Abstract
The Tumor Necrosis Factor (TNF) receptors and their corresponding cytokine ligands have been implicated in many aspects of the biology of immune functions. TNF receptors have key roles during various stages of T cell homeostasis. Many of them can co-stimulate lymphocyte proliferation and cytokine production. Additionally, several TNF cytokines can regulate T cell differentiation, including promoting Th1, Th2, Th17 and more recently the newly described Th9 subset. Four TNF-family cytokines have been identified as regulators for IL-9 production by T cells. OX40L, TL1A and GITRL can promote Th9 formation but can also divert iTreg into Th9, while 4-1BBL seems to inhibit IL-9 production from iTreg and has not been studied for its ability to promote Th9 generation. Regulation of IL-9 production by TNF-family cytokines has repercussions in vivo, including enhancement of anti-tumor immunity and immunopathology in allergic lung and ocular inflammation. Regulating T cell production of IL-9 through blockade or agonism of TNF-family cytokine receptors may be a therapeutic strategy for autoimmune and allergic diseases and in tumor.
Keywords: TNF superfamily cytokines, Th9, Lung inflammation, Tumor immunology
The Tumor Necrosis Factor Cytokine Superfamily
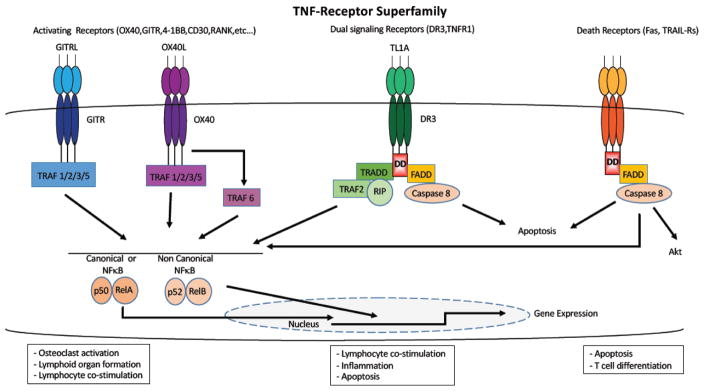
The 19 cytokines in the TNF superfamily cytokines can widely influence the biological function of both innate and adaptive immune cells. TNF cytokines are trimeric molecules [1] originally produced as type-II transmembrane proteins, but can be cleaved off the plasma membrane by metalloproteinases [2–5]. Binding of TNF cytokines to receptors causes receptor oligomerization, promoting the binding of adaptor molecules that in turn recruit signaling complexes which activate NF-κB, AKT and MAP kinase signaling pathways. The downstream effects of these interactions induce cell proliferation and differentiation, or the activation of caspases, leading to programmed cell death (Figure 1). The family of intracellular proteins that serve as adaptors for TNF-receptor signaling were first discovered by their interaction with TNF receptors, hence their name, TNFR-associated factors (TRAFs). However, they have since been implicated in signaling through other types of receptors such as Toll-like receptors (TLR’s [6]) and other families of cytokine receptors [7–10]. Six TRAFs (TRAF1-6 [11–15]) have been identified so far in mammals, while the description of a seven member (TRAF7 [16]) has been disputed due to the lack of the TRAF homology domain that define them. They share a conserved C-terminal homology region that binds to consensus sequences in the cytoplasmic tail of receptors or other signaling molecules [14, 17]. The adaptor molecules connecting the downstream receptor signaling largely defines the signaling potential of TNF receptors. TRAFs are directly associated with the vast majority of TNF receptors, and are requited for activation of MAPK and NF-κB signaling [17]. NF-κB can be activated by two distinct molecular mechanisms, the canonical pathway or the non-canonical pathway [18, 19]. The canonical pathway utilizes the inducible degradation of the inhibitory molecules such as IkBα, which liberates the p65 subunit of NF-κB to translocate to the nucleus, while the non-canonical pathway activates the processing of the p100/RelB subunit of NF-κB into the active p52 form [20]. Two other groups of adaptor molecules, FAS-associated protein with death domain (FADD [21]) and TNF receptor-associated protein with death domain (TRADD [22]), bind the so called death receptor (FAS, TRAIL-R’s) or dual signaling receptors (TNFR1, DR3) respectively. Notably, TRAFs can also interact with TRADD during TNFR1 signaling [23]. Recently, a new concept has emerged suggesting that some TRAFs, such as TRAF2 and TRAF6 can modulate lymphocyte activation upon TCR engagement through activation of the PI3K-Akt pathway [24–28].
