Abstract
Nano-hemostats are synthetic amino acid chains that self-assemble into a scaffold under certain conditions. These have been shown to be effective in stopping bleeding in small animal models of hemorrhage. Proposed mechanisms for their effect are that they form a mesh analogous to the fibrin plug in native hemostasis and that they may potentiate both platelet activation and the coagulation cascade. These may potentially become valuable adjuncts to endoscopic skull base surgery where there is the potential for both major vessel injury and smaller perforator injury to eloquent areas where bipolar cautery may not be suitable. We present a summary of the clinical studies to date and a small pilot study of nano-hemostat in an endoscopic sheep model of major vessel hemorrhage to determine its efficacy in stopping bleeding in this potentially catastrophic complication.
Keywords: endoscopic transsphenoidal surgery, hemorrhage control, nano-medicine, pituitary surgery, internal carotid artery injury
Introduction
“Nano-technology” or “nano-engineering” involves working with structures that are less than 100 nm in size. The challenges that this poses are immense and involve controlling entities on an atomic scale. 1 Given that many disease processes involve damage to cell structures, the ability to administer a substance that either provides a scaffold for ordered cellular regeneration or delivers growth factors that stimulate such recovery is attractive, especially if this scaffold were composed of materials that were able to be broken down without causing long-term damage once their function had expired. 1 2
The Use of Self-Assembling Peptides in Medical Applications
The medical applications of nanotechnology have revolutionized our options for drug delivery, in vivo medical imaging, and biotechnological techniques. 3 Typically these applications utilize amino acid chains that are amenable to self-assembly—that is, forming secondary structures according to their amino acid sequence under certain external conditions. 1 4 They are, as Loo and colleagues described in 2012, “versatile building blocks for fabricating supramolecular architectures.” 4 The development of these has come about through increased understanding of the protein chains found in naturally occurring substances. Peptide self-assembly involves a complex interaction of forces, including hydrogen bonding, ionic forces, hydrophobic forces, van der Waals interactions, and electrostatic forces. 4 Proposed uses for these peptides include drug delivery, scaffold sheaths for neural redevelopment, and, importantly for surgeons, hemostasis.
Multiple different types of self-assembling peptides and their formulations have been described. The feature common to all is that they comprise a dissolvable L-amino acid chain that does not interfere with cellular pathways or promote or inhibit signaling and that is broken down via the body's natural peptidases and enzymes to constituent amino acids after its primary role has been performed. 5 These peptides have been used as hemostatic agents, 5 6 as an adjunct to ophthalmologic surgeries (e.g., corneal endothelial stem cell transplantation) and, intriguingly, as a self-assembling peptide to provide a scaffold or bridge for axonal regeneration in optic nerve reconnection. 1 Theories have also been advanced in the field of oncology that nanostructures could change the extracellular matrix that facilitates cancer metastasis and ensure malignancy remains localized. 7 A peptide chain self-assembled into a β-pleated sheet named RADA-16 has been used to deliver epidermal growth factor, insulin-like growth factor, platelet-derived growth factor, and stromal cell-derived growth factor-1 to postinfarction myocardium to increase the rate of wound healing. 4 The advantage to this method of drug delivery is that amalgamation into a protein sheet renders the drug less susceptible to metabolic processes within the body and allows it to be delivered unaltered to the site of action. 8 Ellis-Behnke and colleagues have also demonstrated the ability to use these nano-fiber scaffolds to repair hamster brain lesions by allowing nerve fibers to reconnect postseverance. 9 10 This is analogous to the role played by myelin sheaths in neural protection and redirection. 10
Self-Assembling Peptides as Nano-hemostats
