor F, Hadida S, Grootenhuis PD, Burton B, Stack JH, Straley KS, Decker CJ, Miller M, McCartney J, Olson ER. Correction of the f508del-cftr protein processing defect in vitro by the investigational drug vx-809. Proc Natl Acad Sci USA. 2011;108:18843–18848. doi: 10.1073/pnas.1105787108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ren HY, Grove DE, De La Rosa O, Houck SA, Sopha P, Van Goor F, Hoffman BJ, Cyr DM. Vx-809 corrects folding defects in cystic fibrosis transmembrane conductance regulator protein through action on membrane-spanning domain 1. Mol Biol Cell. 2013;24:3016–3024. doi: 10.1091/mbc.E13-05-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Accurso FJ, Rowe SM, Clancy J, Boyle MP, Dunitz JM, Durie PR, Sagel SD, Hornick DB, Konstan MW, Donaldson SH. Effect of vx-770 in persons with cystic fibrosis and the g551d-cftr mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Dřevínek P, Griese M, McKone EF, Wainwright CE, Konstan MW. A cftr potentiator in patients with cystic fibrosis and the g551d mutation. N Engl J Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McGarry ME, Nielson DW. Normalization of sweat chloride concentration and clinical improvement with ivacaftor in a patient with cystic fibrosis with mutation s549n. Chest. 2013;144:1376–1378. doi: 10.1378/chest.13-0239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davies JC, Wainwright CE, Canny GJ, Chilvers MA, Howenstine MS, Munck A, Mainz JG, Rodriguez S, Li H, Yen K. Efficacy and safety of ivacaftor in patients aged 6 to 11 6–11 years with cystic fibrosis with a g551d mutation. Am J Respir Crit Care Med. 2013;187:1219–1225. doi: 10.1164/rccm.201301-0153OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sawicki GS, McKone EF, Pasta DJ, Millar SJ, Wagener JS, Johnson CA, Konstan MW. Sustained benefit from ivacaftor demonstrated by combining clinical trial and cystic fibrosis patient registry data. Am J Respir Crit Care Med. 2015;192:836–842. doi: 10.1164/rccm.201503-0578OC. [DOI] [PubMed] [Google Scholar]
- 10.Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA, Rubenstein RC, Pilewski J, Higgins M. Effects of ivacaftor in cf patients with r117h-cftr. Paper presented at: Pediatric Pulmonology; WILEY-BLACKWELL 111 RIVER ST, HOBOKEN 07030–5774, NJ USA. 2014. [Google Scholar]
- 11.De Boeck K, Munck A, Walker S, Faro A, Hiatt P, Gilmartin G, Higgins M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-g551d gating mutation. J Cyst Fibros. 2014;13:674–680. doi: 10.1016/j.jcf.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Moss RB, Flume PA, Elborn JS, Cooke J, Rowe SM, McColley SA, Rubenstein RC, Higgins M. Efficacy and safety of ivacaftor in patients with cystic fibrosis who have an arg117his-cftr mutation: a double-blind, randomised controlled trial. Lancet Respir Med. 2015;3:524–533. doi: 10.1016/S2213-2600(15)00201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cystic fibrosis foundation patient registry. Bethesda Maryland: 2015. 2014 Annual data report. [Google Scholar]
- 14.Van Goor F, Yu H, Burton B, Hoffman BJ. Effect of ivacaftor on cftr forms with missense mutations associated with defects in protein processing or function. J Cyst Fibros. 2014;13:29–36. doi: 10.1016/j.jcf.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 15.Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet. 2015;16:45–56. doi: 10.1038/nrg3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duan N, Kravitz RL, Schmid CH. Single-patient (n-of-1) trials: a pragmatic clinical decision methodology for patient-centered comparative effectiveness research. J Clin Epidemiol. 2013;66:S21. doi: 10.1016/j.jclinepi.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vohra S, Shamseer L, Sampson M, Bukutu C, Schmid CH, Tate R, Nikles J, Zucker DR, Kravitz R, Guyatt G. Consort extension for reporting n-of-1 trials (cent) 2015 statement. BMJ. 2015;350:h1738. doi: 10.1136/bmj.h1738. [DOI] [PubMed] [Google Scholar]