Fig. 1.
TNF-receptors superfamily. The receptors that signal through TRAFs can activate NFκB which will translocate to the nucleus to induce gene expression, they are involved in functions such as osteoclast activation, lymphoid organ formation or lymphocyte co-stimulation. The receptors that signal through TRADD can function as co-stimulators and/or induce inflammation, but they can also recruit FADD and induce apoptosis. The receptors that interact with FADD induce apoptosis, and Fas can also potentiate effector memory T cell differentiation under some circumstances.
The role of Tumor Necrosis Factors in T cell co-stimulation
Activation of T cells upon TCR engagement is rapidly followed by co-stimulatory signaling through CD28-B7 interactions. It has been found that some particular members of the TNFR superfamily emerge as key components to subsequently propagate crucial signals towards survival, proliferation, and differentiation after initial T cell activation. TNF receptors have been involved in various aspect of T cell homeostasis, including a number of them that can provide potent costimulatory signal to activate lymphocytes [29–31]. While some members of the TNF receptors are constitutively expressed, others are strongly upregulated upon T cell activation through TCR. For example, CD27, OX40, 4–1BB, HVEM, CD30, DR3, and GITR positively regulate the survival, differentiation, proliferation and function of CD4+ and CD8+ T cells during immune activation [30, 32]. This list is likely to grow in the future, with recent studies redefining some of the functions that were initially ascribed to certain TNF receptors. For example, FAS, a very well-studied TNF receptor, has been predominantly studied for its role in cell death due to its intracellular death domain portion [33–35]. However, several studies have recently shown that FAS can take part in non-apoptotic functions such as T cell differentiation [36]. In this context, the FAS-controlled T cell differentiation had substantial impact on the efficacy of adoptive tumor immunotherapy [37].
TNF-family cytokine co-stimulation in Th9 development
Since the discovery that IL-4 in combination with TGFβ can promote differentiation of T cells secreting IL-9 in vitro [38], a large number of molecules have been identified as being capable of promoting Th9 generation, including the cytokines IL-25 [39], TSLP [40], IL-33 [41], IL-1, IL-6, Type I IFN, IL-10, IL-21 [42]), calcinotide peptide (calcitonin gene related peptide [43]), cell-fate lineage factors (Jagged-2 [44]), Tec kinase (itk [45]) as well as members of the TNF superfamily (OX40L [46], GITRL [47, 48], TL1A [49]). Other factors such as prostaglandins (Cyclooxygenase-2 [50]), the B7-family member (PDL-2 [51]), or the active form of Vitamin D (1,25-dihidroxyvitamin D3 [52]) have an inhibitory effect on Th9 formation. Here we will focus on the TNF family cytokines that were recently identified as effective regulators of Th9 cell generation, discuss their mechanisms of action, and implications for host defense and pathological immune responses. Although these cytokines use diverse signaling pathways to regulate the development and function of IL-9 producing T cells, they still require TGFβ in combination with either IL-2/STAT5 or IL-4/STAT6 in order to carry out these effects.
OX40-OX40L
In 2012, Xiao et al reported that OX40L (TNFSF4), a member of the TNF family, can promote the generation of IL-9 producing cells [46]. Stimulation of the OX40-OX40L pathway during in vitro Th9 polarization with TGFβ and IL-4 by adding either APC expressing OX40L or an agonist monoclonal antibody to OX40 dramatically increased the amount of IL-9 producing T cells while downregulating FoxP3 and IL-17 under iTreg and Th17 polarizing conditions respectively [46]. OX40 ligation did not alter Smad2/3 phosphorylation, the downstream elements of the TGFβ signaling pathway. Notably, OX40 ligation also did not affect the phosphorylated status of STAT3, STAT5 and STAT6 or the expression of IL-2Rα and IL-4Rα, suggesting that neither the IL-2/STAT5 nor the TGFβ/IL-4 combined axis, which are crucial for Th9 generations were affected by OX40 ligation [46]. The extent of the OX40 enhancement of Th9 polarization was even more potent when using an antigen-specific T cell activation system rather than polyclonal activation. The absence of any IL-9 enhancement by OX40L stimulation in OX40 deficient T cells indicates that it is carried out specifically through the OX40-OX40L pathway [46]. Interestingly, the promotion of Th9 through OX40 signaling is independent of PU.1, a key transcriptional regulator for the induction of Th9 [46]. Similar to other TNF receptors, the downstream signaling of OX40 is through TRAF adaptor molecules, which in turn activate the NF-κB transcription factor complex and a wide array of downstream biological functions [53]. TRAF6 is a member of the TNFR-associated factors (TRAFs) that is crucial for NF-κB activation by OX40. Interestingly, while OX40 ligation can transiently activate the canonical NF-κB pathway, the non-canonical pathway through p52/RelB was shown to play a key role for the Th9 induction by OX40L through TRAF6 [46]. In T cells with a mutation repressing the canonical NF-κB pathway, OX40 co-stimulation could still increase the differentiation of IL-9 producing cells. However, mice lacking the non-canonical NFκ-B subunit p52 were unresponsive to OX40L. In addition, one of the key steps in the p52/RelB pathway, the processing of p100 into p52 by NIK [20], was also required for OX40-mediated enhancement of IL-9 production [46]. These results suggest that while both NFκ-B pathways are activated by OX40, the non-canonical pathway is favored for potentiation of Th9 differentiation.