Coagulation and subsequent hemostasis depends on multiple factors. These include the endothelial cells of blood vessels, platelets, leukocytes, the coagulation cascade, and the milieu of temperature and blood pH. There are also multiple anticoagulant forces and inhibitors. The precipitating event for nonpathologic coagulation initiation is vessel wall damage. This causes activation of endothelial cells and subsequent platelet activation. Platelets roll along the damaged cells and, using von Willebrand factor adhesion to bind to newly exposed subendothelial cells, aggregate in platelet clumps. Post this event, a mesh of platelets and trapped leukocytes form a scaffold for development of a fibrin clot as part of the coagulation pathway. 11 12 13
Several studies have examined the role that these self-assembling peptides may play in hemostasis. 5 6 14 There are a number demonstrating almost immediate hemostasis in small animal models without activation of the coagulation cascade. This is thought to occur as the nano-hemostat scaffold acts in an analogous fashion to fibrin clot, forming a barrier that both holds platelets in situ and provides an activation stimulus, leading to further aggregation. This has been demonstrated on the mammalian brain, spinal cord, liver, kidney, femoral artery, and skin. 5 6 15 16 The peptide solutions have been shown to breakdown via natural enzymatic processes and are nonimmunogenic and nontoxic. 1 In addition, the breakdown products are amino acids that may be used for subsequent tissue repair. 1 5
Nano-hemostat is a particularly attractive concept in skull base surgery, especially when performed endoscopically. Surgeons are constrained by relatively narrow access corridors that are in close proximity to major vascular structures such as the internal carotid artery, anterior cerebral arteries, and basilar artery and venous structures such as the cavernous sinus. 17 18 Injury to these structures carries the potential for devastating and potentially fatal bleeding. 17 18 19 20 21 22 23 24 Much work has been performed to determine the safest way to halt bleeding in this environment and the safest way to train surgeons to operate in this area. 25 26 27 28 29 In this situation, the ideal hemostatic agent should be able to conform to irregular cavities, to not obstruct the surgical field, and to not damage surrounding structures, either through thermal or chemical injury or through expansion and pressure injury to nerves or vessels. 30 31 32 Current methods of hemostasis used in major vessel injury to the skull base include muscle patches, 25 26 27 28 33 Floseal (Baxter Healthcare, Hayward, California, United States), Surgiflo with thrombin (Ethicon, Somerville, New Jersey, United Stated), Tisseel (Baxter Healthcare), 11 34 and hemostatic clips. 33 35 While these are efficacious to varying degrees, they all have their limitations. There can be some delay in harvesting a muscle patch, and it is partially dependent on platelet activation as part of its mechanism of action. 33 Floseal and Tisseel have been shown to wash out of the surgical field rapidly in the case of torrential hemorrhage. 25 26 27 28 Hemostatic clips can be difficult to apply in situations with a tear in an artery at the lateral extremity of the surgical field. 35
Nano-hemostats in Animal Models
d-EAK16
Evidence to support the hypothesis that nano-peptides in hemostasis provide more than a simple physical barrier comes from studies looking at peptide d-EAK16 by Luo et al. 14 Self-assembly of d-EAK16 was timed in a pure water solution and in a living animal model (rabbit liver). It took approximately 16 hours for d-EAK16 to self-assemble in water but approximately 20 seconds to assemble in whole blood. The time to hemostasis in this d-EAK16 group was significantly lower when compared with controls (no treatment) (20 vs. 80–120 seconds). The authors conclude that ions, proteins, enzymes, and “other factors” in blood work cooperatively to cause hemostasis. Their rationale is that normal hemostasis occurs when there is a high concentration of clotting factors held within a localized area whereas the same gross number of coagulation components would be ineffective if spread out over a larger area. d-EAK16 is thought to be triggered by sodium, magnesium, potassium, calcium, iron, and zinc within the circulating plasma to form a stable β-pleated sheet in which platelets and subsequently endogenous coagulation factors become trapped and undergo activation in the usual fashion. This β-pleated sheets then combine to form first short and then long nano-fibers that may then form “tight junctions” impervious to liquids. These peptide chains have been shown to work best as a hemostatic agent when comprised of amino acids of the same chirality. Alternating chirality gave bleeding times up to eight times longer in a rat liver hemorrhage model. 14