- 18.Lillie EO, Patay B, Diamant J, Issell B, Topol EJ, Schork NJ. The n-of-1 clinical trial: the ultimate strategy for individualizing medicine? Per Med. 2011;8:161–173. doi: 10.2217/pme.11.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Accurso FJ, Van Goor F, Zha J, Stone AJ, Dong Q, Ordonez CL, Rowe SM, Clancy JP, Konstan MW, Hoch HE. Sweat chloride as a biomarker of cftr activity: proof of concept and ivacaftor clinical trial data. J Cyst Fibros. 2014;13:139–147. doi: 10.1016/j.jcf.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kerem E, Corey M, Kerem B-s, Rommens J, Markiewicz D, Levison H, Tsui L-C, Durie P. The relation between genotype and phenotype in cystic fibrosis—analysis of the most common mutation (δf508) N Engl J Med. 1990;323:1517–1522. doi: 10.1056/NEJM199011293232203. [DOI] [PubMed] [Google Scholar]
- 21.Veeze HJ, Halley D, Bijman J, De Jongste J, De Jonge H, Sinaasappel M. Determinants of mild clinical symptoms in cystic fibrosis patients. Residual chloride secretion measured in rectal biopsies in relation to the genotype. J Clin Invest. 1994;93:461. doi: 10.1172/JCI116993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green DM, McDougal KE, Blackman SM, Sosnay PR, Henderson LB, Naughton KM, Collaco JM, Cutting GR. Mutations that permit residual cftr function delay acquisition of multiple respiratory pathogens in cf patients. Respir Res. 2010;11(10):1186. doi: 10.1186/1465-9921-11-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–1676. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- 24.Davis PB, Schluchter MD, Konstan MW. Relation of sweat chloride concentration to severity of lung disease in cystic fibrosis. Pediatr Pulmonol. 2004;38:204–209. doi: 10.1002/ppul.20054. [DOI] [PubMed] [Google Scholar]
- 25.Char JE, Wolfe MH, Cho H-j, Park I-H, Jeong JH, Frisbee E, Dunn C, Davies Z, Milla C, Moss RB. A little cftr goes a long way: cftr-dependent sweat secretion from g551d and r117h-5t cystic fibrosis subjects taking ivacaftor. PLos ONE. 2014;9:e88564. doi: 10.1371/journal.pone.0088564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yousef S, Solomon GM, Brody A, Rowe SM, Colin AA. Improved clinical and radiographic outcomes after treatment with ivacaftor in a young adult with cystic fibrosis with the p67l cftr mutation. Chest. 2015;147:e79–e82. doi: 10.1378/chest.14-1198. [DOI] [PubMed] [Google Scholar]
- 27.Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, Rosenstein BJ, Smith AL, Wohl ME. Effect of aerosolized recombinant human dnase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. N Engl J Med. 1994;331:637–642. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- 28.LeGrys VA, Yankaskas JR, Quittell LM, Marshall BC, Mogayzel PJ., Jr Diagnostic sweat testing: the cystic fibrosis foundation guidelines. J Pediatr. 2007;151:85–89. doi: 10.1016/j.jpeds.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Society AT. Standardization of spirometry, 1994 update. Am Respir Crit Care Med. 1995;15:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 30.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general us population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 31.Boyle MP, Bell SC, Konstan MW, McColley SA, Rowe SM, Rietschel E, Huang X, Waltz D, Patel NR, Rodman D. A cftr corrector (lumacaftor) and a cftr potentiator (ivacaftor) for treatment of patients with cystic fibrosis who have a phe508del cftr mutation: a phase 2 randomised controlled trial. Lancet Respir Med. 2014;2:527–538. doi: 10.1016/S2213-2600(14)70132-8. [DOI] [PubMed] [Google Scholar]