This observation was shown to have in vivo relevance, as OX40L transgenic mice have expanded Th9 cells present in their lungs, accompanied by spontaneous pulmonary inflammation with peribronchioloar infiltrates and increased mucin-producing goblet cells [46]. Likewise, triggering OX40 signaling pathway through an agonistic antibody in wild-type mice had similar effect that were abrogated in IL-9 deficient mice [46]. Mice lacking OX40 were resistant to lung pathology after sensitization and challenge with ovalbumin, although this may be due to deficiencies in the development of other T cell subsets such as Th2, whose differentiation is also OX40 dependent [54].
DR3-TL1A
The TNF cytokine TL1A (TNFSF15), which was previously found to promote allergic disease through enhancing Th2 responses [55, 56], was more recently found to promote Th9 polarization [49]. This was discovered when under iTreg conditions (TGFβ and IL-2), TL1A strongly repressed the generation of FoxP3 expressing iTreg. Interestingly, the decline in FoxP3+ T cells was mirrored by an increase in IL-9 producing cells, especially in the presence of APC, effectively diverting iTreg into Th9 [49]. When added to T cells cultured under conditions known to favor Th9 generation (TGFβ and IL-4), TL1A even more dramatically enhanced the generation of Th9 and curtailed the amount of TGFβ required to promote Th9 generation [49]. Th9, Th17 and iTreg cells, which require TGFβ for their differentiation, have the highest levels of DR3 (TNFRSF25), the receptor for TL1A compared to the other effector subsets, indicating that TGFβ might help to enhance responsiveness to TL1A in particular subsets [49]. Ultimately, DR3 expression on activated lymphocytes is downregulated by its ligand [56], indicating a tightly controlled feedback loop. Although DR3-deficient T cells could still be driven to differentiate into Th9 by TGF-beta and IL-4, they were unresponsive to the enhancement of Th9 differentiation by TL1A [49].
TL1A enhances production of a number of cytokines from activated T cells [49, 56–62], which could indirectly contribute to the enhancement of IL-9 production through autocrine signaling. Many cytokines regulate T cell differentiation through the JAK/STAT signaling pathway [63, 64]. Interestingly, even though TL1A enhanced IRF4 accessibility for the IL-9 promoter, its ability to enhance IL-9 production was not through the TGFβ and the STAT6-mediated IL-4 axis, one of the important signaling pathways required for conventional Th9 formation [49]. Rather, TL1A potentiated IL-9 secretion through the other pivotal pathway identified for Th9 polarization, STAT5-mediated activation through IL-2. An intact IL-2 signaling pathway is not sufficient, as T cells must receive a direct signal through DR3 in order to respond to TL1A with enhanced IL-9 production [49]. Unlike OX40L, the mechanism by which TL1A promotes IL-9 production was not dependent on TRAF6 signaling. Rather, DR3 which, like TNFR1, signals through TRADD and TRAF2 [65], might follow a similar mechanism of other TNFR utilizing TRAF2 to induce PI3K activity and Akt activation. However, this has not been investigated. Like OX40L, lack of the transcription factor PU.1 did not affect TL1A induction of IL-9 production, which was also consistent with the fact that TL1A did not influence PU.1 binding to the IL-9 promoter [49].