AC5
AC5 is a proprietary, synthetic nano-hemostat formulation developed by Ellis-Behnke and colleagues, which is currently in preclinical trials. 6 It addresses the potential for hemostasis in the context of anticoagulation. 6 In a noncompressible, full-thickness 4-mm liver punch biopsy model in rats, AC5 achieved hemostasis equivalently in heparinized and nonheparinized rats with a 94% reduction in time to hemostasis compared with saline controls. 6
RADA-16
RADA-16 is a self-assembling peptide of ionic hydrophilic and hydrophobic amino acids. It is 5 nm in length and forms stable β-pleated sheet structures that turn into hydrogels. 2 Sang et al performed a study in which they divided 20 rats in four groups: intracerebral hemorrhage (ICH) without aspiration, ICH with aspiration, ICH with aspiration and saline, and ICH with aspiration and RADA-16 injection. ICH was created via intrastriatal injection of collagenase. After 210 minutes, the hematoma was aspirated and 20 µL of saline or RADA-16 was injected into the ICH cavity. Differences in hematoma volume in aspiration versus saline versus RADA-16 group were not significant. Aspiration significantly reduced inflammatory cells in surrounding cortex on histologic examination. The authors concluded that self-assembling peptide attenuated brain injury; enhanced functional recovery; and reduced cavity volume with a reduction in brain edema, peri-hematoma apoptosis, and inflammatory reaction. 2 This reduction in secondary brain injury is thought to come about by decreasing the leakage of serum proteins into the surrounding parenchyma. Histology showed tight contact between RADA-16 and the cavity wall. 2 More work is required to validate this concept in both the acute and chronic phases of brain inflammatory reactions.
Possible Limitations
While nano-hemostats have been proven to be successful in a small animal model of vessel injury, this may not hold true in larger animal/human models where pressures may be many times that of smaller animals. 6 34 36 37 This pressure may wash the hemostat away from its site of action before it can take effect. Further, the liquid may need to be either impregnated in or held on by a patty or similar. This may remove the advantage of having a clear liquid hemostat—at least in the immediate phase of application. 38 While this has not been seen in nano-hemostats to date, a buildup of abnormal proteins within the central nervous system is the basis of conditions such as dementias and prion diseases. It would be incumbent upon designers of nano-hemostats to ensure that their solution is adequately broken down by the body's enzymatic processes and is not trapped in insoluble form within neurons or astrocytes.
Pilot Study of Nano-hemostat on an Animal Model of Major Vessel Injury Relevant to Skull Base Surgery
Aim
The aim is to determine the feasibility of a nano-hemostat solution in stopping bleeding from major arterial and venous sources and its ease of use in an endoscopic setting.
The peptide chosen was RADA-16 (Arg - Ala - Asp - Ala - Arg - Ala - Asp - Ala - Arg - Ala - Asp - Ala - Arg - Ala - Asp - Ala -NH 2 ), which is known for its propensity to self-assemble and has been used as a nano-hemostat in small animal models. 5 8 16 39 This was sourced from China Peptides as a 70% pure powder.
Method
Ethics approval was obtained from the Animal Ethics Committee of the South Australian Health and Medical Research Institute. Two merino sheep were anaesthetized and placed supine. A midline neck dissection was performed, and left carotid artery was cannulated for an arterial line and the left internal jugular cannulated for a central venous catheter. The right carotid artery and right internal jugular vein were dissected free and a segment of at least 8 cm was cleared of surrounding fascia. An endoscopic training model was placed around the internal jugular. These silicon-based models re-create the sinonasal cavity with the vessel running through a groove at the base of the model. The sheep is then draped and provides a realistic representation of endoscopic surgery ( Fig. 1A, B ).
Fig. 1.