- 32.Clancy J, Rowe SM, Accurso FJ, Aitken ML, Amin RS, Ashlock MA, Ballmann M, Boyle MP, Bronsveld I, Campbell PW. Results of a phase iia study of vx-809, an investigational cftr corrector compound, in subjects with cystic fibrosis homozygous for the f508del-cftr mutation. Thorax. 2012;67:12–18. doi: 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukacs GL, Verkman A. Cftr: folding, misfolding and correcting the δf508 conformational defect. Trends Mol Med. 2012;18:81–91. doi: 10.1016/j.molmed.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flume PA, Liou TG, Borowitz DS, Li H, Yen K, Ordoñez CL, Geller DE. Ivacaftor in subjects with cystic fibrosis who are homozygous for the f508del-cftr mutation. Chest. 2012;142:718–724. doi: 10.1378/chest.11-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilschanski M, Zielenski J, Markiewicz D, Tsui L-C, Corey M, Levison H, Durie PR. Correlation of sweat chloride concentration with classes of the cystic fibrosis transmembrane conductance regulator gene mutations. J Pediatr. 1995;127:705–710. doi: 10.1016/s0022-3476(95)70157-5. [DOI] [PubMed] [Google Scholar]
- 36.Hamosh A, Rosenstein BJ, Cutting GR. Cftr nonsense mutations g542x and w1282x associated with severe reduction of cftr mrna in nasal epithelial cells. Hum Mol Genet. 1992;1:542–544. doi: 10.1093/hmg/1.7.542. [DOI] [PubMed] [Google Scholar]
- 37.Chiba-Falek O, Kerem E, Shoshani T, Aviram M, Augarten A, Bentur L, Tal A, Tullis E, Rahat A, Kerem B. The molecular basis of disease variability among cystic fibrosis patients carrying the 3849+10kb c→t mutation. Genomics. 1998;53:276–283. doi: 10.1006/geno.1998.5517. [DOI] [PubMed] [Google Scholar]
- 38.Van Oene M, Lukacs GL, Rommens JM. Cystic fibrosis mutations lead to carboxyl-terminal fragments that highlight an early biogenesis step of the cystic fibrosis transmembrane conductance regulator. J Biol Chem. 2000;275:19577–19584. doi: 10.1074/jbc.M002186200. [DOI] [PubMed] [Google Scholar]
- 39.Cebotaru L, Rapino D, Cebotaru V, Guggino WB. Correcting the cystic fibrosis disease mutant, a455e cftr. PloS ONE. 2014;9:e85183. doi: 10.1371/journal.pone.0085183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Veit G, Avramescu RG, Perdomo D, Phuan P-W, Bagdany M, Apaja PM, Borot F, Szollosi D, Wu Y-S, Finkbeiner WE. Some gating potentiators, including vx-770, diminish δf508-cftr functional expression. Sci Transl Med. 2014;6:246ra297–246ra297. doi: 10.1126/scitranslmed.3008889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cholon DM, Quinney NL, Fulcher ML, Esther CR, Das J, Dokholyan NV, Randell SH, Boucher RC, Gentzsch M. Potentiator ivacaftor abrogates pharmacological correction of δf508 cftr in cystic fibrosis. Sci Transl Med. 2014;6:246ra296–246ra296. doi: 10.1126/scitranslmed.3008680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seliger VI, Rodman D, Van Goor F, Schmelz A, Mueller P. The predictive potential of the sweat chloride test in cystic fibrosis patients with the g551d mutation. J Cyst Fibros. 2013;12:706–713. doi: 10.1016/j.jcf.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 43.Dekkers JF, Berkers G, Kruisselbrink E, Vonk A, De Jonge HR, Janssens HM, Bronsveld I, van de Graaf EA, Nieuwenhuis EE, Houwen RH. Characterizing responses to cftr-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci Transl Med. 2016;8:344ra384–344ra384. doi: 10.1126/scitranslmed.aad8278. [DOI] [PubMed] [Google Scholar]
- 44.Dekkers JF, Wiegerinck CL, de Jonge HR, Bronsveld I, Janssens HM, de Winter-de Groot KM, Brandsma AM, de Jong NW, Bijvelds MJ, Scholte BJ. A functional cftr assay using primary cystic fibrosis intestinal organoids. Nat Med. 2013;19:939–945. doi: 10.1038/nm.3201. [DOI] [PubMed] [Google Scholar]
- 45.Simsek S, Zhou T, Robinson CL, Tsai S-Y, Crespo M, Amin S, Lin X, Hon J, Evans T, Chen S. Modeling cystic fibrosis using pluripotent stem cell-derived human pancreatic ductal epithelial cells. Stem Cells Transl Med 2015–0276. 2016;5:572–579. doi: 10.5966/sctm.2015-0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 47.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan L-J, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional cftr protein. Nat Biotechnol. 2012;30:876–882. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.