In vivo, TL1A not only markedly increased antigen-specific driven ocular inflammation, but was also able to enhance Th9 driven pathology when injected directly into the eye. TL1A had a similar impact on Th9-driven lung inflammation [49]. Thus, TL1A not only drives expansion of Th9 cells but also intensifies their pathogenicity. Importantly, endogenous DR3 on T cells was required for optimal Th9 function in vivo, as generation of Th9 and maintenance of IL9 expression by antigen-specific T cells was impaired in T cells from DR3 deficient mice [49]. It should be noted that in addition to promoting Th9 differentiation, TL1A also promotes production of pro-allergic effector cytokines, including IL-9, from group 2 innate lymphoid cells, which can promote allergic immunopathology in animal models independently of T or B cells [58, 66, 67]. Thus TL1A appears to coordinately promote allergic response in both innate and adaptive immune cells.
GITR-GITRL
Two recent studies have reported that the Glucocorticoid-induced tumor necrosis factor receptor-related protein (GITR), the TNF-superfamily co-stimulatory receptor for GITR-L, can also promote Il-9 production by T cells [47, 48]. GITR expression was similar among the T cell effector subsets, but the addition of an agonist antibody to GITR (DTA-1) substantially increased Th2 and Th9 cytokines while inhibiting only modestly Th1, Th17 cytokines as well as Foxp3 expression in iTreg [47]. Notably, the IL-9 co-stimulation by GITR ligation was even more pronounced when using cognate antigen and APC [48], a phenomenon that was also seen with OX40L and TL1A. GITR ligation not only co-stimulates IL-9 producing cells under Th9 conditions, but can also divert iTreg to produce IL-9 while reducing FoxP3 expression [47, 48], in a similar manner to TL1A and OX40L. Similar to OX40 ligation, GITR ligation did not alter SMADs or STAT5 under iTreg [48]. GITR co-stimulation to promote Th9 differentiation also required TRAF6, STAT6, but not BATF and PU.1 [47, 48]. GITR ligation strongly activates the expression of the NF-κB molecules contributing to both canonical or non-canonical pathways, and enhanced their translocation to the nucleus, but in contrast to OX40, is dependent on the canonical NF-κB pathway [47]. GITR ligation affected chromatin remodeling, altering histone modification at the FoxP3 and IL-9 promoters. Interestingly, the IL-9 promoter also contains FoxP3 binding sites, with FoxP3 sites highly enriched under iTreg condition and repressed when IL-9 gene expression is induced, suggesting a reciprocal induction of iTreg and Th9. Notably, GITR was unable to enhance Th9 differentiation or recruit p300 to the IL-9 loci in STAT6 deficient T cells [48]. Furthermore, IL-4 signaling is required for optimal Th9 generation, however the autocrine elevation of IL-4 by GITR ligation is not responsible for the enhancement of IL-9 [47]. This is also consistent with the fact that GITR ligation is able to induce IL-9 production under in vitro polarization lacking exogenous IL-4 such as iTreg. Stimulating GITR signaling pathway on either iTreg or Th9 had significant impact in vivo. Adoptively transferred antigen-specific Treg were able to control tumors in a B16 melanoma model when DTA-1 anti-GITR agonistic antibodies were added due to their capacity to secrete IL-9. Furthermore, while GITR ligation also enhanced Th2 responses and Th2 had antitumor activity in this model, Th9 were superior. Th9 cells differentiated in the presence of GITR co-stimulation also improved tumor regression better than conventional Th9. Notably, DTA-1 not only altered Th9 function but also influenced tumor specific CTL responses. Therefore, stimulating the GITR signaling pathway has tremendous effects on regulatory and effector cells as well as other immune subsets in tumor immunity.
4-1BB-4-1BBL
Although 4-1BBL was the first member of the TNF superfamily to be found to influence IL-9 secretion by T cells, this study did not investigate how 4-1BB signaling influences Th9 formation molecular mechanism by which it works [68]. Rather this study focused on the effects of 4-1BB on IL-9 production in Treg in a context of tumor immunity. 4-1BB agonistic antibodies reduced IL-9 production by Treg.