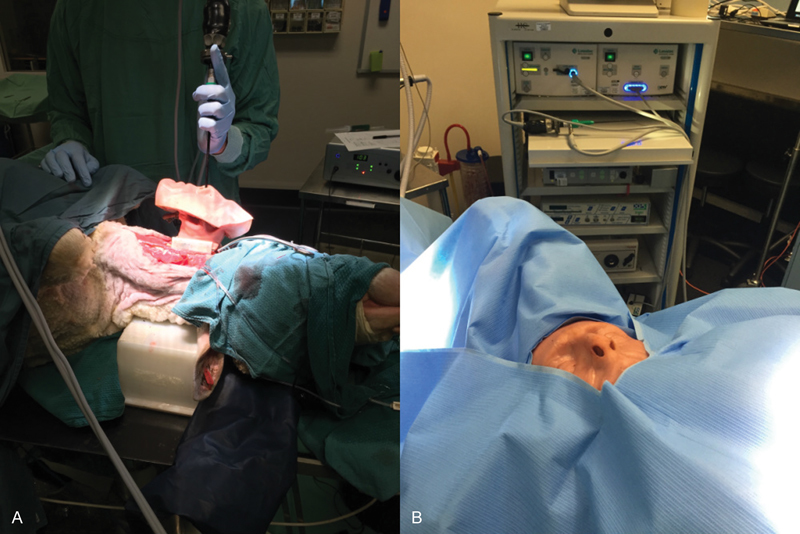
Endoscopic trainer model (A) placed on sheep's carotid artery and (B) draped and ready for use.
RADA-16 nano-hemostat was mixed with saline in a 10% mixture forming a gel at room temperature. This was drawn up into a 10-mL syringe and a blunt cannula attached. Using a 19-gauge needle, a hole was made in the internal jugular and blood allowed to escape for 20 seconds ( Fig. 2A ). RADA-16 was then applied to the jugular injury at the rate of 1 mL/s ( Fig. 2B ). While it slowed the bleeding and provided a semitranslucent membrane over the injury, blood could still be seen escaping from the hole in the jugular vein underneath this for a period of 15 seconds before hemostasis occurred. A proximal injury was subsequently made in the same animals as an untreated control that continued to bleed for 5 minutes until RADA-16 was applied and hemostasis occurred at approximately 15 seconds. The nano-hemostat did not appear to set in a solid fashion but remained as a jelly-like substance when observed for 20 minutes.
Fig. 2.
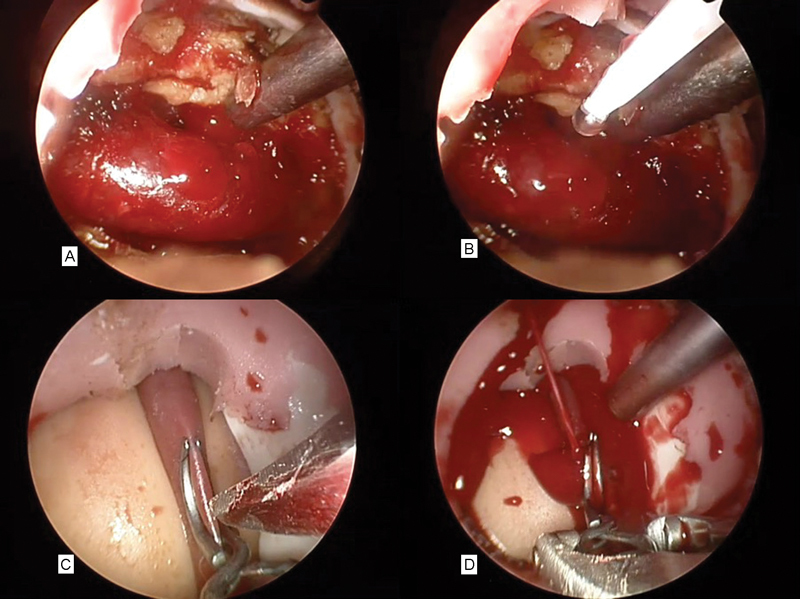
(A) Bleeding from the jugular vein. (B) RADA-16 being applied to the jugular vein. (C) Aneurysm clip across carotid artery and scalpel making linear incision. (D) Arterial hemorrhage from carotid injury.
The endoscopic trainer was then applied to the carotid artery of the same sheep. A curved aneurysm clip was placed half way across the carotid artery and a 4-mm linear incision was made in the wall of carotid artery ( Fig. 2C ). The aneurysm clip was removed and the injury allowed to bleed for 3 seconds ( Fig. 2D ). A sucker was used to control the field, and an identical mix of 10 mL of RADA nano-hemostat was immediately applied to the injury. There was no discernable decrease in the bleeding rate. RADA-16 appeared to be washed away by the pressure of the bleeding (systolic range: 70–90 mm Hg). We were unable to control the bleeding and after a period of 90 seconds were forced to use a crushed muscle patch to stop the bleeding that worked to good effect as previously reported. 25 26 27 28 This was repeated in a second sheep with identical results. Sheep were then humanely killed. The jugular and carotid were dissected out and placed in formalin and scanning electron microscopy (SEM) fixative. A small amount of muscle was also covered in nano-hemostat postmortem and sent for histology and SEM. SEM was performed postpreparation and coating of the samples in platinum. The nano-hemostat could be seen coating the muscle patch and forming a “glue” or matrix with long scaffolds or fibers with a width of 30 to 70 nm. Erythrocytes could be seen trapped within this matrix ( Fig. 3 ).
Fig. 3.
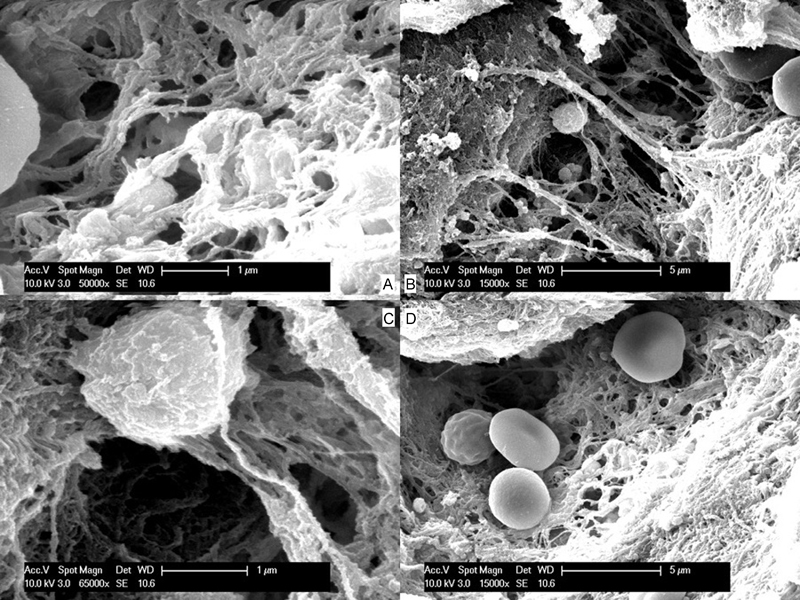
(A) Scanning electron microscope view of RADA-16 scaffold. (B–D) Scanning electron microscope views of erythrocytes and platelets trapped in scaffold of fibrin and RADA-16.
Discussion
This pilot study demonstrates some of the inherent limitations of gels or liquids in high-flow, high-pressure bleeding. While the bleeding from the jugular vein injury could be stopped with this nano-hemostat mixture, this is a comparatively low-flow, low-pressure injury. The carotid artery injury was unable to be controlled with the nano-hemostat, and eventual control was achieved with the conventional option of crushed muscle patch.
There are several questions raised by this small pilot study that may warrant further investigation. The literature is not clear regarding the percentage strength of solution used in previous nano-hemostat studies utilizing RADA-16. 2 5 15 40 41 While these have stopped bleeding in mouse models, this may simply be a reflection of the lower pressure and lower volume of flows in these injuries. The ideal percentage is not yet clear. Our 10% solution provided a solution viscous enough to adhere to the side of the plastic syringe. This was chosen because we were interested in its ability to be applied in an endoscopic scenario, and it appeared to provide a balance between applicability and viscosity.
This pilot study appears to add weight to previously published studies that have described success in bleeding control of small vessels in small animal models using nano-hemostat. 5 15 40 41 Certainly it appeared to have some success controlling a small jugular vein puncture. There may be future avenues worth exploring regarding the use of nano-hemostat in areas where visualization of the bleeding point would be useful such as a venous ooze from the lateral borders of the sphenoid corridor of a pituitary resection or in endoscopic resection of a skull base tumor. Current teaching involves packing these areas and moving the dissection to another region rather than laboriously obtaining control and drying the field with bipolar as this would add inordinate amounts of time to what is already a long operation. It would be advantageous to have a semitranslucent hemostat that would allow the surgeon to see the bleeding point underneath the hemostat rather than having to remove the pack and potentially remove the fibrin/platelet plug/clot in doing so, causing further bleeding.