Integrating TNF cytokines into Th9 biology and therapeutic implications
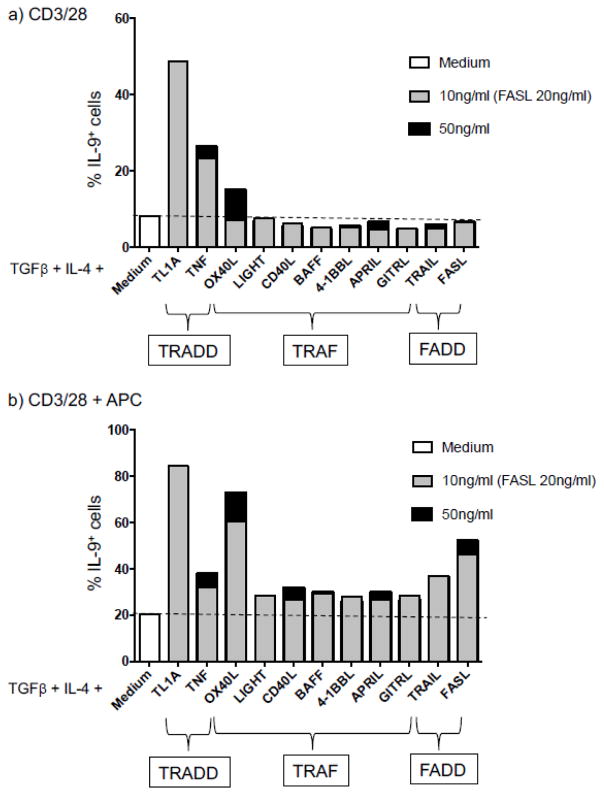
As all of these studies on individual TNF-family cytokines were carried out independently, it is difficult to compare the relative efficiency of each cytokine in promoting Th9 differentiation. To address this, we activated naïve CD4+ T cells under Th9 conditions (IL-4 and TGFβ) and compared the ability of 11 TNF-family cytokines to promote Th9 differentiation in response to activation by CD3/CD28 antibodies with or without irradiated APC. As shown in Figure 2, TL1A was most potent in enhancing Th9 differentiation, with OX40L next in potency. APC enhanced baseline and cytokine-induced Th9 generation as previously reported. Interestingly, in absence of APC, OX40L was not as effective in inducing Th9 compare to TL1A and TNF. Suggesting that OX40L co-stimulatory signal for enhancing IL-9 producing T cells requires additional components produced by APC and/or direct interactions in order to produce its effect. Under the conditions we used, neither GITRL or 4-1BBL enhanced Th9 differentiation, whereas FasL and TRAIL, two other TNF-family cytokines not previously shown to enhance Th9 differentiation, were able to do this in the presence of APC.
Fig. 2.
Mouse naïve CD4+ T cells were cultured in Th9-polarizing conditions (TGFβ + IL-4) in absence or presence of the indicated TNF family cytokines on coated plate with anti-CD3 and anti-CD28 in absence (a) or in presence of APCs (b) and were analyzed for their IL-9 expression by flow cytometry after restimulation with PMA and ionomycin for 4 hrs.
Findings in the past five years have identified the TNF-family cytokines TL1A, OX40L and GITRL as potent co-stimulators of Th9 differentiation. Although the exact molecular mechanisms by which each cytokine potentiates Th9 differentiation are distinct (Table 1), there are commonalities, such as the ability of TNF-family cytokines to divert regulatory T cells into Th9. In vivo, APC are the likely source of TNF costimulatory ligands, providing a mechanism where by signals which activate DC in certain ways may program them to enhance Th9 differentiation through expression of co-stimulatory TNF-family cytokines. TLR and FcR engagement of myeloid cells induce the expression of many TNF-family cytokines, but which signals program APC to do this in vivo remains to be identified. Along these lines, Zhao et al recently showed that Dectin1, a lectin receptor which induces TL1A and OX40L expression in APC [69], enhances Th9 differentiation in a manner partially dependent on these cytokines. Triggering TNF-family receptors in vivo to enhance anti-tumor immunity through Th9 may be a promising avenue to improve the effectiveness of tumor immunotherapy, and conversely blocking Th9-promoting cytokines may be therapeutic in diseases where immunopathology is mediated by T cells secreting IL-9.
Table 1.
Comparison of mechanisms and consequences of Th9 induction by TNF-family cytokines
| Cytokine | TL1A [49] | OX40L[46] | GITRL [47, 48] |
|---|---|---|---|
| Th9 enhancement in vitro | + | + | + |
| iTreg repression | + | + | + |
| TGFβ-SMAD dependent | N/A | − | − |
| IL4/STAT6 dependent | − | − | + |
| IL2/STAT5 dependent | + | − | − |
| PU.1 dependent | − | − | − |
| TRAF6 dependent | − | + | + |
| NF-κB Dependent | N/A | Non-classical | Classical |
| Th9 enhancement in vivo | + | + | + |
| Functional consequences | Enhances Allergic lung, eye disease | Enhances Allergic lung disease | Enhanced anti-tumor immunity |
N/A= Not analyzed
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the U.S. National Institutes of Health.