This pilot study appears to show that nano-hemostat is unlikely to adequately control a high-flow, high-pressure bleed such as that from a carotid artery injury. SEM shows the nano-hemostat forming a matrix in which circulating erythrocytes and platelets are trapped (in much the same way as a fibrin plug is formed), but this only formed in the postmortem samples where there was obviously no pressure washing the nano-hemostat away. For this to work in vivo, the nana-hemostat would need to be held in situ for a prolonged period, possibly as part of a patch or pad. It would also be useful to test this nano-hemostat in a recovery model to determine whether pseudoaneurysms form and whether the scaffold holds for sufficient time for re-endothelialization to occur. Further research is needed to address the optimum formulation and indications for use of this potentially revolutionary new hemostatic agent.
Acknowledgments
This research was funded by the NeuroSurgical Research Foundation, Adelaide. The authors would like to thank Lynnette Waterhouse for assistance devising the scanning electron microscopy protocol to view the nano-hemostat.
References
- 1.Ellis-Behnke R, Jonas J B. Redefining tissue engineering for nanomedicine in ophthalmology. Acta Ophthalmol. 2011;89(02):e108–e114. doi: 10.1111/j.1755-3768.2010.01982.x. [DOI] [PubMed] [Google Scholar]
- 2.Sang L Y, Liang Y X, Li Y et al. A self-assembling nanomaterial reduces acute brain injury and enhances functional recovery in a rat model of intracerebral hemorrhage. Nanomedicine (Lond) 2015;11(03):611–620. doi: 10.1016/j.nano.2014.05.012. [DOI] [PubMed] [Google Scholar]
- 3.Hirst A R, Escuder B, Miravet J F, Smith D K. High-tech applications of self-assembling supramolecular nanostructured gel-phase materials: from regenerative medicine to electronic devices. Angew Chem Int Ed Engl. 2008;47(42):8002–8018. doi: 10.1002/anie.200800022. [DOI] [PubMed] [Google Scholar]
- 4.Loo Y, Zhang S, Hauser C A. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol Adv. 2012;30(03):593–603. doi: 10.1016/j.biotechadv.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Ellis-Behnke R. At the nanoscale: nanohemostat, a new class of hemostatic agent. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2011;3(01):70–78. doi: 10.1002/wnan.110. [DOI] [PubMed] [Google Scholar]
- 6.Csukas D, Urbanics R, Moritz A, Ellis-Behnke R. AC5 Surgical Hemostat™ as an effective hemostatic agent in an anticoagulated rat liver punch biopsy model. Nanomedicine (Lond) 2015;11(08):2025–2031. doi: 10.1016/j.nano.2015.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Ling P M, Cheung S W, Tay D K, Ellis-Behnke R G. Using self-assembled nanomaterials to inhibit the formation of metastatic cancer stem cell colonies in vitro. Cell Transplant. 2011;20(01):127–131. doi: 10.3727/096368910X532783. [DOI] [PubMed] [Google Scholar]
- 8.Khadka D B, Haynie D T. Protein- and peptide-based electrospun nanofibers in medical biomaterials. Nanomedicine (Lond) 2012;8(08):1242–1262. doi: 10.1016/j.nano.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 9.Cho Y, Borgens R B. Polymer and nano-technology applications for repair and reconstruction of the central nervous system. Exp Neurol. 2012;233(01):126–144. doi: 10.1016/j.expneurol.2011.09.028. [DOI] [PubMed] [Google Scholar]