References
- 1.Bodmer JL, Schneider P, Tschopp J. The molecular architecture of the TNF superfamily. Trends Biochem Sci. 2002;27(1):19–26. doi: 10.1016/s0968-0004(01)01995-8. [DOI] [PubMed] [Google Scholar]
- 2.Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385(6618):729–33. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, et al. Mutations within a furin consensus sequence block proteolytic release of ectodysplasin-A and cause X-linked hypohidrotic ectodermal dysplasia. Proc Natl Acad Sci U S A. 2001;98(13):7218–23. doi: 10.1073/pnas.131076098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lum L, et al. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999;274(19):13613–8. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- 5.Powell WC, et al. The metalloproteinase matrilysin proteolytically generates active soluble Fas ligand and potentiates epithelial cell apoptosis. Curr Biol. 1999;9(24):1441–7. doi: 10.1016/s0960-9822(00)80113-x. [DOI] [PubMed] [Google Scholar]
- 6.Hacker H, et al. Immune cell activation by bacterial CpG-DNA through myeloid differentiation marker 88 and tumor necrosis factor receptor-associated factor (TRAF)6. J Exp Med. 2000;192(4):595–600. doi: 10.1084/jem.192.4.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funakoshi-Tago M, et al. TRAF6 is a critical signal transducer in IL-33 signaling pathway. Cell Signal. 2008;20(9):1679–86. doi: 10.1016/j.cellsig.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Sorrentino A, et al. The type I TGF-beta receptor engages TRAF6 to activate TAK1 in a receptor kinase-independent manner. Nat Cell Biol. 2008;10(10):1199–207. doi: 10.1038/ncb1780. [DOI] [PubMed] [Google Scholar]
- 9.Sun D, et al. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat Immunol. 2011;12(9):853–60. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang CH, Murti A, Pfeffer LM. Interferon induces NF-kappa B-inducing kinase/tumor necrosis factor receptor-associated factor-dependent NF-kappa B activation to promote cell survival. J Biol Chem. 2005;280(36):31530–6. doi: 10.1074/jbc.M503120200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishida T, et al. Identification of TRAF6, a novel tumor necrosis factor receptor-associated factor protein that mediates signaling from an amino-terminal domain of the CD40 cytoplasmic region. J Biol Chem. 1996;271(46):28745–8. doi: 10.1074/jbc.271.46.28745. [DOI] [PubMed] [Google Scholar]
- 12.Nakano H, et al. TRAF5, an activator of NF-kappaB and putative signal transducer for the lymphotoxin-beta receptor. J Biol Chem. 1996;271(25):14661–4. doi: 10.1074/jbc.271.25.14661. [DOI] [PubMed] [Google Scholar]
- 13.Regnier CH, et al. Presence of a new conserved domain in CART1, a novel member of the tumor necrosis factor receptor-associated protein family, which is expressed in breast carcinoma. J Biol Chem. 1995;270(43):25715–21. doi: 10.1074/jbc.270.43.25715. [DOI] [PubMed] [Google Scholar]
- 14.Rothe M, et al. A novel family of putative signal transducers associated with the cytoplasmic domain of the 75 kDa tumor necrosis factor receptor. Cell. 1994;78(4):681–92. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 15.Sato T, Irie S, Reed JC. A novel member of the TRAF family of putative signal transducing proteins binds to the cytosolic domain of CD40. FEBS Lett. 1995;358(2):113–8. doi: 10.1016/0014-5793(94)01406-q. [DOI] [PubMed] [Google Scholar]
- 16.Xu LG, Li LY, Shu HB. TRAF7 potentiates MEKK3-induced AP1 and CHOP activation and induces apoptosis. J Biol Chem. 2004;279(17):17278–82. doi: 10.1074/jbc.C400063200. [DOI] [PubMed] [Google Scholar]
- 17.Wajant H, Henkler F, Scheurich P. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 2001;13(6):389–400. doi: 10.1016/s0898-6568(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 18.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132(3):344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Sun SC. Non-canonical NF-kappaB signaling pathway. Cell Res. 2011;21(1):71–85. doi: 10.1038/cr.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao G, Harhaj EW, Sun SC. NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol Cell. 2001;7(2):401–9. doi: 10.1016/s1097-2765(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 21.Chinnaiyan AM, et al. FADD, a novel death domain-containing protein, interacts with the death domain of Fas and initiates apoptosis. Cell. 1995;81(4):505–12. doi: 10.1016/0092-8674(95)90071-3. [DOI] [PubMed] [Google Scholar]