- 10.GhoshMitra S, Diercks D R, Mills N C, Hynds D L, Ghosh S. Role of engineered nanocarriers for axon regeneration and guidance: current status and future trends. Adv Drug Deliv Rev. 2012;64(01):110–125. doi: 10.1016/j.addr.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 11.Yao H H, Hong M K, Drummond K J. Haemostasis in neurosurgery: what is the evidence for gelatin-thrombin matrix sealant? J Clin Neurosci. 2013;20(03):349–356. doi: 10.1016/j.jocn.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Geczy C L. Cellular mechanisms for the activation of blood coagulation. Int Rev Cytol. 1994;152:49–108. doi: 10.1016/s0074-7696(08)62554-1. [DOI] [PubMed] [Google Scholar]
- 13.Tynngård N, Lindahl T L, Ramström S. Assays of different aspects of haemostasis—what do they measure? Thromb J. 2015;13:8. doi: 10.1186/s12959-015-0036-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo Z, Wang S, Zhang S. Fabrication of self-assembling D-form peptide nanofiber scaffold d-EAK16 for rapid hemostasis. Biomaterials. 2011;32(08):2013–2020. doi: 10.1016/j.biomaterials.2010.11.049. [DOI] [PubMed] [Google Scholar]
- 15.Huri E, Beyazit Y, Mammadov R et al. Generation of chimeric “ABS nanohemostat” complex and comparing its histomorphological in vivo effects to the traditional ankaferd hemostat in controlled experimental partial nephrectomy model. Int J Biomater. 2013;2013:949460. doi: 10.1155/2013/949460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song H, Zhang L, Zhao X. Hemostatic efficacy of biological self-assembling peptide nanofibers in a rat kidney model. Macromol Biosci. 2010;10(01):33–39. doi: 10.1002/mabi.200900129. [DOI] [PubMed] [Google Scholar]
- 17.AlQahtani A, Castelnuovo P, Nicolai P, Prevedello D M, Locatelli D, Carrau R L. Injury of the internal carotid artery during endoscopic skull base surgery: prevention and management protocol. Otolaryngol Clin North Am. 2016;49(01):237–252. doi: 10.1016/j.otc.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Biswas D, Daudia A, Jones N S, McConachie N S. Profuse epistaxis following sphenoid surgery: a ruptured carotid artery pseudoaneurysm and its management. J Laryngol Otol. 2009;123(06):692–694. doi: 10.1017/S0022215108002752. [DOI] [PubMed] [Google Scholar]
- 19.Berker M, Aghayev K, Saatci I, Palaoğlu S, Onerci M. Overview of vascular complications of pituitary surgery with special emphasis on unexpected abnormality. Pituitary. 2010;13(02):160–167. doi: 10.1007/s11102-009-0198-7. [DOI] [PubMed] [Google Scholar]
- 20.Davies A, Dale O, Renowden S. Spontaneous rupture of an intra-cavernous internal carotid artery aneurysm presenting with massive epistaxis. J Laryngol Otol. 2011;125(10):1070–1072. doi: 10.1017/S0022215111002040. [DOI] [PubMed] [Google Scholar]
- 21.Gardner P A, Tormenti M J, Pant H, Fernandez-Miranda J C, Snyderman C H, Horowitz M B.Carotid artery injury during endoscopic endonasal skull base surgery: incidence and outcomes Neurosurgery 201373, (2 Suppl Operative)ons261–ons269., discussion ons269–ons270 [DOI] [PubMed] [Google Scholar]
- 22.Gardner P A, Vaz-Guimaraes F, Jankowitz B et al. Endoscopic endonasal clipping of intracranial aneurysms: surgical technique and results. World Neurosurg. 2015;84(05):1380–1393. doi: 10.1016/j.wneu.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 23.Griauzde J, Gemmete J J, Pandey A S, McKean E L, Sullivan S E, Chaudhary N. Emergency reconstructive endovascular management of intraoperative complications involving the internal carotid artery from trans-sphenoidal surgery. J Neurointerv Surg. 2015;7(01):67–71. doi: 10.1136/neurintsurg-2013-010878. [DOI] [PubMed] [Google Scholar]
- 24.Inamasu J, Guiot B H.Iatrogenic carotid artery injury in neurosurgery Neurosurg Rev 20052804239–247., discussion 248 [DOI] [PubMed] [Google Scholar]
- 25.Valentine R, Boase S, Jervis-Bardy J, Dones Cabral J D, Robinson S, Wormald P J. The efficacy of hemostatic techniques in the sheep model of carotid artery injury. Int Forum Allergy Rhinol. 2011;1(02):118–122. doi: 10.1002/alr.20033. [DOI] [PubMed] [Google Scholar]
- 26.Valentine R, Wormald P J. A vascular catastrophe during endonasal surgery: an endoscopic sheep model. Skull Base. 2011;21(02):109–114. doi: 10.1055/s-0031-1275255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Valentine R, Wormald P J. Controlling the surgical field during a large endoscopic vascular injury. Laryngoscope. 2011;121(03):562–566. doi: 10.1002/lary.21361. [DOI] [PubMed] [Google Scholar]
- 28.Valentine R, Wormald P J. Carotid artery injury after endonasal surgery. Otolaryngol Clin North Am. 2011;44(05):1059–1079. doi: 10.1016/j.otc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Solares C A, Ong Y K, Carrau R L et al. Prevention and management of vascular injuries in endoscopic surgery of the sinonasal tract and skull base. Otolaryngol Clin North Am. 2010;43(04):817–825. doi: 10.1016/j.otc.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 30.Arnaud F, Parreño-Sadalan D, Tomori T et al. Comparison of 10 hemostatic dressings in a groin transection model in swine. J Trauma. 2009;67(04):848–855. doi: 10.1097/TA.0b013e3181b2897f. [DOI] [PubMed] [Google Scholar]
- 31.Kessler C M, Ortel T L. Recent developments in topical thrombins. Thromb Haemost. 2009;102(01):15–24. doi: 10.1160/TH09-01-0034. [DOI] [PubMed] [Google Scholar]
- 32.Tomizawa Y. Clinical benefits and risk analysis of topical hemostats: a review. J Artif Organs. 2005;8(03):137–142. doi: 10.1007/s10047-005-0296-x. [DOI] [PubMed] [Google Scholar]
- 33.Padhye V, Valentine R, Paramasivan S et al. Early and late complications of endoscopic hemostatic techniques following different carotid artery injury characteristics. Int Forum Allergy Rhinol. 2014;4(08):651–657. doi: 10.1002/alr.21326. [DOI] [PubMed] [Google Scholar]
- 34.Dyer S R, Bathula S, Durvasula P et al. Intraoperative use of FloSeal with adenotonsillectomy to prevent adverse postoperative outcomes in pediatric patients. Otolaryngol Head Neck Surg. 2013;149(02):312–317. doi: 10.1177/0194599813486253. [DOI] [PubMed] [Google Scholar]
- 35.Padhye V, Murphy J, Bassiouni A, Valentine R, Wormald P J. Endoscopic direct vessel closure in carotid artery injury. Int Forum Allergy Rhinol. 2015;5(03):253–257. doi: 10.1002/alr.21453. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed E M. Hydrogel: preparation, characterization, and applications: a review. J Adv Res. 2015;6(02):105–121. doi: 10.1016/j.jare.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazzeri R, Galarza M, Fiore C, Callovini G, Alfieri A.Use of tissue-glue-coated collagen sponge (TachoSil) to repair minor cerebral dural venous sinus lacerations: technical note Neurosurgery 2015110232–36., discussion 36 [DOI] [PubMed] [Google Scholar]
- 38.Ellis-Behnke R G, Tay D KC, Liang Y X, Zhang S, Schneider G E, So K F. Crystal clear surgery with self-assembling molecules that act as a bio barrier in the brain and intestine (Abstract) Nanomedicine. 2005;1(03):269–270. [Google Scholar]
- 39.Paradís-Bas M, Tulla-Puche J, Zompra A A, Albericio F. RADA-16: a tough peptide—strategies for synthesis and purification. Eur J Org Chem. 2013;26:5871–5878. [Google Scholar]
- 40.Ellis-Behnke R G, Liang Y X, Tay D K et al. Nano hemostat solution: immediate hemostasis at the nanoscale. Nanomedicine (Lond) 2006;2(04):207–215. doi: 10.1016/j.nano.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 41.Heller M, Wei C. Self-assembly peptide prevents blood loss. Nanomedicine (Lond) 2006;2(04):216. doi: 10.1016/j.nano.2006.10.158. [DOI] [PubMed] [Google Scholar]