- 22.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81(4):495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 23.Hsu H, et al. TRADD-TRAF2 and TRADD-FADD interactions define two distinct TNF receptor 1 signal transduction pathways. Cell. 1996;84(2):299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 24.Lee HW, et al. 4-1BB enhances CD8+ T cell expansion by regulating cell cycle progression through changes in expression of cyclins D and E and cyclin-dependent kinase inhibitor p27kip1. Eur J Immunol. 2003;33(8):2133–41. doi: 10.1002/eji.200323996. [DOI] [PubMed] [Google Scholar]
- 25.Lee HW, et al. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–8. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 26.So T, Croft M. Regulation of PI-3-Kinase and Akt Signaling in T Lymphocytes and Other Cells by TNFR Family Molecules. Front Immunol. 2013;4:139. doi: 10.3389/fimmu.2013.00139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soroosh P, et al. Herpesvirus entry mediator (TNFRSF14) regulates the persistence of T helper memory cell populations. J Exp Med. 2011;208(4):797–809. doi: 10.1084/jem.20101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Q, et al. TNF-alpha impairs differentiation and function of TGF-beta-induced Treg cells in autoimmune diseases through Akt and Smad3 signaling pathway. J Mol Cell Biol. 2013;5(2):85–98. doi: 10.1093/jmcb/mjs063. [DOI] [PubMed] [Google Scholar]
- 29.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 30.Croft M. The role of TNF superfamily members in T-cell function and diseases. Nat Rev Immunol. 2009;9(4):271–85. doi: 10.1038/nri2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 32.Richard AC, et al. The TNF-family cytokine TL1A: from lymphocyte costimulator to disease co-conspirator. J Leukoc Biol. 2015;98(3):333–45. doi: 10.1189/jlb.3RI0315-095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krammer PH. CD95(APO-1/Fas)-mediated apoptosis: live and let die. Adv Immunol. 1999;71:163–210. doi: 10.1016/s0065-2776(08)60402-2. [DOI] [PubMed] [Google Scholar]
- 34.Krammer PH. CD95’s deadly mission in the immune system. Nature. 2000;407(6805):789–95. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 35.Trauth BC, et al. Monoclonal antibody-mediated tumor regression by induction of apoptosis. Science. 1989;245(4915):301–5. doi: 10.1126/science.2787530. [DOI] [PubMed] [Google Scholar]
- 36.Strasser A, Jost PJ, Nagata S. The many roles of FAS receptor signaling in the immune system. Immunity. 2009;30(2):180–92. doi: 10.1016/j.immuni.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klebanoff CA, et al. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J Clin Invest. 2016;126(1):318–34. doi: 10.1172/JCI81217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Veldhoen M, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9(12):1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 39.Angkasekwinai P, et al. Regulation of IL-9 expression by IL-25 signaling. Nat Immunol. 2010;11(3):250–6. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yao W, et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity. 2013;38(2):360–72. doi: 10.1016/j.immuni.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blom L, et al. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS One. 2011;6(7):e21695. doi: 10.1371/journal.pone.0021695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong MT, et al. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol Cell Biol. 2010;88(6):624–31. doi: 10.1038/icb.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mikami N, et al. Calcitonin gene-related peptide and cyclic adenosine 5′-monophosphate/protein kinase A pathway promote IL-9 production in Th9 differentiation process. J Immunol. 2013;190(8):4046–55. doi: 10.4049/jimmunol.1203102. [DOI] [PubMed] [Google Scholar]
- 44.Elyaman W, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9-producing T cells. Immunity. 2012;36(4):623–34. doi: 10.1016/j.immuni.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gomez-Rodriguez J, et al. Itk is required for Th9 differentiation via TCR-mediated induction of IL-2 and IRF4. Nat Commun. 2016;7:10857. doi: 10.1038/ncomms10857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xiao X, et al. OX40 signaling favors the induction of T(H)9 cells and airway inflammation. Nat Immunol. 2012;13(10):981–90. doi: 10.1038/ni.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim IK, et al. Glucocorticoid-induced tumor necrosis factor receptor-related protein co-stimulation facilitates tumor regression by inducing IL-9-producing helper T cells. Nat Med. 2015;21(9):1010–7. doi: 10.1038/nm.3922. [DOI] [PubMed] [Google Scholar]
- 48.Xiao X, et al. GITR subverts Foxp3(+) Tregs to boost Th9 immunity through regulation of histone acetylation. Nat Commun. 2015;6:8266. doi: 10.1038/ncomms9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richard AC, et al. The TNF-family ligand TL1A and its receptor DR3 promote T cell-mediated allergic immunopathology by enhancing differentiation and pathogenicity of IL-9-producing T cells. J Immunol. 2015;194(8):3567–82. doi: 10.4049/jimmunol.1401220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li H, et al. Cyclooxygenase-2 inhibits T helper cell type 9 differentiation during allergic lung inflammation via down-regulation of IL-17RB. Am J Respir Crit Care Med. 2013;187(8):812–22. doi: 10.1164/rccm.201211-2073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kerzerho J, et al. Programmed cell death ligand 2 regulates TH9 differentiation and induction of chronic airway hyperreactivity. J Allergy Clin Immunol. 2013;131(4):1048–57. 1057e1–2. doi: 10.1016/j.jaci.2012.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Palmer MT, et al. Lineage-specific effects of 1,25-dihydroxyvitamin D(3) on the development of effector CD4 T cells. J Biol Chem. 2011;286(2):997–1004. doi: 10.1074/jbc.M110.163790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawamata S, et al. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem. 1998;273(10):5808–14. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 54.Salek-Ardakani S, et al. OX40 (CD134) controls memory T helper 2 cells that drive lung inflammation. J Exp Med. 2003;198(2):315–24. doi: 10.1084/jem.20021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fang L, et al. Essential role of TNF receptor superfamily 25 (TNFRSF25) in the development of allergic lung inflammation. J Exp Med. 2008;205(5):1037–48. doi: 10.1084/jem.20072528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Meylan F, et al. The TNF-family receptor DR3 is essential for diverse T cell-mediated inflammatory diseases. Immunity. 2008;29(1):79–89. doi: 10.1016/j.immuni.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jin S, et al. TL1A/TNFSF15 directly induces proinflammatory cytokines, including TNFalpha, from CD3+CD161+ T cells to exacerbate gut inflammation. Mucosal Immunol. 2013;6(5):886–99. doi: 10.1038/mi.2012.124. [DOI] [PubMed] [Google Scholar]
- 58.Meylan F, et al. The TNF-family cytokine TL1A drives IL-13-dependent small intestinal inflammation. Mucosal Immunol. 2011;4(2):172–85. doi: 10.1038/mi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Migone TS, et al. TL1A is a TNF-like ligand for DR3 and TR6/DcR3 and functions as a T cell costimulator. Immunity. 2002;16(3):479–92. doi: 10.1016/s1074-7613(02)00283-2. [DOI] [PubMed] [Google Scholar]
- 60.Prehn JL, et al. Potential role for TL1A, the new TNF-family member and potent costimulator of IFN-gamma, in mucosal inflammation. Clin Immunol. 2004;112(1):66–77. doi: 10.1016/j.clim.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 61.Slebioda TJ, et al. Triggering of TNFRSF25 promotes CD8(+) T-cell responses and anti-tumor immunity. Eur J Immunol. 2011;41(9):2606–11. doi: 10.1002/eji.201141477. [DOI] [PubMed] [Google Scholar]
- 62.Taraban VY, et al. Sustained TL1A expression modulates effector and regulatory T-cell responses and drives intestinal goblet cell hyperplasia. Mucosal Immunol. 2011;4(2):186–96. doi: 10.1038/mi.2010.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ortmann RA, et al. Janus kinases and signal transducers and activators of transcription: their roles in cytokine signaling, development and immunoregulation. Arthritis Res. 2000;2(1):16–32. doi: 10.1186/ar66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annu Rev Immunol. 2010;28:445–89. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chinnaiyan AM, et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274(5289):990–2. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 66.Meylan F, et al. The TNF-family cytokine TL1A promotes allergic immunopathology through group 2 innate lymphoid cells. Mucosal Immunol. 2014;7(4):958–68. doi: 10.1038/mi.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yu X, et al. TNF superfamily member TL1A elicits type 2 innate lymphoid cells at mucosal barriers. Mucosal Immunol. 2014;7(3):730–40. doi: 10.1038/mi.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smith SE, et al. Signals through 4-1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol Immunother. 2011;60(12):1775–87. doi: 10.1007/s00262-011-1075-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao Y, et al. Dectin-1-activated dendritic cells trigger potent antitumour immunity through the induction of Th9 cells. Nat Commun. 2016;7:12368. doi: 10.1038/ncomms12368. [DOI] [PMC free article] [PubMed] [Google Scholar]